Abstract
Introduction The incidence of papillary thyroid microcarcinoma (PTMC) has increased, and its treatment remains controversial.
Objective To identify the clinical and pathological factors predictive of tumor recurrence.
Methods We retrospectively analyzed 2,538 consecutive patients treated for PTMC, most submitted to total thyroidectomy (98%) followed by radioactive iodine (RAI) ablation (51.7%) at a cancer center from 1996 to 2015. The patients were stratified according to the American Thyroid Association (ATA) risk categories (low, intermediate, or high), and the clinicopathological features were evaluated by multivariate Cox regression analysis to identify independent prognostic factors for recurrence.
Results After a mean follow-up of 58 months (range: 3 to 236.5 months), tumor recurrence was diagnosed in 63 (2.5%) patients, mostly in the lymph nodes. Distant metastasis occurred in 2 (0.1%) patients. There were no cancer-related deaths. The multivariate analysis showed that age < 55 years ( p = 0.049; hazard ratio [HR]: 2.54; 95% confidence interval [95%CI]: 0.95 to 0.99), multifocality ( p = 0.032; HR: 1.76; 95%CI: 1.05 to 2.96), and the presence of lymph-node metastasis ( p < 0.001; HR: 3.69; 95%CI: 2.07–6.57) were independent risk factors for recurrence. Recurrence was observed in 29 (1.5%) out of 1,940 low-risk patients, 32 (5.4%) out of 590 intermediate-risk patients, and in 2 (25%) out of 8 high-risk patients.
Conclusions The prognosis of PTMC is excellent, favoring a conservative treatment for most patients. Age < 55 years, multifocality, and node metastasis at diagnosis, as well the ATA staging system effectively predict the risk of recurrence. The presence of these risk factors can help identify patients who should be considered for more aggressive management and more frequent follow-up.
Keywords: thyroid, papillary microcarcinoma, recurrence, prognosis, risk stratification
Introduction
The incidence of papillary thyroid carcinoma (PTC) is increasing worldwide 1 mainly due to the greater number of tumors incidentally discovered after the widespread use of ultrasound-guided fine-needle aspiration (FNA) in patients with suspected thyroid diseases. Most of these tumors are papillary thyroid microcarcinomas (PTMCs), 2 3 4 which are defined by the World Health Organization (WHO) as carcinomas ≤ 1 cm in the greatest dimension. 5 Papillary thyroid microcarcinomas are less aggressive than PTCs > 1 cm, 6 and most thyroid nodules < 1 cm should not undergo FNA.
Several previously-published series have demonstrated excellent outcomes following the therapy for PTMC, with a negligible cancer mortality rate. However, despite the favorable long-term prognosis, cases of locoregional recurrences and even distant metastases have been described. 7 8
There is no consensus regarding the natural history of PTMC, and the treatment options range from observation to total thyroidectomy and neck lymph-node dissection followed by radioactive iodine (RAI) ablation. 9 An active surveillance management approach to low-risk PTMC is a safe and effective alternative to immediate surgical resection. 10 11 The 2015 American Thyroid Association (ATA) guidelines state that “if surgery is chosen for patients with thyroid cancer < 1 cm without extrathyroidal extension and cN0, the initial surgical procedure should be a thyroid lobectomy, unless there are clear indications to remove the contralateral lobe. Thyroid lobectomy alone is sufficient treatment for small, unifocal, intrathyroidal carcinomas in the absence of prior head and neck radiation, familial thyroid carcinoma, or clinically-detectable cervical nodal metastases” 12 . But, due to the high incidence of multifocality and lymph node metastasis in level VI, some authors recommend a total thyroidectomy and concomitant central lymph node dissection (CLND) in patients with clinically node-negative PTMC to avoid a reoperation 13 or reduce the locoregional recurrence rate. 14 However, elective CLND might increase the risk of postoperative complications, especially permanent hypocalcemia. 15 16
The risk of recurrence in PTC can be estimated based on selected clinicopathologic features, such as the presence of extrathyroidal extension, aggressive histologies, vascular invasion, regional metastases, or high levels of postoperative serum thyroglobulin. The 2009 ATA guidelines for the management of thyroid cancer proposed a system to estimate the risk of relapse of PTC based on these clinicopathologic findings. 17 Additional prognostic variables, such as the extent of lymph-node involvement and B-Raf proto-oncogene, serine/threonine kinase (BRAF) mutation profile were included in an updated version of the 2015 ATA risk stratification system 12 ( Table 1 ). However, these additional variables have not been rigorously assessed.
Table 1. Initial American Thyroid Association risk of recurrence classification.
|
Low risk
(all of the following:) |
No local or distant metastases; All macroscopic tumor has been resected; No invasion of locoregional tissues; Tumor does not have aggressive histology (e.g.: tall cell, insular, columnar cell carcinoma, Hurthle cell carcinoma, follicular thyroid cancer); No vascular invasion; No 131 I uptake outside the thyroid bed on the posttreatment scan, if done; Clinical N0 or ≤ 5 pathologic N1 micrometastases (< 0.2 cm);* Intrathyroidal, encapsulated follicular variant of papillary thyroid cancer;* Intrathyroidal, well-differentiated follicular thyroid cancer with capsular invasion and no or minimal (< 4 foci) vascular invasion;* Intrathyroidal, papillary microcarcinoma, unifocal or multifocal, including BRAFV600E mutated (if known).* |
|
Intermediate risk
(any of the following:) |
Microscopic invasion into the perithyroidal soft tissues; Cervical lymph node metastases or 131 I uptake outside the thyroid bed on the posttreatment scan done after thyroid remnant ablation; Tumor with aggressive histology or vascular invasion; Papillary thyroid cancer with vascular invasion;* Clinical N1 or > 5 pathologic N1 with all involved lymph nodes < 3 cm;* Multifocal papillary microcarcinoma with extrathyroidal extension (ETE) and BRAFV600E mutated (if known).* |
|
High risk
(any of the following:) |
Macroscopic (gross ETE) invasion of tumor into the perithyroidal tissues; Incomplete tumor resection; Distant metastases; Postoperative serum thyroglobulin suggestive of distant metastases;* Pathologic N1 with any metastatic lymph node ≥ 3 cm*; Follicular thyroid cancer with extensive vascular invasion (> 4 foci of vascular invasion).* |
Note: *Additional prognostic variables included in the 2015 American Thyroid Association (ATA) risk stratification system.
