Abstract
Background:
The 6-minute walk test (6MWT) is one of the common clinical tests to assess rehabilitation progress and needs in individuals with lower-limb amputation (iLLAs). However, the analysis of this test is mostly limited to the distance parameter. The first aim was to investigate effort intensity and spatiotemporal parameters of 6MWT in iLLAs using inertial measurement units (IMUs) and heart rate (HR) monitor, and second, to assess physical, physiological, and pain-related aspects of fatigue over 6MWT.
Methods:
Eleven unilateral iLLAs (57.91±15.63 years old) participated in this study. To evaluate HR and spatiotemporal parameters over 6MWT, data were classified using 6 intervals of 1 minute each (I1-I6). The pain level of participants was estimated using the visual analog scale (VAS).
Results:
Our results showed that the means of normalized HR gradually increased over 6MWT (I1: 61.59±10.73 HRmax%, I6: 70.15±12.26 HRmax%, P = .003). Variability of HR during the first interval of 6MWT was higher than the others (P < .05). The stance ratio of the gait cycle increased over 6MWT (P < .05). Cadence and speed decreased over 6MWT (P < .05). VAS score after 6MWT was significantly higher than before 6MWT (P = .016).
Conclusion:
In this preliminary study, effort intensity over 6MWT gradually increased because of enhancement of HR. Deterioration of speed and cadence and enhancement of stance ratio over 6MWT imply potential physical aspects of fatigue and instability.
Level of Evidence:
Level III, retrospective comparative study.
Keywords: lower limb amputation, inertial measurement units, heart rate, 6-minute walk test
Introduction
Worldwide prevalence of individuals with lower limb amputation (iLLAs) is rising because of the increasing rate of dysvascular-related disease such as diabetes. 14 Immobility, different aspects of fatigue during walking, and other activities of daily living due to amputation decreased the quality of life among iLLAs. 1,4,29,33
Fatigue has been defined as a decrease in capacity for physical and/or mental activity due to an imbalance in the availability, utilization, and/or restoration of resources. 1 It includes physical, psychological and pain-related aspects caused by acute and/or chronic weakness, illness, treatment or repetitive tasks. 1,2 Fatigue can extensively affect biological functions and decrease their desired capacity. 9,12 For instance, alteration of neuromuscular process and variability of motor output due to fatigue have a destructive effect on movement performance. 6 Alteration of coordination may predispose individuals to poor walking stability and increase fall risk especially in iLLAs owing to the loss of plantarflexors and dorsiflexors, which cannot be compensated by prostheses. 21,29,34
Walking is one of the basic tasks in daily movement, exercise, and physical therapy that requires cardiovascular endurance, muscular strength, coordination, as well as training to familiarize with a new prosthesis. 10,33 Clinicians often evaluate walking performance using various functional tests, such as Timed Up & Go, 2- and 6-minute walk test to preserve mobility, improve independence, and decrease the extensive number of risks and medications. 19,26 The 6-minute walk test (6MWT) has achieved extensive acceptance in different clinical and research settings and populations such as iLLAs owing to its advantages, including daily life activities relevance, as well as submaximal, safe, simple, and reproducible alternative to cardiopulmonary exercise test. 5,19,20 The 6MWT analysis in previous studies was mostly limited to the evaluation of distance. 5,19,26
Body-worn, lightweight, and inexpensive inertial measurement units (IMUs) overcome the traditional motion capture limitations in various environments and allow clinicians to monitor alteration of clinical walking test. 7,15 Combined with IMUs, telemetric heart rate (HR) monitoring could provide useful information about physiological adaptation and intensity of effort in a laboratory-free setting. 16,18,28 Hence, using IMUs and HR monitor along with 6MWT efficiently can improve the quality of clinical analyses. 3,15 Moreover, evaluation of variability (ie, standard deviation [SD]) of spatiotemporal parameters and HR over 6MWT can demonstrate physical and physiological fatigue. 3,6 Among gait spatiotemporal parameters, speed and cadence are robust clinometric parameters that represent gait intensity. 7,11,31 The prolonged stance phase of the gait cycle has been associated with instabilities. 24 Therefore, applying IMUs during 6MWT allow physical therapists to quickly and efficiently quantify these spatiotemporal parameters to assess how and why functional performance of gait is impaired. 3,13,22
Visual analog scale (VAS) assessment before and after 6MWT can represent pain-related fatigue, 1,3 enabling clinicians to identify and follow up the source of fatigue among iLLAs that can disrupt their balance and increase the likelihood of fall. 34 To our knowledge, no study analyzed evaluation of fatigue over a clinical walking test among iLLAs.
