Abstract
Purpose
Tuberculomas can occasionally masquerade as high-grade gliomas (HGG). Evidence from magnetisation transfer (MT) imaging suggests that there is lower protein content in the tuberculoma microenvironment. Building on the principles of chemical exchange saturation transfer and MT, amide proton transfer (APT) imaging generates tissue contrast as a function of the mobile amide protons in tissue’s native peptides and intracellular proteins. This study aimed to further the understanding of tuberculomas using APT and to compare it with HGG.
Method
Twenty-two patients (n = 8 tuberculoma; n = 14 HGG) were included in the study. APT was a 3D turbo spin-echo Dixon sequence with inbuilt B0 correction. A two-second, 2 μT saturation pulse alternating over transmit channels was applied at ±3.5 ppm around water resonance. The APT-weighted image (APTw) was computed as the MT ratio asymmetry (MTRasym) at 3.5 ppm. Mean MTRasym values in regions of interest (areas = 9 mm2; positioned in component with homogeneous enhancement/least apparent diffusion coefficient) were used for the analysis.
Results
MTRasym values of tuberculomas (n = 14; 8 cases) ranged from 1.34% to 3.11% (M = 2.32 ± 0.50). HGG (n = 17;14 cases) showed MTRasym ranging from 2.40% to 5.70% (M = 4.32 ± 0.84). The inter-group difference in MTRasym was statistically significant (p < 0.001). APTw images in tuberculomas were notable for high MTRasym values in the perilesional oedematous-appearing parenchyma (compared to contralateral white matter; p < 0.001).
Conclusion
Tuberculomas demonstrate lower MTRasym ratios compared to HGG, reflective of a relative paucity of mobile amide protons in the ambient microenvironment. Elevated MTRasym values in perilesional parenchyma in tuberculomas are a unique observation that may be a clue to the inflammatory milieu.
Keywords: Amide proton transfer imaging, magnetisation transfer imaging, tuberculoma, chemical exchange saturation transfer
Introduction
Tuberculomas, endemic to the Indian subcontinent, have multifarious neuroimaging phenotypes and can occasionally masquerade as high-grade glial (HGG) neoplasms.1–8 While the mainstay of treatment of tuberculomas is anti-tubercular chemotherapy, surgery with a regimen of chemoradiation forms the front line of management of HGGs.9,10 Considering the digression in their management and prognosis, precise discrimination between these lesions is desirable. Earlier attempts to address the neuroimaging diagnostic challenge that tuberculomas pose have met with varying success. They employed advanced imaging sequences including magnetic resonance (MR) perfusion, spectroscopy, diffusion-weighted imaging and susceptibility-weighted imaging (SWI).11–18 Magnetisation transfer (MT) imaging (a derivative of the chemical exchange saturation transfer (CEST) phenomenon) has generated evidence suggesting that there is lower protein content in the tubercular lesion microenvironment. 13 Building on the principles of CEST and MT, amide proton transfer (APT) imaging generates tissue contrast as a function of the tissue’s native mobile amide protons in intracellular proteins and peptides.
APT imaging involves the application of off-resonance radiofrequency pulses around 3.5 ppm upstream and downstream from the resonance frequency of pure water. This leads to selective saturation of amide protons which subsequently transfer their magnetisation to free water through chemical exchange and dipole interactions leading to loss of signal. The degree of signal loss is a surrogate marker of the protein concentration and extent of macromolecular water interaction.19–21 The sequence does not require exogenously administered contrast. Earlier studies have focussed on various advanced imaging methods such as diffusion tensor imaging (DTI) and MR perfusion, including arterial spin labelling (ASL) for distinguishing gliomas from other neoplastic lesions such as solitary metastases and primary central nervous system (CNS) lymphoma, as well as infective lesions such as tuberculomas.22–25 A study based on ASL and DTI showed significantly higher lesional and perilesional cerebral blood flow and lower mean diffusivity (MD) in gliomas compared to metastases. 22 Another similar study showed significantly lower MD and cerebral blood flow with higher fractional anisotropy (FA) in case of CNS lymphoma. 23 Differences in the peritumoural milieu have been highlighted in various studies which have shown significantly lower peritumoural FA in gliomas compared to metastases 22 and higher FA in the peritumoural parenchyma of low-grade gliomas compared to that of HGGs. 24 The differences in perfusion and diffusion tensor metrics within and outside the lesion reflect variations at the cellular level. Differences in the cellular components involved and their metabolism within and around the lesion lead to a different biochemical milieu in various lesions and hence various degrees of molecular interaction which can be studied through novel imaging techniques such as APT imaging. Over the last decade, many studies have been published on APT imaging in gliomas, very few of which have compared these lesions to infective space-occupying lesions. In view of the frequent occurrence of intracranial tuberculomas in the Indian subcontinent along with a prevalence of multidrug-resistant cases and the diagnostic dilemma that they often pose on imaging, this study aimed to further the understanding of the amide microenvironment in tuberculomas using APT imaging and to compare it with HGGs, which is frequently its imaging look alike. To our knowledge, there is abundant literature on APT imaging in gliomas, with a relative paucity of similar studies in intracranial tuberculomas, thus adding to the significance of our study.
