Abstract
Major depressive disorder (MDD) is a multifactorial psychiatric disorder with obscure pathophysiology. A biomarker-based approach in combination with standardized interview-based instruments is needed to identify MDD subtypes and novel therapeutic targets. Recent findings support the impairment of the mammalian target of rapamycin complex 1 (mTORC1) in MDD. No well-established biomarkers of mTORC1 disease- and treatment-modulated activity are currently available for use in early phase antidepressant drug (AD) development. This review aims to summarize biomarkers of mTORC1 activity in MDD and to suggest how these could be implemented in future early clinical trials on mTORC1 modulating ADs. Therefore, a PubMed-based narrative literature review of the mTORC1 involvement in MDD was performed. We have summarized recent pre-clinical and clinical findings linking the MDD to the impaired activity of several key biomarkers related to mTORC1. Also, cases of restoration of these impairments by classical ADs and novel fast-acting investigational ADs are summarized. The presented biomarkers may be used to monitor pharmacological effects by novel rapid-acting mTORC1-targeting ADs. Based on findings in the peripheral blood mononuclear cells, we argue that those may serve as an ex vivo model for evaluation of mTORC1 activity and propose the use of the summarized biomarkers for this purpose. This could both facilitate the selection of a pharmacodynamically active dose and guide future early clinical efficacy studies in MDD. In conclusion, this review provides a blueprint for the rational development of rapid-acting mTORC1-targeting ADs.
Keywords: rapid-acting antidepressants, pharmacological biomarkers, CNS drug development, biomarker-based drug development, major depressive disorder (MDD), mTORC1
Introduction
Major depressive disorder (MDD) is a multifactorial psychiatric disorder that is primarily characterized by a sustained and pathologically depressed mood and anhedonia and accompanied by disturbances in cognitive function, psychomotor activity, and vegetative symptoms. MDD is one of the top four causes of years lived with disability worldwide, and patients suffering from MDD are at risk of suicidal behavior or death by suicide.1,2 Besides, up to 30% of MDD patients fail to respond to currently registered antidepressant drugs (ADs), a majority of patients experience unacceptable side effects or residual symptoms despite antidepressant treatment, and therapeutic effects with conventional monoaminergic ADs only occur after several weeks. 3 The future development of novel ADs for the treatment of MDD should therefore pursue rapid mood improvement that is associated with a more favorable efficacy and safety profiles compared with currently available drugs.
Currently, the classification of MDD is primarily based on diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders (5th edition; DSM 5). 4 Importantly, the most widely used method to assess the severity of depressive symptoms and the response to pharmacological interventions in virtually all clinical MDD studies is one or a combination of depression severity rating instruments. These include the clinician-rated Montgomery–Åsberg Depression Rating Scale (MADRS) and the Hamilton Rating Scale for Depression (HAMD), and the self-reported Inventory of Depressive Symptomatology (IDS-SR).5–7 These instruments are composed of scoring items that are predominantly derived from the DSM diagnostic criteria, with the severity of each item being rated using a 4- to 6-point Likert-type scale, and ultimately resulting in a total score.5–7 Despite being useful in clinical practice to track treatment response over time, the validity of such phenomenology-based assessments depends on various factors such as rater experience, MDD-mediated recall bias, and unintended non-specific therapeutic effects as a result of interactions with raters or the research team during the study. Moreover, these instruments are not sufficient to distinguish between different biological subtypes or endophenotypes that may underlie the heterogeneous MDD syndrome. Also, as these have been validated using predominantly monoaminergic ADs, they might not be sufficiently sensitive to grasp rapid dynamic central nervous system (CNS) molecular changes induced by drugs with novel mechanisms of action such as N-methyl-D-aspartate receptor (NMDAR) antagonists or α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR) modulators. Clearly, there is a need for biomarkers that complement the current interview-based evaluations, as biomarkers are expected to objectify the effects of novel compounds in early phase psychiatric drug development and ultimately personalized MDD treatments. 8
Although neuroscience has contributed greatly to a better understanding of the neurobiology of psychiatric diseases, the pathophysiology of MDD remains largely obscure. Drug development for MDD is currently still dominated by a phenomenological approach, which ultimately results in the selection of heterogeneous patient populations for inclusion in drug trials and may contribute to the dilution of treatment effects with novel compounds. A biomarker-based approach for MDD presents obvious advantages, such as the identification of patient subgroups that may benefit from drugs with a specific mechanism of action (MOA) and the drug development guided by pharmacological biomarkers.3,9 Several different biomarkers have been proposed, ranging from CNS-based cerebrospinal fluid (CSF) and neuroimaging profiles to peripheral changes involving the neuroendocrine system and inflammatory responses. 9 However, so far, none of these biomarkers have been adequately validated for application in the complementary diagnosis and treatment of MDD, or in drug development programs of novel ADs. 9
An increasing number of molecular findings suggests a relationship between stress-related synaptic dysfunction, one of the putative pathophysiological factors in MDD, and inhibition of the mammalian target of rapamycin complex 1 (mTORC1). 10 Therefore, mTORC1 has recently emerged as a novel surrogate target for emerging rapid-acting ADs, as well as a potential pharmacological and diagnostic biomarker in drug development.11–13 Although mTORC1 activity can readily be quantified in dissected brains of laboratory animals, and molecular and morphological changes can be linked to behavioral response to AD treatment, this approach is not feasible in living human subjects.14,15
This literature review, therefore, aims to summarize the evidence for molecular mTORC1 biomarkers in MDD, and to suggest how these could be implemented as pharmacological biomarkers in future early clinical trials with rapid-acting ADs that target the reversal of stress-related synaptic dysfunction in MDD. Ultimately, development of new ex vivo models may demonstrate the mTORC1-related pharmacological activity of drugs by monitoring relevant biomarkers within the signaling pathway.
mTORC1 involvement in MDD
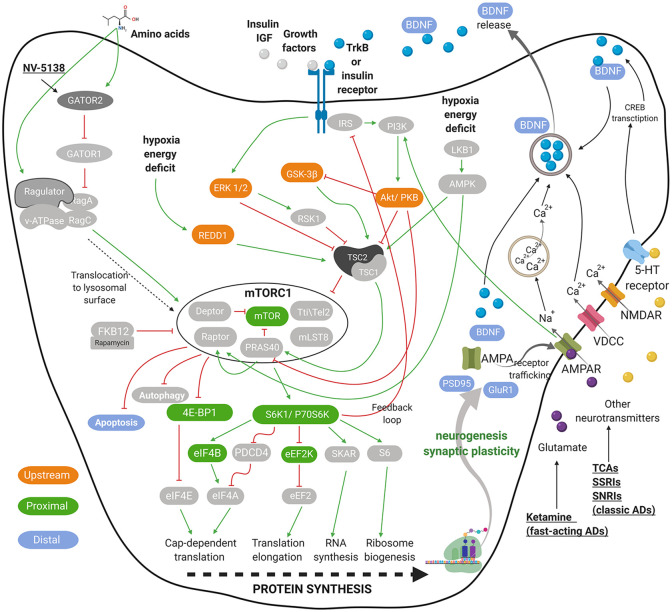
mTOR signaling governs cell growth and metabolism. mTOR complex is divided into two distinct multiprotein complexes named mTORC1 and mTORC2. The signaling of both complexes is intertwined as they share multiple proteins, that is, mLST8, the Tti1/Tel2 complex, and Deptor.16,17 mTORC1 additionally associates with the nonenzymatic scaffolding protein Raptor, the inhibitory PRAS40, which itself is inhibited by Akt, having in total six protein components. 18 mTORC1 plays a central role in controlling cell growth, protein synthesis, lipogenesis, energy metabolism, and autophagy, whereas mTORC2 facilitates cell survival and cytoskeletal organization. 19 Activity of both complexes is modulated by growth factors, and mTORC1 is additionally affected by cellular energy status, oxygen, and nutrients, such as glucose and amino acids.14,19–24 Figure 1 shows a simplified representation of major mTORC1 signaling pathways. Under the scope of this review, only the mTORC1 signaling is discussed in more detail.
Figure 1.
Graphical representation of neuronal mTORC1 signaling. Presented are the key elements that regulate mTORC1 signaling and play a role in depression pathophysiology mediated by impaired synaptogenesis. The crucial inputs regulating mTORC1 activity include amino acids, growth factors, stress, energy and oxygen status, as well as several neurotransmitters and their receptors. A few targets of AD are presented in a simplified form. The green pointed arrowheads indicate activation and the red flat indicate inhibition. mTORC1-related biomarkers, upstream of, close relative to (mTORC1-proximal), farther downstream of the mTORC1 (mTORC1-distal) are indicated in orange, green, and blue, respectively. For all abbreviations, see list of abbreviations.
Figure adapted from previous studies.10,13,14,19–24 The figure was created with Biorender.com.