Accordingly, the objectives of this study were 2-fold: first, to investigate intensity and spatiotemporal (ie, cadence, speed, stance ratio) parameters of 6MWT in iLLAs using IMUs and HR monitor; and second, to assess the physical, physiological, and pain-related aspects of fatigue over the duration of the 6MWT. We hypothesized that (1) the intensity of 6MWT would gradually increase, considering the duration of this test; (2) HR variability would increase as a result of fatigue; (3) spatiotemporal parameters would deteriorate over the 6MWT as well as get increasingly variable as a result of fatigue.
Material and Methods
Participants
We recruited 11 unilateral iLLAs (5 females, 6 males) in the Physical Impairment Rehabilitation Institute (IRDPQ, Quebec City, Quebec, Canada) after 6-week rehabilitation discharge in accordance with the criteria defined by Beausoleil and colleagues. 3 Participants who had cardiorespiratory problems and neuromuscular and/or musculoskeletal disease were excluded from this study. Prosthetic inclusion criteria for the physiotherapists’ team at rehabilitation discharge includes comfortable fit, suspension in transfers, and weightbearing. All the participants signed the informed consent form approved by the ethical committee.
Task and Procedure
The HR of iLLAs over 6MWT was monitored in the clinical setting of the IRDPQ using Polar RS800CX (1000 Hz; Kempele, Finland). The HR connector was attached to the strap after the electrode was humidified. The strap was then tied onto the iLLA’s chest, just below the chest muscles, transmitting data to a watch on the wrist of the participants. Additionally, spatiotemporal parameters (ie, cadence, speed, and stance ratio) were collected during 6MWT by 2 validated IMUs (Physilog 4, Lausanne, Switzerland, 200 Hz) that were placed on top of each shoe. Pain level among our participants was estimated using a visual analog scale (VAS, range: 0-10 cm) before and after the 6MWT.
Data Analysis
The Polar ProTrainer (version 5, NY, USA), RTK Physilog 4 (version 1.1.1; Lausanne, Switzerland), and Matlab 2018a (MathWorks, Natick, MA) software were used for HR and spatiotemporal data extraction and analysis, respectively. The HR and spatiotemporal parameters data were classified into 6 intervals of 1 minute (I1 to I6). Mean and variability (SD) of each interval was computed. The HR was normalized using the age-predicted maximal heart rates (HRmax = 220 – age) to represent the intensity of workload. 19,28
Statistical Analysis
Two nonparametric repeated measures analysis of variance (ANOVA) (nparLD) test with Bonferroni corrections were used to analyze the mean and variability of HR performance and spatiotemporal parameters over 6MWT using R statistical software (version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria), separately. 23 This ANOVA-type statistic controls the Type I error rate even for small sample size and is insensitive to outliers. 23 Instead, to calculate sums of squares, the nparLD ANOVA works with the probability to be “higher than.” Data related to each condition are then compared to the whole data set. The relative treatment effect (RTE) or effect size is this adjusted probability for each condition. Probabilities are adjusted for different group/condition sample sizes. Consequently, RTEs vary between zero and one, with 0.5 as the null hypothesis value. RTEs are proportional to Cohen d when 2 measures are compared. Above 0.5, RTEs are defined as small, medium, and large when their values exceed, respectively, 0.56, 0.64, and 0.71. 32
The independent variable of the repeated measure was the interval (I1-I6) for the HR data. Indeed, intervals (I1-I6) and the legs (amputated leg [AL], non-amputated leg [nAL]) were 2 independent variables for spatiotemporal variables. For all ANOVAs, the nonparametric Wilcoxon tests, using SPSS 24, were calculated for post hoc analysis when a global effect was detected. A significant difference was set at a P level <.05.
Results
Characteristics of participants, amputation level, duration of rehabilitation, HRmax, VAS score, distance of 6MWT, and etiology of amputation are displayed in Table 1. The mean age (±SD) of our participants was 57.91 ± 15.63 years. The mean distance of 6MWT in iLLAs was 321.93 ± 128.04 m. The etiology of amputation was in the following order: vascular (8/11), traumatic (2/11), and tumor (1/11) (Table 1).
