Abstract
Background:
Soluble Tumor Necrosis Factor Weak Inducer of Apoptosis (sTWEAK) has been proposed as a novel biomarker of cardiovascular disease risk. This study compares levels of sTWEAK, sCD163 and the sCD163/sTWEAK ratio in HIV-infected and uninfected patients and their associations with cardiovascular and inflammatory factors.
Methods:
The data for our analysis come from 274 HIV-infected adults and 59 controls. HIV participants were on stable antiretroviral therapy (ART),. Wilcoxon-Mann-Whitney tests were used for comparing markers between HIV-infected participants with HIV viral load <50 copies/mL (aviremic group), HIV-infected participants with detectable viremia (HIV-1 RNA ≥50 copies/mL; viremic group) and HIV negative participants. Multivariable quantile regression analyses were used to assess associations of sTWEAK and sCD163 with other markers of inflammation and carotid intima-media thickness (cIMT).
Results:
Overall, 74% of participants were male; 59% were African Americans; median age was 40 years and CD4 595 cells/mm3. Overall, HIV-infected participants had reduced sTWEAK and increased sCD163 levels compared to HIV-uninfected participants (p=0.0001 for both markers). In addition, these biomarkers were significantly different between HIV-infected viremic and aviremic patients (p≤0.01 for both markers). In multivariable models, sTWEAK and sCD163 in aviremic patients were significantly correlated with common carotid artery IMT (p≤0.05). In HIV-infected aviremic participants, sTWEAK and sCD163 were both associated with IL-6, CD14+CD16+ monocytes (p≤0.02); additionally, sCD163 was associated with D-dimer- (β=−69.5, 0.05), VCAM (β=72.4, p=0.05), TNF RI (β=91.1, p<0.01) and TNF RII (β=87.8, p<0.01).
Conclusions:
HIV-infected participants showed increased systemic inflammatory and monocyte activation markers. Soluble CD163 and sTWEAK levels were associated with carotid intima-media thickness.
Keywords: inflammation, cardiovascular disease, immune activation
Introduction
Tumor Necrosis Factor Weak Inducer of Apoptosis (TWEAK) is a cytokine that belongs to the tumor necrosis factor (TNF) family that is mainly produced by macrophages. TWEAK gets cleaved into a membrane-bound form and an active soluble variant (sTWEAK). TWEAK binds to fibroblasts growth factor-inducible 14 (Fn 14) and is involved in a multitude of signaling inflammatory pathways including angiogenesis and thrombosis[1]. Soluble CD163 (sCD163), a marker of immunomodulation, is cleaved from macrophages during inflammation and has been strongly correlated to coronary atherosclerosis [2]. CD163 has been proposed to act as a TWEAK scavenger receptor [3]. sTWEAK has been proposed as a biomarker in cardiovascular diseases [4]. Reduced levels of sTWEAK have been reported in patients with chronic kidney disease and type II diabetes [5] , and have been associated with coronary artery disease, heart failure, aortic abdominal aneurysm and peripheral artery disease [6-9]. Carotid intima-media thickness (cIMT) is a strong predictor of cardiovascular disease and has also been correlated with sTWEAK in renal patients [10-12] and recently, sTWEAK levels were independently and negatively associated with cIMT in participants with subclinical cardiovascular disease [13].
Despite antiretroviral therapy (ART), HIV-infected adults remain at a higher risk of co-morbidities including cardiovascular disease. Impaired metabolic pathways and persistent inflammation and immune activation are potential contributing factors to these increased co-morbidities. Searching for novel biomarkers to further understand the pathophysiology and predict the risk of cardiovascular disease in asymptomatic HIV-infected patients is imperative. Soluble CD163 is associated with noncalcified plaque in HIV-infected patients [14, 15], however to our knowledge, there is only one small study comparing sTWEAK levels in patients with HIV [16] versus uninfected controls. This study by Beltran et al analyzed levels of sTWEAK in 26 HIV-infected participants, pre and post ART, compared to levels in uninfected controls. Patients with HIV had lower levels of sTWEAK and higher sCD163/sTWEAK ratio; antiretroviral therapy had no effect on sTWEAK levels. Evidence is lacking on the role of sTWEAK on cardiovascular measures and other markers of inflammation and immune activation in HIV-infected patients. Carotid IMT has been used extensively in patients with HIV to understand the relationship between different risk factors and subclinical atherosclerosis. To help us further understand the role of sTWEAK in HIV-infected patients, we analyzed sTWEAK, sCD163 and sCD163/sTWEAK ratio in uninfected controls and HIV-infected adults and the relation of these measurements with cIMT and other systemic inflammatory markers that have been characterized in HIV infection. Several studies have suggested a link between HIV viremia and cardiovascular disease [17, 18]. In this day and age, we believe it is important to consider HIV-infected individuals on ART with suboptimal viral suppression. Patients were therefore further stratified with viral load < or ≥ 50 copies/mL in order to extrapolate our findings to those participants on ART with residual viremia which may have an impact on markers of inflammation and immune activation.