List of abbreviations
| Abbreviation | Definition |
|---|---|
| 4EBP1 | Eukaryotic initiation factor 4E binding protein |
| 5-HT | Serotonin |
| AD | Antidepressant drug |
| Akt | Protein kinase B (PKB) |
| AMPAR | α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor |
| AMPK | AMP-activated protein kinase |
| Bax | Bcl-2-associated X protein |
| Bcl2 | B-cell lymphoma 2 |
| BDNF | Brain-derived neurotrophic factor |
| CNS | Central nervous system |
| CREB | Cyclic adenosine monophosphate response element-binding protein |
| CSF | Cerebrospinal fluid |
| CUS | Chronic unpredictable stress |
| Deptor | DEP-domain containing mTOR-interacting protein |
| DSM 5 | Diagnostic and Statistical Manual of Mental Disorders, 5th edition |
| eEF2K | Eukaryotic elongation factor-2 kinase |
| eIF4B | Eukaryotic translation initiation factor 4B |
| eIF4E | Eukaryotic translation initiation factor 4E |
| ERK | Extracellular signal-regulated kinase |
| FKBP12 | FK506-binding protein of 12 kDa |
| GABA | Gamma aminobutyric acid |
| GATOR | GAP activity toward Rags |
| GluN2B | Glutamate receptor subunit epsilon-2 |
| GluR1 | Glutamate receptor subunit 1 |
| GSK-3β | Glycogen synthase kinase-3 |
| HAMD | Hamilton Rating Scale for Depression |
| HIP | Hippocampus |
| HPA-axis | Hypothalamic-pituitary-adrenal axis |
| IDS-SR | Inventory of Depressive Symptomatology |
| IGF | Insulin-like growth factor |
| IRS | Insulin receptor substrate-1 |
| LKB1 | Liver Kinase B1 |
| MADRS | Montgomery–Åsberg Depression Rating Scale |
| MDD | Major depressive disorder |
| mLST8 | Mammalian lethal with Sec13 protein 8 |
| MOA | Mechanism of action |
| mTOR | Mammalian target of rapamycin |
| mTORC1/2 | Mammalian target of rapamycin complex 1/complex 2 |
| NMDAR | N-methyl-D-aspartate receptor |
| p70S6K | Ribosomal protein S6 kinase |
| p75NTR | To neurotrophin receptor P75 |
| PBMC | Peripheral blood mononuclear cell |
| PDCD4 | Programmed cell death 4 protein |
| PFC | Prefrontal cortex |
| PI3K | Phosphatidylinositol-3-kinase |
| PRAS40 | Proline-rich Akt substrate of 40 kDa |
| PSD95 | Postsynaptic density protein 95 |
| Rag | Ras-related GTPases |
| Raptor | Regulatory-associated protein of TOR |
| REDD1 | Regulated in development and DNA damage responses 1 |
| RSK1 | Ribosomal S6 kinase |
| S6 | Ribosomal protein S6 |
| SKAR | S6K1 aly/REF-like target |
| SNP | Single-nucleotide polymorphism |
| SNRI | Serotonin-norepinephrine re-uptake inhibitor |
| SSRI | Selective serotonin re-uptake inhibitor |
| TCA | Tricyclic antidepressant |
| TrkB | Tropomyosin-related kinase B receptor |
| TSC1/2 | Tuberous sclerosis complexes 1 and 2 |
| Tti1/Tel2 | TELO2 Interacting Protein 1 |
| VDCC | Voltage-gated calcium channel |
mTORC1 is of crucial importance in various CNS-related physiological processes, where it is primarily responsible for the regulation of neuronal development, neurogenesis, and synaptic plasticity.10,25–27 Synaptic plasticity is an adaptive process that regulates synaptic strength, numbers, and density by which neurons and circuits change their excitability and connectivity. Such processes help to adjust to developmental changes, such as aging and environmental stimuli, for example, learning and stress, which influence thoughts, mood, and behavior. 28 It is hypothesized that chronic stress-related mood disorders such as (certain subtypes of) MDD may be associated with loss of synaptic plasticity, which compromises neuronal function.26,29,30 The number of synapses and the cell body size have been found to be significantly decreased postmortem in the prefrontal cortex (PFC) of MDD patients. 31 Such structural neuronal changes are believed to negatively influence modulatory effects and functional connectivity within and between neuro-circuits involved in the physiological regulation of emotion, such as the PFC and subcortical structures, hypothalamus, amygdala, and hippocampus (HIP).32,33 Alongside morphological changes, PFC of postmortem MDD patients also exhibits a significant reduction in expression of mTORC1-dependent translation initiation factors; mTOR, p70S6K, and eIF4B, which may be involved in the regulation of brain function and pathophysiology of MDD.27,34
Animal models of chronic stress that exhibit depressive-like behavior also show neuronal atrophy in the PFC and HIP as a result of reduced expression levels of synaptic proteins.14,22,35 Studies suggest that these changes are associated with stress-induced prolonged excessive extracellular glutamate, which causes excitotoxicity, reduction of spine density, and synaptic strength and overall dendritic atrophy within the PFC.36,37 Moreover, direct inhibition of mTORC1 through overexpression of REDD1 in rodents can induce depressive-like symptoms without exposure to stress. 22 Similar observations are found in MDD patients. Changes in postmortem brain tissue morphology co-occur with decreased levels of phosphorylated (phospho) mTORC1-associated molecules, such as mTOR, p70S6K, and ribosomal protein S6.13,14,22,38,39
These findings indicate a strong connection between stress-related morphological changes in the brain, depressive-like behavior, and reduction of mTORC1 activity. Therefore, mTORC1 signaling may be a putative (indirect) target for monitoring ADs evaluated in a variety of mTORC1-related biomarkers.
The role of mTORC1 in the MOA of novel ADs
Recent pre-clinical discoveries, in the search for novel therapeutic targets, indicate that mTORC1 plays a crucial role in the rapid onset of antidepressant action. One of the emerging rapid-acting ADs, with a MOA relying on mTORC1, is the non-competitive NMDAR antagonist ketamine. 40 Through inhibition of both presynaptic and postsynaptic NMDARs, ketamine blocks the spontaneous activity of GABAergic interneurons, which leads to an acute glutamate release and its binding to pro-synaptogenic AMPARs. Especially, the GluN2B subunit of NMDARs on GABAergic interneurons is proposed to be essential for the rapid antidepressant actions of ketamine. 41 As depicted in Figure 1, this mechanism leads to a subsequent downstream mTORC1 activation, triggering synaptogenesis which contributes to synaptic plasticity resulting in an antidepressant effect.10,26 mTORC1-dependent effect of several investigational ADs with a fast onset of action, including ketamine, has been reported in multiple pre-clinical studies. Table 1 summarizes mTORC1-related molecules altered by chronic stress exposure in pre-clinical studies and the reversed effects after pharmacological treatment. In all cases, the investigational ADs reversed the stress-induced depressive-like behavior.
Table 1.
Potential mTORC1-related biomarkers in animal model of MDD after pharmacological treatment. Summarized are mTORC1-related biomarkers, which are close relative to mTORC1 (mTORC1-proximal), farther downstream to mTORC1 (mTORC1-distal), and upstream to mTORC1. Presented data are based on pre-clinical studies in animals subjected to chronic stress paradigms in which the pharmacological treatment deemed successful, unless stated otherwise.
| References | Biomarker (time after dosing) | |||||
|---|---|---|---|---|---|---|
| mTORC1-proximal | mTORC1-distal | Upstream | AD | Rapid-acting | Drug class/target | |
| Kato and colleagues 13 | mTOR (ph, 1 h) | PSD95 (pr, 24 h) | Ketamine | Yes | NMDA receptor antagonist | |
| p70S6K (ph, 1 h) | GluR1 (pr, 24 h) | |||||
| 4EBP1 (ph, 1 h) | ||||||
| Pazini and colleagues 42 | mTOR (ph, 1 h) | PSD95 (pr, 30 min) | Ketamine | Yes | NMDA receptor antagonist | |
| p70S6K (ph, 1 h) | BDNF (pr) | |||||
| Pazini and colleagues 43 | PSD95 (pr, 1 h) | Ketamine | Yes | NMDA receptor antagonist | ||
| BDNF (pr, 1 h) | ||||||
| Fraga and colleagues 44 | p70S6K (ph, 1 h) | Ketamine | Yes | NMDA receptor antagonist | ||
| Li and colleagues 14 | mTOR (ph, 30 min) | PSD95 (pr, 2 h) | Akt (ph, 1 h) | Ketamine | Yes | NMDA receptor antagonist |
| p70S6K (ph, 30 min) | GluR1 (pr, 2 h) | ERK (ph, 1 h) | ||||
| 4EBP1 (ph, 30 min) | ||||||
| Li and colleagues 35 | PSD95 (pr, 2 days) | Ketamine | Yes | NMDA receptor antagonist | ||
| GluR1 (pr, 2 days) | ||||||
| Liu and colleagues 15 | mTOR (ph, 1 h) | Akt (ph, 1 h) | Ketamine | Yes | NMDA receptor antagonist | |
| p70S6K (ph, 1 h) | ERK (ph, 1 h) | |||||
| GSK-3β (ph, 1 h) | ||||||
| Fukumoto and colleagues 12 | mTOR (ph, 30 min) | Ketamine | Yes | NMDA receptor antagonist | ||
| Tang and colleagues 39 | p70S6K (ph, 2 days) | PSD95 (pr, 2 days) | Akt (ph, 2 days) | Ketamine | Yes | NMDA receptor antagonist |
| 4EBP1 (ph, 2 days) | GluR1 (pr, 2 days) | ERK (ph, 2 days) | ||||
| Liu and colleagues 45 | PSD95 (pr, 2 days) | Ketamine | Yes | NMDA receptor antagonist | ||
| BDNF (pr, 2 days) | ||||||
| Bcl2/Bax (pr, 2 days) | ||||||
| Zhou and colleagues 46 * | mTOR (ph, 30 min) | BDNF (pr, 30 min) | Ketamine | Yes | NMDA receptor antagonist | |
| Yang and colleagues 47 | mTOR (ph, 30 min) | BDNF (pr, 30 min) | Ketamine | Yes | NMDA receptor antagonist | |
| Beurel and colleagues 48 | GSK-3β (ph, 30 min) | Ketamine | Yes | NMDA receptor antagonist | ||
| Autry and colleagues 49 | eEF2K (ph, 30 min) | BDNF (pr, 30 min) | Ketamine | Yes | NMDA receptor antagonist | |
| Silva Pereira and colleagues 50 | BDNF (mRNA, 2 h) | Ketamine | Yes | NMDA receptor antagonist | ||
| Ju and colleagues 51 | BDNF (meth DNA, 23 days) | Ketamine | No | NMDA receptor antagonist | ||
| Pazini and colleagues 42 , Pazini and colleagues 43 | mTOR (ph, 1 h) | PSD95 (pr, 1 h) | Creatine | Yes | Ergogenic guanidine-like compound | |
| p70S6K (ph, 1 h) | BDNF (pr, 1 h) | |||||
| Kato and colleagues 13 | mTOR (ph, 1 h) | PSD95 (pr, 24 h) | NV-5138 | Yes | Leucine analog | |
| p70S6K (ph, 1 h) | GluR1 (pr, 24 h) | |||||
| 4EBP1 (ph, 1 h) | ||||||
| Li and colleagues 14 | mTOR (ph, 1 h) | PSD95 (pr, 6 h) | Akt (ph, 1 h) | Ro 25-6981 | Yes | NMDA receptor NR2B-subunit antagonist |
| p70S6K (ph, 1 h) | GluR1 (pr, 6 h) | ERK (ph, 1 h) | ||||
| 4EBP1 (ph, 1 h) | ||||||
| Fukumoto and colleagues 12 | mTOR (ph, 30 min) | BDNF** (pr, 1 h) | ERK** (ph, 1 h) | (2R,6R)-HNK | Yes | Ketamine metabolite |
| Voleti and colleagues 52 | mTOR (ph, 1 h) | Akt (ph, 1 h) | Scopolamine | Yes | Muscarinic receptor antagonists | |
| p70S6K (ph, 1 h) | ||||||
| Voleti and colleagues 52 | mTOR (ph, 1 h) | Akt (ph, 1 h) | Telenzepine | Yes | Muscarinic receptor antagonists | |
| p70S6K (ph, 2 h) | ERK (ph, 1 h) | |||||
| Fogaça and colleagues 11 | p70S6K (ph, 24 h) | PSD95 (pr, 24 h) | ERK** (ph, 1 h) | d-methadone | Yes | Opioid |
| GluR1 (pr, 24 h) | ||||||
| BDNF** (pr, 1 h) | ||||||
| Autry and colleagues 49 | eEF2K (ph, 30 min) | PSD95 (pr, 30 min) | MK-801/dizocilpine | Yes | NMDA receptor antagonist | |
| BDNF (pr, 30 min) | ||||||
| Suzuki and colleagues 53 | mTOR ** (ph, 1 h) | BDNF** (pr, 1 h) | Akt** (ph, 1 h) | TAK-137 | Yes | AMPA receptor potentiator |
| p70S6K ** (ph, 1 h) | ERK** (ph, 1 h) | |||||
Pr, protein; ph, phosphorylated protein; meth DNA, gene methylation; min, minute; h, hour; d, day; w, week. For all other abbreviations, see list of abbreviations.
