Abstract
Background:
The most common first-line treatment of osteochondral lesions of the talus (OLTs) is microfracture. Although many patients do well with this procedure, a number fail and require reoperation. The mechanism of failure of microfracture is unknown, and to our knowledge there has been no research characterizing failed microfracture regarding histological and inflammatory makeup of these lesions that may contribute to failure.
Purpose:
To characterize the structural and biochemical makeup of failed microfracture lesions.
Study Design:
Case series; Level of evidence, 4.
Methods:
Specimens from 8 consecutive patients with symptomatic OLTs after microfracture who later underwent fresh osteochondral allograft transplantation were analyzed. For each patient, the failed microfracture specimen and a portion of the fresh allograft replacement tissue were collected. The allograft served as a control. Histology of the failed microfracture and the allograft replacement was scored using the Osteoarthritis Research Society International (OARSI) system. Surface roughness was also compared. In addition, tissue culture supernatants were analyzed for 16 secreted cytokines and matrix metalloproteinases (MMPs) responsible for inflammation, pain, cartilage damage, and chondrocyte death.
Results:
The OARSI grade, stage, and total score as well as surface smoothness were significantly worse in the failed microfracture sample, indicating better cartilage and bone morphology for the allografts compared with the failed microfracture lesions. Analyzed cytokines and MMPs were significantly elevated in the microfracture tissue culture supernatants when compared with fresh osteochondral tissue supernatants.
Conclusion:
These data demonstrate a significantly rougher cartilage surface, cartilage and subchondral bone histology that more closely resembles osteoarthritis, and elevated inflammatory cytokines and MMPs responsible for pain, inflammation, cartilage damage, and chondrocyte death when compared with fresh osteochondral allografts used as controls.
Keywords: allograft, failed, inflammation, microfracture, osteochondral lesion, talus
Ankle sprains and fractures are common injuries among athletes and the active population, with studies citing that more than 17% of all injuries in National Collegiate Athletic Association (NCAA) soccer and basketball players involve the ankle and estimating that more than 7% of all injuries in NCAA athletes involving ligamentous ankle injuries. 19,33 In more than 50% of these cases, there was an accompanying osteochondral lesion of the talus (OLT). 22,28,34 With smaller, nondisplaced OLTs typically less than 1.5 cm2, an initial course of nonoperative management is the standard of care; however, many athletes remain symptomatic and require operative intervention.
In these small lesions, microfracture---or bone marrow stimulation---is performed as a means of stimulating healing with a fibrocartilage cap. Although this procedure had a historically high rate of success, recent literature questions the long-term efficacy of this procedure. 29,30 In addition, an in-depth review by Hannon et al 22 of outcomes after microfracture has called into question the literature's reliability and ability to draw meaningful conclusions, as the authors note only 46% of clinical studies reported on the size of the lesion and only 25% performed radiological evaluation on postoperative examination. Specific OLT characteristics have also been tied to higher rates of failure after microfracture, such as lesions located on the shoulder of the talus. 9 However, the mechanism of failure with microfracture is unknown and there have been no studies to our knowledge that have looked into the histological and inflammatory makeup of these lesions that may contribute to the failures.
Prior literature has identified specific cytokines, lipids, and matrix metalloproteinases (MMPs) that create a proinflammatory environment in the joint after ankle fracture that could lead to posttraumatic arthritis. 3,4,27 The purpose of this study was to characterize the structural and biochemical makeup of failed microfracture lesions in an effort to identify potential reasons for continued pain and poor OLT healing after microfracture. Using these same assays, we hypothesized that microfracture creates a similar proinflammatory environment that may negate some of the benefits of the procedure with regard to cartilage healing.
Methods
Eight consecutive patients who were undergoing fresh osteochondral allograft transplantation for failed microfracture were identified and enrolled in this institutional review board—approved study. Discarded intact osteochondral specimens were collected from the patients and analyzed.
Histology
The specimens from each patient included the en bloc excised OLT and the unused, undamaged portion of the fresh allograft replacement that the patient received at the time of surgery (Figure 1). Neither specimen was lavaged prior to collection. The donor patient cohort included 6 male and 2 female patients. All allografts were obtained from RTI Surgical.