Methods
Study Design
This analysis used a cohort of HIV-infected and uninfected participants prospectively enrolled at University Hospitals Case Medical Center, Cleveland, Ohio. The study was approved by the local Institutional Review Board, and written informed consent was provided by all participants. All participants were ≥18 years of age, with HIV-1 infection on stable ART for at least 3 months with cumulative ART duration of at least 6 months.Participants were excluded if they had a history of coronary disease or diabetes, were pregnant or lactating, or had an active infectious or inflammatory condition.
Study evaluations
Blood draws were obtained for measurements of renal and lipid profiles, glucose and insulin levels. Blood was drawn after a 12-hour fast. Additionally, blood was processed and plasma, serum, and peripheral blood mononuclear cells were cryopreserved for measurement of markers of immune activation, systemic inflammation and coagulation as previously described [19, 20].
Inflammation and soluble immune activation markers
Soluble markers of inflammation [interleukin-6 (IL-6), soluble tumor necrosis factor receptors I and II (sTNF-RI and –RII)], soluble vascular cell adhesion molecule (VCAM) were measured by ELISA (R&D Systems, Minneapolis, MN) with the exception of high sensitivity C-reactive protein (hsCRP) which was determined by particle enhanced immunonephelometric assay on a BNII nephelometer (Siemens, Indianapolis, IN, USA). D-Dimer levels were determined by immunoturbidometric assay on a STA-R coagulation analyzer (Diagnostic STago, Inc., Parsippany, NJ). Soluble markers of monocyte activation [soluble CD14 (sCD14) and soluble CD163 (sCD163)] as well as sTWEAK were measured by ELISA (R&D Systems, Minneapolis, MN).
Cellular markers of monocyte and T-cell activation
Monocyte and T-cells were phenotyped by flow cytometry as previously described [20]. CD4+ and CD8+ T-cell activation was defined as co-expression of CD38 and HLA-DR. Monocyte phenotype was determined by the relative expression of CD14, CD16 and surface tissue factor (TF).
Subclinical vascular disease
Mean-mean common carotid artery (CCA) intima media thickness (CCA-IMT) was measured by high resolution ultrasound as described previously [21, 22]. For carotid IMT, a high-resolution B-mode ultrasound scan of the bilateral common carotid arteries was performed using a Philips iU22 ultrasound system with an L9-3 MHz linear array transducer (Philips Healthcare, Andover, MA). R-wave gated still frame images of the distal 1 cm of the common carotid artery (CCA) far wall were obtained at three separate angles bilaterally (anterior, lateral and posterior). CCA IMT was measured offline using semi-automatic edge detection software (Brachial Analyzer for Research; Medical Imaging Applications LLC, Coralville, IA). The mean-mean CCA IMT, mean-max CCA IMT, mean bulb and mean-max carotid bulb were used for analysis. Measurements were taken at three separate angles bilaterally and the average of the six measurements was used for analysis.
Statistical Analysis
The major objective of this study was to compare sTWEAK, sCD163 and sCD163/sTWEAK ratio between HIV-infected participants with HIV viral load less than 50 copies/mL (aviremic group), HIV-infected participants with detectable viremia (HIV-1 RNA >50 copies/mL; viremic group) and HIV negative participants. Secondary objectives were to examine associations between sTWEAK, sCD163, sCD163/sTWEAK and cIMT and markers of systemic inflammation, immune activation and coagulation. Continuous measures were summarized either by mean ± SD or medians (inter-quartile range) depending on the data distribution. Normally distributed data were summarized by mean ± SD and equality of the means in three groups was tested using analysis of variance. Skewed data were summarized by median (inter-quartile range) and equality of the data distributions in three groups are tested using Kruskal Wallis test. The sTWEAK, sCD163 and sCD163/sTWEAK ratio measures were studied graphically using box plots. These variables distributions do not follow Gaussian distribution, and thus we used robust (median) regression approach to assess association between sTWEAK, sCD163 and sCD163/sTWEAK ratio and cIMT, as well as other markers of inflammation. For these, separate regression models were constructed with sTWEAK, sCD163 and sCD163/sTWEAK ratio were as the outcome. For these models, clinically relevant variables as well as markers of inflammation, immune activation and cIMT were considered for inclusion. All the statistical analyses are performed using statistical software Stata 13.0 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX).
Results
Baseline characteristics
Overall, 274 HIV–infected participants and 59 uninfected controls were included in the present analysis. Demographic information and baseline characteristics are displayed in Table 1. Overall, the median age among all participants was 40 years, 74% were male and 59% were African American. Among HIV-infected participants, the median CD4 cell count was 595 cells/mm3. In patients with HIV-1 RNA ≥50 copies/mL, the median viral load was 1860 copies/mL.
Table 1. Baseline Characteristics.