Animals not exposed to stress.
Biomarker measured in cortical cell culture.
In the PFC of animal models, acute ketamine treatment was able to reverse the chronic stress-induced deficits in phospho-mTOR, p70S6K, and 4E-BP1, as well as the expression of PSD95 and glutamate/AMPA receptor subunit, GluR1, responsible for synaptic plasticity.12–15,22,35,39,42 A similar effect on phospho-p70S6K was observed in the HIP alongside an increased dendritic spine density in the dentate gyrus. 44 Ketamine could also lower the level of phospho-eEF2K, which inhibits translation elongation. 49 When administered 1 week prior a corticosterone treatment, ketamine prevented corticosterone-induced deficits of PSD95 and GluR1 in the HIP. 54 The mTORC1-dependent action of ketamine was also demonstrated after pretreatment with a selective mTORC1 inhibitor, rapamycin, which blocked any effect of ketamine on the levels of phospho-mTOR, 4E-BP1, PSD95, and GluR1 as well as on animal behavior, compared with a non-pretreated group.35,55
Besides the rapid increase of phospho-mTOR and PSD95, a single dose of ketamine led to an increase of brain-derived neurotrophic factor (BDNF) in the PFC and the HIP.43,45–47,49,50 Interestingly, this effect was abolished by rapamycin. 43 A long-term ketamine treatment led to less methylation of the BDNF gene in the PFC, which increased its translation and BDNF protein levels. 51
In contrast to ketamine, as a rapidly acting AD, conventional ADs such as sertraline require chronic administration to decrease depressive-like behavior that corresponds to increased BDNF in rodent PFC.56,57 Chronic antidepressant treatment activates a transcription factor CREB which regulates transcription of BDNF. 58
BDNF is essential for neuroplasticity and brain development. It is initially transcribed into two forms, proBDNF and mature BDNF, which activate different processes. proBDNF, expressed constitutively in low levels, binds to neurotrophin receptor P75 (p75NTR) and leads to disrupted plasticity, whereas mature BDNF undergoes neuronal activity dependent release and binds to TrkB. Therewith, mature BDNF positively regulates translation of i.a. synaptic proteins through activation of the PI3K–Akt pathway, followed by activation of mTORC1, as shown in Figure 1. 10 Moreover, proBDNF contains a common single-nucleotide polymorphism (SNP), Val66Met, which hinders its processing to the mature form. A summary of the role of BDNF in the pathophysiology and treatment of MDD is provided in a recent review. 27 As demonstrated by several studies, inhibition of BDNF by infusion of BDNF neutralizing antibodies or a knock-in of Val66Met blocks any antidepressant and molecular effect of investigational ADs.12,13,59 Taken together, a rapid elevation of mature BDNF upon fast-acting AD administration can be considered a potential biomarker signifying mTORC1 activity, given its dependence on mTORC1 and its co-occurrence with other mTORC1-related biomarkers.
In addition, an increase of apoptosis, indicated by i.a. a lower ratio between the pro-apoptotic B-cell lymphoma 2 (Bcl2) and anti-apoptotic Bcl-2-associated X protein (Bax) in the HIP of rats subjected to chronic unpredictable stress (CUS), was alleviated 24 h after ketamine treatment. 45 Considering that energy stress regulates apoptosis through mTORC1 (Figure 1), and direct inhibition of mTORC1 leads to altered neuronal cell death in developing mice, Bcl2: Bax ratio may also be related to mTORC1-mediated action of ketamine.60,61
Stress-induced deficits of several mTORC1-related molecules were also reversed by different investigational drugs that exhibit rapid antidepressant-like effects in pre-clinical animal behavior studies. Similar to ketamine, a leucine analog (NV-5138) and an NMDAR NR2B-subunit antagonist (Ro 25-6981), both increased phospho-mTOR, p70S6K, and 4E-BP1 and expression of PSD95 and GluR1.13,14 Interestingly, the increase in PSD95 and GluR1 was observed with a 5–23 h delay compared with mTOR, p70S6K, and 4E-BP1, confirming that PSD95 and GluR1 are modulated by mTORC1, as depicted in Figure 1.13,14 A similar result was observed after creatine, an ergogenic guanidine-like compound, which increased phospho-mTOR and p70S6K and PSD95 and BDNF protein levels in the HIP.42,43 In a different pre-clinical study, treatment with muscarinic receptor antagonists, either scopolamine or telenzepine also rapidly increased phospho-mTOR and p70S6K. 52 Treatment with d-methadone, an opioid, increased p70S6K, PSD95, and GluR1 in PFC and BDNF levels in in vitro cortical cells. 11 A non-competitive NMDAR antagonist dizocilpine, also known as MK-801, decreased phospho-eEF2K and increased BDNF levels in the same fashion as ketamine in an animal model. 49 A ketamine metabolite, (2R,6R)-HNK, also increased phospho-mTOR in rat PFC and BDNF levels in vitro. 12 Moreover, an AMPAR potentiator (TAK-137) rapidly increased phospho-mTOR and p70S6K and BDNF levels in vitro. 53
Interestingly, the aforementioned investigational ADs targeting mTORC1 also lead to a rapid increase in phosphorylated molecules upstream of the mTORC1, Akt, and ERK in the PFC of animal models and in in vitro experiments.11,12,14,15,39,52,53 The activity of the mTORC1-inhibiting GSK-3β was also found to be decreased after ketamine treatment.15,48 These observations suggest that the signaling pathways linked to mTORC1 are also worth investigating alongside the mTORC1 to better understand the MOA of the novel ADs.
Conclusively, a significant body of experimental evidence provides support for the involvement of mTORC1 in the MOA of investigational rapid-acting ADs. Monitoring of mTORC1 activity through the evaluation of molecular biomarkers could, therefore, provide a helpful insight into novel ADs of which the MOA is not fully elucidated.
CNS-based mTOR-related MDD biomarkers
Postmortem human brain
Animal models have enabled us to thoroughly study the molecular effects of investigational CNS drugs at the cellular level directly in dissected brain tissue combined with behavioral experiments. Whereas evidence for the role of mTORC1 in the activity of ADs can be easily obtained from animals, the same kind of mechanistic data are much more difficult to obtain from humans, where we are limited to postmortem brains and the CSF. Therefore, to date, there are merely a handful of clinical studies focused specifically on mTORC1-related molecules in relation to MDD. Table 2 contains a summary of postmortem studies that indicate differences in levels of mTORC1-related molecules between MDD patients and controls.
Table 2.
mTORC1-related biomarkers detected centrally in postmortem human brain. Summarized are the measured levels of mTORC1 components in postmortem brain tissue of depressive patients.
| References | Biomarker | Tissue | Baseline biomarker | Sample size (m/f) | AD | p * | |
|---|---|---|---|---|---|---|---|
| C/P | C | P | |||||
| Jernigan and colleagues 34 | mTOR | PFC | 0.616 ± 0.055/0.42 ± 0.06 a | 10/2 | 10/2 | Yes/ser, flu, par, amit | 0.0017 |
| Jernigan and colleagues 34 | P70S6K | PFC | 0.54 ± 0.046/0.37 ± 0.04 a | 10/2 | 10/2 | Yes/ser, flu, par, amit | 0.001 |
| Jernigan and colleagues 34 | eIF4B | PFC | 0.78 ± 0.14/0.26 ± 0.10 a | 10/2 | 10/2 | Yes/ser, flu, par, amit | 0.0039 |
| Jernigan and colleagues 34 | Phospho-eIF4B | PFC | 2.18 ± 0.36/1.18 ± 0.32 a | 10/2 | 10/2 | Yes/ser, flu, par, amit | 0.0004 |
| Feyissa and colleagues 62 | PSD95 | PFC | 0.91 ± 0.15/0.54 ± 0.1 a | 11/3 | 11/3 | Yes/ser, flu, par, amit | <0.05 |
| Karege and colleagues 63 | GSK-3β | PFC | 7.6 ± 2.3/11.6 ± 2.5 b | 5/5 | 5/5 | NR | 0.002 |
| Karege and colleagues 63 | Akt | PFC | 27.3 ± 9.0/18.8 ± 5.1 b | 5/5 | 5/5 | NR | 0.002 |
| Martins-de-Souza and colleagues 64 | Akt | Brain | NR | 8/4 | 13/11 | NR | 0.02 |
| Ota and colleagues 22 | REDD1 | PFC | ~1/~1.7 c | NR | NR | NR | 0.012 |
| Dwivedi and colleagues 65 | BDNF | PFC | 1.61 ± 0.39/0.94 ± 0.22 a | 17/4 | 11 | NR | <0.001 |
| Dwivedi and colleagues 65 | BDNF | HIP | 1.71 ± 0.44/1.04 ± 0.20 a | 17/4 | 11 | NR | <0.001 |
| Karege and colleagues 66 | BDNF | PFC | 17.5 ± 3.0/13.8 ± 2.6 d | 8 | 7 | DF | <0.002 |
| Karege and colleagues 66 | BDNF | PFC | 17.5 ± 3.0/17.9 ± 2.9 d | 8 | 7 | Yes/NR | 0.36 NS |
| Karege and colleagues 66 | BDNF | HIP | 24.5 ± 3.6/17.7 ± 2.9 d | 8 | 7 | DF | <0.002 |
| Karege and colleagues 66 | BDNF | HIP | 24.5 ± 3.6/23.3 ± 2.2 d | 8 | 7 | Yes/NR | 0.36 NS |
| Chen and colleagues 67 | BDNF | HIP | ~20/~35 e | 153 | 15 | Yes/NR | 0.09 NS |
| Dwivedi and colleagues 65 | BDNF mRNA | PFC | 606.8 ± 139.5/352.2 ± 133.9 f | 17/4 | 11 | NR | <0.001 |
| Dwivedi and colleagues 65 | BDNF mRNA | HIP | 2049.4 ± 642.8/1075.1 ± 200.6 f | 17/4 | 11 | NR | <0.001 |
C, healthy control; P, patient; m, male; f, female; DF, drug free; NR, not reported; NS, not significant; amit, amitriptyline; ser, sertraline; par, paroxetine; flu, fluoxetine. For all other abbreviations, see list of abbreviations.
