Abstract
BACKGROUND:
The goal of this study was to compare health-related quality of life (HRQL) from diagnosis to 10 years postdiagnosis among breast cancer survivors (BCS) and women without cancer over the same period and to identify BCS subgroups exhibiting different HRQL trajectories.
METHODS:
Our analysis included 141 BCS and 2086 controls from the Study of Women’s Health Across the Nation (SWAN), a multiracial/ethnic cohort study of mid-life women assessed approximately annually from 1995 to 2015. Pink SWAN participants reported no cancer at SWAN enrollment and developed (cases) or did not develop (controls) incident breast cancer after enrollment. We assessed HRQL with SF-36 Mental Component Summary and Physical Component Summary scores. We modeled each as a function of case/control status, years since diagnosis, years since diagnosis squared, and the interaction terms between case/control status and the 2 time variables in linear models. We characterized heterogeneity in postdiagnosis HRQL of cases using group-based trajectories.
RESULTS:
BCS had significantly lower HRQL compared with controls at diagnosis and 1 year postdiagnosis. By 2 years, BCS and controls no longer differed significantly. Among BCS, 2 trajectory groups were identified for both scores. For the Mental Component Summary, 88.4% of BCS had consistently good and 11.6% had very low scores. For the Physical Component Summary, 73.9% had good scores, and 26.1% had consistently low scores. Prediagnosis perceived stress and current smoking were related to being in the low mental trajectory group, and a higher number of comorbidities was related to being in the low physical trajectory group.
CONCLUSION:
Although the majority of BCS have HRQL similar to non-cancer controls after 2 years, subgroups of BCS continue to have low HRQL. Prediagnosis stress, comorbidities, and smoking are vulnerability factors for long-term, low HRQL in BCS.
Keywords: breast, cancer, health-related quality of life, longitudinal, survivors
INTRODUCTION
Breast cancer is the most common cancer among women in the United States, with an estimated 268,600 new cases diagnosed in 2019 and a projected 4.57 million survivors by 2026.1,2 Health-related quality of life (HRQL) is a frequently measured patient-reported outcome and an important endpoint in breast cancer clinical trials.3,4 The Medical Outcomes Study short form (SF-36) is often used to evaluate HRQL among cancer survivors compared with individuals who do not have cancer.5–16 Although numerous studies have reported on HRQL following breast cancer diagnosis, most are cross-sectional,11,12,15,16 and longitudinal reports are often limited to the first year or two after diagnosis.10,13,14 Several studies examine HRQL at multiple time points and extend beyond 5 years after diagnosis,5–7,17–20 but only a few have a comparison group without breast cancer followed for the same length of time.7,18–20
Most of these long-term studies report no long-term difference in HRQL between survivors and controls,7,18,19 while others report lower levels of HRQL among survivors.8,20 Differences may be due to the HRQL measure (the SF-36 or European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30), assessment time periods, and/or participant ages. Furthermore, except for 2 studies,9,17 prior work describes only mean HRQL scores over time, which likely obscures heterogeneity among survivors. Examination of patterns of HRQL over time provides a more nuanced picture and may identify characteristics of breast cancer survivors (BCS) who require intervention. Finally, few studies collected information before diagnosis.6–8 The ability to examine prediagnostic characteristics allows elucidation of risk or protective factors that may signal vulnerable subgroups.
The Study of Women’s Health Across the Nation (SWAN) Breast Cancer Survivors study (Pink SWAN) addresses many limitations of existing research. A substudy within the SWAN longitudinal cohort, Pink SWAN focuses on women who developed incident breast cancer during 20 years of SWAN follow-up. SWAN affords a comparator of women who remained breast cancer free, HRQL measured at multiple timepoints, and a large array of potential prediagnosis predictors of HRQL. The objectives of the present study were to 1) compare longitudinal HRQL in BCS from breast cancer diagnosis to up to 10 years postdiagnosis to HRQL among similarly aged women without cancer over the same period, and 2) among BCS, discern subgroups that exhibit different postdiagnosis HRQL trajectories and identify prediagnosis predictors of subgroup membership.
We hypothesized that BCS would have lower HRQL compared with controls at diagnosis, but their HRQL would improve with time. We also hypothesized that heterogeneous trajectories of HRQL over time would exist among BCS and that prediagnosis comorbidities and psychosocial characteristics would predict HRQL trajectories postdiagnosis.
MATERIALS AND METHODS
Sample and Procedures
SWAN is a multiracial/ethnic cohort study characterizing biological and psychosocial changes occurring during the menopausal transition.21 From 1995 to 1997, 7 clinical sites recruited non-Hispanic white women and women from 1 of 4 racial/ethnic minorities (Black, Japanese, Hispanic, or Chinese). The protocol was approved by Institutional Review Boards at each site. All participants provided written informed consent.
SWAN eligibility included age 42 to 52 years; an intact uterus and at least one ovary; not pregnant, lactating, using oral contraceptives, or receiving hormone therapy; and having a menstrual cycle in the 3 months before screening.21 Participants were assessed in person at baseline and approximately annually through follow-up visit 15 in 2015, using a standardized protocol that included medical, reproductive, and menstrual history; lifestyle and psychosocial factors; physical and psychological symptoms; and anthropometric measurements. Instruments were translated into Spanish, Japanese, and Cantonese.
