Abstract
Background:
Vestibular dysfunction, characterized by nausea, dizziness, imbalance, and/or gait disturbance, represents an important sport-related concussion (SRC) subtype associated with prolonged recovery. Vestibular physical therapy promotes recovery; however, the benefit of earlier therapy is unclear.
Hypothesis:
Earlier vestibular therapy for young athletes with SRC is associated with earlier return to play (RTP), return to learn (RTL), and symptom resolution.
Study Design:
Retrospective cohort study.
Level of Evidence:
Level 3.
Methods:
Patients aged 5 to 23 years with SRC who initiated vestibular rehabilitation therapy (VRT) from January 2019 to December 2019 were included and patient records were reviewed. Therapy initiation was defined as either early, ≤30 days postinjury, or late (>30 days). Univariate comparisons between groups, Kaplan-Meier plots, and multivariate Cox proportional hazard modeling were performed.
Results:
Overall, 23 patients (10 early, 13 late) aged 16.14 ± 2.98 years and 43.5% were male patients. There was no difference between group demographics or medical history. Median initial total and vestibular symptom scores were comparable between groups. The late therapy group required additional time to RTP (110 days [61.3, 150.8] vs 31 days [22.5, 74.5], P = 0.03) and to achieve symptom resolution (121.5 days [71, 222.8] vs 54 days [27, 91], P = 0.02), but not to RTL (12 days [3.5, 26.5] vs 17.5 days [8, 20.75], P = 0.09). Adjusting for age and initial total symptom score, earlier therapy was protective against delayed symptom resolution (P = 0.01).
Conclusion:
This pilot study suggests that initiating VRT within the first 30 days after SRC is associated with earlier RTP and symptom resolution. Further prospective trials to evaluate if even earlier VRT should be pursued to further improve recovery time.
Clinical Relevance:
Clinicians should screen for vestibular dysfunction and consider modifying follow-up schedules after SRC to initiate VRT within a month of injury for improved outcomes.
Keywords: vestibular rehabilitation therapy (VRT), sport-related concussion (SRC), pediatric concussion, vestibular dysfunction
Sport-related concussion (SRC) is a subset of mild traumatic brain injury,4,9 suffered during a sporting activity. Signs and symptoms of SRC are variable and affect a variety of domains.8,14 Given multiple classifications schemes,8,14,24 Collins et al 6 proposed a framework for compartmentalizing concussion into 6 categories based on clinical trajectory and treatment: vestibular, oculomotor, cognitive, posttraumatic migraine, cervical, and anxiety/mood. 6 Symptoms of the vestibular subtype include dizziness, nausea, fogginess, possible balance issues, and exacerbation of symptoms with head movement. 6 Treatment includes comprehensive vestibular rehabilitation therapy (VRT), 6 which is patient-specific but can include targeted gaze-stability training, graded exposure to visually stimulating environments, and dynamic balance training. 19 Multiple studies1-3,5,11,16-19,23 have sought to determine the efficacy of VRT for the treatment of concussed patients. Specifically, adolescents with postconcussion syndrome (PCS) and treated with VRT are nearly 4 times more likely to have medical clearance to return to sports within 2 months of initiating therapy. 22 Despite our current understanding of VRT effectiveness in treating the symptoms of vestibular SRC, the ideal timing of therapy is unknown. We sought to explore the relationship between the timing of therapy and outcome in young athletes after SRC. We hypothesized that earlier therapy initiation would be associated with improved outcomes.
Methods
Study Design and Ethics Statement
A single-institution retrospective cohort study was designed to evaluate the association of timing of VRT initiation and key SRC outcomes of symptom resolution, return to play (RTP), and return to learn (RTL). Before initiation, the investigation received approval and a waiver of informed consent from the institutional review board (No. 192033).
Patient Selection
An internal database of patients who initiated VRT from January 2019 to December 2019 through our concussion center was screened. Inclusion criteria included (1) patients aged 5 to 23 years, (2) a diagnosis of SRC, (3) an evaluation in sport concussion clinic, and (4) having received vestibular therapy. Exclusion criteria included (1) improper age (n = 12), (2) non-SRC (n = 6), and (3) lost to follow-up before achieving any endpoint (n = 8). After application of these criteria, 49 patient records were reviewed, and 23 athletes were included for the purpose of this study.
