Abstract
Background
The SARS-CoV-2 pandemic is affecting millions of people living in rural areas of Low- and Middle-Income Countries and is causing an already anticipated devastating effect on the health and economics of these populations. More information is needed to modify behaviors that may counterbalance the consequences of mass spread of the virus in these underserved communities. This study aimed to identify factors associated with a persistent SARS-CoV-2 seronegative status 1 year after a massive infection outbreak in middle-aged and older adults living in rural Ecuador.
Methods
Individuals enrolled in the Atahualpa Project Cohort as of March 2020 received 5 rounds of tests for determination of SARS-CoV-2 antibodies in blood. Individuals who remained seronegative up to April 2021 were considered “persistently seronegative.” An adjusted Poisson regression model was fitted to estimate the incidence risk ratio of factors directly or inversely associated with a persistent seronegative status.
Results
A total of 673 individuals received baseline tests. Thirty-one declined consent or died and 429 seroconverted, leaving 213 seronegative subjects. Average SARS-CoV-2 incidence rate was 9.87 events (95% C.I.: 8.91-10.83) per 100 person-months of observation. The use of flushing toilet systems (instead of open latrines) increased 1.5 times the possibility of remaining seronegative. Likewise, every additional bedroom in the house increased by 15% the possibility of remaining seronegative. In contrast, every additional person in the house and having high cholesterol levels significantly reduced the possibility of remaining seronegative.
Conclusions
The use of flushing toilet systems and the number of bedrooms in the house directly influenced the possibility of remaining seronegative among individuals living in this rural setting. Study results also demonstrated a sustained transmission of the virus even after a significant proportion of the population has been infected. Our findings reinforce the mass spread of SARS-CoV-2 in rural communities.
Keywords: SARS-CoV-2, COVID-19, cohort study, incidence, rural setting, Ecuador
Introduction
The Coronavirus Disease-2019 (COVID-19), caused by the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), has affected more than 220 million people and has claimed the life of more than 4 million people worldwide. 1 Copious literature has revealed several factors at both the individual and environmental levels that may increase the hazards of getting infected by SARS-CoV-2,2-6 and it is also well-known that the routine practice of biosecurity measures significantly reduces the risk of infection. 7 However, little is known on specific factors that may help individuals to remain free of infection over time, particularly if they live in closed remote settings where community transmission is high and sanitation is insufficient.
SARS-CoV-2 is rapidly spreading across Low-and-Middle-Income-Countries (LMIC), some of which are the main foci of attention of the pandemic nowadays.8-10 With the collusion of illiteracy and poor sanitary resources, the pandemic is currently affecting millions of people living in rural areas of LMIC and is causing an already anticipated devastating effect on the health and economics of these populations.11-14 More information is needed to modify behaviors that may counterbalance the consequences of mass spread of SARS-CoV-2 in these underserved communities.
Atahualpa—a rural Ecuadorian village—was severely struck by the pandemic from March to May 2020, coinciding with the introduction of SARS-CoV-2 in Ecuador. 15 A very high infection rate was initially evidenced by a sudden increase in deaths due to COVID-19, a door-to-door survey demonstrating a SARS-CoV-2 seroprevalence of 45% among middle-aged and older adults, and a subsequent survey indicating an overall incidence rate ratio of 7.4 per 100 person-months of virus exposure.16-18 Following a population-based longitudinal prospective design, this study aimed to identify factors associated with a persistent SARS-CoV-2 seronegative status 1 year after the start of the pandemic in middle-aged and older adults enrolled in the Atahualpa Project Cohort.
