Abstract
Humans are vocal modulators par excellence. This ability is supported in part by the dual representation of the laryngeal muscles in the motor cortex. Movement, however, is not the product of motor cortex alone but of a broader motor network. This network consists of brain regions that contain somatotopic maps that parallel the organization in motor cortex. We therefore present a novel hypothesis that the dual laryngeal representation is repeated throughout the broader motor network. In support of the hypothesis, we review existing literature that demonstrates the existence of network-wide somatotopy and present initial evidence for the hypothesis' plausibility. Understanding how this uniquely human phenotype in motor cortex interacts with broader brain networks is an important step toward understanding how humans evolved the ability to speak. We further suggest that this system may provide a means to study how individual components of the nervous system evolved within the context of neuronal networks.
This article is part of the theme issue ‘Voice modulation: from origin and mechanism to social impact (Part I)’.
Keywords: larynx, somatotopy, motor system, brain evolution, cerebellum, supplementary motor area
1. Introduction
Humans are vocal modulators par excellence. This is usually characterized as the capacity for vocal production learning (VPL), which is the ability to learn to produce novel vocalizations [1]. Few species of mammals, such as cetaceans and bats [2,3], have displayed strong VPL abilities, and none of these species has a close phylogenetic relationship to humans. Monkeys are particularly weak vocal learners [4]. Non-human apes appear to have intermediate VPL, being able to learn certain kinds of limited vocal behaviour from humans [5,6], though there is little evidence of this behaviour in the wild [7]. The human VPL capacity is attributable in part to specialized adaptations in motor cortex that grant voluntary control over the voice. However, complex behavioural abilities such as VPL are not the product of the motor cortex alone but are an emergent property of their interaction with a broader motor network.
Human motor cortex is composed of a band of specialized grey matter along the precentral gyrus and the anterior bank of the precentral sulcus, which is the main source of motor output from the central nervous system. Penfield's seminal neurosurgical studies [8] described the conspicuous somatotopy of the human primary motor cortex (M1), in which the muscles of the foot are represented at one end of the somatotopic map and the muscles of the head represented at the other end [9–11]. Similar somatotopic maps have been described throughout the network of brain areas that control movement, including the cerebellum, supplementary motor area (SMA), basal ganglia (BG) and the middle cingulate cortex (MCC) [12–15].
Penfield's original mapping was uncertain of the somatotopic location of the laryngeal muscles, which control the sound source of the voice. More recent neurosurgical [16,17], molecular genetic [18] and brain imaging studies [19–24] provide compelling evidence that the laryngeal muscles are unusual in being controlled by two distinct loci within the human motor cortex. While other effectors such as the digits of the hand may also have multiple representations in motor cortex, these tend to be contiguous and may represent either subdivision at a finer scale (i.e. muscles of flexion versus extension) or correlated movements with nearby muscles that exert a common influence over shared joints [25–28]. By contrast, the dual laryngeal representations are non-contiguous, being located at opposing ends of the orofacial motor zone—which is a marked deviation from the single larynx area observed in other primates [29,30]. The two representations have therefore been referred to as dorsal and ventral laryngeal motor cortex (dLMC and vLMC). This adaptation has clear implications for the evolution of speech since the neural control of the larynx supports one of the requirements of spoken language [31,32], namely a high degree of control over the voice source beyond the capabilities of other primates [4,33].
Despite extensive searches spanning new world monkeys (primarily Macaca mulatta), old world monkeys (primarily Saimiri sciureus) and all extant genera of great apes including Chimpanzees (Pan troglodytes), Orangutans (Pongo sp.) and Gorillas (Gorilla sp.) [29,34,35], humans appear to be the sole primate with the neural trait of dual larynx motor representation, and much has been written about the possible implications of this phenotype for the evolution of speech [36–41]. Here, we outline a novel hypothesis that this human phenotype is not restricted to the motor cortex but extends throughout a network of somatotopically arranged brain areas that comprise the motor system, including the cerebellum, SMA, BG and MCC and the axonal projections between these regions.
2. Hypothesis: dual larynx motor networks
We hypothesize that each motor region contains two representations of the laryngeal muscles within their respective somatotopic maps: one between the hand and the orofacial muscles, and a second at the end of the orofacial representation (figure 1). This hypothesis is supported by the observations that (i) somatotopic maps throughout the motor network follow a similar ordering of representations from foot to face and (ii) nodes in the motor network project to one another homotopically, suggesting that motor regions beyond motor cortex must have target zones that receive the projections from the dLMC and vLMC. Somatotopic maps in different regions vary in orientation. For instance, somatotopy proceeds dorsoventrally in the motor cortex but antero-posteriorly along with the medial wall. Therefore, it may not be constructive to use the labels dorsal and ventral larynx areas for somatotopic maps beyond motor cortex. We have consequently adopted the convention of referring to larynx somatotopic regions in the MCC, SMA, cerebellum and BG as dLMC-related or vLMC-related to denote their respective positions within the somatotopically arranged motor network.
Figure 1.

Depiction of the dual laryngeal motor network hypothesis. The MCC, SMA and cerebellum are depicted with simplified somatotopic maps for conceptual convenience. The broader motor somatotopy follows the organization of motor cortex, but with idiosyncratic orientations following a different axis in each brain region (BG not shown for simplicity). The hypothesized dLMC-related and vLMC-related networks are shown in orange and purple, respectively.
An alternative hypothesis is that only the dLMC benefits from the gain in function concomitant with support from the broader motor system. Only dLMC is composed of primary motor cortex, while vLMC is likely to be located in a qualitatively different cytoarchitectonic motor region (see a more detailed discussion in §3 below). Moreover, dLMC is a novel phenotype in humans and robustly observed in human functional brain imaging studies, which points towards a prominent role in brain architecture. Therefore, if only one larynx representation is observed in the network of somatotopic maps, then we predict that it will be the dLMC-related locus in a position between the hand and the articulatory muscles. If this turns out to be the case, it will regardless be important to understand the evolution of the dLMC in the context of a broader motor network.
3. A human-specific phenotype in motor cortex
Compared to other primates, lower motor neurons in the human spinal cord and brainstem receive a far greater proportion of their inputs from neocortex. These connections contribute to the dexterity and behavioural flexibility of our species [42–44]. Included in this abundance of cortical efferents is a direct projection to motor neurons in the nucleus ambiguus [36–40], which is a brainstem motor nucleus that controls the muscles of the larynx. Such a direct corticobulbar connection is lacking in monkeys [45], extant but sparse in non-human apes [35], and further elaborated in humans [46,47]. An analogous phenotype distinguishes birds that are strong vocal learners such as songbirds (order Passeriformes), humming birds (order Apodiformes) and parrots (order Psitaciformes) from weaker vocal learners [48,49]. Thus, it appears that multiple phylogenetic lineages with strong VPL abilities have converged on similar neurophenotypes with direct efferent projection from upstream motor areas to voice motor nuclei [50,51].
Evidence for the presence of this direct connection between neocortex and the nucleus ambiguus in humans has come from natural experiments due to cerebrovascular events [46,47], in which large cortical lesions caused the axons of upper motor neurons to degenerate. Tracing the course of these damaged axons against the more intact surrounding white matter allowed the authors to demonstrate the existence of the direct corticobulbar pathway. However, these lesions all resulted from cerebrovascular accidents of the middle cerebral artery (MCA) that can result in widespread damage across the speech-relevant portions of motor cortex (hence the prevalence of speech motor and swallowing disorders following MCA infarcts; [52,53]). Thus, lesion studies provide limited information about the cortical source of the direct pathway.
Researchers using functional neuroimaging to investigate speech motor control initially presumed that the larynx was represented at the ventral-most extent of primary motor cortex [54], in the location that would be expected from the larynx's position within the throat and proximity to the homologous region in non-human primates [29,55,56]. However, later studies demonstrated that the human brain in fact has two separate representations of the larynx, at either end of the orofacial somatotopic map of the precentral gyrus [19–23]. Though the dual larynx representations have not been consistently labelled as such in earlier brain imaging research, they were nonetheless consistently present near the predicted location [54].
The dLMC is located in canonical primary motor cortex in Brodmann Area (BA) 4, which is cytoarchitecturally defined as the region containing a high abundance of giant pyramidal neurons in cortical layer V—these pyramidal neurons are the source of the descending motor pathways of the corticospinal and corticobulbar tracts [57–59].
By contrast, the human vLMC is localized to the most ventral segment of the central sulcus or the lateral segment of the anterior subcentral sulcus [17,18,60]. The localization of the vLMC may be particularly variable due to a high degree of individual variation in the morphology of nearby sulci [60], which may explain why the vLMC escaped notice by many early functional magnetic resonance imaging (fMRI) studies. Unlike its dorsal counterpart, quantitative neuroimaging has also suggested that the vLMC is not located in primary motor cortex [60]. Although no study has both localized the vLMC and performed a cytoarchitectural analysis of the underlying tissue, the location of the vLMC corresponds to BA 43 in the Brodmann atlas. While Brodmann believed that this region most strongly resembled somatosensory cortex based on its cellular composition [57], Vogt believed that it more strongly resembled motor cortex based on the degree of myelination of cortical layer V, which is an indicator of the large myelinated axons that form the efferent motor pathways that carry motor commands to the peripheral nervous system [58,59]. In contrast with the evidence from humans, the larynx representation in non-human primates has been identified in premotor cortex [45], but no separate representation in primary motor cortex has been described. This observation is in line with the theory that primary and premotor cortex together contain one single somatotopic map spanning cytoarchitectural zones [61].
