Abstract
Objective:
ALK rearrangements are an established targetable oncogenic driver in non-small cell lung cancer (NSCLC). This study’s goal was to determine the imaging features of the primary tumor and metastatic patterns in advanced ALK-rearranged (ALK+) NSCLC that may be different from those in EGFR-mutant (EGFR+) or EGFR/ALK-wild-type (EGFR-/ALK-) NSCLC.
Methods:
Patients with advanced ALK+, EGFR+, or EGFR-/ALK- NSCLC were retrospectively identified. Two radiologists concurrently assessed the imaging features of the primary tumor and the distribution metastases in these patients.
Results:
We identified a cohort of 333 patients with metastatic NSCLC (119 ALK+, 116 EGFR+, and 98 EGFR-/ALK-). Compared to EGFR+ and EGFR-/ALK- NSCLC, the primary tumor in ALK+ NSCLC was more likely to be in the lower lobes (ALK+=53%, EGFR+=34%, EGFR-/ALK-=36%; p<0.05), less likely to be subsolid (ALK+=1%, EGFR+=11%, EGFR-/ALK-=8%; p<0.02), and less likely to have air-bronchograms (ALK+=7%, EGFR+=28%, EGFR-/ALK-=29%; p<0.01). ALK+ tumors had higher frequencies of distant nodal metastasis (ALK+=20%, EGFR+=2%, EGFR-/ALK-=9%; p<0.05) and lymphangitic carcinomatosis (ALK+=37%, EGFR+=12%, EGFR-/ALK-=12%; p<0.01) compared to EGFR+ and EGFR-/ALK- tumors, but lower frequency of brain metastasis compared to EGFR+ tumors (ALK+=24%, EGRF+=41%, p=0.01). Although there was no statistically significant difference in the frequencies of bone metastasis among the three groups, sclerotic bone metastases were more common in the ALK+ tumors (ALK+=22%, EGFR+=7%, EGFR-/ALK-=6%; p<0.01).
Conclusion:
Advanced ALK-positive NSCLC is associated with primary tumor imaging features and patterns of metastasis that are different from those of EGFR-mutant or EGFR-/ALK-wild type NSCLC at the time of initial presentation.
INTRODUCTION
The diagnosis and treatment of advanced non-small cell lung cancer (NSCLC) continue to evolve with advances in molecular testing and targeted therapy. Since the discovery of activating mutations in the epidermal growth factor receptor gene (EGFR), which confer sensitivity to EGFR tyrosine kinase inhibitors (TKIs), numerous additional oncogenic driver targets have been identified in NSCLC [1–3]. Occurring in approximately 5% of NSCLC, anaplastic lymphoma kinase (ALK) rearrangements are one of the most common targetable mutations in NSCLC, second only to EGFR mutations [4–6]. Similar to EGFR mutations, ALK rearrangements are more common in younger patients with minimal or no smoking history and adenocarcinoma histology [7–9]. ALK TKIs are highly effective in treating ALK-rearranged (ALK+) NSCLC, and five distinct ALK TKIs have received approval by the United States (US) Food and Drug Administration with alectinib being the current standard initial therapy in advanced ALK+ NSCLC [10–17].
On the basis of the robust efficacy of available targeted therapies, the US and European guidelines recommend routine molecular testing for targetable oncogenic alterations including ALK rearrangements at the initial diagnosis of advanced NSCLC [18, 19]. Despite these recommendations, the real-world adoption of molecular testing guidelines and the testing performance have remained suboptimal. For example, in one retrospective study, the ALK testing rate among advanced nonsquamous NSCLC patients at community practices in the US reached only 66.9% (14,478 of 21,639). Among those patients who did undergo ALK testing, 21.5% (3,290 of 15,320) initiated systemic therapy prior to receiving their ALK testing results [20]. Given the established efficacy of ALK TKIs in ALK+ NSCLC and their impact on patient outcomes, improving the rates of implementation and successful completion of molecular testing and the timely initiation of matched targeted therapy is essential.
Several studies have reported imaging features that can potentially predict the presence of EGFR mutations [21–23]. Whether there are distinct radiologic features associated with ALK+ NSCLC that may help distinguish this subset a priori—potentially helping select patients in whom molecular testing should be prioritized or repeated if initial testing is unsuccessful or inconclusive—remains unknown. Published reports thus far have suggested that ALK+ NSCLC may be associated with solid density of the primary tumor, the presence of lymphangitic carcinomatosis, lymphadenopathy, and increased propensity for metastasis to the pleura and pericardium, but these studies were limited by small cohorts of ALK+ patients [24–30]. Here, we evaluated the pre-treatment imaging of 119 patients with advanced ALK+ NSCLC in order to determine and compare the radiologic features to those of EGFR-mutant (EGFR+) and EGFR/ALK-wild-type (EGFR-/ALK-) NSCLC.
