Abstract
Background: Inappropriate prescribing may lead to medication errors among older adults. Pharmacists can curb the occurrences of these errors by conducting medication reviews. Screening Tool of Older Person’s Prescriptions (STOPP) or Screening Tool to Alert doctors to Right Treatments (START) may curb the incidence of adverse drug reactions and improve medication appropriateness by providing guides about when particular types of medications should be started or stopped. Objective: This study aimed to evaluate the use of STOPP/START to improve the Adapted Medication Appropriateness Index (MAI), to reduce the risk of ADRs (GerontoNet score), and length of stay (LOS). Setting: Geriatric Inpatient Ward, Sanglah General Hospital, Bali, Indonesia. Method: A non-randomized controlled trial was conducted in older adults (>60 years) who were selected consecutively from inpatient units in a tertiary hospital in Bali, Indonesia. The intervention group received medication reviews by pharmacists in collaboration with physicians to assess its appropriateness with STOPP/START criteria on admission and during their stay at the hospital. The control group obtained standard care. Main Outcome Measures: The outcomes were measured using the Adapted MAI, GerontoNet Score, and LOS. Results: Thirty patients in the intervention group and 33 patients in the control group were included in this study. The adapted MAI was 2.97 (2.25) and 9.94 (6.14) with P < .001. The GerontoNet score was 3.33 (2.28) and 5.18 (2.10) with P = .003, LOS was 7.63 (3.00) days and 14.18 (9.97) days with P = .011, respectively. Conclusion: The use of STOPP/START as a tool for medication review improved medication appropriateness and reduced ADR risk and LOS.
Keywords: STOPP/START, adverse drug reactions, drug/medical use evaluation, geriatrics, older adult, medication therapy management
Lay tittle: STOPP-START medication review.
Lay summary: Medication review with STOPP-START is developed for older adults. Chronic diseases and polypharmacy add the burden to elderly health. STOPP-START medication review will help stop unnecessary or harmful medication, propose to start any medicine that needed, and therefore increase the medication appropriate index and patient safety.
Introduction
United Nations Economic and Social Commission for Asia and the Pacific (ESCAP) stated that in 2016, approximately 12.4% of the population in the region was 60 years or older with the increasing projection to more than a quarter or 1.3 billion people by 2050. 1 The percentages vary, however, across regions. By 2050, over a third of the population is expected to be 60 years or older in East and North Asia. In North and Central Asia, 1 in 4 persons will be 60 years or older. The number of older adults in Indonesia will continue to increase, with a population explosion projected at 414% from 1990 to 2025. Indonesia was ranked 4th, after China, India, and the United States, as it has the most populated older adults (around 6% of 267 million people). 2
Older adults often suffer from multiple chronic diseases that need multiple medications. They experience changes in pharmacokinetics and pharmacodynamics due to the physiological aging process and become susceptible to drug-related problems such as drug interactions, adverse drug reactions, poor compliance, and inappropriate medication.3-9 Inappropriate medication can cause medication error, which is mostly preventable10-13 by a collaborative healthcare team’s medication review.14-16
Medication reviews reduce not only the number of medication errors but also increases patient satisfaction and treatment outcomes. 17 In older adults, medication errors occur at different phases of care (prescribing, dispensing, and administration) at a hospital or in a community setting.13,16 Older adults need thorough monitoring and seamless care related to their physiological changes and susceptibility to chronic diseases.15,18,19 Prescribing errors can trigger other errors. Inappropriate Prescribing (IP) is one of the errors triggered by prescribing errors and is considered a severe problem in the pharmacotherapy of older adults, such as adverse drug events (ADEs). 20 ADEs may increase healthcare costs, length of stay (LOS), or hospital readmission.21,22 Pharmacists can play essential roles in the medication reviews.23-25
STOPP/START, Beers criteria, and the Systematic Tool to Reduce Inappropriate Prescribing (STRIP) are examples of tools for medication reviews in older adults. The STOPP/START criteria are more useful to detect potentially inappropriate medication (PIM) in the hospital setting.26-28 Meanwhile, Beers criteria are more suitable for home care or outpatient settings. 28 The STRIP is a better fit for medication reviews in a primary care setting. 29
Although often used in different contexts, there are several disadvantages to Beers’ criteria. First, Beers criteria lacked data on the prevention of ADEs or costs.26,27,30,31 Second, for acute hospital care, STOPP criteria can detect 35% of potentially inappropriate medication (PIM), where a third is related to ADEs. Beers can only detect 25% PIM, where a quarter is related to ADEs. Data showed that the STOPP criteria detected ADEs that contribute to acute hospitalization in older adults 2.8 times more than the Beers criteria. 22 Third, in the daily clinical practice of Indonesia, where this study took place, more than 50% of drugs in the Beers’ criteria are not available, such as trimethobenzamide, metaxalone, amphetamine, methocarbamol, cyclobenzaprine, xaprozin, carisoprodol, and thioridazine.