Protein levels normalized to actin.
Kinase activity expressed in pmol/min/mg of brain tissue.
Fold change relative to control.
Amount of protein expressed in ng/g brain tissue.
Protein immunoreactivity.
mRNA level relative to neuron-specific enolase (NSE) mRNA.
All presented p values are statistically significant unless indicated otherwise.
One prominent postmortem study has reported a significant reduction of mTOR, p70S6K, eIF4B, and phospho-eIF4B in the PFC of MDD patients. 34 In the same group of deceased patients, also the level of synaptic protein PSD95 was lower compared with healthy controls. 62 Moreover, BDNF was lowered in MDD as reported by three independent studies.65–67 BDNF protein level was lowered in the PFC and HIP of drug-free MDD patients relative to healthy control. However, there was no significant difference from patients who received (classic) antidepressant treatment. 66 A similar result was observed in a different study, where a lower BDNF was measured in the HIP of unmedicated MDD patients, compared with medicated ones. 67 BDNF protein, as well as mRNA expression, was also reduced in PFC and HIP of patients in a study, where, unfortunately, the drug type was not reported. 65 The activity of Akt was found to be lowered and the inhibitory GSK-3β and REDD1 increased in depressive patients.22,63,64
As can be seen in Table 2, the common problems with the presented studies are (1) the very narrow window between the biomarker levels measured in patients and controls, and (2) the relatively small sample sizes and not equal male: female ratios due to unavailability of donor tissue. Also, medication use within a study varies between patients, and some studies do not report such information. Therefore, it is not possible to differentiate between true disease-related and drug-related effects.
CSF
Despite that the CSF provides the closest approximation of the CNS and can be obtained from living subjects, to date, no attempts have been made for a systematic evaluation of mTORC1-related molecules in this matrix. The only biomarker related to mTORC1-signaling that has been researched is BDNF, however with inconsistent outcomes. Levels of CSF BDNF in MDD patients were found to be notably lower than in healthy controls. Citalopram treatment led to a reduction in MADRS and HAMD scores, coinciding with trends of increasing CSF BDNF. However, the presented control group was not matched to the patients and the observed changes were not statistically significant. 68 Stronger evidence was presented in a different study, which reported lower BDNF levels in MDD patients and a significant correlation between BDNF levels and severity of depression. 69 In two other studies, no significant differences were found in CSF nor plasma BDNF levels between depressed and control subjects, and no effect of ADs was observed.70,71 The ratio of the BDNF pro-peptide to the total protein level in CSF of male MDD patients was found to be significantly lower than in male controls; however, the mature BDNF levels alone were below the limit of detection. This difference was not found in female subjects, which may be explained by the difference in gene methylation between sexes.72–74
Overall, notwithstanding the limitations, these findings suggest that the activity of mTORC1 in the CNS is impaired in MDD patients. Therefore, it is safe to assume that restoration of mTORC1 activity, upon an AD treatment, signified by relevant biomarkers, could serve as an indication of its molecular MOA.
Peripheral mTOR-related MDD biomarkers
Peripheral blood
Reminiscent to the CNS, a limited number of publications report peripheral alterations in the expression or activity of mTORC1-related molecules in MDD. Table 3 presents studies that report alterations in peripheral levels of mTORC1-related molecules after pharmacological treatment.
Table 3.
mTORC1-related biomarkers in clinical studies. Summarized are mTORC1-related biomarkers, which are close relative to mTORC1 (mTORC1-proximal), farther downstream the mTORC1 (mTORC1-distal), and upstream of mTORC1. Presented data are based on clinical studies in MDD in which the pharmacological treatment deemed successful and resulted in fast mood improvement, unless stated otherwise..
| References | Treatment | Classic AD | Biomarker (time after dosing) | Matrix | Baseline Biomarker | Treatment response | Sample size (m/f) | p | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mTORC1-proximal | mTORC1-distal | Upstream | C/P | Biomarker | Mood | C | P | |||||
| Denk and colleagues 75 | ket | No | mTOR (ph, 100 min) | PBMCs | NA/100% | 500%↑ | MADRS, 4↓ | NA | 1 (f) | NA | ||
| BDI, 3↓ | ||||||||||||
| Haile and colleagues 76 | ket | No | BDNF (pr, 240 min) | Plasma | ~2.5 ng/ml | ~3.5 ng/ml↑ | MADRS, ~12↓ | NA | 15 | 0.03 | ||
| Allen and colleagues 77 | ket | No | BDNF (pr, 1 week) | Serum | ~19/~10 ng/ml | ~15 ng/ml↑ | HAMD, ~10 ↓ | 10/10 | 9/8 | <0.05 a | ||
| <0.001b,c | ||||||||||||
| <0.03 d | ||||||||||||
| Szuster-Ciesielska and colleagues 78 | NA | NA | Apoptosis (Bcl-2/Bax pr) | PBMCs | 0.969 ± 0.05/0.746 ± 0.1 | NA | NA | 15/15 | 14/15 | <0.05b,* | ||
| Eilat and colleagues 79 | NA | NA | Apoptosis (apoptotic cells) | PBMCs | 0.5%–24.85%/1%–27% | NA | NA | 6/1 | 6/1 | <0.05 b | ||
| Amidfar and colleagues 80 | NA | NA | Apoptosis (Bcl2/Bax mRNA) | PBMCs | 1/0.776 ± 0.08; 0.36 ± 0.04 | NA | NA | 15/15 | 13/17; 7/236 | <0.001 e | ||
| <0.001 b | ||||||||||||
PBMC, peripheral blood mononuclear cell; ph, phosphorylated protein; pr, protein; mRNA, gene expression; m, male; f, female; C, control group; P, patient group; V, measured biomarker value; Dep, depression scale score; NA, not applicable; NR, not reported; ket, ketamine; mid, midazolam; escita, escitalopram; cita, citalopram; ser, sertraline; par, paroxetine; ven, venlafaxine; desven, desvenlafaxine; flu, fluoxetine; mir, mirtazapine; clom, clomipramine; traz, trazodone; ago, agomelatine; benzo, benzodiazepines. For all other abbreviations, see list of abbreviations.
~Approximation.
↓Decrease compared with previous state.
↑Increase compared with previous state.
Difference from (baseline) biomarker.
Difference from controls.
Difference from (baseline) mood/depression score.
Correlation between biomarker and mood.
Difference from other patient subgroup.
Measurement performed once.
In a study involving a treatment-resistant depression patient, treatment with ketamine was found to cause an acute increase in phospho-mTOR in peripheral blood mononuclear cell (PBMCs). This change coincided with a decrease in MADRS and HAMD scores. 75 However, this observation should be interpreted with caution as this study was conducted on only one patient, besides lacking a healthy control. 75 Another study, performed in an adequate number of subjects, supports part of previous outcomes by showing increased phospho-GSK-3β in platelet-rich plasma of MDD patients compared with controls. In the same study, the phospho-GSK-3β decreased after monoaminergic AD treatment and correlated with lowered HAMD scores. 81
Several authors showed evidence of accelerated apoptosis in PBMCs of patients with mood disorders, which may be triggered by an impaired activity of mTORC1. Indicators of apoptosis such as elevated expression levels of several cell death–regulating proteins such as Bax in T-lymphocytes and a higher release of reactive oxygen species have been reported in depressed patients relative to healthy controls.78,79 Therewith, the Bcl2: Bax ratio was significantly decreased in PBMCs and specifically CD14+ leukocytes of depressed patients.78,80 Combined with the findings in animal models of chronic stress, where the (mTORC1-activating) ketamine increased the Bcl2: Bax ratio, these observations imply that Bcl2: Bax ratio could serve as an indicator of mTORC1 activity. 45
Despite the lack of well-grounded evidence, the available limited clinical findings suggest that alterations in mTORC1-related signaling can be detected in the periphery and especially in the PBMCs.
BDNF
Being closely related to the regulation of brain function and morphology, BDNF expression has been extensively explored in MDD animal studies. Likewise, numerous attempts have been made to use peripheral BDNF either as a complementary diagnostic biomarker of MDD or to monitor the pharmacological effects of ADs in humans. Due to the positive association to mTORC1 signaling, as described in the context of pre-clinical studies, BDNF could be considered as a marker of mTORC1 activity. Supplementary Table S1 summarizes the evidence of peripheral detection of BDNF found in the literature.
Serum BDNF
The levels of serum BDNF in subjects suffering from MDD were repeatedly reported to be lower compared with healthy controls.29,73,77,82–94 However, several studies did not demonstrate any differences.85,95–98
As shown in Table 4, in the studies in which the effect of ADs was tested, the serum BDNF increased alongside a decrease in HAMD or MADRS scores after treatment with several different classic ADs.29,85,87,89,90,99–101 Also, ketamine (see Table 3) caused an increase of serum BDNF and an amelioration of symptoms in MDD patients. 77 Following the pre-clinical observations, the elevation of serum BDNF required chronic dosing of several weeks with classic ADs, in contrast to ketamine after which BDNF was elevated in serum within 1 week after dosing. 77 As mentioned before, this is accounted by the difference between the relatively fast mTORC1-related BDNF elevation and the slower CREB-mediated elevation. 58
Table 4.
