Abstract
PURPOSE
The objective of this study was to evaluate the effect of thickness reduction and fatigue on the failure load of monolithic zirconia crowns.
MATERIALS AND METHODS
140 CAD-CAM fabricated crowns (3Y-TZP, inCorisTZI, Dentsply-Sirona) with different ceramic thicknesses (2.0, 1.5, 1.0, 0.8, 0.5 mm, respectively, named G2, G1.5, G1, G0.8, and G0.5) were investigated. Dies of a mandibular first molar were made of composite resin. The zirconia crowns were luted with a resin composite cement (RelyX Unicem 2 Automix, 3M ESPE). Half of the specimens (n = 14 per group) were mouth-motion-fatigued (1.2 million cycles, 1.6 Hz, 200 N/ 5 – 55℃, groups named G2-F, G1.5-F, G1-F, G0.8-F, and G0.5-F). Single-load to failure was performed using a universal testing-machine. Fracture modes were analyzed. Data were statistically analyzed using a Weibull 2-parameter distribution (90% CI) to determine the characteristic strength and Weibull modulus differences among the groups.
RESULTS
Three crowns (21%) of G0.8 and five crowns (36%) of G0.5 showed cracks after fatigue. Characteristic strength was the highest for G2, followed by G1.5. Intermediate values were observed for G1 and G1-F, followed by significantly lower values for G0.8, G0.8-F, and G0.5, and the lowest for G0.5-F. Weibull modulus was the lowest for G0.8, intermediate for G0.8-F and G0.5, and significantly higher for the remaining groups. Fatigue only affected G0.5-F.
CONCLUSION
Reduced crown thickness lead to reduced characteristic strength, even under failure loads that exceed physiological chewing forces. Fatigue significantly reduced the failure load of 0.5 mm monolithic 3Y-TZP crowns.
Keywords: Ceramics, Zirconium, Monolithic crowns, Fatigue, Computer-aided design
INTRODUCTION
Nowadays, the fabrication of single crowns is the most common restorative procedure in the U.S.1 According to a current report, the global market of dental crowns and bridges will further increase at a compound annual growth rate (CAGR) of 7.78% to USD 3.8 billion in 2026.2 In recent years, monolithic restorations have gained popularity due to a reduction of technical complications, such as chipping fractures and a lower price, due to a simple fabrication process compared to layered restorations.3,4,5 At this, zirconia and lithium disilicate have become the materials of choice for monolithic applications.6 With recent developments in increasing the translucency of zirconia, its indications expanded to the anterior dentition.7 As the strongest of all dental ceramics,8 zirconia is recommended in stress-bearing areas and allows for non-adhesive cementation.6 Literature shows that monolithic zirconia crowns exhibit considerably higher fracture loads than veneered counterparts and lithium disilicate crown restorations.9,10,11 For veneered zirconia single crowns, a decreased success rate between 5 and 10 years has been reported.4,12 In accordance with previous investigations,13,14 chipping of the veneering ceramic was mentioned to be the most frequent clinical complication.4 One explanation may be related to residual stress within the veneer layer after the cooling procedure, resulting from the low thermal diffusivity of the Y-TZP framework.15,16 Furthermore, a missing cusp supporting anatomical design and parafunctional occlusal forces were mentioned as possible reasons.12 In terms of survival rates, no statistically significant differences between veneered zirconia and metal-ceramic restorations could be observed.4,17 However, significant biological complications following more invasive preparation techniques, especially the loss of abutment tooth vitality, were reported for metal-ceramic crowns.4 Due to a superior fracture strength of zirconia,9,11,18,19 monolithic restorations can be fabricated with reduced layer thicknesses and thus potentially prevent biological complications. Yet, no long-term studies on monolithic zirconia restorations are available. A 3-year short-term study20 recorded survival-rates of 100% for monolithic zirconia single crowns with a minimum occlusal thickness of 0.5 mm. Other studies reported survival rates of 91.5% after 3.5 years21 and 98% after 5 years22 for monolithic zirconia crowns with an occlusal thickness between 0.5 and 1 mm. Whereas some literature is available on reduced thicknesses of 3Y-TZP ceramics,23,24,25,26 the effect of fatigue has still not been extensively addressed.
Zirconia materials appear susceptible to bulk fracture in thin areas.24,26 Consequently, there is still no consensus on how thin restorations can be fabricated,25 and the impact of thermomechanical fatigue on the mechanical properties of thin monolithic zirconia crowns warrants further research. Therefore, the aim of the present study was to evaluate the effect of thickness reduction on the failure load and 5-year in-vitro survival rate of monolithic 3Y-TZP zirconia crowns. The tested null hypotheses were that (i) ceramic layer thickness and (ii) fatigue application do not influence the failure load of posterior 3Y-TZP monolithic zirconia crowns.
