Abstract
Conventional lung cancer treatments include surgery, chemotherapy and radiotherapy; however, these treatments are often poorly tolerated by patients. Cannabinoids have been studied for use as a primary cancer treatment. Cannabinoids, which are chemically similar to our own body’s endocannabinoids, can interact with signalling pathways to control the fate of cells, including cancer cells. We present a patient who declined conventional lung cancer treatment. Without the knowledge of her clinicians, she chose to self-administer ‘cannabidiol (CBD) oil’ orally 2–3 times daily. Serial imaging shows that her cancer reduced in size progressively from 41 mm to 10 mm over a period of 2.5 years. Previous studies have failed to agree on the usefulness of cannabinoids as a cancer treatment. This case appears to demonstrate a possible benefit of ‘CBD oil’ intake that may have resulted in the observed tumour regression. The use of cannabinoids as a potential cancer treatment justifies further research.
Keywords: respiratory medicine, lung cancer (oncology), complementary medicine, cancer intervention, malignant disease and immunosuppression
Background
Lung cancer remains the second most common cancer in the UK. Despite advances in current treatment options, including surgery, chemotherapy, radiotherapy, immunotherapy and targeted cancer drugs, survival rates remain low at around 15% at 5 years from diagnosis.1 Some patients opt for symptom control and even then, the median survival rate without treatment is 7.15 months.2
We report a case of non-small cell lung cancer that was amenable to conventional treatment options. The patient had extensive discussions with the clinicians regarding the potential treatment options and she declined all treatment options offered, so was placed under ‘watch and wait’ surveillance. The patient then chose a non-conventional and unlicensed treatment that appears to have had a positive effect on her disease.
Case presentation
A woman in her 80s first presented to her general practitioner in February 2018 with a cough that had persisted for a few months. A chest radiograph was normal and she was treated with a course of oral antibiotics. Despite this, the cough persisted and a repeat chest radiograph in June 2018 showed a new lesion in the right mid zone of the lung. The differential diagnoses at this point include consolidation, primary lung malignancy or lung metastasis from an unknown primary.
The patient has a background of mild chronic obstructive pulmonary disease (forced expiratory volume in one second: 81% predicted), osteoarthritis and hypertension. She is a current 68 pack-year smoker with no history of alcohol consumption. The patient’s medications are: tiotropium, budesonide/formoterol fumarate and salbutamol inhalers, ramipril, bendroflumethiazide, atorvastatin, aspirin, amlodipine, amitriptyline, lansoprazole and co-codamol.
There was no significant family history of any medical conditions. The results of her general examination were unremarkable with her only being slightly restricted by highly strenous activity, having a World Health Organisation (WHO) performance status grade of 1.
Investigations
The patient underwent a CT scan of the chest in June 2018, which showed a lesion in the right middle lobe of her lung measuring 41 mm at its longest axial diameter. A subsequent positron emission tomography (PET) scan carried out in July 2018 showed this lesion to be avid with a standardised uptake value (SUV) max of 10.5 and a non-specific increased uptake in the head of the right femur. She subsequently underwent a CT-guided lung biopsy and was diagnosed with non-small cell lung carcinoma, not otherwise specified, with a tumour, node and metastases (TNM) staging of T2bN0Mx. Gene mutation testing for anaplastic lymphoma kinase gene and epidermal growth factor receptor gene was negative with<1% of tumour cells expressing programmed death-ligand 1.
Her case was reviewed by the local lung multidisciplinary team (MDT). As she was a potential candidate for treatment with curative intent, further investigations were carried out to look for distant metastases. A CT of the head was normal and an endobronchial ultrasound-guided transbronchial needle aspiration of the subcarinal and left lower paratracheal lymph node showed no evidence of metastases. An MRI of her right thigh showed an abnormal signal in the right femoral head. A bone biopsy showed red marrow hyperplasia with no evidence of malignancy. She also underwent a cardiopulmonary exercise test, which showed a mild VQ mismatch but there was no evidence of cardiovascular or ventilatory limitation.
A repeat CT scan of the chest was carried out in September 2018 once full staging investigations had been completed. This showed that the right middle lobe cancer had reduced to 33 mm but there were new bilateral upper lobe nodules now visible measuring 4 mm in the left apex and 6 mm on the right.
