Abstract
Background:
In recent times, the US-FDA approved istradefylline and opicapone as an adjunct to levodopa/carbidopa for managing the "off" episodes in Parkinson’s disease.
Purpose:
Current meta-analysis was performed to determine the safety and efficacy of these drugs in the management of “off” episodes and to recognize which among them would provide therapeutic benefits clinically.
Methods:
A thorough literature search was performed through the Cochrane Library, PubMed, and clinicaltrials.gov for a period from January 2003 to October 2020, with the following keywords: Istradefylline, KW-6002, opicapone, BIA 9-1067, and Parkinson’s disease. Those randomized, double-blind placebo/active comparator-controlled trials that analyzed the efficacy and safety of istradefylline and opicapone and that were published in the English language were included. In this analysis, the outcomes focused on the least square mean change in “off” time and Unified Parkinson’s Disability Rating Scale (UPDRS) III score from baseline to the end of the study, and the incidence of treatment-emergent adverse events (TEAEs) and dyskinesia.
Results:
Both drugs have shown significant reduction in “off” time duration (mean difference [MD] = –0.70; 95% CI [–1.11, –0.30]; P < 0.001 for istradefylline and MD = –0.85; 95% CI [–1.09, –0.61]; P < .001 for opicapone). Istradefylline showed significant improvement in UPDRS III (MD = –1.56; 95% CI [–2.71, –0.40]; P < .008), but the same was not observed with opicapone (MD = –0.63; 95% CI [–1.42, –0.15]; P < .12). The incidence of TEAEs and dyskinesia reportedly were higher in the intervention group rather than with the placebo, (risk ratio RR =1.11, 95% CI [1.02,1.20] for istradefylline and RR =1.12, 95% CI [1.00,1.25] for opicapone, and for dyskinesia particularly, the incidence was higher with opicapone as compared to istradefylline (RR = 3.47, 95% CI [2.17, 5.57], and RR = 1.77, 95% CI [1.29, 2.44], respectively).
Conclusions:
Both drugs were comparable in efficacy; however, istradefylline seemed to be better in reducing the UPDRS III score. Although the incidence of TEAEs and dyskinesia were higher with both the drugs, the incidence of dyskinesia was more in the opicapone group.
Keywords: Opicapone, Istradefylline, “Off” episodes, Parkinson’s disease
Introduction
Parkinson’s disease (PD), the second most common progressive neurodegenerative disorder, with an annual incidence of 11-19/100000, has affected more than six million people globally.1, 2 The pathological changes like loss of dopaminergic neurons from substantia nigra and accumulation of Lewy bodies in the remaining neurons, along with mitochondrial dysfunction, endoplasmic reticulum stress (ER), altered ER-Golgi transport, and proteotoxicity secondary to accumulation of over-produced α-synuclein, are responsible for bringing about an array of clinical features. 3 The patients of PD experience both motor (bradykinesia, tremors, shuffling gait, postural instability, and muscular rigidity) and nonmotor (flat affect, excessive salivation, staring appearance, autonomic dysfunction, and psychiatric disorder) clinical features during the disease.4, 5
Among the wide variety of pharmacotherapeutic options available to manage a case of PD, like dopamine precursors with peripheral dopa decarboxylase inhibitors, dopamine agonists, anticholinergics, monoamine oxidase B (MAO-B) inhibitors, and catechol-o-methyl transferase (COMT) inhibitors, the levodopa/carbidopa combination therapy stands out to be a gold standard treatment as it has been seen that levodopa can provide persistent mobility benefits. In contrast, carbidopa helps to maintain an appropriate level of levodopa in the substantia nigra.6–8 But with the chronic use of this combination, the therapeutic effects start to wane off (wearing off phenomenon) and gradually get outweighed by the adverse effects like “on-off” fluctuations with or without dyskinesia. 7 This “on-off” phenomenon is clinically symbolized by the absence and reappearance of motor symptoms while the patient is on therapy. 9 Moreover, once these fluctuations deteriorate the quality of life, they become troublesome. To alleviate these symptoms, the drugs which can be utilized as an adjunct can either be a potent dopaminergic agonist (apomorphine) or inhibitor of enzymes that are responsible for degrading dopamine (COMT inhibitors and selective MAO-B inhibitors) or noncompetitive inhibitor of NMDA receptors (amantadine). 10
