Abstract
Purpose:
Genetic testing results are currently obtained approximately 1 year after referral to a medical genetics team for autosomal dominant polycystic kidney disease (ADPKD). We evaluated a mainstream genetic testing (MGT) pathway whereby the nephrology team provided pre-test counseling and selection of patients with suspected ADPKD for genetic testing prior to direct patient interaction by a medical geneticist.
Sources of information:
A multidisciplinary team of nephrologists, genetic counselors, and medical geneticists developed an MGT pathway for ADPKD using current testing criteria for adult patient with suspected ADPKD and literature from MGT in oncology.
Methods:
An MGT pathway was assessed using a prospective cohort and compared to a retrospective cohort of 56 patients with ADPKD who received genetic testing using the standard, traditional pathway prior to implementing the MGT for ADPKD. The mainstream pathway was evaluated using time to diagnosis, diagnostic yield, and a patient survey to assess patient perceptions of the MGT pathway.
Key findings:
We assessed 26 patients with ADPKD using the MGT and 18 underwent genetic testing with return of results. Of them, 52 patients had data available for analysis in the traditional control cohort. The time for return of results using our MGT pathway was significantly shorter with a median time to results of 6 months compared to 12 months for the traditional pathway. We identified causative variants in 61% of patients, variants of uncertain significance in 28%, and 10% had negative testing which is in line with expectations from the literature. The patient surveys showed high satisfaction rates with the MGT pathway.
Limitations:
This report is an evaluation of a new genetic testing pathway restricted to a single, publicly funded health care center. The MGT pathway involved a prospective collection of a limited number of patients with ADPKD with comparison to a retrospective cohort of patients with ADPKD evaluated by standard testing.
Implications:
A MGT pathway using clearly defined criteria and commercially available gene panels for ADPKD can be successfully implemented in a publicly funded health care system to reduce the time required to obtain genetic results.
Keywords: genetic testing, mainstream, autosomal dominant polycystic kidney disease (ADPKD), medical genetics, next generation sequencing (NGS), chronic kidney disease (CKD), kidney failure
Abrégé
Motif:
Actuellement, les résultats du dépistage génétique pour la maladie polykystique rénale autosomique dominante (ADPKD) sont obtenus environ un an après l’aiguillage en médecine génique. Nous avons évalué un parcours de dépistage génétique intégré (DGI) où l’équipe de néphrologie fournit des conseils pré-dépistage et sélectionne les patients soupçonnés d’ADPKD pour un test génétique avant l’interaction directe du patient avec un généticien médical.
Sources:
Une équipe multidisciplinaire constituée de néphrologues, de conseillers en génétique et de généticiens médicaux a développé un parcours de DGI à partir des critères existants pour les patients adultes soupçonnés d’ADPKD et de la littérature portant sur le DGI en oncologie.
Méthodologie:
Le parcours de DGI a été évalué dans une cohorte prospective puis comparé à une cohorte rétrospective de 56 patients atteints d’ADPKD ayant subi un dépistage génétique selon le parcours traditionnel, avant la mise en œuvre d’un parcours de DGI pour l’ADPKD. Le parcours intégré a été évalué en tenant compte du temps requis pour poser le diagnostic, du rendement diagnostique et d’un sondage auprès des patients évaluant leurs perceptions à l’égard du parcours lui-même.
Principaux résultats:
Le parcours de DGI a permis d’évaluer 26 patients atteints d’ADPKD, dont 18 ont subi des tests génétiques avec retour des résultats. Dans la cohorte témoin (dépistage traditionnel), 52 patients disposaient de données disponibles pour l’analyse. Le délai médian pour l’obtention des résultats était significativement plus court avec le parcours de DGI qu’avec le parcours traditionnel (6 mois c. 12 mois). Des variantes causales ont été relevées chez 61 % des patients, 28 % des patients présentaient des variantes de signification incertaine et 10 % ont obtenu des résultats négatifs, ce qui est conforme aux attentes posées par les résultats rapportés dans la littérature. Les sondages menés auprès des patients ont montré des taux de satisfaction élevés à l’égard du parcours de DGI.
Limites:
Ce rapport constitue l’évaluation d’un nouveau parcours de dépistage génétique limitée à un seul centre de soins de santé public. Ce parcours de DGI a été évalué dans une cohorte prospective formée d’un nombre limité de patients atteints d’ADPKD par rapport à une cohorte rétrospective de patients atteints d’ADPKD évalués par la méthode traditionnelle.
Implications:
Un parcours de DGI utilisant des critères clairement définis et des panels génétiques pour l’ADPKD disponible commercialement peut être mis en œuvre avec succès dans un système de santé public et accélérer l’obtention des résultats génétiques.
What was known before
Genetic testing for patients with suspected autosomal dominant polycystic kidney disease (ADPKD) in many centers currently requires an assessment by a medical genetics expert prior to genetic testing. The time to obtain genetic test results can take more than 1 year from the time of referral. A Mainstream Cancer Genetics Initiative successfully implemented a mainstream gene testing process that provides cancer patients fast genetic testing at routine cancer clinic appointments.
What this adds
Our mainstream genetic testing (MGT) pathway for ADPKD is described in sufficient detail with supplementary resources that can be adapted to implement mainstream genetic testing pathways for ADPKD in other center or for other genetic kidney disorders.
