Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common form of non-Hodgkin lymphoma (NHL) representing 30–40% of all cases. 1 It is a heterogeneous B-lymphoid neoplasm that consists of subtypes distinguished by clinical, cytogenetic, and molecular features, with variable outcomes when treated with upfront immunochemotherapy. R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone) is the current standard for first-line immunochemotherapy for DLBCL, with 60–70% of patients being cured by this approach. However, 10–15% of patients have primary refractory disease and a further 20–30% relapse after first-line treatment. 2 The International Prognostic Index (IPI) and age-adjusted IPI are risk stratification tools used since 1993 to identify individuals that will respond poorly to doxorubicin-containing chemotherapy regimens based on clinical variables; age, performance status, tumour stage, number of extranodal sites, and serum LDH level. 3 This prognostic scoring system remains valid in the rituximab era. Biological features of the disease also have prognostic relevance including the cell-of-origin (germinal centre B-cell and activated B-cell, as identified by gene expression profiling),4–6 genetic rearrangements in c-MYC in addition to BCL2 and/or BCL6 (double/triple-hit lymphoma)7–10 and expression of c-myc and Bcl2 in the absence of underlying genetic changes (double expressor lymphoma; Green et al, JCO 2012; Johnson et al, JCO 2012; Horn et al, Blood 2013).
The current standard of care for relapsed/refractory disease for eligible patients remains non cross-reacting relapse therapy with platinum-based or ifosfamide-containing regimens, incorporating an anti-CD20 monoclonal antibody2,11 followed by autologous stem-cell transplantation (ASCT).2,11 Results of the prospective CORAL study, evaluating the efficacy of R-ICE compared to R-DHAP as salvage regimens, demonstrated that only 50% of relapsed/refractory patients were able to undergo ASCT largely due to failure to adequately respond to second line therapy. This was more common among patients with higher secondary age-adjusted IPI score, prior rituximab treatment, and refractory disease/relapse less than 12 months after diagnosis. 11 Other reasons for ineligibility for aggressive approaches include advanced age, comorbidities, and less commonly, failure to collect stem cells. Failure of response to first-line salvage treatment or relapse post ASCT results in extremely poor outcomes. 12 For those patients who could not proceed to ASCT in the CORAL study, median overall survival was 4.4 months from failing response. 13 The curability of these patients with second-line relapse regimens is limited; nevertheless, a minority of relapsed/refractory patients will respond to third-line regimens and may be considered for allogeneic stem cell transplant.13,14
For transplant-ineligible patients with relapsed/refractory disease median overall survival remains very poor at less than 4 months. 15 Treatment options include conventional chemotherapy with or without rituximab, localized radiotherapy, supportive care, or enrolment in clinical trials. Table 1 demonstrates response rates in the trial setting for recently approved therapies in relapsed/refractory and transplant-ineligible patients. These include antibody-drug conjugates: Polatuzumab vedotin and loncastuximab tesirine, tafasitamab (CD19-targeting monoclonal antibody), and selinexor (oral nuclear export inhibitor). Polatuzumab vedotin (targeting CD79b, a B-cell receptor component) combined with Bendamustine and rituximab (BR) has been licenced in some countries based on superior progression free and overall survival results in a randomized phase II trial compared with BR alone, 16 while the results of the POLARIX trial where it is used in the first-line setting alongside R-CHOP are eagerly awaited. The FDA granted accelerated approval of tafasitamab plus lenalidomide, selinexor and more recently loncastuximab tesirine for adult patients with relapsed/refractory DLBCL based on high and durable overall response rates.17–19 Enrolment in clinical trials of novel approaches, including cellular therapies and bispecific antibodies, are becoming increasingly important in targeting this unmet need.
Table 1.
Response rates in the trial setting for recently approved therapies in relapsed/refractory and transplant-ineligible patients.
| Drug combination | Comparator | OR (%) | CR (%) | PFS (median, months) | OS (median, months) |
|---|---|---|---|---|---|
| Polatuzumab Vedotin + Bendamustine + Rituximab
16
n = 40 |
Bendamustine + Rituximab n = 40 |
45 | 40 versus 17.5 | 9.5 versus 3.7 | 12.4 versus 4.7, (median follow-up 22.3 months) |
| Tafasitamab + lenalidomide n = 80 17 |
60 | 43 | 12.1 | Median not reached at 19.6 months follow up, 64% survival at 18 months | |
| Selinexor
18
n = 127 |
28 | 15 | 3.5 | 9.1 | |
| Loncastuximab tesirine
19
n = 145 |
48.3 | 24.1 | 4.9 | 9.9 |
CR, complete response; OR, objective response (defined as the proportion of patients who achieved either complete response or partial response); OS, overall survival; PFS, progression free survival.