Method
Patients
The study population comprised 22 consenting patients (n = 8 tuberculoma; n = 14 glioma) referred to our institution between January 2018 and October 2019, whose clinical examination and preliminary computed tomography imaging suggested the presence of space-occupying lesions. The tuberculomas group comprised eight women who were aged 17–55 years (Mage = 29.5 years), while the HGG group was composed of nine men and five women who were aged 15–60 years (Mage = 36.8 years). The chief presenting complaints were fever, headache, seizures or features of raised intracranial tension. CNS tuberculosis was deemed the aetiology for lesions that responded to antitubercular therapy (size and oedema reduction on serial follow-up imaging) or when the cerebro-spinal fluid examination was confirmatory. Extra-CNS-confirmed tuberculosis was seen in four patients. The diagnosis of HGG was established by histopathological examination of the surgical or stereotactic biopsy specimen according to the World Health Organization (WHO) criteria. 26
Imaging
All MR imaging was performed on a 3T (Ingenia; Philips Medical Systems, Best, The Netherlands) scanner with multi-transmit capabilities.
The standard institutional MR imaging protocol included: fast spin-echo (FSE) T2-weighted (TR = 3000 ms, TE = 80 ms, NEX = 2, slice thickness = 4 mm), fluid-attenuated inversion recovery (FLAIR; TR = 11,000 ms, TE = 120 ms, TI = 2800 ms, NEX = 2, slice thickness = 4 mm), and 3D T1-weighted imaging (TR = 600 ms, TE = 10 ms, NEX = 2, slice thickness = 4 mm), contrast-enhanced 3D T1-weighted (TR = 6.55 ms, TE = 2.94 ms, NEX = 2, voxel size = 1 mm × 0.9 mm × 0.9 mm), 1H MR spectroscopy (TR = 2000 ms, TE = 144 ms, NEX = 128, voxel size = 10 × 10 × 10 mm), diffusion-weighted imaging (TR = 3024 ms, TE = 72, slice thickness = 4mm; matrix size = 112 × 90; directions 32, b-values = 0 and 1000 s2/mm) and multi-echo SWI (TR = 31 ms, minimum TE = 7.2 ms, echo spacing = 6.2 ms, number of echoes = 4, voxel size = 0.6 mm × 0.6 mm × 2 mm).
APT imaging was preceded by standard diffusion-weighted scans as part of the clinical protocol for imaging mass lesions. The APT sequence was a 3D turbo spin-echo Dixon sequence with in-built B0 correction. A two-second, 2 μT saturation pulse that alternated over the transmit channels was applied at ±3.5 ppm around the water resonance. The APT-weighted image (APTw) was computed as the MT ratio asymmetry (MTRasym) at 3.5 ppm. It was expressed as a percentage and mapped to a colour scale.27,28 Scan parameters were: voxel size = 1.8 mm × 1.8 mm × 6 mm, TR/TE = 6120/7.8 ms, TSE factor = 174, SENSE acceleration = 1.6, FH coverage = 60 mm.
Image interpretation
Image analysis and interpretation were done by two neuroradiologists (K.K. and S.J.; combined 18 years of experience). Regions of interest (ROIs; areas = 9 mm2) were positioned on the enhancing component of the lesion. For non-enhancing lesions, care was taken that the ROI was positioned on the segment of the lesion with the least apparent diffusion coefficient. Maximal MTRasym values within this ROI were used for the analysis. Intratumoural/intralesional susceptibility areas and areas of necrosis/cystic changes were avoided.
Intraclass correlation coefficients (ICCs) were used to assess the degree of inter-observer agreement. A non-parametric Mann–Whitney test was used to analyse group-level differences using IBM SPSS Statistics for Windows v23 (IBM Corp., Armonk, NY).
Results
The analysis included 14 tuberculomas and 17 HGGs (WHO grade III/IV). No significant differences were noted in the age distribution between the two groups. The imaging phenotype is summarised in Table 1.
Table 1.
Demographic and mean APT values of the lesion and perilesional parenchyma in tuberculoma and high-grade gliomas.
| Case | Age | Sex | Location | Diagnosis |
Mean APT lesion/core |
Mean APT perilesional parenchyma/rim |
Uninvolved white matter |
ROI (mm2) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OBS1 | OBS2 | OBS1 | OBS2 | OBS1 | OBS2 | ||||||
| 1 | 26 | F | LO | TB | 2.42 | 2.36 | 2.30 | 2.35 | 0.9 | 0.8 | 9 |
| 1 | 26 | F | RT | TB | 2.41 | 2.54 | 2.30 | 2.46 | 0.47 | 0.52 | 9 |
| 1 | 26 | F | RP | TB | 2.69 | 2.60 | 2.40 | 2.5 | 0.47 | 0.52 | 9 |
| 2 | 26 | F | LP | TB | 2.28 | 2.35 | 1.70 | 1.65 | 0.89 | 1.01 | 9 |
| 2 | 26 | F | LP | TB | 1.98 | 2.11 | 1.95 | 2.02 | 0.89 | 1.01 | 9 |