Figure 1.
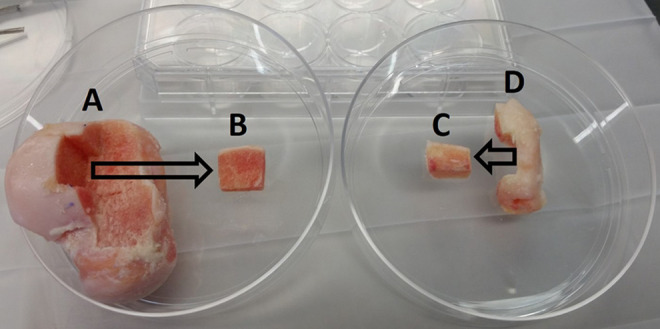
The analyzed allograft section (B) was cut from the donor talus allograft proper (A) at a location immediately adjacent to the portion transplanted to the patient. A portion (C) of the excised osteochondral lesion of the talus (D) was cut for analysis.
The specimens were immediately placed in formalin, decalcified, sectioned, and stained using hematoxylin and eosin and safranin O. The histology sections that were analyzed were chosen from the central portion of both the failed microfracture lesion and the allograft sample. The resultant histological slides were then blinded, randomized, and scored using the Osteoarthritis Research Society International (OARSI) cartilage osteoarthritis (OA) histopathology grading system. 31 In this system, grade is defined as OA depth progression into cartilage, and it is assessed by noting the most advanced grade present within the cartilage, irrespective of its horizontal extent. Stage is defined as the horizontal extent of cartilage involvement, and score is defined as grade multiplied by stage. Therefore, the score represents a combined assessment of OA severity and extent. Three blinded reviewers independently assessed the slides and came to consensus when there was disagreement (S.A., N.A., R.D.). The cartilage surface roughness was also compared between the OLT and allograft cartilage using ImageJ software (National Institutes of Health). 35
Cytokines
Samples of both discarded OLT tissue and to-be-transplanted fresh allograft tissue were weighed before being cultured separately for 48 hours at 37°C and 5% CO2 in 1 mL of serum-free Dulbecco’s modified Eagle medium (Gibco; Thermo Fisher Scientific) containing 5-mL, 100× penicillin/streptomycin (Gibco; Thermo Fisher Scientific) and 25-mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid buffer (Gibco; Thermo Fisher Scientific). The allograft cartilage sample size was roughly matched to the OLT sample size. Media supernatants were collected and stored at –80°C until they were analyzed for 16 different cytokines and MMPs, including interferon (INF)-γ, interleukin (IL)-10, IL-12p70, IL-13, IL-1β, IL-2, IL-4, IL-6, IL-8, tumor necrosis factor (TNF)-α, C-telopeptide fragments of type II collagen (CTX-II), MMP-1, MMP-2, MMP-3, MMP-9, and MMP-10 using human sandwich immunoassays (Meso Scale Discovery) according to the manufacturer’s instructions. Levels of cytokines were normalized to tissue weight prior to statistical analysis.
Statistical Analysis
Paired t tests comparing the OLTs and allografts were performed on OARSI grade, stage, total score, and surface roughness. Significance was set at P < .05. The distribution of all data was assessed. Normally distributed cytokine data levels were analyzed using paired t test. Nonnormally distributed cytokine data (IL-1β, IL-6, IL-2, IL-4, and MMP-2) were compared using the Wilcoxon rank-sum test. Values for measured cytokines that were below the detection limit of the assay were replaced with half of the lower level of detection values supplied by the assay manufacturer. Statistical analysis was performed using JMP 13 (SAS Institute). Post hoc power analysis was performed using an online tool provided by the University of California, San Francisco, 25 and it revealed that this study was sufficiently powered to detect the reported effect sizes for histological, surface roughness, and secretomic analysis outcomes with at least 80% certainty with the exception of CTX-II, MMP-1, MMP-2, and MMP-9. Finally, correlation analysis was performed between the total OARSI score and the measured cytokines. Correlation results are reported as Pearson correlation coefficients except for IL-1β, IL-6, IL-2, IL-4, and MMP-2. Because of the nonnormally distributed data for these cytokines, the Spearman R correlation test was used.