Median values (interquartile range)
| HIV positive ≥50 copies/mL (n=100) |
HIV positive <50 copies/mL (n=174) |
HIV negative (n=59) |
P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 41 (31,49) | 43 (25,51) | 35 (23,44) | 0.01 |
| Male sex | 79% | 78% | 64% | 0.08 |
| African American | 65% | 70% | 41% | <0.01 |
| Metabolic and cardiovascular risk factors | ||||
| Family history of MI | 29% | 25% | 19% | 0.64 |
| Family history of diabetes | 34% | 40% | 15% | 0.01 |
| BMI, kg/m2 | 27 (23,31) | 25 (22,29) | 28 (25,31) | <0.01 |
| Waist Hip Ratio | 0.89 (0.77,0.92) | 0.86 (0.75,0.92) | 0.86 (0.73,0.92) | 0.90 |
| Active Hepatitis C | 8% | 4% | 0% | 0.04 |
| Systolic blood pressure, mm Hg | 122 (110,131) | 122 (114,133) | 120 (110,128) | 0.18 |
| Hypertensive Medication | 26% | 32% | 10% | <0.01 |
| HDL cholesterol, mg/dL | 43 (37,53) | 46 (38,56) | 47 (42,58) | 0.03 |
| LDL cholesterol mg/dL | 99 (78,120) | 95 (75,112) | 118 (91,135) | <0.01 |
| HOMA-IR | 1.54 (0.72,2.31) | 1.99 (1.21,4.07) | 1.32 (0.65,2.38) | <0.01 |
| Current Smoking | 59% | 55% | 19% | <0.01 |
| HIV parameters | ||||
| Current CD4+ count, cells/mm3 | 538 (461,709) | 653 (451,860) | 0.06 | |
| Nadir CD4+ count, cells/mm3 | 107 (322,524) | 198 (80,300) | <0.01 | |
| HIV RNA, copies/mL | 1860 (160,10322) | 20 (20,48) | <0.01 | |
| Current protease inhibitor use | 19% | 46% | <0.01 | |
| Measures of subclinical vascular disease | ||||
| Mean-Mean common carotid artery IMT, mm | 0.64 (0.59,0.69) | 0.64 (0.57,0.73) | 0.59 (0.56,0.68) | 0.02 |
| Mean-Max common carotid artery IMT, mm | 0.80 (0.75,0.90) | 0.81 (0.72,0.91) | 0.78 (0.69,0.88) | 0.22 |
| Mean- mean carotid bulb IMT, mm | 0.72 (0.65,0.81) | 0.74 (0.64,0.89) | 0.67 (0.61,0.79) | 0.06 |
| Mean-Max Carotid Bulb IMT, mm | 1.03 (0.92,1.17) | 0.81 (0.72,0.91) | 0.78 (0.69,0.88) | 0.14 |
| Inflammation and Immune activation | ||||
| hsCRP, μg/mL | 1.15 (0.59,3.50) | 2.15 (0.56,2.30) | 0.86 (0.34,1.82) | <0.01 |
| D-dimer, μg/mL | 0.20 (0.11,0.36) | 0.21 (0.11,0.71) | 0.19 (0.11,0.28) | 0.15 |
| Interleukin 6, pg/mL | 2.61 (1.88,4.14) | 2.16 (1.33,3.76) | 1.81 (1.10,2.72) | <0.01 |
| TNFα- receptor I, Pg/mL | 1261 (1053,1445) | 1253 (944,1762) | 1243 (1087,1394) | 0.79 |
| TNFα- receptor II, Pg/mL | 2888 (2474,3515) | 2332 (1848,2859) | 2295 (2052,2662) | <0.01 |
| VCAM, ng/mL | 843 (653,1069) | 670 (562,812) | 570 (467,714) | <0.01 |
| CD4+CD38+HLADR+T-cells, % | 18 (13,21) | 13 (10,17) | ||
| CD8+CD38+HLADR+T-cells, % | 43 (31,52) | 29 (21,38) | ||
| CD14+CD16+monocytes, % | 20 (18,42) | 23 (18,32) | ||
| CD14dimCD16+monocytes, % | 12 (8,18) | 11 (8,14) | ||
| sCD14, ng/mL | 1558 (1289,1883) | 1997 (1572,2401) | 1221 (1031,1494) | <0.01 |
| sTWEAK, pg/mL | 638 (500,1096) | 850 (541,2539) | 1707 (629,4780) | <0.01 |
| sCD163, ng/mL | 867 (373,1291) | 607 (363,901) | 449 (265; 651) | <0.01 |
| sCD163/sTWEAK | 0.92 (0.33,2.19) | 0.52 (0.17, 1.24) | 0.17 (0.06; 0.61) | <0.01 |
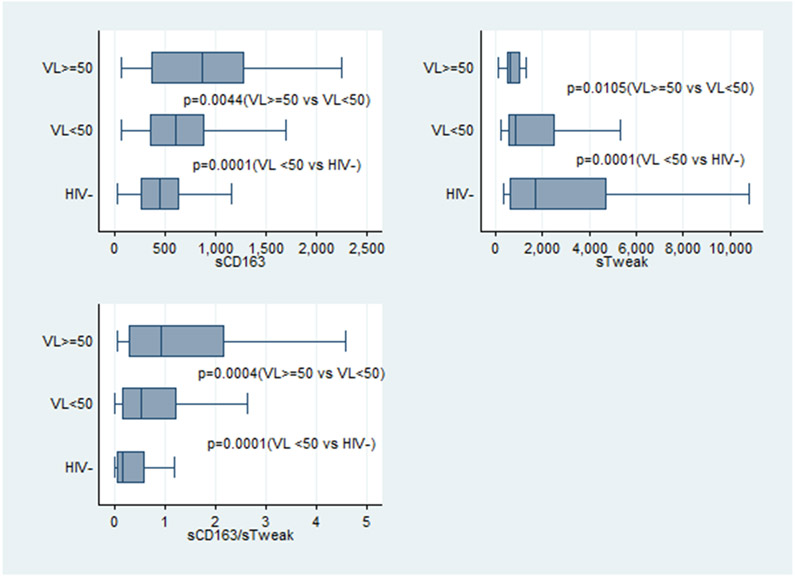
sTWEAK, sCD163 and sCD163/sTWEAK differences
Overall, HIV-infected participants had reduced sTWEAK levels and increased sCD163 levels compared to levels in controls (Fig 1 and Table 2). sTWEAK, sCD163 and sCD163/sTWEAK were significantly different between viremic and aviremic HIV-infected individuals (p≤0.01) and between the aviremic and uninfected participants (p=0.0001). After adjusting for demographic factors that were statistically different between the groups such as age, gender, race, family history of diabetes and current smoking, sTWEAK, sCD163 and sCD163/sTWEAK ratio remained significantly different between HIV-infected and uninfected participants (p≤0.02).