Whether the dLMC and vLMC make separate functional contributions to voice motor control, and what those might be, remains an active area of research. Identifying behaviours that activate one of these regions over the other is challenging, given that the dLMC may be easier to detect than the vLMC. However, electrical stimulation studies in humans have observed that stimulation of the dLMC elicits a vowel-like vocalization, while stimulation of the vLMC elicits grunting [8,16,62]. The dLMC is bounded posteriorly by a putative larynx sensory cortex (LSC) on the posterior central gyrus. This LSC is larger and activates more strongly in professional opera singers than non-singers, suggesting that these individuals make greater use of proprioceptive feedback to guide highly skilled motor control [63,64].
It is not clear whether the vLMC is bounded posteriorly by a sensory zone, analogous to the dLMC. However, the vLMC may itself have some sensory function not matched by its dorsal counterpart. While the vLMC has primarily been localized as a correlate of vocal motor behaviour [17,20–23], activation of this region has also been observed in response to sensory stimulation of the larynx by applying an external puff of air [65]. Somewhat paradoxically, anaesthetizing the larynx does not reduce vLMC activation [19]. A recent cortical parcellation based on multi-modal brain imaging confirms that this region is distinct from both primary motor and primary somatosensory cortex and suggests a combination of sensorimotor functions [66]. Further research on the relationship between the vLMC and the broader motor system may shed further light on its function.
4. The motor system and its somatotopic maps
Motor cortex is the main source of output from the motor system. However, motor control is not the product of M1 alone, but requires a broader motor network that supports complex voluntary movements. This network includes brain regions such as the BG, SMA, cingulate cortex and the cerebellum (figure 2). In this section, we review the existing evidence that each of these brain regions contains its own somatotopic map akin to motor cortex. Intriguingly, the somatotopic maps in the brains of individuals born without one hand undergo a neuroplastic remapping that may occur in parallel across multiple brain regions within this network [67], which may suggest that somatotopic maps across the motor network are driven by common developmental mechanisms.
Figure 2.
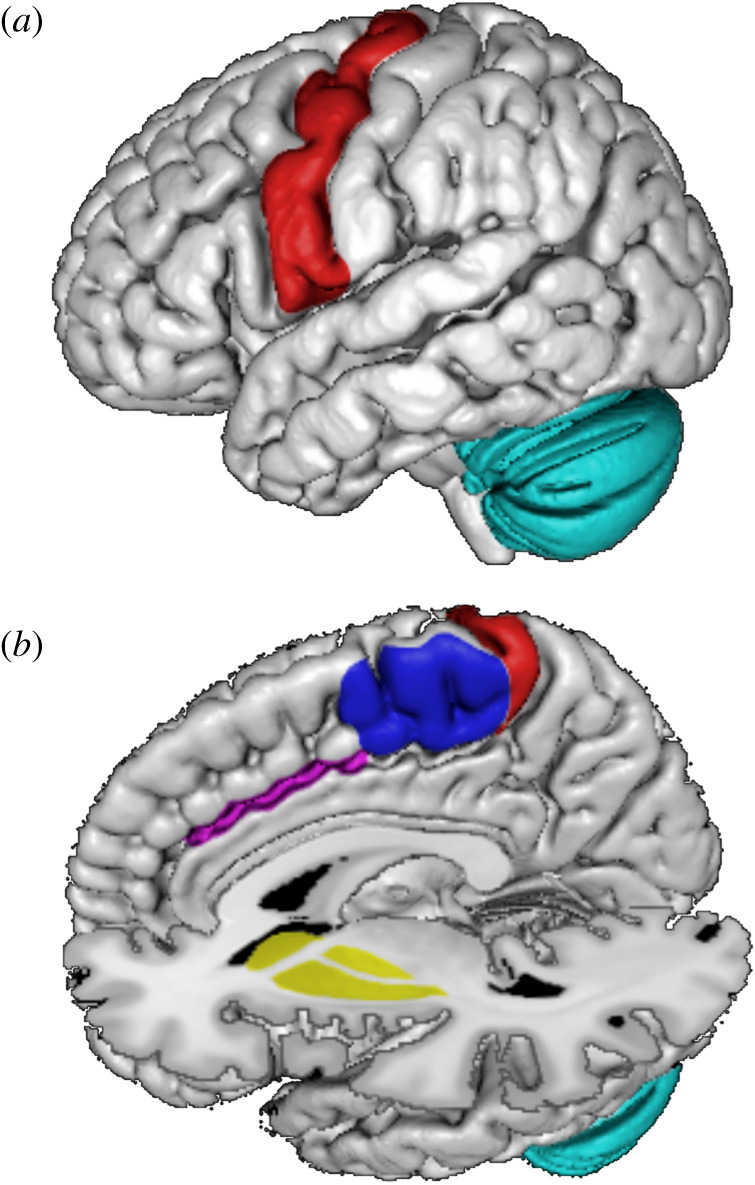
Major components of the motor network. (a) Lateral surface view of MNI152 atlas brain; (b) medial surface view with digital transections at x = 0 and z = 0 showing the motor cortex (red), middle cingulate cortex, (pink), BG (yellow), SMA (blue) and cerebellum (cyan).
(a) . Motor cortex
The somatotopic map in primary motor cortex (BA 4) is well characterized and is sometimes referred to as a homunculus in the brain after its reflection of the physical body. The muscles of the foot are located at one end of the somatotopic map and the muscles of the head located at the other [9–11]. For conceptual convenience, zones within these somatotopic maps are often referred to by simplistic labels based on the effectors with which they are most strongly associated (e.g. M1hand for the predominantly hand-controlling zone). However, at a finer spatial scale, these zones are composed of tessellated fields and individual effectors can be controlled by discontinuous but clustered representations [68]. These representations have been described as either encoding the states of muscles [69,70], the spatial properties of movement vectors [71,72] or ethologically meaningful combinations of effectors that pattern whole movements [61,73], and these levels of encoding are not mutually exclusive [74].
Distinct functional contributions of the dLMC and vLMC remain elusive [75,76]. However, electrical stimulation of these regions in the human brain elicits vowel sounds and grunting, respectively [8,16,62]. These separate behaviours produced by the same ensemble of muscles are suggestive of distinct ethological functions of the dLMC and vLMC, though further evidence is required. It is hoped that an understanding of the connections of these two regions with the broader motor system will begin to elucidate their respective functions.
(b) . Cortico-cerebellar loops
The cerebellum maintains a broad pattern of connections throughout the brain and has some part in a wide range of central nervous system function [77,78]. Among these functions, the cerebellum plays a critical role in making online adjustments that fine-tune movements. The cerebellum receives an efferent copy of motor commands from M1 and compares expected proprioceptive feedback with observed proprioceptive feedback [79–82]. The difference between intended and observed movements produces an error signal that is returned to M1 to implement online corrections to ongoing movements.
The cerebellum contains at least two separate somatotopic maps [83]. The anterior lobe of the cerebellum contains a somatotopic map with the foot located antero-dorsally and the head postero-ventrally, while the posterior lobe has a somatotopic map with the face represented postero-dorsally and the foot antero-ventrally [84–88]. More recent evidence suggests that the anterior lobe may contain an additional somatotopic map along the lateral-to-medial axis [89], though further replication is required.
(c) . Cortico-striatal loops
The SMA and BG form part of the cortico-striatal loop that is involved in motor learning [90,91]. The motoric processing loop of the BG forms a circuit through its various component nuclei including the putamen (a part of the striatum for which this circuit is named), globus pallidus, subthalamic nucleus and substantia nigra, which sends outputs via the thalamus back to the cortex [92]. This circuit receives dopaminergic inputs from reward centres that mediate reinforcement learning [93,94].
The SMA and a region anterior to it called the pre-SMA both contain a distinct set of motor representations, with a clear somatotopy at least in SMA [95]. This somatotopic map spans from the legs posteriorly to the orofacial muscles anteriorly [13,96–99]. The putamen receives inputs from both M1 and the SMA and these inputs retain the somatotopic organization of their sources [15]. Inputs from M1 and the SMA innervate distinct portions of the putamen, and it has therefore been suggested that the putamen may contain two parallel somatotopic maps [100]. Somatotopy may also be retained throughout the entire cortico-striatal loop [101], including the globus pallidus [102,103] and thalamus [104], though on a spatial scale that is inaccessible to current non-invasive brain imaging methodologies.
(d) . Cingulate cortex
The cingulate cortex is nested in the medial surface of the brain following the curvature of the corpus callosum. This brain region combines cognitive, affective and motoric functions for the motivation and initiation of goal-directed behaviours [105–108]. It is divided grossly into the anterior, middle and posterior cingulate cortex (ACC, MCC and PCC, respectively). The MCC has approximate boundaries anteriorly at the genu of the corpus callosum and posteriorly at the marginal sulcus [109–111]. This macro-anatomically defined region itself comprises multiple cytoarchitecturally defined subregions. Of these, area 24c is in the cingulate sulcus, which contains a series of three cingulate motor areas [12,112]. These cingulate motor areas are all involved in action selection, with increasingly complex movement patterns involving the more anterior divisions [95,113,114].
