Abstract
In 2019, a novel coronavirus was identified in Wuhan, China. This strain was classified as a pandemic in early 2020 by the World Health Organization (WHO), rapidly reaching millions of cases worldwide and overwhelming intensive care units. One distinct feature identified in severe SARS-CoV-2 is abnormal and complex coagulation and hematologic abnormalities, including significantly elevated D-dimer and fibrin/fibrinogen values possibly increasing morbidity and mortality in this patient population. Aggressive anticoagulation therapy with appropriate peak anti-Xa level monitoring has produce satisfactory results at our institution. Our intent is to present a case series of our strategy to highlight the benefits of this approach.
Keywords: anticoagulation, SARS-COV-2, severe, peak anti-Xa level, D-dimer
Introduction
It has been theorized that once SARS-CoV-2 infects a patient, viremia ensues and by using the bloodstream, the virus spreads to other parts of the body, gaining access to the lungs, kidneys and gastrointestinal tract possibly using ACE2 receptors. One distinct feature identified in severe SARS-CoV-2 disease is abnormal coagulation parameters and complex hematologic abnormalities, including significantly elevated D-dimer and fibrin/fibrinogen values.1,2 This characteristic may confer higher mortality among patients with severe SARS-CoV-2 infection.1-4
Due to the increased risk for coagulopathy, clinicians are reconsidering their approach to anticoagulation in the intensive care unit (ICU) advocating to aggressively utilize low molecular weight heparin (LMWH) in this patient’s population. Emerging data suggests there may be a benefit from aggressive anticoagulation therapy in patients suffering from severe SARS-CoV-2 disease. 3 At Tongji Hospital of Huazhong University of Science and Technology in Wuhan, Tang and colleagues enrolled 449 patients with SARS-CoV-2 disease. They concluded that anticoagulation therapy with LMWH leads to better outcomes in patients with elevated D-dimers and signs and symptoms of sepsis-induced coagulopathy.3,5,6
Although the definition of severe SARS-CoV-2 disease varies among countries and institutions, the Center for Disease Control (CDC) defines severe disease as patients with radiographic evidence of pneumonia, or acute respiratory distress syndrome (ARDS) or autopsy findings consistent with the either of these 2 conditions. 7
In the setting of lack of medical literature that otherwise may dictate anticoagulation therapy in this novel disease, peak anti-Xa level monitoring to target prophylactic and therapeutic levels could be utilized to guide therapy. The aim of this case series is to describe our experience in critically ill patients with SARS-CoV-2 disease in the ICU.
Case Series Report
Case 1
A 43-year-old Hispanic male (80 kg) presented directly from a cruise ship after testing positive for SARS-CoV-2 with complaints of cough, fevers, and shortness of breath. He arrived on a non-rebreather mask (NRB) at 15 L/min supplementation with oxygen saturation (SpO2) above 90%. Upon admission, a chest x-ray showed multifocal ground opacities with worsening bilateral infiltrates at hospital day (HD) 3 with no signs of pulmonary emboli. The patient was started on an anti-SARS-CoV-2 medication regimen (see Table 1). Enoxaparin 40 mg SQ every 24 h was initiated on HD 1; however, on HD 2, the patient required intubation due to hypoxemia.
Table 1.
Patient Demographic Characteristics.
