Abstract
Objective
We aimed to describe the differences in clinicopathological characteristics and overall survival (OS) between male and female breast cancer patients, and to develop a prognostic nomogram to predict survival in patients with male breast cancer (MBC).
Methods
Using the Surveillance, Epidemiology, and End Results database, we compared age, race, histological type, histological grade, tumor size, lymph node status, metastases, estrogen/progesterone receptor (ER/PR) and HER-2 status between male and female patients, and analyzed their relationships with OS. We established a nomogram and produced a calibration curve to observe its predictive effect.
Results
Age, race, T stage, N stage, bone and lung metastases, and histological type and grade differed between male and female patients. OS in male patients was related to age, tumor size, metastatic site, ER/PR status, and histological grade, but not to race or lymph node status. A nomogram was established, which showed good predictive performance for survival in MBC patients (area under the curve = 0.7).
Conclusion
MBC has a worse prognosis than female breast cancer, mainly characterized by late onset age, late staging, high proportion of invasive non-specific histological types, high histological grade, and luminal breast cancer.
Keywords: Breast cancer, male, clinical feature, prognosis, nomogram, survival
Introduction
Male breast cancer (MBC) is a relatively rare malignant tumor accounting for less than 1.0% of all breast cancers. 1 However, despite its rarity, the incidence of MBC has increased in recent years. 2 , 3 In addition, although breast cancer accounts for a relatively small proportion of all cancers diagnosed in men, it has a high mortality rate, 4 highlighting the need for continued research into MBC.
Many aspects of MBC remain unclear, and its biological characteristics, especially the differences between male and female breast cancer (FBC), should be further studied to determine the relevance of any differences in treatment. There are currently no prospective, international clinical trial results regarding MBC and no diagnostic specifications or guidelines. The diagnosis and treatment of MBC thus currently refer to breast cancer in women; however, MBC has several different characteristics compared with FBC,5,6 and adhering to the treatment protocol for FBC thus remains controversial. Research into MBC groups is thus needed to develop guidelines and recommendations to improve the prognosis of MBC.
Using a large dataset extracted from the Surveillance, Epidemiology, and End Results (SEER) database, we investigated the clinical and prognostic differences between MBC and FBC, and the factors contributing to differences in overall survival (OS).
Patients and Methods
Patient information
We retrospectively analyzed data for patients diagnosed with breast cancer in the SEER database between 2010 and 2015, including age, race, hormone receptor status, tumor size, lymph node status, HER-2 status, pathological type, and histological grade. The inclusion criteria were: (1) clear diagnosis and pathologically confirmed breast cancer; and (2) complete and clear clinicopathological data including age, stage, hormone receptor status, HER-2 status, pathological type, and histological grade. Patients with unclear pathological characteristics were excluded. The reporting of this study conforms to the STROBE statement. 7 We accessed SEER information after signing the SEER research data agreement (username10866-Nov2019). Data were obtained following approved guidelines. The ethics committee of Xi’an International Medical Center Hospital waived the need for patient consent because the subjects were patients who had been researched by the United States Department of Health and Human Services and the data were publicly accessible and deidentified.
Construction and validation of the nomogram
All significant variables in univariate analysis were entered into multivariate Cox proportional hazards analysis. A nomogram based on the results of this model was established to predict 3- and 5-year OS, and a calibration curve was produced to observe the predictive effect of the nomogram.
Statistical analysis
The data were analyzed using SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). Differences between groups were compared by χ2 tests. Survival was analyzed using the Kaplan–Meier method. A multivariate Cox proportional risk model was used to identify the clinical factors associated with OS survival in men and women. The significance level was α = 0.05. R software (version 4.0.3; R Foundation for Statistical Computing, Frank Harrell, USA) was used to construct the nomogram and draw the receiver operating characteristic (ROC) curve. A bootstrap method was used to repeat 1000 samples for internal verification of the rosette. The concordance index (C-index) and area under the ROC curve (AUC) were used to evaluate the differentiation ability of the nomogram, and calibration curves were used to evaluate its calibration.
Results
Clinicopathological characteristics of the patients
According to the inclusion and exclusion criteria, a total of 5484 patients were enrolled in this study, including 670 MBC and 4814 FBC patients. There were significant differences in age, race, T stage, N stage, metastasis, histological type, and histological grade between the two groups (all P < 0.05). Male patients were older, with late clinical stage, poor histological grade, and more invasive non-specific types of histology, mostly luminal breast cancer. Notably, the incidence of MBC was significantly lower than that of FBC among blacks (16.12% vs 24.30%). There were also differences in the proportions of bone and lung metastases between the two groups, but no differences in liver and brain metastases. In addition to differences in luminal type, the HER-2(+) rate was also lower in MBC compared with FBC (Table 1).
Table 1.
Clinicopathological characteristics.
| Group | MBC (n, %) | FBC (n, %) | χ2 | P value |
|---|---|---|---|---|
| Age (years) | ||||
| <35 | 0 (0) | 65 (1.35) | 83.185 | <0.001 |
| 35–59 | 193 (28.8) | 2183 (45.35) | ||
| ≥60 | 477 (71.2) | 2566 (53.30) | ||
| Race | ||||
| White | 529 (78.96) | 3551 (73.76) | 73.286 | <0.001 |
| Black | 108 (16.12) | 1170 (24.30) | ||
| Other | 33 (4.93) | 93 (1.93) | ||
| Histological type | ||||
| Non-specific infiltrating Infiltrating Infiltrating | 569 (84.9) | 3836 (79.68) | 25.001 | <0.001 |
| Other | 101 (15.1) | 978 (20.32) | ||
| Tumor size | ||||
| T1 | 249 (37.1) | 2894 (60.12) | 387.685 | <0.001 |
| T2 | 295 (44.0) | 1438 (29.87) | ||
| T3 | 31 (4.6) | 284 (5.9) | ||
| T4 | 95 (14.1) | 198 (4.11) | ||
| Lymph node status | ||||
| Negative | 220 (32.8) | 3019 (62.71) | 425.558 | <0.001 |
| Positive | 450 (67.2) | 1795 (37.29) | ||
| Distant metastasis | ||||
| Bone | 47 (7.01) | 101 (20.98) | 84.760 | <0.001 |
| Brain | 2 (0.3) | 20 (0.42) | 0.479 | 0.489 |
| Liver | 2 (0.3) | 30 (0.62) | 1.832 | 0.176 |
| Lung | 18 (2.69) | 45 (0.93) | 21.175 | <0.001 |
| ER/PR | ||||
| ER(−)/PR(+) | 3 (0.4) | 74 (1.54) | 167.133 | <0.001 |
| ER(+)/PR(−) | 45 (6.7) | 537 (11.15) | ||
| ER(+)/PR(+) | 599 (89.4) | 3391 (70.44) | ||
| ER(−)/PR(−) | 23 (3.4) | 812 (16.87) | ||
| HER-2 status | ||||
| Negative | 589 (87.9) | 4051 (84.15) | 6.992 | 0.008 |
| Positive | 81 (12.1) | 763 (15.85) | ||
| Histological grade | ||||
| I | 80 (11.94) | 1035 (21.5) | 87.756 | <0.001 |
| II | 331 (49.4) | 2025 (42.06) | ||
| III | 256 (38.21) | 1748 (36.31) | ||
| IV | 3 (0.45) | 6 (0.12) | ||
MBC, male breast cancer; FBC, female breast cancer; ER, estrogen receptor; PR, progesterone receptor.