Figure 1:
sTWEAK, sCD163 and sCD163/sTWEAK ratio in HIV-infected participants and controls
Table 2:
sTWEAK and sCD163 levels between HIV positive participants and HIV negative controls
| HIV positive viral load ≥50 copies/mL (n=100) |
HIV positive viral load < 50 copies/mL (n=174) |
HIV negative (n=59) |
P value | |
|---|---|---|---|---|
| sTWEAK (pg/mL) median (IQR) | 638 (500,1096) | 850 (541,2539) | 1707 (629,4780) | 0.0001 |
| sCD163 (ng/mL) median (IQR) | 867 (373,1291) | 607 (363, 901) | 449 (265; 651) | 0.0001 |
| sCD163/sTWEAK median (IQR) | 0.92 (0.33,2.19) | 0.52 (0.17, 1.24) | 0.17 (0.06; 0.61) | 0.0001 |
Association between sTWEAK, sCD163 and sCD163/sTWEAK and inflammatory and cardiovascular biomarkers
As seen in table 3, separate regression models were constructed in aviremic HIV-infected participants to assess the association between sTWEAK, sCD163, sCD163/sTWEAK and clinically significant variables as well as inflammatory markers and cIMT. In aviremic HIV-infected participants, sTWEAK, sCD163 and sCD163/sTWEAK were all associated with common carotid artery IMT (p≤0.05). Soluble TWEAK, sCD163 and sCD163/sTWEAK were associated with markers of general inflammation, coagulation, endothelial activation as well as T cell activation and monocyte markers. Specifically, both sTWEAK and sCD163 were associated with IL-6 and the proportion of CD14+CD16+ monocytes (p≤0.02); sCD163 levels were also associated with levels of D-dimer, VCAM, TNF RI and RII (p≤0.05).
Table 3:
Multivariable analysis for relationship between inflammatory markers between sTWEAK, sCD163, sTWEAK/sCD163 and inflammatory and cardiovascular markers for HIV-infected patients with VL<50 copies/mL
| sTWEAK | sCD163 | sCD163/sTWEAK | ||||
|---|---|---|---|---|---|---|
| β (SE) | P value | β (SE) | P value | β (SE) | P value | |
| Demographics | ||||||
| Age, years | −125.42 (55.9) | 0.03 | 120.02 (26.80) | <0.01 | 0.20 (0.34) | <0.01 |
| Male sex | 53.19 (217.8) | 0.80 | 16.44 (67.90) | 0.81 | −0.02 (0.23) | 0.91 |
| Metabolic and cardiovascular risk factors | ||||||
| Waist Hip Ratio | −439.67 (972.10) | 0.65 | 988.36 (383.75) | 0.01 | 1.46 (0.74) | 0.05 |
| BMI, kg/m2 | −8.81 (10.59) | 0.41 | 9.31 (6.10) | 0.13 | 0.011 (0.01) | 0.18 |
| Hypertensive Medication | 602.42 (266.75) | 0.03 | −233.96 (137.86) | 0.10 | −0.56 (0.38) | 0.14 |
| HOMA-IR | −6.00 (14.20) | 0.67 | 1.144 (9.09) | 0.90 | 0.15 (0.01) | 0.21 |
| Cholesterol, mg/dL | 26.46 (97.51) | 0.79 | −61.43 (38.32) | 0.11 | −0.04 (0.05) | 0.41 |
| LDL, mg/dL | 96.84 (90.19) | 0.28 | −59.68 (35.58) | 0.09 | −0.06 (0.05) | 0.28 |
| HIV parameters | ||||||
| Nadir CD4+ count, cells/mm3 | 193.16 (116.58) | 0.10 | 0.033 (38.89) | 0.99 | −0.07 (0.06) | 0.20 |
| HIV RNA, copies/mL | −83544.14 (99169.93) | 0.40 | 62948.01 (44852.87) | 0.16 | 127.89 (63.99) | 0.05 |
| Measures of subclinical vascular disease | ||||||
| Mean-Mean common carotid artery IMT, mm | 1806.50 (685.16) | <0.01 | 678.82 (304.98) | 0.03 | −0.75 (0.37) | 0.05 |
| Mean-Max common carotid artery IMT, mm | 1128.18 (555.64) | 0.04 | 776.63 (199.34) | <0.01 | −0.13 (0.33) | 0.70 |
| Mean- mean carotid bulb IMT, mm | 65.76 (281.92) | 0.82 | 165.53 (106.64) | 0.12 | 0.15 (0.22) | 0.52 |
| Mean-Max Carotid Bulb IMT, mm | 371.17 (289.68) | 0.20 | 99.20 (94.18) | 0.29 | 0.04 (0.18) | 0.81 |
| Inflammation and Immune activation | ||||||
| hsCRP, μg/mL | 36.62 (55.62) | 0.51 | 13.31 (22.69) | 0.56 | 0.01 (0.04) | 0.82 |