Among these groups, istradefylline and opicapone have been recently approved by the US-FDA to manage “off” episodes in PD.11–12 With the intermittent levodopa therapy, there occurs an up-regulation of adenosine A2A receptors, contributing to the precipitation-of “off” episodes. Based on this mechanism, the action of istradefylline, a selective A2A receptor antagonist, has been defined in managing the “off” episodes by enhancing the effects of levodopa. 7 While for opicapone, a COMT inhibitor produces its effects by inhibiting the enzyme catechol-o-methyltransferase, which is responsible for degrading levodopa in the periphery, thus increasing its level in Central Nervous System (CNS). 13 Since both these drugs have been introduced recently for managing the same condition, this meta-analysis was conducted to compare and appraise their efficacy and safety in patients of PD who are already on levodopa/carbidopa therapy and are experiencing “off” episodes.
Methods
This meta-analysis was performed as per the guidelines of preferred reporting items for systematic reviews and meta-analyses statement (PRISMA). 14 Furthermore, no ethical approval was required for this review.
Literature Search and Data Extraction
Two authors performed the systematic search (Alok Singh and Dhyuti Gupta) among the databases PubMed, Cochrane library, and Clinical Trial Registry https://clinicaltrials.gov/ for a timeline from January 2003 to October 2020 using the following keywords: “istradefylline, KW-6002, and Parkinson’s disease,” and “opicapone, BIA 9-1067, and Parkinson’s disease.” Other studies were also searched for any missing data, if any.
Patients: Adults with Parkinson’s disease.
Intervention/Comparator: Istradefylline (40 mg/d), opicapone (50 mg/d), placebo/ active comparator. Both drugs were used as an add-on to levodopa/carbidopa, and their maximal recommended doses were compared for their efficacy and safety.
Primary Outcome: Least square mean change in daily “off” time duration from baseline to end-point.
-
Secondary Outcomes:
The least-square mean change in Unified Parkinson’s Disability Rating Scale (UPDRS) part III in the “on” state from baseline to end-point.
Incidence of Treatment-Emergent Adverse Events (TEAE).
Incidence of dyskinesia.
Study Design: Data extraction was performed by two authors. The primary demographic details from all the clinical trials assessing the safety and efficacy of istradefylline or opicapone against placebo/active comparator among PD patients were also noted.
Figure 1. PICOS Criteria.
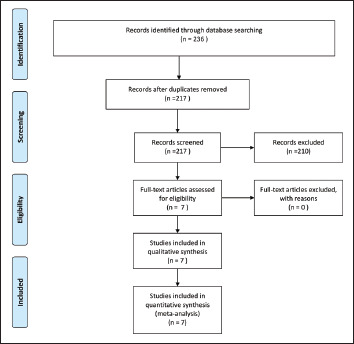
Source: The authors.
Risk of Bias Assessment
Quality assessment of the individual study and the risk of bias was performed as per the preferred reporting items for systematic reviews and meta-analyses statement (PRISMA) guidelines and duly demonstrated in the forest plot.
Statistical Analysis
The authors’ assessed the MD and RR with a confidence interval (CIs) of 95% for continuous and dichotomous variables, respectively. In missing data (standard deviation), the highest values were imputed from other studies for the same parameter. 15 I2 statistics (I2 > 50% indicated heterogeneity) were used to check for heterogeneity among the included studies. 16 This meta-analysis was performed using a random-effect model with Review Manager v5.4 for windows. Moreover, we obtained a funnel plot to assess for any publication bias (of the primary outcome).