BDNF as biomarker in clinical studies. Presented data are based on clinical studies in MDD in which chronic pharmacological treatment with classical ADs deemed successful and resulted in slow mood improvement..
| References | Treatment | Classic AD | BDNF (time after dosing) | Matrix | Baseline biomarker | Treatment response | Sample size (m/f) | p | ||
|---|---|---|---|---|---|---|---|---|---|---|
| C/P | Biomarker | Mood | C | P | ||||||
| Pláteník and colleagues 81 | Escita, ser, par, flu, mir, ven, traz, ago, benzo | Yes | BDNF (pr, 28 ± 15 days*) | Platelet-rich plasma | 2.43/1.62 arbitrary unit | NR↓ (1) | HAMD, 7.6 ± 9.0↓ | 30/66 | 14/51 | <0.05a,b,C |
| Ghosh and colleagues 102 | Flu, desven | Yes | BDNF (pr, 12 weeks*) | Plasma | 775.32 ± 30.38 pg/ml | 850.3 ± 24.92 pg/ml↑ | HAMD, 12.2 ± 4.58; 9.24 ± 3.98↓ | NA | 14/163 | <0.05d,e |
| Kurita and colleagues 103 | NR | NR | BDNF (pr, 8–12 weeks**) | Plasma | 1827 ± 1340 pg/ml | 2402 ± 1 610; 3158 ± 2033 pg/ml↑ | MADRS, 10.9 ± 5.9; 5.0 ± 2.4↓ | NA | 19/19 | <0.001d,e |
| Wolkowitz and colleagues 87 | Escita, ser | Yes | BDNF (pr, 8 weeks*) | Serum | 20.63 ± 4.81/15.28 ± 3.55 ng/ml | 17.46 ± 3.96 ng/ml↑ | HAMD, 14.5 ± 11.2↓ | 15 (m) | 15 (m) | <0.01 b |
| <0.001 d | ||||||||||
| <0.05 e | ||||||||||
| Gonul and colleagues 29 | Cita, ser, par, ven, flu | Yes | BDNF (pr, 8 weeks*) | Serum | 26.8 ± 9.3/20.8 ± 6.7 ng/ml | 33.3 ± 9.89 ng/ml↑ | HAMD, 8.85 ± 3.15↓ | 6/12 | 7/21 | <0.001d,e |
| 0.015 b | ||||||||||
| Aydemir and colleagues 89 | Escita | Yes | BDNF (pr, 6 weeks*) | Serum | 41.16 ± 15.14/27.68 ± 13.74 ng/ml | 38.57 ± 15.30 ng/ml↑ | HAMD, 9.31 ± 6.05↓ | 20 (f) | 20 (f) | 0.002 d |
| <0.001 e | ||||||||||
| 0.010 b | ||||||||||
| Gervasoni and colleagues 99 | Par, ven, clom | Yes | BDNF (pr, 13.5 ± 6 weeks*) | Serum | 26.48 ± 3.6/22.68 ± 3.6 ng/ml | 24.48 ± 3.6 ng/ml↑ | NR | 13/13 | 11/15 | 0.0002 b |
| Molendijk and colleagues 104 | SSRI | Yes | BDNF (pr, NR) | Serum | ~9.5 ng/ml/NA | ~10–~11 ng/ml↑ | NA | 149/233 | 279 (m + f) | 0.001 f |
| Katsuki and colleagues 82 | Mir | Yes | BDNF (pr, 4 weeks*) | Serum | ~7 ng/ml | ~12 ng/ml↑ | NR | NA | 28/56 | <0.05d,e |
| Cattaneo and colleagues 90 | Escita | Yes | BDNF (pr, 12 weeks**) | Serum | 40.92 ± 10.05/30.58 ± 9.13 ng/ml | 41.38 ± 10.49 ng/ml↑ | MADRS, 7.23 ± 5.15↓ | 7/16 | 4/17 | <0.05 b |
| 0.777 d,e | ||||||||||
| Cattaneo and colleagues 90 | Escita | Yes | BDNF (mRNA, 12 weeks**) | PBMCs/leukocytes | 1.01 ± 0.22/0.48 ± 0.18 | 1.02 ± 0.15↑ | MADRS, 7.23 ± 5.15↓ | 7/16 | 4/17 | <0.001 |
| 0.040 d | ||||||||||
| Cattaneo and colleagues 105 | Escita | Yes | BDNF (mRNA, 12 weeks*) | PBMCs/leukocytes | 0.95 ± 0.04/0.71 ± 0.01 | 1.10 ± 0.03↑ | NS | 15/19 | 31/43 | <0.0001b,d |
| <0.0001 d | ||||||||||
| Kimata 69 | NA | NA | BDNF (pr) | CSF | ~70/~15 pg/ml | NA | NA | 8/12 | 8/12 | <0.001 b |
| Pålhagen and colleagues 68 | Cita | Yes | BDNF (pr, 12 weeks) | CSF | 201 ± 55.1/66.5 ± 11.2 pg/ml | ~100 pg/ml↑ | HAMD, 12.9 ± 6.4; MADRS, 13.2 ± 5.7↓ | NR | 7/5 | <0.001 e |
| NR d | ||||||||||
| Martinez and colleagues 70 | Ven | Yes | BDNF (pr, 8 weeks) | CSF | 4.86 ± 2.22/4.28 ± 1.88 pg/ml | NR | HAMD, 10.6 ± 4.3↓ | 13/12 | 8/10 | <0.001 e |
| NS | NSb,d | |||||||||
| Pillai and colleagues 71 | NA | NA | BDNF (pr) | CSF | 7.75 ± 3.08/6.57 ± 3.35 pg/ml | NA | NA | 18 | 29 | NS |
| Mizui and colleagues 73 | NA | NA | BDNF (pr) | CSF | Undetectable | NA | NA | 14/13 | 10/8 | NA |
pr, protein; mRNA, gene expression; m, male; f, female; C, control group; P, patient group; V, measured biomarker value; Dep, depression scale score; NA, not applicable; NR, not reported; ket, ketamine; mid, midazolam; escita, escitalopram; cita, citalopram; ser, sertraline; par, paroxetine; ven, venlafaxine; desven, desvenlafaxine; flu, fluoxetine; mir, mirtazapine; clom, clomipramine; traz, trazodone; ago, agomelatine; benzo, benzodiazepines. For all other abbreviations, see list of abbreviations.
Correlation between biomarker and mood.
Difference from controls.
Studied on Alzheimer’s disease patients with and without depression.
Difference from (baseline) biomarker.
Difference from (baseline) mood/depression score.
Difference in biomarker between treated and untreated patients.
~Approximation.
↓Decrease compared with previous state.
↑Increase compared with previous state.
Measurement performed once.
Measurement performed two times.
The inconsistencies in the BDNF-based discrimination between MDD patients and healthy controls are of concern. These can be attributed to the fact that BDNF is ubiquitously expressed and the circulating protein originates from several sources including neurons, as it can pass the blood-brain barrier, as well as platelets.106,107 BDNF in the blood is mainly stored at high levels in platelets that store and release continuously in plasma and after activation due to a traumatic injury or in serum during clotting. 107
Plasma BDNF
Overall, the literature on BDNF levels in plasma shows the same picture as serum BDNF, with a decreased BDNF level in MDD patients compared with healthy controls.94,108–110 Plasma BDNF was also found increased after chronic AD treatment (Table 4), and as fast as 2 h after a single ketamine administration (Table 3).76,102,103 Interestingly, responders to the treatment had a higher baseline plasma BDNF than treatment-resistant patients.
Contradicting results were reported by other studies, in which no differences in plasma BDNF levels were found between MDD patients and controls.71,111 Moreover, in a different study, ketamine reduced the MADRS scores but did not affect plasma BDNF. 112
BDNF in peripheral cells
In line with the observations based on plasma and serum, BDNF (mRNA) expression was found to be significantly decreased in PBMCs of MDD patients, when compared with healthy controls.90,105,113,114 The same was reported for adult as well as pediatric depressed patients. 115 An 8- and 12-week escitalopram treatment normalized the decreased BDNF expression in leukocytes from MDD patients who responded to the treatment.90,105 In a different study, however, despite reduced neurotrophins, such as glial cell line–derived neurotrophic factor, no significant difference in the expression levels of BDNF mRNA was found between patients with MDD and healthy subjects. 116
It is also worth noting that the epigenetic regulation of the BDNF gene may play an important role in depressive disorders. Two independent studies in MDD patients found a significantly enriched methylation of BDNF promoter, which represses gene transcription, along with impaired BDNF expression in PBMCs.114,117 The same correlations between BDNF methylation and prevalence of MDD, bipolar disorder, or schizophrenia were found in multiple other studies.72,74,117–121 Methylation of the BDNF gene was found to be sex-dependent with a higher degree in male subjects in schizophrenia and bipolar disorders.72,74 Moreover, an elevated BDNF methylation was observed in parallel in the PFC and peripheral muscle tissue of deceased BD patients, which correlated with the values measured in PBMCs of living patients. 74
However, it has to be noted that just like the evaluation of depression severity in most studies, the levels of BDNF were measured at only one timepoint after weeks-long antidepressant therapy. (Continued) Because of this, it cannot be determined whether the used therapy has a slow or rapid effect on BDNF. Moreover, levels of BDNF protein in the periphery differ among sexes and are sensitive to circadian rhythm, which is not accounted in most of the studies, considering that the samples are never obtained longitudinally.71,111,122,123 Also, expression of BDNF is regulated by i.a. glucocorticoid receptors, and depends therewith on activity of the hypothalamic-pituitary-adrenal axis (HPA-axis). 124 The discrepancies between different studies may be also explained by an earlier treatment as most of the enrolled patients used ADs in the past. This makes it even more difficult to distinguish between the drug- and disease-related effects.
Importantly, BDNF blood alterations are not MDD-specific as those are also found in several other disorders such as bipolar disorders or schizophrenia. 90 Still, peripheral BDNF can be used to recognize a common BDNF and mTORC1-mediated pathophysiology shared by disorders in which synaptic plasticity and mTORC1-mediated pharmacological effect play an important role.
Summary and discussion
We aimed to identify and summarize potential molecular biomarkers of mTORC1 activity in MDD and to suggest how these could be implemented in future early clinical trials with rapid-acting ADs that target the reversal of stress-related synaptic dysfunction in MDD.
The current interview-based MDD severity rating instruments are prone to bias and are not sufficiently sensitive to the downstream molecular effects of emerging rapid-acting ADs. Therefore, a complementary biomarker-based approach is needed to unravel neurobiological mechanisms underlying different MDD endophenotypes and to link these to the molecular actions of ADs. Pre-clinical and clinical data support the involvement of mTORC1 signaling in the pathophysiology of MDD, as well as being a molecular target of clinically effective monoaminergic ADs. mTORC1-related molecules could, therefore, serve as potential biomarkers of an mTORC1-related endophenotype in MDD on one hand, and pharmacological biomarkers for antidepressant action of rapid-acting ADs specifically on the other hand. Here, we present and clarify the relationship between mTORC1 and MDD and the effect of AD treatment based on the available evidence.