Patients were grouped by timing of therapy initiation. Early was defined as ≤30 days after injury and late was defined as >30 days. Thirty days was chosen not only to allow for equal groups but also because this is the period after which a diagnosis of PCS would be appropriate. 21 Therefore, this grouping served as a proxy for starting VRT before, or after, the diagnosis of PCS.
Clinical Management
Patients were evaluated initially in our specialty sport concussion clinic. As per our standard practice, referral to additional providers, including physical and occupational therapists, was left to the discretion of the primary treating physician. All referring providers in this study are experienced and board-certified in pediatric sports medicine or neuropsychology. VRT for this study was performed by a single occupational therapist with training in visual processing deficits (posttraumatic vision syndrome) that occur from traumatic brain injury (TBI), as well as in TBI-associated vestibular dysfunction. Typical treatment included the following: gaze stabilization exercises, exercises to address oculomotor dysfunction (convergence insufficiency, saccadic eye movement disorder, accommodative dysfunction, etc), seated vestibular-ocular reflex exercises with progression to include balance loads and exercises to address visual motion sensitivity.7,25 The exercises primarily included adaptation or substitution and also habituation in some circumstances. 25 Each patient was provided with individualized VRT based on his or her symptoms and physical examination.
Data Collection
Manual chart review was performed to obtain the following information: demographics and medical histories, days to concussion clinic, Post-Concussion Symptom Scale (PCSS), days to VRT, and outcomes. Four patients did not have a full PCSS to review and 2 of these did not have initial total scores available.
Outcomes included symptom resolution date, RTP date, and RTL date. Symptom resolution was defined as a PCSS of 0, the concussion specialist stating the patient was “recovered,” or occupational therapist stating the patient was no longer symptomatic and/or successfully discharged from VRT. RTP was defined the date cleared to progress along the graduated RTP protocol. If the patient was cleared to progress, but failed, the RTP date was the initiation of the successful RTP progression (n = 1). RTL was simply defined as returning to school with or without academic accommodations. If available in progress notes, the exact date of return or symptom resolution was recorded. If no exact date was available, the date of the note mentioning the achieved the outcome was used. Two patients were missing partial outcome data. One patient is continuing care and has not had achieved symptom resolution or RTP. One patient had finished their sports career (high school graduate) but achieved symptom resolution.
Statistical Analysis
Categorical variables are presented as frequency and proportion. Continuous variables are presented as median and interquartile range (IQR) given nonparametric distributions. No data were imputed. Differences between the early and late vestibular therapy groups were assessed using Mann-Whitney U, chi-square, and Fischer exact tests. Kaplan-Meier plots were constructed and groups compared using Mantel-Cox log rank tests. Multivariate Cox proportional hazards regression was used to identify important factors associated with achieving each outcome. Statistical significance was determined a priori to be at P < 0.05. Statistical analysis was performed using SPSS Version 21 (IBM Corp).
Results
Overall, 23 patients were included in the study (10 early, 13 late) with an average age of 16.14 ± 2.98 years. The full cohort had median total PCSS score of 47 (IQR: 9.25, 70) and median vestibular score of 2 (IQR: 0, 9). Groups were similar in terms of age, sex, and medical history (Table 1). Patients in both groups presented to concussion clinic at similar times with similar vestibular symptom subset score and total PCSS scores (Tables 2 and 3).
Table 1.