The middle cingulate sulcus contains three distinct motor regions [12,112], each of which contains a somatotopic map with the feet represented posteriorly and the orofacial muscles anteriorly [12,95,115–118]. Somatotopic mapping in the cingulate cortex may be further complicated by the high degree of anatomical variability of this region, since in a subset of human brains the motor regions of the cingulate sulcus are divided across separate cingulate and paracingulate sulci [12,119–121].
(e) . White matter somatotopy
The descending motor pathways that form the corticobulbar and corticospinal outputs from the motor system maintain a clear somatotopic map that is observable in white matter [122–125]. This somatotopy facilitates the mapping of upper motor neurons in primary motor cortex onto their corresponding lower motor neurons in the brainstem and spinal cord. Likewise, the somatotopic maps of M1 in either hemisphere project preferentially to homotopic sites in the opposite hemisphere, retaining ordered somatotopy in the white matter of the corpus callosum [126,127]. At least some of the individual brain regions that make up the motor network also display preferential functional connectivity between somatotopically analogous regions [87,128], maintaining somatotopy in the white matter pathways that connect them [115,129].
5. Initial evidence for dual laryngeal representations in the cerebellum and supplementary motor area
(a) . Cerebellum
We re-analysed an existing fMRI dataset to test whether two distinct representations of the laryngeal muscles can be observed in the cerebellum (see [21] for details on data acquisition). The study was approved by the Central University Research Ethics Committee at the University of Oxford (CUREC, R55787/RE001) in accordance with the regulatory standards of the Code of Ethics of the World Medical Association (Declaration of Helsinki). Twenty participants performed speech movements to localize lips, tongue and laryngeal activity during vocalization. Participants produced non-linguistic utterances overtly, articulating silently, using an isolated vowel, or as covert speech. The LMC was then localized using a factorial model comparing overt speech and vowel production with silent articulation and covert speech. See [21] for a detailed description of the functional paradigm and analysis.
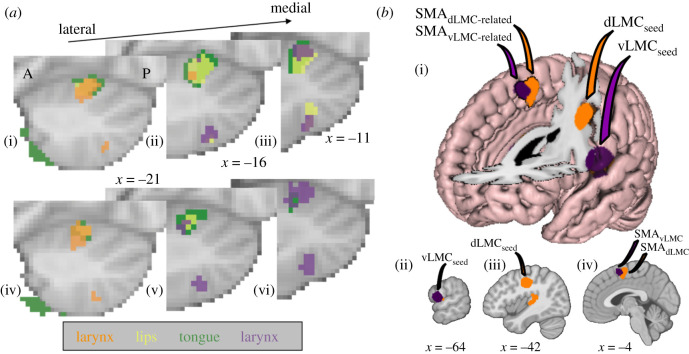
In addition to conventional group-level statistical activation maps, we derived overlap maps of individually thresholded and binarized volumetric maps (figure 3a for details of analysis). A larynx-lip-tongue-larynx pattern can be observed along a lateral/anterior-to-medial/posterior axis. The coordinates of these regions are consistent with lobule VI of the posterior cerebellar lobe [130]. Two distinct activations for the larynx can be observed at the group level (figure 3a(i,ii,iii)) as well as in individual participants (figure 3a(iv,v,vi)). Activations for the lips and the tongue fall in between the two larynx activations as they do in motor cortex, though at the present resolution these activations are largely overlapping. The dLMC-related activation is observed antero-laterally to the articulators while the vLMC-related activation is observed postero-medially. All activations are in close proximity and within the same anatomical lobule.
Figure 3.
Initial evidence for laryngeal motor network somatotopy. (a) Cerebellar task activations during movement of the lips, the tongue and during larynx activity. Shown are sagittal slices of the left hemisphere (A–P: anterior–posterior). Larynx activity is shown in orange and purple to indicate dLMC- and vLMC-related activation, though we note that these are correlated activations derived from the same contrast. (i, ii, iii) Binarized group-level task activations (voxel-wise threshold z > 4, n = 20). (iv, v, vi) Binarized overlap maps (individual maps: voxel-wise threshold of z > 3.1, overlap map: thresholded at n > 10 participants). (b) Results of ALE meta-analysis from the two LMC seed regions displayed on the MNI152 atlas brain. (i) The surface brain is digitally transected sagittally at x = 0, axially at z = 10, and coronally with an oblique slice following the precentral gyrus. (ii, iii, iv) Sagittal slices transecting the two seed regions and the SMA. The dLMC-related SMA (orange) is posterior to the vLMC-related SMA (purple) in line with the expected somatotopy of this region.
Our results are most consistent with one continuous somatotopic map in lobule VI of the cerebellum that contains two distinct laryngeal representations. We note also that additional activations are present at a lower threshold in the remaining lobules, which may reflect additional somatotopic maps [84–88].
(b) . Supplementary motor area
We conducted a meta-analysis of brain imaging studies that activated the dLMC and vLMC to identify brain regions that are co-activated with each larynx area. We searched the BrainMap database [131] for fMRI studies that reported activation within a 5 mm radius sphere of the dLMC (x = −41; y = −16; z = 38) or the vLMC (x = −66; y = −4; z = 14). This search was performed blind to the tasks being performed by the participants and was concerned only with activation within the seed regions [132]. Coordinate tables in Montreal Neurological Institute (MNI) space were retrieved from the database on 4 April 2020 (see electronic supplementary material, S1 and S2). This search yielded 512 foci of activation across 29 participant groups for the dLMC, and 294 foci across 19 participant groups for the vLMC. Each set of activation coordinates was analysed using activation-likelihood estimation (ALE) [133–135] using GingerAle software (v. 3.0.2) with a cluster-level family-wise error rate of p < 0.01 computed with 5000 permutations. Results were visualized using Mango (v. 4.1, Research Imaging Institute, UTHSCSA).
The dLMC-related ALE yielded a network of motor- and auditory-related brain regions including the contralateral dLMC, the superior temporal gyrus (STG), putamen, cerebellum and the SMA (figure 3b and table 1). The vLMC-related ALE yielded a much more restricted network, as expected from the smaller pool of studies in that analysis, including the contralateral vLMC, the insula and the SMA. Both ALEs revealed co-activation with the SMA, but at spatially distinct sites. The dLMC-related SMA was posterior to the vLMC-related SMA. This pattern is consistent with the expected somatotopy of this region and with the previously observed network somatotopy between the SMA and motor cortex [128,129].
Table 1.
Coordinates of peak likelihoods from ALE meta-analysis for seed regions in the dLMC (upper) and vLMC (lower). Brain regions are listed along with their x, y, z coordinates in MNI stereotaxic space and their activation-likelihood estimation scores, which provide a relative measure of confidence.
| brain region | hemisphere | x | y | z | ALE value |
|---|---|---|---|---|---|
| dLMC | |||||
| dLMC [seed] | left | −42 | −16 | 38 | 0.125 |
| dLMC | right | 46 | −12 | 38 | 0.045 |
| SMA | left | −4 | 0 | 56 | 0.041 |
| putamen | right | 26 | 0 | 4 | 0.032 |
| cerebellum | left | −12 | −62 | −20 | 0.032 |
| STG | left | −60 | −14 | 10 | 0.027 |
| vLMC | |||||
| vLMC [seed] | left | −64 | −4 | 14 | 0.098 |
| vLMC | right | 66 | −4 | 22 | 0.024 |
| SMA | left | −2 | 8 | 58 | 0.025 |
| right insula | right | 42 | −6 | 8 | 0.024 |
6. Mechanisms of brain network evolution
We have hypothesized that the human brain has evolved not only a dual representation of the laryngeal muscles in motor cortex, but a dual laryngeal motor network to support it. However, this broader characterization of the phenotype raises important questions about how natural selection may act simultaneously on an entire network of brain regions whose functions are strongly interdependent. Among these questions is how the emergence of a novel pathway overcomes strong allometric constraints, for example, that dictate the relative volume of grey matter to white [136,137], or how individual neural adaptations can be accommodated within the highly conserved organization of neocortex [138,139].
There is some debate about the extent to which evolution is able to influence individual brain regions to form an evolutionary mosaic [140,141] as compared to concerted change over the entire brain [142,143]. While brain area size is highly predictable from overall brain size taken at a broad taxonomical scale (e.g. across mammals), individual brain regions violate this trend when examined at a finer taxonomic scale (e.g. across primates), which is a likely driver of inter-species behavioural differences [42,144].
Pairs of functionally related brain structures have correlated sizes across species even after controlling for brain size, indicating that brain networks may evolve together and at least partially independently of other brain structures [140]. Furthermore, natural selection may be capable of acting on individual brain regions and their corresponding networks due to genetic mechanisms that provide independent regulation of brain region sizes [141]. The primate cortical sheet has not expanded uniformly as brain size increased, with the occipital lobe expanding least and the frontal and temporal lobes expanding most, but this pattern is conserved and species differences appear to be the product of brain size [145].