Pink SWAN cases were women who developed incident breast cancer following SWAN enrollment and had no other prior cancer. Pink SWAN controls had no breast cancer at baseline or during follow-up. Beginning at visit 12, we requested medical records from women reporting incident breast cancer, and the records were reviewed for cancer diagnosis and treatment.
We identified 152 incident cases. Of 110 cases for whom medical records were received, 104 breast cancers were confirmed, a 94.5% agreement between self-report and medical record. Date of diagnosis in BCS was the time “anchor” (ie, time since diagnosis = 0) for analyses. To assign a corresponding date in controls, a date we refer to below as the “pseudo-date of diagnosis,” we randomly assigned a visit as the first postdiagnosis visit such that the distribution of first postdiagnosis visits was comparable in cases and controls. The pseudo-date of diagnosis for controls was a randomly assigned date between the last prediagnosis visit and the first postdiagnosis visit, mirroring the process for cases. A questionnaire assessing treatment-related factors was mailed to BCS who were active in SWAN at follow-up visit 15 (n = 130); 109 responded (83.8%).
Measures
Primary outcome
The SF-36 was used to assess HRQL with the original coding algorithm (raw scores transformed to a 0–100 range).22 The SF-36 is a generic HRQL measure with 2 summary scores: the physical component summary (PCS) and the mental component summary (MCS).23 The PCS and MCS are normalized so that a score of 50 represents the population average.22,23 Using both distributed techniques and anchor-based methods, changes of as few as 2 points are felt to be important differences.24–26 The SF-36 was administered at SWAN follow-ups 6, 8, 10, 12, 13, and 15.
Predictors
Candidate prediagnosis predictors were selected based on variables previously related to HRQL in SWAN27 and other studies of BCS.6,7,9,17 Sociodemographic variables included age at diagnosis (continuous in years), partner status (married or partnered versus nonpartnered) at entry into SWAN, race/ethnicity, and educational attainment (high school or less, some college, ≥4 years college). Race/ethnicity was self-defined by respondents using an open-ended question: “How would you describe your primary racial or ethnic group?” The categories were non-Hispanic white, black, Chinese, Hispanic, or Japanese.
For the predictors summarized below, we selected the last prediagnosis value occurring within 5 years of diagnosis.
Health-related factors included number of medical comorbidities (0, 1, ≥2), menopause status (pre- or perimenopausal vs postmenopausal), and menopause symptoms. Symptoms included sleep problems (any of difficulty falling asleep, staying asleep, and/or early morning awakening ≥3 times/week in past 2 weeks, yes vs no); frequency of vasomotor symptoms in past 2 weeks (none, 1–5 days, 6+ days [ordinal]); and frequency of vaginal dryness over the past 2 weeks (not at all, 1–5 days, 6–8 days, 9–13 days, or every day [ordinal]).
Lifestyle and anthropometric variables included difficulty paying for basics (very or somewhat hard/not hard at all), current cigarette smoking (yes/no), alcohol use (<1 drink/mo, 1–8 drinks/mo, >8 drinks/mo [ordinal]), body mass index in continuous kg/m2, and nonoccupational physical activity.28 Psychosocial factors included perceived stress,29 number of negative life events considered very upsetting from a list of 18 events (none, 1, ≥2) since last study visit, trait anxiety,30 optimism,31 emotional and instrumental social support,32 and presence versus absence of depressive symptoms (score ≥ 16 versus <16 on the Center for Epidemiologic Studies Depression33 scale). Presence/absence of depressive symptoms was examined only in relation to the PCS because of conceptual overlap with the MCS.
Cancer-related variables, collected from parent study surveys, medical records, and self-reported questionnaires completed by survivors, were stage (0, I, II, III), surgery (any/none), chemotherapy (yes/no), radiation (yes/no), and hormonal endocrine therapy (yes/no).
Statistical Analyses
To address the first objective we examined cases and controls. We censored data collected after a recurrence of breast cancer or occurrence of a new primary cancer (among cases), or after an occurrence of an initial cancer (among controls), excluding nonmelanoma skin cancer. Of the 152 cases and 2163 controls meeting Pink SWAN inclusion criteria, 141 cases and 2086 controls had at least 1 postdiagnosis visit at which the SF-36 was measured and were included in these analyses. Linear mixed modeling34 was used to estimate mean MCS and PCS values from 0–10 years post diagnosis date for cases and controls. Based on preliminary examinations of scatterplots and smoothed LOESS plots,35 a quadratic model was used. We modeled PCS and MCS values as a function of group status (case vs control), years since diagnosis (since pseudo-date of diagnosis for controls), years since diagnosis squared, and the interactions of group with linear and quadratic terms years since diagnosis, which allowed patterns over time to differ for cases and controls. The annual mean values of MCS and PCS (years 0–10) were estimated as well as the mean case-control difference at each year; we tested whether this difference was significantly different from 0.
