Abstract
Introduction
Adenomyosis can adversely reduce chances of pregnancy in couples undergoing assisted conception. We aim to evaluate the effect of two different downregulation protocols on the reproductive outcomes in women with moderate and severe adenomyosis undergoing frozen-thawed embryo transfer (FTET).
Methods and analysis
We will conduct a two-armed pragmatic randomised clinical trial comparing modified downregulation with gonadotrophin-releasing hormone (GnRH) analogue for 6 weeks to standard downregulation with GnRH analogue for 1 week prior to FTET. Our primary outcome is clinical pregnancy, defined as a viable intrauterine pregnancy confirmed by ultrasound at greater than 6 weeks gestation, with other secondary reproductive, neonatal and safety outcomes. We aim to randomise 162 patients over 3 years to achieve 80% power for detecting a 20% difference in the primary outcome at 5% significance.
Ethics and dissemination
To date there is no consensus on the optimal protocol for management of subfertile women with adenomyosis. Modified downregulation could improve the clinical pregnancy rate by reducing the endometrial inflammatory reaction and/or myometrial contractility and their impact on uterine receptivity in women with moderate and severe adenomyosis of the uterus undergoing FTET. The MODA trial is designed to offer pragmatic, real-life evaluation of the optimal protocol for downregulation for this population during assisted conception treatments. Our findings will be published in peer-reviewed journals and presented at national and international scientific meetings and congresses. Ethical approval was granted by the NHS Research Ethics Committees (19/LO/1567).
Trial registration number
Keywords: reproductive medicine, subfertility, reproductive medicine
Strengths and limitations of this study.
The MODA trial offers a pragmatic evaluation of two different downregulation protocols used in current practice to optimise the reproductive outcomes of women with adenomyosis.
We will report on established core outcomes of interest for stakeholders in assisted conception and will randomise a large number of women to achieve sufficient power to evaluate the treatments of interest across different participant subgroups.
The intervention is unblinded.
Introduction
Adenomyosis is characterised by the presence of ectopic endometrial glands and stroma located within hypertrophic and hyperplastic myometrium, affecting up to 20.9% of women at reproductive age.1 2 Recent advances in ultrasound and MRI technology have facilitated the diagnosis of adenomyosis with a reported accuracy of up to 81% using two-dimensional and three-dimensional (3D) ultrasound.2
Two meta-analyses have suggested that women with adenomyosis have lower implantation, pregnancy and live birth rates with higher miscarriage rates, thus its impact on fertility is marked.3 4 This impact is directly linked to the severity of the adenomyosis with rate of clinical pregnancy decreasing from 42.7% (95% CI 37.1% to 48.3%) in women with no adenomyosis on ultrasound to 22.9% (95% CI 13.4% to 32.6%) for those with four features and 13.0% (95% CI 2.2% to 23.9%) for those with all seven features.5 Several theories have been suggested to explain its negative impact on fertility, such as abnormal uterotubal transport due to anatomical distortion of the uterine cavity and disturbed uterine peristalsis.6 Ultrastructural myometrial abnormalities may cause hyperperistalsis and increase the intrauterine pressure due to a disturbance in normal myocyte contractility with subsequent loss of normal rhythmic contraction.7 Several molecular alterations have also been noted in the eutopic endometrium of women with adenomyosis. These include increased levels of inflammatory markers, increased oxidative stress, reduced expression of implantation markers, lack of expression of adhesion molecules and changes in the sex steroid hormone pathway, resulting in impaired implantation.6
Treatment with gonadotrophin-releasing hormone (GnRH) analogues for prolonged periods could reduce the amount of inflammatory reaction in the eutopic endometrium of women with adenomyosis.8 It is also possible that prolonged downregulation may improve abnormal uterine peristalsis seen in women with adenomyosis. A study examining the impact of prolonged downregulation in women with adenomyosis undergoing in vitro fertilisation (IVF) treatment first showed increased clinical pregnancy rates a decade ago.9 More recently, three large retrospective studies suggest a significant benefit in the reproductive outcomes of women with adenomyosis following prolonged downregulation prior to frozen embryo transfer, in contrast to one retrospective analysis that showed an adverse impact of prolonged downregulation on pregnancy rates, and another retrospective study that showed no difference in outcomes.10–14 The true benefit of prolonged versus standard downregulation in this population remains imprecise.