SUBJECTS AND METHODS
Patients
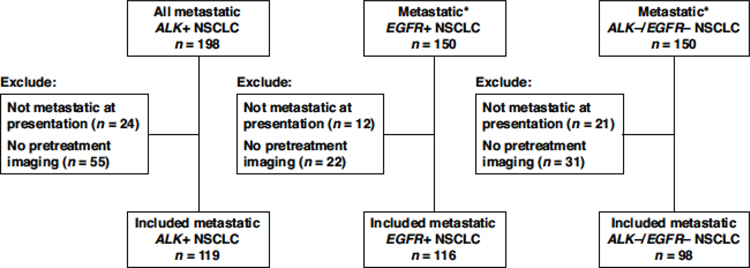
This study was performed under an institutional review board-approved protocol. From a prospectively maintained database of patient with ALK+ NSCLC, we assessed all patients who presented with ALK+ NSCLC to the thoracic oncology clinic of Massachusetts General Hospital (MGH) between January 2013 and December 2018 for eligibility. We included all patients with a) metastatic NSCLC at presentation; b) known ALK rearrangement as determined per local testing using fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), and/or next-generation sequencing (NGS); and c) with pre-treatment imaging available for review. As control groups, we selected a subset of 150 consecutive patients with known metastatic EGFR+ NSCLC and another subset of 150 consecutive patients who were negative for both ALK and EGFR mutations from separate internal databases. We excluded patients 1) without metastatic disease at initial presentation; and 2) those who had any local or systemic therapy prior to the earliest imaging study available. Patient inclusion and exclusion process is summarized in Figure 1. Electronic medical records were retrospectively reviewed to extract clinical and pathologic data, including age, sex, race, smoking history, tumor histology, and disease stage at initial based on 7th edition of the American Joint Committee on Cancer TNM Classification of Malignant Tumors.
Figure 1. Selection of patients with ALK+, EGFR+, and ALK-/EGFR- NSCLC.
*A subset of 150 consecutive patients with metastatic EGFR+ NSCLC and 150 consecutive patients with metastatic ALK-/EGFR- NSCLC were selected from separate databases as control groups.
Imaging review and analysis
Initial imaging studies performed prior to the initiation of cancer treatment were selected for analyses for each patient. Imaging studies reviewed for each patient included CT of the chest, abdomen, and pelvis with or without concurrent fluorodeoxyglucose (FDG)-positron emission tomography (PET) images, and CT and/or MRI of the brain. All imaging was performed at our institution or at another facility with images uploaded into our picture archiving and communications system (PACS; AGFA Impax 6, Mortsel, Belgium). A board-certified radiologist specializing in lung cancer imaging (SRD) and a cardiothoracic imaging fellow (DPM) retrospectively reviewed all imaging concurrently. Findings were determined and recorded by consensus.
The primary tumor, when identifiable, was evaluated for the following features: size, location, solid versus subsolid density, and the presence of other features including air bronchograms, cavities, calcifications, or lymphangitic carcinomatosis. Malignant lymph nodes were confirmed to be malignant with at least one of the following: positive histology, increased uptake on FDG-PET imaging, or malignant behavior based on follow up imaging and were recorded as ipsilateral or contralateral, and as hilar, mediastinal, supraclavicular, or distant (e.g. cervical, axillary, intra-abdominal). All indeterminate lymph nodes were presumed to be benign. Sites examined for metastases included the lungs, pleura, pericardium, liver, adrenals, other visceral organs (e.g. spleen, kidney, etc.), bones, subcutaneous soft tissues, and brain. Brain metastases were identified using CT or magnetic resonance imaging (MRI) of the brain. Other sites of metastases were identified using CT with or without concurrent FDG-PET images. Bone metastases, when present, were further classified as either predominantly lytic versus predominantly blastic or sclerotic. The assessment of bone metastasis was done at a site without fracture.
Statistical analysis
Patient characteristics and imaging features were summarized descriptively. Continuous data were described as median with range, and categorical data were described as frequencies with percentages. The Wilcoxon rank-sum test and Fisher’s exact test were performed to compare continuous and categorical features, respectively. All tests were two-sided. P-values less than 0.05 were considered significant. In order to determine the radiologic predictors of ALK rearrangements as compared to EGFR mutations or lack of EGFR/ALK alterations, multivariable logistic regression models were built with oncogenic driver types as the outcome. The criteria for choosing candidate predictors were p-value <0.05 based on univariate analyses.
RESULTS
Patient characteristics
A total of 333 patients were included in this study (ALK+: 119, EGFR+: 116, EGFR-/ALK-: 98). Table 1 summarizes patient characteristics for all three cohorts. Patients with ALK+ NSCLC were younger at initial diagnosis than patients with EGFR+ or EGFR-/ALK- NSCLC. In this study, ALK+ patients were more likely to be female (56% vs 41%, p=0.03) and more likely to be non-smokers (72% vs 16%, p<0.01) compared to EGFR-/ALK- patients.
Table 1.
Patient characteristics.