MATERIALS AND METHODS
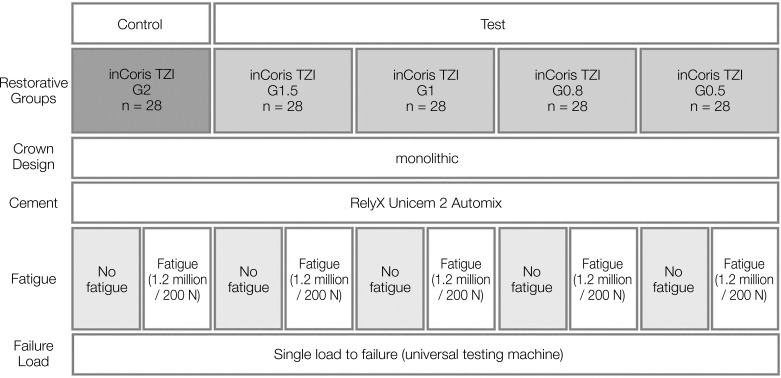
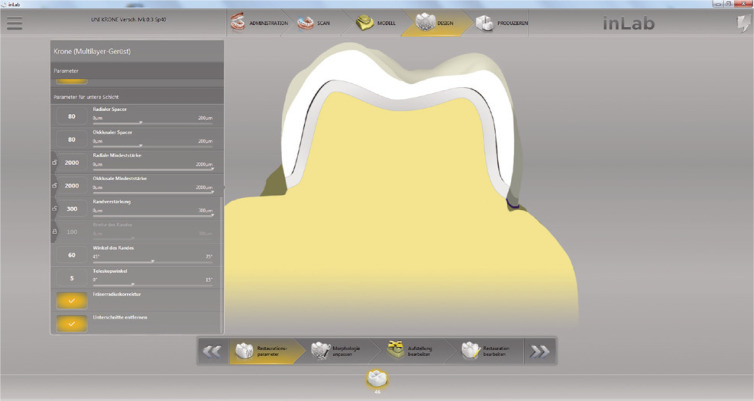
In this in-vitro study a total number of 112 crowns were divided into four groups (n = 28 per group) with different ceramic layer thicknesses (1.5 mm (G1.5), 1.0 mm (G1), 0.8 mm (G0.8), 0.5 mm (G0.5)) (Fig. 1). 28 crowns with a 2.0 mm (G2) ceramic thickness served as control. This control group was already investigated in an earlier study.27 Monolithic crowns of a first mandibular molar (tooth 46 FDI) were fabricated out of a 3Y-TZP zirconia ceramic with a flexural strength of > 900 MPa (inCorisTZI, Dentsply Sirona, Bensheim, Germany).28 All zirconia crowns were designed with a CAD-CAM software (Cerec InLab 4.0, Dentsply Sirona) and a multilayer design was chosen in order to create a separate abutment die and a monolithic crown restoration. By changing the configuration parameters (Fig. 2), the dimension of the die was adjusted depending on the layer thickness of the respective crown, while the occlusal morphology of the crown restoration remained identical. After milling, (inLab MC X5, Dentsply Sirona) crowns were dyed (inCoris TZI Coloring Liquids, Dentsply Sirona) and sintered (inFire HTC speed, Dentsply Sirona) according to manufacturer’s recommendations. A conventional sintering procedure was used at 1510℃ with a dwell time of 120 minutes.28 The initial heating rate was 25℃ per minute until a temperature of 800℃ was reached. Afterwards, the furnace was heated from 800℃ to 1510℃ with a heating rate of 15℃ per minute. For slow cooling, the furnace was programmed to remain closed up to a temperature of 200℃ using a cooling rate of 30℃ per minute. The glaze firing was carried out with a closing time of 4 minutes and a starting temperature of 400℃, followed by a temperature increase of 45℃ per minute until the final temperature of 870℃ was reached. This temperature was maintained for 1 minute, then cooled down to 600℃ until the furnace was opened.
Fig. 1. Test set-up.
Fig. 2. Definition of the different parameters. Yellow = die, grey = varying enlargement of the die, white = monolithic crown (Screenshot, provided by Dentsply Sirona).
112 composite resin master dies were made using a dentin-analog light-curing nanohybrid composite (Tetric Evo Ceram A2 Dentin, Ivoclar Vivadent AG, Schaan, FL; elastic modulus of 8.6 GPa, information provided by manufacturer). Therefore, the dies were duplicated and a plaster replica die was generated for each monolithic crown. Impressions of the plaster dies were made using a polyvinylsiloxane impression material (Affinis, Coltene AG, Altstätten, Switzerland). Subsequently, the negative form was filled up with 1.5 mm thick nanohybrid composite layers (Tetric Evo Ceram) and was light-cured (Polofil Lux, Voco, Cuxhaven, Germany) with approx. 780 mW/cm2 from all directions for 20 seconds.
The resin dies were stored in distilled water in an incubator (IN30, Memmert GmbH+Co KG, Schwabach, Germany) at 37℃ for a minimum of 30 days (mean: 31 days; min: 30 days; max 32 days). Afterwards, specimens were embedded in a cold polymerizing polyester resin (Technovit 4000, Heraeus Kulzer, Hanau, Germany). Resin dies were pretreated with pumice powder (Picodent, Wipperfürth, Germany), rinsed with water, dried, and cleaned with 60% ethanol. Prior to cementation, a layer of MDP-containing primer (Clearfil ceramic primer Plus, Kuraray Noritake, Tokyo, Japan) was applied to the internal surface of the zirconia crowns (no air-particle abrasion was employed). All crowns were then cemented with a resin composite cement (RelyX Unicem 2 Automix, 3M ESPE, Seefeld, Germany) according to manufacturer’s instructions. Excess cement was carefully removed with a scaler after light-curing of 2 seconds (Polofil Lux, Voco, Cuxhaven, Germany). Afterwards, each surface was light-cured for another 20 seconds at around 780 mW/cm2. Finally, all specimens were stored in distilled water for a minimum of 7 days (mean: 8 days; min: 7 days; max 8 days) to allow hydration of the resin cement.
Half of the specimens (G2-F, G1.5-F, G1-F, G0.8-F, G0.5-F) of each group were exposed to mouth-motion fatigue (-F) using a chewing-simulator (CS-4.8, SD Mechatronik, Feldkirchen-Westerham, Germany) with a load of 200 N at 1.6 Hz for 1.2 Mio cycles and simultaneous thermocycling (5℃ to 55℃, dwell time 120 s). This corresponds to a clinical ageing time of 5 years.29 Steatite spheres (Hoechst Ceram Tec, Wunsiedel, Germany) with a diameter of 6 mm served as antagonists. To simulate a lateral masticatory movement, the steatite spheres moved 0.5 mm horizontally from the distolingual cusp towards the central fissure. Subsequently, all crowns of each group underwent single load to failure (SLF) testing in a universal testing machine (Zwick Z010/TN2S, ZwickRoell GmbH & Co KG, Ulm, Germany). The load was applied axially with a metal sphere (r = 3.18 mm) towards the central fossa. The force was applied parallel to the longitudinal axis of the specimen at a test speed of 1.5 mm/min. Failure loads were recorded with a computer software (testXpert II V7.1, ZwickRoell) in Newton [N] for statistical analysis. Finally, all specimens were visually evaluated for failure analysis using a stereo light microscope (SZH 10, Olympus, Hamburg, Germany).