| D-dimer, μg/mL | −10.61 (74.61) | 0.89 | −69.54 (34.62) | 0.05 | −0.00 (0.05) | 0.92 |
| Interleukin 6, pg/mL | 29.21 (7.06) | <0.01 | 6.59 (2.67) | 0.015 | −0.01 (0.00) | 0.11 |
| TNFα- receptor I, Pg/mL | −60.59 (54.96) | 0.27 | 91.07 (25.49) | <0.01 | 0.10 (0.05) | 0.06 |
| TNFα- receptor II, Pg/mL | 89.60 (96.40) | 0.35 | 87.78 (30.48) | <0.01 | −0.00 (0.06) | 0.90 |
| VCAM | −0.04 (100.10) | 1.00 | 72.46 (36.30) | 0.05 | 0.05 (0.07) | 0.45 |
| CD4+CD38+HLADR+T-cells, % | 17.86 (14.33) | 0.22 | 5.08 (6.00) | 0.40 | −0.00 (0.01) | 0.77 |
| CD8+CD38+HLADR+T-cells, % | 172.72 (118.55) | 0.15 | 5.48 (52.81) | 0.92 | −0.13 (0.13) | 0.31 |
| CD14+CD16+monocytes, % | −11.77 (5.15) | 0.02 | 7.93 (2.00) | <0.01 | 0.01 (0.01) | 0.06 |
| CD14dimCD16+monocytes, % | −30.14 (21.82) | 0.17 | 2.50 (8.35) | 0.77 | 0.04 (0.02) | 0.03 |
| sCD14, ng/mL | 11.73 (35.82) | 0.74 | 14.26 (16.23) | 0.38 | 0.2 (0.02) | 0.44 |
| sCD163, ng/mL | 0.54 (0.21) | 0.01 | ||||
All regression coefficients were adjusted for age, sex and race
Among traditional CVD risk factors in aviremic participants, age and hypertension were associated with sTWEAK (p=0.03 for both), and age as well as waist-to-hip ratio with sCD163 (p≤0.01). There were no significant associations between sTWEAK or sCD163 and body mass index, sex, HOMA-IR, LDL- or HDL- cholesterol,(p≥0.09) (see table 3) In aviremic patients, we tested several HIV variables, but only viremia was associated with sCD163/sTWEAK ratio (p=0.04). None of the others variables were significant, including HIV duration, current CD4 count, nadir CD4 count, current protease inhibitor use (p≥0.10).
In HIV-uninfected patients and in HIV-infected viremic patients, no association was found between sTWEAK, sCD163 or sCD163/sTWEAK and carotid artery IMT (p≥0.12).
Discussion
For the first time in HIV-infected participants, we investigated the relationship between sTWEAK levels and early markers of cardiovascular disease. We found that in HIV-infected adults virologically suppressed on ART, sTWEAK, sCD163 and sCD163/sTWEAK ratio were associated with common carotid artery intima media thickness, a surrogate marker of atherosclerosis.
To our knowledge, only one other report has been published on levels of sTWEAK in a small cohort of HIV-infected patients (n=26) versus controls [16]. Participants in this study were ART naive and sTWEAK was measured at baseline and again 48 weeks after ART initiation. Similar to our findings, patients with HIV had reduced sTWEAK levels and increased sCD163 compared to uninfected controls; however, the sTWEAK levels reported were much lower than what we found. In the study by Beltran et al, patients with HIV had sTWEAK median levels of 354 pg/mL compared to 850 pg/mL in our study; and HIV-negative controls median sTWEAK levels of 468 pg/mL compared to 1707 pg/mL reported here. Several factors could explain these differences: participants in their study were slightly younger (37 vs 43 years), had a lower BMI (24 vs 28) and higher levels of baseline viremia (32,050 copies/mL vs 1,860 copies/mL) in addition the kits used were different (the source of our kits was R&D Systems, Minneapolis, MN, while theirs Bender Med Systems, Vienna, Austria).
sTWEAK was significantly higher in aviremic HIV-infected participants compared to viremic participants supporting the hypothesis that ongoing viremia contributes to inflammation and immune activation and highlighting the importance of viral suppression.