A remarkably analogous instance of network-wide brain evolution is found in the song system of parrots. Strong vocal learning abilities have evolved independently in three lineages of birds, and of these, parrots are among the most prodigious vocal learners [50,146]. The avian song system is composed of a series of nuclei, some of which are analogous to structures in the human vocal motor system including the putamen, motor cortex and nucleus ambiguus [18,147], and are regulated by specialized patterns of gene expression [148,149]. The parrot brain is unusual in containing two parallel song systems [150]. Nuclei in the parrot song system are composed of a core that is analogous with the song system of other avian vocal learners, and a surrounding shell that forms a rudimentary second song system. The core and shell song systems form parallel networks; however, only the core sends direct projections to the brainstem motor nucleus that controls the syrinx (i.e. the analogue to mammalian nucleus ambiguus). Chakraborty & Jarvis [151] proposed that such a phenotype could arise by mutations that cause the entire network to duplicate as an ensemble, in line with a previous proposal that the avian song system itself may have evolved as a specialization from a pre-existing limb and body motor network [152].
We suggest that only a relatively minor change to an existing portion of mammalian motor cortex may have been sufficient to evolve a novel laryngeal motor network in humans. We propose that the emergence of novel efferent pathways to the nucleus ambiguus de facto alters the functional significance not only of these cortical neurons in the motor cortex but also the broader network in which they are embedded (figure 4). Given that somatotopic motor networks are defined by the effectors that they control (e.g. M1-hand is that part of motor cortex which projects to hand lower motor neurons in the spinal cord, SMA-hand is that part of the SMA that projects to M1-hand, etc.), modifications to the descending efferent pathways of motor cortex alter the function of corresponding sites throughout the motor network. Hence, we propose that the evolution of novel projections from one or both of the LMCs was sufficient for the emergence of vocal motor networks, thereby acquiring novel functions. Such a mechanism would leverage existing long-range connections in the brain, thereby preserving existing allometric relationships between the grey and white matter volumes and overcoming hard barriers for morphological changes.
Figure 4.
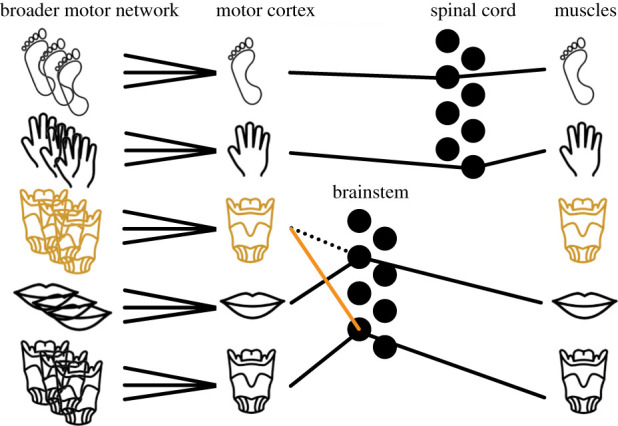
Conceptual depiction of parallel effector-specific circuits feeding from the broader motor network to upper motor neurons in motor cortex and onto lower motor neurons in the brainstem and spinal cord (black circles). We propose that evolutionary changes that add novel downstream targets (orange line) to the efferent motor pathway change the function of the corresponding portion of motor cortex as well as the broader motor networks to which it is connected. The example above depicts a novel projection from a patch of motor cortex to brainstem, which in turn alters the function of the motor network in which it is embedded to support voice motor control. The dotted line indicates that this patch was previously recruited by a different effector.
One mechanism that has been proposed to drive the development of novel laryngeal motor specializations in humans is the evolution of novel patterns of gene expression in the dLMC and vLMC relative to surrounding cortex [18]. This specialization includes genes of the slit and plexin family that encode axon guidance molecules and neuronal growth cone receptors, respectively [153,154]. These genes are likely candidates for a molecular genetic mechanism that may drive the direct projection to nucleus ambiguus in humans. Alternatively, such a specialization may simply arise as a consequence of the increased proportional size of neocortex. Larger brain regions send more axonal projections and compete more effectively for limited dendritic space [155,156]. For example, among mammals, proportionally larger neocortical size is correlated with deeper penetration of the spinal cord by corticospinal axons, which in turn mediates improved manual dexterity [42,43]. Hence, the increased proportional size of human neocortex alone may have been a driving factor in evolving novel vocal motor networks in humans. As cortical expansion increased the total number of corticobulbar axons, they may have invaded novel territory in the nucleus ambiguus, potentially at the expense of other inputs that mediate unlearned vocalizations, such as the periaqueductal grey [157,158].
We note that the human brain has undergone numerous other large-scale structural changes relative to non-human primates [159–164]. The emergence of vocal motor networks is itself not sufficient for the communicative behaviours of humans. Rather, it is part of an ensemble of neural adaptations that support the vocal, auditory, semantic, syntactic and pragmatic faculties that are needed for speech and language, and that may have separate evolutionary histories [31,32,165]. However, we do suggest that the small-scale modification of the corticobulbar outputs of motor cortex may have had large-scale functional implications for the motor network.
7. Summary
We have proposed a novel hypothesis that the dual representation of the laryngeal muscles found in the motor cortex is repeated throughout the motor network. Somatotopic organization is a feature that is found across the network of brain regions that control voluntary movement. Each of these brain regions contains representations of muscle groups following a predictable order based on the plan of the body. These motor regions project preferentially to somatotopically homologous regions (e.g. M1-hand to SMA-hand) to form an extended somatotopic network. Initial evidence suggests that the cerebellum and SMA may also contain dual representations of the larynx, thereby contributing the functions of the cortico-cerebellar and cortico-striatal loops to voice motor control. These findings require further replication and should be extended to other motor regions such as cingulate cortex and the BG. This hypothesis raises important questions about how adaptations at the level of motor cortex may impact the broader network in which it is embedded. We have also discussed brain evolution in search of a parsimonious mechanism for the emergence of this complex phenotype in the human brain.
Acknowledgements
The authors would like to thank Prof. Kate E. Watkins for enlightening discussions on the larynx motor cortex and the speech motor network.
Data accessibility
The fMRI data are publicly available at OpenNeuro under the accession code ds002634 (Dataset: Project_larynx). Processing code for this dataset is publicly available from the Wellcome Centre for Integrative Neuroimaging's GitLab at https://git.fmrib.ox.ac.uk/neichert/project_larynx. Coordinate tables for ALE meta-analyses are provided in the electronic supplementary material.
Authors' contributions
M.B. drafted the general discussion of the manuscript and carried out the meta-analysis of the SMA. N.E. carried out the analysis of the cerebellum, drafted related sections of the manuscript and provided critical edits. C.M. provided critical edits to the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests
Funding
This work was funded by a Research Leadership Award (grant no. RL-2016-013) from The Leverhulme Trust (C.M.) This research was funded in part by the Wellcome Trust. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (grant no. 203139/Z/16/Z) and N.E. was funded by a Wellcome Trust PhD stipendship (grant no. 203730/Z/16/Z). For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
References
- 1.Janik VM, Slater PJB. 2000. The different roles of social learning in vocal communication. Anim. Behav. 60, 1-11. ( 10.1006/anbe.2000.1410) [DOI] [PubMed] [Google Scholar]
- 2.Vernes S, Wilkinson G. 2019. Behaviour, biology, and evolution of vocal learning in bats. Phil. Trans. R. Soc. B 375, 20190061. ( 10.1101/646703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyack PL. 2019. A taxonomy for vocal learning. Phil. Trans. R. Soc. B 375, 20180406. ( 10.1098/rstb.2018.0406) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer J, Hammerschmidt K. 2019. Towards a new taxonomy of primate vocal learning. Phil. Trans. R. Soc. B 375, 20199945. ( 10.1098/rstb.2019.0045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wich SA, et al. 2012. Call cultures in orang-utans? PLoS ONE 7, 1-9. ( 10.1371/journal.pone.0036180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lameira AR, Hardus ME, Mielke A, Wich SA, Shumaker RW. 2016. Vocal fold control beyond the species-specific repertoire in an orangutan. Sci. Rep. 6, 1-10. ( 10.1038/srep30315) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer J. 2021. Primate vocal communication and the evolution of speech. Curr. Dir. Psychol. Sci. 30, 55-60. ( 10.1177/0963721420979580) [DOI] [Google Scholar]