Demographic information and medical history
| Variable | Early Therapy (≤30 d), n = 10 | Late Therapy (>30 d), n = 13 | P |
|---|---|---|---|
| Age at concussion, a y | 15.16 ± 2.16 | 16.90 ± 3.37 | 0.17 (95% CI, 0.14-0.16) |
| Gender (male), b n (%) | 5 (50.0) | 5 (38.5) | 0.69 (95% CI, 0.12-3.32) |
| First concussion, n (%) | 6 (46.2) | 5 (50.0) | 1.00 (95% CI, 0.22-6.08) |
| Medical history, b n (%) | |||
| ADHD | 2 (20) | 0 | 0.18 |
| Learning disability | 0 | 0 | N/A |
| Depression | 1 (10.0) | 3 (23.1) | 0.60 (95% CI, 0.03-4.23) |
| Anxiety | 1 (10.0) | 3 (23.1) | 0.60 (95% CI, 0.03-4.23) |
| Headache | 5 (50.0) | 1 (7.7) | 0.05 (95% CI, 1.10-131) |
| Sleep disorder | 0 | 0 | N/A |
| Meningitis | 0 | 0 | N/A |
| Substance abuse | 0 | 1 (7.7) | 1.00 |
| Seizure disorder | 0 | 0 | N/A |
ADHD, attention-deficit hyperactivity disorder; N/A, not applicable.
Independent t-test P value with means and standard deviation for continuous parametric variables.
P values reported for chi-square or Fischer’s exact test analysis with odds ratio and confidence interval.
Table 2.
Initial symptom information a
| Variable | Early Therapy (≤30 d), n = 10 | Late Therapy (>30 d), n = 13 | P |
|---|---|---|---|
| PCSS total score | 41.5 (9, 78.8) | 48.5 (8, 67.25) | 0.84 (95% CI, −35.0 to 33.0) |
| Vestibular score | 1 (0, 9) | 3 (0, 9.25) | 0.83 (95% CI, −4.0 to 7.0) |
| Nausea/vomiting | 0 (0, 3.5) | 0 (0, 1.5) | 0.71 (95% CI, −1.0 to 3.0) |
| Dizziness | 0 (0, 4.5) | 2 (0, 4) | 0.71 (95% CI, −3.0 to 2.0) |
| Balance | 0 (0, 3.5) | 0.5 (0, 3) | 0.82 (95% CI, −2.0 to 2.0) |
| Ocular score | 1 (0, 5.5) | 1 (0, 2) | 0.46 (95% CI, −1.0 to 5.0) |
| Blurred vision | 1 (0, 5.5) | 1 (0, 2) | 0.46 (95% CI, −1.0 to 5.0) |
PSCC, Post-Concussion Symptom Scale.
Mann-Whitney U test P value and medians with quartiles reported for nonparametric continuous variables.
Table 3.
Time elapsed until vestibular and concussion clinic evaluation a
| Variable | Early Therapy (≤30 d), n = 10 | Late Therapy (>30 d), n = 13 | P |
|---|---|---|---|
| Days to vestibular therapy | 21.5 (17, 28.25) | 74 (37.5, 125) | <0.0001 (95% CI, −71 to −18) |
| Days to concussion clinic | 13 (6.75, 79.5) | 12 (8.5, 61) | 0.88 (95% CI, −30.1 to 33.0) |
Mann-Whitney U test P value and medians with quartiles reported for nonparametric continuous variables.
The groups received similar care in terms of numbers of clinic visits and therapy sessions (Table 4). Table 4 presents the univariate comparison of outcomes between groups. The late therapy group required additional time to RTP (110 days [61.3, 150.8] vs 31 days [22.5, 74.5], P = 0.03), but not to RTL (12 days [3.5, 26.5] vs 17.5 days [8, 20.75], P = 0.09). The early therapy group achieved symptom resolution at a faster rate (54 days [27, 91] vs 121.5 days [71, 222.8], P = 0.02).
Table 4.
Patient outcome data a
| Variable | Early Therapy (≤30 d), n = 10 | Late Therapy (>30 d), n = 13 | P |
|---|---|---|---|
| Total no. of therapies | 2.5 (2, 5.25) | 4 (3, 8) | 0.17 (95% CI, −2.19 to 4.31) |
| No. of follow-ups | 1.5 (1, 4.25) | 3 (2, 7) | 0.17 (95% CI, −2.19 to 4.31) |
| Days to symptom resolution | 54 (27, 91) | 121.5 (71, 222.8) | 0.02 (95% CI, −150.0 to −9.0) |
| Days to RTL | 17.5 (8, 20.75) | 12 (3.5, 26.5) | 0.09 (95% CI, −11.0 to 12.0) |
| Days to RTP | 31 (22.5, 74.5) | 110 (61.3, 150.8) | 0.03 (95% CI, −115.0 to −8.0) |
RTL, return to learn; RTP, return to play.