Unlike TNF-α, which stimulates the innate inflammatory response, TWEAK appears to play an important role in immune modulation. Lower levels of sTWEAK are associated with inflammatory conditions such as rheumatoid arthritis[23] and atherosclerosis [13]. TWEAK is reduced in mice with chronic autoimmune diseases such as systemic lupus erythematosus and autoimmune hemolytic anemia [24]. In addition, studies performed in TWEAK knockout mice suggest that the expression of TWEAK by natural killer cells and macrophages in response to infection helps to downregulate the inflammatory response [25]. Elevated levels of sCD163 has been well documented in HIV [14, 26] and also in other proinflammatory conditions including rheumatoid arthritis [27], lupus [28] and Gaucher disease [29]. Soluble CD163 is known to act as a scavenger receptor for sTWEAK [3]. In vitro studies also suggest that macrophages expressing CD163 are able to internalize sTWEAK [30]. In addition, Fn14, the TWEAK receptor, is almost absent in healthy tissue and gets upregulated during tissue injury, in myocardial infarction and atherosclerotic plaques and binds sTWEAK [9, 31, 32]. Therefore, we hypothesize that the reduced levels of sTWEAK seen in HIV-infected patients could be the result of the upregulation of sCD163 by macrophage activation and/or Fn14 by damaged tissue.
Soluble TWEAK levels are lower in carotid plaques compared to levels in normal arteries [4]. Similar results have been found in plasma samples from patients with carotid stenosis relative to levels from control participants. IMT, a marker of CVD, has been negatively associated with sTWEAK concentrations in asymptomatic participants and in patients with chronic kidney disease [11, 12, 33]. In our cohort of HIV-infected patients who are aviremic, we show for the first time that sTWEAK correlated with carotid bulb and common carotid artery intima media thickness. As seen in renal transplant patients [10], sTWEAK in aviremic participants was positively correlated with IMT. Further supporting the role of sTWEAK as a marker of CVD in HIV, we found that this cytokine was negatively correlated with the inflammatory subset of monocyte (CD14+CD16+) which has been linked to CVD risks and events in HIV-infected [34] and uninfected [35] population. We suspect that the pathogenesis of atherosclerosis in HIV is multifactorial and although TWEAK /Fn14 appear to play a role in vascular remodeling, other members of the TNF family are likely involved promoting both the canonical as well as the non-canonical NF-kappa B pathways [36].
In HIV-uninfected patients, low sTWEAK has also been correlated with other risk factors associated with cardiovascular disease including hemoglobin A1C, central obesity [37], insulin resistance [38], however, in HIV, we did not find a correlation between sTWEAK and HOMA-IR, BMI ,waist hip ratio or cholesterol and LDL.
Levels of sCD163 have been associated with both arterial inflammation [26] and noncalcified plaque [14] in HIV-infected patients; we extend these observations with our finding that sCD163 is associated with cIMT as well as several endothelial and markers of general inflammation.
Chronic inflammation is involved in the development of atherosclerosis. Activation of NF-kappa B pathways leads to the expression of adhesion molecules and inflammatory cells which allows the initiation and perpetuation of the inflammatory response that enables atherosclerosis progression. TWEAK when bound to Fn14 can activate NF-kappa B and promote the inflammatory pathway implicated in atherosclerosis. In mice, anti-TWEAK monoclonal antibody decrease the activation of NF-kappa B [39], decrease macrophage uptake by modified lipids in atherosclerotic plaques [40], and decrease the expression of prothrombotic factors in plaques [41]. These findings suggest that anti-TWEAK treatment could potentially prevent the inflammatory and cellular changes involved in atherosclerosis.
Our study has several strengths, including the comprehensive evaluations of inflammation, immune activation and cardiovascular disease risk. The limitations include the cross-sectional design, therefore we cannot prove causal relationships or exclude the possibility of residual confounding. In addition, because of the study design, the baseline characteristics were significantly different among the three groups including several of the CVD risk factors. Our population was also mostly men and African American, so our findings may not be applicable to other HIV-infected populations.
In conclusion, we show that sTWEAK levels are lower in HIV-infected patients compared to uninfected participants. Our findings also suggest that sTWEAK might have a role in the pathogenesis of atherosclerosis in patients with HIV and could be a novel biomarker of CVD. Large scale longitudinal studies are warranted to determine how Fn 14, sCD163 and sTWEAK interact and their relevance in atherosclerosis pathogenesis in HIV. Finally, the use of targeted therapy towards this axis such as monoclonal antibody and statins could potentially inhibit plaque formation and remodeling and warrant further investigation.
Acknowledgments
The authors would like to thank the patients who participated in this research.