There is abundant evidence of mTORC1-mediated regulation of neuronal development and especially synaptic plasticity.10,25,26 Deficits in synaptic plasticity followed by a decreased number of synapses and cell body size in neuro-circuits involved in the physiological regulation of emotion have been linked to the pathophysiology of MDD. 31 On top of that, postmortem studies in MDD patients show deficits in the expression of several mTORC1-related molecules (see Table 2).22,34,62–65,67,94 Pre-clinical studies on animals exposed to challenges such as CUS confirm those morphological and cell-based findings in humans.13,14,22,35,38,39 Moreover, a multitude of pre-clinical studies demonstrate a clear correlation between antidepressant effects and restoration of the impaired mTORC1 activity in animals after administration of investigational and conventional ADs (see Table 1).12–15,35,39,45–47,49 Together, these findings demonstrate mTORC1 involvement in both the pathophysiology of MDD and the therapeutic effects of not only investigational rapid-acting but also conventional ADs. In vivo quantification of the activity of mTORC1-related molecules could therefore serve as pharmacological biomarkers in clinical AD development.
Based on postmortem studies, mTOR, p70S6K, eIF4B, phospho-eIF4B, PSD95, and BDNF, all lowered in the PFC of MDD patients, could be considered as potential mTORC1-related MDD biomarkers (see Table 2).34,62,65–67 Yet, to be applicable in a clinical trial setting, the biomarkers should be also available in matrixes proximal to the CNS that can be sampled in living subjects, such as CSF.
There is some human evidence indicating that decreased phospho-mTOR in PBMCs has a potential to recognize mTOR-related MDD endophenotypes. 75 Moreover, the occurrence of apoptosis in the PBMCs signified by a lowered Bcl2: Bax ratio could serve the same purpose.78,80 These biomarkers could be also used to monitor the effect of pharmacological treatment in general and rapid-acting antidepressants in particular, provided that the mTORC1 signaling is involved.75,90,105 Obviously, this stresses the need to identify patient subtypes within DSM categories that might benefit from mTORC1 modulation. However, the existing literature does not report any studies focused specifically on mTORC1-related molecules in the CSF, emphasizing the need for such clinical studies.
To date, BDNF is the most prominently researched mTORC1-related molecule with a potential to be used as a peripheral biomarker. However, BDNF levels in peripheral plasma, serum, or PBMCs suffer from high intraindividual variability attributable to many factors such as circadian rhythm, HPA-axis activity, an unidentifiable source of the free circulating protein as well as the possible occurrence of the Val66Met SNP.27,71,90,105–107,111,113–115,117,122–124 Measuring BDNF directly in the periphery without taking into account the factors that contribute to its variability, such as the presence of platelets in serum, is of a low informative value. Therefore, an ex vivo approach is needed.
The findings in the PBMCs suggest that patient- or healthy volunteer (HV)-derived material could be considered as a valuable ex vivo model to study the molecular effect of novel mTORC1-targeting ADs.78,80,90,105,113–115,117 Application of an ex vivo model provides a simplified representation of a complex system, in which any external stimuli, such as HPA-axis activity or circadian rhythm, can be controlled or eliminated. Therefore, in theory, all of the presented biomarkers could be used to study mTORC1 activity ex vivo (see Tables 2 and 3).
We argue that PBMCs may serve as a peripheral model for evaluation of physiological pathways involved in mental pathologies, given the strong physiological resemblance in relevant pathways between circulating immune cells and brain cells.125,126 Also, in a recent drug development program, PBMCs served as a matrix for the evaluation of the pharmacological activity of mTORC1-targeting ADs. 127 Conceptually, the presented mTORC1-related biomarkers (see Tables 2 and 3) can be used to establish an ex vivo challenge model based on HV-derived PBMCs. In such a model, the mechanistic profile of mTORC1 signaling responses to targeted mTORC1 impairment by known inhibitors, such as rapamycin, should be defined. Once established, it can be implemented in early clinical investigation of mTORC1-targeting ADs. Here, the mechanistic profile of mTORC1 signaling responses to such ADs could be defined, following exposure to (CSF-based) pharmacokinetic concentration relevant to in vivo safety studies on HV. Ultimately, ex vivo methodology could contribute to a more precise selection of pharmacologically active dose for early first-in-patient studies.
In conclusion, based on an extensive literature review, we identified several biomarker candidates for monitoring mTORC1 activity in humans. These can be already implemented to study the mechanistic profile of mTORC1 responses to investigational ADs in ex vivo settings. Once validated, these biomarkers may also facilitate both identification of altered mTORC1 signaling in MDD and dynamic pharmacological effects by rapid-acting, novel mTORC1-targeting ADs. From a drug development perspective, such biomarkers can aid selection of ‘mTORC1 impaired’ MDD (sub)populations, to characterize the pharmacodynamics of novel ADs that target mTORC1 on a molecular level, and to ultimately relate pharmacology to behavioral effects in the context of a pathophysiological cascade of events. 128 This approach could be of particular value for early clinical proof-of-pharmacology (safety) and proof-of-mechanism (pharmacodynamic) trials. Here the availability of such biomarkers would facilitate not only an efficient selection of a pharmacodynamically active dose or dosing regimen for proof-of-concept studies but also provide guidance to future efficacy studies in psychiatric patient populations. Herewith, this review is a first step toward the rational development of rapid-acting mTORC1-targeting ADs.
Supplemental Material
Supplemental material, sj-docx-1-tpp-10.1177_20451253211036814 for MTORC1 signaling as a biomarker in major depressive disorder and its pharmacological modulation by novel rapid-acting antidepressants by Tomasz Cholewinski, Diana Pereira, Matthijs Moerland and Gabriel E. Jacobs in Therapeutic Advances in Psychopharmacology
Acknowledgments
We would like to thank Karen Broekhuizen, PhD, who provided medical writing assistance on behalf of the Centre for Human Drug Research, Leiden, The Netherlands.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Tomasz Cholewinski  https://orcid.org/0000-0002-7473-9321
https://orcid.org/0000-0002-7473-9321
Diana Pereira  https://orcid.org/0000-0002-4258-648X
https://orcid.org/0000-0002-4258-648X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tomasz Cholewinski, Centre for Human Drug Research, Leiden, The Netherlands.
Diana Pereira, Centre for Human Drug Research, Leiden, The Netherlands.
Matthijs Moerland, Centre for Human Drug Research, Leiden, The Netherlands.
Gabriel E. Jacobs, Centre for Human Drug Research, Zernikedreef 8, 2333 CL Leiden, The Netherlands.
References
- 1. Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers 2016; 2: 16065. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet 2018; 392: 1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al Shweiki MR, Oeckl P, Steinacker P, et al. Major depressive disorder: insight into candidate cerebrospinal fluid protein biomarkers from proteomics studies. Expert Rev Proteomics 2017; 14: 499–514. [DOI] [PubMed] [Google Scholar]
- 4. Frances AJ, Widiger T. Psychiatric diagnosis: lessons from the DSM-IV past and cautions for the DSM-5 future. Annu Rev Clin Psychol 2012; 8: 109–130. [DOI] [PubMed] [Google Scholar]
- 5. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–389. [DOI] [PubMed] [Google Scholar]
- 6. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rush AJ, Carmody T, Reimitz PE. The Inventory of Depressive Symptomatology (IDS): clinician (IDS-C) and self-report (IDS-SR) ratings of depressive symptoms. Int J Methods Psychiatr Res 2000; 9: 45–59. [Google Scholar]
- 8. Cohen AF, Burggraaf J, van Gerven JM, et al. The use of biomarkers in human pharmacology (Phase I) studies. Annu Rev Pharmacol Toxicol 2015; 55: 55–74. [DOI] [PubMed] [Google Scholar]
- 9. Kennis M, Gerritsen L, van Dalen M, et al. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry 2020; 25: 321–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ignácio ZM, Réus GZ, Arent CO, et al. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol 2016; 82: 1280–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fogaça MV, Fukumoto K, Franklin T, et al. N-Methyl-D-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology 2019; 44: 2230–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukumoto K, Fogaça MV, Liu R-J, et al. Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci USA 2019; 116: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kato T, Pothula S, Liu RJ, et al. Sestrin modulator NV-5138 produces rapid antidepressant effects via direct mTORC1 activation. J Clin Investig 2019; 129: 2542–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li N, Lee B, Liu R-J, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu RJ, Fuchikami M, Dwyer JM, et al. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology 2013; 38: 2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaizuka T, Hara T, Oshiro N, et al. Tti1 and Tel2 are critical factors in mammalian target of Rapamycin complex assembly. J Biol Chem 2010; 285: 20109–20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim D-H, Sarbassov DD, Ali SM, et al. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell 2003; 11: 895–904. [DOI] [PubMed] [Google Scholar]
- 18. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006; 124: 471–484. [DOI] [PubMed] [Google Scholar]
- 19. Laplante M, Sabatini DM. MTOR signaling in growth control and disease. Cell 2012; 149: 274–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol 2014; 24: 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang Y, Kang BN, Tian J, et al. The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation-dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. J Neurosci 2007; 27: 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ota KT, Liu R-J, Voleti B, et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med 2014; 20: 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Page G, Khidir FAL, Pain S, et al. Group I metabotropic glutamate receptors activate the p70S6 kinase via both mammalian target of rapamycin (mTOR) and extracellular signal-regulated kinase (ERK 1/2) signaling pathways in rat striatal and hippocampal synaptoneurosomes. Neurochem Int 2006; 49: 413–421. [DOI] [PubMed] [Google Scholar]