|
|
|
|||||
|---|---|---|---|---|---|---|
| Driver alteration, n(%) |
P-value |
|||||
| Patient Characteristic | All (N=333) | ALK+ (N=119) | EGFR+ (N=116) | EGFR-/ALK- (N=98) | ALK+ vs. EGFR+ | ALK+ vs/ EGFR-/ALK- |
|
| ||||||
| Age, median (range) | 61 (19–90) | 51 (19–84) | 63 (26–90) | 68 (42–84) | <0.01 | <0.01 |
| Age | ||||||
| ≤60 | 162 (49%) | 90 (76%) | 51 (44%) | 21 (21%) | <0.01 | <0.01 |
| >60 | 171 (51%) | 29 (24%) | 65 (56%) | 77 (79%) | ||
| Ethnicity | ||||||
| Asian | 31 (9%) | 14 (12%) | 15 (13%) | 2 (2%) | 0.46 | <0.01 |
| Caucasian | 273 (82%) | 91 (76%) | 93 (80%) | 89 (91%) | ||
| Others | 29 (9%) | 14 (12%) | 8 (7%) | 7 (7%) | ||
| Gender | ||||||
| Female | 187 (56%) | 67 (56%) | 80 (69%) | 40 (41%) | 0.06 | 0.03 |
| Male | 146 (44%) | 52 (44%) | 36 (31%) | 58 (59%) | ||
| Smoking | ||||||
| Ever | 159 (48%) | 33 (28%) | 44 (38%) | 82 (84%) | 0.13 | <0.01 |
| Never | 174 (52%) | 86 (72%) | 72 (62%) | 16 (16%) | ||
Primary tumor features
Table 2 summarizes comparison among the three genotype groups with respect to the CT imaging features of the primary tumor. There was no significant difference in the size of the primary tumor (median largest dimension: ALK+: 45 mm, EGFR+: 48 mm, EGFR-/ALK-: 52 mm; p>0.05). ALK+ tumors were more likely to be in the lower lobes compared to EGFR+ and EGFR-/ALK- tumors (52% vs 34% vs 36%, p<0.05), and less likely to be subsolid in density (1% vs 11% vs 8%, p<0.02) or have air bronchograms (7% vs 28% vs 29%, p<0.01). Cavitation was less common among ALK+ tumors than EGFR-/ALK- tumors (4% vs 12%, p=0.04).
Table 2.
Imaging features of the primary tumor.
|
|
|
|||||
|---|---|---|---|---|---|---|
| Driver alteration, n(%) |
P-value |
|||||
| Tumor Feature | All (N=333) | ALK+ (N=119) | EGFR+ (N=116) | EGFR-/ALK- (N=98) | ALK+ vs. EGFR+ | ALK+ vs EGFR-/ALK- |
|
| ||||||
| Tumor size largest diameter, median (range) | 49 (2–134) | 45 (5–115) | 48 (11–134) | 52 (2–115) | 0.09 | 0.18 |
| Tumor size | ||||||
| ≥3cm | 259 (78%) | 89 (75%) | 95 (82%) | 75 (77%) | 0.21 | 0.87 |
| <3cm | 74 (22%) | 30 (25%) | 21 (18%) | 23 (23%) | ||
| Upper vs lower lobe | ||||||
| Upper lobe | 196 (59%) | 56 (47%) | 77 (66%) | 63 (64%) | <0.01 | 0.01 |
| Lower Lobe | 137 (41%) | 63 (53%) | 39 (34%) | 35 (36%) | ||
| Central vs peripheral | ||||||
| Central | 200 (60%) | 66 (55%) | 83 (72%) | 51 (52%) | 0.01 | 0.68 |
| Peripheral | 133 (40%) | 53 (45%) | 33 (28%) | 47 (48%) | ||
| Solid or not | ||||||
| Solid | 311 (93%) | 118 (99%) | 103 (89%) | 90 (92%) | <0.01 | 0.01 |
| Subsolid | 22 (7%) | 1 (1%) | 13 (11%) | 8 (8%) | ||
| Air bronchograms | ||||||
| No | 264 (79%) | 111 (93%) | 83 (72%) | 70 (71%) | <0.01 | <0.01 |
| Yes | 69 (21%) | 8 (7%) | 33 (28%) | 28 (29%) | ||
| Cavitation | ||||||
| No | 310 (93%) | 114 (96%) | 110 (95%) | 86 (88%) | 0.77 | 0.04 |
| Yes | 23 (7%) | 5 (4%) | 6 (5%) | 12 (12%) | ||
| Tumor calcification | ||||||
| No | 327 (98%) | 119 (100%) | 111 (96%) | 97 (99%) | 0.03 | 0.45 |
| Yes | 6 (2%) | 0 (0%) | 5 (4%) | 1 (1%) | ||
Lymphadenopathy and Metastatic Patterns
Comparison of metastatic patterns among the three tumor genotypes are summarized in Table 3. ALK+ NSCLC were more likely to have intrathoracic nodal disease compared to EGFR+ NSCLC (93% vs 83%; p=0.02) and more likely to have distant nodal metastasis compared to both EGFR+ and EGFR-/ALK- NSCLC (20% vs 2% vs 9%, p<0.05) (Figure 2). ALK+ tumors were more likely to exhibit lymphangitic carcinomatosis than the EGFR+ and EGFR-/ALK- groups (37% vs 12% vs 12%, p<0.01) and less likely to have lung metastases than both groups (19% vs 66% vs 68%, p<0.01) (Figure 3). Compared to EGFR+ tumors, ALK+ tumors were associated with lower frequency of brain metastases (24% vs 41%, p=0.01) and higher frequency of pleural (46% vs 27%, p<0.01) and soft tissue metastases (6% vs 0%, p=0.01) at the time of diagnosis prior to any treatment. Compared to EGFR-/ALK- tumors, ALK+ tumors had lower frequency of adrenal metastases (7% vs 32%, p<0.01) but higher frequency of liver metastases (22% vs 6%, <0.01). While there was no significant difference in the frequencies of bone metastases, ALK+ NSCLC were more likely than EGFR+ and EGFR-/ALK- NSCLC to have sclerotic bone metastases (24% vs 7% vs 6%, p<0.01).
Table 3.
