Abstract
We present the unique case of a 33-year-old male referred to our clinic in search of analgesic options who was found to have a delayed hypersensitivity reaction to ibuprofen manifesting as a maculopapular rash and acute urticaria to acetaminophen. Non-steroidal anti-inflammatory drugs are associated with predictable reactions as well as immunoglobulin E-mediated reactions or T-cell mediated reactions. This case highlights the importance of knowledge of the different types of reactions to non-steroidal anti-inflammatory agents as well as the risk of cross reactivity. Delayed reaction to a single non-steroidal agent is rare; urticaria to acetaminophen is very rare. This is the first report we have found in the literature where one individual has a delayed reaction manifesting as rash to ibuprofen as well as urticaria to acetaminophen. We challenged our patient to aspirin which helped identify that his delayed reaction was only to ibuprofen and urticaria only to acetaminophen. The case also highlights the importance of an oral provocation challenge when no contraindications exist which helped us find that he could take celecoxib and avoid narcotics as initial therapy.
Keywords: Non-steroidal anti-inflammatory drug allergy, ibuprofen allergy, acetaminophen allergy, delayed maculopapular eruption, acute urticaria, rash
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most common analgesics used in the general population and are thought to comprise 20%–25% of all adverse drug reactions. 1 Adverse drug reactions are categorized into type A reactions which are predictable as well as non-predictable type B reactions. 2 Type A drug reactions are due to the pharmacological properties of each drug while type B reactions are independent of the dose of the drug as well as the pharmacological properties.1,3 Type A reactions are more common and comprise 85%–90% of reactions, with an example of a type A reaction to an NSAID being gastritis.1,3 Type B NSAID reactions include but are not limited to NSAID-exacerbated respiratory disease, NSAID-exacerbated cutaneous disease, NSAID-induced urticaria/angioedema or anaphylaxis, single NSAID-induced urticaria/angioedema or anaphylaxis, single NSAID-induced delayed hypersensitivity reactions, drug-induced hyperthermia, drug-induced cutaneous vasculitis, pneumonitis, interstitial nephritis, drug-induced aseptic meningitis, fixed drug eruptions, Stevens–Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), hypersensitivity nodosum, hypersensitivity pneumonitis, contact photoallergic dermatitis, and drug rash with eosinophilia and systemic symptoms.1,3–8 The prevalence of reported drug allergies in adults is approximately 1.9% to NSAIDs; further investigation is recommended to characterize the reaction either as an adverse reaction or a drug hypersensitivity. 7
Case
A 33-year-old male with a history of seizures, as well as SJS to anticonvulsants was referred to allergy clinic for evaluation of drug allergy. One year prior, he had consumed a codeine analgesic after a tooth extraction and developed a delayed maculopapular eruption. Six months prior to being seen in allergy clinic, he had consumed ibuprofen and within 8–10 days had developed a pruritic maculopapular eruption that began on his torso and spread outwards. He was incidentally found to have a gallbladder polyp and required recommendations for safe analgesic options following polypectomy. The patient was scheduled for an ibuprofen challenge in clinic. Prior to the appointment, he had inadvertently challenged himself to ibuprofen, and within 36 hours, he had developed a pruritic maculopapular eruption which was subsequently visualized in clinic as per Figure 1. Thus, we did not perform any further oral provocation challenges on him to ibuprofen. He was treated with oral antihistamines, topical corticosteroids as well as an oral corticosteroid preparation. To further evaluate his allergy, he was given an oral challenge to acetaminophen. Within the literature, multiple protocols exist for oral challenges to acetaminophen, ranging from a single dose challenge to multistep oral challenges.9–11 We reviewed the protocols and made our own modifications in dose and amount of time to monitor. Graded challenge to acetaminophen was undertaken with 325 mg with 30-min increments between the 1% and 10% doses, and 2 hours after the 89% dose. He had an urticarial eruption on his abdomen within 35 min of the last dose as seen in Figure 2. He was treated with diphenhydramine, and the urticaria resolved. He sought an anti-inflammatory medication he would be able to take in lieu of ibuprofen and acetaminophen. At the time of challenge with aspirin, we did not know whether the patient would have NSAID-induced urticaria/angioedema or anaphylaxis reaction or a single NSAID-induced urticaria/angioedema or anaphylaxis reaction, and thus, we chose the former protocol which is slightly more conservative with a lower starting dose. Per protocol, the challenge starts at 40.5 mg, doubling every 90 min to a final dose of 325 mg of aspirin. 12 Although usually well-tolerated, rare reports of reactions to cyclooxygenase-2 (COX-2) inhibitors have occurred in patients with a history of cyclooxygenase-1 (COX-1) reactions. For patient safety, we opted to challenge the patient with celecoxib at a dose of 100 mg with a 60-min observation period followed by 200 mg and a 2-hour observation period to which he had no reactions. 13
Figure 1.

Delayed maculopapular eruption to ibuprofen.
Figure 2.