Results
Literature Search and Study Characteristics
The initial search yielded 236 studies, that is, 57 of istradefylline and 179 of opicapone. On further exploring the clinical trial registry https://clinicaltrials.gov/, we found a total of 11 clinical trials for istradefylline and five for opicapone belonging to different phases. On the other hand, in PubMed, we found a total of ten published clinical trials for istradefylline and nine for opicapone. The inclusion criteria set by the authors were met by four clinical trials of istradefylline and three clinical trials of opicapone.17–23 The reasons for excluding other studies were the pooled analysis of trials, pharmacokinetic studies, different end points, and different doses of the drugs. The relevant characteristics of the included studies have been mentioned in Table 1. The diagnosis of PD among all the included trials was established based on the United Kingdom Parkinson’s Disease Society Criteria, and the majority of the recruited participants were male. 24 In a few studies, the standard deviation (SD) for continuous data was missing; hence these values were imputed from other studies with maximum values being considered. 15
Table 1. Important Characteristics of Included Clinical Trials.
| Study | N | Study Design | No. of Patients in Each Arm | Dose (mg/day) | Age (y) mean ± SD | % Male |
| Lewitt et al. 2008 | 196 | RDBPCT | IST: 130 PLB: 66 |
IST: 40 | IST: 63 ± 9 PLB:64 ± 10 |
59.7 (117/196) |
| Mizuno et al. 2010 | 357 | RDBPCT | IST: 124 PLB: 118 |
IST: 40 | IST: 63.7 ± 8.6 PLB: 65 ± 7.6 |
42.1 (150/357) |
| Pourcheret al. 2012 | 605 | RDBPCT | IST: 152 PLB: 151 |
IST: 40 | IST: 63 ± 9.3 PLB: 63 ± 8.3 |
66.6 (403/605) |
| Mizuno et al. 2013 | 366 | RDBPCT | IST: 123 PLB: 123 |
IST: 40 | IST: 65.7 ± 9 PLB: 65.8 ± 8.6 |
44.3 (162/366) |
| Ferreira et al. 2016 | 599 | RDBPCT | OPC: 115 PLB: 121 |
OPC: 50 | OPC: 63.5 ± 9.2 PLB: 64.3 ± 9.3 |
59.1 (354/599) |
| Lees et al. 2017 | 407 | RDBPCT | OPC: 135 PLB: 147 |
OPC: 50 | OPC: 65.5 ± 8.4 PLB: 61.5 ± 8.9 |
59.5 (242/407) |
| Takeda et al. 2020 | 437 | RDBPCT | OPC: 145 PLB: 147 |
OPC: 50 | OPC: 67.4 ± 7.8 PLB: 68.5 ± 8.6 |
39.8 (174/437) |
Source: The authors.
Abbreviations: RDBPCT: Randomized double-blind placebo-controlled clinical trial; IST: Istradefylline; OPC: Opicapone; PLB: Placebo
Primary Outcome
Based on the funnel plot obtained for the primary outcome, the authors did not observe any publication bias (Figure 2), but heterogeneity was observed (I2 = 95%, heterogeneity P < .00001) among these trials (Figure 3). For the least square mean change in time spent in “off” episodes, both drugs showed a significant reduction from baseline to the end of the study period (MD = –0.70; 95% CI [–1.11, –0.30]; P < 0.001 for istradefylline and MD = –0.85; 95% CI [–1.09, –0.61]; P < .001 for opicapone), with opicapone performing marginally better than istradefylline (P = .56; Figure 3).
Figure 2. Funnel Plot for Studies.