Objective
We aim to determine whether modified downregulation prior to frozen-thawed embryo transfer (FTET) improves the chance of clinical pregnancy in women with moderate and severe adenomyosis.
Methods
Trial design
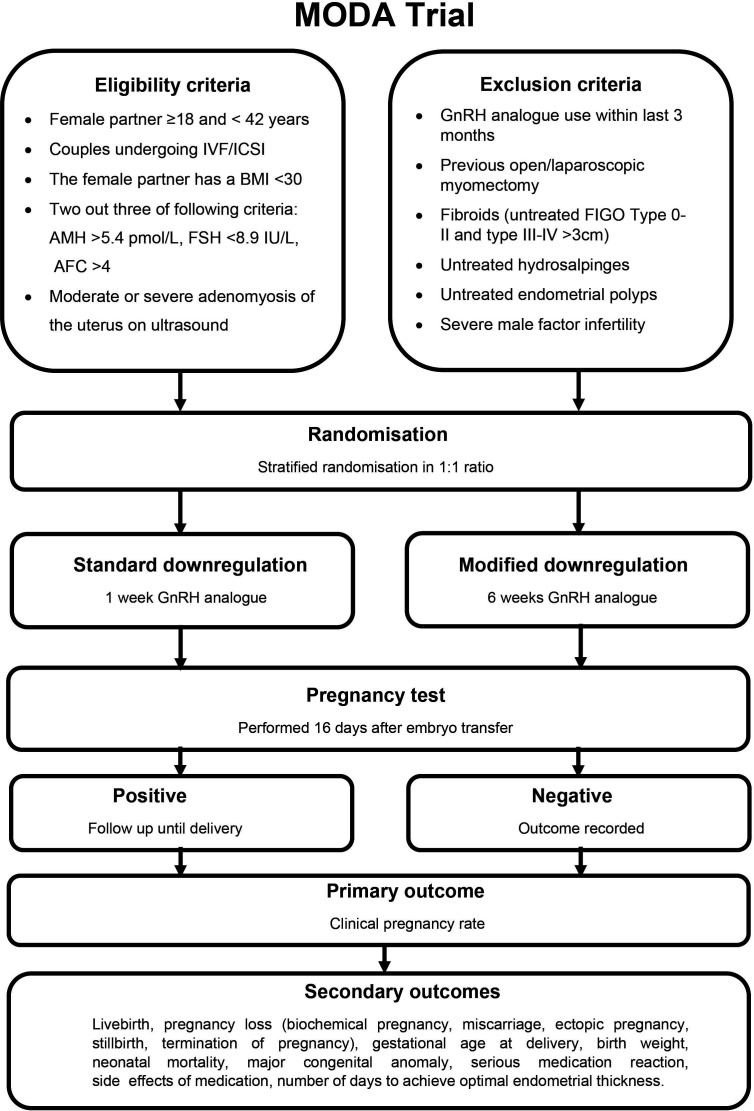
The MODA trial is a pragmatic, multi-site, randomised controlled trial of two parallel arms comparing prolonged downregulation with GnRH analogue for 6 weeks to standard downregulation with GnRH analogue for 1 week prior to FTET, recruiting participants from fertility centres across the UK. Participants will be followed-up until 6 weeks after the pregnancy outcome is determined. The trial design is summarised in figure 1.
Figure 1.
MODA trial study design. AFC, antral follicle count; AMH, anti-müllerian hormone; BMI, body mass index; FIGO, The International Federation of Gynecology and Obstetrics; FSH, follicle stimulating hormone; GnRH, gonadotrophin-releasing hormone; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilisation.
Inclusion criteria
Couples who are undergoing a cycle of IVF/intracytoplasmic sperm injection (ICSI), where a cycle is defined as egg collection following ovarian stimulation.