| 3 | 27 | F | RF | TB | 3.02 | 2.89 | 3.00 | 3.2 | 0.52 | 0.49 | 9 |
| 3 | 27 | F | RP | TB | 1.53 | 1.49 | 1.48 | 1.65 | 0.52 | 0.49 | 9 |
| 4 | 55 | F | LF | TB | 1.97 | 2.23 | 2.20 | 2.37 | 0.28 | 0.30 | 9 |
| 5 | 35 | F | PF | TB | 3.11 | 3.01 | 2.30 | 2.28 | 0.61 | 0.58 | 9 |
| 6 | 17 | F | RF | TB | 2.3 | 1.98 | 2.10 | 2.2 | 0.55 | 0.60 | 9 |
| 6 | 17 | F | RP | TB | 2.25 | 2.35 | 2.30 | 2.45 | 0.55 | 0.60 | 9 |
| 7 | 17 | F | RF | TB | 2.7 | 2.58 | 1.99 | 1.8 | 0.5 | 0.48 | 9 |
| 7 | 17 | F | RP | TB | 2.6 | 2.74 | 2.30 | 2.39 | 0.5 | 0.48 | 9 |
| 8 | 33 | F | RF | TB | 1.34 | 1.4 | 1.68 | 1.56 | 0.67 | 0.70 | 9 |
| 1 | 33 | M | RF | HGG | 4.5 | 3.96 | NA | 0.17 | 0.16 | 9 | |
| 1 | 33 | M | RF | HGG | 5.7 | 5.9 | NA | 0.17 | 0.16 | 9 | |
| 2 | 50 | F | LT | HGG | 5.51 | 5.87 | NA | 0.9 | 0.94 | 9 | |
| 2 | 50 | F | LT | HGG | 4.4 | 3.98 | NA | 0.9 | 0.94 | 9 | |
| 3 | 40 | M | LF | HGG | 2.91 | 3.06 | NA | 0.36 | 0.40 | 9 | |
| 4 | 26 | M | RP | HGG | 4.16 | 4.2 | NA | 0.41 | 0.38 | 9 | |
| 5 | 35 | M | LP | HGG | 5.1 | 5.6 | NA | 0.41 | 0.38 | 9 | |
| 6 | 46 | F | LT | HGG | 4.81 | 4.58 | NA | 0.89 | 1.01 | 9 | |
| 7 | 40 | M | RF | HGG | 3.91 | 4.2 | NA | 0.37 | 0.39 | 9 | |
| 8 | 60 | M | LT | HGG | 4.6 | 4.45 | NA | 0.48 | 0.50 | 9 | |
| 8 | 60 | M | LF | HGG | 4.8 | 5.2 | NA | 0.35 | 0.39 | 9 | |
| 9 | 44 | F | RP | HGG | 3.3 | 3.67 | NA | 0.6 | 0.57 | 9 | |
| 10 | 15 | F | LF | HGG | 2.4 | 2.2 | NA | 0.9 | 0.87 | 9 | |
| 11 | 37 | M | RF | HGG | 4.1 | 4.3 | NA | 0.52 | 0.50 | 9 | |
| 12 | 49 | M | LPO | HGG | 4.3 | 4.7 | NA | 0.9 | 0.94 | 9 | |
| 13 | 26 | M | PF | HGG | 4.5 | 3.98 | NA | 0.22 | 0.26 | 9 | |
| 14 | 15 | F | Brainstem | HGG | 4.5 | 4.9 | NA | 0.7 | 0.68 | 9 | |
APT: amide proton transfer; M: male; F: female; R: right; L: left; T: temporal; F: frontal; O: occipital; P: parietal; TB: tuberculoma; HGG: high-grade glioma; NA: not applicable.
The conventional imaging characteristics of tuberculomas were annotated by T2 hypointensity (n = 14), T1 hyperintensity (n = 10 peripheral, n = 4 diffuse), diffusion restriction (n = 7 peripheral, n = 2 central, n = 2 both peripheral and central, n = 3 absent) and peripheral contrast enhancement (n = 13). Intralesional foci of blooming on SWI were seen in 11 lesions consisting of fine punctate peripheral foci in five, diffuse punctate foci in three and coarser central foci in three. No focus of hyperperfusion relative to the contralateral white matter was seen in any of the lesions. The ICCs for MTRasym values for the lesion core, rim and normal parenchyma in tuberculoma were 0.95 (95% confidence interval (CI) 0.85–0.98), 0.96 (95% CI 0.87–0.98) and 0.95 (95% CI 0.85–0.98), respectively, and those for the core and normal parenchyma in case of glioma were 0.98 (95% CI 0.97–0.99) and 0.93 (95% CI 0.81–0.97), respectively, indicating excellent inter-observer agreement. The MTRasym values of the tuberculomas ranged from 1.34% to 3.11% (M = 2.32 ± 0.50; Figure 1, blue arrow). HGGs had MTRasym values that ranged from 2.40% to 5.70% (M = 4.32 ± 0.84; Figure 1). Note that the colour scale in Figures 1 and 2 have been adopted to the corresponding highest MTRasym values. The group-level difference was statistically significant (p < 0.001). In cases of tuberculomas, the APTw images were remarkable for high MTRasym values, ranging from 1.48% to 3% (M = 2.14 ± 0.38; significant difference compared to contralateral uninvolved white matter, p < 0.001) in the perilesional oedematous-appearing parenchyma (Figure 2, white arrow on the left). Due to the infiltrating front of gliomas, no distinctive lesion–oedema boundary could be ascertained, as perilesional oedematous parenchyma lacking neoplastic tissue cannot be delineated on imaging.
Figure 1.
Multimodal imaging of high-grade glioma (HGG). (a) Axial T2-weighted imaging shows an infiltrative mass lesion centred in the left temporal lobe with mass effect and uncal displacement. (b) T1-weighted post-contrast imaging shows heterogeneous post-contrast enhancement in the lesion. (c) The lesion demonstrates patchy areas of diffusion restriction, as demonstrated in the apparent diffusion coefficient maps. (d) T1-weighted post-contrast image overlaid by the amide proton transfer-weighted (APTw) image demonstrates multifocal areas of high APT ratios in the lesion. The upper limit of the colour scale has been adopted to show the largest APT ratios in the periphery of the lesions (∼4.3 in the index case).