Treatment
The patient was referred to the cardiothoracic surgeons for consideration of a lobectomy, but surgery was declined by the patient following discussions with the surgeons. She was then referred to the oncologists and repeat CT and PET scans were carried out. These restaging scans showed that her cancer had reduced in size (CT: 11 mm reduction; PET: 18 mm reduction; figure 1). The left apical nodule previously seen in the September 2018 CT scan had resolved and the right upper lobe nodule had reduced in size. The patient was offered stereotactic ablative radiotherapy, but she declined this treatment as well. The decision was made to ‘watch and wait’ by carrying out regular CT surveillance of the patient.
Figure 1.
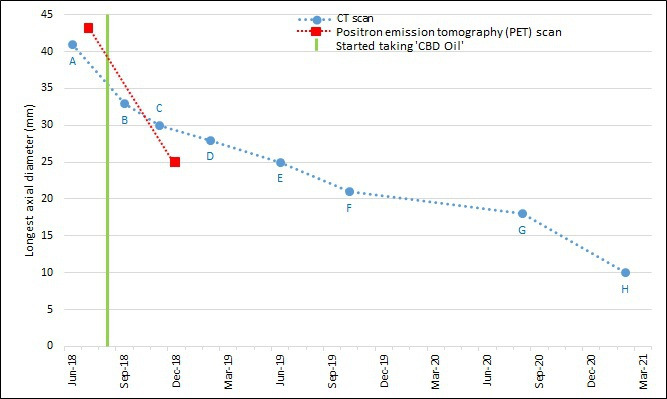
Graph showing longest axial diameter of the tumour (mm) in serial CT scans and two PET scans, and start of self-treatment with ‘CBD oil’. CBD, cannabidiol.
Outcome and follow-up
Regular CT scans were carried out over the following 2.5 years (at intervals of 3–6 months), which showed the lung cancer to be shrinking progressively (figure 2). The initial 41 mm lesion in June 2018 had reduced to 10 mm in February 2021. This reflects an overall 76% reduction in maximum axial diameter, averaging at 2.4% per month over the whole monitoring period.
Figure 2.
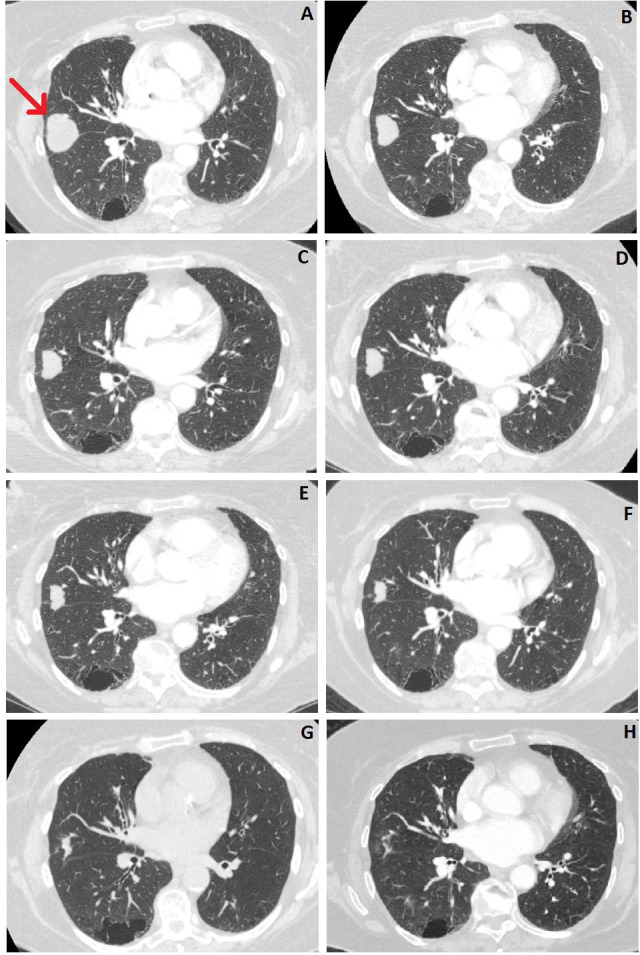
Serial CT scans showing the tumour in the right middle lobe of the lung. (A) June 2018, (B) September 2018, (C) November 2018, (D) February 2019, (E) June 2019, (F) October 2019, (G) August 2020, (H) February 2021. Red arrow indicates the tumour location.