Introduction
Genetic causes of kidney disease are an area of active research as they hold promise to improve patient outcomes through improved prognostication, family screening, family planning, therapeutics, and transplant evaluation.1-6 While genetic kidney diseases are individually rare, together they account for approximately 10% of kidney failure.1,7,8 Improved understanding of genetic kidney diseases, coupled with the reduced cost of sequencing, is making genetic testing in nephrology more common. 9 Nevertheless, multiple barriers need to be addressed for genetic testing to be widely available and maximize its clinical impact. These include cost and coverage issues, pairing of appropriate patients and tests, knowledge gaps for non-geneticist providers, and a limited supply of professionals with genetics expertise.10-14
The Mainstreaming Cancer Genetics Programme was a 5-year initiative in the United Kingdom that made cancer gene testing part of routine care with a mainstream pathway available at a cancer clinic without the involvement of a medical geneticist.15-18 The initiative’s major development was an effective mainstream access model for BRCA1 and BRCA2 testing in patients with breast and ovarian cancer. Prior to the mainstream model, BRCA genetic testing had systematic underuse and inappropriate use contributing lost opportunities for improved cancer care and management.16,19-21 These mainstream genetic testing models were developed by multidisciplinary teams including oncology, medical genetics, and genetic counselors to create a standardized approach allocating consent and testing to a trained oncology team with genetics follow-up as required.15,16,20,21 Mainstreaming programs for genetic testing in oncology have been adopted by many centers with a positive impact on patient care.21-25
ADPKD is the most common inherited kidney disease, and although the prevalence of ADPKD in Canada is unknown, it accounts for 5% of kidney failure in the United States.26-28 ADPKD has well-established Canadian and international guidelines for genetic testing.8,28,29 Ninety percent of patients with ADPKD have an identified variant, 85% of which are within the large and difficult to sequence PKD1 gene while 15% are in PKD2, with most of the identified mutations being private within a specific family.6,30-35 ADPKD shows genetic heterogeneity as variants in multiple other genes have been shown to produce a similar phenotype including GANAB, DNAJB11, HNF1B, VHL, UMOD, MUC1, and others.29,33,35-38 Patients and providers find the results of genetic testing in ADPKD beneficial in prognostication, family and transplant planning, and treatment, and advocate for access to testing.25,29,39-41
Current genetic testing for ADPKD for most centers requires a referral to medical genetics for evaluation, testing, and counseling. Due to a limited number of health-care professionals with expertise in genetics, there can be significant delays in accessing to testing. In this report, we evaluate the implementation of a mainstream genetic testing (MGT) pathway for nephrologists to provide pre-test consent and counseling for ADPKD prior to a formal referral to a medical genetics team. We show the feasibility of a mainstream genetic testing pathway for ADPKD comprising of a clinical work flow partitioned between a nephrology team and a medical genetics team to reduce the wait time for genetic testing.
Purpose of Program
We use an MGT model implemented in oncology as a guide to create and validate an MGT pathway for genetic testing of patients with ADPKD. We assess the time to return of results using our MGT pathway for patients with ADPKD compared to the traditional testing pathway along with the diagnostic yield of the developed gene panels, and patient satisfaction for those who received their testing through the mainstream pathway.
Methods
Patient Ascertainment
Patients were recruited for the prospectively collected mainstream pathway at Alberta Health Services, Calgary, Alberta, Canada from February 2020 to October 2020. All primary nephrologists in the Division of Nephrology at Alberta Health Services in Calgary, Alberta were notified of the MGT for ADPKD by email. Participants were identified and recruited by their primary outpatient nephrologists, and consented by a subsequent nephrologist to the study including the patient surveys. Forty-eight patients with ADPKD based on clinical evaluation and imaging were prospectively recruited to the mainstream pathway of the study. We excluded patients who had features consistent with a syndromic disorder as the medical genetics team felt these patients warranted a pre-testing visit with genetics to determine the optimal genetic tests. Patients or patients with a family member(s) who previously received genetic testing were excluded for cost-effective evaluation by targeted sequencing of the known familial variant. Due to the COVID-19 pandemic, recruitment and testing was halted for 95 days from March 26, 2020 to June 29, 2020. We analyzed a deidentified retrospective cohort of all the patients with ADPKD (n = 56) assessed by the medical genetics team through the existing standard of care pathway between January 1, 2015 through to December 30, 2019 to serve as a historic control. Of these 56 patients, 4 were missing data on the date of return of results and were excluded leaving, 52 patients with complete data who were included for this study. Both the prospective and retrospective studies received approval by the University of Calgary Research Ethics Board.