Mann-Whitney U test P value and medians with quartiles reported for nonparametric continuous variables.
Kaplan-Meier curves were generated to estimate the probabilities of RTP, RTL, and symptom resolution athletes over time for early versus late therapy (Figure 1a-c). Patients in the early VRT group RTP faster (log rank = 4.435, df = 1, P = 0.04) and reached asymptomatic or clinically recovered status earlier (log rank = 3.947, df = 1, P < 0.05). However, the probability of RTL at a given time was not associated with therapy timing (log rank = 0.360, df = 1, P = 0.55).
Figure 1.
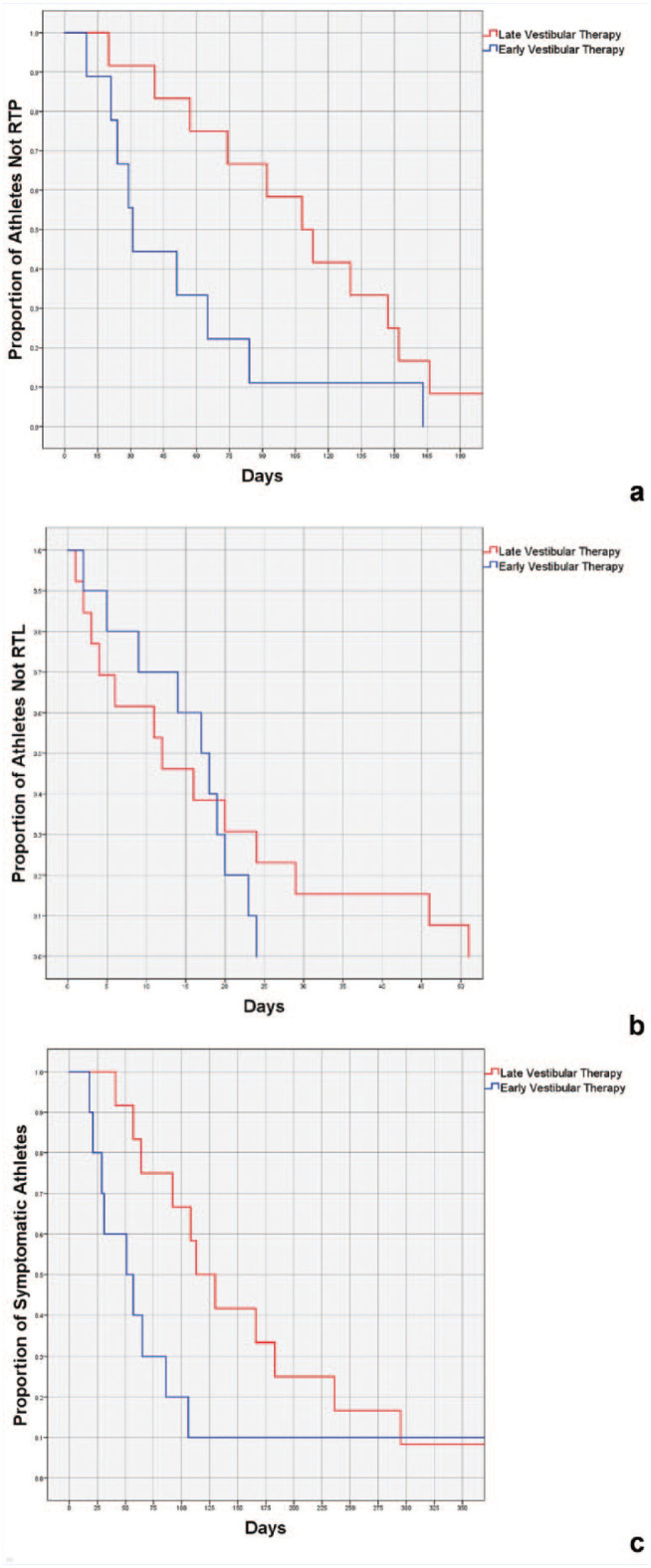
Kaplan-Meier analysis with log rank of (a) proportion of athletes not returning to play (RTP) stratified by number of days since injury, (b) proportion of athletes not returning to learn (RTL) stratified by number of days since injury, and (c) proportion of symptomatic athletes stratified by number of days since injury.