The aim of the present study was to review the characteristics of PTMC at diagnosis in retrospective cohort from a single cancer center, and to identify the clinical and pathological features associated with tumor recurrence. We also evaluated the 2009 ATA risk stratification system for the prediction of recurrence.
Methods
Study Population and Treatment
After obtaining approval form the institutional review board (ethics committee approval number: 2.904.573), we retrospectively reviewed the medical records of 4,085 consecutive patients treated for PTC between January 1996 and December 2015. Among these, we selected all 2,538 patients with PTMC. Only patients with a postoperative pathologic diagnosis of PTC and a maximum tumor diameter of 1 cm were included. Most patients had an initial total or subsequent completion thyroidectomy, which were performed at our institution based on patient preference and clinical criteria such as previous neck irradiation, hypothyroidism, familial predisposition or bilateral nodularity. Many physicians and patients chose bilateral thyroidectomies aiming to simplify the follow-up. Therapeutic lymph node dissection was performed if the clinical involvement was confirmed based on sonographic findings and intraoperative exploration of the central neck compartment. Elective CLND was performed in the presence of extrathyroidal extension. For patients who were pathologically confirmed to have high-risk findings, mainly extrathyroidal extension and cervical lymph node metastasis, routine radioactive iodine (RAI, or I-131) treatment was administered after withdrawal of hormone therapy for at least 4 weeks. Some patients were treated with RAI ablation, with the purpose of facilitating the follow-up or destroying the foci of micrometastatic disease. Diagnostic scintigraphy was performed before the administration of I-131 and 2 to 5 days later. The levels of thyroglobulin (Tg), and anti-Tg antibodies were measured postoperatively just before the RAI treatment. Most PTMC patients received oral therapy with levothyroxine postoperatively, in an attempt to maintain their thyroid-stimulating hormone (TSH) levels below 2.0 mlU/L.
Follow-up
The patients were assessed every 3 months for the first year, every 6 months between the second and fifth years, and every 12 months thereafter at the discretion of the attending physician, based on the risk of the individual patient. The follow-up visits included palpation of the neck, dosage of TSH, Tg and anti-Tg antibody levels, and ultrasound examination of the cervical lymph nodes. Disease recurrence was defined as the first clinical reappearance of the tumor. It included all clinical events reported (local relapses, lymph node metastases, and distant metastases) and those confirmed by imaging modalities, biopsy or surgery.
Prognostic Parameters
Patient characteristics, surgery data, pathological features and postoperative clinical outcomes were retrieved from the medical charts. The pathological characteristics of the thyroidectomy specimens evaluated included: tumor size, extrathyroidal extension, multifocality, aggressive histological variant (such as tall cells, diffuse sclerosing and solid variants), neck lymph-node metastasis, lympho-vascular invasion, and chronic lymphocytic thyroiditis. The patients were classified according to the 2009 ATA risk stratification system as low, intermediate or high risk of recurrence 17 ( Table 1 ). Due to the small number of deaths, the overall survival was not analyzed.
Statistical Methods
The primary endpoint of the study was disease-free survival (DFS). The duration of the of follow-up was calculated as the interval between surgery and death or the last visit to the clinic. The categorical variables were described as the frequency of different categories, and the continuous variables, as means and standard deviations. The Kaplan-Meier method was used to evaluate the DFS. Comparisons between the categorical variables were initially performed using the log-rank technique. The Cox univariate analysis was used to compare the survival analysis, and the significant variables were included in the multivariate Cox model. Values of p < 0.05 were considered statistically significant. All analyses were performed using the Stata (StataCorp LP, College Station, TX, US).
Results
From January 1996 to December 2015, both the number of patients treated for papillary thyroid carcinoma (PTC) and the proportion of papillary thyroid microcarcinomas (PTMCs) increased: 42% (29 out of 69 patients) from 1996 to 2000, 60.8% (262 out of 431) from 2001 to 2005, 61.9% (596 out of 963) from 2006 to 2010, and 63% (1,651 out of 2,622) from 2011 to 2015 ( Fig. 1 ).
Fig. 1.