The STOPP/START criteria are developed based on therapeutic evidence in older adults. Medication reviews with STOPP/START improved rational drug use, prevent adverse drug events, and improve health outcome of older adults. 27 STOPP/START criteria have been used in several hospital settings for older adults with chronic kidney diseases, 32 cardiac diseases, 33 diabetes, 34 psychiatric disorders,35,36 or multiple myeloma. 37 Medication review with STOPP/START can minimize potential prescribing omissions (PPOs),32,34 drug-related problems (DRPs), and potentially inappropriate medications (PIMs).33,34 In the psychiatric setting, STOPP/START criteria reduce adverse drug reaction of anticholinergics, benzodiazepines, antipsychotics, and opioids through deprescribing.35,36
Nevertheless, there are some gaps in the current evidence of the implementation of STOPP/START. Most studies on STOPP/START assessed the prescribing quality and numbers of the drug-related problem. Only a few of them also reported patient-related outcomes. There are 4 randomized clinical trials included in a meta-analysis, only 2 out of 4 measured patient’s length of stay as their secondary outcome, and only one study reported the incidence of selected geriatric syndromes. 37 In Indonesia, where our study took place, evidence on STOPP/START was limited. We found only 2 studies on STOPP/START from 2 hospitals in East Java 38 and South Sumatera. 39
Another gap is that most studies reported that the medication reviews were not conducted collaboratively between pharmacists and physicians. In most studies, pharmacists performed the reviews as a single profession and communicated the results to the physicians. 37 In one study, STOPP/START criteria were applied by a physician without the involvement of pharmacists. 37
Our study aimed to evaluate the effectiveness of STOPP/START as a guide for medication review STOPP/START criteria in an inpatient geriatric care service in Indonesia. The criteria were implemented in a collaborative geriatric team consisting of physicians and pharmacists. We evaluated the adapted Medication Appropriateness Index (MAI), the risk of ADR (GerontoNet score), and the length of stay (LOS) as the outcomes. This study took place in Indonesia, where evidence on STOPP/START was limited. The implementation of STOPP/START criteria is needed because the published clinical practice guidelines usually accommodate recommendations for an adult patient, not specific for geriatric.
Method
Study Context
This study was conducted at a 700-bed tertiary care accredited hospital, Sanglah General Hospital, Denpasar, Bali. The regulation in this hospital stated that the standard care of older adults (>60 years) with 2 or more degenerative chronic diseases and geriatric syndromes were cared for by the geriatric team. This standard care aimed to provide holistic service involving an interprofessional collaborative team consisting of geriatric specialists, pharmacists, nutritionists, physiotherapists, and other consulting physicians. As a part of a collaborative geriatric team, the pharmacists perform medication review.
Study Design
This study was a non-randomized controlled trial. The intervention group received medication reviews using STOPP/START criteria, while the control group received standard care.
In the intervention group, the pharmacist and the physicians applied the STOPP/START criteria in a collaborative fashion. The pharmacists took the patient’s medication history and clinical information, assessed medication appropriateness and potential adverse drug risks, and discussed them with the geriatricians. This medication review is categorized as a type 3 review by The Pharmaceutical Care Network Europe. 40
The primary outcome measure of this study was adapted Medication Appropriateness Index (MAI); the secondary outcomes were GerontoNet ADR risk score and the length of stay (LOS).
The group allocation was based on the different wards: one ward for the intervention group and another for the control group. As each ward had different teams of health professionals, contaminations between groups could be averted. Prior to this study, one of the researchers (MH) introduced STOPP/START to the team members in the intervention group’s ward. The STOPP criteria consist of 65 recommendations to avoid prescribing potentially inappropriate drugs; while the START criteria consist of 22 recommendations to prescribe drug therapy related to their system organ disorders41,42 (Supplemental Appendix 1). One pharmacist for each ward was assigned to conduct medication reviews. The physicians in group interventions have agreed to discuss the pharmacist’s recommendations based on the medication review. The team in the control group, from the other ward, was not aware of STOPP/START medication review. Thus, they used drug use guidelines for adults.
Study Participants
This study population was older adults who were admitted to Sanglah General Hospital, Denpasar. Subjects were selected consecutively according to the following criteria. The inclusion criteria were older adults (>60 years old) who were hospitalized with a non-emergency degenerative disease or non-acute infection sepsis diagnosis, received polypharmacy (with 5-7 drugs),30,31 and used national or district health financing coverage. The exclusion criteria were patients admitted to hospital for chemotherapy or laboratory examinations, or patients who had severe conditions, that is, terminal illness or vegetative conditions. The dropout criteria were a patient’s death during hospital admission, patients’ withdrawal from the study, or discontinuation of care due to self-discharge or referral to other healthcare facilities. For a small effect size (0.2), a trial with 90% power, and 2-sided 5% significance, a minimum of 25 samples per treatment arm were required.42,43
Data Collection
The data of the patients were recorded in a case report form (CRF) throughout their stay in the hospital (from admission until discharge). The medication review was drawn from medication history, patient information, and clinical information. The outcome measurements were adapted Medication Appropriateness Index (MAI), GerontoNet ADR risk score, and length of stay (LOS).
Adapted MAI was assessed from the CRF. The original MAI consisted of 10 items with the scale of 1 through 3. 44 The recently validated and adapted MAI consists of 8 criteria of therapeutic indications, drug selection, dose, route of administration, drug interactions, drug-disease interactions, duration of therapy, and undesirable drug reactions/adverse drug reaction (ADR) with a maximum value of 16 per drug item (Supplemental Appendix 2). 45 Incorrect doses, potential drug interactions, drug interactions possibility with clinical conditions, and potential adverse drug reactions were scored 2. The incorrect routes and duration of therapy were scored 1. Appropriateness in all 8 criteria was scored 0. The MAI assessment was conducted according to Internal Medicine Diagnosis and Therapeutic Guidelines at Sanglah Hospital, the clinical pathway at Sanglah Hospital, Geriatric Dosage Handbook, and British National Formulary.