Advent of CAR-T cells
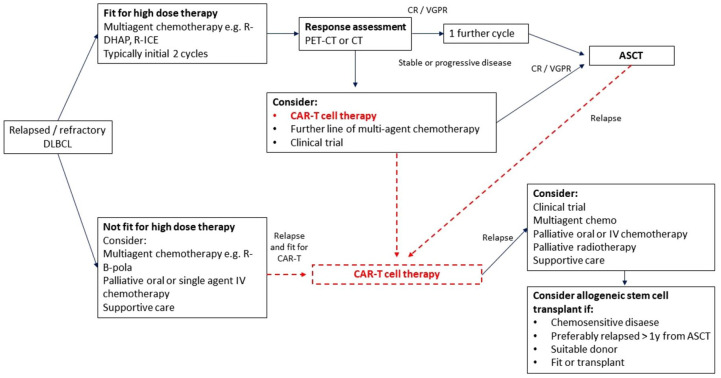
The recent development in genetic engineering of T-cells to express chimeric antigen receptors (CAR-T cells) has led to the availability of an effective new option for patients with relapsed/refractory (R/R) DLBCL associated with high responses rates and durable responses for some patients. CARs are fusion proteins that combine a monoclonal antibody-derived single chain variable fragment recognizing cancer-specific epitopes with a T-cell activation domain derived from the intracellular portion of the T-cell receptor. Second- and third-generation CARs also incorporate co-stimulatory domains such as CD28 and/or 4-1BB. 20 Initial preclinical studies demonstrating the potential for use of CARs in eliminating B-cell malignancies expressing CD19, a ubiquitous B-cell marker, were published in 2003. 21 Since then, multiple single and multicentre trials of anti-CD19 CAR-T cells have demonstrated therapeutic efficacy in R/R B-cell malignancies with a significant number of patients achieving complete and sustained remissions (Table 2).22,23 Axicabtagene ciloleucel (axi-cel) and Tisagenlecleucel are two CAR-T cell products currently licenced for the treatment of R/R DLBCL based on the results of pivotal multicentre trials. The Zuma-1 phase II multicentre study investigating axi-cel therapy, a CD-19 specific CAR containing a CD28 co-stimulatory domain, in patients with R/R large B-cell lymphomas demonstrated a complete response (CR) rate of 58% with a median overall survival of greater than 2 years.24,25 Findings from the international phase II JULIET study of tisagenlecleucel, an anti-CD19 CAR containing a 4-1BB co-stimulatory domain, in patients with R/R DLBCL who were ineligible for or had disease progression after autologous stem-cell transplantation demonstrated a CR rate of 40%, sustained in 79% of these patients at 12 months. 26 A third product, lisocabtagene maraleucel—an anti-CD19 4-1BB CAR, has recently been approved by the FDA following the results in the phase II TRANSCEND study demonstrating high durable CR rates in heavily pretreated R/R DLBCL patients.27,28 A recent meta-analysis evaluating 11 trials of second-generation CAR products in B-cell NHLs reported objective response rates and CR rates of 68% and 46% in 306 patients with R/R DLBCL, mostly anti-CD19 CARs. When compared with results of the recent retrospective SCHOLAR-1 study evaluating outcomes in refractory DLBCL, objective response rate and CR rates to next line of salvage therapy were 26% and 7%, respectively, with a median overall survival of 6.3 months. 29 CAR T-cell therapies therefore hold promise for this cohort of patients. Indeed, since the approval of these treatment the pathway for patients with R/R DLBCL has changed dramatically (Figure 1).
Table 2.
Summary of results of approved CAR-T cell products in phase II trials for relapsed/refractory DLBCL.
| Clinical trial n = enrolled (infused) |
CAR-T product | OR (%) | CR (%) | PFS (median, months) | OS (median, months) | Median turnaround time for manufacturing to delivery/infusion (days) | Relevant toxicity (grade 3 or higher) |
|---|---|---|---|---|---|---|---|
| ZUMA-1
24
n = 111 (101) |
Axicabtagene ciloleucel (Apheresis to infusion efficacy: 99%) |
82 | 58 | 5.8 6 months: 49% 12 months: 44% |
Median not reached. 6 months: 78% 12 months: 59% |
17 | 95% Neutropenia 78% Neurotoxicity 28% CRS 13% |
| JULIET
26
n = 165 (111) |
Tisagenleucel | 52 | 40 | Not reached. Estimated 12 months: 83% |
12 12 months: 49% |
54 | 89% Cytopaenias 16% CRS 22% Neurotoxicity 12% |
| TRANSCEND-001
28
n = 344 (269) |
Lisocabtagene maraleucel (Apheresis to infusion efficacy: 78%) |
73 | 53 | 6.8 6 months: 51.4% 12 months: 44% |
21.1 6 months: 74.7% 12 months: 58% |
37 | 79% Neutropenia 60% Neurotoxicity 10% CRS 2% |
CAR-T, chimeric antigen receptors-T cells; CR, complete response; CRS, cytokine release syndrome; DLBCL, diffuse large B-cell lymphoma; OR, objective response (defined as the proportion of patients who achieved either complete response or partial response); OS, overall survival; PFS, progression free survival.
Figure 1.
Pathway for relapsed/refractory DLBCL.
ASCT, autologous stem cell transplantation; CAR-T, chimeric antigen receptors-T cells; CR, complete response; CT, cell transplantation; DLBCL, diffuse large B-cell lymphoma; PET, positron emmission tomography; R-B-pola, rituximab, Bendamustine, polatuzumab vedotin; R-DHAP, rituximab, dexamethasone, cytarabine, cisplatin; R-ICE, rituximab, ifosfamide, carboplatin, etoposide; VGPR, very good partial response.