Material and Methods
Study Population
Atahualpa is a rural village located in Coastal Ecuador. Inhabitants are homogeneous regarding race/ethnicity (Amerindian ancestry), socioeconomic status, lifestyles, and diet. Almost all men work as artisan carpenters and most women are homemakers. The weather in Atahualpa is hot and dry with 12 daily hours of sunlight year-round. The environment is unpolluted since few people own a motor vehicle and there are no industries within the village or in its neighborhoods. The village has electricity and almost all houses have piped water. However, approximately 20% of houses do not have flushing toilet systems inside the houses and still use open latrines. Latrines consist of small shelters located out of the houses and do not have an incorporated pipe water sink. Atahualpa has a low index of migration rate and the adherence of the population to the Atahualpa Project is high, which makes this village an optimal setting for the conduction of population-based cohort studies. 18
Study Design and Procedures
All individuals actively enrolled in the Atahualpa Project Cohort were monitored as soon as the first case of SARS-CoV-2 infection was confirmed in Ecuador (February 27, 2020), particularly because the patient zero visited rural areas of the Santa Elena Province—where Atahualpa is located—soon after her arrival from Spain (February 14, 2020). 19 Individuals were clinically monitored until the first round of tests for determination of SARS-CoV-2 antibodies was done (May 2020). Those who granted consent for antibody determinations were included in this study. A comprehensive informed consent form was signed by all study participants before enrollment. The study was approved by the Institutional Review Board of Universidad Espiritu Santo (IORG: 0010320; FWA: 00028878), and followed ethical principles stated in the Declaration of Helsinki.
Detection of SARS-CoV-2 IgM and IgG antibodies was performed using a lateral flow antibody test, colloidal gold method, manufactured by BIOHIT Health Care Ltd. (Cheshire, UK) in finger prick blood samples. The manufacturer reports 97.5% sensitivity with 99.5% specificity for IgM, and 97.5% sensitivity with 100% specificity for IgG detection of this kit. As previously detailed, results of those tests were independently reviewed by 2 readers, with excellent Kappa coefficients for interrater agreement (0.91). Discrepancies were resolved by consensus. 17
Individuals who tested positive in May 2020 were administratively censored at that time and no subsequent tests were performed (see below). In contrast, seronegative study participants received subsequent tests for SARS-CoV-2 antibody determinations until they became seropositive. Four subsequent rounds of tests were performed—June 2020, September 2020, January 2021, and April 2021—using the same kits and procedures as previously mentioned. This periodicity—no more than 12 to 16 weeks between tests—was adopted to reduce the possibility that a given subject who got infected after a given round would escape detection by turning seronegative due to a vanished antibody response at the time of the next round. Individuals who became seropositive at any given round were censored at the time of the test, since they could not contribute any more exposed time, and only those who remained seronegative up to April 2021 were considered for inclusion in the group of “persistently seronegative.” In addition, during the follow-up period, all study participants received periodic home visits and clinical examinations.
Covariates of Interest
Covariates were selected if they have been suggested to play a role modifying the susceptibility to SARS-CoV-2 acquisition. These included age, gender, level of education (primary school education or higher), alcohol intake (dichotomized in ≤50 or >50 g/day), the complete set of cardiovascular risk factors (smoking status, diet, physical activity, body mass index, blood pressure, fasting glucose and total cholesterol blood levels, according to the American Heart Association criteria), 20 the waist circumference (as a surrogate of abdominal obesity), sleep quality (assessed by means of the Pittsburgh Sleep Quality Index), 21 and the presence of symptoms of depression (assessed by means of the depression axis of the Depression-Anxiety-Stress Scale-21). 22 These covariates had been updated during a survey conducted 3 months before the start of the pandemic in the village (December, 2019). In addition, social factors of relevance to the pandemic were included as covariates. These included the economic status of the individual (weighted on a Likert scale, were 1 point is less than $100 and 5 points is more than $700 per month, according to the Gijon scale for the measurement of Social Determinants of Health), 23 the number of persons living in the house, bedrooms per house, having a flushing toilet system (as opposed to an open latrine), and home confinement during at least 2 months during the pandemic.
Statistical Analysis
Data analyses were carried out by using STATA version 17 (College Station, TX, USA). In univariate analyses, continuous variables were compared by linear models and categorical variables by the x2 or Fisher exact test as appropriate. For computing person-years of follow-up, we considered the time under surveillance starting on March, 2020 (when clinical surveillance started), and ending the time of the first positive test, death, lost to follow-up or administrative censoring at the end of the study. A Poisson regression model adjusted for all the above-mentioned covariates of interest, was fitted to estimate the incidence risk ratio (IRR) of each of the factors associated with a persistent seronegative status (dependent variable).