Reliability demonstration test (RDT tool, Weibull++v.21, Reliasoft, Tucson, AZ, USA) was used to determine sample size, considering mean time to failure of 3000 N (estimated average load to failure range for groups between 0.5 mm to 2 mm thicknesses), under 90% confidence bounds, using Weibull 2-parameter distribution, assuming Beta value = 5 (estimated average Beta), resulting in a sample size of 11. To account for potential fractures during fatigue, 3 additional samples per group were used, totaling 14 samples per group. Data were statistically analyzed using a Weibull 2-parameter distribution (Weibull++v.21, Reliasoft, Tucson, AZ, USA) (90% CI) to determine the characteristic strength (N) and Weibull modulus (m) differences among groups. Differences among groups were also depicted in a contour plot (characteristic strength vs. Weibull modulus). The probability of survival (reliability) was also plotted to show group survival distribution as a function of load to failure.
RESULTS
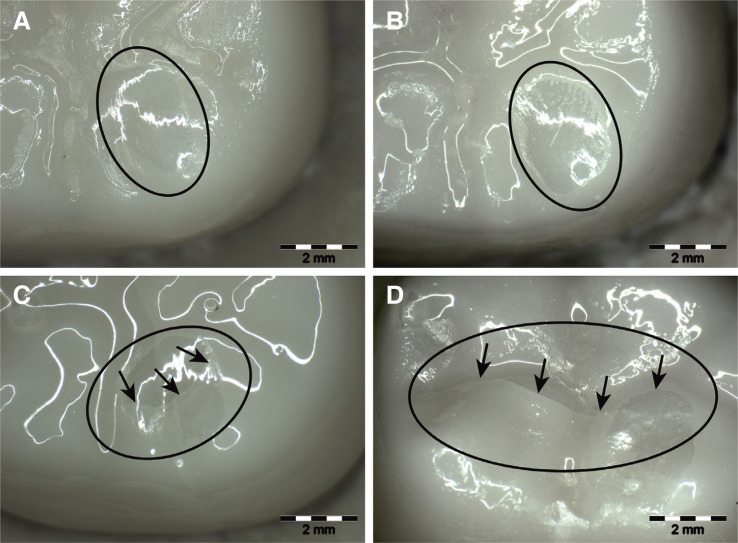
All crowns of G2, G1.5, and G1 presented no failures after 1.2 million fatigue cycles, which corresponds to a clinical 5-year survival.29,30 Three crowns (21%) of G0.8 and five crowns (36%) of G0.5 showed cracks after fatigue. Due to the limited extension of these cracks, all crowns were still exposed to single load to failure (SLF). The observed cracks of G0.8-F and G0.5-F were located in the area of the distolingual cusp, which corresponds with the lateral sliding movement from the distolingual cusp to the central fossa (Fig. 3). After fatigue exposure, all crowns showed superficial wear facets of the glazing material at the distolingual cusp (Fig. 3).
Fig. 3. Monolithic zirconia crowns with different layer thicknesses after loading (1.5 × magnification). The circle shows the attrition caused by the antagonists during fatigue. The arrows mark the cracks. (A) G1.5-F, (B) G1-F, (C) G0.8-F, (D) G0.5-F. Cracks were found in groups 0.8-F and 0.5-F.
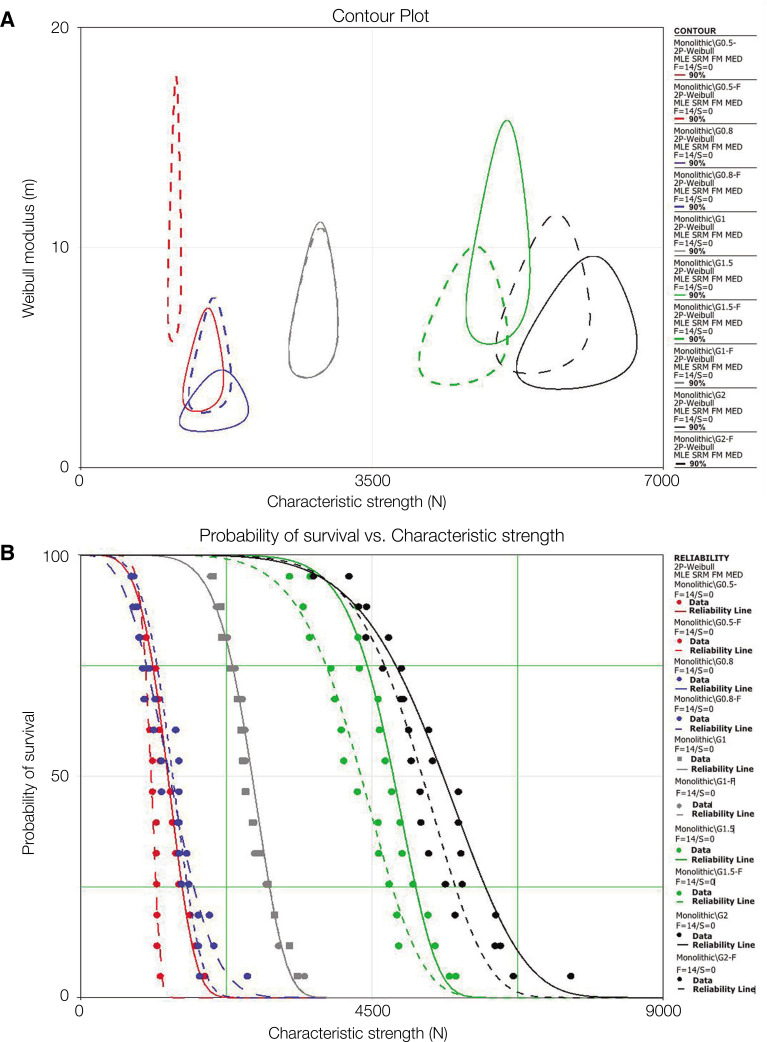
The characteristic strength and Weibull modulus results with differences among groups, based on overlap between upper and lower confidence intervals, are presented in Table 1. Characteristic strength (N) was the highest for G2 compared to G1.5 with significant differences between them. Although decreased characteristic strength values were observed after fatigue in G2-F and G1.5-F, they were not significantly different when compared with their respective non-fatigued counterparts. Intermediate values were observed for G1 and G1-F, followed by significantly lower values for G0.8, G0.8-F, and G0.5, and the lowest for G0.5-F. Weibull modulus was the lowest for G0.8, intermediate for G0.8-F and G0.5, and significantly higher for the remaining groups. Different superscript letters indicate significant differences among groups. Fatigue did not have a statistically significant influence, except for G0.5 (Table 1). A contour plot (Fig. 4A) is presented as an instructive graphical method to depict characteristic strength vs. Weibull modulus significant differences between datasets (no overlap of confidence bounds). Each contoured region represents possible values given both parameter’s combination, and difference at 90% level is detected if contour overlap between groups does not exist.31 Figure 4B depicts the probability of survival (reliability) distribution as a function of characteristic strength.