In addition to CD19, other antigens have been the target of CAR T-cell development for the treatment of lymphoma including CD20, kappa light chain antigen and CD22 in B-cell NHLs. 20 Interpretation of the efficacy of CAR-T products, however, is limited by heterogeneity in trial methodology, CAR-T design and patient selection—including NHL subtype-specific disease variables, prior ASCT, differences in prior lines of therapy and the use of conditioning therapy. The utility in clinical practice of these products is limited by their cost, time to access and eligibility (especially for patients with quickly progressive disease and comorbidities) and toxicity including cytokine release syndrome (CRS) and neurotoxicity reported at rates of as high as 40%. 30 In order to improve CAR-T-cell therapies, reducing the risk of such toxicities is imperative. Furthermore, the majority of patients treated with licenced products relapse with identified possible mechanisms being CD19 negative escape 24 and CAR-T exhaustion. An ongoing trial of a new CD19 CAR-T product aims to mitigate that by using a ‘fast-off’ approach which has a more physiological contact time between the CAR and the target. This approach could reduce toxicity and increase persistence {Claire Roddie, 2020 #1727}
Bispecific antibodies
Bispecific antibodies (BSA) employ a similar mechanism of action to CAR T-cells in that they redirect T-cell effector functions towards cells expressing target cancer-specific epitopes. A forerunner of BSAs was Blinatumomab which was approved by the FDA in 2014 for the treatment of B-acute lymphoblastic leukaemia. It is a bi-specific T-cell engager (BiTE) with two single-chain variable fragments containing antigen binding sites that recognize both CD19 and CD3 T cell receptor complex, resulting in T-cell activation and effector function. 31 However, these molecules lack the Fc region of the antibody. Their small size means that they are filtered by the kidneys which necessitates a continuous infusion as the preferred mode of delivery. This creates many logistical and practical challenges that limits their widespread use. In addition, they lack the potential benefits of a broader activation of the immune system driven by the presence of the Fc receptor. BSAs retain the Fc region of the antibody, while having two different chain variable fragments allowing them to target two different antigens (Figure 2). The retention of the Fc region makes the molecule more stable, having a half-life of 10 days, while it can also be employed to induce a non-T-cell antitumour response by activating complement and other Fc-mediated immune effector cells. They also appear to have a much lower incidence of CRS and neurotoxicity and have demonstrated an overall good safety profile in early phase trials.
Figure 2.
Step 1: Collecting CD3+ T-cell apheresis from the patient is the first step towards making the CAR T product. A minimum of 0.6 × 10*9 and an ideal of 2 × 10*9 cells is usually required. 32 Although the number of cells required is not very high, these patients are often pretreated with steroid, monoclonal antibodies and cytotoxic medication that renders them lymphopenic and this can make harvesting adequate numbers of cells challenging. Despite this, in a cohort of 71 patients, the minimum cell dose was achieved in 97% and the ideal target in 77% of the patients. 32 Step 2: The collected product is washed and the collected T-cells are activated and expanded by various techniques, a process that enables them to obtain a memory phenotype and become less resistance to transduction 33 A lentivirus or retrovirus vector is used to insert the genetic material into the T-cells. Step 3: The genetic material is incorporated into the T-cells’ DNA transforming them into a chimeric receptor T-cells. 34 This genetic material expresses a single chain fragment anti CD19 which is connected to a co-stimulatory transmembrane molecule (either CD28 or 4-1BB) and a CD3-ζ chain which is the cytoplasmic signalling domain that will activate the T-cell. 35 Step 4: The cells are re-infused to the patient. Step 5: Within a few days the CAR-T identify their target, become activated and expand. This can be monitored by flow cytometry as CAR-Ts carry a specific signature, while other markers on their surface determine their status (fatigue, activity, etc). The time of activation, the level of expansion and the persistence in the blood may be determined by the co-stimulatory molecule of each product. 36 Step 5: The CAR-T cells induce a strong immunological response. This immunological response is driven by a cytokine release of TNFa, Interleukin-6, interferon-γ, while it also attracts other cells, mainly macrophages which contribute to the immune response. 37 The tenacity of this phenomenon can determine the severity of cytokine release syndrome which is one of the main CAR-T-related toxicities.
CAR-T, chimeric antigen receptors-T cells; DNA, deoxyribonucleic acid; VH, variable heavy chain; VL variable light chain.
