Abstract
Staphylococcus aureus may contain one or more genes that encode a variety of immunomodulatory pyrogenic toxins (PTs), including the staphylococcal enterotoxins and toxic shock syndrome toxin (TSST). The PTs interact with several cellular targets to produce disease, such as food poisoning and toxic shock syndrome. At present, nine serologically distinct enterotoxins and one immunoreactive form of TSST have been identified and characterized. As isolates of S. aureus are further assessed, it is anticipated that this number will increase. To facilitate screening, a multiplex PCR was designed to simultaneously determine which of these 10 currently known PT genes an individual S. aureus isolate possesses. We show here, using S. aureus isolates with characterized PT phenotypes, that this novel PCR technique reliably detects each of the known PTs in a single reaction.
Staphylococcus aureus is a common pathogen that colonizes and produces disease in a variety of hosts. The ability of this bacterium to successfully persist within this range of hosts is largely due to the expression of a battery of virulence factors which promote adhesion, acquisition of nutrients, and evasion of host immunologic responses (18). Among these is the pyrogenic exotoxin (PT) family, which is comprised of several structurally and biologically related proteins expressed by both S. aureus and Streptococcus pyogenes (22).
PTs, which include toxic shock syndrome toxin (TSST) and the staphylococcal enterotoxins (SEs), are secreted proteins that interact with antigen-presenting cells and T lymphocytes to induce cellular proliferation (12) and high-level cytokine expression (9). This activity does not involve the endocytic processing required for typical antigen presentation but instead occurs by concurrent association with major histocompatibility complex class II molecules of the antigen-presenting cells and the Vβ domain of the lymphocyte T-cell receptor (13). This interaction activates a much greater percentage of the host T-cell repertoire than that induced by antigens presented in the traditional manner (15), explaining the massive cytokine expression and subsequent immunomodulation brought about by these toxins. Proteins which have the capacity to interact with the host immunological system in this manner have been termed superantigens (SAgs), and the PTs are prototypic examples of bacterial SAgs (10).
The SEs and TSST are the causative agents of toxic shock syndrome (6). Additionally, unlike the other members of the PT family, the SEs have the unique ability to induce staphylococcal food poisoning, a common form of gastroenteritis (10). Presently, nine major antigenic types of SEs have been reported (SEA, SEB, SEC, SED, SEE, SEG, SEH, SEI, and SEJ), while only one serotype of TSST (comprised of TSST-1 and TSSTovine) has been described (1, 4, 5, 8, 19, 20, 24, 25). The SEC serotype is heterogenous and contains several antigenic and sequence molecular variants, designated SEC1, SEC2, SEC3, SECbovine, and SECovine. These have been classified on the basis of minor antigenic differences and the animal host with which they are associated (17). Because of the significance of these toxins for public health and food safety, an efficient means for screening is needed. Also, since several of these toxins have been discovered in very recent years, there is reason to believe that as research on pathogenic S. aureus isolates continues, additional SAgs will be described. Identification of novel toxins will require an efficient means to screen isolates for previously described staphylococcal PT genes. Toxigenic isolates that do not harbor genes for currently recognized toxins are likely to express novel SAgs. Therefore, we developed a multiplex PCR procedure which will rapidly and simultaneously assess whether staphylococcal isolates harbor sea, seb, sec, sed, see, seg, seh, sei, and sej, encoding the SEs, and tst, which encodes TSST.
Bacterial strains and DNA isolation.
Developing and testing of the multiplex procedure was accomplished with DNA from the bacterial strains listed in Table 1. Collectively, these isolates contain all of the previously reported PT genes. Staphylococcal genomic DNA was obtained from lysostaphin-treated cells by a previously described process (7). The DNA was extracted with phenol and chloroform and was ethanol precipitated by standard methods (21). The DNA was recovered by centrifugation, vacuum dried, resuspended in 200 μl of pyrogen-free H2O, quantified spectrophotometrically at 260 and 280 nm, and diluted to a final concentration of 10 ng/μl.
TABLE 1.
S. aureus strains used in multiplex PCRs
| Straina | Toxin genotype determined by:
|
Reference | |
|---|---|---|---|
| Previous work | Multiplex PCR | ||
| RN4220 | Nontoxigenic control | Nontoxigenic | 16 |
| FRI913 | sea sec see tst | sea sec see tst | 3, 17 |
| MNHOCH | seb | seb | 7 |
| FRI472 | sed | sed seg sei sej | 16 |
| FRI326 | see | see | 16 |
| FRI572 | seg | seg sei | 19 |
| FRI569 | seh | seh | 23 |
| FRI445 | sei | seg sei | 19 |
| 3169 | tst secbovine | secbovinesed sej tst | 17 |
FRI, Food Research Institute, University of Wisconsin—Madison.