Table 1.
Subjects’ Characteristics, Days of Rehabilitation, Etiology of Amputation, and Pain Level.
| ID | Sex | AMP Level | Age, y |
BMI | Days of Rehab. | HRmax (bpm)a | VAS, Before (/10) | VAS, After (/10) | Distance Over 6MWT | Etiology |
|---|---|---|---|---|---|---|---|---|---|---|
| AMP001 | F | TT | 45 | 23.04 | 216 | 175 | 5.0 | 7.2 | 542.6 | Traumatic |
| AMP002 | M | TT | 51 | N/A | 122 | 169 | 0.0 | 0.0 | 336.6 | Vascular |
| AMP008 | F | TT | 68 | N/A | 121 | 152 | N/A | N/A | 234.6 | Vascular |
| AMP009 | M | TT | 76 | 20.89 | 196 | 144 | 0.0 | 0.0 | 211.2 | Vascular |
| AMP010 | M | TT | 80 | N/A | 87 | 140 | 0.0 | 8.0 | 322.5 | Vascular |
| AMP011 | F | TF | 55 | 38.64 | 122 | 165 | 0.0 | 2.5 | 130.5 | Vascular |
| AMP014 | M | TT | 57 | 21.14 | 145 | 163 | 0.0 | 0.0 | 423.6 | Vascular |
| AMP015 | M | TF | 73 | 25.73 | 108 | 147 | 0.0 | 5.8 | 185.4 | Vascular |
| AMP016 | M | TT | 62 | 26.68 | 122 | 158 | 0.0 | 3.0 | 276.1 | Vascular |
| AMP017 | F | TF | 33 | 19.26 | 184 | 187 | 0.5 | 3.0 | 438.3 | Tumor |
| AMP021 | F | TF | 37 | 21.21 | 315 | 183 | 0.0 | 0.0 | 439.8 | Traumatic |
| Mean ± SD | 5 F, 6 M |
4 TF, 7 TT |
57.91 ± 15.63 |
24.57 ± 6.22 |
158.23 ± 65.65 |
162.09 ± 15.63 |
0.55 ± 1.57 |
2.95 ± 3.10 |
321.93 ± 128.04 |
1 tumor, 2 traumatic, 8 vascular |
Abbreviations: 6MWT, 6-minute walk test; bpm, beats per minute; F, female; HR, heart rate; M, male; TF, transfemoral; TT, transtibial; VAS, visual analog scale.
aAge-predicted maximal heart rate.
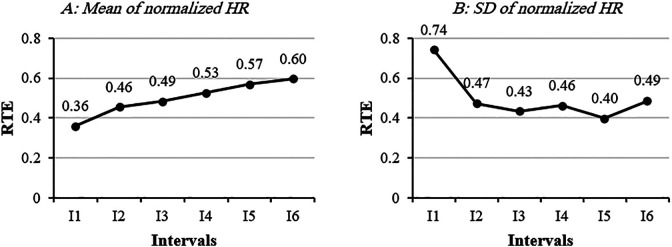
Mean and variability of normalized HR are presented in Table 2. The main effect of “interval” for mean and variability of normalized HR were significant (P < .001, P = .004). Our results showed that the mean of normalized HR gradually increased over 6MWT (I1: 61.59 ± 10.73 HRmax%, I6: 70.15 ± 12.26 HRmax%) (Table 2) as well as their effect size (Figure 1A). All the post hoc pairwise tests for mean of normalized HR were significant (P < .05). Variability of HR during the first interval of the 6MWT was significantly higher than I2, I3, I4, and I5 (P < .01). Effect size of HR variability at I1 was large (RTE = 0.74) (Figure 1B).
Table 2.
Mean and SD of Normalized HR Over 6 Intervals of the 6MWT.
| I1 | I2 | I3 | I4 | I5 | I6 | |
|---|---|---|---|---|---|---|
| HR Mean (HRmax%) | 61.59 ± 10.73 | 64.36 ± 9.73 | 65.78 ± 10.54 | 67.46 ± 11.20 | 69.03 ± 11.73 | 70.15 ± 12.26 |
| HR SD (HRmax%) | 3.04 ± 2.38 | 1.25 ± .22 | 1.21 ± .55 | 1.25 ± .49 | 1.15 ± .60 | 2.06 ± 2.80 |
Abbreviations: 6MWT, 6-minute walk test; HR, heart rate.