- 8.Penfield W, Boldrey E. 1937. Somatic motor and sensory representations in the cerebral cortex of man as studied by electrical stimulation. Brain 60, 389-443. ( 10.1192/bjp.84.352.868-a) [DOI] [Google Scholar]
- 9.Correia JM, Caballero-Gaudes C, Guediche S, Carreiras M. 2020. Phonatory and articulatory representations of speech production in cortical and subcortical fMRI responses. Sci. Rep. 20, 1-14. ( 10.1038/s41598-020-61435-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotze M, Erb M, Flor H, Huelsmann E, Godde B, Grodd W. 2000. fMRI evaluation of somatotopic representation in human primary motor cortex. Neuroimage 11, 473-481. ( 10.1006/nimg.2000.0556) [DOI] [PubMed] [Google Scholar]
- 11.Takai O, Brown S, Liotti M. 2010. Representation of the speech effectors in the human motor cortex: somatotopy or overlap? Brain Lang. 113, 39-44. ( 10.1016/j.bandl.2010.01.008) [DOI] [PubMed] [Google Scholar]
- 12.Amiez C, Petrides M. 2014. Neuroimaging evidence of the anatomo-functional organization of the human cingulate motor areas. Cereb. Cortex 24, 563-578. ( 10.1093/cercor/bhs329) [DOI] [PubMed] [Google Scholar]
- 13.Penfield W, Welch K. 1951. The supplementary motor area of the cerebral cortex: a clinical and experimental study. Arch. Neurol. Psychiatry 66, 289-317. ( 10.1001/archneurpsyc.1951.02320090038004) [DOI] [PubMed] [Google Scholar]
- 14.Glickstein M, Sultan F, Voogd J. 2011. Functional localization in the cerebellum. Cortex 47, 59-80. ( 10.1016/j.cortex.2009.09.001) [DOI] [PubMed] [Google Scholar]
- 15.Nambu A. 2011. Somatotopic organization of the primate basal ganglia. Front. Neuroanat. 5, 1-9. ( 10.3389/fnana.2011.00026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breshears JD, Molinaro AM, Chang EF. 2015. A probabilistic map of the human ventral sensorimotor cortex using electrical stimulation. J. Neurosurg. 123, 340-349. ( 10.3171/2014.11.JNS14889) [DOI] [PubMed] [Google Scholar]
- 17.Bouchard KE, Mesgarani N, Johnson K, Chang EF. 2013. Functional organization of human sensorimotor cortex for speech articulation. Nature 495, 327-332. ( 10.1038/nature11911) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfenning AR, et al. 2014. Convergent transcriptional specializations in the brains of humans and song-learning birds. Science 346, 1256846.1–13. ( 10.1126/science.1256846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kleber B, Zeitouni AG, Friberg A, Zatorre RJ. 2013. Experience-dependent modulation of feedback integration during singing: role of the right anterior insula. J. Neurosci. 33, 6070-6080. ( 10.1523/JNEUROSCI.4418-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belyk M, Pfordresher PQ, Liotti M, Brown S. 2016. The neural basis of vocal pitch imitation in humans. J. Cogn. Neurosci. 28, 621-635. ( 10.1162/jocn_a_00914) [DOI] [PubMed] [Google Scholar]
- 21.Eichert N, Papp D, Mars RB, Watkins KE. 2020. Mapping human laryngeal motor cortex during vocalization. Cereb. Cortex 30, 6254-6269. ( 10.1093/cercor/bhaa182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olthoff A, Baudewig J, Kruse E, Dechent P. 2008. Cortical sensorimotor control in vocalization: a functional magnetic resonance imaging study. Laryngoscope 118, 2091-2096. ( 10.1097/MLG.0b013e31817fd40f) [DOI] [PubMed] [Google Scholar]
- 23.Terumitsu M, Fujii Y, Suzuki K, Kwee IL, Nakada T. 2006. Human primary motor cortex shows hemispheric specialization for speech. Neuroreport 17, 1091-1095. ( 10.1097/01.wnr.0000224778.97399.c4) [DOI] [PubMed] [Google Scholar]
- 24.Belyk M, Brown R, Beal DS, Roebroeck A, McGettigan C, Guldner S, Kotz SA. 2021. Human larynx motor cortices coordinate respiration for vocal-motor control. Neuroimage 239, 118326. ( 10.1016/j.neuroimage.2021.118326) [DOI] [PubMed] [Google Scholar]
- 25.Huber L, et al. 2020. Sub-millimeter fMRI reveals multiple topographical digit representations that form action maps in human motor cortex. Neuroimage 208, 116463. ( 10.1016/j.neuroimage.2019.116463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meier JD, Aflalo TN, Kastner S, Graziano MSA. 2008. Complex organization of human primary motor cortex: a high-resolution fMRI study. J. Neurophysiol. 100, 1800-1812. ( 10.1152/jn.90531.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strick PL, Preston JB. 1982. Two representations of the hand in area 4 of a primate. I. Motor output organization. J. Neurophysiol. 48, 139-149. ( 10.1152/jn.1982.48.1.139) [DOI] [PubMed] [Google Scholar]
- 28.Park MC, Belhaj-Saïf A, Gordon M, Cheney PD. 2001. Consistent features in the forelimb representation of primary motor cortex in rhesus macaques. J. Neurosci. 21, 2784-2792. ( 10.1523/jneurosci.21-08-02784.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leyton S, Sherrington C. 1917. Observations on the excitable cortex of the chimpanzee, organ-utan, and gorilla. Exp. Physiol. 11, 135-222. ( 10.1113/expphysiol.1917.sp000240) [DOI] [Google Scholar]
- 30.Jürgens U. 2002. Neural pathways underlying vocal control. Neurosci. Biobehav. Rev. 26, 235-258. ( 10.1016/S0149-7634(01)00068-9) [DOI] [PubMed] [Google Scholar]
- 31.Jarvis ED. 2019. Evolution of vocal learning and spoken language. Science 366, 50-54. ( 10.1126/science.aax0287) [DOI] [PubMed] [Google Scholar]
- 32.Hauser MD, Chomsky N, Fitch WT. 2002. The faculty of language: what is it, who has it, and how did it evolve? Science 298, 1569-1579. ( 10.1126/science.298.5598.1569) [DOI] [PubMed] [Google Scholar]
- 33.Hayes KJ, Hayes C. 1951. The intellectual development of a home-raised chimpanzee. Proc. Am. Phil. Soc. 95, 105-109. [Google Scholar]
- 34.Jürgens U. 2009. The neural control of vocalization in mammals: a review. J. Voice 23, 1-10. ( 10.1016/j.jvoice.2007.07.005) [DOI] [PubMed] [Google Scholar]
- 35.Kuypers HGJM. 1958. Some projections from the peri-central cortex to the pons and lower brain stem in monkey and chimpanzee. J. Comp. Neurol. 110, 221-255. ( 10.1002/cne.901100205) [DOI] [PubMed] [Google Scholar]
- 36.Belyk M, Brown S. 2017. The origins of the vocal brain in humans. Neurosci. Biobehav. Rev. 77, 177-193. ( 10.1016/j.neubiorev.2017.03.014) [DOI] [PubMed] [Google Scholar]
- 37.Fischer J, Hammerschmidt K. 2011. Ultrasonic vocalizations in mouse models for speech and socio-cognitive disorders: insights into the evolution of vocal communication. Genes Brain Behav. 10, 17-27. ( 10.1111/j.1601-183X.2010.00610.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitch WT. 2011. The evolution of syntax: an exaptationist perspective. Front. Evol. Neurosci. 3, 1-12. ( 10.3389/fnevo.2011.00009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarvis ED. 2004. Learned birdsong and the neurobiology of human language. Ann. N.Y. Acad. Sci. 1016, 749-777. ( 10.1196/annals.1298.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simonyan K, Horwitz B. 2011. Laryngeal motor cortex and control of speech in humans. Neuroscience 17, 197-208. ( 10.1177/1073858410386727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mars RB, Eichert N, Jbabdi S, Verhagen L, Rushworth MFS. 2018. Connectivity and the search for specializations in the language-capable brain. Curr. Opin. Behav. Sci. 21, 19-26. ( 10.1016/j.cobeha.2017.11.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heffner R, Masterton B. 1975. Variation in form of the pyramidal tract and its relationship to digital dexterity. Brain. Behav. Evol. 12, 161-200. ( 10.1159/000124401) [DOI] [PubMed] [Google Scholar]
- 43.Striedter G. 2018. Principles of brain evolution. New York, NY: Sinauer Associates. [Google Scholar]
- 44.Gu Z, et al. 2017. Control of species-dependent cortico-motoneuronal connections underlying manual dexterity. Science 357, 400-404. ( 10.1126/science.aan3721) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonyan K, Jürgens U. 2003. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res. 974, 43-59. ( 10.1016/S0006-8993(03)02548-4) [DOI] [PubMed] [Google Scholar]
- 46.Kuypers HGJM. 1958. Corticobulbar connexions to the pons and lower brain-stem in man. Brain 81, 364-388. ( 10.1093/brain/81.3.364) [DOI] [PubMed] [Google Scholar]