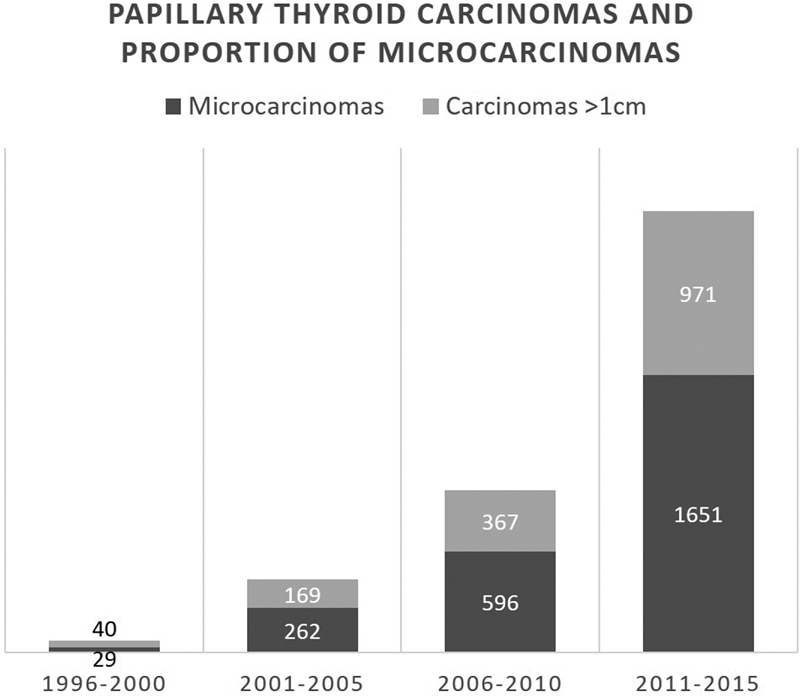
Temporal evolution of papillary thyroid carcinomas and proportion of microcarcinomas.
Among the 4,085 consecutive patients treated for PTC, we included 2,538 patients (62.1%) with PTMC for the present retrospective study. Patients and tumor characteristics are presented in Table 2 .
Table 2. Patients and tumor characteristics.
| Characteristics | Patients (N) | ||
|---|---|---|---|
| Gender | Female | 83% | 2,106 |
| Male | 17% | 432 | |
| Age (years): | Mean (standard deviation) | 44.4 (13.1) | 2,538 |
| Range | 7–85 | ||
| < 55 | 78.3% | 1,696 | |
| ≥ 55 | 21.7% | 470 | |
| Tumor size (mm): | Mean (standard deviation) | 6.1 (9.9) | |
| Range | 0.2–10 | ||
| ≤ 5 mm | 41% | 1,041 | |
| > 5 mm | 59% | 1,497 | |
| Aggressive histology | 3% | 75 | |
| Multifocality | 30.2% | 766 | |
| Extrathyroidal extension | Minor | 14.1% | 357 |
| Gross | 0.2% | 7 | |
| Lympho-vascular invasion | 1.2% | 30 | |
| Lymph-node metastasis | 9.5% | 242 | |
| Chronic lymphocytic thyroiditis | 32.7% | 830 | |
| American Thyroid Association risk stratification category | Low | 76.4% | 1,940 |
| Intermediate | 23.3% | 590 | |
| High | 0.3% | 8 |
Most PTMC (2,292; 90.3%) were nonpalpable nodules incidentally diagnosed during neck radiologic procedures, such as ultrasonography or computed tomography, performed during follow-up due to other cancers or detected through the postoperative pathologic examination of surgical specimens resected for benign thyroid diseases or after prophylactic thyroidectomies.
Almost all patients underwent initial total thyroidectomy (2,394; 94.3%) or completion thyroidectomy (93; 3.7%). Central lymph-node dissection was performed in 212 (8.4%) patients, 53 (2.1%) of whom underwent concomitant lateral dissection. Radioactive iodine was adminidtered postsurgically in 1,311 (51.7%) patients. The dose of iodine ranged from 30 mCi to 425 mCi (mean: 132.2 mCi).
After a mean follow-up of 58 months (range: 3 to 236.5 months), tumor recurrence was diagnosed in 63 (2.5%) patients, mostly in the cervical lymph nodes ( Fig. 2 ). The median time to recurrence was of 58.1 months (range: 3 to 236.5 months; standard deviation: 40.4). There were no cancer-related deaths.
Fig. 2.

Pattern of recurrence of papillary thyroid microcarcinoma.
The log-rank univariate survival analysis showed that male gender ( p = 0.003), age < 55 years ( p = 0.007), tumor size > 5 mm ( p = 0.008), multifocality ( p = 0.001), and the presence of lymph-node metastases at diagnosis ( P < 0.001) were significantly associated with tumor recurrence. However, other pathological factors, such as extrathyroidal extension ( p = 0.126), aggressive histological variants ( p = 0.478), lympho-vascular invasion ( p = 0.175), and chronic lymphocytic thyroiditis ( p = 0.582) did not affect the DFS rate. Multivariate Cox regression analyses revealed that cancer recurrence was independently associated with age < 55 years ( p = 0.049; HR: 2.54; 95%CI: 0.95 to 0.99), multifocality ( p = 0.032; HR: 1.76; 95%CI: 1.05 to 2.96), and the presence of lymph-node metastasis ( p < 0.001; HR: 3.69; 95%CI: 2.07 to 6.57) ( Table 3 ).