Survival
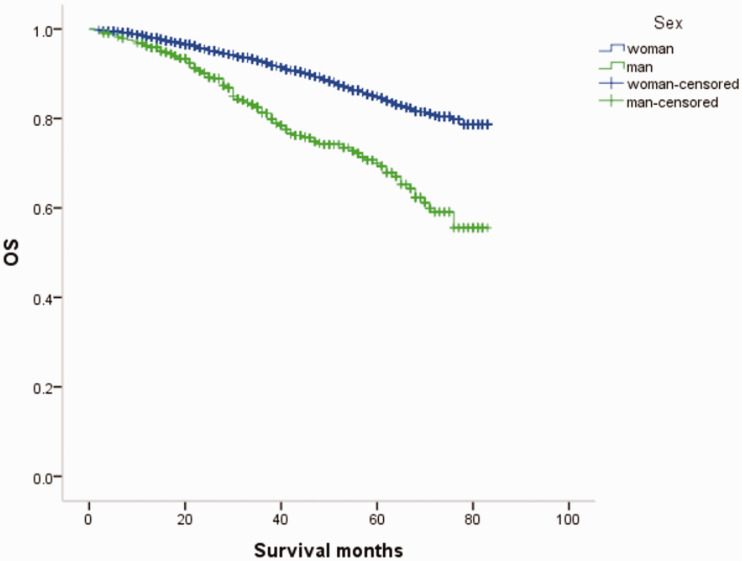
The prognosis in terms of OS was significantly lower in men compared with women with breast cancer (P < 0.05). The 7-year overall survival rates of male and FBC were 77.9% and 89.8%, respectively (Figure 1).
Figure 1.
Male and female breast cancer overall survival (OS) curves.
Prognostic analysis of univariate and multivariate Cox proportional risk models
We conducted univariate analysis of factors that might influence the prognosis of breast cancer, and showed that age, race, tumor size, lymph node metastasis, distant metastasis, histological grading, and estrogen receptor (ER)/progesterone receptor (PR) status affected the prognosis of MBC patients in terms of OS, and also influenced the prognosis of FBC (P < 0.01, Table 2 and Figures 2–19).
Table 2.
Univariate analysis of prognoses of male and female breast cancer.
| Group | MBC |
FBC |
||
|---|---|---|---|---|
| χ2 | P value | χ2 | P value | |
| Age | 5.720 | 0.017 | 6.489 | 0.011 |
| Race | 83.185 | <0.001 | 83.185 | <0.001 |
| Histological type | 0.986 | 0.321 | 0.156 | 0.693 |
| Histological grade | 40.552 | <0.001 | 153.909 | <0.001 |
| Tumor size | 60.741 | <0.001 | 430.876 | <0.001 |
| Lymph node metastasis | 4.030 | 0.045 | 180.184 | <0.001 |
| Distant metastasis | 148.856 | <0.001 | 812.383 | <0.001 |
| ER/PR status | 38.977 | <0.001 | 164.792 | <0.001 |
| HER-2 status | 0.268 | 0.605 | 1.060 | 0.303 |
MBC, male breast cancer; FBC, female breast cancer; ER, estrogen receptor; PR, progesterone receptor.
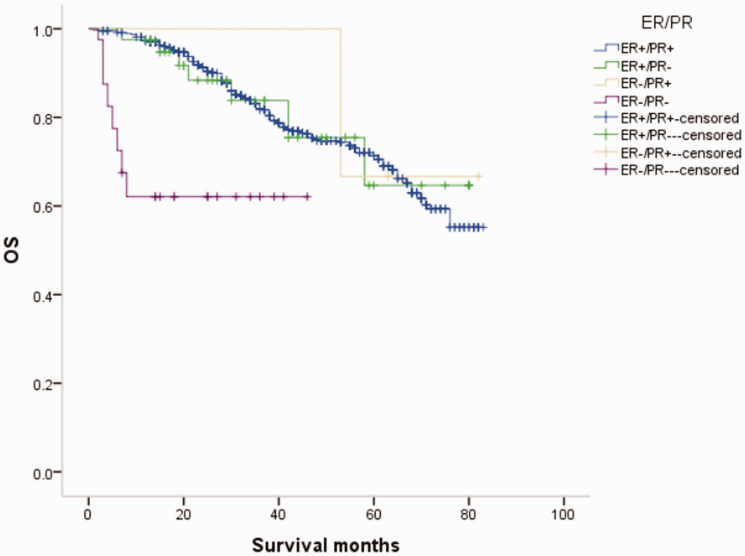
Figure 2.
Overall survival (OS) curves of patients with male breast cancer in relation to estrogen receptor (ER)/progesterone receptor (PR) status.
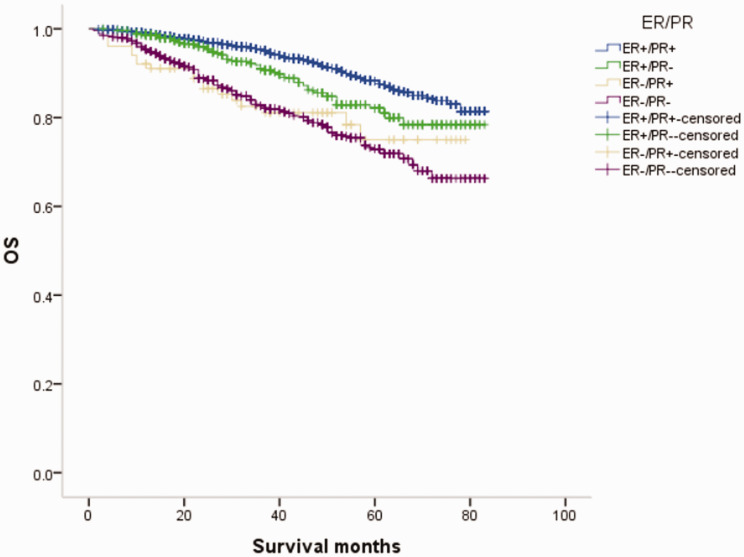
Figure 3.
Overall survival (OS) curves of patients with female breast cancer in relation to estrogen receptor (ER)/progesterone receptor (PR) status.