Sites of metastasis.
|
|
|
|||||
|---|---|---|---|---|---|---|
| Driver alteration, n(%) |
P-value |
|||||
| Metastatic Site | All (N=333) | ALK+ (N=119) | EGFR+ (N=116) | EGFR-/ALK- (N=98) | ALK+ vs. EGFR+ | ALK+ vs/ EGFR-/ALK- |
|
| ||||||
| Thoracic Node | ||||||
| No | 32 (10%) | 8 (7%) | 20 (17%) | 4 (4%) | 0.02 | 0.55 |
| Yes | 301 (90%) | 111 (93%) | 96 (83%) | 94 (96%) | ||
| Intrathoracic | ||||||
| No | 76 (23%) | 34 (29%) | 28 (24%) | 14 (14%) | 0.46 | 0.01 |
| Yes | 257 (77%) | 85 (71%) | 88 (76%) | 84 (86%) | ||
| Lung | ||||||
| No | 166 (50%) | 96 (81%) | 39 (34%) | 31 (32%) | <0.01 | <0.01 |
| Yes | 167 (50%) | 23 (19%) | 77 (66%) | 67 (68%) | ||
| Pleural mets | ||||||
| No | 192 (58%) | 64 (54%) | 85 (73%) | 43 (44%) | <0.01 | 0.17 |
| Yes | 141 (42%) | 55 (46%) | 31 (27%) | 55 (56%) | ||
| Lymphangitic carcinomatosis | ||||||
| No | 263 (79%) | 75 (63%) | 102 (88%) | 86 (88%) | <0.01 | <0.01 |
| Yes | 70 (21%) | 44 (37%) | 14 (12%) | 12 (12%) | ||
| Pericardium | ||||||
| No | 329 (99%) | 116 (97%) | 116 (100%) | 97 (99%) | 0.25 | 0.63 |
| Yes | 4 (1%) | 3 (3%) | 0 (0%) | 1 (1%) | ||
| Extra-thoracic | ||||||
| No | 95 (29%) | 33 (28%) | 32 (28%) | 30 (31%) | >0.99 | 0.66 |
| Yes | 238 (71%) | 86 (72%) | 84 (72%) | 68 (69%) | ||
| Intra-abdominal | ||||||
| No | 233 (70%) | 87 (73%) | 81 (70%) | 65 (66%) | 0.66 | 0.30 |
| Yes | 100 (30%) | 32 (27%) | 35 (30%) | 33 (34%) | ||
| Adrenal | ||||||
| No | 278 (83%) | 111 (93%) | 100 (86%) | 67 (68%) | 0.09 | <0.01 |
| Yes | 55 (17%) | 8 (7%) | 16 (14%) | 31 (32%) | ||
| Liver | ||||||
| No | 277 (83%) | 93 (78%) | 92 (79%) | 92 (94%) | 0.87 | <0.01 |
| Yes | 56 (17%) | 26 (22%) | 24 (21%) | 6 (6%) | ||
| Spleen | ||||||
| No | 328 (98%) | 114 (96%) | 116 (100%) | 98 (100%) | 0.06 | 0.07 |
| Yes | 5 (2%) | 5 (4%) | 0 (0%) | 0 (0%) | ||
| Bone | ||||||
| No | 195 (59%) | 65 (55%) | 67 (58%) | 63 (64%) | 0.69 | 0.17 |
| Yes | 138 (41%) | 54 (45%) | 49 (42%) | 35 (36%) | ||
| Bone type | ||||||
| None | 198 (59%) | 65 (55%) | 68 (59%) | 65 (66%) | <0.01 | <0.01 |
| Lytic | 93 (28%) | 26 (22%) | 40 (34%) | 27 (28%) | ||
| Sclerotic | 42 (13%) | 28 (24%) | 8 (7%) | 6 (6%) | ||
| Brain | ||||||
| No | 227 (68%) | 90 (76%) | 69 (59%) | 68 (69%) | 0.01 | 0.36 |
| Yes | 106 (32%) | 29 (24%) | 47 (41%) | 30 (31%) | ||
| Distant lymph node | ||||||
| No | 298 (89%) | 95 (80%) | 114 (98%) | 89 (91%) | <0.01 | 0.04 |
| Yes | 35 (11%) | 24 (20%) | 2 (2%) | 9 (9%) | ||
| Soft tissue | ||||||
| No | 316 (95%) | 112 (94%) | 116 (100%) | 88 (90%) | 0.01 | 0.31 |
| Yes | 17 (5%) | 7 (6%) | 0 (0%) | 10 (10%) | ||
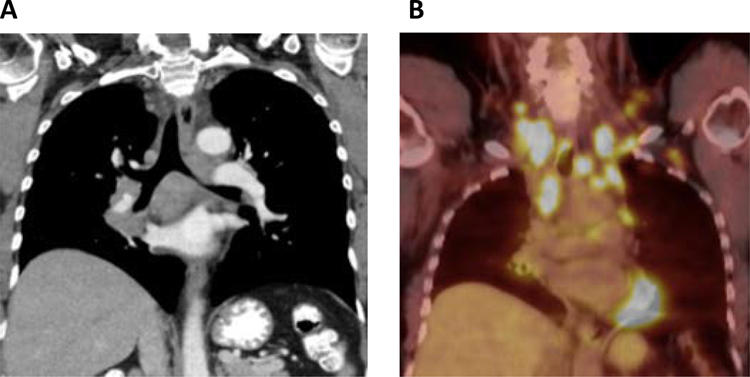
Figure 2. Extensive lymphadenopathy associated with ALK+ lung cancer.