Results
Figure 1 is a flow chart depicting the reasons for excluding study participants at each round of serological testing and their allocation into seronegative or seropositive categories. From 702 individuals actively enrolled in the Atahualpa Project Cohort as of March 2020, 673 (96% coverage) received a baseline test for the detection of SARS-CoV-2 antibodies in May 2020. The average follow-up was 6.8 months (SD: 5.16 months) and the median follow up was 3.02 months (interquartile range: 2.0-13 months). These individuals contributed 383.1 person-years of follow-up (mean: 0.57 ± 0.43 years; range: 0.17-1.08 years).
Figure 1.
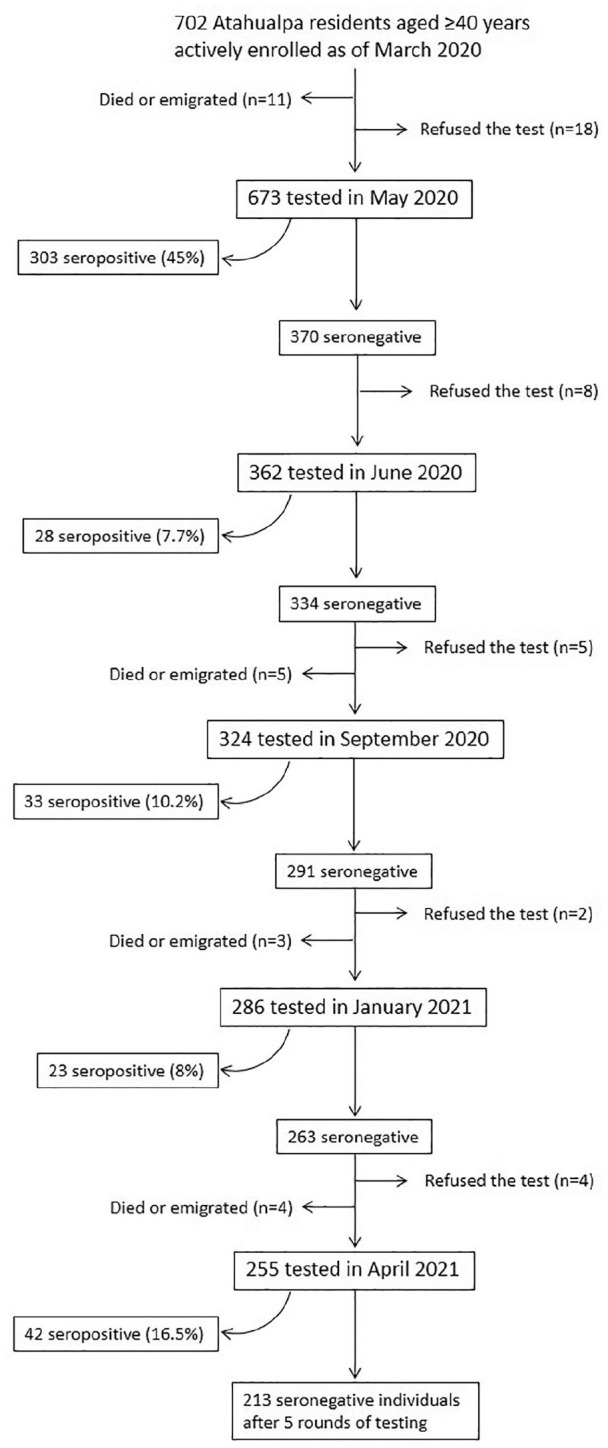
Flow chart depicting the reasons for censoring individuals before the end of the study and the number of persistent seronegative individuals at the administrative censoring date (April, 2021).
A total of 31 individuals were censored before the end of the study because they declined consent, died, or emigrated from Atahualpa. In addition, 429 individuals seroconverted to positive, leaving 213 study participants who remained seronegative by the end of the study. As noticed in the flow chart, with the exception of the first round of tests where 45% of study participants were seropositive, the highest percentage of seroconversion (16.5%) was noticed in the last round of tests (April 2021), which corresponded well with the Ecuadorian scenario, which by that time was going through the second wave of the pandemic. 24 In the second, third, and fourth rounds of test, 7.7%, 10.2%, and 8% of individuals seroconverted, respectively. Average SARS-CoV-2 incidence rate was 9.87 events (95% C.I.: 8.91-10.83) per 100 person months of observation. Overall, 255 study participants received 5 rounds of tests, 31 received 4 rounds, 38 received 3 rounds, 38 received 2 rounds, and the remaining 311 received only 1 round.