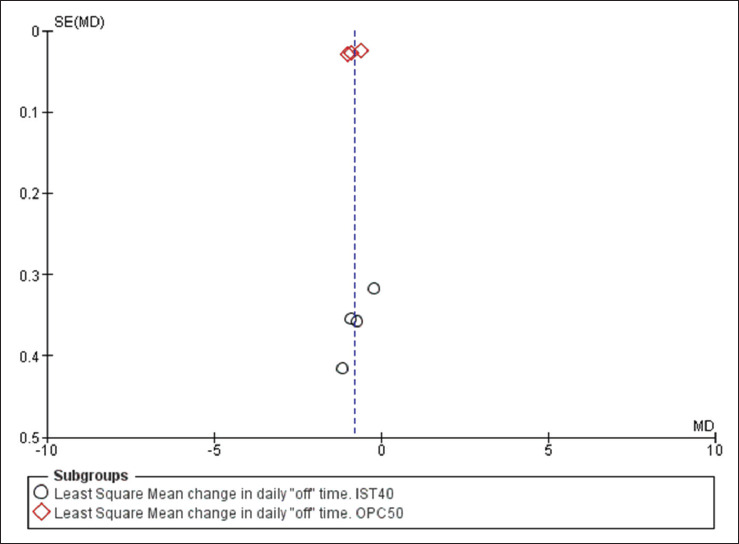
Source: The authors.
Figure 3. Forest Plot for Least Square Mean Reduction in “off’ Time From Baseline to End Point.
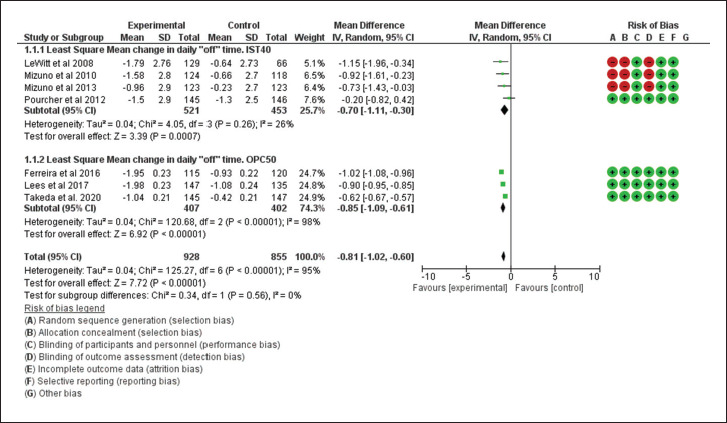
Source: The authors.
Secondary outcomes
For the secondary outcomes, the performance of istradefylline was significantly better than the placebo (MD = –1.56; 95% CI [–2.71, –0.40]; P < .008), but the same cannot be inferred for opicapone (MD = –0.63; 95% CI [–1.42, –0.15]; P < .12). The patients who were randomized to receive istradefylline reported an improvement in the UPDRS III score, but it was insignificant compared to opicapone users (MD: –1.56 vs –0.63; P = .20; Figure 4).
Figure 4. Forest Plot for Least Square Mean Reduction in UPDRS III From Baseline to End Point.
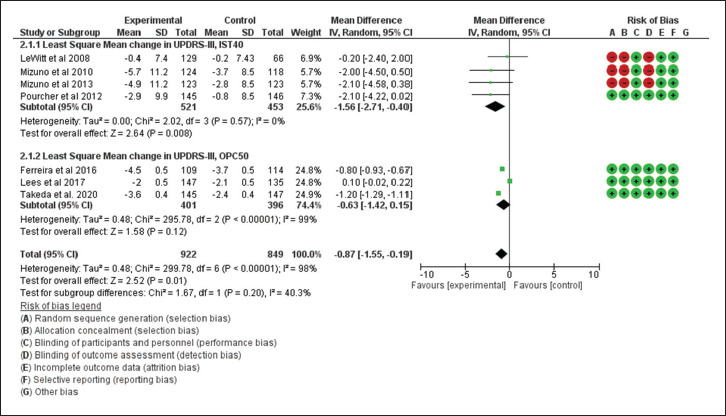
Source: The authors.