Table 1. Characteristic strength (N) and Weibull modulus (m) for the different groups. Different superscript letters indicate significant differences.
| G2 | G2-F | G1.5 | G1.5-F | G1 | G1-F | G0.8 | G0.8-F | G0.5 | G0.5-F | |
|---|---|---|---|---|---|---|---|---|---|---|
| upper limit | 6389.9 | 5895.6 | 5244.9 | 4911.7 | 2969.7 | 2972.89 | 1827.4 | 1691.66 | 1609.35 | 1170.87 |
| Characteristic strength (N) | 5934.2A | 5540.2A,B | 5008.3B,C | 4576.6C | 2783.4D | 2785.16D | 1555.18E | 1533.08E | 1454.88E | 1121.81F |
| lower limit | 5510.9 | 5206.3 | 4782.3 | 4264.3 | 2608.7 | 2609.27 | 1323.5 | 1389.36 | 1315.23 | 1074.81 |
| upper limit | 8.7 | 10.3 | 14.1 | 9 | 10 | 9.84 | 4 | 6.79 | 6.48 | 15.6 |
| Weibull modulus (m) | 6.3A,B | 7.5A,B | 10A | 6.5A,B | 7.2A,B | 7.15A,B | 2.89C | 4.68B | 4.59B | 10.7A |
| lower limit | 4.5 | 5.4 | 7.1 | 4.8 | 5.17 | 5.19 | 2 | 3.22 | 3.26 | 7.39 |
Fig. 4. (A) Two-parameter Weibull contour plot (Weibull modulus [m]) vs. characteristic strength [N] for G2 (black), G1.5 (green) G1.0 (grey), G0.8 (blue) and G0.5 (red) in which solid contour lines represent non-fatigued, and dotted lines represent groups subjected to fatigue prior to single load to failure test. The only group showing no overlap between contours before and after fatigue is G0.5, meaning that fatigue significantly decreased its characteristic strength. (B) Probability of survival vs. characteristic strength shows a general trend in crown survival increase for increased thickness. G1 and G1-F (grey solid and dotted reliability lines, respectively) present an overlap due to similar sample failure distribution.
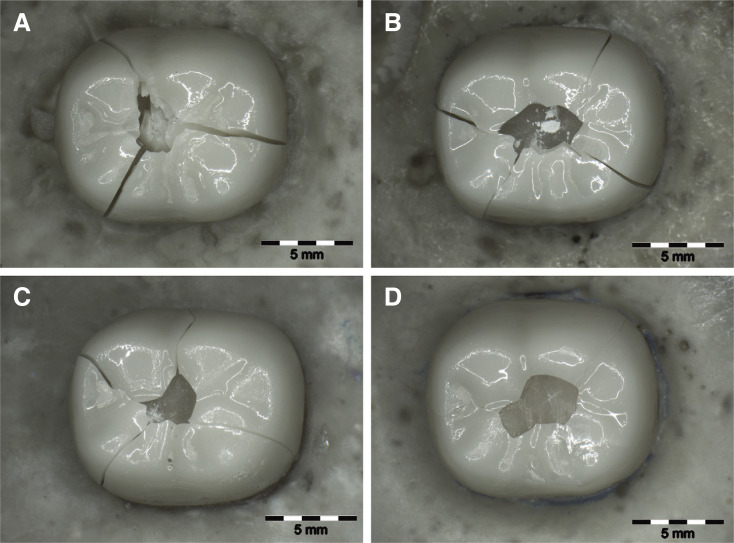
After SLF, all crowns showed catastrophic bulk fractures. Crowns fractured mostly into 2 – 5 main fragments that could be repositioned. No differences could be observed between the individual groups. However, failure analysis revealed that cracks propagated from the occlusal loading point to the cervical crown margin (Fig. 5). The crack origin was mainly located in the distolingual region of the occlusal surface initiating from the area of loading. Fractures within the die material only occurred in groups with a layer thickness of 1.0 mm (G1:0%, G1-F:7.14%), 1.5 mm (G1.5:42.85%, G1.5-F:28.57%) and 2.0 mm (G2:100%, G2-F:100%).
Fig. 5. Monolithic zirconia crowns after single load to failure (1.5 × magnification). (A) G1.5, (B) G1, (C) G0.8, (D) G0.5.
DISCUSSION
The present study showed that all tested crowns withstood failure loads above physiological chewing forces (50 – 250 N physiological, 500 – 900 N parafunctional).26,32 Nevertheless, cracks during fatigue were observed in G0.8 and G0.5. Therefore, the tested null hypothesis was rejected as layer thickness and fatigue application (G0.5) did influence the failure load of posterior 3Y-TZP monolithic zirconia crowns. This can be explained due to the fact that five of the crowns (36%) with fatigue exposure showed cracks after chewing simulation causing a reduction in fracture resistance. It was observed that when the layer thickness was doubled from 0.5 mm to 1.0 mm and from 0.8 mm to 1.5 mm, characteristic strength values increased two- to threefold. Similar observations were made in other in vitro studies.11,26
Considering the significant decrease in characteristic strength observed for crowns of 0.8 mm compared to those of higher thickness and especially evident for the 0.5 mm group subjected to fatigue, it may be suggested that crown thicknesses ≥ 1 mm, supported by dentin, may be less prone to fracture. The clinical longevity in long-term remains uncertain. A reduced layer thickness of 1.0 mm seems to be the limit with no negative influence on the 5-year in vitro survival rate and should thus be preferred. For this reason, most manufacturers do not recommend to reduce the occlusal layer thickness of monolithic zirconia crowns below 1 mm in posterior areas.28,33,34 In contrast, some previous evidence suggests that it can be reduced up to 0.5 mm keeping a sufficient strength to endure maximum chewing forces up to 900 N.19,23,24,25
Previous studies investigated different layer thicknesses of 0.5 to 1.5 mm with different chewing loads and different numbers of masticatory cycles.10,23,26,35 Due to different experimental protocols and test setups, a direct comparison is difficult. Overall, thermomechanical cycling is the most commonly used method for fatigue testing.24,26,36 Some other studies only used mechanical cycling10 or omitted fatigue exposure before failure load testing.19,25 A previous study could show that the flexural strength of 3Y-TZP disc-shaped specimens was predominately affected by mechanical or thermomechanical cycling.37 Therefore, specimens were exposed to mechanical cycling (15,000,000 cycles/3.8 Hz/200 N), thermal cycling (6,000 cycles/5 – 55℃/30 s), and thermomechanical cycling (1.2 million cycles/3.8 Hz/200 N and thermal-cycling at a temperature range from 5℃ to 55℃ for 60 s each). In the present study, half of the specimens were subjected to a similar thermomechanical fatigue protocol (1.2 million cycles/1.6 Hz/ 200 N and thermal-cycling at temperature range from 5℃ to 55℃ for 60 s each).