The mean age of the 673 study participants was 59.2 ± 12.8 years, 381 (57%) were women, 354 (53%) had primary school education only, and 142 (21%) disclosed heavy alcohol intake. Thirty-two individuals (5%) were current smokers, 188 (28%) had a body mass index ≥30 kg/m2, the mean waist circumference was 92.3 ± 10 cm, 48 (7%) had poor physical activity, 38 (6%) had a poor diet, 197 (29%) had blood pressure ≥140/90 mmHg, 152 (23%) had fasting glucose ≥126 mg/dL, 84 (12%) had total cholesterol levels ≥240 mg/dL, 200 (30%) had a poor sleep quality, and 72 (11%) had symptoms of depression. The mean score in the categories of economic status was 3.1 ± 0.7 (from a total of 5 points), the mean number of persons per house (including children and young adults) was 5.5 ± 3.3, and the mean number of bedrooms per house was 2.6 ± 1.1. Flushing toilet systems with closed sewage disposal systems were used by 541 (80%) individuals, and the remaining had open latrines. Overall, 238 (35%) individuals had been confined at home for at least 2 months during the study period.
In unadjusted analyses, individuals who remained seronegative until the end of the study live with fewer persons in the house (P = .010) and used flushing toilet systems more often (P = .024) than those who seroconverted, died or were lost to follow-up. Otherwise, there were no significant differences in the remaining covariates across individuals who persisted seronegative and those who were censored before the end of the study (Table 1). A Poisson regression model, adjusted for all the investigated covariates, disclosed that the use of flushing toilet systems increased 1.5 times the possibility of remaining seronegative, and every additional bedroom in the house increased by 15% the chance of remaining seronegative. In contrast, every additional person in the house reduced by 5% the possibility of remaining seronegative, and having high cholesterol levels reduced 1.5 times the possibility of remaining seronegative (Table 2).
Table 1.
Characteristics of Atahualpa Residents Remaining Seronegative Up to the End of the Study Versus Those Who Seroconverted, Died or Were Lost to Follow-up (Univariate Analysis).
| Finished the study as seronegative (n = 213) | Censored before the end of the study (n = 460) | P-value | |
|---|---|---|---|
| Age, years (mean ± SD) | 59.2 ± 13.2 | 59.2 ± 12.7 | .978 |
| Females, n (%) | 121 (57) | 260 (57) | .945 |
| Primary school education, n (%) | 111 (52) | 243 (53) | .863 |
| Alcohol intake ≥50 g/day, n (%) | 46 (22) | 96 (21) | .829 |
| Smoker, n (%) | 11 (5) | 21 (5) | .734 |
| Body mass index ≥30 kg/m2, n (%) | 59 (28) | 129 (28) | .926 |
| Waist circumference, cm (mean ± SD) | 91.9 ± 8.9 | 92.4 ± 10.4 | .545 |
| Poor physical activity, n (%) | 18 (4) | 30 (7) | .366 |
| Poor diet, n (%) | 12 (6) | 26 (6) | .992 |
| Blood pressure ≥140/90 mmHg, n (%) | 60 (28) | 137 (30) | .669 |
| Fasting glucose ≥126 mg/dL, n (%) | 48 (23) | 104 (23) | .983 |
| Total blood cholesterol ≥240 mg/dL, n (%) | 23 (11) | 61 (13) | .369 |
| Poor sleep quality, n (%) | 69 (32) | 131 (28) | .301 |
| Symptoms of depression, n (%) | 22 (10) | 50 (11) | .833 |
| Economic status, categories (mean ± SD) | 3.1 ± 0.7 | 3.1 ± 0.7 | .918 |
| Number of persons in the house (mean ± SD) | 5.1 ± 2.7 | 5.8 ± 3.5 | .010* |
| Number of bedrooms in the house (mean ± SD) | 2.6 ± 1.1 | 2.5 ± 1.1 | .273 |
| Use of flushing toilet systems, n (%) | 182 (85) | 359 (78) | .024* |
| Confined at home for 2 months, n (%) | 79 (37) | 159 (35) | .524 |
Statistically significant result.