This case was brought to the attention of the local lung MDT meeting in February 2019 when the serial imaging showed a reduction in tumour size despite having received no conventional treatment for her lung cancer. The patient was contacted to discuss her results, at which point she disclosed that she had chosen to take ‘CBD oil’ as an alternative self-treatment for her lung cancer on the advice of a family member since August 2018, shortly after her original diagnosis. The ‘CBD oil’ was sourced from outside the UK. She was consistently taking 0.5 mL of ‘CBD oil’ occasionally two times per day, but normally three times a day, via ingestion. The supplier advised that the main active ingredients of the ‘CBD oil’ used by this patient were Δ9-tetrahydrocannabinol (THC) at 19.5%, CBD at 20.05% and tetrahydrocannabinolic acid (THCA) at 23.8%. The supplier had advised the patient (via her family member) not to take the 'CBD oil' with hot food or drinks as it could result in symptoms of ‘feeling stoned’. The patient reported that she had a reduced appetite since taking ‘CBD oil’ that may or may not be related to the ‘CBD oil’ intake. There were no other changes to her prescribed medications, diet and lifestyle. She was advised to quit smoking, but she declined, and was smoking one pack a week throughout the surveillance period.
Discussion
We report a case of non-small cell lung cancer that was amenable to conventional treatment options. However, after extensive discussions with the patient regarding the potential treatment options, she declined all treatment options offered so was placed on ‘watch and wait’ surveillance. The patient then chose to take ‘CBD oil’ daily that appears to have had a positive effect on her disease.
Cannabis, Cannabis sativa, is a herbaceous flowering plant that has been used as a natural therapeutic agent since ancient times.3 In 1842, cannabis was introduced into modern medicine for its analgesic, sedative, anti-inflammatory, antispasmodic and anticonvulsant effects.4 Cannabinoids are a group of chemicals that are derived from the Cannabis plant, which can directly alter mental state when consumed, which can indirectly lead to physical changes. Of the hundreds of cannabinoids known, the two compounds that have been researched the most are CBD and THC, with THC being a psychoactive compound, whereas CBD is non-psychoactive.
Both of these compounds interact with our body’s endocannabinoid system. The endocannabinoid system is composed of three main parts: the cannabinoid receptors (CB1 and CB2), the endocannabinoids and enzymes. CB1 receptors are found mainly in the brain and central nervous system, and CB2 receptors are found mainly in cells of peripheral organs associated with the immune and haematopoietic system. The endocannabinoids (eg, anandamide and 2-arachidonoylglycerol) are endogenous lipid-based retrograde neurotransmitters that bind to cannabinoid receptors and cannabinoid receptor proteins that are expressed throughout the vertebrate central and peripheral nervous system. The enzymes (eg, fatty acid amide hydrolase and monoacylglycerol acid lipase) are responsible for synthesis and eventual inactivation of the endocannabinoids.5 The endocannabinoid system functions to regulate physiological and cognitive processes, as the endocannabinoids act as neuromodulators. They are involved in a variety of processes, including neuronal function, emotion, feeding and energy metabolism, pain and inflammation, sleep and immune function.6
CBD and THC are chemically similar to our own body’s endocannabinoids. THC has a high affinity to both CB1 and CB2 receptors, whereas CBD has been attributed to work on the enzymes in the endocannabinoid system allowing more endocannabinoids to circulate in the system. These interactions play a role in controlling a cell’s fate by allowing the release of various neurotransmitters, modulating the effects of proteins and nuclear factors that are involved in cell proliferation, differentiation and apoptosis.6
As a result, the endocannabinoid system has been a focus of many research projects as a potential mechanism for drug treatments. It is widely believed that cannabinoids can provide benefits to people suffering from pain, anxiety and sleep disorders, and their use is well established in palliative care settings. A systematic review in 2015 which looked at 79 trials found evidence that using cannabinoids led to a reduction in chronic pain and an improvement in spasticity in multiple sclerosis or paraplegia. There was also some evidence suggesting that cannabinoids can be used to improve a range of other ailments: nausea caused by chemotherapy, sleep disorders, increasing appetite in HIV infection and Tourette syndrome.7 In the UK, National Institute for Health and Care Excellence provides guidance for the prescription of cannabinoids for patients with intractable nausea and vomiting, chronic pain, spasticity and severe treatment-resistant epilepsy.8
In recent years, research has been undertaken to investigate the potential use of cannabinoids as a direct cancer treatment, but no causative relationship has yet been identified. Studies have shown that cannabinoids have an effect on tumour growth, development, invasion, metastasis and angiogenesis.9 However, various studies have come to conflicting conclusions on the specific effect cannabinoids have on cancer cells. In some cases, CBD has been found to have antiproliferative effects, increase apoptosis in cells and inhibit cancer cell migration, invasion and metastasis.10 These effects were seen when studied on lung cancer cells.11–15 Similarly, THC has been shown in some studies to decrease tumour growth, incidences of benign tumours, invasion of cancer cells and metastatic spread,16–19 but it has also been shown to increase proliferation of cancer cells, including lung cancer cells.20 21 Although there is clearly a potential for cannabinoids to be used as a primary or as an adjunct form of cancer treatment, further research is required to identify exactly which compound works against which specific cancer cell type.