To address the second objective, separately for MCS and PCS, we employed group-based trajectory modeling among BCS only36 to identify homogeneous groups of women with distinct group-based trajectories after diagnosis. We used a combination of a statistical criterion (the Bayesian Information Criterion) and judgment (ie, minimum observed group size of 10% and/or distinctively different trajectories) to select the final number of trajectory groups from models with 2 to 7 trajectory groups.
Based on results from the mixed models, both linear and quadratic terms for time since diagnosis were included. For both MCS and PCS, individuals were assigned to the trajectory group for which they had the maximum posterior probability. For all graphic displays, predicted mean MCS and PCS scores over time are shown for the women assigned to a particular trajectory group.
Associations between group membership and covariates were assessed using chi-square tests (for categorical variables) and t tests (for continuous variables). We estimated a multivariate logistic regression model for group membership, including covariates with P < .10 in bivariate analyses.
All analyses were conducted with SAS 9.4.
RESULTS
Sample Characteristics
There were no significant sociodemographic differences between BCS and controls (Table 1). Most BCS were diagnosed at stage I cancer (51.8%); fewer were stage 0 (26.4%) or stage II or III (21.8%). There were no participants with metastatic cancer. Most BCS (97.7%) had surgery, 34.0% had chemotherapy, and 64.5% had radiation. The mean age at diagnosis was 56.5 years (SD, 6.2) for BCS and 57.2 years (SD, 6.1) for controls (P = .19) (data not shown).
TABLE 1.
Key Sociodemographic and Sampling Design Characteristics of Pink SWAN Cases and Controls
| Characteristic | Controls (n = 2086) | Breast Cancer Cases (n = 141) | P |
|---|---|---|---|
|
| |||
| Race/ethnicity | |||
| Non-Hispanic white | 1019 (48.9) | 70 (49.7) | .78 |
| Black | 584 (28.0) | 39 (27.7) | |
| Chinese | 194 (9.3) | 10 (7.1) | |
| Hispanic | 75 (3.6) | 4 (2.8) | |
| Japanese | 214 (10.3) | 18 (12.8) | |
| Study site | |||
| Michigan | 356 (17.1) | 23 (16.3) | .92 |
| Boston | 319 (15.3) | 26 (18.4) | |
| Chicago | 284 (13.6) | 16 (11.4) | |
| Davis | 339 (16.3) | 20 (14.2) | |
| Los Angeles | 379 (18.2) | 26 (18.4) | |
| New Jersey | 104 (5.0) | 8 (5.7) | |
| Pittsburgh | 305 (14.6) | 22 (15.6) | |
| Education | |||
| High school or less | 420 (20.3) | 26 (18.7) | .56 |
| Some college | 677 (32.7) | 41 (29.5) | |
| College or more | 976 (47.1) | 72 (51.8) | |
| Marital status | |||
| Not married/partnered | 657 (31.9) | 48 (34.8) | .48 |
| Married/partnered | 1405 (68.1) | 90 (65.2) | |
All data are presented as n (%).
HRQL Over Time
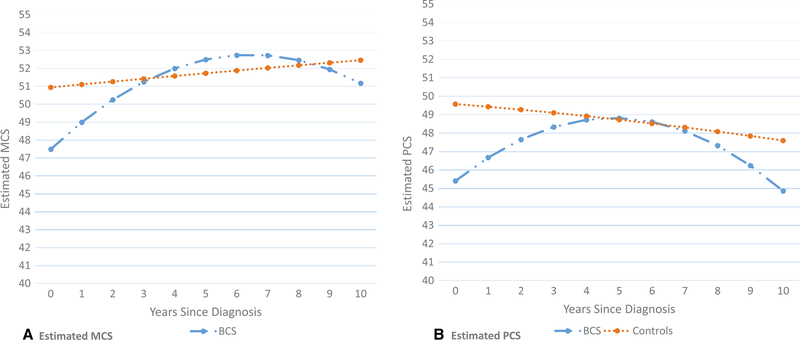
SF-36 values at the last prediagnosis date (data not shown) did not differ significantly between cases and controls; MCS values for cases and controls were 51.9 versus 51.6, respectively (P = .77); PCS values were 49.5 versus 49.6, respectively (P = .90). Figure 1 shows estimated MCS and PCS scores, from diagnosis date (pseudo-date of diagnosis for controls) to 10 years for cases and controls. MCS was significantly lower for BCS compared with controls at diagnosis (P = .013) and 1 year postdiagnosis (P = .036) (Table 2). By 2 years, the MCS values of BCS and controls no longer differed significantly. PCS also was significantly lower for BCS compared with controls at diagnosis (P = .001) and at 1 year postdiagnosis (P = .006). Although PCS improved for BCS, scores began a downward trend at about 7 years, and the difference between BCS and controls approached significance (P = .09) by year 10.
Figure 1.
Estimated mean values of (A) mental component summary (MCS) and (B) physical component summary (PCS) for breast cancer survivors (BCS) and controls over 0 to 10 years postdiagnosis, based on results from repeated measures model including group (BCS vs controls), years since diagnosis (linear term), years since diagnosis squared (quadratic term), and the interaction terms between group and each of the years since diagnosis variables as predictors.
TABLE 2.
