Graphical abstract

Keywords: Coronavirus, siRNA, Inhibitors, Plant Secondary Metabolites
Abstract
The current pneumonia outbreak, which began in early December 2019 near Wuhan City, Hubei Province, China, is caused by a novel corona virus (CoV) known as ‘2019-nCoV’ or ‘2019 novel corona virus or COVID-19′ by the World Health Organization (WHO). Vaccines are available to prevent corona virus contagious infection or to reduce the viral load in body but virus is continuously mutating itself to infect people at severity. In this critical scenario this review provide a compiled study for techniques and tools that can be used to treat corona virus infections and its variants by some modern techniques and natural products such as inhibitors, siRNA technique and plant based approaches. This review focuses on healthy treatment and strategies that can be used effectively to treat the disease globally by reducing the post COVID symptoms.
1. Introduction
The search of viruses that cause upper respiratory infections in humans was in the mid 1960s. The laboratory of Davil Tyrell, Dorothy Hamre and Rober Chanock isolated viruses from humans that could replicate on fragments of tracheal tissues or in human cells in culture. When examined by electron microscopy the enveloped virion appeared to be surrounded by a crown or corona of studded projections and was therefore named corona [1].
The field of Coronavirus research took an abrupt turn when in early 2003, an epidemic of severe and often fatal pneumonia broke out in southeast China, Hong Kong and Vietnam and subsequently spread to Toronto and Canada. Since SARS CoV had not been observed in humans before the 2002–2003 pandemic the virus causing this disease was studied and its genome organization and sequence was very similar to bat species of a diverse group of coronavirus.
World Health Organization recently declared outbreak of pneumonia that began at the beginning of December 2019 in Wuhan City, caused by Coronavirus. COVID-19 has reached to more than 150 nations, including China and has caused WHO to call the disease a worldwide pandemic [4]. India being second largest populated country is worst affected and continues to report a steep increase of new cases. Till April 2021 total number of 15,930,965 and 184,675 deaths were recorded. After reaching almost zero infection phase in January 2021 covid-19 virulent strain again started its death game from February 2021 by manipulating some of its membrane proteins. The analytical data regarding COVID-19 cases and death in India upto march 2021 were listed in Table 1, Fig. 1, Fig. 2 .
Table 1.
Analytical data of covid cases upto March 2021 in India according to WHO official website.
| State | Total Cases Confirmed | Death |
|---|---|---|
| Andaman And Niobar | 5466 | 64 |
| Andhra Pradesh | 976,987 | 7472 |
| Arunachal Pradesh | 17,113 | 56 |
| Assam | 227,473 | 1145 |
| Bihar | 342,059 | 1841 |
| Chandigarh | 35,148 | 421 |
| Chhattisgarh | 574,299 | 6274 |
| Dadra and Nagar Haveli and Daman and Diu | 5422 | 4 |
| Delhi | 905,541 | 12,638 |
| Goa | 69,312 | 926 |
| Gujarat | 428,178 | 5615 |
| Haryana | 371,624 | 3483 |
| Himachal Pradesh | 79,410 | 1219 |
| Jammu and Kashmir | 150,238 | 2071 |
| Jharkhand | 172,315 | 1547 |
| Karnataka | 1,198,644 | 13,646 |
| Kerala | 1,272,645 | 4978 |
| Ladakh | 12,556 | 134 |
| Lakshadweep | 1335 | 1 |
| Maharashtra | 3,960,359 | 6134 |
| Manipur | 29,869 | 378 |
| Meghalaya | 15,116 | 154 |
| Mizoram | 5085 | 12 |
| Madhya Pradesh | 433,704 | 4713 |
| Nagaland | 12,650 | 94 |
| Odisha | 377,464 | 1953 |
| Puducherry | 48,974 | 717 |
| Punjab | 309,316 | 8045 |
| Rajasthan | 438,785 | 3268 |
| Sikkim | 6796 | 136 |
| Tamil Nadu | 1,013,378 | 13,205 |
| Telengana | 367,901 | 1876 |
| Tripura | 34,186 | 394 |
| Uttar Pradesh | 909,405 | 10,159 |
| Uttarakhand | 129,205 | 1919 |
| West Bengal | 678,172 | 10,652 |
Fig. 1.