Reported loads during clinical mastication vary considerably.32,38 As previously described, force peaks of 200 N in the anterior region, 350 N in the posterior, and 1000 N with bruxism occur.38 Many previous investigations used average chewing forces that were considerably lower (between 49 – 80 N) compared to the loads applied in the present test set-up.10,24,39 A recent study determined the test conditions in a preliminary pilot study and finally used a force of 200 N for preloading in a fatigue test set-up.36 In the present study, a chewing force of 200 N was also chosen, since it considers a certain safety load and seems to avoid either too low and too high loads.32,36
Characteristic strength values of this present study are similar to values detected in other studies: In a study investigating different layer thicknesses of monolithic 3Y-TZP zirconia crowns (Lava Frame, 3M ESPE), the following mean fracture load values were measured: 4109 ± 610 N (1.5 mm), 3068 ± 233 N (1.2 mm), 2429 ± 315 N (1.0 mm), 1814 ± 68 N (0.8 mm) and 1308 ± 111 N (0.6 mm).11 Comparing 3Y-TZP zirconia with different alumina contents, fracture load values varied from 450 to 3248 N (Lava Zirconia, 3M ESPE) and 438 to 3487 N for 3Y-TZP with a lower alumina content of 0.1% (Lava Plus, 3M ESPE).26 However, lower values of 691 – 2048 N for monolithic zirconia crowns with layer thicknesses of 2.0, 1.5, 1.0 and 0.5 mm25 and substantially higher values of 5558 ± 522 N (Lava Plus Zirconia, 3M ESPE) for crowns with an occlusal thickness of 0.5 mm19 were found in the literature. However, specimens were partly unexposed to fatigue.19,25 Since also the experimental set-up differs, a direct comparison is not possible. Another previous study compared monolithic 3Y-TZP zirconia crowns (Zikon Biostar HT, Siladent Dr. Böhme & Schöps GmbH, Goslar, Germany) with occlusal layer thicknesses of 0.5 and 0.2 mm and found similar failure load values for 0.5 mm thin crowns (1628 ± 174 N adhesively bonded, 1357 ± 340 N cemented).24
According to manufacturer’s recommendations, an adhesive cementation for 3Y-TZP zirconia crowns is not required, when a sufficient mechanical retention and crown thickness is given.28,40 However, composite resin cements or ceramic primer with special adhesive monomers, like MDP, are recommended for the bonding to zirconia.41 For this reason, an MDP-containing universal primer (Clearfil ceramic primer Plus, Kuraray Noritake) and a self-adhesive dual-curing composite luting cement (RelyX Unicem 2 Automix, 3M ESPE) were used in this study. But, cementation procedures are controversially discussed in the present literature.40,42
As a limitation of this in vitro study, it has to be stated that dentin-analog resin dies instead of extracted human molars were used, which do not exactly represent the structure of a real tooth. On the other hand, extracted teeth are difficult to standardize due to age and size difference.43 The applied composite resin reveals a similar elastic modulus (Tetric Evoceram: 8.6 GPa) compared to human dentin (10 – 16 GPa), and was also applied in comparable studies.15,19,26,44,45 A thermomechanical cycling protocol followed by a static test (SLF) was chosen. This commonly used method24,26,46 obtains reliable information and allows ranking of all-ceramic systems, according to given variables such as thicknesses.
Clinical failures are usually based on slow crack growth mechanisms,47 which lead to a continued reduction of initial strength over the years.48 Two important crack mechanisms for bulk fractures are reported: firstly, radial cracks caused by tensile stress propagating from the intaglio to the occlusal surface and secondly, cracks caused by contact stress at the occlusal surface propagating downwards.49 After SLF, the crack origins were mainly located at the distolingual area of the occlusal surface. This can be attributed to a change of the ceramic structure after fatigue exposure caused by the sliding movements at the distolingual cusp to the central fossa. A recently published clinical study found a grain transformation in the occlusal loading areas of monolithic zirconia crowns after two years in situ.50 In the present study, all crowns showed catastrophic bulk fractures after SLF. The observed cracks propagated from the occlusal loading point to the cervical crown margins. This fracture pattern initiating from the occlusal loading points has also been reported in clinical12,50 and in vitro studies.11,25,51
In terms of failure mode, no differences could be observed among the groups of this study. Interestingly, all dies of G0.5 and G0.8 remained intact, while the dies of G2, G1.5, and G1 showed fractures. In these groups, partial and longitudinal fractures occurred and a few dies even showed bulk/catastrophic failures. The simulated abutment teeth/ dies of G0.8 and G0.5 were thicker than the dies of G2, G1.5, and G1. This might explain why no die fractures occurred in G0.8 and G0.5. Furthermore, it should be considered that in the present experimental set-up, stiff zirconia specimens were loaded by using a force of 200 N. According to a previous investigation,52 the velocity of the weight and the resulting impulse depend on the stiffness of the specimen material. For stiff specimens, several impacts /‘bouncing’ were detected leading to substantial measurement errors. Therefore, low impact velocities are recommended, especially for stiff specimen loading, and were applied in the present study.52 Axial loading might produce several uncontrolled contact points.53 Therefore, other studies used a tin foil distributing the force equally to the cusps.9,11,19,39 Another investigation applied an inverse V-shaped two-plane intender for achieving multiple contacts more equally and in order to reproduce more clinically relevant fractures.54 Some authors suggest that an angulation of the specimens could better represent clinical scenarios, due to the more complex stress state caused by sliding actions.9,26 Other authors used an off-axis-loading of 30° for the investigation of flat specimens in order to simulate cusp inclination.55,56 However, in the present study, a sliding component was achieved by the lateral movement of the steatite sphere during fatigue testing.