- 47.Iwatsubo T, Kuzuhara S, Kanemitsu A. 1990. Corticofugal projections to the motor nuclei of the brainstem and spinal cord in humans. Neurology 40, 309-312. ( 10.1212/WNL.40.2.309) [DOI] [PubMed] [Google Scholar]
- 48.Wild JM. 1993. Descending projections of the songbird nucleus robustus archistriatalis. J. Comp. Neurol. 338, 225-241. ( 10.1002/cne.903380207) [DOI] [PubMed] [Google Scholar]
- 49.Wild JM, Williams MN, Suthers RA. 2000. Neural pathways for bilateral vocal control in songbirds. J. Comp. Neurol. 426, 413-426. () [DOI] [PubMed] [Google Scholar]
- 50.Petkov CI, Jarvis ED. 2012. Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Front. Evol. Neurosci. 4, 1-24. ( 10.3389/fnevo.2012.00012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nieder A, Mooney R. 2019. The neurobiology of innate, volitional and learned vocalizations in mammals and birds. Phil. Trans. R. Soc. B 375, 20190054. ( 10.1098/rstb.2019.0054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theys C, van Wieringen A, Sunaert S, Thijs V, De Nil LF.. 2011. A one year prospective study of neurogenic stuttering following stroke: incidence and co-occurring disorders. J. Commun. Disord. 44, 678-687. ( 10.1016/j.jcomdis.2011.06.001) [DOI] [PubMed] [Google Scholar]
- 53.Heinsius T, Bogousslavsky J, Van Melle G.. 1998. Large infarcts in the middle cerebral artery territory: etiology and outcome patterns. Neurology 50, 341-350. ( 10.1212/WNL.50.2.341) [DOI] [PubMed] [Google Scholar]
- 54.Ludlow CL. 2005. Central nervous system control of the laryngeal muscles in humans. Respir. Physiol. Neurobiol. 147, 205-222. ( 10.1016/j.resp.2005.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hast MH, Fischer JM, Wetzel AB, Thompson VE. 1974. Cortical motor representation of the laryngeal muscles in Macaca mulatta. Brain 73, 229-240. ( 10.1016/0006-8993(74)91046-4) [DOI] [PubMed] [Google Scholar]
- 56.Jürgens U. 1976. Projections from the cortical larynx area in the squirrel monkey. Exp. Brain Res. 25, 401-411. ( 10.1007/BF00241730) [DOI] [PubMed] [Google Scholar]
- 57.Brodmann K. 1909. Localisation in the cerebral cortex, 3rd edn. New York, NY: Springer. [Google Scholar]
- 58.Vogt O. 1910. Die myeloarchitektonische Felderung des menschlichen Stirnhirns. J. für Psychol. und Neurol. 15, 221-232. [Google Scholar]
- 59.Judaš M, Cepanec M. 2010. Oskar Vogt: the first myeloarchitectonic map of the human frontal cortex. Transl. Neurosci. 1, 72-94. ( 10.2478/v10134-010-0005-z) [DOI] [Google Scholar]
- 60.Eichert N, Watkins KE, Mars RB, Petrides M. 2020. Morphological and functional variability in central and subcentral motor cortex of the human brain. Brain Struct. Funct. 226, 263-279. ( 10.1007/s00429-020-02180-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graziano MSA, Taylor CSR, Moore T. 2002. Complex movements evoked by microstimulation of precentral cortex. Neuron 34, 841-851. ( 10.1016/S0896-6273(02)00698-0) [DOI] [PubMed] [Google Scholar]
- 62.Foerster O. 1931. The cerebral cortex in man. Lancet 218, 309-312. ( 10.1016/S0140-6736(00)47063-7) [DOI] [Google Scholar]
- 63.Kleber B, Veit R, Birbaumer N, Gruzelier J, Lotze M. 2010. The brain of opera singers: experience-dependent changes in functional activation. Cereb. Cortex 20, 1144-1152. ( 10.1093/cercor/bhp177) [DOI] [PubMed] [Google Scholar]
- 64.Kleber B, Veit R, Moll CV, Gaser C, Birbaumer N, Lotze M. 2016. Voxel-based morphometry in opera singers: increased gray-matter volume in right somatosensory and auditory cortices. Neuroimage 133, 477-483. ( 10.1016/j.neuroimage.2016.03.045) [DOI] [PubMed] [Google Scholar]
- 65.Miyaji H, Hironaga N, Umezaki T, Hagiwara K, Shigeto H, Sawatsubashi M, Tobimatsu S, Komune S. 2014. Neuromagnetic detection of the laryngeal area: sensory-evoked fields to air-puff stimulation. Neuroimage 88, 162-169. ( 10.1016/j.neuroimage.2013.11.008) [DOI] [PubMed] [Google Scholar]
- 66.Glasser MF, et al. 2016. A multi-modal parcellation of human cerebral cortex. Nature 536, 171-178. ( 10.1038/nature18933) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hahamy A, Makin TR. 2019. Remapping in cerebral and cerebellar cortices is not restricted by somatotopy. J. Neurosci. 39, 9328-9342. ( 10.1523/JNEUROSCI.2599-18.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krubitzer LA, Seelke AMH. 2012. Cortical evolution in mammals: the bane and beauty of phenotypic variability. Proc. Natl Acad. Sci. USA 109, 10 647-10 654. ( 10.1073/pnas.1201891109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mussa-Ivaldi FA. 1988. Do neurons in the motor cortex encode movement directions? An alternative hypothesis. Neurosci. Lett. 91, 106-111. ( 10.1016/0304-3940(88)90257-1) [DOI] [PubMed] [Google Scholar]
- 70.Woolsey CN, Erickson TC, Gilson WE. 1979. Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J. Neurosurg. 51, 476-506. ( 10.3171/jns.1979.51.4.0476) [DOI] [PubMed] [Google Scholar]
- 71.Kakei S, Hoffman DS, Strick P. 1999. Muscle and movement representations in the primary motor cortex. Science 285, 2136-2139. ( 10.1126/science.285.5436.2136) [DOI] [PubMed] [Google Scholar]
- 72.Georgopoulos AP, Kettner RE, Schwartz AB. 1988. Primate motor cortex and free arm movements to visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal population. J. Neurosci. 8, 2928-2937. ( 10.1089/scd.2011.0674) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rizzolatti G, Luppino G. 2001. The cortical motor system. Neuron 31, 889-901. ( 10.1080/1059924X.2010.512854) [DOI] [PubMed] [Google Scholar]
- 74.Graziano MSA. 2016. Ethological action maps: a paradigm shift for the motor cortex. Trends Cogn. Sci. 20, 121-132. ( 10.1016/j.tics.2015.10.008) [DOI] [PubMed] [Google Scholar]
- 75.Belyk M, Lee YS, Brown S. 2018. How does human motor cortex regulate vocal pitch in singers? R. Soc. Open Sci. 5, 172208. ( 10.1098/rsos.172208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belyk M, Brown S. 2014. Somatotopy of the extrinsic laryngeal muscles in the human sensorimotor cortex. Behav. Brain Res. 270, 364-371. ( 10.1016/j.bbr.2014.05.048) [DOI] [PubMed] [Google Scholar]
- 77.Koziol L, et al. 2014. Consensus paper: the cerebellum's role in movement and cognition. Cerebellum 13, 151-177. ( 10.1016/j.pestbp.2011.02.012.Investigations) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Buckner RL. 2013. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron 80, 807-815. ( 10.1016/j.neuron.2013.10.044) [DOI] [PubMed] [Google Scholar]
- 79.Scott S. 2004. Optimal feedback control and the neural basis of volitional motor control. Nat. Rev. Neurosci. 5, 532-546. ( 10.1038/nrn1427) [DOI] [PubMed] [Google Scholar]
- 80.Wolpert DM, Kawato M. 1998. Multiple paired forward and inverse models for motor control. Neural Netw. 11, 1317-1329. ( 10.1016/S0893-6080(98)00066-5) [DOI] [PubMed] [Google Scholar]
- 81.Wolpert DM, Ghahramani Z, Jordan MI. 1995. An internal model for sensorimotor integration. Science 269, 1880-1882. ( 10.1126/science.7569931) [DOI] [PubMed] [Google Scholar]
- 82.Ishikawa T, Tomatsu S, Izawa J, Kakei S. 2016. The cerebro-cerebellum: could it be loci of forward models? Neurosci. Res. 104, 72-79. ( 10.1016/j.neures.2015.12.003) [DOI] [PubMed] [Google Scholar]
- 83.Manni E, Petrosini L. 2004. A century of cerebellar somatotopy: a debated representation. Nat. Rev. Neurosci. 5, 241-249. ( 10.1038/nrn1347) [DOI] [PubMed] [Google Scholar]
- 84.Grodd W, Hülsmann E, Lotze M, Wildgruber D, Erb M. 2001. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum. Brain Mapp. 13, 55-73. ( 10.1002/hbm.1025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rijntjes M, Buechel C, Kiebel S, Weiller C. 1999. Multiple somatotopic representations in the human cerebellum. Neuroreport 10, 3653-3658. ( 10.1097/00001756-199911260-00035) [DOI] [PubMed] [Google Scholar]
- 86.Boillat Y, Bazin P, van der Zwaag W.. 2020. Whole-body somatotopic maps in the cerebellum revealed with 7T fMRI. Neuroimage 211, 116624. ( 10.1016/j.neuroimage.2020.116624) [DOI] [PubMed] [Google Scholar]
- 87.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Thomas Yeo BT. 2011. The organization of the human cerebellum estimated by intrinsic functional connectivity. J. Neurophysiol. 106, 2322-2345. ( 10.1152/jn.00339.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mottolese C, Richard N, Harquel S, Szathmari A, Sirigu A, Desmurget M. 2013. Mapping motor representations in the human cerebellum. Brain 136, 330-342. ( 10.1093/brain/aws186) [DOI] [PubMed] [Google Scholar]