Mainstream Genetic Testing Pathway Design
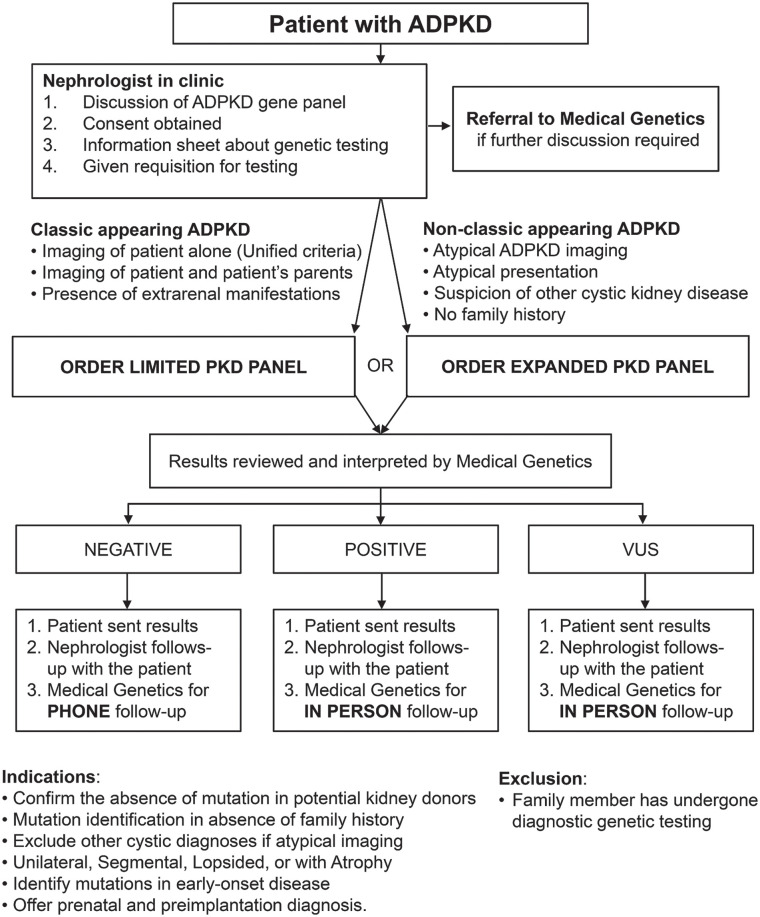
The MGT pathway was designed by a multidisciplinary team comprised of nephrologists, medical geneticists and genetic counselors. The group worked together over multiple sessions to adapt a standardized patient-centered approach from the oncology literature to work for patients with ADPKD. The MGT pathway involved multiple iterations with input from all pathway design team members (M.D.E., L.C.J., P.S., J.L., and J.C.). Resource utilization, test funding, patient flow, administrative support, and changes to clinical workflows for both the nephrology and medical genetics teams were taken into consideration. The final mainstreaming pathway divided the workflow such that the nephrology team carried out counseling, consent, and arranged the sample collection without the direct assistance of the medical genetics team; ordering the test, interpretation and return of results to patients and their care providers were done by the medical genetics team similar to the workflow described in the oncology literature (Figure 1).15,20,42,43 Unlike prior studies of genetic testing in nephrology, our mainstream pathway utilized a split work-load model, which aimed to free up the limited resources and time of our medical genetics team by removing their direct patient involvement during pre-test counseling, while still ensuring interpretation and return of results consistent with current best practices in nephrology.4,44,45 Study visits for consent and testing discussions were offered virtually over the telephone or in person based on participant preference.
Figure 1.
Mainstream genetic testing pathway for autosomal dominant polycystic kidney disease (ADPKD) testing in adult nephrology patients.
Note. Pathway developed by a team of nephrologists, medical geneticists and genetic counselors to facilitate ordering of a polycystic kidney disease (PKD) genetic panel by nephrologists prior to assessment patients by the medical genetics team.
A counseling checklist was developed for the nephrology team that outlined the benefits and limitations of genetic testing as required for informed consent (Supplementary Figure 1). Patient information handouts were developed to explain the testing, and genetic testing reports were developed for patients, and their health-care providers (Supplementary Figures 2–4). If patients had further questions prior to testing, the option of speaking with the medical genetics team was available. Once a patient consented to genetic testing, the nephrology team made a referral to the medical genetics team who ordered testing, then reviewed and returned the testing results. Expenses for testing were obtained through existing routine clinical coverage by Alberta’s Genetic Resource Centre.
To simulate a mainstream model, the primary nephrologists obtained consent for our research nephrologists to contact their patients. The research nephrologist then phoned or met patients in-person to obtain full study consent and subsequently counseled the patient on genetic testing for ADPKD, provided information about genetic testing, selected the appropriate gene panel, and arranged for genetic testing. In the event that the patient requested further discussion prior to genetic testing, a referral was sent to the medical genetics team.
Gene Panel Design
Blueprint Genetics (Helsinki, Finland) was used for the clinical gene panel testing in this study. We offered two panels based on clinical phenotype that were designed based on a review of the literature. Patients could collect a sample via salivary testing or whole blood at their discretion.
A limited polycystic kidney disease (PKD) panel was used for patients with classic appearing ADPKD where there was typical Mayo Class 1 imaging, positive family history, or the presence of typical ADPKD-related extrarenal manifestations and tested for both the PKD1 and PKD2 genes (Figure 1). This was chosen based on known diagnostic rates in well characterized patients of approximately 90% in these two genes, balanced with the lower likelihood of non-PKD1/PKD2 variants in these classic appearing cases.30,36,46 An extended PKD panel was developed for patients with non-classic appearing ADPKD defined by atypical kidney imaging (Mayo Class 2), absence of a family history, atypical clinical presentation for ADPKD, or where there was suspicion for another genetic cystic kidney disease, as non-PKD1/PKD2 variants have been shown to cause atypical imaging features, less prominent PKD, and are associated with other extra-kidney manifestations (Figure 1).33,36,47 The extended PKD panel consisted of genes known to cause typical and atypical PKD: PKD1, PKD2, COL4A1, DNAJB11, GANAB, HNF1B, REN, and UMOD. This panel was designed collaboratively by the study nephrologists and medical geneticists, with the intent of capturing those genes where the phenotype can appear similar to PKD while excluding genes with autosomal recessive inheritance, clear non-kidney related symptoms, such as Alagille syndrome, where more specific genetic testing could be employed and genes where ADPKD was a highly atypical presentation. The results were reviewed by the medical genetics team to order additional testing for rare genes not included in the testing panels if there were concerns.