Broadly referring to the treatment-emergent adverse events (TEAEs), the users of drugs, that is, istradefylline and opicapone, reported more TEAEs than those who were on placebo adjunct (RR = 1.11, 95% CI [1.02, 1.2] for istradefylline and RR = 1.12, 95% CI [1.00, 1.25] for opicapone). However, the risk was still comparable among these drugs (P = 0.88; Figure 5). Furthermore, the incidence of dyskinesia among the active drug users in trials was more as compared to the placebo (RR = 1.77, 95% CI [1.29, 2.44] for istradefylline and RR = 3.47, 95% CI [2.17, 5.57] for opicapone), with the patient randomized to receive opicapone reporting more significantly of it than those who received istradefylline (P = .02; Figure 6). A comparison of different outcomes is presented in Table 2.
Figure 5. Forest Plot for Incidence of Treatment-emergent Adverse Events.
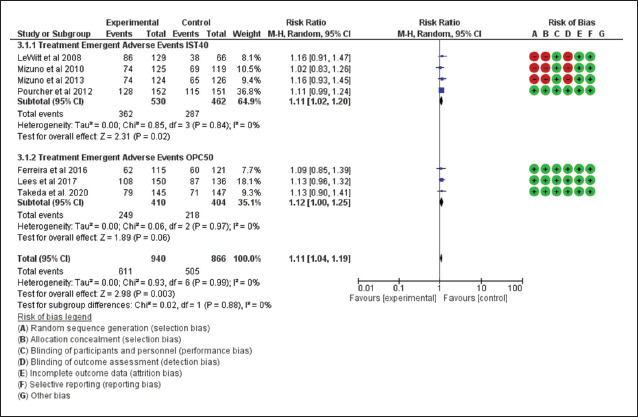
Source: The authors.
Figure 6. Forest Plot for Incidence of Dyskinesia.
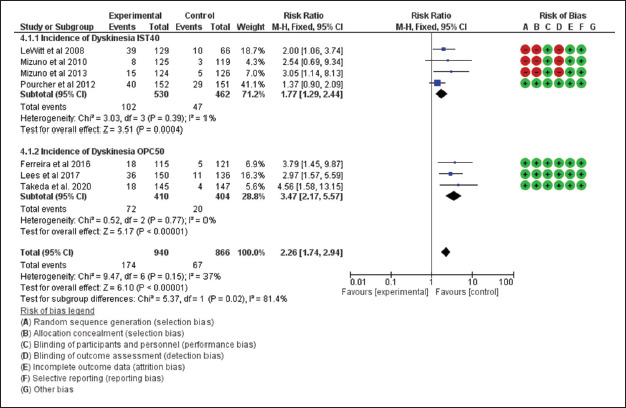
Source: The authors.
Table 2. Comparison Between Istradefylline and Opicapone for Different Outcomes.
| S. No. | Outcomes | IST40 | OPC50 | P-Value |
| 1. | The least-square mean change in daily "off" time duration from baseline to end-point (MD compared to placebo) | –0.70 | –0.85 | .56 |
| 2. | Least square mean change in Unified Parkinson’s Disability Rating Scale (UPDRS) part III in the “on” state from baseline to end-point. (MD compared to placebo) | –1.56 | –0.63 | .2 |
| 3. | Incidence of treatment-emergent adverse events (RR) | 1.11 | 1.12 | .88 |
| 4. | Incidence of dyskinesia (RR) | 1.77 | 3.47 | .02* |
Source: The authors.
Note: * statistically significant.