The female partner is ≥18 and <42 years of age.
The female partner has a body mass index <30 kg/m2.
Two out of three of the following criteria are met: anti-müllerian hormone (AMH) >5.4 pmol/L, follicle stimulating hormone (FSH) <8.9 IU/L, antral follicle count (AFC) >4.15
Moderate or severe adenomyosis of the uterus diagnosed on ultrasound scan.
Both partners are willing and able to provide written informed consent.
Exclusion criteria
Concurrent and/or recent involvement in other research that is likely to interfere with the intervention within the previous 3 months of study enrolment.
Previous open or laparoscopic myomectomy.
Uterine fibroids (untreated International Federation of Gynecology and Obstetrics (FIGO) type 0-I-II and type III-IV fibroids >3 cm).
Untreated endometrial polyps.
Untreated hydrosalpinges.
Use of GnRH analogues within previous 3 months.
Severe male factor infertility (sperm count <2×106/mL, use of surgically retrieved spermatozoa).
Couples who in the opinion of the researcher by virtue of language or learning impairment would be unable to give fully informed consent to the study.
Outcomes
The primary outcome of the study is clinical pregnancy, defined as a viable intrauterine pregnancy confirmed by ultrasound at greater than 6 weeks gestation.
Our secondary outcomes will include live birth, pregnancy loss (biochemical pregnancy, miscarriage, ectopic pregnancy, stillbirth, termination of pregnancy), gestational age at delivery, birth weight, neonatal mortality, major congenital anomaly, serious medication reaction, frequency and severity of medication side effects, number of days to achieve optimal endometrial thickness.
Enrolment
Enrolment of participants will involve coordinated registration and allocation of participant trial numbers by the trial coordinator. All participants will be asked by a member of the research team to complete a written consent at least 24 hours after providing the trial’s participant information sheet (see model consent form in online supplemental file). All consents will be recorded in the medical notes including signatures from both partners undergoing the IVF/ICSI procedure. A member of the research team will explain that participants are under no obligation to enter the trial and that they can withdraw at any time during the trial, without having to give a reason. No trial procedures will be conducted prior to the participant giving consent by signing the consent form.
bmjopen-2021-050248supp001.pdf (430.1KB, pdf)
Once consent has been granted a transvaginal ultrasound scan (TVS) will be performed by a member of the research team. The scan will be performed in a systematic fashion starting from the uterus in longitudinal plane with measurement of the endometrial thickness. The probe is then rotated to the transverse plane and the uterus scanned from the cervix to the fundus with any uterine pathologies noted and measured in three orthogonal planes. A 3D ultrasound volume is then obtained and saved starting with the uterus in longitudinal view making sure to include all uterine tissue in the 3D volume sweep. Any congenital or acquired uterine anomalies are diagnosed according to published diagnostic criteria. Adenomyosis is diagnosed according to a standardised diagnostic criteria2 and graded for severity according to the number of adenomyosis features present (assign a score of 1 for each of: (1) asymmetrical myometrial thickening, (2) parallel shadowing, (3) myometrial cysts, (4) irregular endometrial–myometrial junction, (5) linear striations, (6) hyperechoic islands, (7) adenomyoma). Patients with four or more features of adenomyosis are considered to have moderate or severe adenomyosis.5 Videosonography for a period of 4 minutes will be performed and recorded to assess uterine peristalsis. The operator will then sweep to the adnexae, starting from the left to identify and measure the ovaries in three orthogonal planes and document the antral follicle count. Each ovary is examined for the presence of cysts as well as for mobility and tenderness by gentle pressure with the ultrasound probe. Once the ovaries have been assessed the operator examines the pouch of Douglas for the presence of free fluid as well as any evidence of endometriosis such as obliteration, endometriotic nodules and endometriomas, as previously described.16 Once the ultrasound scan is concluded all information is added to the clinical database (REDCap - Research Electronic Data Capture, Vanderbilt University).