Funding:
The work was supported by the National Institutes of Health (R56HL126526 and R56HL126539 to GAM and R00HL108743 to NF). Technical support was provided by the Center for AIDS Research, Case Western Reserve University (P30 AI36219).
Disclaimer:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Preliminary results from this study were presented at CROI in Boston, MA, February 2016. This trial is registered at clinicaltrials.gov (NCT01218802).
Footnotes
Conflicts of Interest:
GAM served as a consultant for Bristol-Myers Squibb, Viiv/GlaxoSmithKline, Pfizer, Gilead, and ICON. She received research grants from Gilead, GSK, BMS, and Astra Zeneca. NF served as a paid consultant for Gilead Science.
REFERENCES
- 1.Vendrell J, Chacon MR. TWEAK: A New Player in Obesity and Diabetes. Frontiers in immunology. 2013;4:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aristoteli LP, Moller HJ, Bailey B, Moestrup SK, Kritharides L. The monocytic lineage specific soluble CD163 is a plasma marker of coronary atherosclerosis. Atherosclerosis. 2006;184(2):342–7. [DOI] [PubMed] [Google Scholar]
- 3.Bover LC, Cardo-Vila M, Kuniyasu A, et al. A previously unrecognized protein-protein interaction between TWEAK and CD163: potential biological implications. Journal of immunology (Baltimore, Md : 1950). 2007;178(12):8183–94. [DOI] [PubMed] [Google Scholar]
- 4.Blanco-Colio LM, Martin-Ventura JL, Munoz-Garcia B, et al. Identification of soluble tumor necrosis factor-like weak inducer of apoptosis (sTWEAK) as a possible biomarker of subclinical atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2007;27(4):916–22. [DOI] [PubMed] [Google Scholar]
- 5.Kralisch S, Ziegelmeier M, Bachmann A, et al. Serum levels of the atherosclerosis biomarker sTWEAK are decreased in type 2 diabetes and end-stage renal disease. Atherosclerosis. 2008;199(2):440–4. [DOI] [PubMed] [Google Scholar]
- 6.Jelic-Ivanovic Z, Bujisic N, Spasic S, et al. Circulating sTWEAK improves the prediction of coronary artery disease. Clinical biochemistry. 2009;42(13-14):1381–6. [DOI] [PubMed] [Google Scholar]
- 7.Chorianopoulos E, Rosenberg M, Zugck C, et al. Decreased soluble TWEAK levels predict an adverse prognosis in patients with chronic stable heart failure. European journal of heart failure. 2009;11(11):1050–6. [DOI] [PubMed] [Google Scholar]
- 8.Martin-Ventura JL, Lindholt JS, Moreno JA, et al. Soluble TWEAK plasma levels predict expansion of human abdominal aortic aneurysms. Atherosclerosis. 2011;214(2):486–9. [DOI] [PubMed] [Google Scholar]
- 9.Moreno JA, Dejouvencel T, Labreuche J, et al. Peripheral artery disease is associated with a high CD163/TWEAK plasma ratio. Arteriosclerosis, thrombosis, and vascular biology. 2010;30(6):1253–62. [DOI] [PubMed] [Google Scholar]
- 10.Turkmen K, Tonbul HZ, Erdur FM, et al. Soluble TWEAK independently predicts atherosclerosis in renal transplant patients. BMC nephrology. 2013;14:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan SB, El-demery AB, Ahmed AI, Abukhalil RE. Soluble TWEAK and cardiovascular morbidity and mortality in chronic kidney disease patients. Arab journal of nephrology and transplantation. 2012;5(1):27–32. [PubMed] [Google Scholar]
- 12.Valdivielso JM, Coll B, Martin-Ventura JL, et al. Soluble TWEAK is associated with atherosclerotic burden in patients with chronic kidney disease. Journal of nephrology. 2013;26(6): 1105–13. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Laso V, Sastre C, Valdivielso JM, et al. Soluble TWEAK levels predict the presence of carotid atherosclerotic plaques in subjects free from clinical cardiovascular diseases. Atherosclerosis. 2015;239(2):358–63. [DOI] [PubMed] [Google Scholar]
- 14.Burdo TH, Lo J, Abbara S, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. The Journal of infectious diseases. 2011;204(8):1227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitch KV, Srinivasa S, Abbara S, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. The Journal of infectious diseases. 2013;208(11):1737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beltran LM, Munoz Hernandez R, de Pablo Bernal RS, et al. Reduced sTWEAK and increased sCD163 levels in HIV-infected patients: modulation by antiretroviral treatment, HIV replication and HCV co-infection. PloS one. 2014;9(3):e90541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. Journal of the American College of Cardiology. 2008;52(7):569–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emery S, Neuhaus JA, Phillips AN, et al. Major clinical outcomes in antiretroviral therapy (ART)-naive participants and in those not receiving ART at baseline in the SMART study. The Journal of infectious diseases. 2008;197(8):1133–44. [DOI] [PubMed] [Google Scholar]