Results
The mean age of the patients was 43 years (range, 24-59 years). This cohort included 3 men and 5 women. The mean age of the donor allograft patients was 27 years (range, 16-37 years), and the mean time from donor death to implantation was 40 days (range, 28-49 days). Demographics of the sample are shown in Table 1.
Table 1.
Demographics of Donor and Recipient Patients
| Recipient Age, y | Recipient Sex | Donor Age, y | Donor Sex | Days to Implantation |
|---|---|---|---|---|
| 40 | Male | 25 | Male | 29 |
| 49 | Male | 24 | Male | 42 |
| 37 | Female | 37 | Male | 39 |
| 48 | Female | 32 | Female | 41 |
| 46 | Female | 26 | Female | 44 |
| 24 | Female | 35 | Male | 49 |
| 59 | Male | 19 | Male | 35 |
| 37 | Female | 16 | Male | 45 |
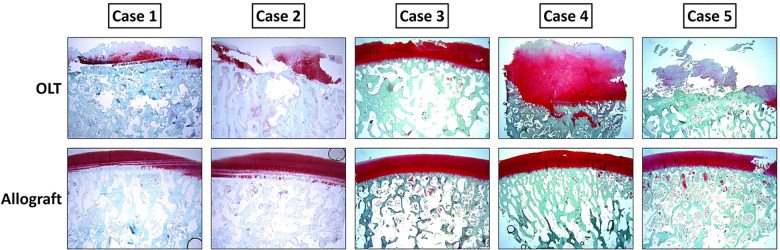
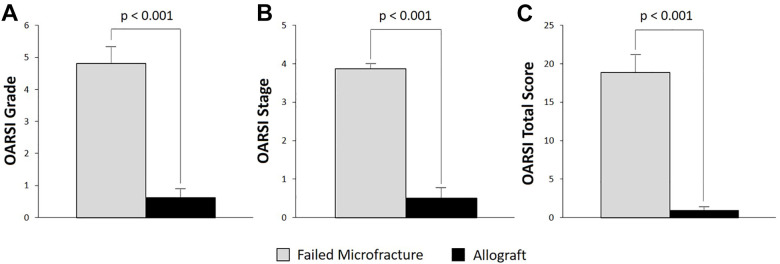
Gross histological inspection of the samples demonstrated cartilage erosion and subchondral bone destruction in the failed microfracture OLTs and an intact cartilage surface in the allografts (Figure 2). The OARSI grade, stage, and total score were significantly worse in the resected OLTs that failed microfracture compared with the healthy allograft controls (Figure 3), indicating worse cartilage and bone morphology. The mean grade for the resected failed microfracture samples was 5. However, 4 of 8 samples had a score of 6. Scores of 5 and 6 correspond to osteochondral damage consisting of deformation of the subchondral bone with subchondral sclerosis and areas of reparative fibrocartilage. The mean stage for the failed microfracture samples was 4 (Figure 3), which indicates more than 50% involvement of the grade in any particular sample. In contrast, half of the allograft samples had grade and stage scores of 0, indicating normal cartilage and bone. Regarding surface roughness, the calculated mean and standard deviation for the Ra value for the failed microfracture OLTs was 192 ± 41 versus 97 ± 16 for the allograft samples. This difference was significant (P < .001). For example, the Ra value for the OLT from case 4 in Figure 2 was 192 and for the corresponding allograft the Ra value was 95.
Figure 2.
Safranin O histology of 5 representative cases demonstrating the patients’ osteochondral lesion of the talus (OLT) (top row) and the transplanted fresh talus allografts (bottom row). The disrupted cartilage with bone erosion can be seen in the OLT images. Intact cartilage and bone can be seen in the allograft images. Note sectioning artifacts in allografts cases 1, 2, and 5. This is not cartilage damage.
Figure 3.