According to the supplier of our patient’s ‘CBD oil’, the active ingredients were roughly equal amounts of THC and CBD. There was also a high amount of THCA, which is the precursor to THC. THCA can be converted into the THC molecule in a process called decarboxylation, which is achieved by using heat. However, our patient was advised by the supplier to not take her 'CBD oil' with a hot drink as it would activate a much higher level of the psychoactive THC compound, so we can assume that the CBD and THC levels stayed roughly equal.
There has previously been a similar case reported, which also showed evidence of tumour reduction after taking ‘CBD oil’, in a patient of a similar demographic with lung cancer (adenocarcinoma).22 However, in this case, the only active component was CBD. In both cases, the patients did not change their lifestyle, medications or diet; and the self-administration of the ‘CBD oil’ seems to be the only explanation for the radiological improvement of their known lung cancer. Due to each case involving a different selection of cannabinoids, it is difficult to conclude if the THC in our case contributed to the lung cancer reduction, or if it was just the CBD component that had a positive effect.
The specific dosage of ‘CBD oil’ that our patient ingested was not consistent, as she would only take 'CBD oil' when she had another family member present as a safety precaution. This means that on some days, she only took 0.5 mL two times per day rather than three times per day. As well as making our patient’s self-treatment harder to replicate, this also serves to highlight the fact that ‘CBD oil’ as a self-administered medical treatment is still viewed by many people as requiring more caution when using than prescribed medications.
We are aware of the limitations of this case report. We are unable to confirm the full ingredients of the ‘CBD oil’ that the patient was taking or to provide information on which of the ingredient(s) may be contributing to the observed tumour regression. Although there appears to be a relationship between the intake of ‘CBD oil’ and the observed tumour regression, we are unable to conclusively confirm that the tumour regression is due to the patient taking ‘CBD oil’.
Existing cancer treatments could have severe side effects, both physically and mentally. This is why our patient decided on non-conventional self-treatment. The limited number of case reports appear to show that ‘CBD oil’ can have positive effects on tumour reduction. More research is needed to identify the actual mechanism of action, administration pathways, safe dosages, its effects on different types of cancer and any potential adverse side effects when using cannabinoids.
The potential for cannabinoids to be used as an alternative to augment or replace conventional primary cancer treatments definitely justifies further research.
Patient’s perspective.
I was not very interested in traditional cancer treatments as I was worried about the risks of surgery, and I saw my late husband suffer through the side effects of radiotherapy. My relative suggested that I should try ‘cannabidiol (CBD) oil’ to treat my cancer, and I have been taking it regularly ever since. I am ‘over the moon’ with my cancer shrinking, which I believe was caused by the ‘CBD oil’. I am tolerating it very well and I intend to take this treatment indefinitely.
Learning points.
A clinician treating a patient with cancer needs to be aware that the patient may be taking non-conventional and unlicensed treatment without the clinician’s knowledge.
It is important to take into account a patient’s choices relating to potential side effects when discussing treatment options, and to keep an open mind of the potential benefits of non-conventional treatments.
Both oncologists and patients would welcome a cancer treatment option with minimal side effects to replace or augment the current cancer treatments.