In recent years, increased translucency of zirconia could be achieved by increasing the yttria content or the cubic phase (4Y-PSZ and 5Y-PSZ).7 Literature shows that these translucent zirconia materials are associated with lower mechanical properties because phase transformation toughening is absent due to predominance of cubic phase, which makes them more prone to failure.36,57,58,59 However, it is expected that in the future, zirconia materials will be further enhanced, regarding translucency, fluorescence, and strength. Further investigations on failure load, pretreatments, and thermomechanical longevity of 4Y- and 5Y-TZP crowns should be addressed in the future.
CONCLUSION
Within the limitations of this study, the following can be concluded: layer thickness has a statistically significant influence on the characteristic strength of 3Y-TZP monolithic zirconia crowns. All crowns with a layer thickness above 1.0 mm withstood a simulated 5-year clinical application without fracture. Yet, all groups achieved characteristic strength values that exceed physiological chewing forces. A preparation design resulting in a reduction for a ceramic layer thickness of 1.0 mm can be recommended. Further laboratory and clinical studies are necessary to confirm these results.
Footnotes
This study was supported by Dentsply Sirona.
References
- 1.The American College of Prosthodontics. Facts & Figures 2021. [Accessed May 6, 2021]. Available from: https://www.gotoapro.org/facts-figures/
- 2.Grand View Research Inc. Dental crowns & bridges market size worth $3.8 Billion By 2026 June 2019. [Accessed May 6, 2021]. Available from: https://www.grandviewresearch.com/press-release/global-dental-crowns-bridges-market .
- 3.Zhao K, Pan Y, Guess PC, Zhang XP, Swain MV. Influence of veneer application on fracture behavior of lithium-disilicate-based ceramic crowns. Dent Mater. 2012;28:653–660. doi: 10.1016/j.dental.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Sailer I, Makarov NA, Thoma DS, Zwahlen M, Pjetursson BE. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs) Dent Mater. 2015;31:603–623. doi: 10.1016/j.dental.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 5.Makhija SK, Lawson NC, Gilbert GH, Litaker MS, Mc-Clelland JA, Louis DR, Gordan VV, Pihlstrom DJ, Meyerowitz C, Mungia R, McCracken MS, National Dental PBRN Collaborative Group Dentist material selection for single-unit crowns: Findings from the National Dental Practice-Based Research Network. J Dent. 2016;55:40–47. doi: 10.1016/j.jdent.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma L, Guess PC, Zhang Y. Load-bearing properties of minimal-invasive monolithic lithium disilicate and zirconia occlusal onlays: finite element and theoretical analyses. Dent Mater. 2013;29:742–751. doi: 10.1016/j.dental.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Lawn BR. Novel zirconia materials in dentistry. J Dent Res. 2018;97:140–147. doi: 10.1177/0022034517737483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Amleh B, Lyons K, Swain M. Clinical trials in zirconia: a systematic review. J Oral Rehabil. 2010;37:641–652. doi: 10.1111/j.1365-2842.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- 9.Johansson C, Kmet G, Rivera J, Larsson C, Vult Von Steyern P. Fracture strength of monolithic all-ceramic crowns made of high translucent yttrium oxide-stabilized zirconium dioxide compared to porcelain-veneered crowns and lithium disilicate crowns. Acta Odontol Scand. 2014;72:145–153. doi: 10.3109/00016357.2013.822098. [DOI] [PubMed] [Google Scholar]
- 10.Lameira DP, Buarque e Silva WA, Andrade e Silva F, De Souza GM. Fracture strength of aged monolithic and bilayer zirconia-based crowns. Biomed Res Int. 2015;2015:418641. doi: 10.1155/2015/418641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun T, Zhou S, Lai R, Liu R, Ma S, Zhou Z, Longquan S. Load-bearing capacity and the recommended thickness of dental monolithic zirconia single crowns. J Mech Behav Biomed Mater. 2014;35:93–101. doi: 10.1016/j.jmbbm.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Miura S, Yamauchi S, Kasahara S, Katsuda Y, Fujisawa M, Egusa H. Clinical evaluation of monolithic zirconia crowns: a failure analysis of clinically obtained cases from a 3.5-year study. J Prosthodont Res. 2021;65:148–154. doi: 10.2186/jpr.JPOR_2019_643. [DOI] [PubMed] [Google Scholar]
- 13.Kontonasaki E, Rigos AE, Ilia C, Istantsos T. Monolithic zirconia: an update to current knowledge. optical properties, wear, and clinical performance. Dent J (Basel) 2019;7:90. doi: 10.3390/dj7030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyazaki T, Nakamura T, Matsumura H, Ban S, Kobayashi T. Current status of zirconia restoration. J Prosthodont Res. 2013;57:236–261. doi: 10.1016/j.jpor.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Guess PC, Zavanelli RA, Silva NR, Bonfante EA, Coelho PG, Thompson VP. Monolithic CAD/CAM lithium disilicate versus veneered Y-TZP crowns: comparison of failure modes and reliability after fatigue. Int J Prosthodont. 2010;23:434–442. [PubMed] [Google Scholar]