Abbreviations: IST: Istradefylline; OPC: Opicapone; MD: Mean difference; RR: Risk ratio
Discussion
The present meta-analysis includes two recently approved drugs for “off” episodes in PD. Our study incorporates randomized clinical trials, which were mostly of high quality. We found that certain biases might have existed, that is, selection and detection bias in few studies (mentioned in forest plot).17, 19, 20 The possible explanations for biases were inappropriate information provided in the text regarding the method of randomization, allocation concealment, and outcome assessment. In few studies, SD was not mentioned, imputed from other studies, and the maximum value was taken, which is the simplest method of imputation. 15 In general, all the included trials had a low number of participants (196–605). Although PD is the most common movement disorder of neurodegenerative nature, the absolute incidence is low, that is, 11–19/100000 per year, which may be one of the reasons for the low number of participants. 25 Clinically, the “on-off” phenomenon starts manifesting after a few years of treatment; this may also be a contributing factor in relatively fewer participants. Most of the participants of the included trials were male and were in the sixth decade of their life that follows the usual epidemiological trend. 25
For the efficacy outcomes, both drugs showed a significant reduction in “off” time and an improvement in UPDRS III score from baseline to end-point as compared to placebo. The reduction in “off” time by opicapone was slightly higher than by istradefylline. Reduction in "off" time indicates that both drugs restored the effectiveness of levodopa/carbidopa, which was compromised due to ongoing neurodegeneration, though by different mechanisms. UPDRS is used to assess the symptoms of Parkinson’s and is divided into four segments, in which part III deals with the motor symptoms of PD. The adenosine antagonist, that is, istradefylline, had better improvement than opicapone in UPDRS III “on” state. The adenosine receptor heterodimerizes with the dopamine receptor (D2), thereby modulates dopamine response and motor behavior. 7 Chronic levodopa therapy possibly up-regulates the adenosine receptor and may be responsible for the precipitation of “off” episodes. Istradefylline may prevent heterodimerization, leading to increased dopaminergic activity and possibly explaining the better UPDRS III score. The effects of istradefylline (40 mg) were similar to the previous meta-analysis.26–29 On the other hand, an increase in levodopa level in CNS by opicapone may be responsible for the significant reduction in “off” time; however, the same was not observed with UPDRS III. The positive correlation of levodopa and improvement in UPDRS III has been shown in previous studies.30–32 As opicapone’s primary mechanism involves increased availability of levodopa into the CNS, it was expected to improve UPDRS III significantly. This unanticipated finding may be attributable to a severe loss of dopaminergic neurons to the extent that even levodopa and opicapone could not improve UPDRS III along with lack of any modulatory effect of opicapone on DRs. More clinical studies are needed to substantiate this finding.
Both drugs had a similar incidence of TEAEs, but the incidence of dyskinesia was more with opicapone, and it was statistically significant. Increased incidence of dyskinesia with opicapone may be attributable to the increased availability of levodopa in CNS, and this effect lacks in istradefylline. This also agrees that istradefylline is less effective in reducing “off” time duration and has less dyskinesia. As “off” episodes are usually encountered after five to seven years of therapy with ongoing neurodegeneration, the drugs offer nonphysiologic replacement of dopamine possibly due to the increase in levodopa, which is responsible for its beneficial effects, is also responsible for the dyskinesia as the replacement is nonphysiologic. The development of dyskinesia may be a limiting factor in the wide acceptance of opicapone.
Both drugs had common adverse effects of dopamine excess in CNS, that is, hallucination(s) and impulse control disorder; however, hallucinations are more common with istradefylline. Hallucinations with istradefylline have been reported in every clinical trial, but only one clinical trial reported hallucination with opicapone.17–20, 23 Further on carefully observing clinical trials of istradefylline, the hallucination appears to be dose-dependent. This fact has also been substantiated in the real-world study of istradefylline. 33 In addition to these adverse effects, opicapone had peripheral adverse effects of dopamine excess, that is, hypotension and arrhythmias, which can be dangerous in the geriatric population. Both drugs must be avoided in case of severe hepatic impairment, and dose reduction is required in moderate hepatic dysfunction. The opicapone is to be taken without food, whereas istradefylline can be taken with or without food.34, 35
Limitations
This study has got certain limitations, that is, a relatively fewer number of studies were included, and the included studies were of relatively less duration (12–14 weeks). The missing SD in some of the studies was imputed from other studies.