Figure 1.
Relative treatment effect of mean and SD of normalized heart rate over 6 intervals of the 6-minute walk test (6MWT).
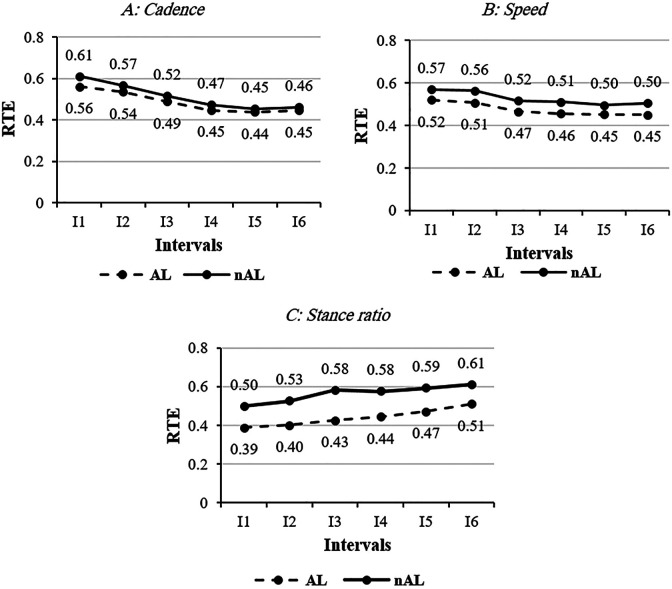
The main effect of “interval” was significant on cadence (P = .011), speed (P = .047), and stance ratio during 6MWT (P = .005). Interval post hoc showed mean of cadence in I1 (AL: 100.16 ± 20.18; nAL: 103.07 ± 18.7 steps/min) and I2 (AL: 99.39±20.41; nAL: 101.88±19.44 steps/min) were higher than I3 (AL: 97.65 ± 21.29; nAL: 99.99 ± 20.86 steps/min), I4 (AL: 97.31 ± 21.10; nAL: 99.65 ± 20.38 steps/min), I5 (AL: 97.09 ± 20.79; nAL: 99.00 ± 20.40 steps/min), and I6 (AL: 96.76 ± 21.93; nAL: 98.63 ± 21.32 steps/min) during 6MWT (P < .05). Effect size of cadence in 6 intervals in both sides are illustrated in Figure 2A. Our findings also demonstrated that walking speed over 6MWT slowly decreased. Mean of speed in I1 (0.99 ± 0.36 m/s) were 1.04, 1.04, 1.05, and 1.06 times higher than I3, I4, I5, and I6 in the AL, respectively (P < .05). Walking speed in I1 and I2 (1.06 ± 0.36 m/s) were higher than I3 (1.01 ± 0.37 m/s), I4 (1.00 ± 0.36 m/s), I5 (0.99 ± 0.37 m/s), and I6 (0.99 ± 0.39 m/s) in nAL (P < .03) (Figure 2B).
Figure 2.
Relative treatment effect of selected spatiotemporal parameters over the 6-minute walk test (6MWT) in both legs.
Additionally, stance ratio of the gait cycle gradually increased over 6MWT in AL and nAL. Mean of stance ratio in I5 (62.98 ± 2.92 gait cycle%) and I6 (63.39 ± 3.27 gait cycle%) were significantly longer than I1, I2, I3, and I4 (61.99 ± 3.35, 62.17 ± 3.47, 62.39 ± 3.28, 62.66 ± 3.22 gait cycle%) in AL. Likewise, stance ratio in I5 (64.40 ± 4.08 gait cycle%) and I4 (64.20 ± 3.66 gait cycle%) were longer than I1 (63.33 ± 2.63 gait cycle%) and I2 (63.59 ± 3.15 gait cycle%) in nAL, respectively (P = .042) (Figure 1C). There was not any significant difference for the main effect of “leg” and interaction of interval by leg for mean of selected spatiotemporal parameters. Indeed, variability of cadence, speed, and stance ratio over 6MWT and between 2 legs were almost similar. There was one technical error, where an IMU placed on the nAL did not work for 1 participant.