Estimated Values of MCS and PCS by Year Since Diagnosis
| Years Since Diagnosis | Estimated Case, Mean (SE) | Estimated Control, Mean (SE) | Estimated Case-Control Difference (SE) | P for Difference |
|---|---|---|---|---|
|
| ||||
| MCS | ||||
| 0 | 47.5 (1.3) | 50.9 (0.3) | −3.4 (1.3) | .013 |
| 1 | 49.0 (0.9) | 51.0 (0.2) | −2.0 (1.0) | .036 |
| 2 | 50.3 (0.8) | 51.2 (0.2) | −1.0 (0.8) | .239 |
| 3 | 51.3 (0.8) | 51.4 (0.2) | −0.1 (0.8) | .882 |
| 4 | 52.0 (0.8) | 51.5 (0.2) | 0.5 (0.9) | .590 |
| 5 | 52.5 (0.9) | 51.7 (0.2) | 0.8 (0.9) | .370 |
| 6 | 52.7 (0.9) | 51.8 (0.2) | 0.9 (0.9) | .322 |
| 7 | 52.7 (0.9) | 52.0 (0.2) | 0.7 (0.9) | .423 |
| 8 | 52.5 (1.0) | 52.1 (0.2) | 0.3 (1.0) | .744 |
| 9 | 51.9 (1.3) | 52.3 (0.3) | −0.3 (1.3) | .814 |
| 10 | 51.2 (1.7) | 52.4 (0.4) | −1.2 (1.8) | .499 |
| PCS | ||||
| 0 | 45.4 (1.2) | 49.6 (0.3) | −4.2 (1.3) | .001 |
| 1 | 46.7 (1.0) | 49.4 (0.2) | −2.7 (1.0) | .006 |
| 2 | 47.7 (0.8) | 49.2 (0.2) | −1.6 (0.9) | .076 |
| 3 | 48.3 (0.9) | 49.0 (0.2) | −0.7 (0.9) | .434 |
| 4 | 48.7 (0.9) | 48.8 (0.2) | −0.1 (0.9) | .904 |
| 5 | 48.8 (0.9) | 48.6 (0.2) | 0.2 (0.9) | .853 |
| 6 | 48.6 (0.9) | 48.5 (0.2) | 0.2 (1.0) | .860 |
| 7 | 48.1 (0.9) | 48.3 (0.2) | −0.1 (1.0) | .894 |
| 8 | 47.3 (1.0) | 48.1 (0.2) | −0.7 (1.0) | .494 |
| 9 | 46.2 (1.2) | 47.8 (0.3) | −1.6 (1.3) | .205 |
| 10 | 44.9 (1.6) | 47.6 (0.4) | −2.8 (1.6) | .089 |
Abbreviations: MCS, mental component summary; PCS, physical component summary.
The estimated values were derived from a repeated measures model including group (case vs control), years since diagnosis, years since diagnosis squared, and the 2 interaction terms between group and years since diagnosis and group and years since diagnosis squared.
P values < .05 are bolded.
HRQL Trajectories
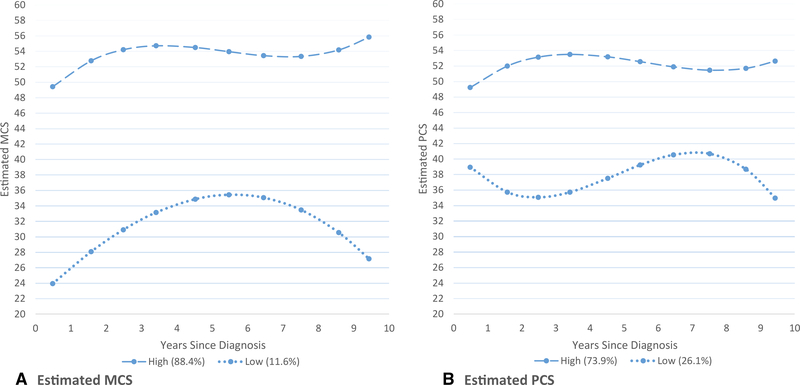
Figure 2 shows group-based trajectory results for BCS. The 2-trajectory model was selected for both MCS and PCS. MCS scores improved in the first few years postdiagnosis for both trajectory groups. The high group was larger (88.4%), with a rise in the first few years postdiagnosis and remaining above the scale’s midpoint of 50. A small group of women (11.6%) had very low MCS at diagnosis. Although their MCS improved considerably in the first few years postdiagnosis, a downward trend began at about 6 years.
Figure 2.
Estimated mean values of (A) mental component summary (MCS) and (B) physical component summary (PCS) for breast cancer survivors (BCS) by trajectory group (high and low), where the means are derived from a group-based trajectory model with 2 groups and including linear and quadratic terms for years since diagnosis.
There were 73.8% of BCS in the high PCS trajectory group, with improvement in the first few years postdiagnosis, then remaining high. The low PCS trajectory group (26.2%) had a downward trend in the first few years postdiagnosis, then improved to slightly above their diagnosis levels, and declined again at about 7 years.
We found no distinct trajectory groups (for either PCS or MCS) in the controls.