The GerontoNet score was an assessment of the ADR risk in the older adults consisting of the number of drugs, the previous ADR experienced, presence of heart failure, liver disease, presence of 4 or more comorbidities, and presence of kidney failure. 46 Data for The GerontoNet score assessment was taken from the case report form (CRF). The data of patients’ length of stay (LOS) were also recorded from the medical record.
Before the data collection, we ensured inter-rater reliability for the assessment of MAI and GerontoNet score. We aimed for a good agreement of the Kappa value >0.6. For the MAI assessment, we measured the Kappa agreement by comparing 2 pharmacists and 2 doctors. The MAI inter-rater reliability test showed good agreement between pharmacists (Kappa 0.868), and moderate agreement between pharmacists and doctors (Kappa 0.747 and 0.620). For the GerontoNet score, the Kappa agreement was obtained by comparing the assessment of the 3 pharmacists with the Kappa of 0.630 and 0.759, showing moderate agreement.
Data Analysis
These outcome variables (MAI, GerontoNet ADR risk score, LOS) were drawn from case report form (CRF) and assessed by 2 pharmacists who had been previously trained in clinical pharmacy. An independent party removed patient identity and group allocation information. Thus, both assessors were not aware of the group allocation.
The outcome measure in this study, that is, adapted MAI, GerontoNet score, and LOS were analyzed with independent t-test or Mann-Whitney test, if the data were not normally distributed. Categorical data were analyzed with the chi-square test.
Ethical Consideration
The ethical clearance of the study (No. 678/UN.14.2/Litbang/2013) was granted by the independent ethical committee. The study was approved by the hospital director board and in concordance with the Indonesian Law for the Protection of Personal Data and the Declaration of Helsinki. Information were provided to the participants before they agreed to join the study and signed the informed consent forms. Participants are free to withdraw their participation throughout the study period. To ensure confidentiality, patients’ identities were removed from the analysis and the reports.
Results
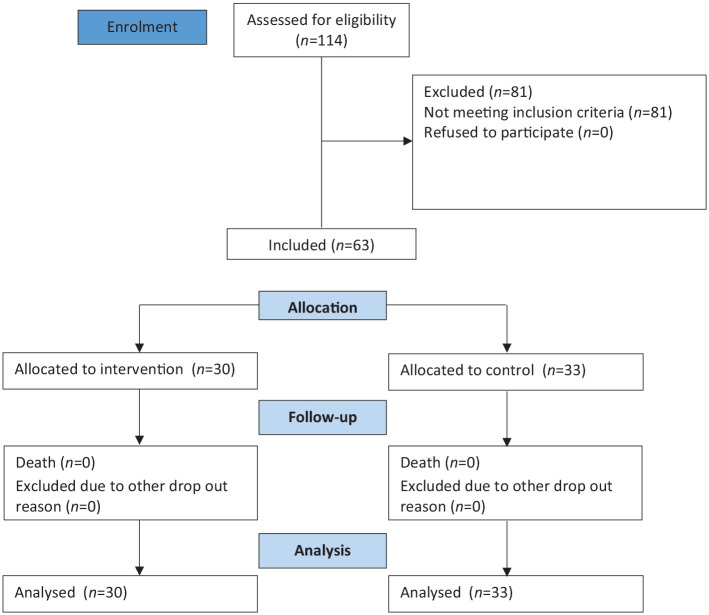
There are 144 elderly patients in the 3 months of study periods. One hundred and forty-four older patients were transferred from the emergency unit to the inpatient ward. Only 63 patients met the inclusion criteria: 30 patients in the intervention group and 33 other patients in the control group. All patients were followed up until each of them was discharged. None of them dropped out of the study. The flow of the participant is presented in Figure 1.
Figure 1.
Participant flow diagram.
The characteristics of the subjects are presented in Table 1. In this study, all patients were non-emergency cases; nor an acute state of any comorbidities, including acute infection(s). The mean age of the subjects in the intervention group was significantly higher than one in the control group. Gender, number of co-morbidity, and the number of polypharmacy were comparable in both groups. More than 30% of the patients had cardiovascular disease, respiratory disease, or renal impairment. Sixteen percent of patients had 7 drugs during their stay at the hospital.
Table 1.