Patient Survey
Two patient surveys were developed based on surveys used to assess the mainstreaming genetics pathways within oncology with modifications to accommodate the context and work-flow of this study. 15 The surveys consisted of 5-point Likert questions and free space questions for more general feedback. The first survey was given to the patient after their mainstreaming counseling and consent session with the nephrologist to obtain patient feedback about their interaction with the nephrologist, the developed resources, and understanding of testing. The survey was done in person or if the visit occurred virtually via mail. The second survey was sent to patients after their follow-up visit with the medical genetics team and aimed to gather patient feedback about how the counseling with the nephrologist prepared them for the results, how the medical genetics session was, and the overall satisfaction with the mainstream pathway. These surveys were sent only to patients within the MGT pathway with pre-printed and pre-paid postage to maintain anonymity. The questions were categorized into 4 themes based on the consensus of our group. The 4 categories included the study interaction, quality of written information, impact of results, and overall process. Survey question and linked themes are included in Supplementary Figure 5.
Outcomes
The primary outcome was the time from patient referral to for ADPKD genetic testing to the return of genetic test results to the patient. The primary outcome compared a prospectively collected cohort using our MGT pathway with a retrospectively collected control cohort subjected to genetic testing though the traditional, standard-of-care pathway. Our secondary outcomes included the diagnostic yield of gene panel testing, specific genetic diagnoses and survey-based patient outcomes that assessed the patient satisfaction specific to the MGT pathway.
Statistical Analysis
Descriptive and comparative analysis of the prospective and retrospective cohorts, and the survey data was done in R 4.0.3. Time to return of results were compared using Kaplan Meier survival analysis performed in R using the survival 3.0-7 and survminer 0.4.8 packages. 48
Key Findings
Time to Return of Results for ADPKD Genetic Testing
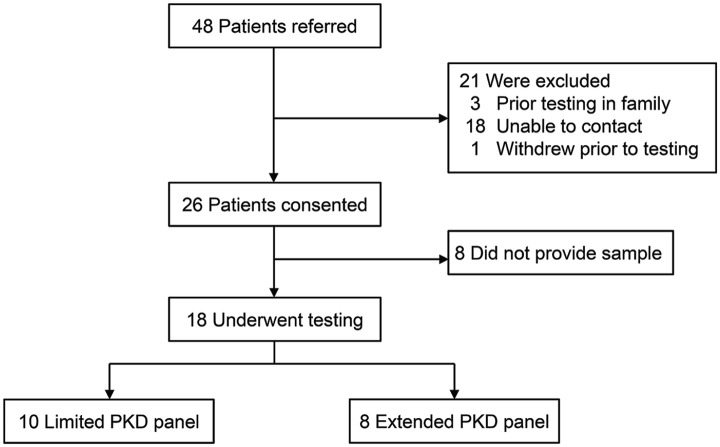
In total, 48 patients were referred to our study from their primary nephrologist and 26 consented to genetic testing for the MGT pathway. Patients underwent counseling and test selection by the study nephrologists using the MGT pathway during the 10-month recruitment phase (Figure 2). Eighteen patients submitted a sample for genetic testing and had their results returned with the mainstream pathway. Ten patients received the limited PKD panel, 8 the extended PKD panel. For the prospective MGT cohort, the median time from referral to the study to return of results was 184 days (95% confidence interval [CI]: 110-307 days), including the 95-day period of inactivity due to the COVID-19 pandemic that affected 12 participants with returned results (Figure 3A). The retrospective control cohort containing 52 patients with ADPKD who underwent genetic testing using the traditional pathway had a median time from referral to medical genetics to return of results of 368 days (95% CI: 327-493 days). The prospective mainstream cohort was significantly faster than the traditional control (log-rank test, P < 0.001) (Figure 3A). The timing to specific clinical visits in the traditional control and the mainstream pathway are shown in Figure 3B. The nominal time to the pretest counseling visit and for the receipt and return of results were shorter in the mainstream cohort compared to the traditional cohort.
Figure 2.
Referrals and inclusion in the analysis for polycystic kidney disease (PKD) genetic testing.
Note. Patients were referred by their primary nephrologists for PKD genetic testing. In total, 10 patients were tested using the limited PKD panel and 8 with the extended PKD panel.
Figure 3.