Discussion
Knowing there is a lack of information regarding intensity and variability of HR and spatiotemporal parameters in iLLAs during common walking test (ie, 6MWT) among iLLAs, we aimed to evaluate intensity and spatiotemporal parameters over 6MWT using HR monitor and IMUs in iLLAs as well as investigate physical, physiological, and pain-related aspects of fatigue during this clinical test.
The travelled distance over 6MWT was 321.93 ± 128.04 m among our participants. The average distance of the 6MWT in Lin and colleagues’ study 19 was 544.6 ± 64.5 m. This difference might be due to the cause of amputation in their participants, which was mostly (9/13) traumatic, whereas the main cause of amputation in our study was vascular (72%). Raya and colleagues 25 and Shields and colleagues 29 reported that vascular amputees have poorer ambulatory, general functioning, and well-being because of a longer duration of amputation. Cahalin and colleagues reported that 6MWT distance can predict oxygen uptake and short-term event-free survival among patients with heart failure. 5 Their results showed that a distance of 6MWT inferior to 300 m increased the likelihood of death within 6 months. Our participants did not have any cardiovascular disease, but 5 of them walked less than 300 m, which may be due to pain-related fatigue (based on VAS scores). Moreover, the distance covered in 6MWT of 7 iLLAs in this study was less than the typical community ambulation requirements (eg, going to the supermarket) in a large city (342 m). 27 By finding and decreasing the source of fatigue, clinicians would be able to increase daily activity among iLLAs.
Intensity of activity in first (I1: 61.59 ± 10.73 HRmax%) and second (I2: 64.36 ± 9.73 HRmax%) intervals of 6MWT was of low to moderate range (50-65 HRmax%) in iLLAs. 10,33 The intensity of the last 4 intervals of 6MWT was in the range of moderate to high (65-85 HRmax%) among our participants (Table 2). 10,33 This finding confirmed our first hypothesis about the gradual HR increase over the 6MWT. This rapid increase in HR during 6MWT might be due to stress, induced by pain, and/or duration of the test. 1 Although Beausoleil et al 3 did not normalize HR data in iLLAs, the increase of HR over the 6MWT was in agreement with our finding.
Our results showed that HR variability in the first interval (I1) of the 6MWT, with a large effect size (RTE = 0.74), was higher than in the other intervals (Table 2, Figure 1B). Therefore, our second hypothesis about increased HR variability was rejected. High HR variability at the first interval of 6MWT could be due to a “white coat effect” 17 —defined as the anxiety associated with clinic visit that affects several physiological processes of the human body, such as increased blood pressure, increased muscle tension, and changes in HR and HR variability. 30 Diminished HR variability after the first interval (I1) of 6MWT might reflect reduction of the mental stress of clinic visit.
Our results showed that the number of steps per minute (cadence), walking speed, and their effect size (RTE) over 6MWT decreased in iLLAs (Figure 2A, B). Consequently, the stance ratio and its effect size (RTE) increased during 6MWT (Figure 2C). Intensity of walking during the first interval (I1) of 6MWT was moderate based on cadence (100 steps/min) that continuously diminished until the end of the 6MWT. 31 However, normalized HR as an indicator of workload over this test increased in iLLAs. 10,16,33 Therefore, our third hypothesis about deterioration of spatiotemporal parameters over 6MWT was confirmed and might be due to the negative effect of physiological and pain-related fatigue on physical performance. Our finding was in agreement with the study of Devan and colleagues 8 that reported fatigue, both mental and physical, affected the “uneven movements” in iLLAs and might alter the mechanical loading of the spine during functional activities and contribute to the ongoing low back pain. Variability of cadence, speed, and stance ratio during the 6MWT were almost similar and could indicate that fatigue did not change stability of walking in iLLAs during this test. This finding was in disagreement with Wong and colleagues 34 and might be due to the shorter duration of 6MWT in our study in comparison with that of the 30-minute walking test.
Limitations of this study were the small sample size and the heterogeneity of both level and cause of amputation that could limit the generalization of our result. However, we are confident that this preliminary study provides practical insight for clinicians to apply lightweight and user-friendly IMUs and HR monitor to precisely analyze fatigue and movement strategy during the walking test among iLLAs.