- 16.Swain MV, Mercurio V, Tibballs JE, Tholey M. Thermal induced deflection of a porcelain-zirconia bilayer: Influence of cooling rate. Dent Mater. 2019;35:574–584. doi: 10.1016/j.dental.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 17.Vigolo P, Mutinelli S. Evaluation of zirconium-oxide-based ceramic single-unit posterior fixed dental prostheses (FDPs) generated with two CAD/CAM systems compared to porcelain-fused-to-metal single-unit posterior FDPs: a 5-year clinical prospective study. J Prosthodont. 2012;21:265–269. doi: 10.1111/j.1532-849X.2011.00825.x. [DOI] [PubMed] [Google Scholar]
- 18.Beuer F, Stimmelmayr M, Gueth JF, Edelhoff D, Naumann M. In vitro performance of full-contour zirconia single crowns. Dent Mater. 2012;28:449–456. doi: 10.1016/j.dental.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura K, Harada A, Inagaki R, Kanno T, Niwano Y, Milleding P, Örtengren U. Fracture resistance of monolithic zirconia molar crowns with reduced thickness. Acta Odontol Scand. 2015;73:602–608. doi: 10.3109/00016357.2015.1007479. [DOI] [PubMed] [Google Scholar]
- 20.Bömicke W, Rammelsberg P, Stober T, Schmitter M. Short-term prospective clinical evaluation of monolithic and partially veneered zirconia single crowns. J Esthet Restor Dent. 2017;29:22–30. doi: 10.1111/jerd.12270. [DOI] [PubMed] [Google Scholar]
- 21.Gunge H, Ogino Y, Kihara M, Tsukiyama Y, Koyano K. Retrospective clinical evaluation of posterior monolithic zirconia restorations after 1 to 3.5 years of clinical service. J Oral Sci. 2018;60:154–158. doi: 10.2334/josnusd.17-0176. [DOI] [PubMed] [Google Scholar]
- 22.Solá-Ruiz MF, Baixauli-López M, Roig-Vanaclocha A, Amengual-Lorenzo J, Agustín-Panadero R. Prospective study of monolithic zirconia crowns: clinical behavior and survival rate at a 5-year follow-up. J Prosthodont Res. 2021;65:284–290. doi: 10.2186/jpr.JPR_D_20_00034. [DOI] [PubMed] [Google Scholar]
- 23.Tekin YH, Hayran Y. Fracture resistance and marginal fit of the zirconia crowns with varied occlusal thickness. J Adv Prosthodont. 2020;12:283–290. doi: 10.4047/jap.2020.12.5.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weigl P, Sander A, Wu Y, Felber R, Lauer HC, Rosentritt M. In-vitro performance and fracture strength of thin monolithic zirconia crowns. J Adv Prosthodont. 2018;10:79–84. doi: 10.4047/jap.2018.10.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorrentino R, Triulzio C, Tricarico MG, Bonadeo G, Gherlone EF, Ferrari M. In vitro analysis of the fracture resistance of CAD-CAM monolithic zirconia molar crowns with different occlusal thickness. J Mech Behav Biomed Mater. 2016;61:328–333. doi: 10.1016/j.jmbbm.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Nordahl N, Vult von Steyern P, Larsson C. Fracture strength of ceramic monolithic crown systems of different thickness. J Oral Sci. 2015;57:255–261. doi: 10.2334/josnusd.57.255. [DOI] [PubMed] [Google Scholar]
- 27.Gierthmuehlen P, Rübel A, Stampf S, Spitznagel F. Effect of reduced material thickness on fatigue behavior and failure load of monolithic CAD/CAM PICN molar crowns. Int J Prosthodont. 2019;32:71–74. doi: 10.11607/ijp.5946. [DOI] [PubMed] [Google Scholar]
- 28.Dentsply Sirona GmbH. inCoris TZI 2021. [Accessed May 6, 2021]. Available from: https://manuals.sirona.com/de/digitale-zahnheilkunde/cad-cam-material/incoris-tzi .
- 29.Kern M, Strub JR, Lü XY. Wear of composite resin veneering materials in a dual-axis chewing simulator. J Oral Rehabil. 1999;26:372–378. doi: 10.1046/j.1365-2842.1999.00416.x. [DOI] [PubMed] [Google Scholar]
- 30.DeLong R, Sakaguchi RL, Douglas WH, Pintado MR. The wear of dental amalgam in an artificial mouth: a clinical correlation. Dent Mater. 1985;1:238–242. doi: 10.1016/S0109-5641(85)80050-6. [DOI] [PubMed] [Google Scholar]
- 31.Abernethy R. The New Weibull Handbook. Reliability and statistical analysis for predicting life, safety, survivability, risk, cost and warranty claims. 5th ed. North Palm Beach (FL): 2006. [Google Scholar]
- 32.Ferrario VF, Sforza C, Zanotti G, Tartaglia GM. Maximal bite forces in healthy young adults as predicted by surface electromyography. J Dent. 2004;32:451–457. doi: 10.1016/j.jdent.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 33.Ivoclar Vivadent GmbH. IPS e.max ZirCAD Labside - Gebrauchsinformation 2019. Provided by manufacturer. [Accessed October 21, 2021].
- 34.VITA Zahnfabrik H. Rauter GmbH. VITA YZ Solutions-Working Instructions 2019. [Accessed May 6, 2021]. Available from: https://www.vita-zahnfabrik.com/de/Technician-Solutions/CAD/CAM/Geruestkonstruktionen/Vollanatomische-Bruecken/VITA-YZ-HT-25899,27568.html .