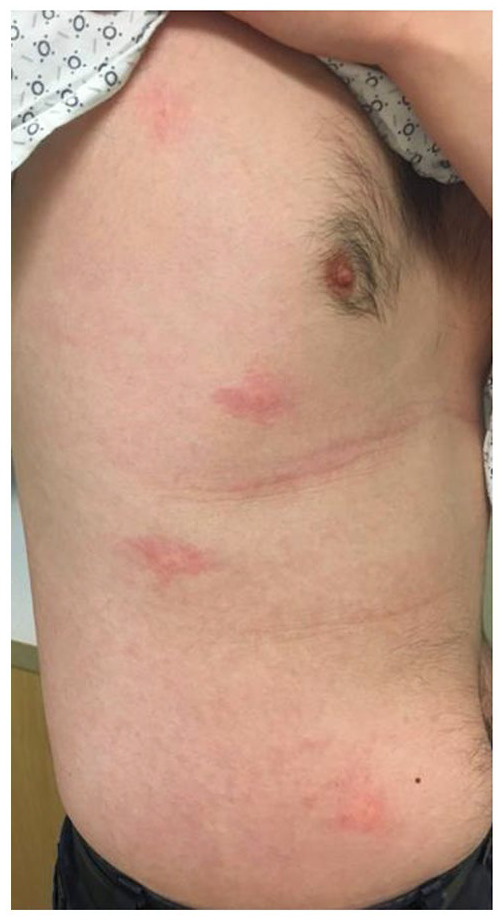
Urticarial eruption after consumption of acetaminophen.
The laboratory results at the initial visit were as follows: eosinophils 600 cells/μL (normal range = 0–500 cells/μL); complete blood count, basic metabolic panel, liver function tests, tryptase, and immunoglobulin E (IgE) were within the normal range. They were repeated at the time of his maculopapular eruption and were non-contributory.
Discussion
NSAIDs’ mechanism of action is via COX-1 inhibition, which primarily targets prostaglandins. In NSAID-induced urticaria/angioedema or anaphylaxis, susceptible individuals without any underlying urticaria/angioedema will have symptoms consisting of urticaria, angioedema, and/or anaphylaxis to at least two different classes of NSAIDs distinguished by chemical structure.12,14 In such patients, a strong non-cross-reacting COX-1 inhibitor should cause symptoms as well.1,3,12,14 Aspirin may be used for the provocation challenge if not the implicated agent; otherwise, another strong COX-1 inhibitor of a different class should be used.12,14 Similarly, in single NSAID-induced urticaria/angioedema or anaphylaxis, provocation challenge with a strong non-cross-reacting COX-1 inhibitor, such as aspirin, is usually undertaken as a negative result will strengthen the diagnosis.12,14 Symptoms may occur within seconds to 1 hour after ingestion with pathophysiology thought to be a type 1 hypersensitivity reaction in which affected individuals may react to the same group of chemicals due to a specific epitope.14,15 Although studies have been done to find nonirritating skin test concentrations of acetaminophen, the validity of skin testing is unknown at this point in time, and there is no standard skin testing concentration. 7 Thus, the gold standard is to evaluate individuals with a history of a concerning type 1 hypersensitivity reaction to acetaminophen with an oral-based provocation challenge, which is what we performed in our patient. 7 However, skin testing may be a useful adjunct in diagnosis of single NSAID-induced urticaria/angioedema or anaphylaixs with select NSAIDs where nonirritating concentrations have been determined. 15
For single NSAID-induced delayed hypersensitivity reactions, reactions occur after 24-hours of exposure to the implicated agent with symptoms ranging from a maculopapular eruption, fixed drug eruptions, photodermatitis, delayed urticaria, and less frequently drug-induced hypersensitivity reactions, acute generalized exanthematous pustulosis, TEN, SJS, as well as organ-specific reactions (pneumonitis and nephritis).1,14 Immediate epicutaneous skin testing is used when individuals have a suspected type 1 hypersensitivity or IgE-mediated reaction. It is not useful for delayed rashes such as our patient’s maculopapular eruption to ibuprofen that developed 36–48 hours after he inadvertently challenged himself. The use of delayed intradermal testing and patch testing may be considered to delineate delayed immunological drug allergies, but the tests are challenging due to the lack of standard guidelines in methods of testing as well as concerns of drug stability. 16 Although delayed intradermal tests could have been considered in this case, the patient had already performed the gold standard of oral provocation when he inadvertently challenged himself prior to the clinic appointment. 16 If he had not challenged himself, patch testing to the NSAID of interest in 10% petrolatum may be performed. 15 The nonirritating concentration for delayed intradermal testing to NSAIDs is 0.1 mg/mL. 15 As opioids such as codeine are mast cell degranulators, one would have to be cautious in interpretation of their delayed intradermal results. 16 Our patient was not interested in opioid analgesics, and thus, we did not pursue that route.