Mean OARSI (A) grade, (B) stage, and (C) total score of the osteochondral lesion of the talus and allografts. A higher score means more cartilage and bone destruction. The transplanted allografts demonstrated significantly better OARSI grades, stages, and total scores. Error bars represent SEM. OARSI, Osteoarthritis Research Society International.
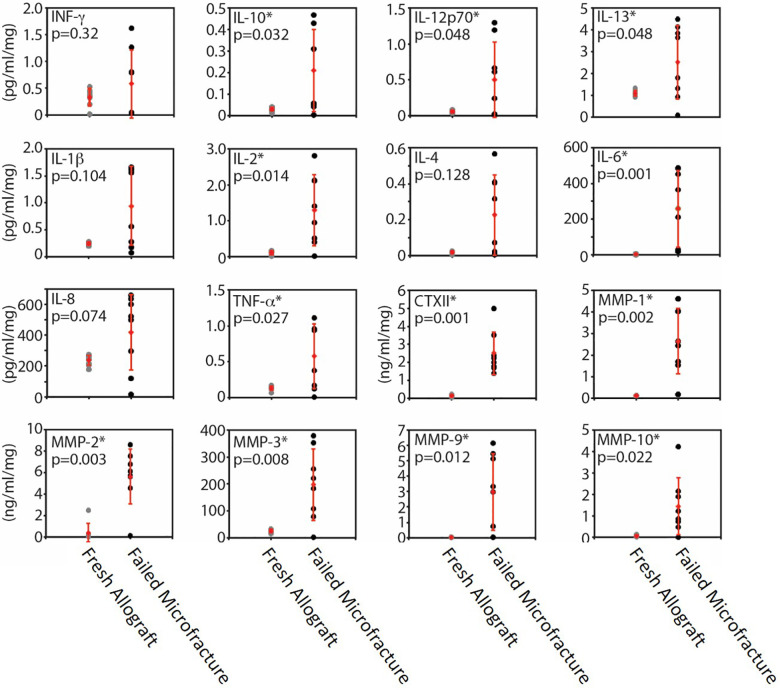
Secretomic analysis demonstrated that the level of all measured cytokines and MMPs except INF-γ, IL-1β, IL-4, and IL-8 were significantly higher in the microfracture OLT tissue culture supernatants compared with allograft supernatants (Figure 4), indicating there was inflammatory cytokine and degradative MMP production from the failed microfracture samples. It should be noted that all values for MMP-9 for the fresh allograft group fell below the lower limit of detection.
Figure 4.
Cytokine and MMP levels for cultured fresh allograft (gray dots) and failed microfracture (black dots) tissues. Mean (diamonds) and SD (error bars) are shown in red for both groups. Asterisk denotes significant difference between groups (P < .05). CTX-II, C-telopeptide fragments of type II collagen; IL, interleukin; INF, interferon; MMP, matrix metalloproteinase; TNF, tumor necrosis factor.
Correlation analyses between the OARSI total score and the measured cytokines revealed a significant positive correlation for TNF-α, IL-2, IL-6, IL-10, IL-13, MMP-1, MMP-2, MMP-3, MMP-9, MMP-10, and CTX-II.
Discussion
The primary findings of this study are that failed microfracture-treated lesions had arthritic-like histology with subchondral plate sclerosis and deformation, rough surface features, and produced inflammatory cytokines and MMPs, which are known to be both pain generators and to degrade cartilage extracellular matrix. 38 More specifically, OARSI grade and surface roughness, as defined by Ra value, were significantly worse in the failed microfracture group, and secretomic analysis showed significantly higher levels of the analyzed factors in the failed microfracture group in 75% (12 of 16). This is the first study to our knowledge to identify the histological and secretomic characteristics of failed microfracture of the talus. Moreover, this study was able to compare these characteristics with fresh human osteochondral allografts, which are an ideal control second only to the patient’s own healthy cartilage and subchondral bone, which would be unethical to obtain outside of the required preparation for allograft implantation. In addition, as represented in these cases, osteochondral allograft transplantation often represents the next step in treatment.