Epcoritamab is a BSA against CD20 and CD3 which utilizes the DuoBody® technology. DuoBody® technology is used as a platform to speed up academic and commercial manufacturing of bispecific antibodies. 38 It involves the manufacturing of two separate monoclonal antibodies which are combined to form the final product. Epcoritamab has shown promising preclinical efficacy with high rates of in vitro cytotoxicity activity against malignant B-cells from patients with non-Hodgkin lymphomas including DLBCL. 39 In a phase I/II trial, it was shown to have an overall response rate (ORR) of 66.7%. Most importantly, patients who already had CAR-T therapy have responded to this BSA with no reported grade 3 or above toxicity. Further evaluation of this agent is underway. 40
Glofitamab is BSA targeting CD20 and CD3, but instead of using a 1:1 format, it facilitates bivalent binding to CD20 and monovalent binding to CD3 in a 2:1 format. Recent data from a phase I trial evaluating glofitamab in R/R B-cell non-Hodgkin lymphoma demonstrated an overall response rate of 65.7%, with a complete response in 57.1% of patients dosed at the recommended phase II dose. 84.1% of patients maintained CR with a maximum of 27.4 months. The most common adverse event was CRS occurring in >50% of patients, but this was manageable with only 3.5% of patients experiencing grade 3 or 4 CRS. Despite this glofitamab had good tolerability (only five patients withdrew because of adverse events). 41
Mosunutuzumab (M) is a fully humanized IgG1 BSA targeting CD20 and CD3. A phase I/IB study evaluated the efficacy and safety of Mosunetuzumab in R/R NHL patients as a single agent. 42 In aggressive NHL, 22/119 (18.6%) achieved a CR with 15/22 (68.2%) of those achieving a durable remission. In addition, expansion of previously administered CAR-Ts after administration of Mosunetuzumab was detected indicating that the ability to bind to CD3 may not only activate native T-cells, but also CAR-T cells that retain their TCR. Preliminary data from the ongoing GO40515 (NCT03677141) study evaluating combination of M-CHOP in R/R and newly diagnosed patients with DLBCL confirms high response rates and a promising tolerability profile. 43 ORR and CR rates in patients with R/R NHL were 86% and 71% and in newly diagnosed patients were 96% and 85%, respectively. No patients had grade ⩾3 CRS or neurotoxicity. Other combinations, such as M with polatuzumab vedotin, are now currently being investigated.
Odronextamab is another CD20/CD3 BSA using a fully humanized IgG4 platform. A phase I study (NCT02290951) and updated safety and efficacy data from this study demonstrate durable CRs that extend to patients refractory to CAR-T therapy (Table 3). In 127 heavily pre-treated patients with R/R Non-Hodgkin lymphoma, Grades 3 and 4 CRS were reported in only nine patients and resolved with supportive measures. In the higher dose groups, CR rates of 60% were observed in patients with R/R DLBCL with median response duration of 10.3 months. 44
Table 3.
Summary of response rates and relevant toxicities of bispecific antibody products in clinical trials as single agents for B-cell non-Hodgkin lymphomas.
| Bispecific antibody | OR (%) | CR (%) | PFS (median, months) | OS (median, months) | Relevant toxicity (grade 3 or higher) |
|---|---|---|---|---|---|
| Epcoritamab
40
n = 67 |
66.7 | 33.3 | NA | NA | No grade 3 or higher CRS Transient neurotoxicity 3% |
| Glofitamab
41
n = 171 |
53.8 | 36.8 | 2.9 in aggressive NHL | NA | 56.7% Neutropenia 25.1% CRS 3.5% |
| Mosunutuzumab
42
n = 119 |
34.7 | 18.6 | NA | NA | Neurotoxicity 3.2% CRS 1.4% |
| Odronextamab
44
n = 127 |
60% (No prior CAR-T) 33.3% (Prior CAR-T) |
60% (No prior CAR-T) 23.8% (Prior CAR-T) |
11.1(No prior CAR-T) 2.5 (Prior CAR-T) |
NA | Neurotoxocity 3.9% CRS 7.1% |
CAR-T, chimeric antigen receptors-T cells; CR, complete response; CRS, cytokine release syndrome; NA, not available; NHL, non-Hodgkin lymphoma; OR, objective response (defined as the proportion of patients who achieved either complete response or partial response); OS, overall survival; PFS, progression free survival.
Overall, there are many promising BSAs which have demonstrated an excellent safety profile with promising response rates in early-phase clinical trials. Interestingly, their ability to bind to CD3+ T-cells means that they could have a synergistic effect with CAR-T cells that retain their native TCR. It is reasonable to expect that some of these results will be replicated in larger phase III trials which could lead to their regulatory approval.