| Patients | Case:1 | Case:2 | Case:3 |
|---|---|---|---|
| Age | 43 | 27 | 53 |
| Sex | Male | Male | Male |
| Comorbidities | Hypertension | None | Hypertension, diabetes |
| Anti COVID-19 treatment | Day 1: HCQ 400 mg orally every 12 h + Azithro
500 mg IV × 1 Day 2: HCQ 200 mg orally every 12 h for 4 days + Azithro 250 mg orally daily × 4 days |
||
| PTT | 33.7 (seconds) | 36.7 (seconds) | 37.0 (seconds) |
| INR | 1.1 | 1.3 | 1.3 |
| WBC | 9.8 × 103/uL | 4.0 × 103/uL | 6.4 × 103/uL |
| Platelet | 305,000/mm3 | 159,000/mm3 | 209,000/mm3 |
| Lymphocytes | 2% to 11% | 10.5% to 13.4% | 11.1% to 43.4% |
| CRP* | 2 to 23.54 mg/dL | 28.60 to 34.96 mg/dL | 0.53 to 10.36 vmg/dL |
| Ferritin | 1215 to 3326 ng/mL | 580 to 19512 ng/mL | 610 to 1171 ng/mL |
Note. HCQ = Hydroxychloroquine; Azithro = Azithromycin; INR = International normalized ratio; PTT = partial thromboplastin time. Reference ranges as follows: PTT: 25.4 to 34.9 s, platelets 150,000 to 450,000 per mm3, ferritin 30 to 400 ng/mL
C-reactive protein 0 to 0.8 mg/dL. Lymphocytes 20% to 45%.
On HD 3, the PaO2:FiO2 (partial pressure of arterial oxygen: fraction of inspired oxygen) ratio was 63 while in supine position on maximal ventilatory settings of 100% FiO2. Manual proning was performed for 16 h a day, which improved his PaO2:FiO2 ratio to 115 while in prone position. On HD 5, the supine PaO2:FiO2 ratio was 101 on 70% FiO2.
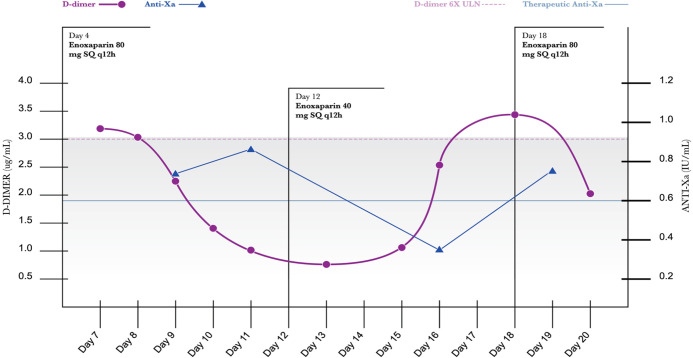
A peak anti-Xa level was drawn on HD 5 to determine if the patient was appropriately anticoagulated with the prophylactic enoxaparin dose. Exactly 4 h after the administration of enoxaparin, the heparin peak anti-Xa was undetectable (<0.10 IU/mL) and the decision was made to initiate therapeutic enoxaparin at a dose of 1 mg/kg (80 mg) subcutaneous (SQ) injection every 12 h due to an elevated D-dimer level (3.17 ug/mL on HD 8) (see Table 2 for anti-Xa level reference).
Table 2.
Anti-Xa Level Goals.
| Anti-Xa Level Targets (Peak anti-Xa levels are drawn 4 h after administration of 3rd dose) | |
|---|---|
| Prophylaxis | 0.2 to 0.4 IU/mL |
| Treatment | 0.6 to 1.2 IU/mL |
Thromboelastography (TEG) noted a prolonged maximal amplitude (MA) of 76.2 mm, indicative of hypercoagulability. The D-dimer trended downward throughout the course of therapeutic anticoagulation (see Figure 1).
Figure 1.
D-Dimer/Anti-Xa level.
After the 5th HD of therapeutic anticoagulation, the patient’s supine PaO2:FiO2 ratio improved to 170 on 50% FiO2, and the D-dimer decreased to 2.23 ug/mL. The peak anti-Xa level was within goal therapeutic range (0.72 IU/mL). After 2 HD of D-dimer readings less than 3 ug/mL, the enoxaparin was decreased to 40 mg (0.5 mg/kg) SQ q12h. The peak anti-Xa level was appropriate at 0.35 IU/mL on this dosing regimen. On HD 16, manual proning was discontinued. The patient was extubated on HD 17 to NRB mask and initiated on an intravenous methylprednisolone taper. One day after extubation, the D-dimer increased to 3.48 ug/mL, and therapeutic anticoagulation was reinitiated at the previous dosage (1mg/kg SQ every 12 h). The patient was transferred out of the ICU on HD 22 and the enoxaparin was discontinued due to melena which could have been exacerbated by the initiation of aspirin on the day of extubation. On HD 24, an echocardiogram was performed with no significant findings. The patient was discharged to rehab on HD 28 with no mental deficit.