Single NSAID-induced urticaria/angioedema or anaphylaxis reaction and single NSAID-induced delayed hypersensitivity reactions are uncommon, with immediate acetaminophen hypersensitivity comprised mostly of case reports.4,7,14 We did investigate excipients as a potential etiology, and found that he had tolerated these excipients in foods as well as medications such as eye drops, corticosteroids, antihistamines with no immediate or delayed reactions which reassured us that his reactions were not to the excipients. 17
Conclusion
We present a rare case of a patient with ibuprofen-delayed hypersensitivity reaction as well as an acetaminophen immediate hypersensitivity reaction. In these difficult cases, it is important to check for aspirin sensitivity to see whether the sensitivity encompasses all NSAIDs or only one specific NSAID. It is important for providers to be vigilant when prescribing analgesics given the high degree of cross reactivity in COX-1 inhibitors. However, as seen in this patient, as well as others, the cross reactivity may extend beyond the level of COX inhibition. We found that our patient was able to tolerate aspirin and potentially other NSAIDs, which increased his repertoire of available over the counter pain medications. Drug provocation challenges are critical in clarifying whether patients may be candidates for other NSAIDs, weak COX-1 inhibitors such as acetaminophen, and COX-2 selective inhibitors such as celecoxib.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Asal Gharib Naderi  https://orcid.org/0000-0001-6295-3972
https://orcid.org/0000-0001-6295-3972
References
- 1. Woessner KM, Castells M. NSAID single-drug-induced reactions. Immunol Allergy Clin North Am 2013; 33(2): 237–249. [DOI] [PubMed] [Google Scholar]
- 2. Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology and Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol 2010; 105(4): 259–273. [DOI] [PubMed] [Google Scholar]
- 3. Walters KM, Woessner KM. An overview of nonsteroidal anti-inflammatory drug reactions. Immunol Allergy Clin North Am 2016; 36(4): 625–641. [DOI] [PubMed] [Google Scholar]
- 4. Wöhrl S. NSAID hypersensitivity – recommendations for diagnostic work up and patient management. Allergo J Int 2018; 27(4): 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Musselman ME, Saely S. Diagnosis and treatment of drug-induced hyperthermia. Am J Health Syst Pharm 2013; 70(1): 34–42. [DOI] [PubMed] [Google Scholar]
- 6. Guzman AK, Balagula Y. Drug-induced cutaneous vasculitis and anticoagulant-related cutaneous adverse reactions: insights in pathogenesis, clinical presentation, and treatment. Clin Dermatol 2020; 38(6): 613–628. [DOI] [PubMed] [Google Scholar]
- 7. Broyles AD, Banerji A, Castells M. Practical guidance for the evaluation and management of drug hypersensitivity: general concepts. J Allergy Clin Immunol Pract 2020; 8(Suppl. 9): S3–S15. [DOI] [PubMed] [Google Scholar]
- 8. Bianchi L, Marietti R, Hansel K, et al. Drug-induced aseptic meningitis to ibuprofen: the first case confirmed by positive patch test. Contact Dermatitis 2020; 82(6): 405–406. [DOI] [PubMed] [Google Scholar]
- 9. de Paramo BJ, Gancedo SQ, Cuevas M, et al. Paracetamol (acetaminophen) hypersensitivity. Ann Allergy Asthma Immunol 2000; 85: 508–511. [DOI] [PubMed] [Google Scholar]
- 10. Rojas-Pérez-Ezquerra P, Sánchez-Morillas L, Gómez-Traseira C, et al. Selective hypersensitivity reactions to acetaminophen: a 13-case series. J Allergy Clin Immunol Pract 2014; 2(3): 343–345. [DOI] [PubMed] [Google Scholar]
- 11. Yilmaz O, Ertoy Karagol IH, Bakirtas A, et al. Challenge-proven nonsteroidal anti-inflammatory drug hypersensitivity in children. Allergy 2013; 68(12): 1555–1561. [DOI] [PubMed] [Google Scholar]
- 12. Laidlaw TM, Cahill KN. Current knowledge and management of hypersensitivity to aspirin and NSAIDs. J Allergy Clin Immunol Pract 2017; 5(3): 537–545. [DOI] [PubMed] [Google Scholar]
- 13. Kim YJ, Lim KH, Kim MY, et al. Cross-reactivity to acetaminophen and celecoxib according to the type of nonsteroidal anti-inflammatory drug hypersensitivity. Allergy Asthma Immunol Res 2014; 6(2): 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kowalski ML, Asero R, Bavbek S, et al. Classification and practical approach to the diagnosis and management of hypersensitivity to nonsteroidal anti-inflammatory drugs. Allergy 2013; 68(10): 1219–1232. [DOI] [PubMed] [Google Scholar]
- 15. Stingeni L, Bianchi L, Tramontana M, et al. Skin tests in the diagnosis of adverse reactions. G Ital Dermatol Venereol 2020; 155(5): 602–621. [DOI] [PubMed] [Google Scholar]
- 16. Phillips EJ, Bigliardi P, Bircher AJ, et al. Controversies in drug allergy: testing for delayed reactions. J Allergy Clin Immunol 2019; 143(1): 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Caballero ML, Krantz MS, Quirce S, et al. Hidden dangers: recognizing excipients as potential causes of drug and vaccine hypersensitivity reactions. J Allergy Clin Immunol Pract 2021; 9(8): 2968–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]