After the scan and once eligibility is confirmed participants will be asked to confirm if they want to participate in the trial. Those that confirm their consent will proceed with FTET preceded by the downregulation protocol allocated at randomisation. If an eligible participant undergoes a fresh transfer they will be followed through their cycle to determine pregnancy outcome and will be offered the opportunity to participate in MODA for their subsequent FTET.
Randomisation
Participants will be randomised using an online sequence generation and allocation system (www.sealedenvelope.com) in a ratio of 1:1, using stratified randomisation to adjust for age (age <37, age ≥37), with permuted blocks of random sizes. The block sizes will not be disclosed to ensure concealment. Randomisation will be performed by a member of the University College London Hospital (UCLH) Reproductive Medicine Unit administration team, independent to the research team. Participants will be informed of their assigned treatment group and this will be recorded on the Trial Subject Enrolment Log. The principal investigator (PI) will hold the randomisation list. It is not feasible to perform blinding in our setting.
Procedures
Standard downregulation protocol
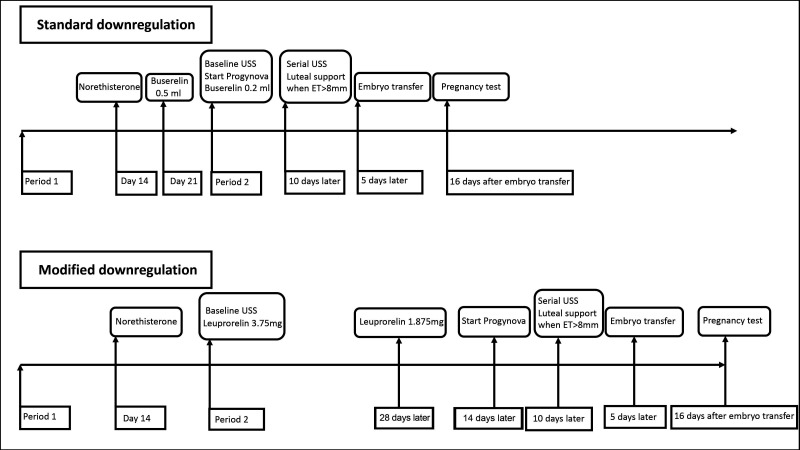
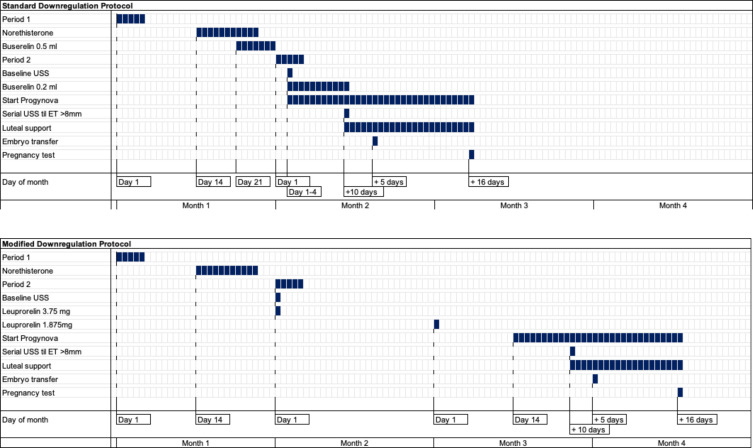
Participants allocated to the standard downregulation protocol will start Norethisterone on day 14 of the downregulation cycle and continue for 11 days, followed by Buserelin 0.5 mL subcutaneously from day 21. A baseline scan will be performed between days one and four of their bleed, Buserelin reduced to 0.2 mL and they will commence Progynova 2 mg three times daily orally. Serial scanning will be performed from day 10 until an endometrial thickness of greater than 8 mm is achieved, followed by luteal support with Cyclogest 400 mg two times daily vaginally or rectally and Lubion 25 mg two times daily subcutaneously. Blastocyst embryo transfer will be performed with a minimum morphological quality of B–C on the appropriate day for embryo age (figures 2 and 3).
Figure 2.
Standard and modified downregulation protocol overview. ET, embryo transfer; USS, ultrasound scan.
Figure 3.