Cox proportional hazards regression models adjusted for age at concussion and PCSS total score were created to evaluate the association of days from injury to therapy with outcomes (Table 5). Days to therapy was not significantly associated with RTP (hazard ratio [HR], 0.995; 95% CI, 0.99-1.00; P = 0.18) or RTL (HR, 1.00; 95% CI, 0.99-1.00; P = 0.68). Days to therapy was found to be an independent risk factor for remaining symptomatic (HR, 0.988; 95% CI, 0.98-0.99; P = 0.01) where the longer it took to initiate therapy, the less likely the athlete was to become asymptomatic at a given time.
Table 5.
Cox proportional hazards regression models
| Return to Play | Return to Learn | Symptom Resolution | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Full model | — | — | 0.11 | — | — | 0.40 | — | — | 0.01 |
| Days to therapy | 0.995 | 0.99-1.00 | 0.18 | 1.00 | 0.99-1.00 | 0.68 | 0.988 | 0.98-0.99 | 0.01 |
| Age at concussion | 0.995 | 0.83-1.10 | 0.53 | 0.936 | 0.80-1.10 | 0.41 | 0.948 | 0.82-1.10 | 0.47 |
| Total PCSS | 0.978 | 0.96-1.00 | 0.05 | 0.993 | 0.98-1.01 | 0.34 | 0.979 | 0.96-1.00 | 0.07 |
HR, hazard ratio; PCSS, Post-Concussion Symptom Scale.
Discussion
In this pilot study evaluating early versus late VRT after SRC, we hypothesized that those who received earlier therapy would have better outcomes (symptom improvement, RTP, RTL) compared with those who started late. Our findings support this notion, as those who started therapy late (ie, more than 30 days postinjury) took longer to RTP and achieve symptom resolution.
Importance of VRT and Optimizing Timing
Prior literature1-3,5,12,17-19,22,23 concerning VRT for SRC remains limited. Murray et al 17 conducted a meta-analysis to determine the efficacy of VRT in SRC. They concluded that VRT is associated with earlier medical clearance for RTP after SRC. 17 Two randomized controlled trials have demonstrated the effectiveness of VRT after SRC. Schneider et al 22 found athletes receiving VRT plus cervical spine physiotherapy were more likely to be medically cleared for sport within 8 weeks from the initiation of treatment compared with those who received range of motion physical therapy alone (73% vs 7%). Of note, it is unclear how impactful cervical spine physiotherapy is in helping athletes to RTP. 22 Reneker et al 20 compared children 10 to 23 years old receiving comprehensive physical therapy to sham therapy. Ultimately, the therapy group was medically cleared to RTP earlier (15.5 vs 26 days) and achieved symptom resolution at a faster rate (13.5 vs 17 days). 20 The physical therapy as described by Reneker et al 20 was more comprehensive including musculoskeletal therapy in addition to VRT. Therefore, it would be difficult to isolate the specific benefits attributable solely to VRT.
The present study represents an initial attempt to primarily address the question of optimal therapy timing to achieve faster RTP and symptom resolution. Using the same definition of RTP as Schneider et al 22 (ie, the initiation of a graded-RTP protocol), our results suggest initiating VRT within 30 days of injury is associated with better outcomes. Specifically, 50% of athletes in the early group achieved RTP by day 31, while this result was not achieved until over day 105 for the late group. As both groups were equivalent across all domains (Table 1), our results suggest that earlier VRT referral was the only notable difference between groups and the primary driver of the improved outcomes. Interestingly, the same effect of early VRT referral was not seen on RTL. Our definition of RTL is liberal and while vestibular symptoms may not prevent returning, they may result in school performance detriments. 13 Future studies should consider more granular academic outcomes to assess the effectiveness of VRT on successful RTL.