Table 2.
Poisson Regression Model Showing Factors Associated With a Persistent Seronegative Status Among Atahualpa Residents.
| Persistent seronegative status | IRR § | 95% confidence interval | P-value |
|---|---|---|---|
| Age | 1.01 | 0.99-1.01 | .976 |
| Gender (being female) | 1.06 | 0.82-1.36 | .666 |
| Primary school education | 1.06 | 0.85-1.33 | .612 |
| Alcohol intake ≥50 g/day | 1.16 | 0.86-1.58 | .338 |
| Smoker | 1.21 | 0.73-2.00 | .460 |
| Body mass index ≥30 kg/m2 | 0.87 | 0.66-1.15 | .336 |
| Waist circumference | 1.01 | 0.99-1.02 | .499 |
| Poor physical activity | 1.16 | 0.78-172 | .455 |
| Poor diet | 0.99 | 0.65-1.52 | .956 |
| Blood pressure ≥140/90 mmHg | 0.86 | 0.68-1.09 | .208 |
| Fasting glucose ≥126 mg/dL | 0.88 | 0.70-1.12 | .292 |
| Total blood cholesterol ≥240 mg/dL | 0.65 | 0.49-0.86 | .003* |
| Poor sleep quality | 1.08 | 0.86-1.33 | .540 |
| Symptoms of depression | 0.89 | 0.65-1.22 | .469 |
| Economic status | 0.90 | 0.78-1.04 | .165 |
| Number of persons in the house | 0.95 | 0.92-0.98 | .006* |
| Number of bedrooms in the house | 1.15 | 1.01-1.30 | .029* |
| Use of flushing toilet systems | 1.53 | 1.20-1.95 | .001* |
| Confined at home for 2 months | 1.18 | 0.94-1.49 | .147 |
Incidence risk ratio.
Statistically significant result.
Discussion
This prospective study, conducted in a cohort of community-dwelling middle-aged and older adults living in a rural Ecuadorian setting, demonstrates several factors positively or negatively influencing a persistent SARS-CoV-2 seronegative status among individuals living in a village severely struck by the pandemic. On the other hand, study results show a sustained transmission after a significant proportion of the population has already been infected, confirming previous worries of disseminated spread of this infection in remote Latin American settings.25,26 At the end of the study, the accumulated percentage of seropositive individuals was almost 64% (Figure 1), which is close to the 70% infection rate that has been suggested for reaching herd immunity. Similar seroprevalence rates have been observed in other underserved rural communities, providing further support to our findings. 27
In-house crowding was inversely associated with the probability of remaining seronegative. This is an expected finding since social distancing is usually not taken into account and individuals do not wear a face mask within the house, exposing other family members to the virus. This is probably why home confinement was not associated with a persistent seronegative status since this individual protection is overcome by the presence of other household members who go out of the house for working or social gatherings. In this line, the number of bedrooms per house was a protective factor since the lower the number of individuals sleeping in the same bedroom (or even in the same bed), the lower the risk of being infected. This has previously been demonstrated in a Brazilian study that demonstrated that social inequities—including crowded households—contribute to the spread of SARS-CoV-2 in rural settings. 9
As noticed, the number of bedrooms in the house was significantly associated with a persistent seronegative status in the multivariate Poisson regression model but not in unadjusted analysis. This is because the univariate association between bedrooms and seronegativity ignores the number of persons in the house. Nonetheless, there is an association between bedrooms and persons, and the association with seroprevalence is quite different for the 2 variables in question. As the number of people increases, seronegativity decreases, while as the number of bedrooms increases, seronegativity increases. In a multivariate model the significance of adding 1 bedroom is greater if there are more than 5 persons in the bedroom (average number of persons). In other words, the number of bedrooms in the house is evaluated at the means of any other variables in the model at a fixed point, which is 5.4 persons per bedroom in this case.