Standard and modified downregulation protocol daily schedule. ET, embryo transfer; USS, ultrasound scan.
Modified downregulation protocol
Participants allocated to the modified downregulation protocol will have a baseline scan between day 1 and 4 of their bleed and will administer Leuprorelin acetate 3.75 mg subcutaneously, followed by Leuprorelin acetate 1.875 mg subcutaneously 28 days later. They will commence Progynova 2 mg three times daily orally 14 days later. Serial scanning will be performed from day 10 until an endometrial thickness of greater than 8 mm is achieved, followed by luteal support with Cyclogest 400 mg two times daily vaginally or rectally and Lubion 25 mg two times daily subcutaneously. Blastocyst embryo transfer will be performed with a minimum morphological quality of B–C on the appropriate day for embryo age (figures 2 and 3).
Discontinuation/withdrawal of participants
In consenting to participate in the trial, participants are consenting to intervention, assessments, follow-up and data collection.
A participant may be withdrawn from the trial whenever continued participation is no longer in the participant’s best interests, but the reasons for doing so must be recorded. Reasons for discontinuing the trial may include:
Intercurrent illness.
Participants withdrawing consent.
Persistent non-compliance to protocol requirements.
The decision to withdraw a participant from treatment will be recorded in the electronic case report form (eCRF) and medical notes.
Patient and public involvement
Patients were involved as research partners in all aspects of the study including identifying the original research question, in revising study design and in confirming acceptability of study monitoring methods and the intervention to be administered.
Modification of the protocol
Any modifications to the protocol which may impact on the conduct of the study, potential benefit of the patient or may affect patient safety, including changes of study objectives, study design, patient population, sample sizes, study procedures or significant administrative aspects will require a formal amendment to the protocol. Such amendment will be agreed on by the chief investigator (CI) and research team. They will be approved by the Research Ethics Committee (REC) prior to implementation.
Data and trial management
Data will be collected on trial specific eCRFs. All eCRFs will be completed and signed by staff that are listed on the site staff delegation log and authorised by the CI/PI to perform this duty. The CI/PI is responsible for the accuracy of all data reported in the CRF. The study is compliant with the requirements of General Data Protection Regulation (2016/679) and the Data Protection Act (2018). All investigators and study site staff will comply with the requirements of the General Data Protection Regulation (2016/679). Personal data will be held on the password protected database. We will adopt the NHS Code of Confidentiality and will allocate a unique participant identification number to ensure participant anonymity at data handling and analysis stage. Access to this will be restricted to the PI. Anonymised data will be stored for 20 years after completion of the trial, after which time data will be disposed of using confidential information trust destruction procedures.
MODA will have a Trial Management Group (TMG) that will include the CI and trial staff to oversee the everyday trial’s conduct (table 3, trial personnel). The TMG will meet regularly four times a year to review recruitment figures, serious adverse events (SAEs) and substantial amendments to the protocol prior to submission to the REC. We will identify an independent Trial Steering Committee (TSC), which has overall responsibility for the conduct of the study. The study will be supervised on a day-to-day basis by the TMG, who will report to the TSC. We will also identify an independent Data Monitoring Committee (DMC) to provide advice on data management and safety aspects of the trial. The DMC will meet 6 monthly to review interim analyses, or as necessary to address any issues.
Recording and reporting of adverse events
Each adverse event (AE) will be assessed for severity, causality, seriousness and expectedness. All AEs will be recorded with clinical symptoms and accompanied with a simple, brief description of the event, including dates as appropriate. All SAEs will be recorded in the medical records and the eCRF, and the sponsor’s AE log. The AE log of SAEs will be reported to the sponsor at least two times per year. SAEs will be reported to the sponsor within 5 days of becoming aware of the event.
Sample size
Our previous observational studies suggest a clinical pregnancy rate of 42.7% in women with mild adenomyosis compared with 22.9% with moderate/severe adenomyosis.7 We theorise that the modified protocol will improve the chance of clinical pregnancy to similar levels in those with mild disease. We need to randomise 162 patients over 3 years to achieve 80% power for detecting a 20% difference in the primary outcome across those groups at 5% significance.