Case 2
A 27-year-old Indonesian male (67 kg) with no past medical history arrived to the hospital requiring oxygen supplementation via an NRB mask at 15 L/min from a cruise ship with SpO2 88% to 90%. He tested positive for SARS-CoV-2 and was initiated on the anti-COVID 19 medication regimen (see Table 1) and enoxaparin 40 mg SQ every 24 h. On HD 1, the patient required intubation due to worsening mentation and hypoxia. The following day, he required maximal ventilatory support of 100% FiO2 resulting in a PaO2:FiO2 of 64. Manual proning similar to the previous case was instituted. The peak anti-Xa level resulted in an appropriate prophylactic value of 0.33 IU/mL, however, no therapeutic anticoagulation was initiated.
On HD 10, despite efforts of proning, neuromuscular blockade, and nitric oxide, the patients PaO2:FiO2 continued to decline on FiO2 of 100% to 60 while supine and 72 while in prone position. His D-dimer value resulted in greater than 20 ug/mL (SARS-CoV-2 protocol did not include daily D-dimer at the time of admission). The patient continued to deteriorate in the following 48 h developing acute renal failure, fevers of 104 degrees Fahrenheit, required multiple vasopressors and a do-not-resuscitate order was placed after discussion with his next of kin. The patient was not initiated on therapeutic anticoagulation during his hospital course. Despite efforts of the multi-disciplinary team, the patient expired on HD 12.
Case 3
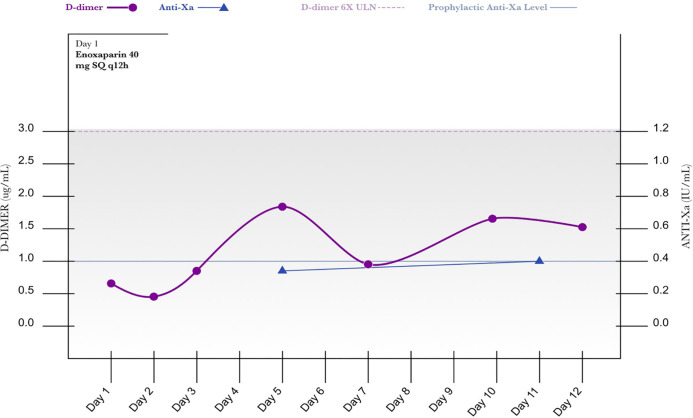
A 53-year-old male (65.3 kg) presented with complaints of fever, shortness of breath, malaise, and tested positive for SARS-CoV-2. He was placed on high-flow nasal cannula, but became tachypneic with a respiratory rate of 40 to 42 breaths per minute requiring intubation and transfer to the intensive care unit. Patient was initiated on a COVID-19 related regimen (see Table 1) and intravenous hydrocortisone 50 mg every 6 h. Based on initial D-dimer of 0.65 upon admission, a more aggressive dose of enoxaparin 40 mg SQ every 12 h was started. His PaO2:FiO2 ratio on HD 2 was 195 on 40% FiO2. The D-dimer remained less than 3 ug/mL throughout his ICU stay and an appropriate peak anti-Xa level of 0.38 IU/mL was observed on HD 4 with the previously mentioned enoxaparin regimen. He was extubated on HD 7, transferred out of the intensive care unit on HD 13 and discharged to rehab on HD 18 with no mental deficit (see Figure 2).
Figure 2.
D-Dimer/Anti-Xa level.