Table 3. Univariate and multivariate cancer-recurrence logistic regression analyses of patients with papillary thyroid microcarcinoma.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Hazard ratio (95% confidence interval) | p -value | Hazard ratio (95% confidence interval) | p -value | |
| Male gender | 2.30 (1.32–4.02) | 0.003 | 1.68 (0.94–3.02) | 0.081 |
| Age < 55 years | 3.50 (1.41–8.74) | 0.007 | 2.54 (0.95–0.99) | 0.049 |
| Tumor size > 5 mm | 1.15 (1.04–1.27) | 0.008 | 1.07 (0.95–1.19) | 0.203 |
| Multifocality | 2.33 (1.42–3.83) | 0.001 | 1.76 (1.05–2.96) | 0.032 |
| Extrathyroidal extension | 1.61 (0.87–2.98) | 0.126 | 0.88 (0.45–1.71) | 0.706 |
| Aggressive histology | 1.52 (0.21–2.09) | 0.478 | 1.45 (0.45–1.71) | 0.531 |
| Lympho-vascular invasion | 2.05 (0.725–5.86) | 0.175 | 1.22 (0.43–3.51) | 0.708 |
| Lymph-node metastasis | 5.56 (3.31–9.34) | < 0.001 | 3.69 (2.07–6.57) | < 0.001 |
| Chronic thyroiditis | 0.86 (0.51–1.46) | 0.582 | 0.98 (0.57–1.67) | 0.936 |
According to the 2009 ATA risk stratification system, the patients were classified as low (1,940 - 76.4%), intermediate (590 - 23.3%) or high-risk (8 - 0.3%). Recurrence was observed in 29 (1.5%) out of 1,940 low-risk patients, 32 (5.4%) out of 590 intermediate-risk patients, and in 2 (25%) out of 8 high-risk patients. The probability of 5-year DFS was significantly lower in high-risk (84.9%) than in intermediate (94%) and low-risk (98.8%) patients ( Fig. 3 ).
Fig. 3.

Kaplan-Meier recurrence estimates based on American Thyroid Association risk categories.
Discussion
The incidence of PTMC is rising, mostly due to the increased presurgical diagnosis of incidental nonpalpable tumors. 18 The prevalence of occult PTMC in autopsy specimens is high, ranging from 11.3% to 35.6%, 19 20 depending on the extent of the histologic examination of the thyroid gland. A similar high frequency of incidental PTMCs is observed in 7.2% of thyroid glands surgically resected for benign diseases or after prophylactic thyroidectomies. 21 In the present series, PTMCs were mainly incidental cancers detected during neck radiologic procedures or after postoperative pathologic examination of surgical specimens resected for benign thyroid diseases. As we know, incidental PTMC has different clinical characteristics and a much lower recurrence rate than non-incidental PTMC. 22
The 2015 ATA guidelines recommend initial unilateral lobectomy for PTMC, with subsequent long-term surveillance. In properly selected low-risk patients, the extent of the initial thyroid surgery probably has little impact on disease-specific survival. While recurrence rates can be a little high in patients submitted to unilateral thyroidectomy, salvage therapy is quite effective in the few patients that have recurrence. Besides, the surgical risks of two-stage thyroidectomy (lobectomy followed by completion thyroidectomy) are similar to those of a near-total or total thyroidectomy. 12 Instead of unilateral resection, some investigators favor total thyroidectomy as an appropriate initial treatment for some cases of PTMC, with the advantages of providing lower local recurrence because of the removal of all potential foci in both lobes, improving the sensitivity of thyroglobulin as a tumor marker, and enabling the use of RAI in the detection of metastasis and recurrence during follow-up. 23 24 25 26 27 28 It is important to note that thyroid lobectomy is associated with an increased risk of recurrence, but not mortality, compared with total thyroidectomy for PTMC. 29 In our institution, most patients were submitted to total thyroidectomy, based on patient preference and clinical criteria such as previous neck irradiation, hypothyroidism, familial predisposition, bilateral nodularity, or as a strategy to simplify the follow-up. Most authors, 30 31 but not all, 32 33 agree that postthyroidectomy RAI ablation is not beneficial in reducing cancer recurrence in PTMC patients. Otherwise, RAI ablation can make the follow-up easier by improving the sensitivity of Tg and anti-Tg antibodies. 34
In general, recurrence of cancer is not frequently observed following thyroidectomy for PTMC. In a meta-analysis including 6,839 PTMC patients, Yi et al. 35 found a low recurrence rate (2.8%), very similar to ours (2.5%). However, recurrence rates as high as 14.3% and 19% have been described in the literature. 36 37 As expected, most of our patients had recurrence in the lymph nodes: 42.9% exclusively in level VI; 28.6% in the lateral neck levels; and 25.4% in both central and lateral compartment nodes. Recurrence is directly associated with the risk of regional spread. For PTMC patients, the incidence of occult lymph node metastasis is as high as 33%, and these occur mainly in the central compartment of the neck. 38 However, microscopic nodal disease is rarely of clinical importance, since it often remains quiescent or the subsequent RAI administration ablates these occult foci. Therefore, we do not routinely perform elective CLND in most PTMC patients. Meta-analyses revealed that lymph-node metastases in PTMC patients are associated with the male gender, younger age (< 45 years), larger tumor size (> 5 mm), multifocality, extrathyroidal extension, and lymphovascular invasion, but not with thyroid bilaterality and chronic lymphocytic thyroiditis. 38 39
Several clinicopathologic factors have been described to predict the recurrence of PTMC 40 : tumor diameter > 5 mm or 7 mm, 41 42 younger age, 43 44 45 the male gender, 42 43 multifocality, 37 42 45 46 capsular invasion, 47 or absence of tumor capsule, 37 extrathyroid extension, 37 43 45 47 48 lymph node metastases, 43 44 46 47 48 49 50 the CLNM ratio (number of metastasized and removed nodes at the first operation) > 0.5, 41 51 aggressive histological variants, 37 52 mutated BRAF, 53 and non-incidental diagnosis. 54 Our data showed that younger age (< 55 years), multifocality, and presence of lymph node metastases were independently associated with tumor recurrence in PTMC patients. Additionally, the male gender and maximum tumor diameter > 5 mm also increased the risk of recurrence on the univariate analysis. In our cohort, most PTMCs (14.1%) presented minor extrathyroidal extension compared with only 0.2% of those with gross extension to the perithyroidal tissues. Similarly, some authors previously showed that gross but not minimal extrathyroidal extension is a significant factor associated with tumor recurrence in PTC patients. 55 56 57 Other pathological factors, such as aggressive histology and lympho-vascular invasion, did not affect the DFS rate, probably due the small number of cases. Finely, chronic lymphocytic thyroiditis was found in one third of PTMC patients, with no impact on cancer recurrence. Actually, previous publications found that lymphocytic thyroiditis resulted in a decreased risk of lymph-node metastases. 58 59
Although PTMC is generally associated with an excellent prognosis, some patients will experience poor outcomes. An analysis of 46,662 patients with PTMC from the Surveillance, Epidemiology, and End Results (SEER) program (1983–2015) showed 5-year, 10-year, and 20-year probabilities of death of 0.3%, 0.6%, and 1.4% respectively. Older age at diagnosis, male gender, tumor extension and lymph-node involvement were related to the cumulative incidence of death. 60 Metastatic PTMCs have been described in the literature. The location of the distant metastases was primarily pulmonary, and most patients, 47 61 but not all, 62 had lymph node involvement on the initial presentation. In our series, two patients presented metastases in the lungs and bones, with no cancer-related deaths.