A 61-year-old female never smoker presented with a solid right lower lobe mass associated with extensive mediastinal, hilar, supraclavicular, cervical, and left axillary lymphadenopathy, and was later found to have ALK+ NSCLC. Representative coronal slices of (A) CT and (B) PET imaging are shown.
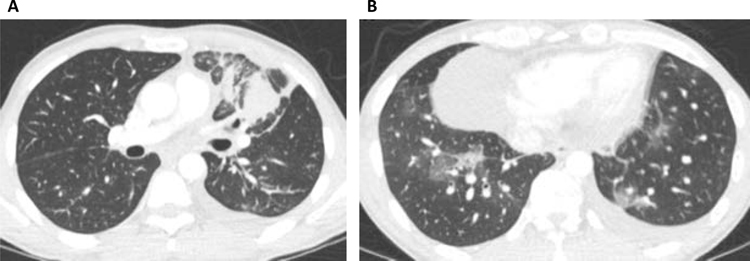
Figure 3. Lymphangitic carcinomatosis associated with ALK+ lung cancer.
Representative axial CT images are taken from a 43-year-old male never smoker who presented with (A) dominant left upper lobe mass with surrounding lymphangitic carcinomatosis and (B) additional areas of lymphangitic carcinomatosis in the lower lobes.
Multivariable logistic regression models
Based on multivariate analysis, younger age at diagnosis, the absence of air bronchograms in the primary tumor, the absence of lung metastasis, the absence of brain metastasis, and the presence of lymphangitic carcinomatosis, pleural metastasis, sclerotic bone metastasis, or distant lymph node metastasis were significant predictors of whether patients had ALK+ vs EGFR+ NSCLC (Table 4; Figure 4A). Younger age at diagnosis, non-smoking history, the absence of air bronchograms, the absence of lung metastasis, the absence of adrenal metastasis, and the presence of lymphangitic carcinomatosis, liver metastasis, or sclerotic bone metastasis were significant predictors of whether patients had ALK+ vs EGFR-/ALK- NSCLC (Table 4; Figure 4B).
Table 4.
Multivariable models for ALK+ vs. EGFR+ NSCLC and vs. EGFR-/ALK- NSCLC.
| Predictor (vs. EGFR+) | OR (95% CI) | P-value | |
|
| |||
| Age at diagnosis | >60 vs ≤60 | 0.19 (0.08 – 0.43) | <0.01 |
|
| |||
| Air bronchograms | Yes vs No | 0.11 (0.03 – 0.42) | <0.01 |
| Lung metastasis | Yes vs No | 0.08 (0.03 – 0.19) | <0.01 |
| Pleural metastasis | Yes vs No | 2.99 (1.31 – 6.83) | 0.01 |
| Lymphangitic carcinomatosis | Yes vs No | 5.63 (2.06 – 15.35) | <0.01 |
| Bone metastasis | Lytic vs None | 1 (0.42 – 2.39) | >0.99 |
| Sclerotic vs None | 4.04 (1.27 – 12.87) | 0.02 | |
| Brain metastasis | Yes vs No | 0.34 (0.15 – 0.76) | 0.01 |
| Distant lymphadenopathy | Yes vs No | 18.43 (3.53 – 96.13) | <0.01 |
|
| |||
| Predictor (vs. EGFR-/ALK-) | OR (95% CI) | P-value | |
|
| |||
| Age at diagnosis | >60 vs <=60 | 0.16 (0.06 – 0.43) | <0.01 |
| Smoke status | Never vs Ever | 12.15 (4.24 – 34.81) | <0.01 |
| Air bronchograms | Yes vs No | 0.07 (0.02 – 0.34) | <0.01 |
| Lung metastasis | Yes vs No | 0.07 (0.02 – 0.21) | <0.01 |
| Lymphangitic carcinomatosis | Yes vs No | 5.22 (1.5 – 18.23) | 0.01 |
| Adrenal metastasis | Yes vs No | 0.13 (0.03 – 0.56) | 0.01 |
| Liver metastasis | Yes vs No | 8.26 (1.79 – 38.15) | 0.01 |
| Bone metastasis | Lytic vs None | 1.58 (0.49 – 5.15) | 0.45 |
| Sclerotic vs None | 10.84 (1.67 – 70.28) | 0.01 | |
Abbreviations: OR, odds ratio; CI, confidence interval
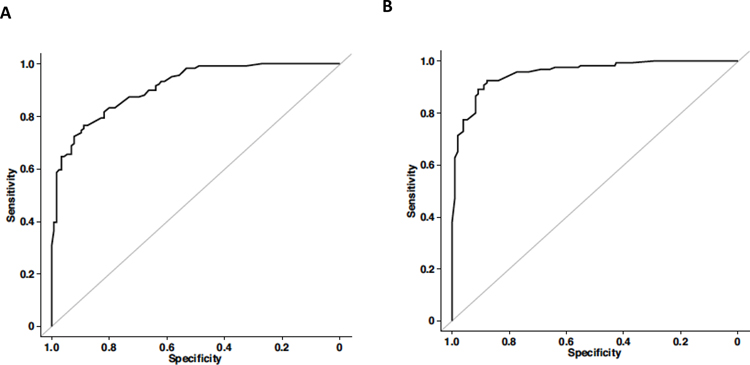
Figure 4.