PCR primer design and amplification of bacterial DNA.
Nucleotide sequences for each of the PT genes were obtained from GenBank by using their specific accession numbers (Table 2). The sequences were compared and evaluated by using Genetics Computer Group (Madison, Wis.) computer software to identify nucleotide sequences unique to each gene. With the exception of the seb-sec primer set, which produces a 643-bp amplification product common to both seb and sec, all primer sets were designed to anneal to unique regions and generate amplification products that would allow identification of each PT gene based on the molecular weight of its PCR product (Table 2). To discriminate whether the 643-bp seb-sec amplification product is indicative of either seb or sec, a separate 5′ sec primer was designed that works in combination with the 3′ seb-sec primer to produce a 283-bp amplification product unique to sec, including the bovine variant (see below). Additionally, to ensure that toxin-negative samples were interpreted correctly and that a sufficient quantity of PCR template DNA was present, the samples were also tested by PCR with a primer set that anneals to the S. aureus 16S rRNA gene that generates a 228-bp amplicon during the amplification process (Table 2).
TABLE 2.
Staphylococcal toxin-specific primers used for multiplex PCR
| Gene | Primer sequence (5′ and 3′)a | GenBank accession no.b | Locationc | Sized |
|---|---|---|---|---|
| sea | GCA GGG AAC AGC TTT AGG C | M18970 | 126–144 | 520 |
| GTT CTG TAG AAG TAT GAA ACA CG | 646–624 | |||
| seb-sec | ATG TAA TTT TGA TAT TCG CAG TG | M11118 (seb) | 28–48 | 643 |
| TGC AGG CAT CAT ATC ATA CCA | 690–670 | |||
| sec | CTT GTA TGT ATG GAG GAA TAA CAA | X05815 | 407–430 | 283 |
| TGC AGG CAT CAT ATC ATA CCA | 690–670 | |||
| sed | GTG GTG AAA TAG ATA GGA CTG C | M28521 | 368–389 | 384 |
| ATA TGA AGG TGC TCT GTG G | 752–734 | |||
| see | TAC CAA TTA ACT TGT GGA TAG AC | M21319 | 446–468 | 170 |
| CTC TTT GCA CCT TAC CGC | 616–599 | |||
| seg | CGT CTC CAC CTG TTG AAG G | AF064773 | 317–335 | 327 |
| CCA AGT GAT TGT CTA TTG TCG | 644–624 | |||
| seh | CAA CTG CTG ATT TAG CTC AG | U11702 | 245–264 | 360 |
| GTC GAA TGA GTA ATC TCT AGG | 603–583 | |||
| sei | CAA CTC GAA TTT TCA ACA GGT AC | AF064774 | 325–347 | 465 |
| CAG GCA GTC CAT CTC CTG | 790–773 | |||
| sej | CAT CAG AAC TGT TGT TCC GCT AG | AF053140 | 471–493 | 142 |
| CTG AAT TTT ACC ATC AAA GGT AC | 612–590 | |||
| tst | GCT TGC GAC AAC TGC TAC AG | J02615 | 48–67 | 559 |
| TGG ATC CGT CAT TCA TTG TTA A | 606–587 | |||
| 16S rRNA | GTA GGT GGC AAG CGT TAT CC | X68417 | 545–564 | 228 |
| CGC ACA TCA GC GTC AG | 773–758 |
Primer sequences are given in the 5′→3′ direction. For each primer pair the sequence of the 5′ primer is provided first (above), followed by that of the 3′ primer (below).
Staphylococcal PT gene identical to designation in first column unless otherwise noted in parentheses.
Location of primer sequence within open reading frame using the nucleotide numbering indicated in GenBank.
Predicted PCR product size generated by using the indicated gene-specific primer pair. For instance, the sea primer pair produces a 520-bp amplicon when the gene encoding SEA is present in the reaction mixture.