Graph showing death cases where maximum cases are in Karnataka and least in Sikkim (Scale of axis in hundred).
Fig. 2.
Graph showing total positive cases of COVID till March 2021 as per WHO official website.
 SARS-CoV-2 genome analysis revealed that around 80% of its genome is similar to other SARS-CoV strains and 96% similar to Bat Coronavirus strain [2]. Rational Drug designing [3] and Repurposing of drugs [4] are the two strategies that are being used in discovering the drugs against target or by identifying new uses of known and existing drugs [2], [5].
SARS-CoV-2 genome analysis revealed that around 80% of its genome is similar to other SARS-CoV strains and 96% similar to Bat Coronavirus strain [2]. Rational Drug designing [3] and Repurposing of drugs [4] are the two strategies that are being used in discovering the drugs against target or by identifying new uses of known and existing drugs [2], [5].
1.1. Coronavirus Genome
Coronavirus is spherical shaped completely enveloped particle studied with clubbed spikes of diameter 120–160 nm with coiled helical nucleocapsid shell having linear single strand RNA positive stand of 27–32 kb size whose 5′-terminal is capped with 3′poly (A) tail.
Six to nine genes code for more than 20 proteins, the replicase genes are directly translated from genomic RNA. Frame shift joins two big open reading frames, and these genes are translated as polyproteins, which are then cleaved into 14–16 separate proteins. Other genes are translated from multiple 3′- co terminal, sub genomic mRNAs which form three to four envelop proteins (HE,S,E,M), one nucleocapsid protein (N) and four to six non-structural or accessory proteins.
There is diversity of hosts for coronavirus. Humans are infected by Human Coronavirus 229E,OC43, NL63,HKU-1; SARS coronvirus. Coronavirus causes up to 30% of common colds in humans, as well as SARS (Severe Acute Respiratory Syndrome) and pneumonia in humans, with a 10% death rate. Direct touch, aerosol and faecal oral transfer, and contact with contaminated surfaces are all the ways the virus transmits between hosts.
Interference RNA (RNAi) is a natural antiviral defense system in plants, fungi, and invertebrates that is mediated by short interfering RNAs produced from viral genomes or replicative intermediates. The ability of siRNA to fight viruses was initially established against respiratory syncitial virus (RSV). RNAi-based drugs tend to be a promising treatment option for serious viral infections for which there are currently no successful vaccines, such as Ebola or emerging viruses.
2. Infection mechanism and inhibitors for coronavirus
COVID19 has spikes like glycoprotein arranged in a form of crown thus called “corona” [5] attached with the receptors of the host epithelial cells. Other than this various host proteases helps in the entry of virus. There are many ways to prevent the entry the virus into the host cells like by blocking the attachment of viral glycoprotein into the host cell receptors, blocking viral replication or by destroying the viral machinery. Many antiviral drugs are being in use to effectively block one of the ways of viral infection [6], [7]. Docking and simulation studies revealed many drugs that can be used against this contagious disease. Studies showed some drugs have better binding and specificity against the targets of SARS- CoV-2 virus. In this review article, focus has been made on the strategies and finding inhibitors against COVID19 targets.
The Spike (S) protein of SARS-CoV-2 entered in host cells through the receptor binding domain (RBD) of Angiotensin Converting Enzyme (ACE2) which specifically expressed in mucosal epithelial cells [8], [9]. ACE2 receptors number increased in patients having diabetes or hypertension and by the administrationof drugs like captopril or Losartan which are ACE2 blockers [10]. This further leads to the increase in risk of infection rate of COVID19. The cryo-EM and crystal structures of the ACE2 complex SARS-CoV-2 RBD have recently been solved and provide major structural guidance for the inhibitor design [9]. The interaction of SARS-CoV-2 and ACE2 is necessary for virus entry and this can be used strategically to block interaction and entry [11]. Various protein analogues, monoclonal antibodies and small molecules are identified that can inhibit ACE2. Small molecules or drugs act better than protein analogues or peptides. Catalytic site of ACE2 is docked with around 14,000 compounds through virtual screening which ends in identification of NAAE (N-(2-amino-ehtyl)-1aziridine-ethanamine). It shows a dose dependent inhibition of catalytic activity of ACE2 [12].Chloroquine which is an antimalarial drug also shown to block interaction of SARS-CoV-2 and ACE2 [13]. It interfere the mechanism with the terminal glycosylation of ACE2 and also promotes increment in pH for fusion of host cells with virus [14].