Patient Characteristics.
| Characteristic | Intervention group (N = 30) |
Control group (N = 33) |
P value |
|---|---|---|---|
| Age (mean) a | 72.5 (9.2) | 67.8 (4.8) | .01 |
| Gender | .12 | ||
| Male | 17 (56.7%) | 25 (75.8%) | |
| Female | 13 (43.3%) | 8 (24.2%) | |
| Comorbidity | |||
| Cardiovascular disease | 13 (43.3%) | 22 (66.7%) | |
| Neurological disorders | 3 (10%) | 4 (12.1%) | |
| Gastrointestinal disorders | 12 (40%) | 7 (21.2%) | |
| Respiratory diseases | 14 (46.7%) | 10 (30.3%) | |
| Musculoskeletal disorders | 2 (6.7%) | 1 (3.0%) | |
| Endocrine diseases | 9 (30%) | 8 (24.2%) | |
| Renal impairment | 9 (30%) | 11 (33.3%) | |
| Urogenital diseases | 2 (6.7%) | 6 (18.2%) | |
| Liver impairment | 2 (6.7%) | 0 (0%) | |
| Number of comorbid per patient | |||
| 1 | 5 (16.7%) | 8 (24.2%) | .76 |
| 2 | 16 (53.3%) | 16 (48.5%) | |
| ≥3 | 9 (30%) | 9 (27.3%) | |
| Polypharmacy (number of medication) | |||
| 5 | 18 (60%) | 22 (66.7%) | .85 |
| 6 | 7 (23.3%) | 6 (18.2%) | |
| 7 | 5 (16.7%) | 5 (15.2%) | |
Age was reported in mean (SD).
The baseline MAI differences between the intervention 3.30 (2.09) and the control group 3.27 (2.50) were not statistically significant (P > .05). Whereas the MAI assessment of the patient at the time of discharge differed significantly between the two groups: 2.97 (2.25) in the intervention group and 9.94 (6.14) in the control group (Table 2). Within 3 months, there were 43 recommendations for STOPP criteria and 49 recommendations for START criteria (Table 3). As agreed, physicians in the intervention groups discussed and accepted pharmacists’ recommendations in the intervention group; the prescribers in the intervention group accepted the pharmacist recommendation. Forty-four percent (19/43) of accepted STOPP recommendations were stopping the use of calcium channel blockers in a patient with chronic constipation (10/43) and substitute systemic corticosteroids with an inhaled corticosteroid in COPD patients (9/43). There were 10 out of 49 accepted START recommendations to initiate aspirin or clopidogrel in patients with atherosclerotic coronary and sinus rhythm.
Table 2.
The Improvement Outcome From Admission to Discharge.
| Variables | Intervention group (N = 30) | Control group (N = 33) | P value |
|---|---|---|---|
| Medication Appropriateness Index score (mean) | <.001 | ||
| Before intervention | 3.3 (2.1) | 3.3 (2.5) | |
| After intervention | 3.0 (2.3) | 9.9 (6.1) | |
| GerontoNet score (mean) | 3.3 (2.3) | 5.2 (2.1) | .003 |
| 0-2 | 13 (43.3%) | 4 (12.1%) | |
| 3-5 | 11 (36.7%) | 13 (39.4%) | |
| >5 | 6 (20.0%) | 16 (48.5%) | |
| Length of stay (mean) | 7.6 days (3.0) | 14.2 days (10.0) | .011 |
| 0-7 | 17 (56.7%) | 13 (39.4%) | |
| 8-14 | 12 (40.0%) | 8 (24.2%) | |
| 15-21 | 1 (3.3%) | 3 (9.1%) | |
| >21 | 0 (0%) | 9 (27.3%) |
Table 3.
STOPP and START Recommendation.
| STOPP recommendation (N a = 30) | START recommendation (N a = 30) | ||
|---|---|---|---|
| Total (n) | 33 | Total (n) | 47 |
| A. Cardiovascular system | 17 | A. Cardiovascular system | 27 |
| A01 | 1 | A02 | 1 |
| A02 | 1 | A03 | 8 |
| A03 | 1 | A04 | 6 |
| A04 | 1 | A05 | 3 |
| A08 | 10 | A06 | 3 |
| A12 | 2 | A07 | 2 |
| A17 | 1 | A08 | 2 |
| B. Central nervous system (B02) | 2 | B. Central nervous system | 5 |
| B01 | 2 | ||
| B02 | 3 | ||
| C. Gastrointestinal system (C05) | 1 | C. Gastrointestinal system | 0 |
| D. Respiratory system (D02) | 6 | D. Respiratory system (D01) | 2 |
| E. Musculoskeletal system | 4 | E. Musculoskeletal system | 4 |
| E01 | 1 | E02 | 1 |
| E02 | 3 | E03 | 3 |
| F. Urogenital system | 0 | F. Endocrine system | 11 |
| F01 | 4 | ||
| F02 | 3 | ||
| F03 | 1 | ||
| F04 | 3 | ||
| G. Endocrine system (G02) | 1 | ||
| H. Drugs that adversely affect those prone to falls | 2 | ||
| H01 | 1 | ||
| H03 | 1 | ||
N was the number of patients in the intervention group.
There was also a significant difference in the GerontoNet score of the two groups at the time of discharge: 3.33 (2.28) in the intervention group and 5.18 (2.10) in the control group (the higher score indicates a higher risk of ADR as shown in (Table 2). The length of stay of the patient admitted in the study period is 14.2 (10.0) days (control group), longer than the length of stay of patients in the intervention group 7.6 (3.0) days (Table 2). The ADR occurrence in the control group is higher than one in the intervention group (Table 4).
Table 4.