- 24. Sofer A, Lei K, Johannessen CM, et al. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol 2005; 25: 5834–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perluigi M, Di Domenico F, Butterfield DA. mTOR signaling in aging and neurodegeneration: at the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol Dis 2015; 84: 39–49. [DOI] [PubMed] [Google Scholar]
- 26. Abdallah CG, Adams TG, Kelmendi B, et al. Ketamine’s mechanism of action: a path to rapid-acting antidepressants. Depress Anxiety 2016; 33: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Duman RS, Deyama S, Fogaça MV. Role of BDNF in the pathophysiology and treatment of depression: activity-dependent effects distinguish rapid-acting antidepressants. Eur J Neurosci 2021; 53: 126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Citri A, Malenka RC. Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 2008; 33: 18–41. [DOI] [PubMed] [Google Scholar]
- 29. Gonul AS, Akdeniz F, Taneli F, et al. Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci 2005; 255: 381–386. [DOI] [PubMed] [Google Scholar]
- 30. Karolewicz B, Cetin M, Aricioglu F. Beyond the glutamate N-methyl D-aspartate receptor in major depressive disorder: the mTOR signaling pathway/majör depresif bozuklukta glutamat N-metil-D-aspartat reseptörlerinin ötesi: mTOR sinyal yolağı. Klin Psikofarmakol Bülteni/Bull Clin Psychopharmacol 2011; 21: 1–6. [Google Scholar]
- 31. Kang HJ, Voleti B, Hajszan T, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 2012; 18: 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beer JS, Lombardo MV, Bhanji JP. Roles of medial prefrontal cortex and orbitofrontal cortex in self-evaluation. J Cogn Neurosci 2010; 22: 2108–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. MacQueen G, Frodl T. The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 2011; 16: 252–264. [DOI] [PubMed] [Google Scholar]
- 34. Jernigan CS, Goswami DB, Austin MC, et al. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1774–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li N, Liu R-J, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry 2011; 69: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Popoli M, Yan Z, McEwen BS, et al. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat Rev Neurosci 2011; 13: 22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry 2013; 73: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chandran A, Iyo AH, Jernigan CS, et al. Reduced phosphorylation of the mTOR signaling pathway components in the amygdala of rats exposed to chronic stress. Prog Neuropsychopharmacol Biol Psychiatry 2012; 40: 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang J, Xue W, Xia B, et al. Involvement of normalized NMDA receptor and mTOR-related signaling in rapid antidepressant effects of Yueju and ketamine on chronically stressed mice. Sci Rep 2015; 5: 13573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abdallah CG, Averill LA, Krystal JH. Ketamine as a promising prototype for a new generation of rapid-acting antidepressants. Ann NY Acad Sci 2015; 1344: 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gerhard DM, Pothula S, Liu R-J, et al. GABA interneurons are the cellular trigger for Ketamine’s rapid antidepressant actions. J Clin Investig 2020; 130: 1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pazini FL, Cunha MP, Rosa JM, et al. Creatine, similar to ketamine, counteracts depressive-like behavior induced by corticosterone via PI3K/Akt/mTOR pathway. Mol Neurobiol 2016; 53: 6818–6834. [DOI] [PubMed] [Google Scholar]
- 43. Pazini FL, Rosa JM, Camargo A, et al. MTORC1-dependent signaling pathway underlies the rapid effect of creatine and ketamine in the novelty-suppressed feeding test. Chem Biol Interact 2020; 332: 109281. [DOI] [PubMed] [Google Scholar]
- 44. Fraga DB, Costa AP, Olescowicz G, et al. Ascorbic acid presents rapid behavioral and hippocampal synaptic plasticity effects. Prog Neuropsychopharmacol Biol Psychiatry 2020; 96: 109757. [DOI] [PubMed] [Google Scholar]
- 45. Liu W, Wang J, Xie Z, et al. Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology 2016; 233: 405–415. [DOI] [PubMed] [Google Scholar]
- 46. Zhou W, Wang N, Yang C, et al. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry 2014; 29: 419–423. [DOI] [PubMed] [Google Scholar]
- 47. Yang C, Hu YM, Zhou Z-Q, et al. Acute administration of ketamine in rats increases hippocampal BDNF and mTOR levels during forced swimming test. Ups J Med Sci 2013; 118: 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry 2011; 16: 1068–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Autry AE, Adachi M, Nosyreva E, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011; 475: 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Silva Pereira V, Elfving B, Joca SRL, et al. Ketamine and aminoguanidine differentially affect Bdnf and Mtor gene expression in the prefrontal cortex of adult male rats. Eur J Pharmacol 2017; 815: 304–311. [DOI] [PubMed] [Google Scholar]
- 51. Ju LS, Yang JJ, Lei L, et al. The combination of long-term ketamine and extinction training contributes to fear erasure by Bdnf methylation. Front Cell Neurosci 2017; 11: 100–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Voleti B, Navarria A, Liu RJ, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavioral responses. Biol Psychiatry 2013; 74: 742–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Suzuki A, Murakami K, Tajima Y, et al. TAK-137, an AMPA receptor potentiator with little agonistic effect, produces antidepressant-like effect without causing psychotomimetic effects in rats. Pharmacol Biochem Behav 2019; 183: 80–86. [DOI] [PubMed] [Google Scholar]
- 54. Camargo A, Dalmagro AP, de Souza MM, et al. Ketamine, but not guanosine, as a prophylactic agent against corticosterone-induced depressive-like behavior: possible role of long-lasting pro-synaptogenic signaling pathway. Exp Neurol 2020; 334: 113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Abelaira HM, Réus GZ, Ignácio ZM, et al. Effects of ketamine administration on mTOR and reticulum stress signaling pathways in the brain after the infusion of rapamycin into prefrontal cortex. J Psychiatr Res 2017; 87: 81–87. [DOI] [PubMed] [Google Scholar]
- 56. Altar CA, Whitehead RE, Chen R, et al. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry 2003; 54: 703–709. [DOI] [PubMed] [Google Scholar]
- 57. Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 1995; 15: 7539–7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gass P, Riva MA. CREB, neurogenesis and depression. Bioessays 2007; 29: 957–961. [DOI] [PubMed] [Google Scholar]
- 59. Lepack AE, Fuchikami M, Dwyer JM, et al. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 2014; 18: pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cloëtta D, Thomanetz V, Baranek C, et al. Inactivation of mTORC1 in the developing brain causes microcephaly and affects gliogenesis. J Neurosci 2013; 33: 7799–7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shaw RJ, Kosmatka M, Bardeesy N, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci USA 2004; 101: 3329–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Feyissa AM, Zyga A, Stockmeier CA, et al. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry 2008; 33: 70–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Karege F, Perroud N, Burkhardt S, et al. Alteration in kinase activity but not in protein levels of protein kinase B and Glycogen Synthase kinase-3β in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry 2007; 61: 240–245. [DOI] [PubMed] [Google Scholar]
- 64. Martins-de-Souza D, Guest PC, Harris LW, et al. Identification of proteomic signatures associated with depression and psychotic depression in post-mortem brains from major depression patients. Transl Psychiatry 2012; 2: e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dwivedi Y, Rizavi HS, Conley RR, et al. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 2003; 60: 804–815. [DOI] [PubMed] [Google Scholar]
- 66. Karege F, Vaudan G, Schwald M, et al. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Mol Brain Res 2005; 136: 29–37. [DOI] [PubMed] [Google Scholar]
- 67. Chen B, Dowlatshahi D, MacQueen GM, et al. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 2001; 50: 260–265. [DOI] [PubMed] [Google Scholar]
- 68. Pålhagen S, Qi H, Mårtensson B, et al. Monoamines, BDNF, IL-6 and corticosterone in CSF in patients with Parkinson’s disease and major depression. J Neurol 2010; 257: 524–532. [DOI] [PubMed] [Google Scholar]
- 69. Kimata H. Differential modulation of cerebrospinal fluid neurotrophins in patients with atopic dermatitis who attempted suicide. J Clin Psychiatry 2005; 66: 1193–1194. [DOI] [PubMed] [Google Scholar]
- 70. Martinez JM, Garakani A, Yehuda R, et al. Proinflammatory and ‘resiliency’ proteins in the CSF of patients with major depression. Depress Anxiety 2012; 29: 32–38. [DOI] [PubMed] [Google Scholar]
- 71. Pillai A, Bruno D, Sarreal AS, et al. Plasma BDNF levels vary in relation to body weight in females. PLoS ONE 2012; 7: e39358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ikegame T, Bundo M, Sunaga F, et al. DNA methylation analysis of BDNF gene promoters in peripheral blood cells of schizophrenia patients. Neurosci Res 2013; 77: 208–214. [DOI] [PubMed] [Google Scholar]
- 73. Mizui T, Hattori K, Ishiwata S, et al. Cerebrospinal fluid BDNF pro-peptide levels in major depressive disorder and schizophrenia. J Psychiatr Res 2019; 113: 190–198. [DOI] [PubMed] [Google Scholar]
- 74. Stenz L, Zewdie S, Laforge-Escarra T, et al. BDNF promoter I methylation correlates between post-mortem human peripheral and brain tissues. Neurosci Res 2015; 91: 1–7. [DOI] [PubMed] [Google Scholar]
- 75. Denk MC, Rewerts C, Holsboer F, et al. Monitoring ketamine treatment response in a depressed patient via peripheral mammalian target of Rapamycin activation. Am J Psychiatr 2011; 168: 751–752. [DOI] [PubMed] [Google Scholar]
- 76. Haile CN, Murrough JW, Iosifescu DV, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 2014; 17: 331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Allen AP, Naughton M, Dowling J, et al. Serum BDNF as a peripheral biomarker of treatment-resistant depression and the rapid antidepressant response: a comparison of ketamine and ECT. J Affect Disord 2015; 186: 306–311. [DOI] [PubMed] [Google Scholar]
- 78. Szuster-Ciesielska A, Słotwińska M, Stachura A, et al. Accelerated apoptosis of blood leukocytes and oxidative stress in blood of patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32: 686–694. [DOI] [PubMed] [Google Scholar]
- 79. Eilat E, Mendlovic S, Doron A, et al. Increased apoptosis in patients with major depression: a preliminary study. J Immunol 1999; 163: 533–534. [PubMed] [Google Scholar]