Practical Barriers to Early VRT
Concussions are taxonomized according to the clinical profile and subdivided into cognitive/fatigue, vestibular, ocular, migraine, and anxiety/mood subtypes. 22 Although the common symptoms for each subtype often overlap and “fitting” a patient into a subset can be exceedingly difficult. For example, while vestibular concussion is treated with VRT, a migraine-predominant concussion may necessitate referral to a headache specialist and behavioral regulation. 22
Understanding how VRT may be “resource limited” stresses the importance of tools for screening and properly identifying those patients who would benefit. Specific to the vestibular-SRC, tools are available, such as the Vestibular-Ocular Motor Screening, to aid in diagnosis.16,23
Even with optimal screening and identification of those athletes requiring VRT, a specialty trained clinician is required.
Limitations
First, this is a single-center pilot study with a limited sample size, and the results should be corroborated by a multi-institutional effort. Second, this is a retrospective study and outcome variables of interest were defined based on the availability of data. Furthermore, treatment was at the discretion of the treating therapist, and referral was determined to be necessary by a concussion center provider. This may introduce selection bias as to which patients were referred early, yet the biopsychosocial characteristics were similar between groups, suggesting that there were no systematic biases were introduced. Latent variables that better explain the association between early therapy and improved outcomes may exist; however, many possible confounding variables were equally distributed between the groups. Third, the Cox regression analysis should be interpreted within the constraints of this small pilot study—increased risk of overfitting and/or type II error. In a larger sample, additional confounders may have been added to the models such as medical history variables known to be associated with prolonged recovery (eg, attention-deficit hyperactivity disorder, mood disorders, migraine).10,15 Finally, RTL usually starts with a trial of half days within the week of concussion regardless of symptom severity. As such, a study comparing return to “full days” and academic performance would be better suited to evaluate the benefit of early VRT on education outcomes.
Conclusion
This pilot study suggests that delaying VRT initiation, specifically more than 30 days postinjury is associated with prolonged times to RTP and achievement of symptom resolution. Early recognition of vestibular-SRC and consideration of short interval follow-up for these patients with VRT referral within the first month postinjury appears beneficial.
Acknowledgments
REDCap, used for study data management, is supported by NCATS/NIH grant UL1 TR000445.
Footnotes
The authors report no potential conflicts of interest in the development and publication of this article.
References
- 1. Alsalaheen BA, Mucha A, Morris LO, et al. Vestibular rehabilitation for dizziness and balance disorders after concussion. J Neurol Phys Ther. 2010;34:87-93. [DOI] [PubMed] [Google Scholar]
- 2. Alsalaheen BA, Whitney SL, Marchetti GF, et al. Relationship between cognitive assessment and balance measures in adolescents referred for vestibular physical therapy after concussion. Clin J Sport Med. 2016;26:46-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown L, Camarinos J. The role of physical therapy in concussion rehabilitation. Semin Pediatr Neurol. 2019;30:68-78. [DOI] [PubMed] [Google Scholar]
- 4. Cardenache R. The Relationship Between the Glasgow Coma Scale and the Functional Independence Measure in a Sample of Traumatic Brain Injury Patients in a Neurorehabilitation Setting [Doctoral dissertation]. Pepperdine University; 2009. [Google Scholar]
- 5. Cheever KM, McDevitt J, Tierney R, Wright WG. Concussion recovery phase affects vestibular and oculomotor symptom provocation. Int J Sports Med. 2018;39:141-147. [DOI] [PubMed] [Google Scholar]