Predictors of MCS and PCS Trajectory Group Membership Over Time Among BCS
In bivariate analyses of prediagnosis characteristics with MCS trajectory grouping (Table 3), smoking, social support, and perceived stress met our criteria of P < .10 for inclusion in the multivariable model. These variables were tested in a multivariable logistic regression model, adjusted for race/ethnicity and age at diagnosis (Table 3). Two characteristics remained statistically significantly associated with low MCS group membership: current smoking (P = .04; the estimated odds ratio [OR] for being in lower MCS group for current smokers compared to non-smokers was close to 5) and higher perceived stress (P = .02; OR for 1-unit increase in stress score 1.27).
TABLE 3.
Bivariate and Logistic Regression Results for MCS Trajectory Group
| Bivariate Associations by Trajectory Group |
Logistic Regression Modelb |
|||||
|---|---|---|---|---|---|---|
| Prediagnosis Predictora | Low Group (n = 16) | High Group (n = 122) | P | Parameter Estimate (SD) | P | OR (95% CI) |
|
| ||||||
| Age at diagnosis, y, mean (SD) | 55.0 (5.1) | 57.5 (6.2) | .12 | −0.05 (0.05) | .32 | 0.95 (0.85–1.05) |
| Race/ethnicity, n (%) | .54 | .79 | ||||
| Non-Hispanic white | 8 (50.0) | 59 (48.4) | Reference | – | – | |
| Black | 4 (25.0) | 35 (28.7) | −0.08 (0.67) | .90 | 0.88 (0.20–3.99) | |
| Chinese | 2 (12.5) | 8 (6.6) | 0.84 (0.87) | .34 | 2.21 (0.30–16.36) | |
| Hispanic | 1 (6.3) | 3 (2.5) | 0.42 (1.32) | .75 | 1.46 (0.06–36.30) | |
| Japanese | 1 (6.3) | 17 (13.9) | −1.22 (1.04) | .24 | 0.28 (0.02–3.25) | |
| Current smoking, n (%) | 4 (25.0) | 12 (9.8) | .09 | 1.59 (0.79) | .04 | 4.88 (1.04–22.91) |
| Social support, mean (SD) | 10.4 (4.1) | 13.4 (2.8) | .01 | −0.13 (0.10) | .20 | 0.88 (0.72–1.07) |
| Perceived stress, mean (SD) | 10.1 (4.0) | 6.9 (2.7) | .006 | 0.24 (0.10) | .02 | 1.27 (1.04–1.55) |
Abbreviations: MCS, mental component summary; OR, odds ratio.
The following variables did not meet the P < .10 criterion for inclusion in the multivariable logistic regression: education, partner status, difficulty paying for basics, overall health, number of comorbidities, body mass index, menopause status, vaginal dryness, physical activity, alcohol use, sleep problems, trait anxiety, optimism, stressful life events, cancer stage, cancer surgery, chemotherapy, radiation therapy.
Predicts odds of membership in the low trajectory group.
P values < .05 are bolded.
In bivariate analyses of PCS groups, prediagnosis characteristics meeting the P < .10 criteria for inclusion in the multivaraible model were: education, difficulty paying for basics, number of comorbidities, body mass index, perceived stress, optimism, and race/ethnicity (Table 4). On multivariable analysis, only the number of comorbidies (P = .048) remained significantly related to group membership (Table 4). Compared to those with no comordibities, those with 1 comorbidity had an estimated OR of 2.2 for being in the low trajectory group and those with 2 or more comorbidities had an OR of 4.85 for being in the low trajectory group.
TABLE 4.
Bivariate and Logistic Regression Results for PCS Trajectory Group
| Bivariate Associations |
Logistic Regression Modelb |
|||||
|---|---|---|---|---|---|---|
| Prediagnosis Predictora | Low Group (n = 36) | High Group (n = 102) | P | Parameter Estimate (SE) | P | OR (95% CI) |
|
| ||||||
| Age at diagnosis, y, mean (SD) | 57.5 (6.8) | 57.1 (5.9) | .77 | 0.0002 (0.04) | .99 | 1.0 (0.92–1.09) |
| Race/ethnicity, n (%) | .09 | .49 | ||||
| Non-Hispanic white | 13 (36.1) | 54 (52.9) | Reference | – | – | |
| Black | 12 (33.3) | 27 (26.5) | −0.33 (0.49) | .51 | 1.48 (0.48–4.58) | |
| Chinese | 4 (11.1) | 6 (5.9) | 0.61 (0.67) | .37 | 3.76 (0.75–18.82) | |
| Hispanic | 3 (8.3) | 1 (1.0) | 0.74 (1.08) | .49 | 4.31 (0.29–63.55) | |
| Japanese | 4 (11.1) | 14 (13.7) | −0.30 (0.66) | .65 | 1.52 (0.32–7.33) | |
| Education, n (%)c | .015 | −0.42 (0.33)c | .20 | 0.65 (0.34–1.26) | ||
| High school or less | 11 (31.4) | 15 (14.9) | ||||
| Some college | 13 (37.1) | 27 (26.7) | ||||
| College or more | 11 (31.4) | 59 (58.4) | ||||
| Very/somewhat hard to pay for basics, n (%) | 15 (41.7) | 21 (20.6) | .01 | 0.63 (0.52) | .23 | 1.88 (0.67–5.27) |
| No. of comorbidities, n (%)c | .006 | 0.79 (0.40) | .048 | 2.20 (1.01–4.81) | ||
| 0 | 4 (11.1) | 22 (21.6) | ||||
| 1 | 6 (16.7) | 38 (37.3) | ||||
| ≥2 | 26 (72.2) | 42 (41.2) | ||||
| Body mass index, mean (SD) | 32.9 (9.0) | 28.1 (7.1) | .002 | 0.04 (0.03) | .22 | 1.04 (0.98–1.11) |
| Perceived stress, mean (SD) | 8.0 (3.1) | 7.0 (2.9) | .09 | 0.10 (0.08) | .21 | 1.11 (0.95–1.29) |
| Optimism, mean (SD) | 11.8 (3.4) | 13.0 (3.1) | .06 | −0.12 (0.08) | .12 | 0.88 (0.76–1.03) |
Abbreviations: PCS, physical component summary; OR, odds ratio.