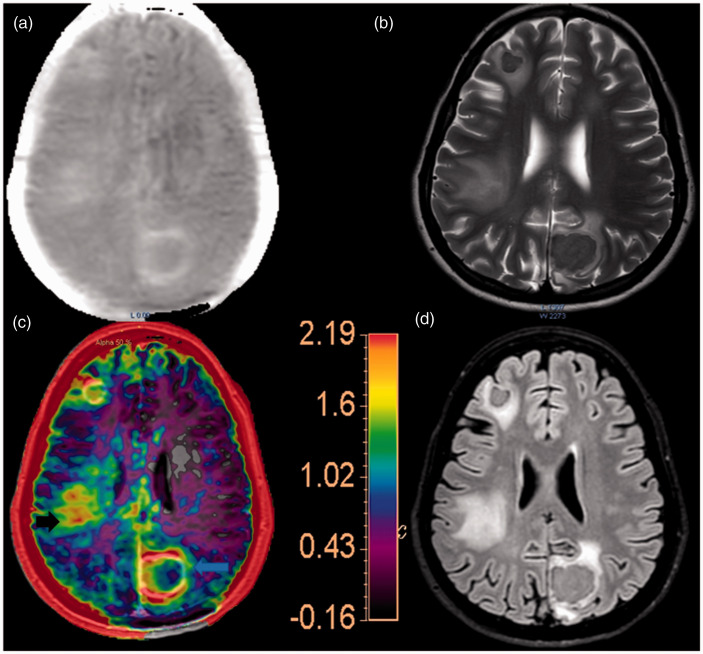
Figure 2.
Tuberculoma. (a) APTw imaging shows elevated magnetisation transfer ratio asymmetry (MTRasym) in the periphery of the lesions in the left posterior parasagittal and right inferior frontal region. (b) T2-weighted imaging shows a T2 hypointense circumscribed lesion. (c) T1-weighted post-contrast image overlaid by the APTw image. The upper limit of the colour scale has been assigned to depict the largest MTRasym. Remarkably, the perilesional oedematous parenchyma in the right inferior parietal region has MTRasym approaching that of the lesions. (d) Axial fluid-attenuated inversion recovery imaging shows perilesional oedema.
Discussion
Typical mammalian cells are composed of 18% proteins by weight. 29 Broadly, cellular proteins may be categorised as bound proteins (having solid-like characteristics whose protons have very short T2 (on the scale of μs) and mobile proteins and peptides (whose protons show relatively longer T2 (on the scale of ms)). While MT imaging seeks to probe the former, APTw imaging targets the latter for imaging contrast. 29 The conceptual roots of APT imaging can be traced to the work of Ward et al. who first proposed a molecular approach denominated CEST. 30 The contrast in this imaging approach exploited the mechanisms of saturation transfer between macromolecular exchangeable protons to water for an enhanced detection sensitivity for molecules. 30 The Z spectrum, the plot of the intensity of the signal of bulk water at differing offset frequencies of the saturation prepulse, is considered the macromolecular signature of the voxel. First proposed by Zhou et al., APT imaging is a relatively nascent imaging approach that draws upon CEST and MT imaging propositions.31–33 APT sequence typically incorporates a long saturation pulse (0.5–2 seconds; to saturate more water protons) and an ensuing image acquisition component (usually 2D/3D; gradient echo/spin echo/echo planar imaging). APT imaging contrast is based on a non-invasive in vivo assessment of spin exchange between the tissue bulk water and amide protons of mobile endogenous proteins and peptides chains (which have their signature resonance frequency at 3.5 ppm downfield that of water in the Z spectrum). Quantitatively, the notation for the APT effect is given by the asymmetry of the MT ratio (MTRasym at 3.5 ppm; MTRasym (3.5 ppm) = ratio of the difference in signal intensities 3.5 ppm downfield and upfield of water resonance, respectively ((S−3.5ppm)–(S3.5ppm)) to that of the unsaturated signal intensity S0). 31 Aside from the water-exchangeable amide proton moieties, other physicochemical determinants (e.g. T1 of water and pH) influence the rate of spin exchange and are thus detected indirectly through the changes in the bulk water signal used in MR imaging. This pH dependence of the signal and contrast was the first described characteristic of this sequence. 32 Other influences on the APTw signal include the spin-lattice relaxation rate of tissue water R1w ( = 1/T1w) saturation time/pulse length(tsat) and upfield nuclear Overhauser enhancement effect. For practical considerations, however, higher APTw signal (as denoted by a higher MTRasym value) is reflective of the high magnitude of exchange, thereby implying greater mobile protein concentration. The cell-biological significance of APTw imaging resides in the fact that native cellular protein-related information is acquired indirectly via bulk water signal, thereby expanding the scope of molecular MR imaging to the level of proteins. Lately, this modality has shown promise in glioma evaluation34–36 and assessment of brain maturation.33,37 Brain maturation and myelination have seen an exponential decrease in the APTw signal (white matter > grey matter), reaching up to 0.18 (fitted MTR at 3.5 ppm) in the putamen and 0.87 (fitted MTR at 3.5 ppm) in the frontal white matter, with no significant changes in the latter part of the first decade. 37
Proteomic profiles of the gliomas have been shown to vary considerably according to their WHO grades. Subcellular protein fractions are markedly increased in various brain tumours compared to the normal brain parenchyma. 38 Qualitatively, differential protein expression among varying glioma grades has been confirmed by two-dimensional polyacrylamide gel electrophoresis(2D-PAGE) and mass spectrometry (MS).39,40 In vivo proton MR spectroscopy visible mobile macromolecular proton signal is higher in neoplasms of the brain compared to normal neuroparenchyma and in fact corresponds to tumour grade. 41 In a proteomic analysis of a murine model of 9L gliosarcoma, Yan et al. demonstrated a significant difference in the cytosolic protein composition of the lesion compared to the normal parenchyma (0.88 ± 0.12 vs. 0.63 ± 0.12 μg/μL; p < 0.05) and a compatible increase in the APT signal. Hitherto, the rationale for adopting APT imaging in glioma characterisation21,42 has been the premise that synthesis of mobile (cytosolic) proteins covaries with the tumoural proliferative activity. 43 High-grade tumours, with a commensurate increase in cytosolic protein synthesis and concentrations, thus demonstrate high APT signal 42 – a finding reprised in our study population.