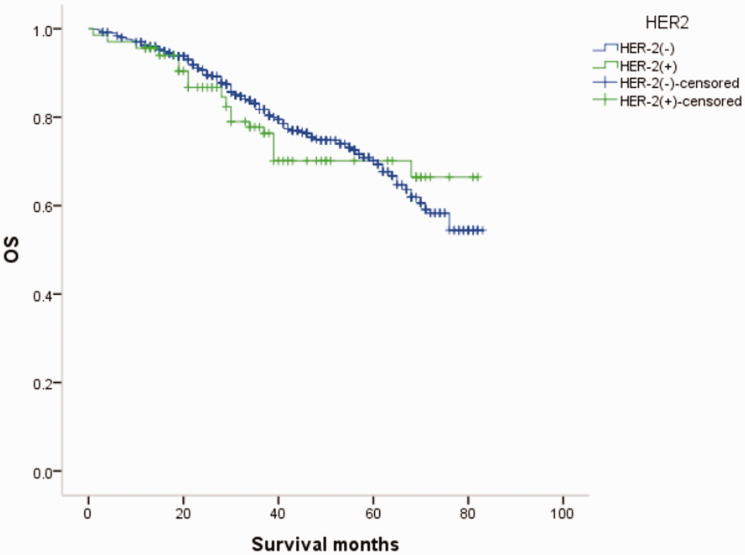
Figure 4.
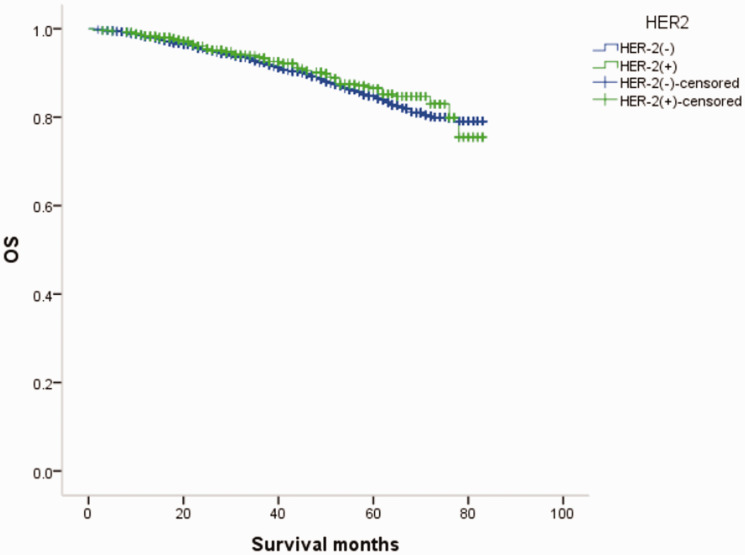
Overall survival (OS) curves of patients with male breast cancer in relation to HER-2 status.
Figure 5.
Overall survival (OS) curves of patients with female breast cancer in relation to HER-2 status.
Figure 6.
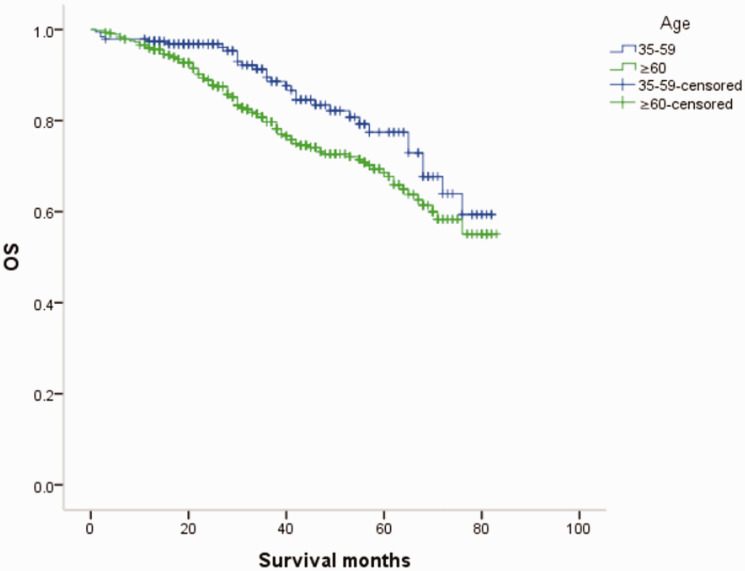
Overall survival (OS) curves of patients with male breast cancer in relation to age.
Figure 7.
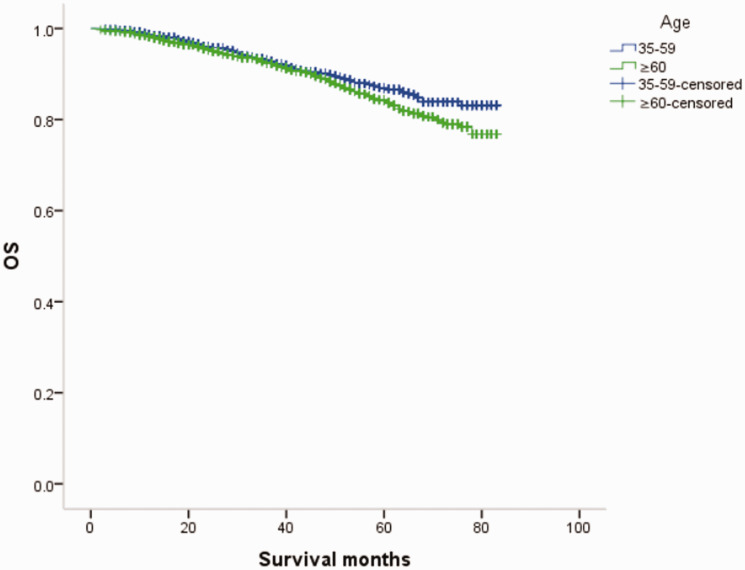
Overall survival (OS) curves of patients with female breast cancer in relation to age.
Figure 8.