BSAs versus CAR-T cells
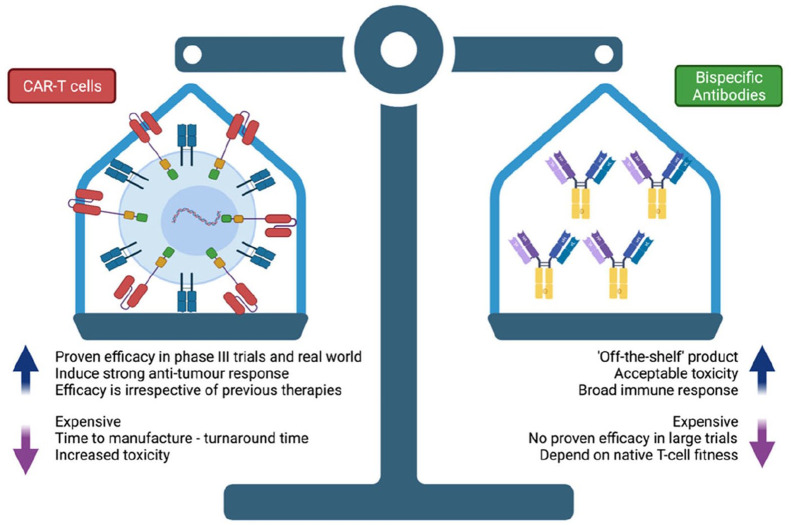
Figure 4 summarises the benefits and limitiation sof CAR-T and BSAs. In a retrospective evaluation, the relapse rate after axi-cel or tisagenlecleucel for R/R DLBCL patients was 55% at a median follow-up of 9 months. 45 Mechanisms postulated for progression through CAR T-cell therapy include resistance mediated by loss of target antigen, in this case CD19, and lack of CAR-T persistence due to exhaustion or poor expansion. The development of bispecific antibodies targeting CD20 antigen (pan B-cell surface protein) may offer an additional line of treatment in the event of CAR T-cell resistance/relapse or even as adjunctive treatment. Clinical trials of anti-CD20/CD3 bispecific antibody products are ongoing with promising results as mentioned above. These drugs hold promise for R/R disease, including in the setting of relapse after CAR-T therapy as preliminary results suggest that bispecific antibodies may help overcome therapeutic resistance/exhaustion of CAR-T cells and augment their antitumour activity. The incidence of adverse events leading to treatment withdrawal in these studies was low and the incidence of cytokine release syndrome was mostly of grade 1–2 severity. In addition to promising efficacy and favourable tolerability, bispecific antibodies do not require individualized manufacturing, allowing for quicker access for patients with limited prognosis or faster relapsing disease that is difficult to control in the time required to manufacture autologous CAR-T cells. Currently, however, there is longer follow up data available for CAR-T cells allowing a degree of confidence that a significant number of patients enjoy durable remissions. 25 In addition, the responses appear to be similar for older patients (>65 years old) where other treatment options such as an autologous transplant may not be available. 46 While the BSA data is promising, longer follow up is required to determine the durability of remissions.
Figure 4.
Benefits and limitations of CAR-T cells and bispecific antibodies.
CAR-T, chimeric antigen receptors-T cells.
The future of the new therapies
R-CHOP has maintained its status as standard of care for the first line treatment of DLBCL for at least two decades. Despite many additional trials no other drug combination has so far been proven more efficacious or safer 42 {Nowakowski, 2021 #1655} {Bartlett, 2019 #1672}. However, with a CR rate of 70–80% there are some patients that could potentially benefit from newer drug developments. DLBCL is usually a type of lymphoma that presents aggressively and requires urgent treatment. R-CHOP is a regimen that can be given quickly and therefore it is likely that any new treatment will be used in combination with some of what constitutes R-CHOP. In addition, R-CHOP is a relatively inexpensive regimen that can be manufactured widely, while clinicians have vast amounts of experience using it to treat patients with B-cell malignancies.
Many of the 20% of patients who are primary-refractory to R-CHOP are double hit, or double-expressing lymphomas. For this category of patients, a more aggressive approach in the first-line setting by combining bispecific antibodies with R-CHOP or using R-CHOP as a bridge to CAR-T therapy may be a useful strategy; currently being investigated in the ZUMA-12 trial. 47 Initial results from this trial incorporating data from 12 patients show 80% CR rate with acceptable toxicity, but more results are awaited. In an elderly population with comorbidities, these treatments may become an attractive up-front alternative to cytotoxic chemotherapy.
A more feasible initial strategy is to use these therapies as second-line agents in the relapsed setting (Figure 3). Currently, second-line therapies have poor outcomes. Autologous stem cell transplantation can lead to durable remissions and cures for patients who remain chemosensitive at relapse and are young and fit enough to tolerate an intensive chemotherapy-based approach. In refractory cases or transplant-ineligible patients the overall prognosis remains poor with limited treatment options available. CAR-T cells have currently been approved in the United Kingdom in the third-line setting. There are two phase III trials currently active comparing CAR-T therapy to standard of care in R/R DLBCL following Rituximab plus Anthracycline-containing chemotherapy (NCT03391466, NCT03570892). Rates of grade 3 or higher neurotoxicity or CRS with CAR-T cells remain low with more than 50% of patients developing neither. 48 In the age group of above 70 years, where transplant would not usually be an option, CAR-T cells or BSAs offer an efficacious and tolerable alternative following R-CHOP failure. However, logistical and institutional challenges are significant hurdles to the wider adoption of CAR-T cells, while the associated toxicity and relatively long turnaround time may prevent some patients from having them, giving the advantage to ‘off-the-shelf’ BSAs. Other combination treatments with other novel agents such as Venetoclax have showed some positive initial results in phase I and are under investigation for this setting {Paolo Caimi, 2018 #1728}.
Figure 3.
Bispecific antibodies comparison to Bispecific T-cell Engager. BiTEs target a tumour-specific antigen and CD3 which is present in T-cells ‘bringing them’ together and facilitating an immune response by the T-cell towards the tumour. BSAs work in a similar manner, but they also retain the Fc receptor which enables them to induce a broader immune response by other immune effector cells as well. Their larger size makes them less prone to renal excretion avoiding the need for a continuous infusion that BiTEs require.
BiTE, bi-specific T-cell engager; BSA, bispecific antibodies.