Tuberculomas are polymorphic lesions and the most common manifestation of neuroparenchymal tuberculosis.10,18,44,45 The parenchymal ‘tubercle’ (the ‘Rich focus’). an early step in the pathogenesis of CNS tuberculosis, is a granulomatous response to the bacilli. 10 Its rupture and subsequent inoculation into the subarachnoid space is indeed the source of tubercular meningitis. Tuberculomas are presumed to arise from conglomeration and enlargement of these microtubules without subarachnoid rupture.44,46 The typical tuberculoma shows caseous necrosis at the centre, with granulomatous inflammation in the periphery. The inflammatory cell population is composed of Langerhans multinucleated giant cells, eosinophilic histiocytes and lymphoplasmacytic lineages. 47 The T2-weighted imaging signal intensity has earlier been reported to mirror the stage in evolution (non-caseation, caseating with the central solid area, caseating with the central liquid area or calcifying) of the granulomatous response, with the signal determinants being macrophages, cellular infiltrates, fibrosis and lipid contents in tuberculomas.48,49 The origins of the T1 signal in the centre and periphery have been ascribed to variations in the protein component in the central necrosis and inflammatory layer peripherally. 50 While tuberculomas with solid caseation are usually T2 hypointense with nodular or rim enhancement on post-contrast images, HGGs are predominantly hyperintense or show mixed signal intensity on T2-weighted images with heterogenous enhancement and large areas of necrosis. The degree of diffusion restriction is higher in glioma compared to tuberculoma. However, tuberculomas can be T2 hyperintense in the presence of central liquefaction. Hence, on conventional imaging, the features of tuberculoma and glioma frequently overlap, often requiring advanced imaging methods.
To the best of our knowledge, no prior work has investigated APT imaging in tuberculomas. Regarding APT (essentially considered an offshoot of MT imaging) in tuberculomas, we reflect on work by Gupta et al. on MT imaging and histopathological correlation in tuberculomas.13,51 The value of pre-contrast MT spin-echo imaging for accurate delineation of tubercular granulomas had been established earlier by Gupta et al. 13 Subsequently, it was recognised that the MT hyperintense rim of tuberculomas was indeed histopathologically and geographically equivalent to the cellular/inflammatory component and Langerhans giant cells. 51 This peripheral inflammatory cellular component had a lower MT ratio compared to the core necrotic components. The T2 and MT-T1 hypointense core sections demonstrated caseous necrosis. It is intuitive to draw parallels and extrapolate findings from MT imaging to its complementary conceptual adjunct, namely, APT imaging. All our cases of tuberculomas had T2/T1 signal corresponding to caseation in the centre, with no significant increased signal in the APTw maps in the centre of the lesion. While tuberculomas in our study had a periphery with higher APT ratios compared to the uninvolved parenchyma, the net increase was significantly smaller compared to the markedly raised APT in HGG. In contrast to HGGs, tuberculomas have earlier been described to have lesional microenvironments, with a relative increase in lipid components and possibly reduced concentration of peptides. 13 This lipid predominance has been shown to result in a relatively smaller MT ratio in tuberculomas compared to granulomas due to neurocysticercosis. 13 We posit that the observed intergroup difference in APTw–MTRasym values are indeed reflective of this inherent difference in mobile peptide composition in the tubercular granulomatous medium compared to HGGs. We propose that although the central caseation may be rich in extracellular proteinaceous content, the APTw imaging visible mobile peptide pool is fractional. Quantification of the fluid content of aspirates for protein, viscosity and cell count was performed by Gupta et al. 52 Two lesions in their study demonstrated cellularity of 17,300 and 45,300 cells/mm3, viscosity of 4.2 and 6.5 cP, and protein content of 35 and 56 mg/mL. To the best of our knowledge, no proteomic analysis of the peptide composition of tuberculoma exists.
Higher APT values (compared to uninvolved white matter) have been reported in the peritumoural FLAIR signal changes in gliomas, reiterating the notion of tumour + oedema in the vicinity of the contrast-enhancing component.31,53 While it is easy to attribute the APTw signal as emanating from cytosolic amides in infiltrating gliomatous neoplasms, the perilesional high signal in the ‘vasogenic’ oedema in the context of metastases is a stand-out feature on APTw imaging. 50 Blood–brain barrier (BBB) dysfunction with a consequent leakiness of intravascular proteins to the extracellular space has been proposed as the mechanism to account for the above phenomenon.31,54 High MTRasym ratios in the ‘vasogenic’ perilesional oedema in tuberculomas in our study are again noteworthy. We presuppose that the mechanisms operating are indeed similar to those culminating in BBB dysfunction. Reflecting on the prior work by Gupta et al., 51 it was noted in their study that the boundary of the T2 hypointense component of the tuberculoma lesions failed to reveal the complete extent of the lesion, likely in part due to perilesional oedema. The anatomical extent of the hypointense tuberculomas as evidenced on T2-weighted imaging was systematically smaller compared to the MT-T1 image abnormality (MT-T1 image matched the dimensions of the final surgical specimen corroborating with lesion margins). It reiterates the between-sequence variability in delineating the ‘true extent’ of the tubercular lesion and the potential additive value of APTw imaging. We note that MT and APTw imaging generate contrast by interrogating different aspects (relatively immobile macromolecules and free proteins/polypeptides) of the tissue. 33 The molecular information provided by these sequences is likely complementary rather than identical. It remains to be seen which component of the inflammatory milieu in the perilesional parenchyma contributes to the changes in MTRasym.