Adverse Drug Event.
| Intervention group (N = 30) |
Control group (N = 33) |
P value | |
|---|---|---|---|
| Total (n) | 3 | 13 | .017 |
| Cough | 0 (0%) | 1 (3.0%) | |
| Blood pressure increase >20 mm Hg | 1 (3.3%) | 3 (9.1%) | |
| Blood pressure <100 mm Hg | 0 (0%) | 1 (3.0%) | |
| Gastrointestinal bleeding | 0 (0%) | 2 (6.1%) | |
| Hyperkalemia | 1 (3.3%) | 2 (6.1%) | |
| Hypokalemia | 0 (0%) | 2 (6.1%) | |
| Hypersensitivity | 2 (6.7%) | 0 (0%) | |
| Constipation | 0 (0%) | 2 (6.1%) |
Discussion
The prevalence of potentially inappropriate medication (PIM) prescribed in older adults is high.47,48 The older adults, frequently with multiple comorbidities, obtain prescriptions from multiple physicians. Consequently, this polypharmacy leads to more harms than benefits that may require further hospital admission. 49
Hospital pharmacy practice for clinical pharmacy services includes medication review50,51 and the pharmacists should provide an evidenced-based therapeutic recommendation for the health care team. In Indonesia, a pharmacist-led medication review process is the standard care of pharmaceutical services, 52 but the implementation varied across hospitals. For older adults, the pharmacists use STOPP/START criteria as a guideline to stop or start medication. This study showed that the use of STOPP/START criteria offered several beneficial outcomes, such as increasing medication appropriateness, minimizing adverse drug events, and decreasing the length of stay. Besides medication appropriateness review during the patient stay at the hospital, the clinical pharmacy role should expand to reconciliation medication on admission and discharge.
Our study showed that the use of STOPP/START could improve the MAI during hospital treatment. The result was consistent with other studies on medication review using STOPP/START.28,37,41,44 One study of the medication review effectiveness (using STOPP/START) on MAI showed an improved MAI score of up to 2.8 (95% CI 2.2-3.8) in the intervention group with the absolute risk reduction of 35.7% (95% CI 26.3-44.9). 51
Onder et al in Italy validated the GerontoNet score as a tool to assess ADR risk in older adults. The number of drugs and previous ADR events history was the strongest predictors of subsequent ADR, followed by heart failure, liver disease, 4 or more comorbidities, and kidney failure. The Gerontonet score in the Onder et al study showed that the receiver operating characteristic (ROC) curve for predicting ADR risk is 0.71 (95% CI, 0.68-0.73). 46 Our study proved that medication review with STOPP/START could improve the GerontoNet score associated with an increased risk of occurrence ADR in older adults during hospital admission. The number of participants who experienced ADR in the control group was almost 4 times the same as in the intervention group.
The length of stays of inpatient in the hospital was varied, related to any factor, such as transfer and discharge delay time, the number of diagnosis and severity, the number of adverse drug event, including the insurance type. 53 Our study showed that the average LOS in the control group (14.2 ± 10.0 days) was longer than the intervention group (7.6 ± 3.0 days), with the mean LOS difference of 6.55 days. A descriptive and exploratory analysis from a database of (53 965) patients admitted to a tertiary general university hospital in South Korea showed that the patients’ median length of hospital stay was 9 days for geriatric center admissions. 53 In Vetrano et al study, average LOS in patients admitted through elective admission was 12.0 (6.7) days. 54 LOS is an outcome related to hospitalization costs. 55 Research showed that every 1-day reduction in LOS could save about 3% of the total hospital costs. 56 Reduction in length of hospital stay (LOS) is a potential strategy to optimize resource consumption and reduce health care costs. Nevertheless LOS can be influenced by other factors other than comorbidity and polypharmacy, such as the frequency of adverse drug events. 57 The ADE occurrence in the control group was significantly higher than in the intervention group, and therefore, it might have contributed to the difference of LOS between groups.
The education for pharmacists and physicians regarding the implementation of the STOPP/START criteria for medication review for all older adults is essential and urgent. 58 The use of STOPP/START should also be supported by inter-professional collaboration in the geriatric team to positively impact professional satisfaction, patient satisfaction, health care quality, and patient outcome. 59 The prescribers in the intervention group followed all the recommendations because there is an agreement before the study. In a real setting, the accepted rate may vary. The sound collaboration and intensive discussions between the pharmacists and the physicians may improve this acceptance rate.
Study Limitations
This study has several limitations. First, randomization was not conducted because the patient allocation was based on admission to the ward. This approach was selected to mask the intervention and to ensure that the healthcare team in the control group were not aware of the implementation of STOPP/START criteria. We minimized confounding by applying the inclusion and exclusion criteria to ensure that both groups were comparable. Unfortunately, patients in intervention groups were significantly older than one in control groups. When patients were getting older, they were more susceptible to comorbidities and polypharmacy.3,4,8,9 Consequently, we might expect a worse health outcome in the intervention groups. Contradictory, our studies showed that the adapted MAI, GerontoNet, and LOS in the intervention group were better than the control group.
Second, the descriptive data showed differences between groups in the type of comorbidities. For example, the percentage of patients with cardiovascular diseases in the control group was higher than the intervention groups, while the percentage of patients with respiratory diseases and gastrointestinal disorders were higher in the intervention groups. Nevertheless, the overall number of comorbidities and polypharmacy was not significantly different. There are 63 patients; every patient had 1 to 5 comorbidities that may interplay to influence the health outcomes. Studies showed that the strongest predictor of LOS among older adults was polypharmacy. Other predictors reported in different studies were pressure ulcers, cerebrovascular disease, and dementia. 54
Third, we calculated our sample based on the MAI, as the primary outcome. We did not power our study to the GerontoNet score and LOS. Many variables are influencing the length of stay. Further research with a larger sample size to minimize the uncertainty of the length of stay result is needed.