Conclusion
Practically, the “off” phenomena can be considered a change in a PD patient's clinical state where motor and/or nonmotor symptoms appear or worsen, resulting in functional disability. Amongst the various classes of drugs available to manage PD, nondopaminergic drugs can be utilized to manage “off” episodes with the long-term levodopa/carbidopa therapy, and a detrimental effect eventually develops, that being “on-off” fluctuations. This manifestation is characterized by an unpredictable switch from being free of any PD symptoms (on phase) to experiencing worsening symptoms and functional disability (off phase).7, 36 A few drugs amongst the group mentioned earlier have been tried to resolve this complication (like COMT inhibitors, selective MAO-B inhibitors, and apomorphine), but either due to incomplete effectiveness or owing to unfavorable administration regime and associated adverse effects, there is still a gap in the therapy of PD. 7
In such a scenario, both the newly approved drugs may appear promising in alleviating the phenomena of “yo-yoing,” as both have been approved for the same condition, in conjunction with levodopa/carbidopa therapy. Moreover, they have shown a reduction in duration of “off” time, which was comparatively better with opicapone. While on the other hand, istradefylline showed a better response in improving UPDRS III. Both the drugs had relatable/comparable TEAEs, but where opicapone was associated with a significantly higher incidence of dyskinesia, istradefylline was observed to produce hallucinations. Considering all these facts, istradefylline does appear to be superior to opicapone, yet this finding needs to be corroborated as a few of the included studies had certain biases and missing data. Despite the limitations, both drugs appear to be effective in managing “off” episodes in PD.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
ORCID iD: Alok Singh  https://orcid.org/0000-0001-6720-2756
https://orcid.org/0000-0001-6720-2756
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Statement
The study does not require ethical approval.
References
- 1.Sveinbjornsdottir S. The clinical symptoms of Parkinson's disease. J Neurochem 2016; 139: 318–324. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2016 Parkinson's Disease collaborators. Global, regional, and national burden of Parkinson's disease, 1990–2016: Asystematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17(11): 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganguly U, Chakrabarti SS, Kaur U, et al. Alpha-synuclein, proteotoxicity, and Parkinson's disease: Search for neuroprotective therapy. Curr Neuropharmacol 2018; 16(7): 1086–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim SD, Allen NE, Canning CG, et al. Parkinson disease. Handb Clin Neurol 2018; 159: 173–193. [DOI] [PubMed] [Google Scholar]
- 5.Capriotti T and Terzakis K.. Parkinson disease. Home Healthc Now. 2016; 34(6): 300–307. [DOI] [PubMed] [Google Scholar]
- 6.Hayes MT. Parkinson’s disease and parkinsonism. Am J Med 2019; 132(7): 802–807. [DOI] [PubMed] [Google Scholar]
- 7.Sahoo AK, Gupta D, Singh A.. Istradefylline: A novel drug for ‘off’ episodes in Parkinson’s disease. Drugs Ther Perspect 2020; 36: 208–212. [Google Scholar]
- 8.Armstrong MJ, Okun MS.. Diagnosis and treatment of parkinson disease: A review. JAMA 2020; 323(6), 548–560. [DOI] [PubMed] [Google Scholar]
- 9.Müller T. Safinamide: An add-on treatment for managing Parkinson’s disease. Clin Pharmacol 2018; 10: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vijiaratnam N and Foltynie T.. Therapeutic strategies to treat or prevent off episodes in adults with Parkinson’s Disease. Drugs 2020; 80: 775–796. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food & Drug Administration. Novel Drug Approvals for 2019. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2019 (accessed October 27, 2020).
- 12.U.S. Food & Drug Administration. Novel Drug Approvals for 2020. https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020 (accessed October 27, 2020).