The multiplex PCR was performed in a 50-μl volume with the Gibco BRL Taq DNA polymerase system (Life Technologies, Inc., Rockville, Md.) containing the following: 1× Taq polymerase buffer, 4 mM MgCl2, 300 nM concentrations of each of the primers listed in Table 2, 400 μM concentrations of deoxynucleoside triphosphates, 5 U of Taq polymerase, and 50 ng of staphylococcal DNA. Bulk solutions containing the Taq buffer, MgCl2, deoxynucleoside triphosphates, and multiplex PCR primer mix at the appropriate concentrations were prepared for the desired number of reactions. Aliquots of the staphylococcal template DNA (50 ng in 5 μl) from each test strain were individually placed into 500-μl thin-walled PCR tubes. Afterwards, 40 μl of the bulk solution was added to each tube containing the template DNA and covered with 50 μl of mineral oil. These tubes were subsequently incubated for 10 min (95°C), during which (after the initial 3 min) 5 U of Taq polymerase (in 5 μl [total volume] of 1× Taq buffer) was added to each reaction. Following this “hot-start” procedure, DNA was amplified in an Amplitron II thermocycler (Barnstead Thermolyne Co., Dubuque, Iowa) by 15 cycles of 95°C for 1 min, 68°C for 45 s, and 72°C for 1 min and 16 cycles of 95°C for 1 min, 64°C for 45 s, and 72°C for 1 min. The reaction was terminated with a 10-min incubation at 72°C.
PCR products were resolved by electrophoresis in 1.5% agarose (0.5× Tris boric acid, EDTA) gels at 100 V (constant voltage) and visualized on a transilluminator with a charged coupled device camera and the Molecular Analyst software (Bio-Rad, Hercules, Calif.). Product sizes were determined by using the 1 Kb Plus DNA molecular weight ladder (Life Technologies, Inc.).
Genetic analysis of clinical strains using the multiplex PCR procedure.
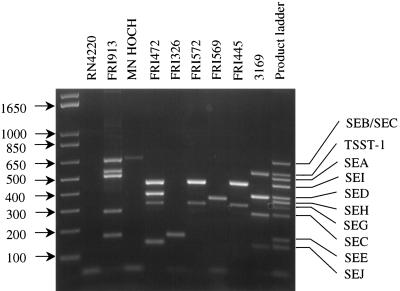
Analyses using DNA obtained from the staphylococcal isolates demonstrated that all primer pairs produced amplification products consistent with their predicted sizes. For example, amplification of DNA from FRI913, known to produce SEA, SEC, SEE and TSST-1 (3), generated bands indicative of sea (520 bp), sec (643 and 283 bp), see (170 bp), and tst (559 bp) (Fig. 1). Similarly, amplification of DNA obtained from S. aureus MN HOCH (7), FRI472 (16), FRI326 (16), FRI572 (19), FRI569 (24), and FRI445 (19) produced PCR products consistent with the toxin genes that had previously been reported for these strains (Table 1 and Fig. 1). As expected, while amplification of DNA obtained from the nontoxigenic strain RN4220 consistently produced the 16S rRNA gene amplicon (results not shown), it failed to produce any SE-related PCR product when subjected to the multiplex PCR analysis. Since a single isolate may contain multiple toxin genes, we assessed the number of genes which could be simultaneously detected with this process under these particular reaction conditions. Using DNA pooled from several isolates (FRI913, FRI472, and FRI569), we demonstrated that it was possible to simultaneously generate PCR products representative of all 10 PT genes, as well as the 16S rRNA gene, in a single reaction (results not shown).
FIG. 1.
Agarose gel electrophoresis of the multiplex PCR amplification products from analysis of bacterial test strains. Lanes contain amplification products of DNA isolated from strains designated at top. Product identification was facilitated by direct comparison to the 1 Kb Plus (Gibco BRL) molecular weight ladder (far left lane) and a PT gene amplification product ladder prepared by pooling reaction products generated in individual reactions (far right lane).
By design, amplification of DNA obtained from strains producing SEC were expected to result in the production of the 643- and 283-bp amplification products indicative of sec, like that observed for FRI913 (Fig. 1). However, strains known to express SECbovine did not generate both amplicons when tested by this procedure. Amplification of DNA obtained from the SECbovine+ strain 3169 (Fig. 1) (17) resulted in the production of the 283-bp amplicon but did not produce the 643-bp seb-sec PCR product typically observed in analyses of SEB- or SEC-expressing isolates (MN HOCH and FRI913, respectively) (Fig. 1). This finding, which was also observed when DNA isolated from another known SECbovine-producing S. aureus isolate was tested (results not shown), suggests that the gene encoding SECbovine has unique attributes not shared by other sec genes (see below). The 5′ seb-sec primer was designed to anneal to these genes at a conserved sequence within the region encoding the signal peptides of SEB and SEC. Since amplification of DNA derived from SECbovine producers does not result in the production of the 643-bp amplicon, it is likely that the region of the gene encoding the SECbovine signal peptide is comprised of a nucleotide sequence dissimilar to that of the other sec variants. At present, the nucleotide sequence within this portion of the SECbovine gene is not available in GenBank and, therefore, cannot be directly compared to analogous regions of the other sec variants.