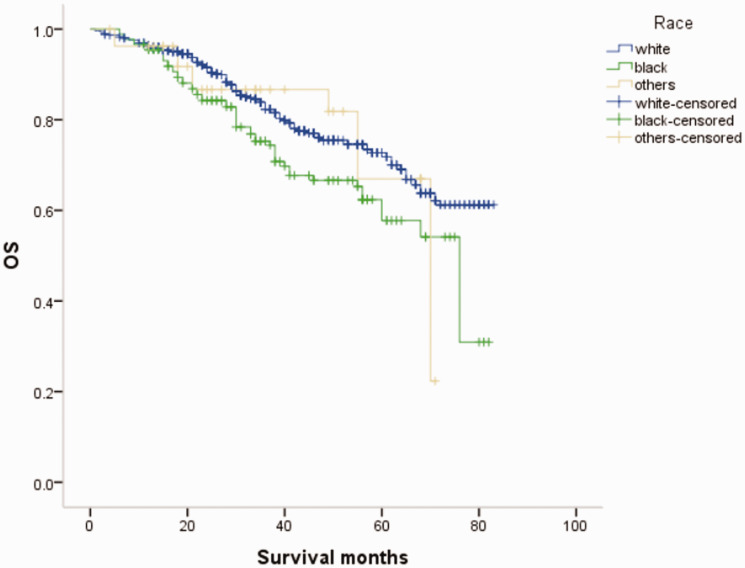
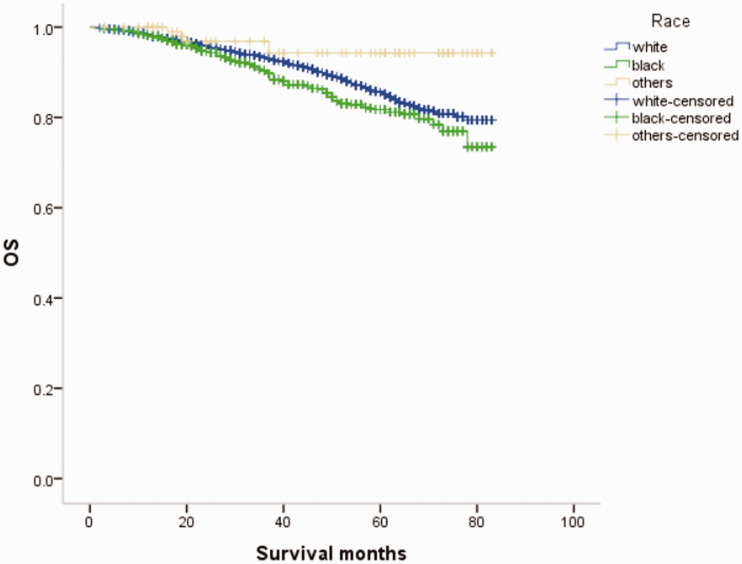
Overall survival (OS) curves of patients with male breast cancer in relation to race.
Figure 9.
Overall survival (OS) curves of patients with female breast cancer in relation to race.
Figure 10.
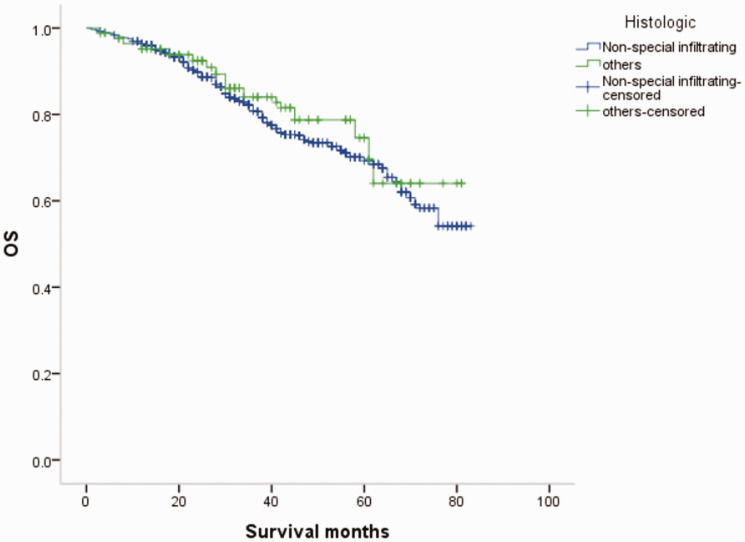
Overall survival (OS) curves of patients with male breast cancer in relation to histological type.
Figure 11.
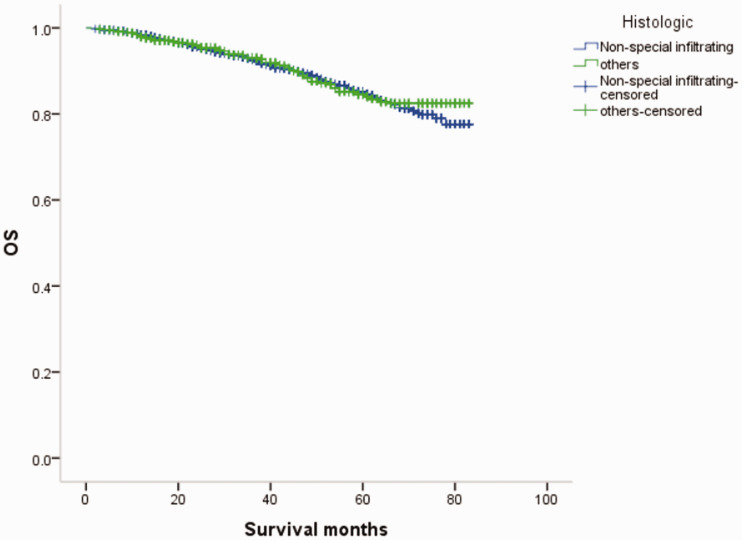
Overall survival curves (OS) of patients with female breast cancer in relation to histological type.
Figure 12.
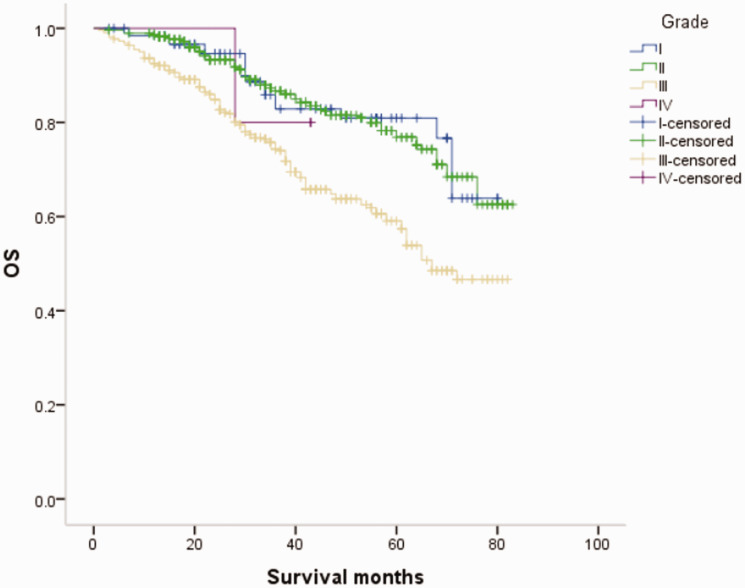
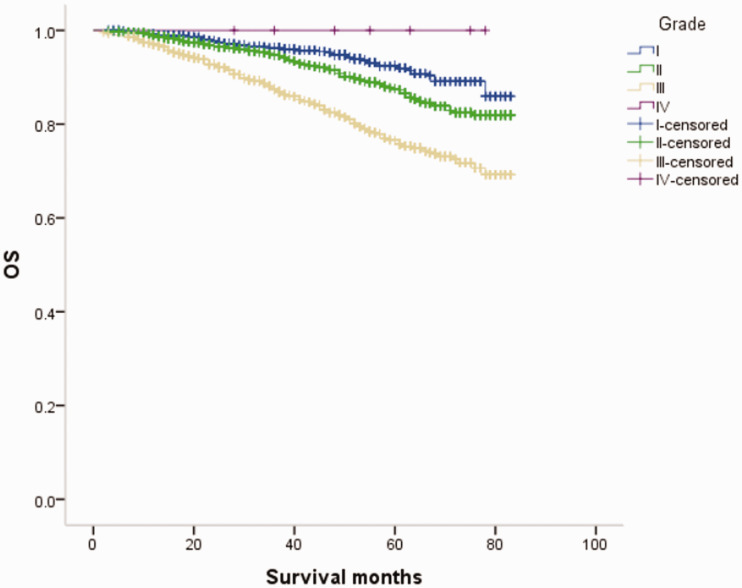
Overall survival (OS) curves of patients with male breast cancer in relation to histological grade.