After nonoperative management has failed, microfracture is a technique commonly used for small OLT lesions owing to the relatively quick return to sport and ability for the patient to regain high levels of activity postoperatively. 37 However, a recent systematic review found that only 88% of patients who underwent microfracture were able to return to sport at any level, and only 79% were able to return to prior levels of activity. 37 Even less is known about how well elite athletes return and, more troubling, the long-term success of this procedure in this specific population. 24 There is previous magnetic resonance imaging (MRI) evidence that microfracture does not adequately address the damaged subchondral bone and that the fibrocartilage replacement tissue deteriorates quickly over time—a process that may also be expedited in high-level athletes based on the repetitive high-impact activities. 14,24,36 Our study adds to the literature, as it demonstrates that, at least with failed microfracture, the subchondral bone is sclerotic and deformed. It is difficult to know whether these characteristics were present before microfracture, but these abnormalities do demonstrate that, in failed microfracture, subchondral bone does not remodel to normal.
Choi et al 10 looked at a series of 120 microfracture-treated ankles and found 6.7% progressed to osteochondral transplant, 18.4% were deemed clinical failures, and there was an overall increase in failure over time. In a series of 434 patients, lesions on the talar shoulder had a clinical failure after microfracture in 27.2% of cases compared with 12.5% outside of the shoulder, which is significantly less but still far from ideal for a population looking to return to high-level activities. 9 Hunt and Sherman 23 looked at a cohort of 37 ankles with a mean follow-up of 66 months and found that only 46% had a good or excellent result after microfracture. In a long-term follow-up study on microfracture-treated lesions, Ferkel et al 14 looked at second-look arthroscopy findings and reported that 35% of patients had worsened outcomes according to the qualitative Weber system. Becher et al 7 looked at MRIs of patients with microfractures postoperatively and found that both the structure and the surface of the repair tissue were neither intact nor homogeneous. Although second-look arthroscopy and postoperative MRI provide benefit in the identification of failed microfracture lesions, they do not provide insight into the pathogenesis as the histology and cytokine analyses presented in this study were able to do.
Beyond the concerning effects that the inflammatory microenvironment may have on microfracture-treated cartilage and on the healing of this regenerative tissue, there is also evidence to suggest further long-term joint damage could result. Prior studies have identified the proinflammatory milieu of synovial fluid at various time points after ankle fracture, which have been proposed as a contributing factor to posttraumatic arthritis. 3,4 These same studies have shown that the inflammatory environment persists well beyond fracture healing, as many of the cytokines and MMPs remain elevated in the synovial fluid. 3 Many of the same cytokines and MMPs that were elevated in the fracture studies were also found to be elevated in our microfracture samples, suggesting that microfracture may create a microenvironment similar in nature to a traumatic fracture that is detrimental to long-term healing and outcomes. Although this study did not investigate long-term microenvironment changes of the microfracture samples, it opens an additional avenue for potential research.
In cases where microfracture fails, particularly in larger lesions, fresh allograft transplantation is a promising option with a growing number of case series reporting relatively high rates of success for improved pain and function in OLT lesions. § In addition, a recent study compared the short-term success of femoral condylar autograft with fresh allograft for OLTs and concluded that both methods resulted in improved function and pain relief with similar rates, but the use of fresh allograft eliminated the risk of complications from autograft surgery. 5 Further, fresh talar allografts provide osteochondral tissue from the same anatomic location. Although ankle cartilage is hyaline and similar in overall composition to knee cartilage, ankle cartilage thickness, cell alignment, permeability, and water and proteoglycan content differ significantly from that seen in the knee and result in ankle cartilage being stiffer and more resistant to mechanical deformation. Ankle cartilage is also more metabolically active than knee cartilage and is less susceptible to proinflammatory mediators. 26 Further, the use of fresh allograft tissue allows the surgeon more freedom to better match the shape of the implant tissue to the lesion because fresh allografts are not limited to smaller, cylindrical implants traditionally obtained with autografts. 11,12 This is especially helpful with defects involving a larger area of the talar dome.