Conclusion
Use of IMUs and HR monitoring provides more details for clinical walking tests in iLLAs to analyze the various aspects of fatigue during walking performance in order to preserve mobility and independence as a main goal of rehabilitation.
Variability of HR at the first minute of 6MWT was higher than during the rest of the test and reflects the high level of stress at the test or initial startup stress response. Workload gradually increased over 6MWT in iLLAs. However, intensity of activity based on cadence and speed decreased, which could be due to physiological or pain-related fatigue.
Acknowledgments
Authors would like to appreciate Jean Leblond, PhD, a statistician at CIRRIS, for his valuable assistance, and the physiotherapy amputation team at the IRDPQ center (Isabelle Paradis, Catherine Verret, Marthe Dubé). We would also like to thank all participants for their time.
Footnotes
Ethics Approval: All the participants signed the informed consent form approved by the ethical committee of IRDPQ (2016-489).
Ethical approval for this study was obtained from Quebec Physical Impairment Rehabilitation Institute (2016-489).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. ICMJE forms for all authors are available online.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this research was provided by Fonds de Recherche Québec-Santé (grant number 32640).
ORCID iDs: Hananeh Younesian, PhD,  https://orcid.org/0000-0002-7695-2417
https://orcid.org/0000-0002-7695-2417
Thomas Legrand, PhD,  https://orcid.org/0000-0001-9995-3976
https://orcid.org/0000-0001-9995-3976
References
- 1. Aaronson LS, Teel CS, Cassmeyer V, et al. Defining and measuring fatigue. Image. 1999;31(1):45–50. [DOI] [PubMed] [Google Scholar]
- 2. Barofsky I, Legro MW. Definition and measurement of fatigue. Rev Infect Dis. 1991;13:S94–S97. [DOI] [PubMed] [Google Scholar]
- 3. Beausoleil S, Miramand L, Turcot K. Evolution of gait parameters in individuals with a lower-limb amputation during a six-minute walk test. Gait Posture. 2019;72:40–45. [DOI] [PubMed] [Google Scholar]
- 4. Boutoille D, Féraille A, Maulaz D, Krempf M. Quality of life with diabetes-associated foot complications: comparison between lower-limb amputation and chronic foot ulceration. Foot Ankle Int. 2008;29(11):1074–1078. [DOI] [PubMed] [Google Scholar]
- 5. Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–332. [DOI] [PubMed] [Google Scholar]
- 6. Cortes N, Onate J, Morrison S. Differential effects of fatigue on movement variability. Gait Posture. 2014;39(3):888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Della Croce U, Cereatti A, Mancini M. Gait parameters estimated using inertial measurement units. In: Handbook of Human Motion. Springer International Publishing; 2018:245–265. [Google Scholar]
- 8. Devan H, Carman AB, Hendrick PA, Ribeiro DC, Hale LA. Perceptions of low back pain in people with lower limb amputation: a focus group study. Physiotherapy. 2015;101 (Suppl 1):e314. [DOI] [PubMed] [Google Scholar]
- 9. Enoka RM, Stuart DG. Neurobiology of muscle fatigue. J Appl Physiol. 1992;72(5):1631–1648. [DOI] [PubMed] [Google Scholar]
- 10. Esquenazi A, DiGiacomo R. Rehabilitation after amputation. J Am Podiatr Med Assoc. 2001;91(1):13–22. [DOI] [PubMed] [Google Scholar]
- 11. Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign.” J Geriatr Phys Ther. 2009;32(2):2–5. [PubMed] [Google Scholar]
- 12. Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–1789. [DOI] [PubMed] [Google Scholar]
- 13. Horak F, King L, Mancini M. Role of body-worn movement monitor technology for balance and gait rehabilitation. Phys Ther. 2015;95(3):461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imam B, Miller WC, Finlayson HC, Eng JJ, Jarus T. Incidence of lower limb amputation in Canada. Can J Public Health. 2017;108(4):374–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iosa M, Picerno P, Paolucci S, Morone G. Wearable inertial sensors for human movement analysis. Expert Rev Med Devices. 2016;13(7):641–659. [DOI] [PubMed] [Google Scholar]