The 2017 tumor, node, metastasis (TNM) staging system of the American Joint Cancer Committee/Union for International Cancer Control (AJCC/UICC) is adequately used to predict disease-specific mortality. 63 Since death is uncommon following the management of PTMC patients, we also use the ATA clinicopathologic staging system to provide initial estimates of the risk of recurrence and thus improve clinical decision-making. 64 As expected, most of our PTMC patients were classified as 2009 ATA low-risk (76.4%) or intermediate-risk of relapse (23.3%). The risk of recurrence in PTMC patients was very low and effectively predicted by the ATA staging system. Moreover, while the TNM and ATA staging systems can be used to guide the initial therapeutic and diagnostic strategy decisions, it is important to recognize that initial risk estimates may be refined as new information is accumulated during the first two years of follow-up monitoring. 65 As an example, in patients with successfully-treated PTC (postoperative undetectable s-Tg levels and no evidence of disease on whole-body iodine scan after total thyroidectomy and RAI ablation), recurrence-free survival did not differ between patients classified as high-risk and those classified as low-risk based on TNM stage at diagnosis. 66 Thus, further prospective studies are required to investigate the impact of this dynamic risk assessment on ATA initial-risk estimates.
Some limitations of this retrospective study are mainly related to selection bias. Recommendations on treatment and on the intensity and frequency of follow-up visits and tests varied from patient to patient, based on individual surgeons and patient preferences, and not on an institutional protocol. This would lead to an increase in the diagnosis of recurrent disease in intermediate- to high-risk patients in comparison with the less rigorous testing paradigm often used in low-risk patients. Furthermore, important prognostic variables included in the updated version of the 2015 ATA risk stratification system, such as the number and dimension of lymph-node metastases, were not assessed in the present study. Finally, a median follow-up period of 58 months may be short, as some patients with a less aggressive disease may manifest clinically-significant recurrence many years following the initial therapy.
Conclusion
In summary, our data confirm that clinical and pathological factors such as age < 55 years, multifocality, and the presence of lymph-node metastasis at diagnosis are good initial predictors of recurrence in PTMC patients. Further, our data confirm that the ATA recurrence staging system effectively predicts recurrence, thus providing valuable information that can help individualize the clinical management and follow-up for PTMC patients.
Footnotes
Conflict of Interests The authors have no conflict of interests to declare.
References
- 1.Wiltshire J J, Drake T M, Uttley L, Balasubramanian S P. Systematic Review of Trends in the Incidence Rates of Thyroid Cancer. Thyroid. 2016;26(11):1541–1552. doi: 10.1089/thy.2016.0100. [DOI] [PubMed] [Google Scholar]
- 2.Roti E, degli Uberti E C, Bondanelli M, Braverman L E. Thyroid papillary microcarcinoma: a descriptive and meta-analysis study. Eur J Endocrinol. 2008;159(06):659–673. doi: 10.1530/EJE-07-0896. [DOI] [PubMed] [Google Scholar]
- 3.Danish Thyroid Cancer Group . Londero S C, Krogdahl A, Bastholt L. Papillary thyroid microcarcinoma in Denmark 1996-2008: a national study of epidemiology and clinical significance. Thyroid. 2013;23(09):1159–1164. doi: 10.1089/thy.2012.0595. [DOI] [PubMed] [Google Scholar]
- 4.A study from The Danish Thyroid Cancer Group – DATHYRCA (part of the DAHANCA organization) . Reinke R, Mathiesen J S, Larsen S R. Incidental and Non-incidental Papillary Thyroid Microcarcinoma in Denmark 1996-2015: A national study on incidence, outcome and thoughts on active surveillance. Cancer Epidemiol. 2019;60:46–50. doi: 10.1016/j.canep.2019.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Hedinger C, Williams E, Sobin L. 2nd ed. Verlag: Springer; 1988. Histologic types of thyroid tumors in World Health Organization histological classification of tumors; pp. 9–10. [Google Scholar]
- 6.Zheng W, Wang X, Rui Z, Wang Y, Meng Z, Wang R. Clinical features and therapeutic outcomes of patients with papillary thyroid microcarcinomas and larger tumors. Nucl Med Commun. 2019;40(05):477–483. doi: 10.1097/MNM.0000000000000991. [DOI] [PubMed] [Google Scholar]
- 7.Hay I D, Grant C S, van Heerden J A. Papillary thyroid microcarcinoma: a study of 535 cases observed in a 50-year period. Surgery. 1992;112:1139Y1146. [PubMed] [Google Scholar]
- 8.Noguchi S, Yamashita H, Murakami N. Small carcinomas of the thyroid a long-term follow-up of 867 patients. Arch Surg. 1996;131(02):187–191. doi: 10.1001/archsurg.1996.01430140077021. [DOI] [PubMed] [Google Scholar]