Although studies are needed to compare long-term outcomes, these differences in cartilage characteristics are the reason fresh allograft was used in this study as the comparison arm. Fresh talar allograft tissue may prove advantageous for certain OLTs because it is better suited for the application, with near-normal cartilage and subchondral bone histology and minimal production of inflammatory, pain, and cartilage-degrading cytokines and MMPs.
As outlined previously, the decision to use fresh allograft as the control arm in this study was based on 2 primary factors. While healthy talar cartilage surrounding the microfracture-treated lesions presented a local source of presumed healthy, patient-specific comparison tissue, there were concerns about the ability to obtain enough sample for analysis. Although undamaged cartilage is removed during preparation for allograft implantation and could be used as the control, the quantity is generally limited and can be compromised in the removal process. In using the remaining allograft samples, we were able to obtain large, undamaged specimens of standardized sizes that were ideal for testing. Second, as the allograft was being transplanted into the patient, we felt it was important to establish the baseline histological and cytokine profile of the replacement tissue that we were deeming of high enough quality to use as long-term replacement tissue, which we felt was important and added to the foundational understanding of this procedure. Future work may be able to establish the histological and cytokine profiles of the healthy surrounding tissue but will require a standardization process of tissue removal and handling to ensure quality specimens for testing.
Regarding allograft quality and the potential alterations of the sample microenvironment during processing, we are confident that the uniform nature of the methodology helps mitigate some of these concerns. As Goodfriend et al 15 pointed out in their study on the state of fresh allograft handling in the United States, each distributor uses their own proprietary processing technique and each of the major players in the space uses a different storage medium. Although it is impossible to know how the specific processing strategy used by our supplier had affected the samples, the use of only 1 graft source in this study provided consistency across the analysis. What may be more important to the findings would be any comorbidities of the donor patients that could lead to variations of the sample microenvironment at baseline; however, it was not possible to obtain this information. Despite these potential concerns, we are confident in our results, as the cytokine and histology data are consistent throughout allograft samples.
While we do not suggest that small OLT lesions be treated initially with fresh allograft transplantation, this study highlights factors that may contribute to the failure of some microfracture procedures. In addition, this study may serve to identify avenues for future research for early intervention along with the microfracture procedure to enhance clinical outcomes.
The limitations of this study include small sample size, unknown histology of the OLT lesions prior to microfracture, and no comparison with successful microfracture lesions. Although future research can address the sample size and histology of lesions prior to microfracture, there is unlikely to be an avenue for analysis of successful microfracture lesions outside of retrieval of cartilage after unrelated procedures.
Conclusion
The current study is the first to our knowledge to provide histologic and secretomic evidence demonstrating insufficiencies of osteochondral tissue after failed microfracture. These data demonstrate a significantly rougher cartilage surface, cartilage and subchondral bone histology that more closely resembles osteoarthritis, and elevated inflammatory cytokines and MMPs responsible for pain, inflammation, cartilage damage, and chondrocyte death when compared with fresh osteochondral allografts used as controls.
Footnotes
Final revision submitted April 5, 2021; accepted May 19, 2021.
One or more of the authors has declared the following potential conflict of interest or source of funding: N.G. has received hospitality payments from Smith & Nephew. J.A.N. has received research support from Acumed, Breg, and Integra; consulting fees from Acumed, DTMedTech, Exactech, Mirus, OrthoFix, Stryker, Treace Medical, and Wright Medical; speaking fees from Treace Medical and Trimed; nonconsulting fees from Pacira Pharmaceuticals; and royalties from Exactech and Wright Medical; and has stock/stock options in Bristol-Myers Squibb. M.E.E. has received research support from Medartis; education payments from SouthTech Orthopedics; consulting fees from Exactech, Medartis, OrthoFix, RTI Surgical, Stryker, Treace Medical, and TriMed; speaking fees from Exactech; nonconsulting fees from Integra LifeSciences; honoraria from In2Bones; and royalties from Exactech. S.B.A. has received education payments from Arthrex; consulting fees from 4WEB, Conventus, Embody, Exactech, FH Orthopedics, MedShape, OrthoFix, Ortho Solutions, RTI Surgical, Sonoma Orthopedics, and Stryker; nonconsulting fees from Arthrex and Terumo BCT; honoraria from In2Bones; and hospitality payments from Medline Industries; and has stock/stock options in MedShape, Restor3d, and Tyber Medical. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Duke Health Institutional Review Board (Pro00033747).