A study conducted in early 2020s showed that a human neutralising antibody specific to SARS-CoV named CR3022 has an ability to bind to the SARS-CoV-2 S protein and block its binding to ACE2 receptor [15]. Furthermore, inhibition of other targets involved in ACE2 receptor mediated endocytosis also used as strategy. Like, a well-known positive regulator of receptor endocytosis is AP2-associated protein kinase 1 (AAK1). A baricitinib (AAK1) inhibitor is known to reduce the ability of COVID virus entry by inhibiting endocytosis mediated by the ACE2 receptor [16]. Intense computational approaches are in use to find lead compounds that can specifically target the virus.
Although currently after the approval of ICMR many existing antiviral and other drugs are in use to treat COVID19 patients.
-
•
Remdesivir is most popularly used broad spectrum antiviral drug which targets viral proteins and prevent copying, replication as well viral development. They are similar to adenosine and causes irreversible chain termination [17], [18]. It transforms into active triphosphate and competes for the ATP needed for replication and thus to terminate the RNA synthesis process [19].
-
•
Lopinavir and Ritonavir drugs belongs to protease inhibitor class and are undergoing for COVID19 clinical trials. They affect secondary metabolism by self-metabolizing and thus effective against COVID19 [20].
-
•
Kevzara is a monoclonal antibody which is FDA approved for rheumatoid arthritisand work against Interleukin-6 receptors. It inhibits inflammatory response in the lungs of severely infected COVID19 patients [21].
-
•
Favipiravir (T-705; 6-fluoro-3 hydroxy-2-pyrazine carboxamide) is an antiviral drug that inhibits the RNA dependent RNA polymerase enzyme of RNA viruses. In China, patients with COVID-19 were recovered by using a mixture of drugs like anti-viral drugs (for SARS, Ebola and AIDS), Remdisivir (Ebola), Flavipiravir (Japan-Avigan/Ebola-developed) and a Lopinaviras well Ritonavir (anti-AIDS) combinationChloroquine and Azithromycin (antibiotics) along with anti malarial and (anti-TB) pyrimidine. Out of these, the most powerful combination of Flavipiravir, along with chloroquine, Pyrimidine and Azithromycin combined with normal care [22].
-
•
Galidesivir is a drug that blocks RNA. It undergoes in clinical trials on COVID19 patients. It converted into active triphosphate form by kinases found in cells and thus introduced into the forming chains of RNA strand. It leads to chain termination [23].
-
•
Ruxolitinib is an inhibitor of JAK mediated pathway and is related with IL-6 signalling. Clinical trials were already carried out inpatient withCOVID-19. It is an immune modulator which reduces the pneumonia symptoms caused by deregulated immune response [24], [25].
-
•
Bemcentinib drug is in Phase II clinical trial and an AXL kinase inhibitor. It has antiviral activity and shown to be safe and well-tolerated in COVID19 patients [26].
-
•
Convalescent Plasma therapy was approved on August 23, 2020 by FDA. The antibodies generated in recovered patients from COVID19 are transferred to infected patients which have severe complications. This therapy works well and safe too.
-
•
Ivermectin is an anti-parasitic drug. An in-vitro laboratory study by scientists at Monash University in Melbourne, Australia showed that it is effective against COVID19 virus but it can only be approved after human trials.
-
•
Leronlimab is a CCR5 antagonist which prevents the 'cytokine storm' in somepatients of COVID-19 treated in hospital of New York [26].
-
•
REGN-COV2 is the combination of two monoclonal antibodies (REGN10933 and REGN10987) reduces the viral load as per study conducted on September 29, 2020 [27].
-
•
RLF-100 that inhibits pro-inflammatory cytokines is a vasoactive intestinal polypeptide (VIP) which is also known as aviptadil. On August 6, 2020, Relief Therapeutics announced on 6 august 2020 for granting permission to RLF-100 as an Investigational New Drug (IND) for inhalation by patients to prevent the respiratory failure [28] (Table 2 ).