Adenomyosis has an estimated prevalence of 20.9% in benign gynaecology patients,2 we estimate higher prevalence among women seeking assisted conception with a history of subfertility. Given our yearly 750 cycles of IVF/ICSI, we estimate a recruitment period of 3 years to achieve our target sample size.
Analysis
Participants with missing data and non-compliers, including participants who decide to withdraw from the study or do not follow their assigned protocol, will be excluded from our analysis. We will perform univariate and multivariate analyses to compare the primary outcome of both groups and report using risk ratio and 95% CIs for dichotomous outcomes as well as mean difference with SD for continuous outcomes.
Ethics and dissemination
The findings of this study will be published in peer-reviewed journals and presented at national and international scientific meetings and congresses. No later than 3 years after the collection of the 6 weeks post pregnancy outcome data, we will deliver a completely deidentified data set to an appropriate data archive for sharing purposes. Ethical approval was granted by the NHS Research Ethics Committees (UK IRAS integrated research application system; reference 19/LO/1567). MODA is registered online with ClinicalTrials.gov. See tables 1–3 for WHO trial registration data, protocol version history and trial personnel.
Table 1.
WHO trial registration data
| Data category | Information |
| Primary registry and trial identifying number | ClinicalTrials.gov NCT03946722 |
| Date of registration in primary registry | 13 May 2019 |
| Source of monetary or material support | Dimitrios Mavrelos (CI) |
| Primary sponsor | University College London |
| Contact for public queries | DM, SL (dimitrios.mavrelos@nhs.net, sanialatif@nhs.net) University College London Hospital, UK |
| Contact for scientific queries | DM, SL (dimitrios.mavrelos@nhs.net, sanialatif@nhs.net) University College London Hospital, UK |
| Scientific title | Modified downregulation for women with moderate/severe adenomyosis of the uterus prior to frozen-thawed embryo transfer. |
| Descriptive title | The effectiveness of modified downregulation for women with moderate and severe adenomyosis of the uterus prior to frozen-thawed embryo transfer (MODA) study protocol: a pragmatic randomised controlled trial. |
| Public title | MODA |
| Countries of recruitment | UK |
| Health condition or problem studied | Adenomyosis |
| Intervention(s) | Active comparator: prolonged downregulation for 6 weeks. |
| Placebo comparator: standard downregulation for 1 week. | |
| Key inclusion and exclusion criteria | Ages eligible for study: ≥18 years to <42 years Sexes eligible for study: female Accepts healthy volunteers: no |
| Inclusion criteria: women with moderate/severe adenomyosis undergoing IVF treatment, body mass index <30 kg/m2, two out of three of the following criteria are met: AMH >5.4 pmol/L, FSH <8.9 IU/L, antral follicle count >4. | |
| Exclusion criteria: previous open or laparoscopic myomectomy, uterine fibroids (untreated FIGO type 0-I-II and type III-IV fibroids >3 cm), untreated endometrial polyps, untreated hydrosalpinges, use of GnRH analogues within previous 3 months, severe male factor infertility (sperm count <2×106/mL, use of surgically retrieved spermatozoa). | |
| Study type | Interventional |
| Allocation: randomised intervention model. Parallel assignment masking: unblinded. | |
| Primary purpose: to determine effectiveness of modified downregulation. | |
| Phase III | |
| Date of first enrolment | October 2020 |
| Target sample size | 162 |
| Recruitment status | Recruiting |
| Primary outcome | Clinical pregnancy rate |
| Key secondary outcomes | Live birth, pregnancy loss (biochemical pregnancy, miscarriage, ectopic pregnancy, stillbirth, termination of pregnancy), gestational age at delivery, birth weight, neonatal mortality, major congenital anomaly, serious medication reaction, frequency and severity of medication side effects, number of days to achieve optimal endometrial thickness. |
AMH, anti-müllerian hormone; BMI, body mass index; CI, chief investigator; FIGO, The International Federation of Gynecology and Obstetrics; FSH, follicle stimulating hormone; GnRH, gonadotrophin-releasing hormone; ICSI, intracytoplasmic sperm injection; IVF, in vitro fertilisation.