References
- 1. Adams SB, Dekker TJ, Schiff AP, Gross CP, Nunley JA, Easley ME. Prospective evaluation of structural allograft transplantation for osteochondral lesions of the talar shoulder. Foot Ankle Int. 2018;39(1):28–34. [DOI] [PubMed] [Google Scholar]
- 2. Adams SB, Jr, Viens NA, Easley ME, Stinnett SS, Nunley JA, II. Midterm results of osteochondral lesions of the talar shoulder treated with fresh osteochondral allograft transplantation. J Bone Joint Surg Am. 2011;93(7):648–654. [DOI] [PubMed] [Google Scholar]
- 3. Adams SB, Leimer EM, Setton LA, et al. Inflammatory microenvironment persists after bone healing in intra-articular ankle fractures. Foot Ankle Int. 2017;38(5):479–484. [DOI] [PubMed] [Google Scholar]
- 4. Adams SB, Setton LA, Bell RD, et al. Inflammatory cytokines and matrix metalloproteinases in the synovial fluid after intra-articular ankle fracture. Foot Ankle Int. 2015;36(11):1264–1271. [DOI] [PubMed] [Google Scholar]
- 5. Ahmad J, Jones K. Comparison of osteochondral autografts and allografts for treatment of recurrent or large talar osteochondral lesions. Foot Ankle Int. 2016;37(1):40–50. [DOI] [PubMed] [Google Scholar]
- 6. Al-Shaikh RA, Chou LB, Mann JA, Dreeben SM, Prieskorn D. Autologous osteochondral grafting for talar cartilage defects. Foot Ankle Int. 2002;23(5):381–389. [DOI] [PubMed] [Google Scholar]
- 7. Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):656–663. [DOI] [PubMed] [Google Scholar]
- 8. Berlet GC, Hyer CF, Philbin TM, Hartman JF, Wright ML. Does fresh osteochondral allograft transplantation of talar osteochondral defects improve function? Clin Orthop Relat Res. 2011;469(8):2356–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi WJ, Choi GW, Kim JS, Lee JW. Prognostic significance of the containment and location of osteochondral lesions of the talus: independent adverse outcomes associated with uncontained lesions of the talar shoulder. Am J Sports Med. 2013;41(1):126–133. [DOI] [PubMed] [Google Scholar]
- 10. Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974–1980. [DOI] [PubMed] [Google Scholar]
- 11. Cinque ME, Kennedy NI, Moatshe G, et al. Osteochondral allograft transplants for large trochlear defects. Arthrosc Tech. 2017;6(5):e1703–e1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Demange M, Gomoll AH. The use of osteochondral allografts in the management of cartilage defects. Curr Rev Musculoskelet Med. 2012;5(3):229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El-Rashidy H, Villacis D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: a retrospective review. J Bone Joint Surg Am. 2011;93(17):1634–1640. [DOI] [PubMed] [Google Scholar]
- 14. Ferkel RD, Zanotti RM, Komenda GA, et al. Arthroscopic treatment of chronic osteochondral lesions of the talus: long-term results. Am J Sports Med. 2008;36(9):1750–1762. [DOI] [PubMed] [Google Scholar]
- 15. Goodfriend B, Essilfie AA, Jones IA, Vangsness CT, Jr. Fresh osteochondral grafting in the United States: the current status of tissue banking processing. Cell Tissue Bank. 2019;20(3):331–337. [DOI] [PubMed] [Google Scholar]
- 16. Gortz S, De Young AJ, Bugbee WD. Fresh osteochondral allografting for osteochondral lesions of the talus. Foot Ankle Int. 2010;31(4):283–290. [DOI] [PubMed] [Google Scholar]
- 17. Gross AE, Agnidis Z, Hutchison CR. Osteochondral defects of the talus treated with fresh osteochondral allograft transplantation. Foot Ankle Int. 2001;22(5):385–391. [DOI] [PubMed] [Google Scholar]
- 18. Gross CE, Adams SB, Jr, Easley ME, Nunley JA. Role of fresh osteochondral allografts for large talar osteochondral lesions. Instr Course Lect. 2016;65:301–309. [PubMed] [Google Scholar]