Challenges
Toxicity
CAR T-cell therapy is limited by toxicity mediated by the release of cytokines from activated immune cells (cytokine release syndrome, CRS) and neurotoxicity. 30 The management has become easier with use of specialized centres delivering therapy and clinical experience, but still remains a significant challenge. Long-term toxicity, including prolonged neutropenia, is being recognized as another notable adverse event. The mechanism for that remains elusive. Of note, B-cell aplasia and hypogammaglobinaemia are seen in much lower rates than in paediatric B-ALL. This may indicate the lack of persistence of CAR-T cells when used in DLBCL.
Cost
Perhaps the most significant problems limiting success in the scalability of autologous CAR T-cell therapies are the cost and manufacturing processes and facilities required to produce patient-specific engineered cells. An estimated cost of US$400,000 per CAR-T treatment makes them extremely costly for wealthy nations and unreachable for the developing world. 49 A big part of this sum comes from the expensive manufacturing of viral vectors. However, the logistical difficulties of creating one product for one patient greatly inflates this cost. Commercialization of CAR T-cells is therefore limited by the availability of cost-effective GMP manufacturing platforms to produce treatments in a timely manner and at scale. Automated cell therapy production platforms such as CliniMACS Prodigy – an integrated cell processing device used to develop virus-specific T-cells, simplification and standardisation of quality control measures, and testing and tracking of products will allow for greater accessibility with reduction in costs. 50 The academic centres that use the CliniMACS Prodigy platform have been able to produce comparable products that are immediately available to their patients at a much lower price. 51 BSAs will likely be less expensive given their off-the-shelf nature, but judging by the Blinatumumab pricing of US$178,000 per year, they are likely to be far more expensive than conventional chemotherapy.
Availability
Another big hurdle with the use of CAR-T cells is the turnaround time between order and administration of treatment. Autologous CAR-Ts require T-cell apheresis and off-site manufacturing. The length of time taken from leukapheresis to reinfusion of the engineered product can often take multiple weeks, during which time a patient with relapsed/refractory disease may deteriorate and become too unwell for CAR T-cell treatment, given its toxicities. Even in trial settings, where turnaround time is much quicker and patients are generally fitter, around 10% of patients die while waiting to receive CAR therapy.52,53 This number is likely to be much higher in the real world. Although the manufacturing process will hopefully become more efficient, it is unlikely that it will be reduced to less than 3–4 weeks. There have been a wide range of bridging therapies designed to help prevent rapid progression of lymphoma during CAR-T cell manufacturing, but there is no definitive evidence in terms of which therapy is better, while concerns about the effect on the fitness of CAR-T cells have been raised. There is emerging evidence that radiotherapy can be a very good bridging therapy, while also improving the efficacy and safety of CAR-T cells. 54
Universal CAR-T
A universal, ‘off-the-shelf’, CAR T-cell will eliminate most of the delay in the manufacturing process and can potentially reduce the production cost. Attempts to mitigate the risk of graft versus host disease associated with allogenic cell therapies have included use of non-αβ T-cells such as NK cells or γδ T cells for generation of CAR T-cells, which have shown promise in the preclinical setting. No such products have been approved yet, but early phase clinical trials have proven that such an approach can potentially be effective. 55 Other than being easily available, this approach can go a long way towards addressing many of the current challenges with CAR-T cell therapy, while additionally providing an option for patients who do not achieve satisfactory T-cell apheresis due to previous lymphodepleted chemotherapies or impaired T-cell health. Cord-blood derived NK cells do not require HLA matching and so could be used as an off-the-shelf product. 56 Initial in vitro and murine models demonstrated their efficacy against CD19 malignancies 57 which led to the first product to be used in a human trial. It is engineered to express IL-15 to boost expansion and caspase 9 as an off-switch in the event of unacceptable toxicity alongside the anti-CD19 receptor. The first phase I/II trial of 11 patients has shown that CAR-NK cells are safe and efficacious in the management of CD19 positive malignancies. 46 Larger studies are in the pipeline to build upon this highly successful trial.
Conclusion
CAR-T therapy is an established therapy for R/R DLBCL, but improved cellular products with reduced toxicity, lower cost, and improved availability are the key to the wider adoption of this therapy. Upcoming trial results may indicate that they are the best option following R-CHOP, but the real-world large scale uptake would still be problematic if these challenges are not overcome. Bispecific antibodies offer a more ‘off-the-shelf’ solution and the results of larger phase II and phase III trials are awaited. They could be an addition to current regimens or an option for patients who have failed all other available treatments.
Acknowledgments
The Biorender software was used for the creation of Figures 2–4. All authors acknowledge the Oxford Centre for Haematology.
Footnotes
Author contributions: All authors contributed equally to the writing of this manuscript.
Conflict of interest statement: A.R. received conference fees from Gilead. G.C. received honoraria from Roche, Takeda, Gilead Sciences, Pfizer, Novartis, Daiichi Sankyo, Incyte, Celleron Therapeutics, MSD Oncology, BeiGene, and ADC Therapeutics; played consulting or advisory role in Roche, Takeda, Incyte, Pfizer, MSD, Celgene, Beigene, Daiichi Sankyo, Celleron Therapeutics, and ADC Therapeutics; as speakers’ bureau in Roche, Takeda, Novartis, and Gilead Sciences; received research funding from MSD Oncology, Celgene, Celleron Therapeutics, Bristol-Myers Squibb, and Amgen; received travel and accommodations expenses from Roche and Takeda.