The following variables did not meet the P < .10 criterion for inclusion in the multivariable logistic regression: partner status, menopause status, vasomotor symptoms, vaginal dryness, physical activity, current smoking, alcohol use, sleep problems, social support, trait anxiety, stressful life events, cancer stage, cancer surgery, chemotherapy, radiation therapy.
Predicts odds of membership in the low trajectory group.
Treated as single ordinal variable (3 ordered levels) in the multivariable model.
P values < .05 are bolded.
We also examined models that included prediagnosis levels of MCS or PCS as covariates. However, we did not include these in final models due to considerable collinearity with the covariates of interest.
DISCUSSION
Comparing HRQL between BCS and controls during the 10-year period after breast cancer diagnosis, BCS overall had lower HRQL than controls for up to 2 years postdiagnosis. After 2 years, HRQL did not differ significantly between groups. Group-based trajectory analyses among cases, however, identified 2 patterns of HRQL over time for both MCS and PCS, with one group consistently doing well and a smaller group reporting considerably lower HRQL over the follow-up. In the present study, we were able to examine an array of prediagnosis factors as possible predictors of postdiagnosis HRQL. Perceived stress was a significant predictor of being in the low group for both MCS and PCS. Smoking emerged as an additional predictor of being in the low MCS group, and comorbidities were predictive of being in the low PCS group. None of the cancer-related variables was related to trajectory group membership.
Other studies comparing BCS and controls have also found significant HRQL differences between cancer survivors and noncancer controls for up to 2 years after diagnosis,7,8,10,37 although several longitudinal studies extending beyond 2 years found that differences in HRQL between BCS and controls waned over time.11,18,19 We also found that differences initially diminished with time, but that there was a tendency for the groups to again diverge as time from breast cancer increases. In particular, a downward trend in PCS among BCS was greater than that of controls at 7 years postdiagnosis, consistent with several other longitudinal studies that extended 5 to 10 years postdiagnosis.6,8,20 One possible explanation for this downward trend is the increasing evidence of late effects resulting from cancer and its treatment that may appear more than 5 years posttreatment, such as cardiac, respiratory, or musculoskeletal problems.38,39 It is also possible that some of this decline could reflect prediagnosis onset of symptoms among cases that were subsequently censored due to a new cancer diagnosis or a recurrence of breast cancer.
In contrast to analysis of sample means over time, trajectory analyses provide a more nuanced view of longitudinal patterns of HRQL. For both MCS and PCS, the majority of women showed improvement in the first few years postdiagnosis that was maintained 10 years out. However, for both MCS and PCS, there were groups of survivors with values well below the scale midpoint of 50. As little as a 2-point difference in MCS or PCS score is considered to represent a clinically meaningful change.24–26 In this study, the low MCS group showed a highly meaningful improvement in the first 5 years after diagnosis, but then a decline that almost returns to the immediate postdiagnosis level. The low PCS group improves after the first few years, but then shows a meaningful decline further from diagnosis. Two other studies used latent growth curve analyses among BCS, though neither of these studies extends 10 years postdiagnosis.9,17 With extended follow-up, the present study was able to show that those survivors not doing well postdiagnosis may even begin a second downward pattern 6 years postdiagnosis. These declines do not reflect known cancer recurrence or a new cancer, since we censored data at both of these occurrences; however, unknown recurrence or new cancer prior to decline is possible.
Furthermore, the present study is able to identify prediagnosis factors related to trajectory group. Higher perceived stress prediagnosis was a significant predictor of MCS and PCS. Other studies have also identified prediagnosis perceived stress or stressful life events as important predictors of postdiagnosis HRQL6,40,41 and suggest that techniques to reduce stress (eg, relaxation techniques, mindfulness) may be useful interventions in this vulnerable group. Prediagnosis smoking also predicted membership in the low MCS group. Others have shown a positive association between cigarette smoking and depression and anxiety.42 Having 2 comorbidities at the time of diagnosis, which was a predictor of being in the low PCS group, has been recognized as an important predictor of HRQL.17,43 None of the cancer- or treatment-related variables was a significant determinant of either PCS or MCS.