- 80. Amidfar M, Kim YK, Scaini G, et al. Evidence for additionally increased apoptosis in the peripheral blood mononuclear cells of major depressive patients with a high risk for suicide. Am J Med Genet B Neuropsychiatr Genet 2018; 177: 388–396. [DOI] [PubMed] [Google Scholar]
- 81. Pláteník J, Fišar Z, Buchal R, et al. GSK3β, CREB, and BDNF in peripheral blood of patients with Alzheimer’s disease and depression. Prog Neuropsychopharmacol Biol Psychiatry 2014; 50: 83–93. [DOI] [PubMed] [Google Scholar]
- 82. Katsuki A, Yoshimura R, Kishi T, et al. Serum levels of brain-derived neurotrophic factor (BDNF), BDNF gene Val66Met polymorphism, or plasma catecholamine metabolites, and response to mirtazapine in Japanese patients with major depressive disorder (MDD). CNS Spectr 2012; 17: 155–163. [DOI] [PubMed] [Google Scholar]
- 83. Naveen GH, Varambally S, Thirthalli J, et al. Serum cortisol and BDNF in patients with major depression-effect of yoga. Int Rev Psychiatry 2016; 28: 273–278. [DOI] [PubMed] [Google Scholar]
- 84. Nomoto H, Baba H, Satomura E, et al. Serum brain-derived neurotrophic factor levels and personality traits in patients with major depression. BMC Psychiatry 2015; 15: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ristevska-Dimitrovska G, Shishkov R, Gerazova VP, et al. Different serum BDNF levels in depression: results from BDNF studies in FYR Macedonia and Bulgaria. Psychiatr Danub 2013; 25: 123–127. [PubMed] [Google Scholar]
- 86. Yoshida T, Ishikawa M, Niitsu T, et al. Decreased serum levels of mature brain-derived neurotrophic factor (BDNF), but not its precursor proBDNF, in patients with major depressive disorder. PLoS ONE 2012; 7: e42676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wolkowitz OM, Wolf J, Shelly W, et al. Serum BDNF levels before treatment predict SSRI response in depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35: 1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Aydemir Ö, Deveci A, Taskin OE, et al. Serum brain-derived neurotrophic factor level in dysthymia: a comparative study with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31: 1023–1026. [DOI] [PubMed] [Google Scholar]
- 89. Aydemir C, Yalcin ES, Aksaray S, et al. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuropsychopharmacol Biol Psychiatry 2006; 30: 1256–1260. [DOI] [PubMed] [Google Scholar]
- 90. Cattaneo A, Bocchio-Chiavetto L, Zanardini R, et al. Reduced peripheral brain-derived neurotrophic factor mRNA levels are normalized by antidepressant treatment. Int J Neuropsychopharmacol 2010; 13: 103–108. [DOI] [PubMed] [Google Scholar]
- 91. Chiou Y-J, Huang T-L. Serum brain-derived neurotrophic factors in Taiwanese patients with drug-naïve first-episode major depressive disorder: effects of antidepressants. Int J Neuropsychopharmacol 2017; 20: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Huang X, Huang X, Zhou Y, et al. Association of serum BDNF levels with psychotic symptom in chronic patients with treatment-resistant depression in a Chinese Han population. Psychiatry Res 2017; 257: 279–283. [DOI] [PubMed] [Google Scholar]
- 93. Karege F, Perret G, Bondolfi G, et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res 2002; 109: 143–148. [DOI] [PubMed] [Google Scholar]
- 94. Karege F, Bondolfi G, Gervasoni N, et al. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry 2005; 57: 1068–1072. [DOI] [PubMed] [Google Scholar]
- 95. Gustafsson G, Lira CM, Johansson J, et al. The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry Res 2009; 169: 244–248. [DOI] [PubMed] [Google Scholar]
- 96. Kotan Z, Sarandöl E, Kırhan E, et al. Serum brain-derived neurotrophic factor, vascular endothelial growth factor and leptin levels in patients with a diagnosis of severe major depressive disorder with melancholic features. Ther Adv Psychopharmacol 2012; 2: 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Skibinska M, Kapelski P, Rajewska-Rager A, et al. Brain-derived neurotrophic factor (BDNF) serum level in women with first-episode depression, correlation with clinical and metabolic parameters. Nord J Psychiatry 2018; 72: 191–196. [DOI] [PubMed] [Google Scholar]
- 98. Wysokiński A. Serum levels of brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3) in depressed patients with schizophrenia. Nord J Psychiatry 2016; 70: 267–271. [DOI] [PubMed] [Google Scholar]
- 99. Gervasoni N, Aubry J-M, Bondolfi G, et al. Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology 2005; 51: 234–238. [DOI] [PubMed] [Google Scholar]
- 100. Huang T-L, Lee C-T, Liu Y-L. Serum brain-derived neurotrophic factor levels in patients with major depression: effects of antidepressants. J Psychiatr Res 2008; 42: 521–525. [DOI] [PubMed] [Google Scholar]
- 101. Bus BA, Molendijk ML, Penninx BJ, et al. Determinants of serum brain-derived neurotrophic factor. Psychoneuroendocrinology 2011; 36: 228–239. [DOI] [PubMed] [Google Scholar]
- 102. Ghosh R, Gupta R, Bhatia MS, et al. Comparison of efficacy, safety and brain derived neurotrophic factor (BDNF) levels in patients of major depressive disorder, treated with fluoxetine and desvenlafaxine. Asian J Psychiatr 2015; 18: 37–41. [DOI] [PubMed] [Google Scholar]
- 103. Kurita M, Nishino S, Kato M, et al. Plasma brain-derived neurotrophic factor levels predict the clinical outcome of depression treatment in a naturalistic study. PLoS ONE 2012; 7: e39212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Molendijk ML, Bus BA, Spinhoven P, et al. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry 2011; 16: 1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cattaneo A, Gennarelli M, Uher R, et al. Candidate genes expression profile associated with antidepressants response in the GENDEP study: differentiating between baseline ‘predictors’ and longitudinal ‘targets’. Neuropsychopharmacology 2013; 38: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pan W, Banks WA, Fasold MB, et al. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacol 1998; 37: 1553–1561. [DOI] [PubMed] [Google Scholar]
- 107. Fujimura H, Altar CA, Chen R, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost 2002; 87: 728–734. [PubMed] [Google Scholar]
- 108. Chu C-L, Liang C-K, Chou M-Y, et al. Decreased plasma brain-derived neurotrophic factor levels in institutionalized elderly with depressive disorder. J Am Med Dir Assoc 2012; 13: 434–437. [DOI] [PubMed] [Google Scholar]
- 109. Lee BH, Kim H, Park SH, et al. Decreased plasma BDNF level in depressive patients. J Affect Disord 2007; 101: 239–244. [DOI] [PubMed] [Google Scholar]
- 110. Piccinni A, Veltri A, Costanzo D, et al. Decreased plasma levels of brain-derived neurotrophic factor (BDNF) during mixed episodes of bipolar disorder. J Affect Disord 2015; 171: 167–170. [DOI] [PubMed] [Google Scholar]
- 111. Kenna HA, Reynolds-May M, Stepanenko A, et al. Blood levels of brain derived neurotrophic factor in women with bipolar disorder and healthy control women. J Affect Disord 2014; 156: 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Machado-Vieira R, Yuan P, Brutsche N, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-Methyl-D-aspartate antagonist. J Clin Psychiatry 2009; 70: 1662–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Lee BH, Kim YK. BDNF mRNA expression of peripheral blood mononuclear cells was decreased in depressive patients who had or had not recently attempted suicide. J Affect Disord 2010; 125: 369–373. [DOI] [PubMed] [Google Scholar]
- 114. Roy B, Shelton RC, Dwivedi Y. DNA methylation and expression of stress related genes in PBMC of MDD patients with and without serious suicidal ideation. J Psychiatr Res 2017; 89: 115–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Pandey GN, Dwivedi Y, Rizavi HS, et al. Brain-derived neurotrophic factor gene and protein expression in pediatric and adult depressed subjects. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 645–651. [DOI] [PubMed] [Google Scholar]
- 116. Otsuki K, Uchida S, Watanuki T, et al. Altered expression of neurotrophic factors in patients with major depression. J Psychiatr Res 2008; 42: 1145–1153. [DOI] [PubMed] [Google Scholar]
- 117. D’Addario C, Dell'Osso B, Galimberti D, et al. Epigenetic modulation of BDNF gene in patients with major depressive disorder. Biol Psychiatry 2013; 73: e6–e7. [DOI] [PubMed] [Google Scholar]
- 118. Carlberg L, Scheibelreiter J, Hassler MR, et al. Brain-derived neurotrophic factor (BDNF)-epigenetic regulation in unipolar and bipolar affective disorder. J Affect Disord 2014; 168: 399–406. [DOI] [PubMed] [Google Scholar]
- 119. D’Addario C, Dell’Osso B, Palazzo MC, et al. Selective DNA methylation of BDNF promoter in bipolar disorder: differences among patients with BDI and BDII. Neuropsychopharmacology 2012; 37: 1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Dell’Osso B, D’Addario C, Carlotta Palazzo M, et al. Epigenetic modulation of BDNF gene: differences in DNA methylation between unipolar and bipolar patients. J Affect Disord 2014; 166: 330–333. [DOI] [PubMed] [Google Scholar]
- 121. Kang HJ, Kim JM, Bae KY, et al. Longitudinal associations between BDNF promoter methylation and late-life depression. Neurobiol Aging 2015; 36: 1764.e1–1764.e7. [DOI] [PubMed] [Google Scholar]
- 122. Piccinni A, Marazziti D, Del Debbio A, et al. Diurnal variation of plasma brain-derived neurotrophic factor (BDNF) in humans: an analysis of sex differences. Chronobiol Int 2008; 25: 819–826. [DOI] [PubMed] [Google Scholar]
- 123. Cain SW, Chang AM, Vlasac I, et al. Circadian rhythms in plasma brain-derived neurotrophic factor differ in men and women. J Biol Rhythms 2017; 32: 75–82. [DOI] [PubMed] [Google Scholar]
- 124. de Kloet ER, Joëls M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci 2005; 6: 463–475. [DOI] [PubMed] [Google Scholar]
- 125. Gladkevich A, Kauffman HF, Korf J. Lymphocytes as a neural probe: potential for studying psychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry 2004; 28: 559–576. [DOI] [PubMed] [Google Scholar]
- 126. Iga J, Ueno S, Ohmori T. Molecular assessment of depression from mRNAs in the peripheral leukocytes. Annals of medicine 2008; 40: 336–342. [DOI] [PubMed] [Google Scholar]
- 127. Lago SG, Tomasik J, van Rees GF, et al. Drug discovery for psychiatric disorders using high-content single-cell screening of signaling network responses ex vivo. Sci Adv 2019; 5: eaau9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. van der Doef TF, Zaragoza Domingo S, Jacobs GE, et al. New approaches in psychiatric drug development. Eur Neuropsychopharmacol 2018; 28: 983–993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tpp-10.1177_20451253211036814 for MTORC1 signaling as a biomarker in major depressive disorder and its pharmacological modulation by novel rapid-acting antidepressants by Tomasz Cholewinski, Diana Pereira, Matthijs Moerland and Gabriel E. Jacobs in Therapeutic Advances in Psychopharmacology