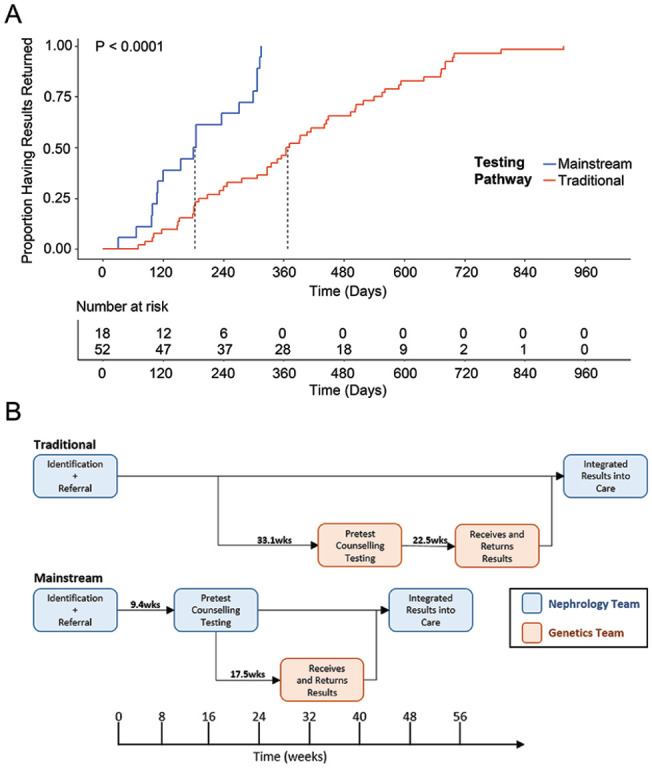
Comparison of turnaround time for results and diagnostic rates for the mainstream pathway to current standards. (A) Time to return of genetic results with median denoted for traditional and mainstream testing pathways. P-value per log-rank test. (B) Temporal schematic of specific actions that occur within each pathway with times between steps noted in weeks.
Diagnostic Rates Using the ADPKD Panels
We identified a diagnostic pathogenic or likely pathogenic variant in 11 of 18 patients (61%); 7 truncating PKD1 variants, 3 non-truncating PKD1 variants, and 1 PKD2 variant. Five patients (28%) had a variant of uncertain significance (VUS) which were rare missense changes within PKD1 and 2 patients (11%) had negative results. Eleven of the identified variants have not been described in the literature. The specific mutations and American College of Medical Genetics (ACMG) Classifications are provided in Supplementary Table 1. The genetic results led to cascade testing of family members in 1 case and segregation analysis in 2 cases to confirm a de novo variant. All the VUS cases are able to be reclassified if segregation and phasing data becomes available.
Patient Survey Results
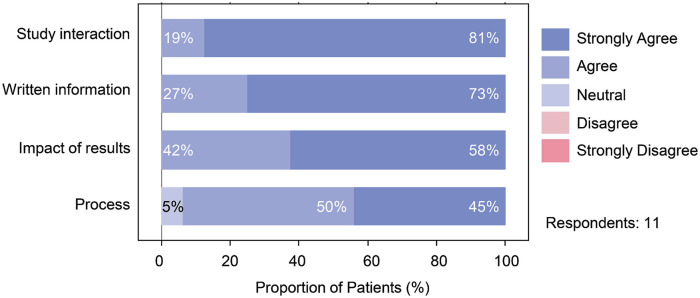
Altogether, 11 patients responded to the surveys. Survey results showed patients were satisfied with the MGT pathway we developed in all 4 major survey themes (Figure 4). No patient concerns were raised within the free text boxes, with most saying the information and process left them satisfied or very satisfied. Overall the patients felt that the information they received based on the developed checklists and information sheet was appropriate for the setting.
Figure 4.
Patient survey results categorized by major theme addressed in each question.
Note. Patient responses to the mainstreaming genetic testing for PKD for the categories of study interaction, written information, impact of results and process. PKD = polycystic kidney disease.
Limitations
Our study had several limitations. The single-center nature may limit generalizability to other centers and health systems, especially given that we had access to clinical quality genetic testing for all included patients that was fully funded by our universal health care system. The use of a historic control sample for comparisons introduces the risk of confounding based on the standard of care at time that testing was offered compared to the standardized mainstream cohort. We did not collect significant characteristic data between the two groups so there could be an unmeasured imbalance between the groups hat has led to the difference in time to return results that we have seen. The small sample size of patients who underwent testing means there were fewer genetic results to analyze than we had planned. Nonetheless, our diagnostic rates are consistent with the existing literature. The surveys used were not developed using a Delphi process and did not undergo pilot testing prior to their use broadly within the study which limits our ability to generalize their answers beyond those who returned a survey.
Implications
We have successfully developed and implemented an efficient, mainstream approach to genetic testing in patients with ADPKD that leverages a collaboration between nephrology and medical genetics teams that may be suitable for other publicly funded centers. We developed new resources for nephrologists to carry out pretest counseling and consent, selection of the appropriate testing panel, and arrangement for sample collection. Integration of the medical genetics team for ordering tests, interpretation of results and follow-up for the mainstream pathway minimized deviation from standard practice while educating nephrologists and expediting return of genetic testing results. One of the main reasons for the significant time to return results in the traditional cohort is a limited supply of medical genetics experts, leading to long wait times and delays (Figure 3B). By eliminating the wait time associated with the initial clinic visit for assessment by medical genetics, the mainstream pathway returned results to patients faster and minimized the direct patient counseling by the genetics team for patients without an identified variant. The survey data showed patients are comfortable with the pathway and in the ability of nephrologists without specialized genetics training to provide pre-testing care, which aligns with patient experiences in mainstreaming in other fields, but our data does not allow the direct comparison to the patient experiences in the historic cohort who received testing through the traditional pathway.15,21
Our mainstream pathway for ADPKD genetic testing has a number of features that lend it scalability and generalizability. The use of commercially available gene panels allows any center with public funding to implement our mainstream pathway genetic testing without the need for in-house sequencing and interpretation capabilities. Moreover, the genetic testing has flexibility as new genes can easily be added to panels as they are validated as causative genes. The use of general nephrologists for pretest care reduces the burden for the medical genetics team from significant involvement with patients without an identified mutation, allowing them to focus on patients with positive and VUS results. The mainstream pathway has the potential to be adapted to other conditions for which there are known genetic causes as the educational materials and the gene panels can be modified as appropriate while the core workflow remains constant.