- 16. Karvonen J, Vuorimaa T. Heart rate and exercise intensity during sports activities. Sports Med. 1988;5(5):303–311. [DOI] [PubMed] [Google Scholar]
- 17. Lantelme P, Milon H, Gharib C, Gayet C, Fortrat JO. White coat effect and reactivity to stress: cardiovascular and autonomic nervous system responses. Hypertension. 1998;31(4):1021–1029. [DOI] [PubMed] [Google Scholar]
- 18. Laukkanen RM, Virtanen PK. Heart rate monitors: state of the art. J Sports Sci. 1998;16(Suppl 1):3–7. [DOI] [PubMed] [Google Scholar]
- 19. Lin SJ, Bose NH. Six-minute walk test in persons with transtibial amputation. Arch Phys Med Rehabil. 2008;89(12):2354–2359. [DOI] [PubMed] [Google Scholar]
- 20. Lin SJ, Winston KD, Mitchell J, et al. Physical activity, functional capacity, and step variability during walking in people with lower-limb amputation. Gait Posture. 2014;40(1):140–144. [DOI] [PubMed] [Google Scholar]
- 21. Major M, Twiste M, Kenney L, Howard D. The effects of prosthetic ankle stiffness on stability of gait in people with trans-tibial amputation. J Rehabil Res Dev. 2016;53(6):839–852. [DOI] [PubMed] [Google Scholar]
- 22. Mariani B, Rouhani H, Crevoisier X, Aminian K. Quantitative estimation of foot-flat and stance phase of gait using foot-worn inertial sensors. Gait Posture. 2013;37(2):229–234. [DOI] [PubMed] [Google Scholar]
- 23. Noguchi K, Gel YR, Brunner E, Konietschke F. nparLD: an R software package for the nonparametric analysis of longitudinal data in factorial experiments. J Stat Softw. 2012;50(12). [Google Scholar]
- 24. Perry J, Davids JR. Gait analysis: normal and pathological function. J Pediatr Orthop. 1992;12(6):815. [Google Scholar]
- 25. Raya MA, Gailey RS, Fiebert IM, Roach KE. Impairment variables predicting activity limitation in individuals with lower limb amputation. Prosthet Orthot Int. 2010;34(1):73–84. [DOI] [PubMed] [Google Scholar]
- 26. Reid L, Thomson P, Besemann M, Dudek N. Going places: does the two-minute walk test predict the six-minute walk test in lower extremity amputees? J Rehabil Med. 2015;47(3):256–261. [DOI] [PubMed] [Google Scholar]
- 27. Robinett CS, Vondran MA. Functional ambulation velocity and distance requirements in rural and urban communities: a clinical report. Phys Ther. 1988;68(9):1371–1373. [DOI] [PubMed] [Google Scholar]
- 28. Scherr J, Wolfarth B, Christle JW, Pressler A, Wagenpfeil S, Halle M. Associations between Borg’s rating of perceived exertion and physiological measures of exercise intensity. Eur J Appl Physiol. 2013;113(1):147–155. [DOI] [PubMed] [Google Scholar]
- 29. Shields C, Thorp H, Hendry G, Jayakaran P. Health-related quality of life in persons with dysvascular and traumatic lower limb amputation—a systematic review. Physiotherapy. 2015;101: e673. [Google Scholar]
- 30. Taelman J, Vandeput S, Spaepen A, Van Huffel S. Influence of mental stress on heart rate and heart rate variability. In: Vander Sloten J, Verdonck P, Nyssen M, Haueisen J, eds. European Conference of the International Federation for Medical and Biological Engineering: ECIFMBE 2008 23–27 November 2008 Antwerp, Belgium. Springer; 2009:1366–1369. [Google Scholar]
- 31. Tudor-Locke C, Craig CL, Brown WJ, et al. How many steps/day are enough? For adults. Int J Behav Nutr Phys Act. 2011;8(1):79.21798015 [Google Scholar]
- 32. Vargha A, Delaney HD. A critique and improvement of the CL common language effect size statistics of McGraw and Wong. J Educ Behav Stat. 2000;25(2):101–132. [Google Scholar]
- 33. Wilhoite S, Williams S, Cook J, Ryan G. Rehabilitation, guidelines, and exercise prescription for lower limb amputees. Strength Cond J. 2020;42(2):95–102. [Google Scholar]
- 34. Wong DWC, Lam WK, Yeung L, Lee WC. Does long-distance walking improve or deteriorate walking stability of transtibial amputees? Clin Biomech. 2015;30(8):867–873. [DOI] [PubMed] [Google Scholar]