Table 2.
Protocol version history
| Version number | Date | Protocol update finalised by : | Reasons for update |
| 1 | 3 April 2019 | N/A | N/A |
| 2 | 1 January 2020 | Novin Fard | Hospital trust formulary update; Triptorelin changed to Leuprorelin. |
Table 3.
Trial personnel
| Chief investigator | Mr Dimitrios Mavrelos |
| Consultant Obstetrician & Gynaecologist and Subspecialist in Reproductive Medicine | |
| Reproductive Medicine Unit University College London Hospital |
|
| Sponsor representative | Pushpsen Joshi |
| Joint Research Office University College London |
|
| pushpsen.joshi1@nhs.net | |
| Student | Miss Sania Latif Clinical Research Fellow in Reproductive Medicine Reproductive Medicine Unit University College London Hospital sanialatif@nhs.net |
| Statistician | Dr George Ploubidis Professor of Population Health and Statistics Director of Research and Chief Statistician Department of Social Science University College London g.ploubidis@ucl.ac.uk |
Discussion
Women with moderate/severe adenomyosis undergoing assisted conception are known to have a reduced clinical pregnancy rate; there is currently no consensus regarding their treatment and the existing literature is conflicting. Retrospective studies have identified benefit in prolonged downregulation for these patients however these are subject to selection and publication bias and therefore may not offer a realistic assessment of this protocol modification.10–12 Others have reported a reduction in pregnancy rates with prolonged downregulation but again this is retrospective data which may have underestimated the positive effect of the intervention.13
One retrospective study showed increased clinical pregnancy rates in women with adenomyosis following GnRH agonist treatment for 2–3 months prior to frozen embryo transfer, whereas no benefit was noted in women who had prolonged downregulation prior to fresh embryo transfer.11 The hyperoestrogenic state following controlled ovarian stimulation in a fresh embryo transfer cycle is postulated to diminish the effect of GnRH agonist pretreatment. A higher dose and longer duration of gonadotrophins were required during controlled ovarian stimulation to overcome the prolonged downregulation effect.11 Another retrospective study in which women with adenomyosis received 3 months downregulation with GnRH analogue prior to fresh embryo transfer found a reduction in clinical pregnancy rates, with a significantly lower endometrial thickness in the pretreatment group that did not fall pregnant.13 However, prolonged downregulation for a less extended duration of 6 weeks prior to FTET has previously been shown to improved clinical pregnancy rates, without any negative effects on endometrial preparation.10 We are therefore evaluating a similar protocol in the MODA trial.
Coexisting undiagnosed endometriosis is a substantial confounding factor, as not all patients with adenomyosis undergo surgical diagnostic procedures. There is also potential for variability in the diagnosis of adenomyosis by ultrasound. In the MODA trial we have only included fertility centres with an expert ultrasound operator and will review ultrasound images to ensure quality of diagnosis. This approach should also reduce the risk of undiagnosed moderate or severe endometriosis as this can be reliably diagnosed on transvaginal ultrasound. Randomisation should ensure confounding due to endometriosis coexistence is balanced. We will report the frequency of ultrasound diagnosed endometriosis in the control and treatment arm of the study.
There is an urgent need to prospectively evaluate prolonged downregulation as an option for couples with adenomyosis undergoing assisted conception both to confirm or refute the validity of this approach. The MODA trial is designed to offer pragmatic, real-life evaluation of the optimal use of downregulation in affected women. Our modified downregulation protocol is a practical, simple and readily available treatment option that could help women with adenomyosis to improve their chances of conception.