This study has several limitations. Although the sample was racially and ethnically diverse, small samples in some groups precluded separate analyses. Furthermore, the SWAN sample was recruited primarily from large urban areas; BCS from rural areas were thus underrepresented, and our findings may not be generalizable to such women. SWAN was unable to obtain medical records for all BCS, although there was high agreement between self-report and medical records. In addition, MCS and PCS data points became more sparse at the tail end of years since diagnosis (ie, at 9 to 10 years postdiagnosis), as reflected in the larger standard errors in Table 2. Although caution is warranted when interpreting predicted values at the upper end of the interval, sensitivity analyses revealed that the data at 9 to 10 years were not unusually influential in our analyses, and that none of our findings/ conclusions changed when we went out to only 8 years postdiagnosis rather than 10 years. Finally, as in all longitudinal studies, study attrition is not random. It is likely that some of the 11 BCS who were excluded from analyses because they lacked postdiagnosis SF-36 data, may have had lower HRQL than the analytic sample.
Strengths of Pink SWAN include frequent, prospective HRQL measures over 10 years of post–breast cancer follow-up, a noncancer control group, and prediagnosis data. Thus, we were able to identify characteristics of a group of BCS with meaningful declines in HRQL after 6 years, following initial improvements. Cigarette smoking and high levels of stress—both modifiable characteristics—and at least 2 comorbidities prediagnosis represent vulnerability factors for long-term low HRQL postdiagnosis. These findings underscore the importance of an integrative approach to the treatement of at-risk women with breast cancer—those with preexisting psychological or physical conditions, or modifiable health behaviors. Attending to mental health care, both during and after the initial phases of cancer treatment, and being vigilant to the potential negative consequences of medical comorbidities on postdiagnosis HRQL may afford better medical and functional outcomes.
FUNDING SUPPORT
SWAN is supported by the National Institutes of Health (NIH) National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the Office of Research on Women’s Health (ORWH) (grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, and U01AG012495). Pink SWAN is supported by the National Cancer Institute (NCI) (grant R01CA199137). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NCI, NIA, NINR, ORWH or the NIH.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Rachel Hess has received personal fees from Astellas. The other authors made no disclosures.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2019. American Cancer Society; 2019. [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. [DOI] [PubMed] [Google Scholar]
- 3.Bottomley A, Reijneveld JC, Koller M, Flechtner H, Tomaszewski KA, Greimel E. Current state of quality of life and patient-reported outcomes research. Eur J Cancer. 2019;121:55–63. [DOI] [PubMed] [Google Scholar]
- 4.Lemieux J, Goodwin PJ, Bordeleau LJ, Lauzier S, Theberge V. Quality-of-life measurement in randomized clinical trials in breast cancer: an updated systematic review (2001–2009). J Natl Cancer Inst. 2011;103:178–231. [DOI] [PubMed] [Google Scholar]
- 5.Ganz PA, Desmond KA, Leedham B, Rowland JH, Meyerowitz BE, Belin TR. Quality of life in long-term, disease-free survivors of breast cancer: a follow-up study. J Natl Cancer Inst. 2002;94:39–49. [DOI] [PubMed] [Google Scholar]
- 6.Jones SM, LaCroix AZ, Li W, et al. Depression and quality of life before and after breast cancer diagnosis in older women from the Women’s Health Initiative. J Cancer Surviv. 2015;9:620–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsen RV, Frederiksen K, Larsen MB, et al. The impact of a breast cancer diagnosis on health-related quality of life. A prospective comparison among middle-aged to elderly women with and without breast cancer. Acta Oncol. 2016;55:720–727. [DOI] [PubMed] [Google Scholar]
- 8.Trentham-Dietz A, Sprague BL, Klein R, et al. Health-related quality of life before and after a breast cancer diagnosis. Breast Cancer Res Treat. 2008;109:379–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helgeson VS, Snyder P, Seltman H. Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychol. 2004;23:3–15. [DOI] [PubMed] [Google Scholar]
- 10.Stover AM, Mayer DK, Muss H, Wheeler SB, Lyons JC, Reeve BB. Quality of life changes during the pre- to postdiagnosis period and treatment-related recovery time in older women with breast cancer. Cancer. 2014;120:1881–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peuckmann V, Ekholm O, Rasmussen NK, et al. Health-related quality of life in long-term breast cancer survivors: nationwide survey in Denmark. Breast Cancer Res Treat. 2007;104:39–46. [DOI] [PubMed] [Google Scholar]
- 12.Helgeson VS, Tomich PL. Surviving cancer: a comparison of 5-year disease-free breast cancer survivors with healthy women. Psychooncology. 2005;14:307–317. [DOI] [PubMed] [Google Scholar]
- 13.Kroenke CH, Rosner B, Chen WY, Kawachi I, Colditz GA, Holmes MD. Functional impact of breast cancer by age at diagnosis. J Clin Oncol. 2004;22:1849–1856. [DOI] [PubMed] [Google Scholar]