Specialized clinics focusing on genetic kidney disease have been previously described but they differ from the pathway presented here because they have relied heavily on genetic counselors to do the pre-testing counseling and consent.43,45,49,50 These approaches may limit access to genetic testing as they rely heavily on professionals with significant knowledge in genetics, who are in short supply. Recent data shows nephrologists are enthusiastic about genetic testing and are most comfortable with consenting for genetic testing, ordering testing, and integrating results into clinical care. Often challenging is selecting the right test, interpreting the results, and choosing the appropriate patients to test. 14 The pathway we have developed allows nephrologists to perform the tasks they feel best suited to perform, while addressing the more challenging aspects through standardization and direct integration of medical genetics. It should be noted that mainstreaming approaches for hereditary breast and ovarian cancers have been shown to, at times, deviate from guideline based standards of care. 51 However, the clinical tools developed for the mainstreaming pathway in this study directly address many of the cited shortfalls, including a session for pre-test counseling, discussion of familial and management implications, discussion of variant result terminology and the inclusion of a pedigree. These tools are included in the Supplementary Figures to help others implement this mainstream pathway.
Patients with kidney disease have identified genetic testing as an important aspect of their care and recognize the impact a molecular diagnosis can have on prognosis, management, transplant, and family planning. 25 Patients have advocated for timely and broad access to genetic testing and the pathway we present allows for both these goals to be met. 41 Our pathway can serve as a flexible and efficient model to build off of in the future as the utility of genetic testing grows and is integrated into the care of a broader set of patients.
The overall positive diagnostic rate in our pathway of 61% was lower than expected, and the VUS rate (28%) was higher than expected.30-34 We compared our diagnostic rates to two contemporary papers that also utilize ACGM consensus definitions for variant interpretation.30,35,52 By comparison, Schönauer et al had a diagnostic rate of 81% pathogenic (P) or likely pathogenic (LP) with 9% VUS and 10% negative cases, while Mantovani et al had a diagnostic rate of 61% P or LP with 13% VUS, 35% negative.30,35 This shows the patient selection criteria, gene panels, and the use of commercially available genetic testing in our mainstream pathway produce similar diagnostic outcomes when compared to specialized research testing environments which is important if it is to be applied in other centers and for other genetic kidney diseases. The VUS identified in our study are all missense variants within PKD1 where in silico analysis and population level data suggest pathogenicity. With segregation analysis and phasing data they all have the opportunity to be reclassified to pathogenic or likely pathogenic. A reclassification toward pathogenicity would align our diagnostic rate with the existing literature that has relied heavily on segregation data for these types of variants.30-34 This shows the importance of the mainstream pathway’s integrated approach to genetic testing as the VUS results will require follow-up and further testing by the medical genetics team that is likely beyond the clinical scope of a non-genetics expert.
In summary, we describe a mainstream pathway for genetic testing of patients with ADPKD that led to significantly faster return of results compared to a historic control, with a reasonable variant identification rate. The pathway is designed to be scalable and flexible and we hope it can be adapted to other centers and genetic conditions to improve the care of patients with kidney disease.
Supplemental Material
Supplemental material, sj-docx-1-cjk-10.1177_20543581211055001 for Mainstreaming Genetic Testing for Adult Patients With Autosomal Dominant Polycystic Kidney Disease by Mark D. Elliott, Leslie C. James, Emily L. Simms, Priyana Sharma, Louis P. Girard, Kim Cheema, Meghan J. Elliott, Julie L. Lauzon and Justin Chun in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-2-cjk-10.1177_20543581211055001 for Mainstreaming Genetic Testing for Adult Patients With Autosomal Dominant Polycystic Kidney Disease by Mark D. Elliott, Leslie C. James, Emily L. Simms, Priyana Sharma, Louis P. Girard, Kim Cheema, Meghan J. Elliott, Julie L. Lauzon and Justin Chun in Canadian Journal of Kidney Health and Disease
Acknowledgments
Special thanks to Dr. Daniel Muruve, Dr. Nairne Scott-Douglas, Dr. Jeffrey Ma, Dr. Pietro Ravani, Dr. Elena Qirjazi, Dr. Sophia Chou, and Dr. Adam Bass for helpful discussions and coordinating the launch of this initiative.
Footnotes
Ethics Approval and Consent to Participate: All participants provided their informed consent for a study protocol that was approved by the University of Calgary Research Ethics Board.
Consent for Publication: All authors have given their consent for publication of this article.