The present study demonstrates that using a closed flushing toilet system (as opposed to an open latrine) is a strong protective factor against SARS-CoV-2 infection. As previously mentioned, about 20% of houses in Atahualpa still have open latrines. 17 Latrines consist of non-ventilated small shelters located at the backyards that do not have an incorporated pipe water sink or any other disinfecting fluids. In addition, toilet papers are often disposed in open waste bins or on the floor of the cabin. Several studies have demonstrated persistence of SARS-CoV-2 shedding in feces.28-30 However, it is not known whether these viral particles are viable and how long they will be infective in latrine walls. It has been postulated that under specific circumstances SARS-CoV-2 can remain viable in fomites for several weeks. 31 A study from rural China suggested that the use of pit latrines may be a source of fecal transmission of SARS-CoV-2, either by direct contagion of humans with contaminated feces or through intermediate animal hosts. 32 Also, an editorial comment published early in the pandemic alerted about the potential role of alternative sources of viral transmission—including fecal-oral contamination—in rural areas of LMIC. 33 A recent case-control study conducted in Atahualpa showed an adjusted odds ratio of 4.82 (95% C.I.: 1.38-16.8) for the presence of SARS-CoV-2 RNA in open latrines when compared to closed flushing toilet systems, providing support for the potential role of latrines as reservoirs of SARS-CoV-2 RNA. 34 While the way excreta is disposed may be assumed as a surrogate of economic disparities, this may not apply to the study population, inasmuch as income status of individuals living in houses with closed flushing toilet systems and open latrines are similar. Cultural factors and lack of basic home facilities may account for the use of latrines in some houses.
Cardiovascular risk factors have shown to enhance the severity of COVID-19 expression, but their role in making individuals more prone to SARS-CoV-2 infection is in debate. A recent study based on more than 9000 individuals enrolled in the UK Biobank disclosed several factors associated with an increased risk of infection, but also revealed important interactions between them. 35 In the current study, most of the investigated cardiovascular risk factors were not associated with a modification in the risk of being infected by the virus. A notable exception was the presence of high total blood cholesterol, which was inversely associated with the possibility of remaining seronegative. As previously mentioned, this inverse association was only noticed in the multivariate Poisson regression model but not in unadjusted analysis. The lack of significant association between high cholesterol and seronegativity found in unadjusted analysis ignores the relative weight of other cardiovascular risk factors that also had an effect (non-significant, though) on seronegativity, such as smoking, the waist circumference, and poor physical activity.
While pathogenetic mechanisms involved in the association between high cholesterol levels and susceptibility to SARS-CoV-2 infection has not fully elucidated thus far, it has been shown that cholesterol-enriched lipid rafts in the cell membrane may favor the entrance of the virus into host cells, explaining the inverse relationship between high cholesterol levels and seronegativity in an endemic milieu.36,37
Major strengths of this study are the high coverage and unbiased inclusion of long-term participants in the Atahualpa Project Cohort, in whom several risk factors and conditions have been investigated. Other strengths include the homogeneous characteristics of the village and the study population, the systematic follow-up of study participants by field personnel acquainted with the population, the low numbers of dropouts, and the use of a reliable test for detection of SARS-CoV-2 antibodies in blood. Nevertheless, the study has limitations. The study population was limited to middle-aged and older adults and we missed information about the infection status of younger villagers and how it could have influenced the overall seroprevalence of adults as well as patterns of aggrupation. As mentioned, the test we used is reported to be highly reliable, but we cannot rule out misclassifications due to false positive or false negative results, 38 or the possibility of cross-reactions with other viral agents. 39 In addition, the study only involved one village and our results may be different to realities in other remote settings of LMIC countries.
In summary, this study discloses factors associated with a persistent SARS-CoV-2 seronegative status among individuals living in a remote rural village struck by the pandemic, as well as a sustained transmission of the virus even after a significant proportion of the population has already been infected. Our findings reinforce the fears of spread of SARS-CoV-2 in rural Latin America and suggest a contributory role of fecal-oral transmission. Retrieved information from this well-established cohort provide grounds for the conduction of further studies aimed to assess long-term dynamics of SARS-CoV-2 infection in a closed rural setting.