Lately, work by Debnath et al. has investigated the APTw contrast in neoplastic and infective mass lesions, with an emphasis on methods of normalisation and ROI selection. 55 They observed that inter-subject variability of APTw contrast was substantially reduced when the normalisation was performed with the reference signal at a negative offset frequency and APTw contrast in normal-looking white matter. Histogram parameters offered greater discrimination of neoplastic mass lesions compared to mean APTw contrast. While a similar trend of APTw signal difference between neoplasms and infections was noted as in our study, the infection group investigated in their study was heterogenous in its composition, including fungal infections and abscess. 55
While evidence for alternative means of discriminating tuberculomas based on metabolite patterns (lower choline on MR spectroscopy) and histoarchitecture (higher apparent diffusion coefficient) exist, 2 APTw imaging represents a unique modality that probes an altogether different facet of the lesion pathophysiology, namely, the amide milieu. We wish to underscore its potential adjunctive value.
The modality is not without its limitations. Erroneous high APTw signal is frequently noted in areas of haemorrhage and loci of protein-rich cystic changes composed of protein-rich content.56–59 While we have excluded foci of haemorrhage and cystic changes, the potential contributions of these influences (when present at levels below the resolution of the imaging modality) on the APTw signal should be borne in mind. As stated earlier, the APTw SI can be modulated by the tissue pH, and brain tumours are noted for a reversal of pH gradient across the cell. 60 Proton exchange rate is base catalysed in the ranges of physiological pH, with fluctuations in the exchange speed evident with alterations in pH. Prior microelectrode-based work on human brain tumours has highlighted that tumour microenvironments may harbour a lower pH (up to 5.9, with a mean approximately 6.8, while normal neuroparenchyma has a pH of approximately 7.1). Other investigators have observed pH higher than normal brain tissue in murine models of glioma from 31P NMR spectra. 61 While its quantitative effects on APTw signal are uncertain, the possible confounding effect of the altered pH in the pathological tissue should be considered while interpreting APTw imaging in these conditions. We acknowledge the limitations of the study in terms of the small sample size and non-availability of histopathological data for all cases of tuberculomas.
To conclude, we demonstrate that tuberculomas show significantly lower MTRasym ratios compared to HGGs, reflective of a relative paucity of mobile amide protons in the ambient microenvironment. This finding holds adjunctive and discriminatory value in the Indian subcontinent where a diagnostic dilemma between these two entities frequently arises. Perilesional elevated MTRasym values in the tuberculomas are a novel observation that may point towards the inflammatory milieu.
Conflict of interests
The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Footnotes
Funding: The authors disclosed the receipt of the following financial support for the research, authorship, and/or publication of this article: Science and Engineering Research Board (SERB) Sanction order number- EEQ/2018/000682 dated 20th March, 2019. The grant was sanctioned at the request of Dr. Atchayaram Nalini who is a coauthor.
ORCID iDs: Karthik Kulanthaivelu https://orcid.org/0000-0002-1585-8769
Shumyla Jabeen https://orcid.org/0000-0002-3816-4313
References
- 1.Suslu HT, Bozbuga M, Bayindir C. Cerebral tuberculoma mimicking high grade glial tumor. Turk Neurosurg 2011; 21: 427–429. [DOI] [PubMed] [Google Scholar]
- 2.Peng J, Ouyang Y, Fang W-D, et al. Differentiation of intracranial tuberculomas and high grade gliomas using proton MR spectroscopy and diffusion MR imaging. Eur J Radiol 2012; 81: 4057–4063. [DOI] [PubMed] [Google Scholar]
- 3.Dash C, Singla R, Garg K, et al. Hypothalamic chiasmatic tuberculoma mimicking glioma. Childs Nerv Syst 2016; 32: 233–235. [DOI] [PubMed] [Google Scholar]
- 4.Kim J-K, Jung T-Y, Lee K-H, et al. Radiological follow-up of a cerebral tuberculoma with a paradoxical response mimicking a brain tumor. J Korean Neurosurg Soc 2015; 57: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Algahtani HA, Aldarmahi AA, Algahtani AY, et al. Tumour-like presentation of central nervous system tuberculosis: a retrospective study in Kingdom of Saudi Arabia. J Taibah Univ Med Sci 2014; 9: 143–150. [Google Scholar]
- 6.Fath-Ordoubadi F, Lane RJM, Richards PG. Histological surprise: callosal tuberculoma presenting as malignant glioma. J Neurol Neurosurg Psychiatry 1997; 63: 98–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mukherjee S, Das R, Begum S. Tuberculoma of the brain – a diagnostic dilemma: magnetic resonance spectroscopy a new ray of hope. J Assoc Chest Phys 2015; 3: 3. [Google Scholar]
- 8.Bernaerts A, Vanhoenacker FM, Parizel PM, et al. Tuberculosis of the central nervous system: overview of neuroradiological findings. Eur Radiol 2003; 13: 1876–1890. [DOI] [PubMed] [Google Scholar]
- 9.Nayak L, Reardon DA. High-grade gliomas. Continuum (Minneap Minn) 2017; 23: 1548–1563. [DOI] [PubMed] [Google Scholar]
- 10.Schaller MA, Wicke F, Foerch C, et al. Central nervous system tuberculosis: etiology, clinical manifestations and neuroradiological features. Clin Neuroradiol 2019; 29: 3–18. [DOI] [PubMed] [Google Scholar]