- 9.Nabhan F, Ringel M D. Thyroid nodules and cancer management guidelines: comparisons and controversies. Endocr Relat Cancer. 2017;24(02):R13–R26. doi: 10.1530/ERC-16-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito Y, Miyauchi A, Oda H. Low-risk papillary microcarcinoma of the thyroid: A review of active surveillance trials. Eur J Surg Oncol. 2018;44(03):307–315. doi: 10.1016/j.ejso.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Miyauchi A, Ito Y, Oda H. Insights into the Management of Papillary Microcarcinoma of the Thyroid. Thyroid. 2018;28(01):23–31. doi: 10.1089/thy.2017.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haugen B R, Alexander E K, Bible K C. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(01):1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi L, Chen J H, Wang S T, Xiong Y Q, Huang T. Treatment for papillary thyroid microcarcinoma. Contemp Oncol (Pozn) 2013;17(01):20–23. doi: 10.5114/wo.2013.33769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su H, Li Y. Prophylactic central neck dissection and local recurrence in papillary thyroid microcarcinoma: a meta-analysis. Rev Bras Otorrinolaringol (Engl Ed) 2019;85(02):237–243. doi: 10.1016/j.bjorl.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.So Y K, Seo M Y, Son Y I. Prophylactic central lymph node dissection for clinically node-negative papillary thyroid microcarcinoma: influence on serum thyroglobulin level, recurrence rate, and postoperative complications. Surgery. 2012;151(02):192–198. doi: 10.1016/j.surg.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Ywata de Carvalho A, Chulam T C, Kowalski L P. Long-term Results of Observation vs Prophylactic Selective Level VI Neck Dissection for Papillary Thyroid Carcinoma at a Cancer Center. JAMA Otolaryngol Head Neck Surg. 2015;141(07):599–606. doi: 10.1001/jamaoto.2015.0786. [DOI] [PubMed] [Google Scholar]
- 17.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer . Cooper D S, Doherty G M, Haugen B R. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 18.Kaliszewski K, Zubkiewicz-Kucharska A, Kiełb P, Maksymowicz J, Krawczyk A, Krawiec O. Comparison of the prevalence of incidental and non-incidental papillary thyroid microcarcinoma during 2008-2016: a single-center experience. World J Surg Oncol. 2018;16(01):202. doi: 10.1186/s12957-018-1501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto Y, Maeda T, Izumi K, Otsuka H. Occult papillary carcinoma of the thyroid. A study of 408 autopsy cases. Cancer. 1990;65(05):1173–1179. doi: 10.1002/1097-0142(19900301)65:5<1173::aid-cncr2820650524>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Harach H R, Franssila K O, Wasenius V M. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer. 1985;56(03):531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.de Matos P S, Ferreira A P, Ward L S. Prevalence of papillary microcarcinoma of the thyroid in Brazilian autopsy and surgical series. Endocr Pathol. 2006;17(02):165–173. doi: 10.1385/ep:17:2:165. [DOI] [PubMed] [Google Scholar]
- 22.Mehanna H, Al-Maqbili T, Carter B. Differences in the recurrence and mortality outcomes rates of incidental and nonincidental papillary thyroid microcarcinoma: a systematic review and meta-analysis of 21 329 person-years of follow-up. J Clin Endocrinol Metab. 2014;99(08):2834–2843. doi: 10.1210/jc.2013-2118. [DOI] [PubMed] [Google Scholar]
- 23.Karatzas T, Vasileiadis I, Kapetanakis S, Karakostas E, Chrousos G, Kouraklis G. Risk factors contributing to the difference in prognosis for papillary versus micropapillary thyroid carcinoma. Am J Surg. 2013;206(04):586–593. doi: 10.1016/j.amjsurg.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Hay I D, Hutchinson M E, Gonzalez-Losada T.Papillary thyroid microcarcinoma: a study of 900 cases observed in a 60-year period Surgery 200814406980–987., discussion 987–988 [DOI] [PubMed] [Google Scholar]
- 25.Ito Y, Miyauchi A, Jikuzono T. Risk factors contributing to a poor prognosis of papillary thyroid carcinoma: validity of UICC/AJCC TNM classification and stage grouping. World J Surg. 2007;31(04):838–848. doi: 10.1007/s00268-006-0455-0. [DOI] [PubMed] [Google Scholar]
- 26.Cappelli C, Castellano M, Braga M. Aggressiveness and outcome of papillary thyroid carcinoma (PTC) versus microcarcinoma (PMC): a mono-institutional experience. J Surg Oncol. 2007;95(07):555–560. doi: 10.1002/jso.20746. [DOI] [PubMed] [Google Scholar]
- 27.Giordano D, Gradoni P, Oretti G, Molina E, Ferri T. Treatment and prognostic factors of papillary thyroid microcarcinoma. Clin Otolaryngol. 2010;35(02):118–124. doi: 10.1111/j.1749-4486.2010.02085.x. [DOI] [PubMed] [Google Scholar]
- 28.Ross D S, Litofsky D, Ain K B. Recurrence after treatment of micropapillary thyroid cancer. Thyroid. 2009;19(10):1043–1048. doi: 10.1089/thy.2008.0407. [DOI] [PubMed] [Google Scholar]