Footnotes
Authors’ Contributions: OHD: study design, manuscript drafting; AFC: data collection and analysis; BYR: study coordinator, data collection and analysis; RMM: statistical analysis, significant intellectual contribution to manuscript content.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Study supported by Universidad Espíritu Santo—Ecuador. The sponsor had no role in the design of the study, nor in the collection, analysis, and interpretation of data.
ORCID iD: Oscar H. Del Brutto  https://orcid.org/0000-0003-1917-8805
https://orcid.org/0000-0003-1917-8805
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. https://www.worldometers.info/coronavirus/. Accessed September 7, 2021.
- 2. de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General practitioners research and surveillance centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lewis NM, Chu VT, Ye D, et al. Household transmission of severe acute respiratory coronavirus-2 in the United States. Clin Infect Dis. 2021;73:1805-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fan VS, Dominitz JA, Eastment MC, et al. Risk factors for testing positive for SARS-CoV-2 in a national US healthcare system. Clin Infect Dis. 2020;ciaa1624. doi: 10.1093/cid/ciaa1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vena A, Berruti M, Adessi A, et al. Prevalence of antibodies to SARS-CoV-2 in Italian adults and associated risk factors. J Clin Med. 2020;9:2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yadav R, Acharjee A, Salkar A, et al. Mumbai mayhem of COVID-19 pandemic reveals important factors that influence susceptibility to infection. EClinical Med. 2021;35:100841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html. Accessed September 7, 2021.
- 8. Ballesteros N, Muñoz M, Patiño LH, et al. Deciphering the introduction and transmission of SARS-CoV-2 in the Colombian Amazon Basin. PLoS Negl Trop Dis. 2021;15:e0009327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Souza CDF, do Carmo RF, Machado MF. The burden of COVID-19 in Brazil is greater in areas with high social deprivation. J Travel Med. 2020;27:taaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joshi A, Kaur H, Krishna LN, et al. Tracking COVID-19 burden in India using SMAART RAPID tracker. Online J Public Health Inform. 2021;13:e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cimerman S, Chebabo A, Cunha CAD, Rodríguez-Morales AJ. Deep impact of COVID-19 in the healthcare of Latin America: the case of Brazil. Braz J Infect Dis. 2020;24:93-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meneses-Navarro S, Freyermuth-Enciso MG, Pelcastre-Villafuerte BE, Campos-Navarro R, Meléndez-Navarro DM, Gómez-Flores-Ramos L. The challenges facing indigenous communities in Latin America as they confront the COVID-19 pandemic. Int J Equity Health. 2020;19:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhao Z, Li X, Liu F, Zhu G, Ma C, Wang L. Prediction of the COVID-19 spread in African countries and implications for prevention and control: a case study in South Africa, Egypt, Algeria, Nigeria, Senegal and Kenya. Sci Total Environ. 2020;729:138959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Caicedo-Ochoa Y, Rebellón-Sánchez DE, Peñaloza-Rallón M, Cortés-Motta HF, Méndez-Fandiño YR. Effective reproductive number estimation for initial stage of COVID-19 pandemic in Latin American countries. Internet J Infect Dis. 2020;95:316-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marquez S, Prado-Vivar B, Guadalupe JJ, et al. Genome sequencing of the first SARS-CoV-2 reported from patients with COVID-19 in Ecuador. medRxiv. 2020. doi: 10.1101/2020.06.11.20128330. [DOI] [Google Scholar]
- 16. Del Brutto OH, Costa AF, Mera RM, Recalde BY, Bustos JA, García HH. SARS-CoV-2-related mortality in a rural Latin American population. Internet J Infect Dis. 2020;99:226-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Del Brutto OH, Costa AF, Mera RM, Recalde BY, Bustos JA, García HH. SARS-CoV-2 in rural Latin America. A population-based study in coastal Ecuador. Clin Infect Dis. 2021;73:314-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Del Brutto OH, Costa AF, Mera RM, Recalde BY, Bustos JA, García HH. Late incidence of SARS-CoV-2 infection in a highly-endemic remote rural village. A prospective population-based cohort study. Pathog Glob Health. 2020;114:457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. https://www.comunicacion.gob.ec/se-registra-el-primer-caso-de-coronavirus-en-ecuador/ Accessed September 7, 2021.