Figure 13.
Overall survival (OS) curves of patients with female breast cancer in relation to histological grade.
Figure 14.
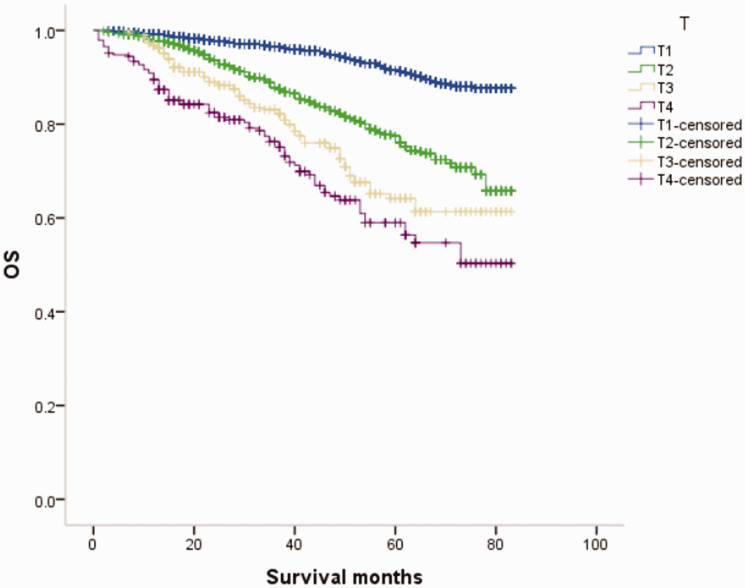
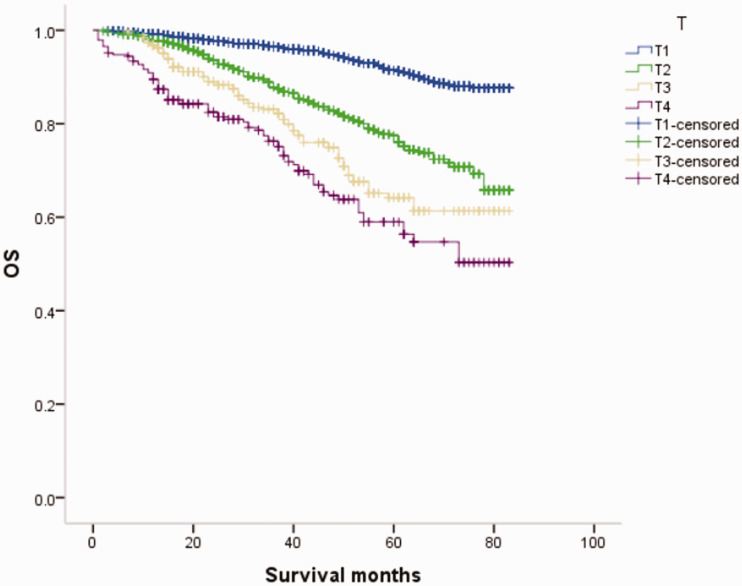
Overall survival (OS) curves of patients with male breast cancer in relation to T stage.
Figure 15.
Overall survival (OS) curves of patients with female breast cancer in relation to T stage.
Figure 16.
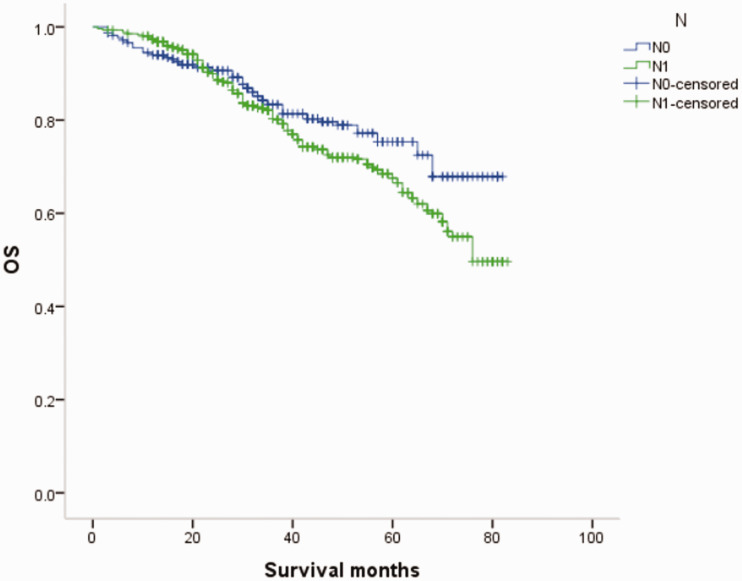
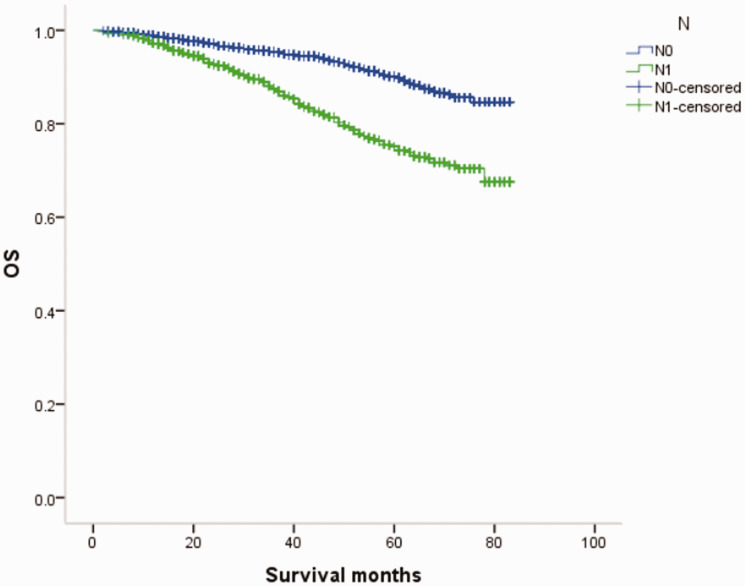
Overall survival (OS) curves of patients with male breast cancer in relation to N stage.