Further research is needed to focus on the potential use of cannabinoids as a primary form of cancer treatment.
The endocannabinoid system controls a wide range of physiological and neurological processes and, therefore, it can be a challenge to isolate a particular response in the context of primary treatment for a specific disease.
Footnotes
Contributors: Supervised by and patient was under the care of BY. Report was written by KLL and BY. Imaging and related reporting by EC.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
References
- 1.Cancer Research UK . Cancer incidence for common cancers. Available: https://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared [Accessed 26 Mar 2021].
- 2.Wao H, Mhaskar R, Kumar A, et al. Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev 2013;2:10. 10.1186/2046-4053-2-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russo EB. Cannabis and cannabinoids: pharmacology, toxicology, and therapeutic potential. Routledge, 2013: 478. [Google Scholar]
- 4.Iversen LL. The science of marijuana. Oxford University Press, 2001: 304. [Google Scholar]
- 5.De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol 2004;141:765–74. 10.1038/sj.bjp.0705666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 2006;58:389–462. 10.1124/pr.58.3.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015;313:2456–73. 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence (NICE) . Cannabis-based medicinal products NICE guideline [NG144], 2019. Available: https://www.nice.org.uk/guidance/ng144 [Accessed 27 Mar 2021].
- 9.Cridge BJ, Rosengren RJ. Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Manag Res 2013;5:301–13. 10.2147/CMAR.S36105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 2003;4:873–84. 10.1038/nrn1247 [DOI] [PubMed] [Google Scholar]
- 11.Ramer R, Bublitz K, Freimuth N, et al. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. Faseb J 2012;26:1535–48. 10.1096/fj.11-198184 [DOI] [PubMed] [Google Scholar]
- 12.Ramer R, Rohde A, Merkord J, et al. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm Res 2010;27:2162–74. 10.1007/s11095-010-0219-2 [DOI] [PubMed] [Google Scholar]
- 13.Ramer R, Merkord J, Rohde H, et al. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem Pharmacol 2010;79:955–66. 10.1016/j.bcp.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 14.Ramer R, Heinemann K, Merkord J, et al. COX-2 and PPAR-γ confer cannabidiol-induced apoptosis of human lung cancer cells. Mol Cancer Ther 2013;12:69–82. 10.1158/1535-7163.MCT-12-0335 [DOI] [PubMed] [Google Scholar]
- 15.Haustein M, Ramer R, Linnebacher M, et al. Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Biochem Pharmacol 2014;92:312–25. 10.1016/j.bcp.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 16.Munson AE, Harris LS, Friedman MA, et al. Antineoplastic activity of cannabinoids. J Natl Cancer Inst 1975;55:597–602. 10.1093/jnci/55.3.597 [DOI] [PubMed] [Google Scholar]
- 17.National Toxicology Program . NTP Toxicology and Carcinogenesis Studies of 1-Trans-Delta(9)-Tetrahydrocannabinol (CAS No. 1972-08-3) in F344 Rats and B6C3F1 Mice (Gavage Studies). Natl Toxicol Program Tech Rep Ser 1996;446:1–317. [PubMed] [Google Scholar]
- 18.Ramer R, Hinz B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J Natl Cancer Inst 2008;100:59–69. 10.1093/jnci/djm268 [DOI] [PubMed] [Google Scholar]
- 19.Preet A, Ganju RK, Groopman JE. Delta9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene 2008;27:339–46. 10.1038/sj.onc.1210641 [DOI] [PubMed] [Google Scholar]
- 20.Hart S, Fischer OM, Ullrich A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res 2004;64:1943–50. 10.1158/0008-5472.CAN-03-3720 [DOI] [PubMed] [Google Scholar]
- 21.McKallip RJ, Nagarkatti M, Nagarkatti PS. Delta-9-Tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J Immunol 2005;174:3281–9. 10.4049/jimmunol.174.6.3281 [DOI] [PubMed] [Google Scholar]
- 22.Sulé-Suso J, Watson NA, van Pittius DG, et al. Striking lung cancer response to self-administration of cannabidiol: a case report and literature review. SAGE Open Med Case Rep 2019;7:2050313X1983216. 10.1177/2050313X19832160 [DOI] [PMC free article] [PubMed] [Google Scholar]