Finally, we selected patients consecutively in 3 months in a single hospital in Bali, Indonesia. Thus, the result of the sample represented the target population, which might be homogeneous in the term of culture, within the study period. Further study for a more extended period is needed to avoid potential seasonal variation 60 or to explore the possibility of generalization in other population or cultural contexts.
Conclusion
Medication review with STOPP/START criteria can improve the adapted Medication Appropriateness Index and reducet ADR risks and the length of stay. There is a need to introduce STOPP/START criteria to pharmacists and physicians through continuing educational support. A better collaboration of pharmacists and physicians in the clinical practice is essential to improve the quality of daily care for older adults.
Supplemental Material
Supplemental material, Appendix for STOPP-START Medication Review: A Non-Randomized Trial in an Indonesian Tertiary Hospital to Improve Medication Appropriateness and to Reduce the Length of Stay of Older Adults by Fauna Herawati, Ida Bagus Nyoman Maharjana, Tuty Kuswardhani and Astrid Pratidina Susilo in Hospital Pharmacy
Acknowledgments
The authors wish to thank: the clinical pharmacist Anita Irawan, S.Farm., M.Farm., Apt. for her support in the process of medication review, the geriatric team of the Sanglah General Hospital, and the patients involved in the study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Fauna Herawati  https://orcid.org/0000-0002-8355-955X
https://orcid.org/0000-0002-8355-955X
Supplemental Material: Supplemental material for this article is available online.
References
- 1. United Nations ESCAP, Social Development Division. 2016 Population Data Sheet. United Nations ESCAP; September 9, 2016. revision. [Google Scholar]
- 2. Populationof.Net. Indonesia population. Accessed May 27, 2019. https://www.populationof.net/indonesia/
- 3. Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. Washington, DC: McGraw-Hill Companies, Inc.; 2009. [Google Scholar]
- 4. Rahmawati F, Nurrochmah H, Wasilah R, Sulaiman SAS. Potentiality of drug-drug interaction in hospitalized geriatric patients in a private hospital, Yogyakarta, Indonesia. Asian J Pharm Clin Res. 2010;3(3):191-194. [Google Scholar]
- 5. Kulkarni V, Bora SS, Sirisha S, Saji M, Sundaran S. A study on drug–drug interactions through prescription analysis in a South Indian teaching hospital. Ther Adv Drug Saf. 2013;4(4):141-146. doi: 10.1177/2042098613490009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bucşaa C, Farcaşa A, Cazacua I, et al. How many potential drug–drug interactions cause adverse drug reactions in hospitalized patients?. Eur J Intern Med. 2013;24(1):27-33. [DOI] [PubMed] [Google Scholar]
- 7. Dumbreck S, Flynn A, Nairn M, et al. Drug-disease and drug-drug interactions: systematic examination of recommendations in 12 UK national clinical guidelines. BMJ. 2015;350:h949. doi: 10.1136/bmj.h949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186. doi: 10.1016/j.cger.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 9. Teka F, Teklay G, Ayalew E, Teshome T. Potential drug–drug interactions among elderly patients admitted to medical ward of Ayder Referral Hospital, Northern Ethiopia: a cross sectional study. BMC Res Notes. 2016;9(1):431. doi: 10.1186/s13104-016-2238-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Keers RN, Williams SD, Cooke J, Ashcroft DM. Causes of medication administration errors in hospitals: a systematic review of quantitative and qualitative evidence. Drug Saf. 2013;36(11):1045-1067. doi: 10.1007/s40264-013-0090-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tariq RA, Scherbak Y. Medication errors. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2019. Accessed May 25, 2020. https://www.ncbi.nlm.nih.gov/books/NBK519065/ [Google Scholar]
- 12. Wheeler AJ, Scahill S, Hopcroft D, Stapleton H. Reducing medication errors at transitions of care is everyone’s business. Aust Prescr. 2018;41(3):73-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Metsӓlӓ E, Vaherkoski U. Medication errors in elderly acute care—a systematic review. Scand J Caring Sci. 2014;28(1): 12-28. doi: 10.1111/scs.12034 [DOI] [PubMed] [Google Scholar]
- 14. da Silva BA, Krishnamurthy M. The alarming reality of medication error: a patient case and review of Pennsylvania and National data. J Community Hosp Intern Med Perspect. 2016;6(4):31758. doi: 10.3402/jchimp.v6.31758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lavan AH, Gallagher PF, O’Mahony D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin Interv Aging. 2016;11:857-866. doi: 10.2147/CIA.S80280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986. doi: 10.1002/14651858.CD008986.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kianmehr N, Mofidi M, Saidi H, Hajibeigi M, Rezai M. What are patients’ concerns about medical errors in an Emergency Department?. Sultan Qaboos Univ Med J. 2012;12(1):86-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bahrani L, Eriksson T, Höglund P, Midlöv P. The rate and nature of medication errors among elderly upon admission to hospital after implementation of clinical pharmacist-led medication reconciliation. Eur J Hosp Pharm. 2014;21(3): 156-160. [Google Scholar]