Figure 17.
Overall survival (OS) curves of patients with female breast cancer in relation to N stage.
Figure 18.
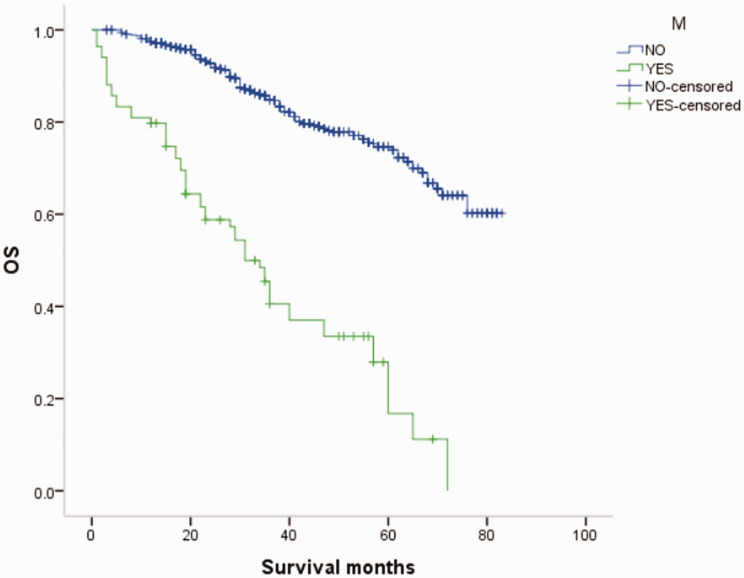
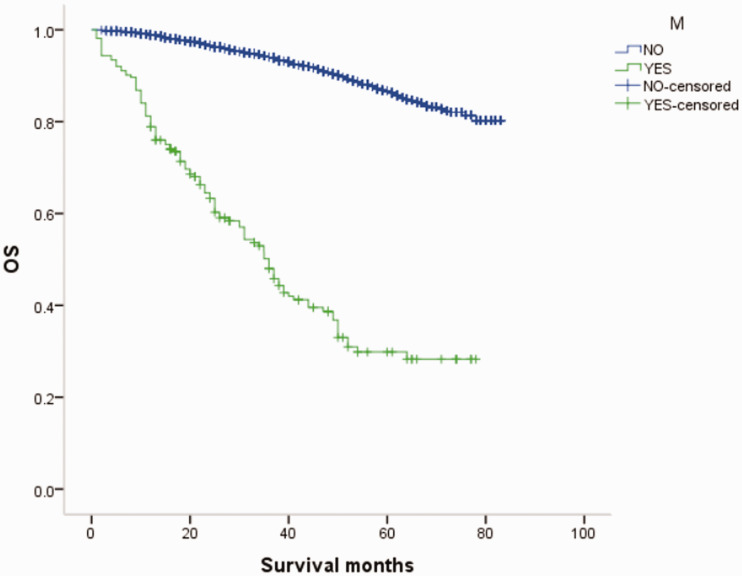
Overall survival (OS) curves of patients with male breast cancer in relation to M stage.
Figure 19.
Overall survival (OS) curves of patients with female breast cancer in relation to M stage.
Significant factors from univariate analysis of OS were included in a Cox proportional risk model for multivariate analysis, which identified age, tumor size, metastatic site, ER/PR status, and histological grade as independent prognostic factors affecting OS of MBC patients. Compared with MBC, OS of FBC patients was related to the above factors, and also to race and lymph node metastasis (P < 0.01, Tables 3 and 4)
Table 3.
Multivariate Cox regression analysis of male breast cancer patients.
| Group | Regression coefficient | P | HR | 95% CI |
|---|---|---|---|---|
| Age | 0.627 | 0.001 | 1.872 | (1.278, 2.734) |
| Tumor size | 0.277 | <0.001 | 1.319 | (1.178, 1.477) |
| Distant metastasis | 1.496 | <0.001 | 4.463 | (3.225, 6.174) |
| ER/PR | 0.374 | <0.001 | 1.453 | (1.214, 1.740) |
| Histological grade | 0.448 | <0.001 | 1.565 | (1.271, 1.928) |
HR, hazard ratio; CI, confidence interval, ER, estrogen receptor; PR, progesterone receptor.
Table 4.
Multivariate Cox regression analysis of female breast cancer patients.
| Group | Regression coefficient | P | HR | 95% CI |
|---|---|---|---|---|
| Age | 0.414 | <0.001 | 1.513 | (1.279, 1.789) |
| Race | −0.174 | 0.014 | 0.841 | (0.731, 0.966) |
| Tumor size | 0.367 | <0.001 | 1.443 | (1.329, 1.567) |
| Lymph node metastasis | 0.427 | <0.001 | 1.533 | (1.296, 1.812) |
| Distant metastasis | 1.656 | <0.001 | 5.240 | (4.201, 6.535) |
| ER/PR | 0.252 | <0.001 | 1.287 | (1.212, 1.367) |
| Histological grade | 0.207 | 0.001 | 1.229 | (1.090, 1.387) |
HR, hazard ratio; CI, confidence interval, ER, estrogen receptor; PR, progesterone receptor.
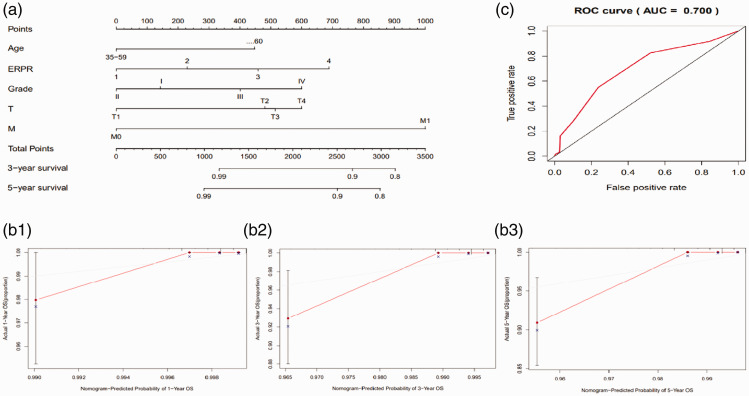
Nomogram and area under the curve (AUC)
We established a nomogram based on the patients’ age, ER/PR status, tumor grade, and T and M stages (Figure 20a). C-index and calibration plots were produced to observe the predictive effect of the nomogram. Internal validation using a bootstrap with 1000 resamplings revealed that the nomogram performed well for discrimination, with a C-index of 0.733. The deviation between the calibration curve and the oblique line representing the actual survival probability was small (Figures 20b1–3), indicating that the predictive accuracy of the nomogram was high. The predictive ROC curve for all clinical indicators also showed that this prognostic model had a good predictive performance for survival in MBC patients (AUC = 0.7, Figure 20c).