Table 2.
Structure of inhibitors use to treat Covid infection in human body.
| S. No. | Compound name | Structure |
|---|---|---|
| Ramdisivir | 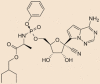 |
|
| Lopinavir | 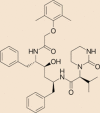 |
|
| Favipiravir | 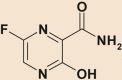 |
|
| Galidesivir | 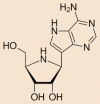 |
|
| Ruxolitinib |  |
|
| Ivermectin | 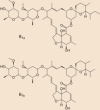 |
|
3. Production of siRNA for cornavirus inhibition
Although there are many approaches being developed to prepare antiviral siRNA. Automated solid phase synthesis is used to create ssRNA molecules, which contains 2′-hydroxyl protecting groups that provide ribonucleoside phosphoramitides. For synthesis of siRNA, M envelope gene would be most beneficial approach. After the synthesis step RNA duplexes are formed when cognate siRNAs hybridize.
SiRNA therapeutics can be administered locally or systematically via intravenous injection, depending on the target tissue. However siRNA which is unprotected are prone to frequent degradation by ubiquitous endo and exonucleases as they are undetectable in the blood after 10 min of administration. Because of phosphate backbone siRNA have strong anionic charge which is unable to diffuse negatively charged cellular receptors and induce IFN responses or other off target effects. The prepared siRNA can be delivered using viral and non viral systems, when siRNA binds to the positively charged or lipid molecule, it can approach the cell membrane close enough to be internalized via micropinocytosis or the clathrin dependent pathway. In the case of coronavirus infections, the human respiratory tract can be easily achieved by inhaling an aerosol, indicating a plausible route for antiviral administration (Fig. 3 ).
Fig. 3.
Showing Diagram of siRNA technique for inhibition of the viral RNA in host cells.
4. Potential plant based technology to treat corona
Years before the COVID-19 outbreak, a group of Queensland University of Technology researchers sequenced the DNA of a native tobacco plant (Nicotiana benthamiana). They used the plant's genome sequencing to build a vaccine for COVID-19. The plant is a suitable choice as a biofactory because it has 60,000 genes, which is twice as many as an ordinary plant, and it has ability to generate large quantities of high-quality vaccines and antibodies [29].
British American Tobacco company with its biotech subsidiary company in the US, Kentucky Bio Processing (KBP) is in its final stage of developing vaccine from plants for COVID-19 and is currently persuing its pre-clinical testing. Researchers at KBP developed a potential antigen by cloning a part of genetic sequence of SARS-CoV-2. Then this antigen was inserted into tobacco plants for its reproduction. The production time is much faster as the components of vaccine accumulate in six weeks than the several months in conventional methods [30].
A Canadian Biopharmaceutical Company Medicago after obtaining the SARs-CoV-2 genetic sequence have successfully developed a Virus Like Particle (VLP) of coronavirus in 20 days using proprietary plant-based technology [35].
Plant Secondary Metabolites (PSMs) used from ages can be a potential source of natural antiviral compounds could be effective, safer, and more cost effective as compared to orthodox drugs [31].
Alkaloids are one of the class of natural organic compounds which comprises of several groups based on their heterocyclic ring, such as imidazoles, quinolizidines, indoles, piperidines, pyrrolozidines, quinolizidines, isoquinolinepurines and tropanes [32].
Many alkaloids showed anti-SARS activity by the inhibition of protease enzyme,RNA synthesis and protein synthesis are emetine, Ipecac, Macetaxime, tylophorine and 7- methoxycryptopleurine [33].
Saponins (amphipathic glycosides) are another class of PSMs found in plants that have antiviral activities against Newcastle disease virus (NDV), Simian (SA-11) virus, Murine norovirus (MNV), and Feline calicivirus (FCV), RSV, VSV, HSV-1, HSv-2, HIV-1, Epstein–Barr virus (EBV), (SA-11) and human (HCR3) rotaviruses, Dengue virus and Influenza virus. ‘Plants produces different types of terpenes among which five carbon isoprene are the largest and most diversed group of PSMs. They are classified as monoterpenes, diterpenes, triterpenes, sesterterpenes, hemiterpenes and sesqueterpenes [34].