- 29.Zheng W, Li J, Lv P, Chen Z, Fan P. Treatment efficacy between total thyroidectomy and lobectomy for patients with papillary thyroid microcarcinoma: A systemic review and meta-analysis. Eur J Surg Oncol. 2018;44(11):1679–1684. doi: 10.1016/j.ejso.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Hu G, Zhu W, Yang W, Wang H, Shen L, Zhang H. The Effectiveness of Radioactive Iodine Remnant Ablation for Papillary Thyroid Microcarcinoma: A Systematic Review and Meta-analysis. World J Surg. 2016;40(01):100–109. doi: 10.1007/s00268-015-3346-4. [DOI] [PubMed] [Google Scholar]
- 31.Kwon H, Jeon M J, Kim W G. Lack of Efficacy of Radioiodine Remnant Ablation for Papillary Thyroid Microcarcinoma: Verification Using Inverse Probability of Treatment Weighting. Ann Surg Oncol. 2017;24(09):2596–2602. doi: 10.1245/s10434-017-5910-7. [DOI] [PubMed] [Google Scholar]
- 32.Yang T, Zheng S Y, Jiao J, Zou Q, Zhang Y. Radioiodine remnant ablation in papillary thyroid microcarcinoma: a meta-analysis. Nucl Med Commun. 2019;40(07):711–719. doi: 10.1097/MNM.0000000000001018. [DOI] [PubMed] [Google Scholar]
- 33.Mihailovic J, Stefanovic L, Stankovic R. Influence of initial treatment on the survival and recurrence in patients with differentiated thyroid microcarcinoma. Clin Nucl Med. 2013;38(05):332–338. doi: 10.1097/RLU.0b013e3182872ed2. [DOI] [PubMed] [Google Scholar]
- 34.Soydal C, Araz M, Ozkan E, Arslantaş E, Kucuk O N, Aras G. Assessment of recurrence rates in papillary thyroid microcarcinoma patients with and without histopathological risk factors after radioiodine ablation treatment. Nucl Med Commun. 2015;36(02):109–113. doi: 10.1097/MNM.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 35.Yi D, Song P, Huang T, Tang X, Sang J. A meta-analysis on the effect of operation modes on the recurrence of papillary thyroid microcarcinoma. Oncotarget. 2017;8(04):7148–7156. doi: 10.18632/oncotarget.12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao X, Zhang X, Zhang Y, Hua W, Maimaiti Y, Gao Z. Is papillary thyroid microcarcinoma an indolent tumor?: A retrospective study on 280 cases treated with radioiodine. Medicine (Baltimore) 2016;95(40):e5067. doi: 10.1097/MD.0000000000005067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ardito G, Revelli L, Giustozzi E. Aggressive papillary thyroid microcarcinoma: prognostic factors and therapeutic strategy. Clin Nucl Med. 2013;38(01):25–28. doi: 10.1097/RLU.0b013e318279bc65. [DOI] [PubMed] [Google Scholar]
- 38.Qu N, Zhang L, Ji Q H. Risk Factors for Central Compartment Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Meta-Analysis. World J Surg. 2015;39(10):2459–2470. doi: 10.1007/s00268-015-3108-3. [DOI] [PubMed] [Google Scholar]
- 39.Liu L S, Liang J, Li J H. The incidence and risk factors for central lymph node metastasis in cN0 papillary thyroid microcarcinoma: a meta-analysis. Eur Arch Otorhinolaryngol. 2017;274(03):1327–1338. doi: 10.1007/s00405-016-4302-0. [DOI] [PubMed] [Google Scholar]
- 40.Ruggiero R, Pirozzi R, Gualtieri G. Overview on surgical management of papillary thyroid microcarcinoma. G Chir. 2019;40(02):81–87. [PubMed] [Google Scholar]
- 41.Wang X, Lei J, Wei T, Zhu J, Li Z. Clinicopathological characteristics and recurrence risk of papillary thyroid microcarcinoma in the elderly. Cancer Manag Res. 2019;11:2371–2377. doi: 10.2147/CMAR.S198451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y, Xu L, Wang J. Clinical predictors of lymph node metastasis and survival rate in papillary thyroid microcarcinoma: analysis of 3607 patients at a single institution. J Surg Res. 2018;221:128–134. doi: 10.1016/j.jss.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Yu X M, Wan Y, Sippel R S, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? An analysis of 18,445 cases. Ann Surg. 2011;254(04):653–660. doi: 10.1097/SLA.0b013e318230036d. [DOI] [PubMed] [Google Scholar]
- 44.Pedrazzini L, Baroli A, Marzoli L, Guglielmi R, Papini E. Cancer recurrence in papillary thyroid microcarcinoma: a multivariate analysis on 231 patients with a 12-year follow-up. Minerva Endocrinol. 2013;38(03):269–279. [PubMed] [Google Scholar]
- 45.Siddiqui S, White M G, Antic T. Clinical and Pathologic Predictors of Lymph Node Metastasis and Recurrence in Papillary Thyroid Microcarcinoma. Thyroid. 2016;26(06):807–815. doi: 10.1089/thy.2015.0429. [DOI] [PubMed] [Google Scholar]
- 46.Chow S M, Law S C, Chan J K, Au S K, Yau S, Lau W H. Papillary microcarcinoma of the thyroid-Prognostic significance of lymph node metastasis and multifocality. Cancer. 2003;98(01):31–40. doi: 10.1002/cncr.11442. [DOI] [PubMed] [Google Scholar]
- 47.Mercante G, Frasoldati A, Pedroni C. Prognostic factors affecting neck lymph node recurrence and distant metastasis in papillary microcarcinoma of the thyroid: results of a study in 445 patients. Thyroid. 2009;19(07):707–716. doi: 10.1089/thy.2008.0270. [DOI] [PubMed] [Google Scholar]