Figure 20.
Nomogram predicting 3- and 5-year overall survival (OS) in patients with breast cancer and receiver operating characteristic (ROC) curve. (a) Nomogram used to predict OS. (b) Calibration plots of 1-, 3-, and 5-year OS predicted by the nomogram. (c) Area under the receiver operating characteristic curve (AUC, ROC) of OS predicted by the nomogram.
ER, estrogen receptor; PR, progesterone receptor.
Discussion
Many factors affect the epidemiology and risk factors of MBC.5,6,8–10 The incidence of MBC varies among regions with the highest incidence in Africa, followed by Europe and America, and the lowest incidence in Asia. 11 The average age of onset of MBC is higher than that of FBC, without the characteristic bimodal incidence. 12 In this study, 71.2% of patients with MBC were ≥60 years old compared with 53.3% of females with breast cancer, consistent with previous reports.12,13 Notably, both MBC and FBC were more common among whites, but FBC was significantly more common than MBC among blacks (24.30% vs. 16.13%).
MBC and FBC have been reported to show similar histological and pathological characteristics, with the most common histological type being non-specific invasive ductal carcinoma (64.0%–93.0%). 14 In this study, 569 of the 670 cases (84.9%) of MBC were invasive non-specific breast cancer, consistent with the previous literature. The proportion of ER(+) and HER-2(−) cases was higher among MBC than FBC patients, similar to that in postmenopausal and elderly women.5,15The International Male Breast Cancer Program study reexamined 1483 MBC specimens and showed that 99% were ER(+), 82% were PR(+), 97% were androgen receptor(+), and only 9% were HER-2(+). In addition, 42% were luminal A type, 49% were luminal B type HER-2(−), 9% were luminal B type HER-2(+), and <1% were triple-negative breast cancer.5,16 In the current study, 89.4% of MBCs were ER(+)/PR(+), 3.4% were ER(−)/PR(−), and 12.1% were HER-2(+), while the equivalent incidences for FBC were 70.44%, 16.87%, and 15.85%, respectively.
The prognosis of MBC is worse than that for FBC. The current survival analysis accordingly showed that men with breast cancer had a significant survival disadvantage compared with women with breast cancer, with 7-year OS rates of 77.9% and 89.8% (P < 0.05), respectively, for patients diagnosed over the same period. The poorer prognosis of MBC may be because independent prognostic factor analysis showed that MBC was usually diagnosed at a late stage, with high T stage, high rates of lymph node, lung, and bone metastases, and high histological grade, which are often associated with a poor prognosis. In addition, MBC patients with late onset age tend to have more complications, including heart and vascular diseases, and high mortality after treatment. 17 MBC patients also have an increased risk of developing secondary primary cancers,18–20and the mortality rate from second primary breast cancer or other causes, including prostate, colon, lung, and contralateral breast cancer, is higher in men than in women. 20 Tamoxifen has demonstrated a survival benefit in men with advanced breast cancer, and observational studies of adjuvant tamoxifen therapy have also suggested a survival benefit; 21 however, the efficacy of aromatase inhibitors for MBC remains unclear and may be lower than in women.22,23This may be one factor leading to the poorer prognosis of MBC compared with postmenopausal FBC. MBC is often an exclusion criterion in cancer clinical trials and MBC patients are generally under-represented in clinical studies. Existing treatment methods for MBC thus usually refer to FBC, despite differences in biological behaviors, which might also affect the prognosis of MBC.
Univariate analysis showed that age, race, tumor size, lymph node metastasis, distant metastasis, histological grade, and ER/PR status significantly affected the prognosis of MBC patients (P < 0.05), and were also prognostic factors affecting OS among FBC patients. Domestic and foreign guidelines, as well as the results of large clinical studies, recommend anti-HER2 adjuvant therapy or palliative treatment for female patients with HER-2(+) breast cancer. 24 However, most studies of anti-HER-2 therapy in HER-2(+) MBC patients have been case reports with mixed results. 25 , 26 In this study, the prognoses of both MBC and FBC were unrelated to HER-2 status. We considered that anti-HER-2 therapy might significantly improve the prognosis of these patients and thus weaken the influence of HER-2 index on prognosis. Based on this conjecture, although no relevant guidelines have specified anti-HER-2 therapy for HER-2(+) MBC patients, this may represent an effective treatment for MBC patients with positive HER-2 expression.
Interestingly, the current multivariate analysis showed that lymph node metastasis and race were not predictive of survival in MBC, in contrast to FBC, despite the large number of patients analyzed (n = 670 for MBC). Because there is less breast tissue in men, the breast is closer to the chest wall, and there is an abundant lymphatic duct network under the nipple and areola. This means that breast cancer cells can penetrate the breast tissue more easily and invade the regional lymph nodes, leading to axillary lymph node metastasis. 27 Tumor size and lymph node metastasis are independent prognostic factors affecting OS and disease-free survival in women with breast cancer, and these factors can be used as independent predictors of a poor prognosis in women with breast cancer, with more lymph node metastases associated with a worse prognosis. The effect of lymph node metastasis on the prognosis of MBC has mostly been studied in small samples and the results have differed.28–30 Prospective studies with large samples are thus needed to determine the effect of lymph node metastasis on the prognosis of MBC. Further multicenter clinical studies with large sample sizes are also required to explore the clinical characteristics and molecular biological mechanisms responsible for the occurrence and development of MBC, with the aim of expanding screening for specific breast cancers in high-risk men. Efforts should also be made to increase the inclusion of men in expanded clinical breast cancer trials, to improve people’s understanding of the differences between male and FBCs, and to develop safe, effective, standardized, and individualized comprehensive treatment plans according to the patient’s condition, clinical stage, histological grade, and molecular type.
The current study had some limitations, including a lack of data on the Ki-67 proliferation index and on patient treatment. In addition, we used internal rather than external validation.
Conclusions
MBC is closely related to FBC but has some differences, including late onset age, diagnosis at a relatively late stage and higher histological grade, and poorer prognosis, associated with age, tumor size, distant metastasis, histological grade, and ER/PR status. Based on the above risk factors, we developed a nomogram to predict 3- and 5-year OS in men with breast cancer. This nomogram revealed good discrimination and calibration, and may thus be helpful for predicting individual survival among MBC patients.