Availability of Data and Materials: Data and materials are not available for this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Meghan J. Elliott  https://orcid.org/0000-0002-5434-2917
https://orcid.org/0000-0002-5434-2917
Justin Chun  https://orcid.org/0000-0002-3820-7192
https://orcid.org/0000-0002-3820-7192
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Groopman EE, Marasa M, Cameron-Christie S, et al. Diagnostic utility of exome sequencing for kidney disease. N Engl J Med. 2019;380:142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cameron-Christie S, Wolock CJ, Groopman E, et al. Exome-based rare-variant analyses in CKD. J Am Soc Nephrol. 2019;30(6):1109-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Connaughton DM, Kennedy C, Shril S, et al. Monogenic causes of chronic kidney disease in adults. Kidney Int. 2019;95(4):914-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jayasinghe K, Quinlan C, Stark Z, et al. Renal genetics in Australia: kidney medicine in the genomic age. Nephrology (Carlton). 2019;24(3):279-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mann N, Braun DA, Amann K, et al. Whole-exome sequencing enables a precision medicine approach for kidney transplant recipients. J Am Soc Nephrol. 2019;30(2):201-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cornec-Le Gall E, Audrézet MP, Rousseau A, et al. The PROPKD score: a new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2016;27(3):942-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Devuyst O, Knoers NVAM, Remuzzi G, et al. Rare inherited kidney diseases: challenges, opportunities, and perspectives. Lancet. 2014;383:1844-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rasouly HM, Groopman EE, Heyman-Kantor R, et al. The burden of Candidate pathogenic variants for kidney and genitourinary disorders emerging from exome sequencing. Ann Intern Med. 2019;170:11-21. [DOI] [PubMed] [Google Scholar]
- 9. Cocchi E, Nestor JG, Gharavi AG. Clinical genetic screening in adult patients with kidney disease. Clin J Am Soc Nephrol. 2020;15:1497-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gordon EJ, Wicklund C, Lee J, Sharp RR, Friedewald J. A national survey of transplant surgeons and nephrologists on implementing apolipoprotein L1 (APOL1) genetic testing into clinical practice. Prog Transpl. 2019;29:26-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabatello M, Milo Rasouly H. The ethics of genetic testing for kidney diseases. Nat Rev Nephrol. 2020;16(11):619-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delikurt T, Williamson GR, Anastasiadou V, Skirton H. A systematic review of factors that act as barriers to patient referral to genetic services. Eur J Hum Genet. 2015;23(6):739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. White S, Jacobs C, Phillips J. Mainstreaming genetics and genomics: a systematic review of the barriers and facilitators for nurses and physicians in secondary and tertiary care. Genet Med. 2020;22(7):1149-1155. [DOI] [PubMed] [Google Scholar]
- 14. Jayasinghe K, Quinlan C, Mallett AJ, et al. Attitudes and practices of Australian nephrologists toward implementation of clinical genomics. Kidney Int Rep. 2020;6:272-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. George A, Riddell D, Seal S, et al. Implementing rapid, robust, cost-effective, patient-centred, routine genetic testing in ovarian cancer patients. Sci Rep. 2016;6:29506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hallowell N, Wright S, Stirling D, Gourley C, Young O, Porteous M. Moving into the mainstream: healthcare professionals’ views of implementing treatment focussed genetic testing in breast cancer care. Fam Cancer. 2019;18(3):293-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scheinberg T, Young A, Woo H, Goodwin A, Mahon KL, Horvath LG. Mainstream consent programs for genetic counseling in cancer patients: a systematic review. Asia Pac J Clin Oncol. 2021;17:163-177. doi: 10.1111/ajco.13334. [DOI] [PubMed] [Google Scholar]
- 18. Kemp Z, Turnbull A, Yost S, et al. Evaluation of cancer-based criteria for use in mainstream BRCA1 and BRCA2 genetic testing in patients with breast cancer. JAMA Netw Open. 2019;2:e194428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eccleston A, Bentley A, Dyer M, et al. A cost-effectiveness evaluation of germline BRCA1 and BRCA2 testing in UK women with ovarian cancer. Value Health. 2017;20(4):567-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bennett CL, Burke SE, Burton H, Farndon PA. A toolkit for incorporating genetics into mainstream medical services: learning from service development pilots in England. BMC Health Serv Res. 2010;10:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rahman B, Lanceley A, Kristeleit RS, et al. Mainstreamed genetic testing for women with ovarian cancer: first-year experience. J Med Genet. 2019;56(3):195-198. [DOI] [PubMed] [Google Scholar]
- 22. Kentwell M, Dow E, Antill Y, et al. Mainstreaming cancer genetics: a model integrating germline BRCA testing into routine ovarian cancer clinics. Gynecol Oncol. 2017;145(1):130-136. [DOI] [PubMed] [Google Scholar]
- 23. Nilsson MP, Nilsson ED, Borg Å, Brandberg Y, Silfverberg B, Loman N. High patient satisfaction with a simplified BRCA1/2 testing procedure: long-term results of a prospective study. Breast Cancer Res Treat. 2019;173(2):313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Høberg-Vetti H, Bjorvatn C, Fiane BE, et al. BRCA1/2 testing in newly diagnosed breast and ovarian cancer patients without prior genetic counselling: the DNA-BONus study. Eur J Hum Genet. 2016;24(6):881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Odland D. A patient perspective on genetic testing for ADPKD: the lack of complete genetic information, especially early in the course of the disease, is harming adult autosomal dominant polycystic kidney disease (ADPKD) patients. Clin J Am Soc Nephrol. 2021;16:671-673. doi: 10.2215/CJN.14051119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saran R, Robinson B, Abbott KC, et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75:A6-A7. [DOI] [PubMed] [Google Scholar]