- 6. Collins MW, Kontos AP, Reynolds E, Murawski CD, Fu FH. A comprehensive, targeted approach to the clinical care of athletes following sport-related concussion. Knee Surg Sports Traumatol Arthrosc. 2014;22:235-246. [DOI] [PubMed] [Google Scholar]
- 7. Gurley JM, Hujsak BD, Kelly JL. Vestibular rehabilitation following mild traumatic brain injury. NeuroRehabilitation. 2013;32:519-528. [DOI] [PubMed] [Google Scholar]
- 8. Halstead ME, Walter KD, Moffatt K. Sport-related concussion in children and adolescents. Pediatrics. 2018;142:e20183074. [DOI] [PubMed] [Google Scholar]
- 9. Ianof JN, Freire FR, Calado VTG, et al. Sport-related concussions. Dement Neuropsychol. 2014;8:14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iverson GL, Gardner AJ, Terry DP, et al. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med. 2017;51:941-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kontos AP, Deitrick JM, Collins MW, Mucha A. Review of vestibular and oculomotor screening and concussion rehabilitation. J Athl Train. 2017;52:256-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kontos AP, Sufrinko A, Sandel N, Emami K, Collins MW. Sport-related concussion clinical profiles: clinical characteristics, targeted treatments, and preliminary evidence. Curr Sports Med Rep. 2019;18:82-92. [DOI] [PubMed] [Google Scholar]
- 13. Lumba-Brown A, Yeates KO, Sarmiento K, et al. Centers for Disease Control and Prevention guideline on the diagnosis and management of mild traumatic brain injury among children. JAMA Pediatr. 2018;172:e182853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport—the 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br J Sports Med. 2017;51:838-847. [DOI] [PubMed] [Google Scholar]
- 15. Miller JH, Gill C, Kuhn EN, et al. Predictors of delayed recovery following pediatric sports-related concussion: a case-control study. J Neurosurg Pediatr. 2016;17:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mucha A, Fedor S, DeMarco D. Vestibular dysfunction and concussion. Handb Clin Neurol. 2018;158:135-144. [DOI] [PubMed] [Google Scholar]
- 17. Murray DA, Meldrum D, Lennon O. Can vestibular rehabilitation exercises help patients with concussion? A systematic review of efficacy, prescription and progression patterns. Br J Sports Med. 2017;51:442-451. [DOI] [PubMed] [Google Scholar]
- 18. Nagib S, Linens SW. Vestibular rehabilitation therapy improves perceived disability associated with dizziness postconcussion. J Sport Rehabil. 2019;28:764-768. [DOI] [PubMed] [Google Scholar]
- 19. Park K, Ksiazek T, Olson B. Effectiveness of vestibular rehabilitation therapy for treatment of concussed adolescents with persistent symptoms of dizziness and imbalance. J Sports Rehabil. 2018;27:485-490. [DOI] [PubMed] [Google Scholar]
- 20. Reneker JC, Hassen A, Phillips RS, Moughiman MC, Donaldson M, Moughiman J. Feasibility of early physical therapy for dizziness after a sports-related concussion: a randomized clinical trial. Scand J Med Sci Sports. 2017;27:2009-2018. [DOI] [PubMed] [Google Scholar]
- 21. Rose SC, Fischer AN, Heyer GL. How long is too long? The lack of consensus regarding the post-concussion syndrome diagnosis. Brain Inj. 2015;29:798-803. [DOI] [PubMed] [Google Scholar]
- 22. Schneider KJ, Meeuwisse WH, Nettel-Aguirre A, et al. Cervicovestibular rehabilitation in sport-related concussion: a randomised controlled trial. Br J Sports Med. 2014;48:1294-1298. [DOI] [PubMed] [Google Scholar]
- 23. Storey EP, Wiebe DJ, D’Alonzo BA, et al. Vestibular rehabilitation is associated with visuovestibular improvement in pediatric concussion. J Neurol Phys Ther. 2018;42:134-141. [DOI] [PubMed] [Google Scholar]
- 24. Sussman ES, Pendharkar AV, Ho AL, Ghajar J. Mild traumatic brain injury and concussion: terminology and classification. Handb Clin Neurol. 2018;158:21-24. [DOI] [PubMed] [Google Scholar]
- 25. Whitney SL, Alghwiri AA, Alghadir A. An overview of vestibular rehabilitation. Handb Clin Neurol. 2016;137:187-205. [DOI] [PubMed] [Google Scholar]