(A) ROC curve for the multivariable logistic regression model for ALK+ vs EGFR+ NSCLC. (B) ROC curve for the multivariable logistic regression model for ALK+ vs EGFR-/ALK- NSCLC.
DISCUSSION
This is the largest study to date to systematically assess the imaging features and metastatic patterns of ALK+ NSCLC. We found that ALK+ NSCLC has some imaging features and patterns of metastasis that are distinct compared to those of EGFR+ and EGFR-/ALK- NSCLC. In our cohort, ALK+ tumors were more likely be in the lower lobes compared to EGFR+ or EGFR-/ALK- tumors, and were less likely to be subsolid in density or have air bronchograms. Additionally, ALK+ tumors were more likely to be associated with absence of lung metastases and presence of lymphangitic carcinomatosis, distant nodal metastases, and sclerotic bone metastasis compared to EGFR+ or EGFR-/ALK- tumors.
Of note, virtually all of the primary tumors in ALK+ NSCLC evaluated in this study presented as solid masses or nodules. While most of the primary tumors in EGFR+ and EGFR-/ALK- NSCLC were also solid in density, there were increased frequencies of subsolid density and presence of air-bronchograms in these molecular subsets of tumors. The association between EGFR+ tumors and subsolid density and air bronchograms has been reported [23, 31, 32], although the mechanism behind these differences is unclear. ALK+ tumors in our study were also more likely to be in the lower lobes compared to the EGFR+ or EGFR-/ALK- tumors. While the propensity for the lower lobe location has been suggested for nonsmokers (vs upper lobe location for smokers) [33], the impact of the driver oncogene on the primary tumor location has not previously been reported. It has been suggested that lower lobe tumors may be associated with poorer prognoses; however, these studies did not include oncogene-driven tumors treated with targeted therapy [34, 35].
Prior smaller studies have suggested the propensity of ALK+ NSCLC for lymphangitic carcinomatosis [28, 30]. Its association with sclerotic bone metastases observed in our cohort, however, is a novel finding. Historically, sclerotic metastases have been considered relatively rare compared to lytic metastases in treatment-naïve NSCLC [36, 37]. It is also noteworthy that ALK+ NSCLC had decreased frequency of lung metastases compared to EGFR+ or EGFR-/ALK- NSCLC. EGFR+ NSCLC can be associated with an increased frequency of “miliary” lung metastases [38, 39], which may partially account for this difference. These differences may potentially have larger prognostic implications in patients, as metastases are the primary determinants of mortality in NSCLC [40, 41].
Current guidelines recommend testing for the most common targetable molecular alterations in NSCLC including ALK rearrangements, and treatment with targeted TKIs is only indicated in those who test positive for the targetable mutations [18, 19]. Without further study and validation, imaging cannot replace molecular testing in determining the presence of ALK rearrangements in NSCLC, the distinct radiologic features described herein may potentially help identify patients who may benefit from prioritized testing or re-testing following an initial non-diagnostic or inconclusive result. Available assays for ALK rearrangement detection include IHC, FISH, and NGS. The latter two, especially NGS, can be time-consuming. There are diagnostic pathways that have been suggested to reduce time to diagnosis and to expedite initiation of targeted TKIs when appropriate [42–44]. Patients with clinical and imaging findings that suggest the presence of an ALK rearrangement (or other oncogene subsets such as EGFR+ NSCLC) could benefit from being triaged towards these expedited pathways. Additionally, conflicting ALK testing results can be seen using different diagnostic methods; for example, a patient with a negative ALK FISH result may then be found to have an ALK+ tumor by IHC or NGS testing and go on to benefit from ALK TKIs [45–49]. The presence of compelling clinical and radiologic features may help determine which patients should be re-tested using an alternative diagnostic method.
This study had several limitations. Due to its retrospective, single-institution nature, the findings herein may not be generalizable. While this study evaluated the largest cohort of patients with ALK+ NSCLC, the sample size still remained relatively small. Other oncogene subsets such as ROS1- or RET-rearranged lung cancer or BRAF-mutant lung cancer were not examined in this study. In addition, the EGFR-/ALK- cohort may be heterogeneous as it may include many different other mutational subsets other than ALK or EGFR. Due to these limitations, it remains unknown if there are imaging features of ALK-rearranged NSCLC that overlap with those of other mutational subgroups other than EGFR, and further study may be helpful in defining these features. Finally, while our findings suggested distinct imaging features that may be helpful in distinguishing ALK+ NSCLC from EGFR+ or EGFR-/ALK- NSCLC, we were not able to elucidate the biologic mechanisms underlying these differences, and further study is needed to explore why certain oncogenes exhibit particular metastatic tropism or primary tumor characteristics.
CONCLUSIONS
This is the largest study to date to assess the imaging features and metastatic patterns of advanced ALK+ NSCLC. Our findings suggest that ALK+ tumors have certain imaging features and patterns of metastasis that are distinct compared to EGFR+ or EGFR-/ALK- NSCLC. Although these radiologic features cannot substitute for appropriate molecular testing to detect oncogenic driver gene alterations such as ALK rearrangements, they may nevertheless assist in selecting those patients who are most likely to benefit from expedited genotyping, or from repeat testing following an initial non-diagnostic result.
Contributor Information
Dexter P. Mendoza, Department of Radiology, Massachusetts General Hospital, Boston, MA.
Jessica J. Lin, Massachusetts General Hospital Cancer Center and Department of Medicine, Massachusetts General Hospital, Boston, MA.