- 14.Baker F, Denniston M, Haffer SC, Liberatos P. Change in health-related quality of life of newly diagnosed cancer patients, cancer survivors, and controls. Cancer. 2009;115:3024–3033. [DOI] [PubMed] [Google Scholar]
- 15.Broeckel JA, Jacobsen PB, Balducci L, Horton J, Lyman GH. Quality of life after adjuvant chemotherapy for breast cancer. Breast Cancer Res Treat. 2000;62:141–150. [DOI] [PubMed] [Google Scholar]
- 16.Champion VL, Wagner LI, Monahan PO, et al. Comparison of younger and older breast cancer survivors and age-matched controls on specific and overall quality of life domains. Cancer. 2014;120:2237–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durá-Ferrandis E, Mandelblatt JS, Clapp J, et al. Personality, coping, and social support as predictors of long-term quality-of-life trajectories in older breast cancer survivors: CALGB protocol 369901 (Alliance). Psychooncology. 2017;26:1914–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu T, Ennis M, Hood N, Graham M, Goodwin PJ. Quality of life in long-term breast cancer survivors. J Clin Oncol. 2013;31:3540–3548. [DOI] [PubMed] [Google Scholar]
- 19.Klein D, Mercier M, Abeilard E, et al. Long-term quality of life after breast cancer: a French registry-based controlled study. Breast Cancer Res Treat. 2011;129:125–134. [DOI] [PubMed] [Google Scholar]
- 20.Koch L, Jansen L, Herrmann A, et al. Quality of life in long-term breast cancer survivors—a 10-year longitudinal population-based study. Acta Oncol. 2013;52:1119–1128. [DOI] [PubMed] [Google Scholar]
- 21.Sowers MF, Crawford S, Sternfeld B, et al. Design, survey sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press. 2000:175–188. [Google Scholar]
- 22.Ware JE, Snow KK, Kosinski M, Gandek B, Institute. NEMCHH. SF-36 Health Survey: Manual and Interpretation Guide. Health Institute, New England Medical Center; 1993. [Google Scholar]
- 23.Ware JE, Kosinski M, Dewey JE, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Quality Metric, Inc; 2000. [Google Scholar]
- 24.Hays RD, Farivar SS, Liu H. Approaches and recommendations for estimating minimally important differences for health-related quality of life measures. COPD. 2005;2:63–67. [DOI] [PubMed] [Google Scholar]
- 25.Desikan R, Mason HL, Rupp MT, Skehan M. Health-related quality of life and healthcare resource utilization by COPD patients: a comparison of three instruments. Qual Life Res. 2002;11:739–751. [DOI] [PubMed] [Google Scholar]
- 26.Samsa G, Edelman D, Rothman ML, Williams GR, Lipscomb J, Matchar D. Determining clinically important differences in health status measures: a general approach with illustration to the Health Utilities Index Mark II. PharmacoEconomics. 1999;15:141–155. [DOI] [PubMed] [Google Scholar]
- 27.Avis NE, Colvin A, Bromberger JT, Hess R. Midlife predictors of health-related quality of life in older women. J Gerontol A Biol Sci Med Sci. 2018;73:1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sternfeld B, Ainsworth BE, Quesenberry CP. Physical activity patterns in a diverse population of women. Prev Med. 1999;28:313–323. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 30.Spielberger CD, Gorsuch RL, Lushene RE. STAI Manual for the Stait-Trait Anxiety Inventory (“Self-Evaluation Questionnaire”). Consulting Psychologists Press; 1970. [Google Scholar]
- 31.Scheier MF, Carver CS. Optimism, coping, and health: assessment and implications of generalized outcome expectancies. Health Psychol. 1985;4:219–247. [DOI] [PubMed] [Google Scholar]
- 32.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991:705–714. [DOI] [PubMed] [Google Scholar]
- 33.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 34.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Wiley; 2004. [Google Scholar]
- 35.Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 36.Nagin DS. Group-Based Modeling of Development 3. Harvard University Press; 2005. [Google Scholar]
- 37.Michael YL, Kawachi I, Berkman LF, Holmes MD, Colditz GA. The persistent impact of breast carcinoma on functional health status: prospective evidence from the Nurses’ Health Study. Cancer. 2000; 89:2176–2186. [DOI] [PubMed] [Google Scholar]
- 38.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112(11 suppl):2577–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Runowicz CD, Leach CR, Henry NL, et al. American Cancer Society/American Society of Clinical Oncology breast cancer survivorship care guideline. CA Cancer J Clin. 2016;66:43–73. [DOI] [PubMed] [Google Scholar]
- 40.Golden-Kreutz DM, Thornton LM, Wells-Di Gregorio S, et al. Traumatic stress, perceived global stress, and life events: prospectively predicting quality of life in breast cancer patients. Health Psychol. 2005;24:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beatty L, Lee C, Wade TD. A prospective examination of perceived stress as a mediator of the relationship between life-events and QOL following breast cancer. Br J Health Psychol. 2009;14:789–804. [DOI] [PubMed] [Google Scholar]
- 42.Fluharty M, Taylor AE, Grabski M, Munafo MR. The association of cigarette smoking with depression and anxiety: a systematic review. Nicotine Tob Res. 2017;19:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fu MR, Axelrod D, Guth AA, et al. Comorbidities and quality of life among breast cancer survivors: a prospective study. J Pers Med. 2015;5:229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]