Acknowledgment
The authors thank the managers of the SEER database for making the data available.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Xinli Wang https://orcid.org/0000-0002-7061-998X
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2020; 70: 7–30. DOI: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ottini L. Male breast cancer: a rare disease that might uncover underlying pathways of breast cancer. Nat Rev Cancer 2014; 14: 643–644. DOI: 10.1038/nrc3806. [DOI] [PubMed] [Google Scholar]
- 3.Lee EG, Jung SY, Lim MC, et al. Comparing the characteristics and outcomes of male and female breast cancer patients in Korea: Korea Central Cancer Registry. Cancer Res Treat 2020; 52: 739–746. DOI: 10.4143/crt.2019.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cancer Facts & Figures 2012. American Cancer Society (ACS), http://www.cancer.org/Research/CancerFactsFigures/CancerFactsFigures/cancer-facts-figures-2012 (2012, accessed.
- 5.Cardoso F, Bartlett JMS, Slaets L, et al. Characterization of male breast cancer: results of the EORTC 10085/TBCRC/BIG/NABCG International Male Breast Cancer Program. Ann Oncol 2018; 29(2): 405–417. DOI: 10.1093/annonc/mdx651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piscuoglio S, Ng CK, Murray MP, et al. The genomic landscape of male breast cancers. Clin Cancer Res 2016; 22(16): 4045–4056. DOI: 10.1158/1078-0432.Ccr-15-2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. DOI: 10.1590/s1135-57272008000300002. [DOI] [PubMed] [Google Scholar]
- 8.Greif JM, Pezzi CM, Klimberg VS, et al. Gender differences in breast cancer: analysis of 13,000 breast cancers in men from the National Cancer Data Base. Ann Surg Oncol 2012; 19: 3199–3204. DOI: 10.1245/s10434-012-2479-z. [DOI] [PubMed] [Google Scholar]
- 9.Vera-Badillo FE, Templeton AJ, De Gouveia P, et al. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst 2014; 106: djt319. DOI: 10.1093/jnci/djt319. [DOI] [PubMed] [Google Scholar]
- 10.Daly MB, Pilarski R, Berry M, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J Natl Compr Canc Netw 2017; 15: 9–20. DOI: 10.6004/jnccn.2017.0003. [DOI] [PubMed] [Google Scholar]
- 11.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30. DOI: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 12.Johansson I, Killander F, Linderholm B, et al. Molecular profiling of male breast cancer-lost in translation? Int J Biochem Cell Biol 2014; 53: 526–535. DOI: 10.1016/j.biocel.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y, Heller SL, Moy L. Male breast cancer in the age of genetic testing: an opportunity for early detection, tailored therapy, and surveillance. Radiographics 2018; 38: 1289–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Korde LA, Zujewski JA, Kamin L, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol 2010; 28: 2114–2122. DOI: 10.1200/jco.2009.25.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nilsson C, Johansson I, Ahlin C, et al. Molecular subtyping of male breast cancer using alternative definitions and its prognostic impact. Acta Oncol 2013; 52: 102–109. DOI: 10.3109/0284186X.2012.711952. [DOI] [PubMed] [Google Scholar]
- 16.Chavez-Macgregor M, Clarke CA, Lichtensztajn D, et al. Male breast cancer according to tumor subtype and race: a population-based study. Cancer 2013; 119: 1611–1617. DOI: 10.1002/cncr.27905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson AE, Coopey SB, Spring LM, et al. Management and outcomes of men diagnosed with primary breast cancer. Breast Cancer Res Treat 2021; 188: 561–569. DOI: 10.1007/s10549-021-06174-y. [DOI] [PubMed] [Google Scholar]
- 18.Mangone L, Ferrari F, Mancuso P, et al. Epidemiology and biological characteristics of male breast cancer in Italy. Breast Cancer 2020; 27: 724–731. doi: 10.1007/s12282-020-01068-1. [DOI] [PubMed] [Google Scholar]
- 19.Auvinen A, Curtis RE, Ron E. Risk of subsequent cancer following breast cancer in men. J Natl Cancer Inst 2002; 94: 1330–1332. DOI: 10.1093/jnci/94.17.1330. [DOI] [PubMed] [Google Scholar]
- 20.Hemminki K, Scélo G, Boffetta P, et al. Second primary malignancies in patients with male breast cancer. Br J Cancer 2005; 92: 1288–1292. DOI: 10.1038/sj.bjc.6602505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goss PE, Reid C, Pintilie M, et al. Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955–1996. Cancer 1999; 85: 629–639. DOI: 10.1002/(sici)1097-0142(19990201)85: 3<629: : aid-cncr13>3.0.co; 2-v. [DOI] [PubMed] [Google Scholar]
- 22.Harlan LC, Zujewski JA, Goodman MT, et al. Breast cancer in men in the United States: a population-based study of diagnosis, treatment, and survival. Cancer 2010; 116: 3558–3568. DOI: 10.1002/cncr.25153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eggemann H, Ignatov A, Smith BJ, et al. Adjuvant therapy with tamoxifen compared to aromatase inhibitors for 257 male breast cancer patients. Breast Cancer Res Treat 2013; 137: 465–470. DOI: 10.1007/s10549-012-2355-3. [DOI] [PubMed] [Google Scholar]
- 24.McCullough AE, Dell'orto P, Reinholz MM, et al. Central pathology laboratory review of HER2 and ER in early breast cancer: an ALTTO trial [BIG 2-06/NCCTG N063D (Alliance)] ring study. Breast Cancer Res Treat 2014; 143: 485–492. DOI: 10.1007/s10549-013-2827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi H, Kimura M, Yoshimoto N, et al. A case of HER2-positive male breast cancer with lung metastases showing a good response to trastuzumab and paclitaxel treatment. Breast Cancer 2009; 16: 136–140. DOI: 10.1007/s12282-008-0060-1. [DOI] [PubMed] [Google Scholar]
- 26.Daniels IR, Layer GT. Gynaecomastia. Eur J Surg 2001; 167: 885–892. DOI: 10.1080/110241501753361550. [DOI] [PubMed] [Google Scholar]
- 27.Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med 2002; 137: 678–687. DOI: 10.7326/0003-4819-137-8-200210150-00013. [DOI] [PubMed] [Google Scholar]
- 28.El-Tamer MB, Komenaka IK, Troxel A, et al. Men with breast cancer have better disease-specific survival than women. Arch Surg 2004; 139: 1079. DOI: 10.1001/archsurg.139.10.1079. [DOI] [PubMed] [Google Scholar]
- 29.Iorfida M, Bagnardi V, Rotmensz N, et al. Outcome of male breast cancer: a matched single-institution series. Clin Breast Cancer 2014; 14: 371–377. DOI: 10.1001/archsurg.139.10.1079. [DOI] [PubMed] [Google Scholar]
- 30.Sarmiento S, McColl M, Musavi L, et al. Male breast cancer: a closer look at patient and tumor characteristics and factors that affect survival using the National Cancer Database. Breast Cancer Research and Treatment 2020; 180: 471–479. DOI: 10.1007/s10549-020-05556-y. [DOI] [PubMed] [Google Scholar]