- 11.Parry AH, Wani AH, Shaheen FA, et al. Evaluation of intracranial tuberculomas using diffusion-weighted imaging (DWI), magnetic resonance spectroscopy (MRS) and susceptibility weighted imaging (SWI). Br J Radiol 2018; 91: 20180342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales H, Alfaro D, Martinot C, et al. MR spectroscopy of intracranial tuberculomas: a singlet peak at 3.8 ppm as potential marker to differentiate them from malignant tumors. Neuroradiol J 2015; 28: 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta RK, Kathuria MK, Pradhan S. Magnetization transfer MR imaging in CNS tuberculosis. Am J Neuroradiol 1999; 20: 867–875. [PMC free article] [PubMed] [Google Scholar]
- 14.Haris M, Gupta RK, Husain M, et al. Assessment of therapeutic response in brain tuberculomas using serial dynamic contrast-enhanced MRI. Clin Radiol 2008; 63: 562–574. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee S, Saini J, Kesavadas C, et al. Differentiation of tubercular infection and metastasis presenting as ring enhancing lesion by diffusion and perfusion magnetic resonance imaging. J Neuroradiol 2010; 37: 167–171. [DOI] [PubMed] [Google Scholar]
- 16.Gupta R. Magnetization transfer MR imaging in central nervous system infections. Ind J Radiol Imag 2002; 12: 51. [Google Scholar]
- 17.Gupta RK, Haris M, Husain N, et al. Relative cerebral blood volume is a measure of angiogenesis in brain tuberculoma. J Comput Assist Tomogr 2007; 31: 335–341. [DOI] [PubMed] [Google Scholar]
- 18.Patkar D, Narang J, Yanamandala R, et al. Central nervous system tuberculosis: pathophysiology and imaging findings. Neuroimaging Clin N Am 2012; 22: 677–705. [DOI] [PubMed] [Google Scholar]
- 19.Zhou J, Heo H-Y, Knutsson L, et al. APT-weighted MRI: techniques, current neuro applications, and challenging issues: APTw MRI for neuro applications. J Magn Reson Imaging 2019; 50: 347–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakata A, Okada T, Yamamoto Y, et al. Addition of amide proton transfer imaging to FDG-PET/CT improves diagnostic accuracy in glioma grading: a preliminary study using the continuous net reclassification analysis. AJNR Am J Neuroradiol 2018; 39: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi YS, Ahn SS, Lee S-K, et al. Amide proton transfer imaging to discriminate between low- and high-grade gliomas: added value to apparent diffusion coefficient and relative cerebral blood volume. Eur Radiol 2017; 27: 3181–3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Razek AA, Talaat M, El-Serougy L, et al. Differentiating glioblastomas from solitary brain metastases using arterial spin labeling perfusion- and diffusion tensor imaging-derived metrics. World Neurosurg 2019; 127: e593–598. [DOI] [PubMed] [Google Scholar]
- 23.Razek AA, El-Serougy L, Abdelsalam M, et al. Differentiation of primary central nervous system lymphoma from glioblastoma: quantitative analysis using arterial spin labeling and diffusion tensor imaging. World Neurosurg 2019; 123: e303–309. [DOI] [PubMed] [Google Scholar]
- 24.El-Serougy L, Abdel Razek AA, Ezzat A, et al. Assessment of diffusion tensor imaging metrics in differentiating low-grade from high-grade gliomas. Neuroradiol J 2016; 29: 400–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Razek AA, Talaat M, El-Serougy L, et al. Clinical applications of arterial spin labeling in brain tumors. J Comput Assist Tomogr 2019; 43: 525–532. [DOI] [PubMed] [Google Scholar]
- 26.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016; 131: 803–820. [DOI] [PubMed] [Google Scholar]
- 27.Togao O, Keupp J, Hiwatashi A, et al. Amide proton transfer imaging of brain tumors using a self-corrected 3D fast spin-echo Dixon method: comparison with separate B0 correction. Magn Reson Med 2017; 77: 2272–2279. [DOI] [PubMed] [Google Scholar]
- 28.Togao O, Hiwatashi A, Keupp J, et al. Amide proton transfer imaging of diffuse gliomas: effect of saturation pulse length in parallel transmission-based technique. PLoS One 2016; 11: e0155925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou J. Amide proton transfer imaging of the human brain. Methods Mol Biol 2011; 711: 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST). J Magn Reson 2000; 143: 79–87. [DOI] [PubMed] [Google Scholar]
- 31.Kamimura K, Nakajo M, Yoneyama T, et al. Amide proton transfer imaging of tumors: theory, clinical applications, pitfalls, and future directions. Jpn J Radiol 2019; 37: 109–116. [DOI] [PubMed] [Google Scholar]
- 32.Zhou J, Payen J-F, Wilson DA, et al. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med 2003; 9: 1085–1090. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Y, Wang X, Zhao X. Magnetization transfer and amide proton transfer MRI of neonatal brain development. Biomed Res Int 2016; 2016: 3052723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Togao O, Hiwatashi A, Yamashita K, et al. Grading diffuse gliomas without intense contrast enhancement by amide proton transfer MR imaging: comparisons with diffusion- and perfusion-weighted imaging. Eur Radiol 2017; 27: 578–588. [DOI] [PubMed] [Google Scholar]