Marguerite M. Rooney, Massachusetts General Hospital Cancer Center and Department of Medicine, Massachusetts General Hospital, Boston, MA.
Tianqi Chen, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA.
Lecia V. Sequist, Massachusetts General Hospital Cancer Center and Department of Medicine, Massachusetts General Hospital, Boston, MA.
Alice T. Shaw, Massachusetts General Hospital Cancer Center and Department of Medicine, Massachusetts General Hospital.
Subba R. Digumarthy, Department of Radiology, Massachusetts General Hospital, Boston, MA.
REFERENCES
- 1.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–1500 [DOI] [PubMed] [Google Scholar]
- 2.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306–13311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129–2139 [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Hsu PP, Awad MM, Engelman JA. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat Rev Cancer 2013;13:772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378–381 [DOI] [PubMed] [Google Scholar]
- 6.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561–566 [DOI] [PubMed] [Google Scholar]
- 7.Camidge DR, Kono SA, Flacco A, et al. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res Off J Am Assoc Cancer Res 2010;16:5581–5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong DW-S, Leung EL-H, So KK-T, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723–1733 [DOI] [PubMed] [Google Scholar]
- 9.Fukui T, Yatabe Y, Kobayashi Y, et al. Clinicoradiologic characteristics of patients with lung adenocarcinoma harboring EML4-ALK fusion oncogene. Lung Cancer Amst Neth 2012;77:319–325 [DOI] [PubMed] [Google Scholar]
- 10.Shaw A, Peters S, Mok T, et al. Alectinib Versus Crizotinib in Treatment-Naive Advanced ALK Positive Non-Small Cell Lung Cancer (NSCLC): primary Results of the Global Phase III ALEX Study. J Clin Oncol Conf 2017 Annu Meet Am Soc Clin Oncol ASCO U S 2017;35 [Google Scholar]
- 11.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non–Small-Cell Lung Cancer. N Engl J Med 2017;377:829–838 [DOI] [PubMed] [Google Scholar]
- 12.Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet Lond Engl 2017;390:29–39 [DOI] [PubMed] [Google Scholar]
- 13.Soria J-C, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. The Lancet 2017;389:917–929 [DOI] [PubMed] [Google Scholar]
- 14.Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim D, Tiseo M, Ahn M, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: a Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490–2498 [DOI] [PubMed] [Google Scholar]
- 16.Camidge DR, Kim HR, Ahn M-J, et al. Brigatinib versus Crizotinib in ALK-Positive Non–Small-Cell Lung Cancer. N Engl J Med 2018;379:2027–2039 [DOI] [PubMed] [Google Scholar]
- 17.Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol 2018;19:1654–1667 [DOI] [PubMed] [Google Scholar]
- 18.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol 2018;29:iv192–iv237 [DOI] [PubMed] [Google Scholar]
- 19.National Comprehensive Cancer Network. NCCN practice guidelines in oncology: Non-small cell lung cancer. National Comprehensive Cancer Network. [Google Scholar]
- 20.Illei PB, Wong W, Wu N, et al. ALK Testing Trends and Patterns Among Community Practices in the United States. JCO Precis Oncol 2018;1–11 [DOI] [PubMed] [Google Scholar]
- 21.Digumarthy SR, Padole AM, Gullo RL, Sequist LV, Kalra MK. Can CT radiomic analysis in NSCLC predict histology and EGFR mutation status? Medicine (Baltimore) 2019;98:e13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Cai W, Wang Y, Liao M, Tian S. CT and clinical characteristics that predict risk of EGFR mutation in non-small cell lung cancer: a systematic review and meta-analysis. Int J Clin Oncol 2019 [DOI] [PubMed] [Google Scholar]
- 23.Cheng Z, Shan F, Yang Y, Shi Y, Zhang Z. CT characteristics of non-small cell lung cancer with epidermal growth factor receptor mutation: a systematic review and meta-analysis. BMC Med Imaging 2017;17:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miao Y, Zhu S, Li H, et al. Comparison of clinical and radiological characteristics between anaplastic lymphoma kinase rearrangement and epidermal growth factor receptor mutation in treatment naïve advanced lung adenocarcinoma. J Thorac Dis 2017;9:3927–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou JY, Zheng J, Yu ZF, et al. Comparative analysis of clinicoradiologic characteristics of lung adenocarcinomas with ALK rearrangements or EGFR mutations. Eur Radiol 2015;25:1257–1266 [DOI] [PubMed] [Google Scholar]
- 26.Rizzo S, Petrella F, Buscarino V, et al. CT Radiogenomic Characterization of EGFR, KRAS, and ALK Mutations in Non-Small Cell Lung Cancer. Eur Radiol 2016;26:32–42 [DOI] [PubMed] [Google Scholar]
- 27.Park J, Kobayashi Y, Urayama KY, Yamaura H, Yatabe Y, Hida T. Imaging Characteristics of Driver Mutations in EGFR, KRAS, and ALK among Treatment-Naïve Patients with Advanced Lung Adenocarcinoma. PloS One 2016;11:e0161081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi C-M, Kim MY, Hwang HJ, Lee JB, Kim WS. Advanced adenocarcinoma of the lung: comparison of CT characteristics of patients with anaplastic lymphoma kinase gene rearrangement and those with epidermal growth factor receptor mutation. Radiology 2015;275:272–279 [DOI] [PubMed] [Google Scholar]