- 89.Schlerf JE, Verstynen TD, Ivry RB, Spencer RMC. 2010. Evidence of a novel somatopic map in the human neocerebellum during complex actions. J. Neurophysiol. 103, 3330-3336. ( 10.1152/jn.01117.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehéricy S, Benali H. 2009. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 199, 61-75. ( 10.1016/j.bbr.2008.11.012) [DOI] [PubMed] [Google Scholar]
- 91.Lehéricy S, Benali H, Van de Moortele PF, Pélégrini-Issac M, Waeschter T, Ugurbil K, Doyon J.. 2005. Distinct basal ganglia territories are engaged in early and advanced motor sequences. Proc. Natl Acad. Sci. USA 102, 12 566-12 571. ( 10.1073/pnas.0502762102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Postuma RB, Dagher A. 2006. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex 16, 1508-1521. ( 10.1093/cercor/bhj088) [DOI] [PubMed] [Google Scholar]
- 93.Houk JC, Wise SP. 1995. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb. Cortex 5, 95-110. ( 10.1093/cercor/5.2.95) [DOI] [PubMed] [Google Scholar]
- 94.Doya K. 1999. What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Netw. 12, 961-974. ( 10.1016/S0893-6080(99)00046-5) [DOI] [PubMed] [Google Scholar]
- 95.Picard N, Strick PL. 1996. Motor areas of the medial wall: a review of their location and functional activation. Cereb. Cortex 6, 342-353. ( 10.1093/cercor/6.3.342) [DOI] [PubMed] [Google Scholar]
- 96.Cauda F, Giuliano G, Federico D, Sergio D, Sacco K. 2011. Discovering the somatotopic organization of the motor areas of the medial wall using low-frequency BOLD fluctuations. Hum. Brain Mapp. 32, 1566-1579. ( 10.1002/hbm.21132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fontaine D, Capelle L, Duffau H. 2002. Somatotopy of the supplementary motor area: evidence from correlation of the extent of surgical resection with the clinical patterns of deficit. Neurosurgery 50, 297-303. ( 10.1227/00006123-200202000-00011) [DOI] [PubMed] [Google Scholar]
- 98.Gould HJ, Cusick CG, Pons TP, Kaas JH. 1986. The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J. Comp. Neurol. 247, 297-325. ( 10.1002/cne.902470303) [DOI] [PubMed] [Google Scholar]
- 99.Fried I, Katz A, McCarthy G, Sass KJ, Williamson P, Spencer SS, Spencer DD. 1991. Functional organization of human supplementary motor cortex studied by electrical stimulation. J. Neurosci. 11, 3656-3666. ( 10.1523/JNEUROSCI.11-11-03656.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takada M, Tokuno H, Nambu A, Inase M. 1998. Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: segregation versus overlap of input zones from the primary motor cortex, the supplementary motor area, and the premotor cortex. Exp. Brain Res. 120, 114-128. ( 10.1007/s002210050384) [DOI] [PubMed] [Google Scholar]
- 101.Alexander GE, DeLong MR, Strick PL. 1986. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357-381. ( 10.1146/annurev.ne.09.030186.002041) [DOI] [PubMed] [Google Scholar]
- 102.Baker KB, Lee JYK, Mavinkurve G, Russo GS, Walter B, DeLong MR, Bakay RAE, Vitek JL. 2010. Somatotopic organization in the internal segment of the globus pallidus in Parkinson's disease. Exp. Neurol. 222, 219-225. ( 10.1016/j.expneurol.2009.12.030) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taha JM, Favre J, Baumann TK, Burchiel KJ. 1996. Characteristics and somatotopic organization of kinesthetic cells in the globus pallidus of patients with Parkinson's disease. J. Neurosurg. 85, 1005-1012. ( 10.3171/jns.1996.85.6.1005) [DOI] [PubMed] [Google Scholar]
- 104.Vitek JL, Ashe J, DeLong MR, Alexander GE. 1994. Physiologic properties and somatotopic organization of the primate motor thalamus. J. Neurophysiol. 71, 1498-1513. ( 10.1152/jn.1994.71.4.1498) [DOI] [PubMed] [Google Scholar]
- 105.Paus T. 2001. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat. Rev. Neurosci. 2, 417-424. ( 10.1038/35077500) [DOI] [PubMed] [Google Scholar]
- 106.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA, Rosen BR. 2002. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl Acad. Sci. USA 99, 523-528. ( 10.1073/pnas.012470999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holroyd CB, Yeung N. 2012. Motivation of extended behaviors by anterior cingulate cortex. Trends Cogn. Sci. 16, 122-128. ( 10.1016/j.tics.2011.12.008) [DOI] [PubMed] [Google Scholar]
- 108.Devinsky O, Morrell MJ, Vogt BA. 1995. Contributions of anterior cingulate cortex to behaviour. Brain 118, 279-306. ( 10.1093/brain/118.1.279) [DOI] [PubMed] [Google Scholar]
- 109.Petrides M, Pandya DN. 2007. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J. Neurosci. 27, 11 573-11 586. ( 10.1523/JNEUROSCI.2419-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palomero-Gallagher N, Vogt BA, Schleicher A, Mayberg HS, Zilles K. 2009. Receptor architecture of human cingulate cortex: evaluation of the four-region neurobiological model. Hum. Brain Mapp. 2355, 2336-2355. ( 10.1002/hbm.20667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vogt BA, Vogt L, Farber NB, Bush G. 2005. Architecture and neurocytology of monkey cingulate gyrus. J. Comput. Neurosci. 485, 218-239. ( 10.1002/cne.20512.Architecture) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dum RP, Strick PL. 2002. Motor areas in the frontal lobe of the primate. Physiol. Behav. 77, 677-682. ( 10.1016/S0031-9384(02)00929-0) [DOI] [PubMed] [Google Scholar]
- 113.Shima K. 1998. Role for cingulate motor area cells in voluntary movement selection based on reward. Science 282, 1335-1338. ( 10.1126/science.282.5392.1335) [DOI] [PubMed] [Google Scholar]
- 114.Mueller VA, Brass M, Waszak F, Prinz W. 2007. The role of the preSMA and the rostral cingulate zone in internally selected actions. Neuroimage 37, 1354-1361. ( 10.1016/j.neuroimage.2007.06.018) [DOI] [PubMed] [Google Scholar]
- 115.Morecraft RJ, van Hoesen GW.. 1992. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J. Comp. Neurol. 322, 471-489. ( 10.1002/cne.903220403) [DOI] [PubMed] [Google Scholar]
- 116.Turken AU, Swick D. 1999. Response selection in the human anterior cingulate cortex. Nat. Neurosci. 2, 920-924. ( 10.1038/13224) [DOI] [PubMed] [Google Scholar]
- 117.Paus T, Petrides M, Evans AC, Meyer E. 1993. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: a positron emission tomography study. J. Neurophysiol. 70, 453-469. ( 10.1152/jn.1993.70.2.453) [DOI] [PubMed] [Google Scholar]
- 118.Hutchins KD, Martino AM, Strick PL. 1988. Corticospinal projections from the medial wall of the hemisphere. Exp. Brain Res. 1, 667-672. ( 10.1007/BF00248761) [DOI] [PubMed] [Google Scholar]
- 119.Crosson B, et al. 1999. Activity in the paracingulate and cingulate sulci during word generation: an fMRI study of functional anatomy. Cereb. Cortex 9, 307-316. ( 10.1093/cercor/9.4.307) [DOI] [PubMed] [Google Scholar]
- 120.Paus T, Tomaiuolo F, Otaky N, Petrides M, Atlas J, Morris R, Evans AC. 1996. Human cingulate and paracingulate sulci: pattern, variability, assymmetry, and probabilistic map. Cereb. Cortex 6, 207-214. ( 10.1093/cercor/6.2.207) [DOI] [PubMed] [Google Scholar]
- 121.Loh KK, Procyk E, Neveu R, Lamberton F, Hopkins WD, Petrides M, Amiez C. 2020. Cognitive control of orofacial motor and vocal responses in the ventrolateral and dorsomedial human frontal cortex. Proc. Natl Acad. Sci. USA 117, 4994-5005. ( 10.1073/pnas.1916459117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pan C, Peck KK, Young RJ, Holodny AI. 2012. Somatotopic organization of motor pathways in the internal capsule: a probabilistic diffusion tractography study. Am. J. Neuroradiol. 33, 1274-1280. ( 10.3174/ajnr.A2952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bertrand G, Blundell J, Musella R. 1965. Electrical exploration of the internal capsule and neighbouring structures during stereotaxic procedures. J. Neurosurg. 22, 333-343. ( 10.3171/jns.1965.22.4.0333) [DOI] [PubMed] [Google Scholar]
- 124.Yim SH, Kim JH, Han ZA, Jeon S, Cho JH, Kim GS, Choi SA, Lee JH. 2013. Distribution of the corticobulbar tract in the internal capsule. J. Neurol. Sci. 334, 63-68. ( 10.1016/j.jns.2013.07.015) [DOI] [PubMed] [Google Scholar]
- 125.Duerden EG, Finnis KW, Peters TM, Sadikot AF. 2011. Three-dimensional somatotopic organization and probabilistic mapping of motor responses from the human internal capsule. J. Neurosurg. 114, 1706-1714. ( 10.3171/2011.1.JNS10136) [DOI] [PubMed] [Google Scholar]
- 126.Wahl M, Lauterbach-Soon B, Hattingen E, Jung P, Singer O, Volz S, Klein JC, Steinmetz H, Ziemann U. 2007. Human motor corpus callosum: topography, somatotopy, and link between microstructure and function. J. Neurosci. 27, 12 132-12 138. ( 10.1523/JNEUROSCI.2320-07.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van den Heuvel MP, Pol HEH. 2010. Specific somatotopic organization of functional connections of the primary motor network during resting state. Hum. Brain Mapp. 31, 631-644. ( 10.1002/hbm.20893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Matsumoto R, Nair DR, LaPresto E, Bingaman W, Shibasaki H, Lüders HO. 2007. Functional connectivity in human cortical motor system: a cortico-cortical evoked potential study. Brain 130, 181-197. ( 10.1093/brain/awl257) [DOI] [PubMed] [Google Scholar]