- 19. Gulbrandsen M, Hartigan DE, Patel KA, Makovicka JL, Tummala SV, Chhabra A. Ten-year epidemiology of ankle injuries in men’s and women’s collegiate soccer players. J Athl Train. 2019;54(8):881–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94(12):1105–1110. [DOI] [PubMed] [Google Scholar]
- 21. Hahn DB, Aanstoos ME, Wilkins RM. Osteochondral lesions of the talus treated with fresh talar allografts. Foot Ankle Int. 2010;31(4):277–282. [DOI] [PubMed] [Google Scholar]
- 22. Hannon CP, Smyth NA, Murawski CD, et al. Osteochondral lesions of the talus: aspects of current management. Bone Joint J. 2014;96-B(2):164–171. [DOI] [PubMed] [Google Scholar]
- 23. Hunt SA, Sherman O. Arthroscopic treatment of osteochondral lesions of the talus with correlation of outcome scoring systems. Arthroscopy. 2003;19(4):360–367. [DOI] [PubMed] [Google Scholar]
- 24. Hurley ET, Shimozono Y, McGoldrick NP, Myerson CL, Yasui Y, Kennedy JG. High reported rate of return to play following bone marrow stimulation for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2721–2730. [DOI] [PubMed] [Google Scholar]
- 25. Institute UCaTS. Sample size calculators for designing clinical research. Accessed November 26, 2020. https://sample-size.net/.
- 26. Kraeutler MJ, Kaenkumchorn T, Pascual-Garrido C, Wimmer MA, Chubinskaya S. Peculiarities in ankle cartilage. Cartilage. 2017;8(1):12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leimer EM, Pappan KL, Nettles DL, et al. Lipid profile of human synovial fluid following intra-articular ankle fracture. J Orthop Res. 2017;35(3):657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Leontaritis N, Hinojosa L, Panchbhavi VK. Arthroscopically detected intra-articular lesions associated with acute ankle fractures. J Bone Joint Surg Am. 2009;91(2):333–339. [DOI] [PubMed] [Google Scholar]
- 29. Murawski CD, Foo LF, Kennedy JG. A review of arthroscopic bone marrow stimulation techniques of the talus: the good, the bad, and the causes for concern. Cartilage. 2010;1(2):137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murawski CD, Kennedy JG. Prolongation of T2 stratification after microfracture does not indicate normal cartilage. Cartilage. 2011;2(4):399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. [DOI] [PubMed] [Google Scholar]
- 32. Raikin SM. Fresh osteochondral allografts for large-volume cystic osteochondral defects of the talus. J Bone Joint Surg Am. 2009;91(12):2818–2826. [DOI] [PubMed] [Google Scholar]
- 33. Roos KG, Kerr ZY, Mauntel TC, Djoko A, Dompier TP, Wikstrom EA. The epidemiology of lateral ligament complex ankle sprains in National Collegiate Athletic Association sports. Am J Sports Med. 2017;45(1):201–209. [DOI] [PubMed] [Google Scholar]
- 34. Saxena A, Eakin C. Articular talar injuries in athletes: results of microfracture and autogenous bone graft. Am J Sports Med. 2007;35(10):1680–1687. [DOI] [PubMed] [Google Scholar]
- 35. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Seow D, Yasui Y, Hutchinson ID, Hurley ET, Shimozono Y, Kennedy JG. The subchondral bone is affected by bone marrow stimulation: a systematic review of preclinical animal studies. Cartilage. 2019;10(1):70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steman JAH, Dahmen J, Lambers KTA, Kerkhoffs GMMJ. Return to sports after surgical treatment of osteochondral defects of the talus: a systematic review of 2347 cases. Orthop J Sports Med. 2019;7(10):2325967119876238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yasuda T. Cartilage destruction by matrix degradation products. Mod Rheumatol. 2006;16(4):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]