The multiplex PCR process described in this report reliably detects the genes for all staphylococcal PTs reported as of April 1999. Moreover, this technique was shown to be able to detect at least four different SE genes (producing five separate PCR products) in a single bacterial isolate. The fact that it can simultaneously detect all 10 genes in a pooled sample of DNA ensures that this procedure can both confirm the presence of PT genes previously associated with a particular strain and detect other currently known toxin genes within the isolate. For instance, in the present study S. aureus FRI472, previously described as a SED producer (1), consistently generated a 384-bp PCR product, confirming the presence of the sed gene within its genome. However, amplicons with molecular sizes of 143, 327, and 465 bp were also produced, indicating that FRI472 contains genes encoding SEJ, SEG, and SEI, respectively, as well (Fig. 1). This observation is consistent with the recent report by Zhang et al. (25), who determined that the SEJ determinant is present on the same plasmid as the SED determinant. Similarly, we also showed that other isolates, such as S. aureus FRI572 and FRI445, carry toxin genes not previously associated with these isolates (Fig. 1).
This work has produced a system that expands the capabilities of the multiplex PCR procedures previously developed by several other investigators (2, 11, 14, 23). Most notably, the system described in this report reliably, rapidly, and simultaneously detects each of the 10 currently described staphylococcal toxin determinants, including the most recently described seg (19), sei (19), and sej (25) genes. Additionally, in a single reaction, the process generates amplicons that allow easy discrimination of the determinants an isolate carries, regardless of the number of PT genes carried by the isolate. These features allow this procedure to be applied in the clinical setting for epidemiological studies or to guide therapeutic strategies. This efficient method of screening isolates for PT genes could also facilitate the identification of additional genes encoding novel, yet-undescribed toxins.
Acknowledgments
This work was supported by the funds provided by Public Health Service grant AI28401, the United Dairymen of Idaho, and the Idaho Agriculture Experiment Station (G.A.B.).
We thank Claudia Deobald for her assistance in the development of this assay. We also thank Patrick Schlievert (at the University of Minnesota), Amy Wong (at the Food Research Institute at the University of Wisconsin), and Merlin Bergdoll (also at the Food Research Institute) for kindly providing many of the strains used in this work.
REFERENCES
- 1.Bayles K W, Iandolo J J. Genetic and molecular analyses of the gene encoding staphylococcal enterotoxin D. J Bacteriol. 1989;171:4799–4806. doi: 10.1128/jb.171.9.4799-4806.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker K, Roth R, Peters G. Rapid and specific detection of toxinogenic Staphylococcus aureus: use of two multiplex PCR enzyme immunoassays for amplification and hybridization of staphylococcal enterotoxin genes, exfoliative toxin genes, and toxic shock syndrome toxin 1 gene. J Clin Microbiol. 1998;36:2548–2553. doi: 10.1128/jcm.36.9.2548-2553.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergdoll, M. S. 1988. Personal communication.
- 4.Betley M J, Mekalanos J J. Nucleotide sequence of the type A staphylococcal enterotoxin gene. J Bacteriol. 1988;170:34–41. doi: 10.1128/jb.170.1.34-41.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohach G A. Staphylococcal enterotoxins B and C. Structural requirements for superantigenic and enterotoxigenic activities. Prep Biochem Biotechnol. 1997;27:79–110. doi: 10.1080/10826069708000072. [DOI] [PubMed] [Google Scholar]
- 6.Bohach G A, Fast D J, Nelson R D, Schlievert P M. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 7.Bohach G A, Kreiswirth B N, Novick R P, Schlievert P M. Analysis of toxic shock syndrome isolates producing staphylococcal enterotoxins B and C1 with use of southern hybridization and immunologic assays. Rev Infect Dis. 1989;11(Suppl.1):S75–S81. doi: 10.1093/clinids/11.supplement_1.s75. [DOI] [PubMed] [Google Scholar]