- 129.Rech F, Herbet G, Moritz-Gasser S, Duffau H. 2016. Somatotopic organization of the white matter tracts underpinning motor control in humans: an electrical stimulation study. Brain Struct. Funct. 221, 3743-3753. ( 10.1007/s00429-015-1129-1) [DOI] [PubMed] [Google Scholar]
- 130.Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. 2009. A probabilistic MR atlas of the human cerebellum. Neuroimage 46, 39-46. ( 10.1016/j.neuroimage.2009.01.045) [DOI] [PubMed] [Google Scholar]
- 131.Laird AR, Lancaster JL, Fox PT. 2005. BrainMap: the social evolution of a human brain mapping database. Neuroinformatics 3, 65-77. ( 10.1385/ni:3:1:065) [DOI] [PubMed] [Google Scholar]
- 132.Robinson JL, Laird AR, Glahn DC, Lovallo WR, Fox PT. 2011. Meta-analytic connectivity modeling: delineating the functional connectivity of the human amygdala. Hum. Brain Mapp. 31, 173-184. ( 10.1002/hbm.20854.Meta-analytic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. 2002. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage 16, 765-780. ( 10.1006/nimg.2002.1131) [DOI] [PubMed] [Google Scholar]
- 134.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. 2012. Activation likelihood estimation meta-analysis revisited. Neuroimage 59, 2349-2361. ( 10.1016/j.neuroimage.2011.09.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Eickhoff SB, Nichols TE, Laird AR, Hoffstaedter F, Amunts K, Fox PT, Bzdok D, Eickhoff CR. 2016. Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 137, 70-85. ( 10.1016/j.neuroimage.2016.04.072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhang K, Sejnowski TJ. 2000. A universal scaling law between gray matter and white matter of cerebral cortex. Proc. Natl Acad. Sci. USA 97, 5621-5626. ( 10.1073/pnas.090504197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bush EC, Allman JM. 2003. The scaling of white matter to gray matter in cerebellum and neocortex. Brain. Behav. Evol. 61, 1-5. ( 10.1159/000068880) [DOI] [PubMed] [Google Scholar]
- 138.Krubitzer L. 1995. The organization if neocortex in mammals: are species differences really so different? Trends Cogn. Neurosci. 18, 408-417. ( 10.1016/0166-2236(95)93938-T) [DOI] [PubMed] [Google Scholar]
- 139.Krubitzer L. 2009. In search of a unifying theory of complex brain evolution. Ann. N. Y. Acad. Sci. 1156, 44-67. ( 10.1111/j.1749-6632.2009.04421.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Barton RA, Harvey PH. 2000. Mosaic evolution of brain structure in mammals. Nature 405, 1055-1058. ( 10.1038/35016580) [DOI] [PubMed] [Google Scholar]
- 141.Hager R, Lu L, Rosen GD, Williams RW. 2012. Genetic architecture supports mosaic brain evolution and independent brain–body size regulation. Nat. Commun. 3, 8-12. ( 10.1038/ncomms2086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Finlay BL, Darlington RB. 1995. Linked regularities in the development and evolution of mammalian brains. Science 268, 1578-1584. ( 10.1126/science.7777856) [DOI] [PubMed] [Google Scholar]
- 143.Finlay BL, Darlington RB, Nicastro N. 2001. Developmental structure in brain evolution. Behav. Brain Sci. 24, 263-278. ( 10.1017/S0140525X01003958) [DOI] [PubMed] [Google Scholar]
- 144.de Winter W, Oxnard CE.. 2001. Evolutionary radiations and convergences in the structural organization of mammalian brains. Nature 409, 710-714. ( 10.1038/35055547) [DOI] [PubMed] [Google Scholar]
- 145.Chaplin TA, Yu H, Soares JGM, Gattass R, Rosa MGP. 2013. A conserved pattern of differential expansion of cortical areas in simian primates. J. Neurosci. 33, 15 120-15 125. ( 10.1523/JNEUROSCI.2909-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pepperberg IM. 2010. Vocal learning in grey parrots: a brief review of perception, production, and cross-species comparisons. Brain Lang. 115, 81-91. ( 10.1016/j.bandl.2009.11.002) [DOI] [PubMed] [Google Scholar]
- 147.Jarvis E, et al. 2005. Avian brains and a new understanding of vertebrate brain evolution. Nat. Rev. Neurosci. 6, 151-159. ( 10.1038/nrn1606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Matsunaga E, Okanoya K. 2008. Expression analysis of cadherins in the songbird brain: relationship to vocal system development. J. Comp. Neurol. 508, 329-342. ( 10.1002/cne.21676) [DOI] [PubMed] [Google Scholar]
- 149.Matsunaga E, Okanoya K. 2009. Vocal control area-related expression of neuropilin-1, plexin-A4, and the ligand semaphorin-3A has implications for the evolution of the avian vocal system. Dev. Growth Differ. 51, 45-54. ( 10.1111/j.1440-169X.2008.01080.x) [DOI] [PubMed] [Google Scholar]
- 150.Chakraborty M, et al. 2015. Core and shell song systems unique to the parrot brain. PLoS ONE 10, e0118496. ( 10.1371/journal.pone.0118496) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chakraborty M, Jarvis ED. 2015. Brain evolution by brain pathway duplication. Phil. Trans. R. Soc. B 370, 20150056. ( 10.1098/rstb.2015.0056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Feenders G, Liedvogel M, Rivas M, Zapka M, Horita H, Hara E, Wada K, Mouritsen H, Jarvis ED. 2008. Molecular mapping of movement-associated areas in the avian brain: a motor theory for vocal learning origin. PLoS ONE 3, e1768. ( 10.1371/journal.pone.0001768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dickson BJ. 2002. Molecular mechanisms of axon guidance. Science 298, 1959-1964. ( 10.1126/science.1072165) [DOI] [PubMed] [Google Scholar]
- 154.Chisholm A, Tessier-Lavigne M. 1999. Conservation and divergence of axon guidance mechanisms. Curr. Opin. Neurobiol. 9, 603-615. ( 10.1016/S0959-4388(99)00021-5) [DOI] [PubMed] [Google Scholar]
- 155.Deacon TW. 1989. The neural circuitry underlying primate calls and human language. Hum. Evol. 4, 367-401. ( 10.1007/BF02436435) [DOI] [Google Scholar]
- 156.Deacon TW. 1990. Rethinking mammalian brain evolution. Integr. Comp. Biol. 30, 629-705. ( 10.1093/icb/30.3.629) [DOI] [Google Scholar]
- 157.Jürgens U, Pratt R. 1979. Role of the periaqueductal grey in vocal expression of emotion. Brain Res. 167, 367-378. ( 10.1016/0006-8993(79)90830-8) [DOI] [PubMed] [Google Scholar]
- 158.Jürgens U, Pratt R. 1979. The cingular vocalization pathway in the squirrel monkey. Exp. Brain Res. 510, 499-510. ( 10.1007/BF00239145) [DOI] [PubMed] [Google Scholar]
- 159.Balezeau F, Wilson B, Gallardo G, Dick F, Hopkins W, Anwander A, Friederici AD, Griffiths TD, Petkov CI. 2020. Primate auditory prototype in the evolution of the arcuate fasciculus. Nat. Neurosci. 23, 611-614. ( 10.1038/s41593-020-0623-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rilling JK, Glasser MF, Preuss TM, Ma X, Zhao T, Hu X, Behrens TEJ. 2008. The evolution of the arcuate fasciculus revealed with comparative DTI. Nat. Neurosci. 11, 426-428. ( 10.1038/nn2072) [DOI] [PubMed] [Google Scholar]
- 161.Rilling JK, Glasser MF, Jbabdi S, Andersson J, Preuss TM. 2012. Continuity, divergence, and the evolution of brain language pathways. Front. Evol. Neurosci. 3, 1-6. ( 10.3389/fnevo.2011.00011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Eichert N, Robinson EC, Bryant KL, Jbabdi S, Li L, Krug K, Watkins KE, Mars RB. 2019. Cross-species cortical alignment identifies different types of neuroanatomical reorganization in higher primates. eLife 9, e53232. ( 10.7554/eLife.53232) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Petrides M, Pandya DN. 2002. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 16, 291-310. ( 10.1046/j.1460-9568.2002.02090.x) [DOI] [PubMed] [Google Scholar]
- 164.Petrides M, Tomaiuolo F, Yeterian EH, Pandya DN. 2012. The prefrontal cortex: comparative architectonic organization in the human and the macaque monkey brains. Cortex 48, 46-57. ( 10.1016/j.cortex.2011.07.002) [DOI] [PubMed] [Google Scholar]
- 165.Fitch WT. 2018. The biology and evolution of speech: a comparative analysis. Annu. Rev. Lingustics 4, 255-279. ( 10.1146/annurev-linguistics-011817-045748) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The fMRI data are publicly available at OpenNeuro under the accession code ds002634 (Dataset: Project_larynx). Processing code for this dataset is publicly available from the Wellcome Centre for Integrative Neuroimaging's GitLab at https://git.fmrib.ox.ac.uk/neichert/project_larynx. Coordinate tables for ALE meta-analyses are provided in the electronic supplementary material.