Discussion
We began investigating possible explanations of refractory oxygenation despite optimal interventions, and factors leading to an increased mortality. A plethora of scientists have postulated theories to explain the coagulation phenomenon induced by severe SARS-CoV-2 disease, yet the pathophysiology remains complex and multifactorial.3,7
COVID-19 Associated Coagulopathy (CAC) 8 is one of the hallmark complications of SARS-CoV-2. Profound inflammation and elevation of IL-6 and IL-1, tumor necrosis factor undoubtedly contribute to severity of clinical presentation and poor patient’s outcomes. 9 Kuba and Zhang et al suggest that activation of endothelial cells may represent a unique pathogenesis of this disease, possibly contributing to severe lung injury.10,11 After disruption of endothelial cells by the SARS-CoV-2 virus, it is believed an up-regulation of thrombin generation occurs both systemically and locally in vital organs such as the lungs.12,13 Dysregulation of thrombin generation is further exacerbated by decreased fibrinolysis leading to increased fibrin deposition. Refractory oxygenation can also contribute to a hypercoagulable state due to an increase in blood viscosity. 14 Additionally, a case series of 4 autopsies of SARS-CoV-2 infected patients found small, firm thrombi in sections of peripheral parenchyma and vessel thrombus formation in lung periphery.15,16
A recent prospective study determined D-dimer and fibrin/fibrinogen degradation products were significantly higher in patients with severe SARS-CoV-2 infection compared to those with milder forms of disease, and that routine monitoring may be helpful for early identification in severe disease.4,6 D-dimer, an acute phase reactant, is a sign of excessive coagulation when elevated and in addition to peak anti-Xa levels, may be useful laboratory values to trend in order to predict the likelihood of thrombosis and monitor disease progression.
In a single-center, retrospective study of 81 patients with SARS-CoV-2, 25% of patients developed venous thromboembolism (VTE). Researchers noted that a D-dimer value of 3 ug/mL was associated with a sensitivity of 70%, specificity of 96.7% and positive predictive value of 87.5% for predicting VTE. 3 Klok and colleagues suggest a cumulative incidence of thrombosis of 31% in 184 ICU patients with severe SARS-CoV-2 disease. Despite this data, initiation of therapeutic anticoagulation without evidence of active VTE remains controversial. 17
Treating severe SARS-CoV-2 patients with unfractionated heparin (UFH) has been shown to decrease mortality in 1 retrospective analysis conducted at the Tongii Medical Center in Wuhan, China. In those with a D-dimer exceeding 3 ug/mL, a 19.6% reduction in mortality was noted in patients treated with anticoagulation for a maximum of 7 days. However, in the 99 patients that received heparin, 94 received LMWH at a dosage of 40 to 60 mg of enoxaparin per day, compared to 5 patients receiving UFH at a dosage of 10,000 to 15,000 units per day, leaving uncertainty in the optimal product, dosing, duration and frequency. 3
Based on the recent literature, we took into consideration that conventional prophylactic dosing may be suboptimal to prevent thromboembolism. Additionally, in those with a D-dimer greater than 3 ug/mL, therapeutic anticoagulation without a VTE diagnosis may have a role based on a high positive predictive value in previous studies. 5
At Broward Health District, we established an anticoagulation protocol in an effort to aggressively treat critically ill patients affected with SARS-CoV-2, based on the D-dimer value. Patients described in this case series did not require consent for treatment and per our institution guidelines this is IRB (Institutional Review Board) exempt. Our approach has been to fully anticoagulate those with D-dimer levels 6 times above the upper normal limit (3 ug/mL). For those without a significantly elevated D-dimer but less than 6 times the upper normal limit (<3 ug/mL), we used high prophylactic doses of LMWH (see Table 3). It is important to highlight that none of the patients described in this case series receive remdesivir or tosilizumab due to their unavailability at the time. The CDC and NIH recommended against the use of steroids for SARS-CoV-2 at the time of treatment., however, steroids were used in 2 cases based on physician discretion. 18
Table 3.
Anticoagulation Protocol.
| Anticoagulation protocol for patients with severe SARS-CoV-2 infection | ||
|---|---|---|
| D-dimer* | Medication | Dose/Route |
| D-dimer > 3 ug/ml | LMWH (enoxaparin) | 1 mg/kg subcutaneous every 12 h |
| D-dimer < 3 ug/ml | LMWH (enoxaparin) | 40 mg subcutaneous every 12 h |
| Patients with CrCl < 30 ml/min | Heparin 7500 units Q8H | |
Normal D-dimer level (0.27-0.48 mcg/ml). D-dimer orders should be placed daily as part of our SARS-CoV-2 protocol.