- 35.Jiang S, Yu H, Wang X, et al. Molecular MRI differentiation between primary central nervous system lymphomas and high-grade gliomas using endogenous protein-based amide proton transfer MR imaging at 3 Tesla. Eur Radiol 2016; 26: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su C, Zhao L, Li S, et al. Amid proton transfer (APT) and magnetization transfer (MT) MRI contrasts provide complimentary assessment of brain tumors similarly to proton magnetic resonance spectroscopy imaging (MRSI). Eur Radiol 2019; 29: 1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang H, Kang H, Zhao X, et al. Amide proton transfer (APT) MR imaging and magnetization transfer (MT) MR imaging of pediatric brain development. Eur Radiol 2016; 26: 3368–3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kökoǧlu E. RNA, DNA and total protein levels in subcellular fractions of human brain tumors. Cancer Lett 1987; 34: 67–71. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Zhuang Z, Okamoto H, et al. Proteomic profiling distinguishes astrocytomas and identifies differential tumor markers. Neurology 2006; 66: 733–736. [DOI] [PubMed] [Google Scholar]
- 40.Hobbs SK, Shi G, Homer R, et al. Magnetic resonance image-guided proteomics of human glioblastoma multiforme. J Magn Reson Imaging 2003; 18: 530–536. [DOI] [PubMed] [Google Scholar]
- 41.Howe FA, Barton SJ, Cudlip SA, et al. Metabolic profiles of human brain tumors using quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 2003; 49: 223–232. [DOI] [PubMed] [Google Scholar]
- 42.Su C, Liu C, Zhao L, et al. Amide proton transfer imaging allows detection of glioma grades and tumor proliferation: comparison with Ki-67 expression and proton MR spectroscopy imaging. Am J Neuroradiol 2017; 38: 1702–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan K, Fu Z, Yang C, et al. Assessing amide proton transfer (APT) MRI contrast origins in 9 L gliosarcoma in the rat brain using proteomic analysis. Mol Imaging Biol 2015; 17: 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khatri GD, Krishnan V, Antil N, et al. Magnetic resonance imaging spectrum of intracranial tubercular lesions: one disease, many faces. Pol J Radiol 2018; 83: e524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Torres C, Riascos R, Figueroa R, et al. Central nervous system tuberculosis. Top Magn Reson Imaging 2014; 23: 173–189. [DOI] [PubMed] [Google Scholar]
- 46.Rock RB, Olin M, Baker CA, et al. Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev 2008; 21: 243–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kleinschmidt-DeMasters BK, Beckham JD, Tyler KL. 23 – Infec tions and inflammatory disorders. In: Perry A and Brat DJ (eds) Practical surgical neuropathology: a diagnostic approach. 2nd ed. Philadelphia: Elsevier, 2018, pp.547–579. [Google Scholar]
- 48.Gupta RK, Pandey R, Khan EM, et al. Intracranial tuberculomas: MRI signal intensity correlation with histopathology and localised proton spectroscopy. Magn Reson Imaging 1993; 11: 443–449. [DOI] [PubMed] [Google Scholar]
- 49.Gupta RK, Kumar S. Central nervous system tuberculosis. Neuroimaging Clin N Am 2011; 21: 795–814, vii–viii. [DOI] [PubMed] [Google Scholar]
- 50.Kim TK, Chang KH, Kim CJ, et al. Intracranial tuberculoma: comparison of MR with pathologic findings. AJNR Am J Neuroradiol 1995; 16: 1903–1908. [PMC free article] [PubMed] [Google Scholar]
- 51.Gupta RK, Husain N, Kathuria MK, et al. Magnetization transfer MR imaging correlation with histopathology in intracranial tuberculomas. Clin Radiol 2001; 56: 656–663. [DOI] [PubMed] [Google Scholar]
- 52.Gupta RK, Prakash M, Mishra AM, et al. Role of diffusion weighted imaging in differentiation of intracranial tuberculoma and tuberculous abscess from cysticercus granulomas – a report of more than 100 lesions. Eur J Radiol 2005; 55: 384–392. [DOI] [PubMed] [Google Scholar]
- 53.Wen Z, Hu S, Huang F, et al. MR imaging of high-grade brain tumors using endogenous protein and peptide-based contrast. Neuroimage 2010; 51: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu H, Lou H, Zou T, et al. Applying protein-based amide proton transfer MR imaging to distinguish solitary brain metastases from glioblastoma. Eur Radiol 2017; 27: 4516–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Debnath A, Gupta RK, Singh A. Evaluating the role of amide proton transfer (APT)–weighted contrast, optimized for normalization and region of interest selection, in differentiation of neoplastic and infective mass lesions on 3T MRI. Mol Imaging Biol 2020; 22: 384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Togao O, Yoshiura T, Keupp J, et al. Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro-Oncology 2014; 16: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park JE, Kim HS, Park KJ, et al. Pre- and posttreatment glioma: comparison of amide proton transfer imaging with MR spectroscopy for biomarkers of tumor proliferation. Radiology 2016; 278: 514–523. [DOI] [PubMed] [Google Scholar]
- 58.Jeong H-K, Han K, Zhou J, et al. Characterizing amide proton transfer imaging in haemorrhage brain lesions using 3T MRI. Eur Radiol 2017; 27: 1577–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang M, Hong X, Chang C-F, et al. Simultaneous detection and separation of hyperacute intracerebral hemorrhage and cerebral ischemia using amide proton transfer MRI: MRI detection of intracerebral hemorrhage. Magn Reson Med 2015; 74: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ray KJ, Simard MA, Larkin JR, et al. Tumor pH and protein concentration contribute to the signal of amide proton transfer magnetic resonance imaging. Cancer Res 2019; 79: 1343–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ross BD, Higgins RJ, Boggan JE, et al. 31P NMR spectroscopy of the in vivo metabolism of an intracerebral glioma in the rat. Magn Reson Med 1988; 6: 403–417. [DOI] [PubMed] [Google Scholar]