- 13.Fabbri M, Ferreira JJ, Lees A, et al. Opicapone for the treatment of Parkinson's disease: A review of a new licensed medicine. Mov Disord 2018; 33: 1528–1539. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
- 16.Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21(11): 1539–1558. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno Y, Kondo T.. istradefylline study group. Adenosine A2A receptor antagonist istradefylline reduces daily OFF time in Parkinson's disease. Mov Disord 2013; 28(8): 1138–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pourcher E, Fernandez HH, Stacy M, et al. Istradefylline for Parkinson's disease patients experiencing motor fluctuations: Results of the KW-6002-US-018 study. Parkinsonism Relat Disord 2012; 18(2): 178–184. [DOI] [PubMed] [Google Scholar]
- 19.Mizuno Y, Hasegawa K, Kondo T, et al. Clinical efficacy of istradefylline (KW-6002) in Parkinson's disease: A randomized, controlled study. Mov Disord 2010 July 30; 25(10): 1437–1443. [DOI] [PubMed] [Google Scholar]
- 20.LeWitt PA, Guttman M, Tetrud JW, et al. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces "off" time in Parkinson's disease: A double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol 2008; 63(3): 295–302. [DOI] [PubMed] [Google Scholar]
- 21.Takeda A, Takahashi R, Tsuboi Y, et al. Randomized, controlled study of opicapone in Japanese Parkinson's patients with motor fluctuations. Mov Disord 2021; 36(2): 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lees AJ, Ferreira J, Rascol O, et al. Opicapone as adjunct to Levodopa therapy in patients with Parkinson disease and motor fluctuations: A randomized clinical trial. JAMA Neurol 2017; 74(2): 197–206. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira JJ, Lees A, Rocha JF, et al. Opicapone as an adjunct to levodopa in patients with Parkinson's disease and end-of-dose motor fluctuations: A randomised, double-blind, controlled trial. Lancet Neurol 2016; 15(2): 154–165. [DOI] [PubMed] [Google Scholar]
- 24.Hughes AJ, Daniel SE, Kilford L, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992; 55(3): 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balestrino R and Schapira AHV.. Parkinson disease. Eur J Neurol 2020. January; 27(1): 27–42. [DOI] [PubMed] [Google Scholar]
- 26.Sako W, Murakami N, Motohama K, et al. The effect of istradefylline for Parkinson's disease: A meta-analysis. Sci Rep 2017. December 21; 7(1): 18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen W, Wang H, Wei H, et al. Istradefylline, an adenosine A2A receptor antagonist, for patients with Parkinson's Disease: A meta-analysis. J Neurol Sci 2013. January 15; 324(1–2): 21–28. [DOI] [PubMed] [Google Scholar]
- 28.Tao Y and Liang G.. Efficacy of adenosine A2A receptor antagonist istradefylline as augmentation for Parkinson's disease: A meta-analysis of randomized controlled trials. Cell Biochem Biophys 2015; 71(1): 57–62. [DOI] [PubMed] [Google Scholar]
- 29.Zhu C, Wang G, Li J, et al. Adenosine A2A receptor antagonist istradefylline 20 versus 40 mg/day as augmentation for Parkinson's disease: A meta-analysis. Neurol Res 2014; 36(11): 1028–1034. [DOI] [PubMed] [Google Scholar]
- 30.Goetz CG, Thelen JA, MacLeod CM, et al. Blood levodopa levels and unified Parkinson's disease rating scale function: With and without exercise. Neurology 1993; 43(5): 1040–1042. [DOI] [PubMed] [Google Scholar]
- 31.Schade S, Sixel-Döring F, Ebentheuer J, et al. Acute levodopa challenge test in patients with de novo Parkinson's disease: Data from the DeNoPa cohort. Mov Disord Clin Pract 2017; 4(5): 755–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pieterman M, Adams S, Jog M.. Method of levodopa response calculation determines strength of association with clinical factors in Parkinson disease. Front Neurol 2018; 9: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh A. Implications of istradefylline induced hallucinations. Encephale 2021;47(2):179-180. [DOI] [PubMed] [Google Scholar]
- 34.Highlights of prescribing information (istradefylline). https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022075s000lbl.pdf (accessed October 29, 2020).
- 35.Highlights of prescribing information (opicapone). https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212489s000lbl.pdf (accessed October 29, 2020).
- 36.Liang T -W, Weissfeld T, Bober T.. New developments in the treatment of Parkinson's disease: Bridging the gap between medical and surgical therapy for PD (Article 4). JHN J; 11(2). : 28–31 [Google Scholar]