Use Actual Body Weight and round up to nearest vial size. CrCl = Creatinine Clearance.
After initiation of therapeutic anticoagulation, a steady decline in the D-dimer was observed as shown in Figures 1 and 2. Additionally, aggressive prophylactic anticoagulation (enoxaparin dose of 40 mg SQ every 12 h) resulted in D-dimers consistently below 3 ug/mL with appropriate peak anti-Xa levels.
The peak anti-Xa assay is a useful monitoring parameter to determine the degree of anticoagulant activity of LMWH by assessing the function of a specific coagulation cofactor, factor Xa. 7 Due to the hypercoagulable state, we analyzed peak anti-Xa values 4 h after administration to determine the efficacy of LMWH.
Tang and colleagues suggested a higher dose of LMWH could be considered in non-Asian patients with severe SARS-CoV-2. 5 After instituting our anticoagulation protocol, positive clinical outcomes, including survival and extubation, were noted among cases 1 and 3. Anticoagulation was discontinued in 1 case due to melena; however, no intervention was required. As suggested in medical literature, it appears that anticoagulation in patients with CAC and elevated D-dimer (cases 1 and 3, described herein) correlates to better prognostic outcomes.
Formal studies are warranted to investigate the benefit of adjusting LMWH dosing based on D-dimer values, and the utility of peak anti-Xa monitoring in patients infected with SARS-CoV-2. 15 Further evaluation is required to determine whether these observations were the result of anticoagulation therapy or the result of other management strategies. Our results were consistent with previous studies suggesting that higher D-dimer levels, without appropriate anticoagulation, as with case 2, leads to poor outcomes.19,20
Conclusion
Peak anti-Xa level monitoring could be a useful tool to monitor the effects of LMWH in patients with severe SARS-COV-2 disease. To our knowledge, this is the first report of utilization of an aggressive anticoagulation approach based on D-dimer levels and additional monitoring of anticoagulation using peak anti-Xa values. Larger, randomized studies are warranted to confirm our results.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Francis J. Zamora  https://orcid.org/0000-0001-7705-9092
https://orcid.org/0000-0001-7705-9092
References
- 1. Zhu N, Zhang D, Wang W, et al. China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hui DS, Azhar EI, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health – the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gupta N, Zhao YY, Evans CE. The stimulation of thrombosis by hypoxia. Thromb Res. 2019; 181:77-83. [DOI] [PubMed] [Google Scholar]
- 7. National Center for Health Statistics, & Centers for Disease Control and Prevention. (2020). https://www.cdc.gov/sars/guidance/b-surveillance
- 8. Wang YD, Zhang SP, Wei QZ, et al. COVID-19 complicated with DIC: 2 cases report and literatures review. Zhonghua Xue Ye Xue Za Zhi. 2020;41(3):245-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323(11):1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky A. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulation in patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116-1120. [DOI] [PubMed] [Google Scholar]
- 13. Lin L, Lu LF, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microb Infect. 2020;9(1):727-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tang N, Li D, Wang X, Sun Z, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mucha SR, Dugar S, McCrae K, et al. Coagulopathy in COVID-19. Cleve Clin J Med. 2020;87(8):461-468. [DOI] [PubMed] [Google Scholar]
- 16. Lillicrap D, et al. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thromb Haemost. 2020;18:786-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) Cases in the U.S. (2020, March 3). https://www.cdc.gov/coronavirus/2019-ncov/cases-in-us.html
- 18. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020;191:145-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vera-Aguilera J, Yousef H, Beltran-Melgarejo D, et al. Clinical scenarios for discordant peak anti-Xa. Adv Hematol. 2016: 4054806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fox SE, Akmatbekov A, Harbert J, et al. Pulmonary and cardiac pathology in covid-19: the first autopsy series from New Orleans. Chemrxiv Pre-Print. 2020. 10.1101/2020.04.06.20050575 [DOI] [PMC free article] [PubMed] [Google Scholar]