- 19. Pepa PA, Langley-DeGroot MH, Rule OS. Prescribing cascade in a geropsychiatric patient: a slippery slope. J Geriatr Ment Health. 2018;5(1):62-64. doi: 10.4103/jgmh.jgmh_17_17 [DOI] [Google Scholar]
- 20. Morimoto T, Gandhi TK, Seger AC, Hsieh TC, Bates DW. Adverse drug events and medication errors: detection and classification methods. Qual Saf Health Care. 2004;13(4):306-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klopotowska JE, Wierenga PC, Stuijt CCM, et al. Adverse drug events in older hospitalized patients: results and reliability of a comprehensive and structured identification strategy. PLoS ONE. 2013;8(8):e71045. doi: 10.1371/journal.pone.0071045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nachtigall A, Heppner HJ, Thürmann PA. Influence of pharmacist intervention on drug safety of geriatric inpatients: a prospective, controlled trial. Ther Adv Drug Saf. 2019;10:2042098619843365. doi: 10.1177/2042098619843365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rollason V, Vogt N. Reduction of polypharmacy in the elderly: a systematic review of the role of the pharmacist. Drugs Aging. 2003;20(11):817-832. doi: 10.2165/00002512-200320110-00003 [DOI] [PubMed] [Google Scholar]
- 24. Hailu BY, Berhe DF, Gudina EK, Gidey K, Getachew M. Drug related problems in admitted geriatric patients: the impact of clinical pharmacist interventions. BMC Geriatr. 2020;20(13):1-8. doi: 10.1186/s12877-020-1413-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gallagher PF, O’Mahony D. STOPP (Screening Tool of Older Persons potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37(6):673-679. [DOI] [PubMed] [Google Scholar]
- 26. O’Mahony D, O’Sullivan D, Byrne S, O’Connor MN, Ryan C, Gallagher P. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing. 2015;44(2):213-218. doi: 10.1093/ageing/afu145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Varallo FR, Capucho HC, Planeta CS, Mastroianni PC. Safety assessment of potentially inappropriate medications (PIM) use in older people and the factors associated with hospital admission. J Pharm Pharmaceu Sci. 2011;14(2):283-290. [DOI] [PubMed] [Google Scholar]
- 28. Fick DM, Cooper JW, Wade WE, Waller JL, Maclean JR, Beers MH. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med. 2003;163(22):2716-2724. [DOI] [PubMed] [Google Scholar]
- 29. Meulendijk MC, Spruit MR, Drenth-van Maanen AC, et al. Computerized decision support improves medication review effectiveness: an experiment evaluating the STRIP assistant’s usability. Drugs Aging. 2015;32(6):495-503. doi: 10.1007/s40266-015-0270-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gallagher PF, O’Connor MN, O’Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89(6):845-854. [DOI] [PubMed] [Google Scholar]
- 31. Chang CB, Chen JH, Wen CJ, et al. Potentially inappropriate medications in geriatric outpatients with polypharmacy: application of six sets of published explicit criteria. Brit J Clin Pharmacol. 2011;72(3):482-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parker K, Bull-Engelstad I, Benth JŠ, et al. Effectiveness of using STOPP/START criteria to identify potentially inappropriate medication in people aged ≥ 65 years with chronic kidney disease: a randomized clinical trial. Eur J Clin Pharmacol. 2019;75(11):1503-1511. doi: 10.1007/s00228-019-02727-9 [DOI] [PubMed] [Google Scholar]
- 33. Mekdad SS, Alsayed AA. Quality Improvement project to reduce drug-related problems (DRPs) and potentially inappropriate medications (PIMs) in Geriatrics Cardiac Clinic in Saudi Arabia. Can Geriatr J. 2019;22(2):49-54. doi: 10.5770/cgj.22.338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williams S, Miller G, Khoury R, Grossberg GT. Rational deprescribing in the elderly. Ann Clin Psychiatry. 2019;31(2):144-152. [PubMed] [Google Scholar]
- 35. Umıt EG, Baysal M, Bas V, Asker I, Kırkızlar O, Demır AM. Polypharmacy and potentially inappropriate medication use in older patients with multiple myeloma, related to fall risk and autonomous neuropathy. J Oncol Pharm Pract. 2019;26(1):43-50. doi: 10.1177/1078155219835303 [DOI] [PubMed] [Google Scholar]
- 36. Siripala UGS, Premadasa SPK, Samaranayake NR, Wanigatunge CA. Usefulness of STOPP/START criteria to assess appropriateness of medicines prescribed to older adults in a resource-limited setting. Int J Clin Pharm. 2019;41(2):525-530. doi: 10.1007/s11096-019-00786-7 [DOI] [PubMed] [Google Scholar]
- 37. Hill-Taylor B, Walsh KA, Stewart S, Hayden J, Byrne S, Sketris IS. Effectiveness of the STOPP/START (Screening Tool of Older Persons’ potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: systematic review and meta-analysis of randomized controlled studies. J Clin Pharm Ther. 2016;41(2):158-169. doi: 10.1111/jcpt.12372 [DOI] [PubMed] [Google Scholar]
- 38. Julaiha S. Identifikasi potentially inappropriate medications (PIMs) berdasarkan kriteria STOPP START pada pasien geriatri rawat inap di RS Advent Bandar Lampung. Jurnal Analis Kesehatan. 2018;7(1):657-665. [Google Scholar]
- 39. Fitriah N. Analisis penggunaan obat pada pasien geriatri Rawat Jalan Poli Jantung RSD Dr. Soebandi Jember dengan Metode STOPP START [undergraduate theses]. Jember: Universitas Jember; 2018. [Google Scholar]