GS: No conflicts of interest
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Gina Sangha  https://orcid.org/0000-0002-8433-8994
https://orcid.org/0000-0002-8433-8994
Graham P. Collins  https://orcid.org/0000-0002-8803-4234
https://orcid.org/0000-0002-8803-4234
Contributor Information
Alexandros Rampotas, Oxford University Hospitals NHS Foundation Trust, Oxford, UK.
Gina Sangha, Oxford University Hospitals NHS Foundation Trust, Oxford, UK.
Graham P. Collins, Oxford University Hospitals NHS Foundation Trust, Churchill Hospital, Old Road, Headington, Oxford OX3 7LE, UK.
References
- 1. Rodriguez-Abreu D, Bordoni A, Zucca E. Epidemiology of hematological malignancies. Ann Oncol 2007; 18(Suppl. 1): i3–i8. [DOI] [PubMed] [Google Scholar]
- 2. Chaganti S, Illidge T, Barrington S, et al. Guidelines for the management of diffuse large B-cell lymphoma. Br J Haematol 2016; 174: 43–56. [DOI] [PubMed] [Google Scholar]
- 3. International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med 1993; 329: 987–994. [DOI] [PubMed] [Google Scholar]
- 4. Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002; 346: 1937–1947. [DOI] [PubMed] [Google Scholar]
- 5. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 2000; 403: 503–511. [DOI] [PubMed] [Google Scholar]
- 6. Thieblemont C, Briere J, Mounier N, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol 2011; 29: 4079–4087. [DOI] [PubMed] [Google Scholar]
- 7. Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013; 121: 4021–4031; quiz 4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akyurek N, Uner A, Benekli M, et al. Prognostic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer 2012; 118: 4173–4183. [DOI] [PubMed] [Google Scholar]
- 9. Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 2010; 28: 3360–3365. [DOI] [PubMed] [Google Scholar]
- 10. Rosenwald A, Bens S, Advani R, et al. Prognostic significance of MYC rearrangement and translocation partner in diffuse large B-cell lymphoma: a study by the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol 2019; 37: 3359–3368. [DOI] [PubMed] [Google Scholar]
- 11. Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010; 28: 4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagle SJ, Woo K, Schuster SJ, et al. Outcomes of patients with relapsed/refractory diffuse large B-cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol 2013; 88: 890–894. [DOI] [PubMed] [Google Scholar]
- 13. Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B-cell lymphoma who fail second-line salvage regimens in the International CORAL study. Bone Marrow Transplant 2016; 51: 51–57. [DOI] [PubMed] [Google Scholar]
- 14. Glass B, Hasenkamp J, Wulf G, et al. Rituximab after lymphoma-directed conditioning and allogeneic stem-cell transplantation for relapsed and refractory aggressive non-Hodgkin lymphoma (DSHNHL R3): an open-label, randomised, phase 2 trial. Lancet Oncol 2014; 15: 757–766. [DOI] [PubMed] [Google Scholar]
- 15. Kansara RR, Savage KJ, Vila D, et al. Outcome in unselected patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) following R-CHOP when stem cell transplantation is not feasible. Blood 2014; 124: 3069. [Google Scholar]
- 16. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2020; 38: 155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salles G, Duell J, González Barca E, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol 2020; 21: 978–988. [DOI] [PubMed] [Google Scholar]
- 18. Kalakonda N, Maerevoet M, Cavallo F, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol 2020; 7: e511–e522. [DOI] [PubMed] [Google Scholar]
- 19. Caimi PF, Ai W, Alderuccio JP, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2021; 22: 790–800. [DOI] [PubMed] [Google Scholar]
- 20. Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 2018; 15: 31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brentjens RJ, Latouche JB, Santos E, et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med 2003; 9: 279–286. [DOI] [PubMed] [Google Scholar]
- 22. Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015; 33: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kochenderfer JN, Somerville RPT, Lu T, et al. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor T cell therapy. Mol Ther 2017; 25: 2245–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017; 377: 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol 2019; 20: 31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019; 380: 45–56. [DOI] [PubMed] [Google Scholar]
- 27. Abramson JS, Palomba ML, Gordon LI, et al. High durable CR rates in relapsed/refractory (R/R) aggressive B-NHL treated with the CD19-directed CAR T cell product JCAR017 (TRANSCEND NHL 001): defined composition allows for dose-finding and definition of pivotal cohort. Blood 2017; 130(Suppl. 1): 581.28584136 [Google Scholar]
- 28. Abramson JS, Gordon LI, Palomba ML, et al. Updated safety and long term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol 2018; 36(Suppl. 15): 7505. [Google Scholar]
- 29. Crump M, Neelapu SS, Farooq U, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017; 130: 1800–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Al-Mansour M, Al-Foheidi M, Ibrahim E. Efficacy and safety of second-generation CAR T-cell therapy in diffuse large B-cell lymphoma: a meta-analysis. Mol Clin Oncol 2020; 13: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loffler A, Kufer P, Lutterbüse R, et al. A recombinant bispecific single-chain antibody, CD19 x CD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 2000; 95: 2098–2103. [PubMed] [Google Scholar]