- 35.Baladhandayutham B, Lawson NC, Burgess JO. Fracture load of ceramic restorations after fatigue loading. J Prosthet Dent. 2015;114:266–271. doi: 10.1016/j.prosdent.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Skjold A, Schriwer C, Gjerdet NR, Oilo M. Effect of artificial aging on high translucent dental zirconia: simulation of early failure. Eur J Oral Sci. 2020;128:526–534. doi: 10.1111/eos.12739. [DOI] [PubMed] [Google Scholar]
- 37.Cotes C, Arata A, Melo RM, Bottino MA, Machado JP, Souza RO. Effects of aging procedures on the topographic surface, structural stability, and mechanical strength of a ZrO2-based dental ceramic. Dent Mater. 2014;30:e396–e404. doi: 10.1016/j.dental.2014.08.380. [DOI] [PubMed] [Google Scholar]
- 38.Rosentritt M, Behr M, Gebhard R, Handel G. Influence of stress simulation parameters on the fracture strength of all-ceramic fixed-partial dentures. Dent Mater. 2006;22:176–182. doi: 10.1016/j.dental.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 39.Elsayed A, Meyer G, Wille S, Kern M. Influence of the yttrium content on the fracture strength of monolithic zirconia crowns after artificial aging. Quintessence Int. 2019;50:344–348. doi: 10.3290/j.qi.a42097. [DOI] [PubMed] [Google Scholar]
- 40.Blatz MB, Vonderheide M, Conejo J. The effect of resin bonding on long-term success of high-strength ceramics. J Dent Res. 2018;97:132–139. doi: 10.1177/0022034517729134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lawson NC, Khajotia S, Bedran-Russo AK, Frazier K, Park J, Leme-Kraus A, Urquhart O Council on Scientific Affairs. Bonding crowns and bridges with resin cement: An American Dental Association Clinical Evaluators Panel survey. J Am Dent Assoc. 2020;151:796–797.e2. doi: 10.1016/j.adaj.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 42.Guess PC, Zhang Y, Kim JW, Rekow ED, Thompson VP. Damage and reliability of Y-TZP after cementation surface treatment. J Dent Res. 2010;89:592–596. doi: 10.1177/0022034510363253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fernández-Estevan L, Millan-Martínez D, Fons-Font A, Agustín-Panadero R, Román-Rodríguez JL. Methodology in specimen fabrication for in vitro dental studies: Standardization of extracted tooth preparation. J Clin Exp Dent. 2017;9:e897–e900. doi: 10.4317/jced.54020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lawson NC, Jurado CA, Huang CT, Morris GP, Burgess JO, Liu PR, Kinderknecht KE, Lin CP, Givan DA. Effect of surface treatment and cement on fracture load of traditional zirconia (3y), translucent zirconia (5y), and lithium disilicate crowns. J Prosthodont. 2019;28:659–665. doi: 10.1111/jopr.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coelho PG, Silva NR, Bonfante EA, Guess PC, Rekow ED, Thompson VP. Fatigue testing of two porcelain-zirconia all-ceramic crown systems. Dent Mater. 2009;25:1122–1127. doi: 10.1016/j.dental.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Guess PC, Schultheis S, Wolkewitz M, Zhang Y, Strub JR. Influence of preparation design and ceramic thicknesses on fracture resistance and failure modes of premolar partial coverage restorations. J Prosthet Dent. 2013;110:264–273. doi: 10.1016/S0022-3913(13)60374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y, Sailer I, Lawn BR. Fatigue of dental ceramics. J Dent. 2013;41:1135–1147. doi: 10.1016/j.jdent.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Lawn B. Long-term strength of ceramics for biomedical applications. J Biomed Mater Res B Appl Biomater. 2004;69:166–172. doi: 10.1002/jbm.b.20039. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Y, Kim JW, Bhowmick S, Thompson VP, Rekow ED. Competition of fracture mechanisms in monolithic dental ceramics: flat model systems. J Biomed Mater Res B Appl Biomater. 2009;88:402–411. doi: 10.1002/jbm.b.31100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koenig V, Bekaert S, Dupont N, Vanheusden A, Le Goff S, Douillard T, Chevalier J, Djaker N, Lamy de la Chapelle M, Amiard F, Dardenne N, Wulfman C, Mainjot A. Intraoral low-temperature degradation of monolithic zirconia dental prostheses: results of a prospective clinical study with ex vivo monitoring. Dent Mater. 2021;37:1134–1149. doi: 10.1016/j.dental.2021.03.008. [DOI] [PubMed] [Google Scholar]
- 51.Guilardi LF, Pereira GKR, Giordani JC, Kleverlaan CJ, Valandro LF, Rippe MP. Effect of zirconia surface treatment, resin cement and aging on the load-bearing capacity under fatigue of thin simplified full-contour Y-TZP restorations. J Mech Behav Biomed Mater. 2019;97:21–29. doi: 10.1016/j.jmbbm.2019.04.050. [DOI] [PubMed] [Google Scholar]
- 52.Rues S, Huber G, Rammelsberg P, Stober T. Effect of impact velocity and specimen stiffness on contact forces in a weight-controlled chewing simulator. Dent Mater. 2011;27:1267–1272. doi: 10.1016/j.dental.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Øilo M, Kvam K, Tibballs JE, Gjerdet NR. Clinically relevant fracture testing of all-ceramic crowns. Dent Mater. 2013;29:815–823. doi: 10.1016/j.dental.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 54.Nakamura K, Ankyu S, Nilsson F, Kanno T, Niwano Y, Vult von Steyern P, Örtengren U. Critical considerations on load-to-failure test for monolithic zirconia molar crowns. J Mech Behav Biomed Mater. 2018;87:180–189. doi: 10.1016/j.jmbbm.2018.07.034. [DOI] [PubMed] [Google Scholar]
- 55.Santana T, Zhang Y, Guess P, Thompson VP, Rekow ED, Silva NR. Off-axis sliding contact reliability and failure modes of veneered alumina and zirconia. Dent Mater. 2009;25:892–898. doi: 10.1016/j.dental.2009.01.093. [DOI] [PubMed] [Google Scholar]
- 56.Bonfante EA, Coelho PG, Guess PC, Thompson VP, Silva NR. Fatigue and damage accumulation of veneer porcelain pressed on Y-TZP. J Dent. 2010;38:318–324. doi: 10.1016/j.jdent.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Kontonasaki E, Giasimakopoulos P, Rigos AE. Strength and aging resistance of monolithic zirconia: an update to current knowledge. Jpn Dent Sci Rev. 2020;56:1–23. doi: 10.1016/j.jdsr.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang F, Inokoshi M, Batuk M, Hadermann J, Naert I, Van Meerbeek B, Vleugels J. Strength, toughness and aging stability of highly-translucent Y-TZP ceramics for dental restorations. Dent Mater. 2016;32:e327–e337. doi: 10.1016/j.dental.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 59.Rosentritt M, Preis V, Behr M, Strasser T. Fatigue and wear behaviour of zirconia materials. J Mech Behav Biomed Mater. 2020;110:103970. doi: 10.1016/j.jmbbm.2020.103970. [DOI] [PubMed] [Google Scholar]