- 27. Levy M, Feingold J. Estimating prevalence in single-gene kidney diseases progressing to renal failure. Kidney Int. 2000;58(3):925-943. [DOI] [PubMed] [Google Scholar]
- 28. Soroka S, Alam A, Bevilacqua M, et al. Updated Canadian expert consensus on assessing risk of disease progression and pharmacological management of autosomal dominant polycystic kidney disease [published online ahead of print October 12, 2018]. Can J Kidney Health Dis. doi: 10.1177/2054358118801589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chapman AB, Devuyst O, Eckardt K-U, et al. Autosomal dominant polycystic kidney disease (ADPKD): executive summary from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. 2015;88(1):17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schönauer R, Baatz S, Nemitz-Kliemchen M, et al. Matching clinical and genetic diagnoses in autosomal dominant polycystic kidney disease reveals novel phenocopies and potential candidate genes. Genet Med. 2020;22(8):1374-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cornec-Le Gall E, Audrézet MP, Chen JM, et al. Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol. 2013;24(6):1006-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rossetti S, Consugar MB, Chapman AB, et al. Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2007;18(7):2143-2160. [DOI] [PubMed] [Google Scholar]
- 33. Iliuta I-A, Kalatharan V, Wang K, et al. Polycystic kidney disease without an apparent family history. J Am Soc Nephrol. 2017;28:2768-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Audrézet MP, Cornec-Le Gall E, Chen JM, et al. Autosomal dominant polycystic kidney disease: comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum Mutat. 2012;33(8):1239-1250. [DOI] [PubMed] [Google Scholar]
- 35. Mantovani V, Bin S, Graziano C, et al. Gene panel analysis in a large cohort of patients with autosomal dominant polycystic kidney disease allows the identification of 80 potentially causative novel variants and the characterization of a complex genetic architecture in a subset of families. Front Genet. 2020;11:464. doi: 10.3389/fgene.2020.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cornec-Le Gall E, Torres VE, Harris PC. Genetic complexity of autosomal dominant polycystic kidney and liver diseases. J Am Soc Nephrol. 2018;29(1):13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lanktree MB, Haghighi A, di Bari I, Song X, Pei Y. Insights into autosomal dominant polycystic kidney disease from genetic studies. Clin J Am Soc Nephrol. 2021;16:790-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harris PC, Torres VE. Polycystic kidney disease, autosomal dominant. In: Adam MP, Ardinger HH, Pagon RA, et al., eds. GeneReviews®. Seattle: University of Washington, Seattle; 1993. http://www.ncbi.nlm.nih.gov/books/NBK1246/. Accessed 11 January 2021. [Google Scholar]
- 39. Simms RJ, Travis DL, Durkie M, Wilson G, Dalton A, Ong ACM. Genetic testing in the assessment of living related kidney donors at risk of autosomal dominant polycystic kidney disease. Transplantation. 2015;99:1023-1029. [DOI] [PubMed] [Google Scholar]
- 40. Groopman EE, Rasouly HM, Gharavi AG. Genomic medicine for kidney disease. Nat Rev Nephrol. 2018;14:83-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Verma M. Genetic testing is beneficial to the entire family. Clin J Am Soc Nephrol. 2020;15:1224-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mallett A, Corney C, McCarthy H, Alexander SI, Healy H. Genomics in the renal clinic—translating nephrogenetics for clinical practice. Hum Genomics. 2015;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mallett A, Fowles LF, McGaughran J, Healy H, Patel C. A multidisciplinary renal genetics clinic improves patient diagnosis. Med J Aust. 2016;204:58-59. [DOI] [PubMed] [Google Scholar]
- 44. Nestor JG, Marasa M, Milo-Rasouly H, et al. Pilot study of return of genetic results to patients in adult nephrology. Clin J Am Soc Nephrol. 2020;15:651-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thomas CP, Freese ME, Ounda A, et al. Initial experience from a renal genetics clinic demonstrates a distinct role in patient management. Genet Med. 2020;22:1025-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pei Y, Obaji J, Dupuis A, et al. Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol. 2009;20(1):205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Irazabal MV, Rangel LJ, Bergstralh EJ, et al. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. J Am Soc Nephrol. 2015;26(1):160-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. https://www.R-project.org/. [Google Scholar]
- 49. Lundquist AL, Pelletier RC, Leonard CE, et al. From theory to reality: establishing a successful kidney genetics clinic in the outpatient setting. Kidney360. 2020;1:1099-1106. doi: 10.34067/KID.0004262020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alkanderi S, Yates LM, Johnson SA, Sayer JA. Lessons learned from a multidisciplinary renal genetics clinic. QJM. 2017;110:453-457. [DOI] [PubMed] [Google Scholar]
- 51. Vadaparampil ST, Scherr CL, Cragun D, Malo TL, Pal T. Pre-test genetic counseling services for hereditary breast and ovarian cancer delivered by non-genetics professionals in the state of Florida. Clin Genet. 2015;87:473-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cjk-10.1177_20543581211055001 for Mainstreaming Genetic Testing for Adult Patients With Autosomal Dominant Polycystic Kidney Disease by Mark D. Elliott, Leslie C. James, Emily L. Simms, Priyana Sharma, Louis P. Girard, Kim Cheema, Meghan J. Elliott, Julie L. Lauzon and Justin Chun in Canadian Journal of Kidney Health and Disease
Supplemental material, sj-docx-2-cjk-10.1177_20543581211055001 for Mainstreaming Genetic Testing for Adult Patients With Autosomal Dominant Polycystic Kidney Disease by Mark D. Elliott, Leslie C. James, Emily L. Simms, Priyana Sharma, Louis P. Girard, Kim Cheema, Meghan J. Elliott, Julie L. Lauzon and Justin Chun in Canadian Journal of Kidney Health and Disease