- 19.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. The Journal of infectious diseases. 2014;209(8):1156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58(4):588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Longenecker CT, Jiang Y, Orringer CE, et al. Soluble CD14 is independently associated with coronary calcification and extent of subclinical vascular disease in treated HIV infection. AIDS (London, England). 2014;28(7):969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longenecker CT, Funderburg NT, Jiang Y, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV medicine. 2013;14(6):385–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dharmapatni AA, Smith MD, Crotti TN, et al. TWEAK and Fn14 expression in the pathogenesis of joint inflammation and bone erosion in rheumatoid arthritis. Arthritis research & therapy. 2011;13(2):R51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chicheportiche Y, Fossati-Jimack L, Moll S, Ibnou-Zekri N, Izui S. Down-regulated expression of TWEAK mRNA in acute and chronic inflammatory pathologies. Biochemical and biophysical research communications. 2000;279(1):162–5. [DOI] [PubMed] [Google Scholar]
- 25.Maecker H, Varfolomeev E, Kischkel F, et al. TWEAK attenuates the transition from innate to adaptive immunity. Cell. 2005;123(5):931–44. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. Jama. 2012;308(4):379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsushita N, Kashiwagi M, Wait R, et al. Elevated levels of soluble CD163 in sera and fluids from rheumatoid arthritis patients and inhibition of the shedding of CD163 by TIMP-3. Clinical and experimental immunology. 2002;130(16):156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakayama W, Jinnin M, Makino K, et al. CD163 expression is increased in the involved skin and sera of patients with systemic lupus erythematosus. European journal of dermatology : EJD. 2012;22(4):512–7. [DOI] [PubMed] [Google Scholar]
- 29.Adly AA, Ismail EA, Ibraheem TM. Macrophage-derived soluble CD163 level in young patients with Gaucher disease: relation to phenotypes, disease severity and complications. International immunopharmacology. 2015;24(2):416–22. [DOI] [PubMed] [Google Scholar]
- 30.Moreno JA, Munoz-Garcia B, Martin-Ventura JL, et al. The CD163-expressing macrophages recognize and internalize TWEAK: potential consequences in atherosclerosis. Atherosclerosis. 2009;207(1):103–10. [DOI] [PubMed] [Google Scholar]
- 31.Munoz-Garcia B, Martin-Ventura JL, Martinez E, et al. Fn14 is upregulated in cytokine-stimulated vascular smooth muscle cells and is expressed in human carotid atherosclerotic plaques: modulation by atorvastatin. Stroke; a journal of cerebral circulation. 2006;37(8):2044–53. [DOI] [PubMed] [Google Scholar]
- 32.Kim SH, Lee WH, Kwon BS, et al. Tumor necrosis factor receptor superfamily 12 may destabilize atherosclerotic plaques by inducing matrix metalloproteinases. Japanese circulation journal. 2001;65(2):136–8. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz MI, Sonmez A, Ortiz A, et al. Soluble TWEAK and PTX3 in nondialysis CKD patients: impact on endothelial dysfunction and cardiovascular outcomes. Clinical journal of the American Society of Nephrology : CJASN. 2011;6(4):785–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baker JV, Hullsiek KH, Singh A, et al. Immunologic predictors of coronary artery calcium progression in a contemporary HIV cohort. AIDS (London, England). 2014;28(6):831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rogacev KS, Cremers B, Zawada AM, et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. Journal of the American College of Cardiology. 2012;60(16):1512–20. [DOI] [PubMed] [Google Scholar]
- 36.Blanco-Colio LM. TWEAK/Fn14 Axis: A Promising Target for the Treatment of Cardiovascular Diseases. Frontiers in immunology. 2014;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz-Lopez A, Bullo M, Chacon MR, et al. Reduced circulating sTWEAK levels are associated with metabolic syndrome in elderly individuals at high cardiovascular risk. Cardiovascular diabetology. 2014;13:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kralisch S, Ziegelmeier M, Bachmann A, et al. Serum levels of the atherosclerosis biomarker sTWEAK are decreased in type 2 diabetes and end-stage renal disease. Atherosclerosis. 2008;199(2):440–4. [DOI] [PubMed] [Google Scholar]
- 39.Munoz-Garcia B, Moreno JA, Lopez-Franco O, et al. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) enhances vascular and renal damage induced by hyperlipidemic diet in ApoE-knockout mice. Arteriosclerosis, thrombosis, and vascular biology. 2009;29(12):2061–8. [DOI] [PubMed] [Google Scholar]
- 40.Schapira K, Burkly LC, Zheng TS, et al. Fn14-Fc fusion protein regulates atherosclerosis in ApoE−/− mice and inhibits macrophage lipid uptake in vitro. Arteriosclerosis, thrombosis, and vascular biology. 2009;29(12):2021–7. [DOI] [PubMed] [Google Scholar]
- 41.Munoz-Garcia B, Madrigal-Matute J, Moreno JA, et al. TWEAK-Fn14 interaction enhances plasminogen activator inhibitor 1 and tissue factor expression in atherosclerotic plaques and in cultured vascular smooth muscle cells. Cardiovascular research. 2011;89(1):225–33. [DOI] [PubMed] [Google Scholar]