- 8.Couch J L, Soltis M T, Betley M J. Cloning and nucleotide sequence of the type E staphylococcal enterotoxin gene. J Bacteriol. 1988;170:2954–2960. doi: 10.1128/jb.170.7.2954-2960.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fast D J, Schlievert P M, Nelson R D. Nonpurulent response to toxic shock syndrome toxin 1-producing Staphylococcus aureus. Relationship to toxin stimulated production of tumor necrosis factor. J Immunol. 1988;140:949–953. [PubMed] [Google Scholar]
- 10.Jablonski L M, Bohach G A. Staphylococcus aureus. In: Doyle M P, Beuchat L P, Montville T J, editors. Food microbiology: fundamentals and frontiers. Washington, D.C: ASM Press; 1997. pp. 353–375. [Google Scholar]
- 11.Johnson W M, Tyler S D, Ewan E P, Ashton F E, Pollard D R, Rozee K R. Detection of genes for enterotoxins, exfoliative toxins, and toxic shock syndrome toxin 1 in Staphylococcus aureus by the polymerase chain reaction. J Clin Microbiol. 1991;29:426–430. doi: 10.1128/jcm.29.3.426-430.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotb M. Bacterial pyrogenic exotoxins as superantigens. Clin Microbiol Rev. 1995;8:411–426. doi: 10.1128/cmr.8.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavoie P M, Sekaly R P, Thibodeau J, Denis F. Interaction of superantigens with MHC class II molecules. In: Leung D Y, Huber B T, Schlievert P, editors. Superantigens: molecular biology, immunology, and relevance to human disease. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 61–83. [Google Scholar]
- 14.Lee S U, Quesnell M, Fox L K, Yoon J W, Park Y H, Davis W C, Falk D, Deobald C F, Bohach G A. Characterization of staphylococcal bovine mastitis isolates using polymerase chain reaction. J Food Prot. 1998;61:1384–1386. doi: 10.4315/0362-028x-61.10.1384. [DOI] [PubMed] [Google Scholar]
- 15.Leung D Y, Huber B T, Schlievert P. Historical perspectives of superantigens and their biological activities. In: Leung D Y, Huber B T, Schlievert P, editors. Superantigens: molecular biology, immunology, and relevance to human disease. New York, N.Y: Marcel Dekker, Inc.; 1997. pp. 1–13. [Google Scholar]
- 16.Louch H A, Eck M L, Miller K J. Osmoadaptation by Staphylococcus aureus; analysis of several strains linked to food poisoning outbreaks. J Food Prot. 1997;60:139–143. doi: 10.4315/0362-028X-60.2.139. [DOI] [PubMed] [Google Scholar]
- 17.Marr J C, Lyon J D, Roberson J R, Lupher M, Davis W C, Bohach G A. Characterization of novel type C staphylococcal enterotoxins: biological and evolutionary implications. Infect Immun. 1993;61:4254–4262. doi: 10.1128/iai.61.10.4254-4262.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monday S R, Bohach G A. Properties of Staphylococcus aureus enterotoxins and toxic shock syndrome toxin-1. In: Alouf J E, Freer J H, editors. The comprehensive sourcebook of bacterial protein toxins. 2nd ed. London, England: Academic Press; 1999. pp. 589–610. [Google Scholar]
- 19.Munson S H, Tremaine M T, Betley M J, Welch R A. Identification and characterization of staphylococcal enterotoxin types G and I from Staphylococcus aureus. Infect Immun. 1998;66:3337–3348. doi: 10.1128/iai.66.7.3337-3348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray D L, Prasad G S, Earhart C A, Leonard B A B, Kreiswirth B N, Novick R P, Ohlendorf D H, Schlievert P M. Immunobiologic and biochemical properties of mutants of toxic shock syndrome toxin-1. J Immunol. 1994;152:87–95. [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 22.Schlievert P M, Bohach G A, Ohlendorf D H, Stauffacher C V, Leung D Y, Murray D L, Prasad G S, Earhart C A, Jablonski L M, Hoffmann M L, Chi Y I. Molecular structure of Staphylococcus and Streptococcus superantigens. J Clin Immunol. 1995;15:4S–10S. doi: 10.1007/BF01540887. . (Erratum, 16:126, 1996.) [DOI] [PubMed] [Google Scholar]
- 23.Schmitz F J, Steiert M, Hofmann B, Verhoef J, Hadding U, Heinz H P, Kohrer K. Development of a multiplex-PCR for direct detection of the genes for enterotoxin B and C, and toxic shock syndrome toxin-1 in Staphylococcus aureus. J Med Microbiol. 1998;47:335–340. doi: 10.1099/00222615-47-4-335. [DOI] [PubMed] [Google Scholar]
- 24.Su Y C, Wong A C. Identification and purification of a new staphylococcal enterotoxin, H. Appl Environ Microbiol. 1995;61:1438–1443. doi: 10.1128/aem.61.4.1438-1443.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Iandolo J J, Stewart G C. The enterotoxin D plasmid of Staphylococcus aureus encodes a second enterotoxin determinant (sej) FEMS Microbiol Lett. 1998;168:227–233. doi: 10.1111/j.1574-6968.1998.tb13278.x. [DOI] [PubMed] [Google Scholar]