- 20. Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic impact goal through 2020 and beyond. Circulation. 2010;121:586-613. [DOI] [PubMed] [Google Scholar]
- 21. Buysse DJ, Reynolds Cf, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193-213. [DOI] [PubMed] [Google Scholar]
- 22. Osman A, Wong JL, Bagge CL, Freedenthal S, Gutierrez PM, Lozano G. The depression anxiety stress scales-21 (DASS-21): further examination of dimensions, scale reliability, and correlates. J Clin Psychol. 2012;68:1322-1338. [DOI] [PubMed] [Google Scholar]
- 23. Cabrera González D, Menéndez Caicoya A, Fernández Sánchez A, et al. Evaluación de la fiabilidad y validez de una escala de valoración social en el adulto. Aten Primaria. 1999;23:434-440. [PubMed] [Google Scholar]
- 24. https://www.worldometers.info/coronavirus/country/ecuador/. Accessed September 7, 2021.
- 25. Burki T. COVID-19 in Latin America. Lancet Infect Dis. 2020;20(5):547-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Amigo I. Indigenous communities in Brazil fear pandemic’s impact. Science. 2020;368:352. [DOI] [PubMed] [Google Scholar]
- 27. Sagara I, Woodford J, Kone M, et al. Rapidly increasing SARS-CoV-2 seroprevalence and limited clinical disease in three Malian communities: a prospective cohort study. medRxiv. doi: 10.1101/2021.04.26.21256016. Posted, April 29, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol. 2020;92:833-840. [DOI] [PubMed] [Google Scholar]
- 29. Wong MC, Huang J, Lai C, Ng R, Chan FKL, Chan PKS. Detection of SARS-CoV-2 RNA in fecal specimens of patients with confirmed COVID-19: a meta-analysis. J Infect. 2020;81:e31-e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park SK, Lee CW, Park DI, et al. Detection of SARS-CoV-2 in fecal samples from patients with asymptomatic and mild COVID-19 in Korea. Clin Gastroenterol Hepatol. 2021;19:1387-1394.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riddell S, Goldie S, Hill A, Eagles D, Drew TW. The effect of temperature on persistence of SARS-CoV-2 on common surfaces. Virol J. 2020;17(1):145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu L, Hu J, Hou Y, Tao Z, Chen Z, Chen K. Pit latrines may be a potential risk in rural China and low-income countries when dealing with COVID-19. Sci Total Environ. 2021;761:143283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller MJ, Loaiza JR, Takyar A, Gilman RH. COVID-19 in Latin America: novel transmission dynamics for a global pandemic? PLoS Negl Trop Dis. 2020;14:e0008265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Del Brutto OH, Costa AF, Mera RM, et al. SARS-CoV-2 RNA in swabbed samples from latrines and flushing toilets: a case–control study in a rural Latin American setting. Am J Trop Med Hyg. 2021;104:1045-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Scalsky RJ, Chen YJ, Desai K, O’Connell JR, Perry JA, Hong CC. Baseline cardiometabolic profiles and SARS-CoV-2 infection in the UK biobank. PLoS One. 2021;16:e0248602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Radenkovic D, Chawla S, Pirro M, Sahebkar A, Banach M. Cholesterol in relation to COVID-19: should we care about it? J Clin Med. 2020;9:1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kočar E, Režen T, Rozman D. Cholesterol, lipoproteins, and COVID-19: basic concepts and clinical applications. Biochim Biophys Acta Mol Cell Biol Lipids. 2021;1866:158849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Castro R, Luz PM, Wakimoto MD, Veloso VG, Grinsztejn B, Perazzo H. COVID-19: a meta-analysis of diagnostic test accuracy of commercial assays registered in Brazil. Braz J Infect Dis. 2020;24:180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spinicci M, Bartoloni A, Mantella A, Zammarchi L, Rossolini GM, Antonelli A. Low risk of serological cross-reactivity between dengue and COVID-19. Mem Inst Oswaldo Cruz. 2020;115:e200225. [DOI] [PMC free article] [PubMed] [Google Scholar]