- 32. Allen ES, Stroncek DF, Ren J, et al. Autologous lymphapheresis for the production of chimeric antigen receptor T cells. Transfusion 2017; 57: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vormittag P, Gunn R, Ghorashian S, et al. A guide to manufacturing CAR T cell therapies. Curr Opin Biotechnol 2018; 53: 164–181. [DOI] [PubMed] [Google Scholar]
- 34. Hollyman D, Stefanski J, Przybylowski M, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother 2009; 32: 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang X, Rivière I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncolytics 2016; 3: 16015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheng Z, Wei R, Ma Q, et al. In vivo expansion and antitumor activity of coinfused CD28- and 4-1BB-engineered CAR-T cells in patients with B cell leukemia. Mol Ther 2018; 26: 976–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Giavridis T, Van Der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018; 24: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Engelberts PJ, Hiemstra IH, De Jong B, et al. DuoBody-CD3xCD20 induces potent T-cell-mediated killing of malignant B cells in preclinical models and provides opportunities for subcutaneous dosing. EBioMedicine 2020; 52: 102625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Van Der Horst HJ, De Jonge B, Hiemstra IH, et al. Epcoritamab induces potent anti-tumor activity against malignant B-cells from patients with DLBCL, FL and MCL, irrespective of prior CD20 monoclonal antibody treatment. Blood Cancer J 2021; 11: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hutchings M, Mous R, Clausen MR, et al. Subcutaneous epcoritamab induces complete responses with an encouraging safety profile across relapsed/refractory B-cell non-Hodgkin lymphoma subtypes, including patients with prior CAR-T therapy: updated dose escalation data. Blood 2020; 136: 45–46. [Google Scholar]
- 41. Hutchings M, Morschhauser F, Iacoboni G, et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol 2021; 39: 1959–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schuster SJ, Bartlett NL, Assouline S, et al. Mosunetuzumab induces complete remissions in poor prognosis non-Hodgkin lymphoma patients, including those who are resistant to or relapsing after chimeric antigen receptor T-cell (CAR-T) therapies, and is active in treatment through multiple lines. Blood 2019; 134(Suppl. 1): 6.31273004 [Google Scholar]
- 43. Phillips TJ, Olszewski AJ, Munoz J, et al. Mosunetuzumab, a novel CD20/CD3 bispecific antibody, in combination with CHOP confers high response rates in patients with diffuse large B-cell lymphoma. Blood 2020; 136(Suppl. 1): 37–38. [Google Scholar]
- 44. Bannerji R, Allan JN, Arnason JE, et al. Odronextamab (REGN1979), a human CD20 x CD3 bispecific antibody, induces durable, complete responses in patients with highly refractory B-cell non-Hodgkin lymphoma, including patients refractory to CAR T therapy. Blood 2020; 136: 42–43. [Google Scholar]
- 45. Wudhikarn K, Pennisi M, Garcia-Recio M, et al. DLBCL patients treated with CD19 CAR T cells experience a high burden of organ toxicities but low nonrelapse mortality. Blood Adv 2020; 4: 3024–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu E, Marin D, Banerjee P, et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med 2020; 382: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Neelapu SS, Dickinson M, Ulrickson ML, et al. Interim analysis of ZUMA-12: a phase 2 study of axicabtagene ciloleucel (Axi-Cel) as first-line therapy in patients (Pts) with high-risk large B cell lymphoma (LBCL). Blood 2020; 136(Suppl. 1): 49. [Google Scholar]
- 48. Gajra A, Zettler ME, Phillips EG, Jr, et al. Neurological adverse events following CAR T-cell therapy: a real-world analysis. Immunotherapy 2020; 12: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 49. Leech AA, Dusetzina SB. Cost-effective but unaffordable: the CAR-T conundrum. J Natl Cancer Inst 2018; 111: 644–645. [DOI] [PubMed] [Google Scholar]
- 50. Zhang W, Jordan KR, Schulte B, et al. Characterization of clinical grade CD19 chimeric antigen receptor T cells produced using automated CliniMACS Prodigy system. Drug Des Devel Ther 2018; 12: 3343–3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Castella M, Boronat A, Martín-Ibáñez R, et al. Development of a novel anti-CD19 chimeric antigen receptor: a paradigm for an affordable CAR T cell production at academic institutions. Mol Ther Methods Clin Dev 2019; 12: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017; 377: 2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018; 378: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Minn I, Rowe SP, Pomper MG. Enhancing CAR T-cell therapy through cellular imaging and radiotherapy. Lancet Oncol 2019; 20: e443–e451. [DOI] [PubMed] [Google Scholar]
- 55. Qasim W, Zhan H, Samarasinghe S, et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med 2017; 9: eaaj2013. [DOI] [PubMed] [Google Scholar]
- 56. Liu E, Tong Y, Dotti G, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018; 32: 520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rotolo A, Caputo VS, Holubova M, et al. Enhanced anti-lymphoma activity of CAR19-iNKT cells underpinned by dual CD19 and CD1d targeting. Cancer Cell 2018; 34: 596–610.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]