Supplementary Material
Footnotes
Contributors: DM conceived of the study. SL, BW, NB, TL, ES, EY, PS and DM initiated and revised the study design, drafted and revised the manuscript critically and contributed to refinement of the study protocol. SL, BW, NB, TL, ES, EY, PS and DM approved the final manuscript and are in agreement to be accountable for all aspects of the work.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Bird CC, McElin TW, Manalo-Estrella P. The elusive adenomyosis of the uterus-revisited. Am J Obstet Gynecol 1972;112:583–93. 10.1016/0002-9378(72)90781-8 [DOI] [PubMed] [Google Scholar]
- 2.Naftalin J, Hoo W, Pateman K, et al. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod 2012;27:3432–9. 10.1093/humrep/des332 [DOI] [PubMed] [Google Scholar]
- 3.Vercellini P, Consonni D, Dridi D, et al. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod 2014;29:964–77. 10.1093/humrep/deu041 [DOI] [PubMed] [Google Scholar]
- 4.Younes G, Tulandi T. Effects of adenomyosis on in vitro fertilization treatment outcomes: a meta-analysis. Fertil Steril 2017;108:483–90. 10.1016/j.fertnstert.2017.06.025 [DOI] [PubMed] [Google Scholar]
- 5.Mavrelos D, Holland TK, O'Donovan O, et al. The impact of adenomyosis on the outcome of IVF-embryo transfer. Reprod Biomed Online 2017;35:549–54. 10.1016/j.rbmo.2017.06.026 [DOI] [PubMed] [Google Scholar]
- 6.Vannuccini S, Tosti C, Carmona F, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online 2017;35:592–601. 10.1016/j.rbmo.2017.06.016 [DOI] [PubMed] [Google Scholar]
- 7.Mehasseb MK, Bell SC, Pringle JH, et al. Uterine adenomyosis is associated with ultrastructural features of altered contractility in the inner myometrium. Fertil Steril 2010;93:2130–6. 10.1016/j.fertnstert.2009.01.097 [DOI] [PubMed] [Google Scholar]
- 8.Khan KN, Kitajima M, Hiraki K, et al. Cell proliferation effect of GnRH agonist on pathological lesions of women with endometriosis, adenomyosis and uterine myoma. Hum Reprod 2010;25:2878–90. 10.1093/humrep/deq240 [DOI] [PubMed] [Google Scholar]
- 9.Tremellen K, Russell P. Adenomyosis is a potential cause of recurrent implantation failure during IVF treatment. Aust N Z J Obstet Gynaecol 2011;51:280–3. 10.1111/j.1479-828X.2010.01276.x [DOI] [PubMed] [Google Scholar]
- 10.Niu Z, Chen Q, Sun Y, et al. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol Endocrinol 2013;29:1026–30. 10.3109/09513590.2013.824960 [DOI] [PubMed] [Google Scholar]
- 11.Park CW, Choi MH, Yang KM, et al. Pregnancy rate in women with adenomyosis undergoing fresh or frozen embryo transfer cycles following gonadotropin-releasing hormone agonist treatment. Clin Exp Reprod Med 2016;43:169–73. 10.5653/cerm.2016.43.3.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou X, Xing J, Shan H. The impact of adenomyosis on IVF in infertile women with normal ovarian reserve following long or ultra-long GnRH-agonist treatment. Reprod Biomed 2020;41:845–53. [DOI] [PubMed] [Google Scholar]
- 13.Chen M, Luo L, Wang Q, et al. Impact of gonadotropin-releasing hormone agonist pre-treatment on the cumulative live birth rate in infertile women with adenomyosis treated with IVF/ICSI: a retrospective cohort study. Front Endocrinol 2020;11:318. 10.3389/fendo.2020.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lan J, Wu Y, Wu Z, et al. Ultra-Long GnRH agonist protocol during IVF/ICSI improves pregnancy outcomes in women with adenomyosis: a retrospective cohort study. Front Endocrinol 2021;12:495. 10.3389/fendo.2021.609771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence (NICE) . Clinical guideline CG156 fertility: assessment and treatment for people with fertility problems 2017.
- 16.Holland TK, Cutner A, Saridogan E, et al. Ultrasound mapping of pelvic endometriosis: does the location and number of lesions affect the diagnostic accuracy? A multicentre diagnostic accuracy study. BMC Womens Health 2013;13:13–43. 10.1186/1472-6874-13-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-050248supp001.pdf (430.1KB, pdf)