- 40. Pharmaceutical Care Network Europe. Statement on Medication Review 2013. Zuidlaren: PCNE; 2013. [Google Scholar]
- 41. Gallagher PF, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83. [DOI] [PubMed] [Google Scholar]
- 42. O’Connor MN, Gallagher P, O’Mahony D. Inappropriate prescribing criteria, detection and prevention. Drugs Aging. 2012;29(6):437-452. [DOI] [PubMed] [Google Scholar]
- 43. Whitehead AL, Julious SA, Cooper CL, Campbell MJ. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25(3):1057-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hanlon JT, Schmader KE. The medication appropriateness index at 20: where it started, where it has been, and where it may be going. Drugs Aging. 2013;30(11):893-900. doi: 10.1007/s40266-013-0118-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nair NP, Chalmers L, Peterson GM, Bereznicki BJ, Castelino RL, Bereznicki LR. Hospitalization in older patients due to adverse drug reactions—the need for a prediction tool. Clin Interv Aging. 2016; 11:497-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Onder G, Petrovic M, Tangiisuran B, et al. Development and validation of a score to assess risk of adverse drug reactions among in-hospital patients 65 years or older. Arch Intern Med. 2010;170(13):1142-1148. [DOI] [PubMed] [Google Scholar]
- 47. Page RL, 2nd, Linnebur SA, Bryant LL, Ruscin JM. Inappropriate prescribing in the hospitalized elderly patient: defining the problem, evaluation tools, and possible solutions. Clin Interv Aging. 2010;5:75-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tokuda Y. Polypharmacy, inappropriate prescribing and adverse drug events in Japan. J Gen Fam Med. 2016;17(1):3-4. [Google Scholar]
- 49. Pérez T, Moriarty F, Wallace E, McDowell R, Redmond P, Fahey T. Prevalence of potentially inappropriate prescribing in older people in primary care and its association with hospital admission: longitudinal study. BMJ. 2018;363:k4524. doi: 10.1136/bmj.k4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Silva ROS, Macêdo LA, Santos GAD, Jr, Aguiar PM, de Lyra DP., Jr. Pharmacist-participated medication review in different practice settings: service or intervention? An overview of systematic reviews. PLoS ONE. 2019;14(1):e0210312. doi: 10.1371/journal.pone.0210312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Geurts MM, Talsma J, Brouwers JR, de Gier JJ. Medication review and reconciliation with cooperation between pharmacist and general practitioner and the benefit for the patient: a systematic review. Br J Clin Pharmacol. 2012;74(1):16-33. doi: 10.1111/j.1365-2125.2012.04178.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Indonesian Ministry of Health. Standar pelayanan kefarmasian di rumah sakit. Jakarta, Indonesia: Indonesian Ministry of Health; 2016. Accessed July 26, 2019. https://www.persi.or.id/images/regulasi/permenkes/pmk722016.pdf [Google Scholar]
- 53. Baek H, Cho M, Kim S, Hwang H, Song M, Yoo S. Analysis of length of hospital stay using electronic health records: a statistical and data mining approach. PLoS ONE. 2018;13(4):e0195901. doi: 10.1371/journal.pone.0195901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vetrano DL, Landi F, De Buyser SL, et al. Predictors of length of hospital stay among older adults admitted to acute care wards: a multicentre observational study. Eur J Intern Med. 2014;25:56-62. [DOI] [PubMed] [Google Scholar]
- 55. Bailey JG, Davis PJ, Levy AR, Molinari M, Johnson PM. The impact of adverse events on health care costs for older adults undergoing nonelective abdominal surgery. Can J Surg. 2016;59(3):172-179. doi: 10.1503/cjs.013915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Barnes DE, Palmer RM, Kresevic DM, et al. Acute care for elders units produced shorter hospital stays at lower cost while maintaining patients’ functional status. Health Aff (Millwood). 2012;31(6):1227-1236. doi: 10.1377/hlthaff.2012.0142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Calderón-Larrañaga A, Poblador-Plou B, González-Rubio F, Gimeno-Feliu LA, Abad-Díez JA, Prados-Torres A. Multimorbidity, polypharmacy, referrals, and adverse drug events: are we doing things well?. Br J Gen Pract. 2012;62(605): e821-e826. doi: 10.3399/bjgp12X659295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Francis J, Abraham S. Clinical pharmacists: bridging the gap between patients and physicians. Saudi Pharm J. 2014; 22(6):600-602. doi: 10.1016/j.jsps.2014.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tsakitzidis G, Timmermans O, Callewaert N, et al. Outcome indicators on interprofessional collaboration interventions for elderly. Int J Integr Care. 2016;16(2):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mathieson K. Making sense of biostatistics: types of nonprobability sampling. J Clin Res Best Pract. 2014;10(10):1-2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix for STOPP-START Medication Review: A Non-Randomized Trial in an Indonesian Tertiary Hospital to Improve Medication Appropriateness and to Reduce the Length of Stay of Older Adults by Fauna Herawati, Ida Bagus Nyoman Maharjana, Tuty Kuswardhani and Astrid Pratidina Susilo in Hospital Pharmacy