- 29.Nakada T, Okumura S, Kuroda H, et al. Imaging Characteristics in ALK Fusion-Positive Lung Adenocarcinomas by Using HRCT. Ann Thorac Cardiovasc Surg Off J Assoc Thorac Cardiovasc Surg Asia 2015;21:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halpenny DF, Riely GJ, Hayes S, et al. Are there imaging characteristics associated with lung adenocarcinomas harboring ALK rearrangements? Lung Cancer Amst Neth 2014;86:190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai J, Shi J, Soodeen-Lalloo AK, et al. Air bronchogram: A potential indicator of epidermal growth factor receptor mutation in pulmonary subsolid nodules. Lung Cancer Amst Neth 2016;98:22–28 [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Kim J, Qu F, et al. CT Features Associated with Epidermal Growth Factor Receptor Mutation Status in Patients with Lung Adenocarcinoma. Radiology 2016;280:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamnik S, Uehara C, da Silva VV. Location of lung carcinoma in relation to the smoking habit and gender. J Bras Pneumol Publicacao Of Soc Bras Pneumol E Tisilogia 2006;32:510–514 [DOI] [PubMed] [Google Scholar]
- 34.Lee HW, Lee C-H, Park YS. Location of stage I-III non-small cell lung cancer and survival rate: Systematic review and meta-analysis. Thorac Cancer 2018;9:1614–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kudo Y, Saji H, Shimada Y, et al. Do tumours located in the left lower lobe have worse outcomes in lymph node-positive non-small cell lung cancer than tumours in other lobes? Eur J Cardio-Thorac Surg Off J Eur Assoc Cardio-Thorac Surg 2012;42:414–419 [DOI] [PubMed] [Google Scholar]
- 36.Haghighatkhah HR, Sanei Taheri M, Kharrazi SMH, Ghazanfari Amlashi D, Haddadi M, Pourabdollah M. An Unusual Case of Pulmonary Adenocarcinoma with Multiple and Extraordinary Metastases. Iran J Radiol 2012;9:93–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali Mohammed Hammamy R, Farooqui K, Ghadban W. Sclerotic Bone Metastasis in Pulmonary Adenocarcinoma. Case Rep Med 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okuma Y, Kashima J, Watanabe K, Homma S. Survival analysis and pathological features of advanced non-small cell lung cancer with miliary pulmonary metastases in patients harboring epidermal growth factor receptor mutations. J Cancer Res Clin Oncol 2018;144:1601–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsu F, Nichol A, Toriumi T, Caluwe AD. Miliary metastases are associated with epidermal growth factor receptor mutations in non-small cell lung cancer: a population-based study. Acta Oncol 2017;56:1175–1180 [DOI] [PubMed] [Google Scholar]
- 40.Putila J, Remick SC, Guo NL. Combining clinical, pathological, and demographic factors refines prognosis of lung cancer: a population-based study. PloS One 2011;6:e17493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chansky K, Sculier J-P, Crowley JJ, et al. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 2009;4:792–801 [DOI] [PubMed] [Google Scholar]
- 42.Cheema PK, Menjak IB, Winterton-Perks Z, et al. Impact of Reflex EGFR/ALK Testing on Time to Treatment of Patients With Advanced Nonsquamous Non–Small-Cell Lung Cancer. J Oncol Pract 2016;13:e130–e138 [DOI] [PubMed] [Google Scholar]
- 43.DiStasio M, Chen Y, Rangachari D, Costa DB, Heher YK, VanderLaan PA. Molecular Testing Turnaround Time for Non-Small Cell Lung Cancer in Routine Clinical Practice Confirms Feasibility of CAP/IASLC/AMP Guideline Recommendations: A Single-center Analysis. Clin Lung Cancer 2017;18:e349–e356 [DOI] [PubMed] [Google Scholar]
- 44.Dagogo-Jack I, Azzolli CG, Fintelmann F, et al. Clinical Utility of Rapid EGFR Genotyping in Advanced Lung Cancer. JCO Precis Oncol 2018;1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shan L, Jiang P, Xu F, et al. BIRC6-ALK, a Novel Fusion Gene in ALK Break-Apart FISH-Negative Lung Adenocarcinoma, Responds to Crizotinib. J Thorac Oncol 2015;10:e37–e39 [DOI] [PubMed] [Google Scholar]
- 46.Peled N, Palmer G, Hirsch FR, et al. Next-generation sequencing identifies and immunohistochemistry confirms a novel crizotinib-sensitive ALK rearrangement in a patient with metastatic non-small-cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 2012;7:e14–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ren S, Hirsch FR, Varella-Garcia M, et al. Atypical negative ALK break-apart FISH harboring a crizotinib-responsive ALK rearrangement in non-small-cell lung cancer. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 2014;9:e21–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J-M, Choi Y-L, Won J-K, et al. A dramatic response to crizotinib in a non-small-cell lung cancer patient with IHC-positive and FISH-negative ALK. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 2012;7:e36–e38 [DOI] [PubMed] [Google Scholar]
- 49.Li W, Zhang J, Guo L, Chuai S, Shan L, Ying J. Combinational Analysis of FISH and Immunohistochemistry Reveals Rare Genomic Events in ALK Fusion Patterns in NSCLC that Responds to Crizotinib Treatment. J Thorac Oncol 2017;12:94–101 [DOI] [PubMed] [Google Scholar]