- 48.Chéreau N, Buffet C, Trésallet C. Does extracapsular extension impact the prognosis of papillary thyroid microcarcinoma? Ann Surg Oncol. 2014;21(05):1659–1664. doi: 10.1245/s10434-013-3447-y. [DOI] [PubMed] [Google Scholar]
- 49.Baek S K, Jung K Y, Kang S M. Clinical risk factors associated with cervical lymph node recurrence in papillary thyroid carcinoma. Thyroid. 2010;20(02):147–152. doi: 10.1089/thy.2008.0243. [DOI] [PubMed] [Google Scholar]
- 50.Lee J, Song Y, Soh E Y. Central lymph node metastasis is an important prognostic factor in patients with papillary thyroid microcarcinoma. J Korean Med Sci. 2014;29(01):48–52. doi: 10.3346/jkms.2014.29.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lombardi C P, Bellantone R, De Crea C. Papillary thyroid microcarcinoma: extrathyroidal extension, lymph node metastases, and risk factors for recurrence in a high prevalence of goiter area. World J Surg. 2010;34(06):1214–1221. doi: 10.1007/s00268-009-0375-x. [DOI] [PubMed] [Google Scholar]
- 52.Ghossein R, Ganly I, Biagini A, Robenshtok E, Rivera M, Tuttle R M. Prognostic factors in papillary microcarcinoma with emphasis on histologic subtyping: a clinicopathologic study of 148 cases. Thyroid. 2014;24(02):245–253. doi: 10.1089/thy.2012.0645. [DOI] [PubMed] [Google Scholar]
- 53.Chen Y, Sadow P M, Suh H. BRAF(V600E) Is Correlated with Recurrence of Papillary Thyroid Microcarcinoma: A Systematic Review, Multi-Institutional Primary Data Analysis, and Meta-Analysis. Thyroid. 2016;26(02):248–255. doi: 10.1089/thy.2015.0391. [DOI] [PubMed] [Google Scholar]
- 54.Lo C Y, Chan W F, Lang B H, Lam K Y, Wan K Y. Papillary microcarcinoma: is there any difference between clinically overt and occult tumors? World J Surg. 2006;30(05):759–766. doi: 10.1007/s00268-005-0363-8. [DOI] [PubMed] [Google Scholar]
- 55.Radowsky J S, Howard R S, Burch H B, Stojadinovic A. Impact of degree of extrathyroidal extension of disease on papillary thyroid cancer outcome. Thyroid. 2014;24(02):241–244. doi: 10.1089/thy.2012.0567. [DOI] [PubMed] [Google Scholar]
- 56.Jin B J, Kim M K, Ji Y B, Song C M, Park J H, Tae K. Characteristics and significance of minimal and maximal extrathyroidal extension in papillary thyroid carcinoma. Oral Oncol. 2015;51(08):759–763. doi: 10.1016/j.oraloncology.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 57.Shin J H, Ha T K, Park H K. Implication of minimal extrathyroidal extension as a prognostic factor in papillary thyroid carcinoma. Int J Surg. 2013;11(09):944–947. doi: 10.1016/j.ijsu.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 58.Qu H, Sun G R, Liu Y, He Q S. Clinical risk factors for central lymph node metastasis in papillary thyroid carcinoma: a systematic review and meta-analysis. Clin Endocrinol (Oxf) 2015;83(01):124–132. doi: 10.1111/cen.12583. [DOI] [PubMed] [Google Scholar]
- 59.Vita R, Ieni A, Tuccari G, Benvenga S. The increasing prevalence of chronic lymphocytic thyroiditis in papillary microcarcinoma. Rev Endocr Metab Disord. 2018;19(04):301–309. doi: 10.1007/s11154-018-9474-z. [DOI] [PubMed] [Google Scholar]
- 60.Wang K, Xu J, Li S, Liu S, Zhang L. Population-based study evaluating and predicting the probability of death resulting from thyroid cancer among patients with papillary thyroid microcarcinoma. Cancer Med. 2019;8(16):6977–6985. doi: 10.1002/cam4.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baudin E, Travagli J P, Ropers J. Microcarcinoma of the thyroid gland: the Gustave-Roussy Institute experience. Cancer. 1998;83(03):553–559. doi: 10.1002/(sici)1097-0142(19980801)83:3<553::aid-cncr25>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 62.Godbert Y, Henriques-Figueiredo B, Cazeau A L. A papillary thyroid microcarcinoma revealed by a single bone lesion with no poor prognostic factors. Case Rep Endocrinol. 2013;2013:719304. doi: 10.1155/2013/719304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tuttle R M, Morris L F, Haughen B R. Springer; New York: 2017. Thyroid - Differentiated and Anaplastic Carcinoma; p. 873. [Google Scholar]
- 64.Stefanova D I, Bose A, Ullmann T M. Does the ATA Risk Stratification Apply to Patients with Papillary Thyroid Microcarcinoma? World J Surg. 2020;44(02):452–460. doi: 10.1007/s00268-019-05215-4. [DOI] [PubMed] [Google Scholar]
- 65.Tuttle R M, Tala H, Shah J. Estimating risk of recurrence in differentiated thyroid cancer after total thyroidectomy and radioactive iodine remnant ablation: using response to therapy variables to modify the initial risk estimates predicted by the new American Thyroid Association staging system. Thyroid. 2010;20(12):1341–1349. doi: 10.1089/thy.2010.0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Verburg F A, Stokkel M P, Düren C. No survival difference after successful (131)I ablation between patients with initially low-risk and high-risk differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2010;37(02):276–283. doi: 10.1007/s00259-009-1315-6. [DOI] [PubMed] [Google Scholar]