However many phytochemicals show significant antiviral potency against Coronavirus infection some are: Aescin isolated from Aesculus hippocastanum and reserpine isolated from various Rauwolfia species [37], bothe have significant anti CoV activity [38]. Ginsenoside-Rb1, one of the pharmacologically active components of Panax ginseng [43], was reported against SARS-CoV infection [36]. Cowmarin rich compounds are shown to have great potential of eliminating Coronavirus [38]. It has been noticed that lycorine extracted from Lycoris radiata have anti-SARS-CoV [39]. Latest study of repurposing of clinically approved drugs for treatment of COVID-19 showed that cepharanthine, a bisbenzylisoquinoline an type of alkaloid from tubers of Stephania japonica, showed a potent inhibition of a 2019-nCoV-related infection. Dihydrotanshinone is a major lipophilic compound isolated from Salviae Miltiorrhiza. A recent study showed that dihydrotanshinone exerted inhibitor effects against virus entery [40].
Saikosaponin B2, is isolated from Bupleuri Radix which inhibits the viral attachment and block viral penetration into cells. It also interferes in early stages of virus development such as replication, virus absorption and penetration [41].
All these natural compounds possess antiviral effects against the coronavirus. These compounds concentration ranges from micrograms to milligrams per mililiter or from nanomoles to micromoles per liter. These natural compounds are reported to invade various viral targets such as spike [S] glycoproteins (Surface proteins), coronavirus main proteinase-chymotrypsin-like protease (3CLpro), the papain-like proteases (PLpro), RNA-dependent RNA polymerases (RdRp), and nucleocapsid (N) proteins [42] (Fig. 4 ).
Fig. 4.
Showing structure of various different natural products proved effective against Coronavirus infection.
5. Conclusion
40% of world’s entire population is severely affected by coronavirus. There are many other drugs which are currently being used in the treatment of COVID19 which effect body in one or more other ways. The side effects caused due to these drugs adds to post Csovid symptoms which are likely due to low immune responses like sore throats, weakness, joint pain and most recently mucormycosis which is creating troubles in India.
In recent years, CoVs have been linked to a number of human infectious disease epidemics, notably SARS in 2002–2003 and MERS in 2012. Human coronaviruses HKU1, OC43, NL63, and 229E are also linked to the disease. when dealing with respiratory illnesses the SARS-CoV-caused epidemic outbreak in the years 2002–2003, 8422 patients were impacted in 29 countries around the world. The MERS-CoV is a virus that has been circulating in the United States. SARS-CoV-2, the virus that caused the COVID-19 outbreak, has a sequence that is distinct from the six other coronavirus subtypes and can be classed as a beta-coronavirus. The number of COVID-19 instances is fast increasing, with over 200 countries affected by early April 2020. COVID-19 patients typically had a significant reduction in lymphocyte counts as well as new pulmonary infiltrates on chest radiography. Furthermore, a CT scan revealed that 97 percent of COVID-19 patients verified by RT-PCR test developed pneumonia. The symptoms of COVID-19, on the other hand, did not improve after three days of antibiotic treatment.
There is a great challenge ahead in drug discovery as it needs patience, intense research, time and money. To look forward for better treatments siRNA and plant based technologies would prove better and reliable. Nowadays nano molecules are also under investigation for the treatment of viral infection. This provides a new line to think and work. Keeping current scenario in picture, need of hour is multi targeted drug using intense informatic tools. Rational Drug designing can also show promising result in this regard. A biotechnological approach is a rapid and eco-friendly way which offers a desired amount of secondary metabolites against SARS-CoV-2. In addition to this plant metabolomics an emerging technology are now used as a effective tool for discovering novel drugs from potential plant resources. Finally, for novel drug discovery, advanced and fast acting extraction, purification, and characterization techniques for plant metabolites, as well as multidisciplinary expertise and funding are very essential.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Siyu Xiu, Alexej Dick, Han Ju, Sako Mirzaie, Fatemeh Abdi, Simon Cocklin, Peng Zhan, and Xinyong Liu, Inhibitors of SARS-CoV‑2 Entry: Current and Future Opportunities, 10.1021/acs.jmedchem.0c00502. [DOI] [PMC free article] [PubMed]
- 2.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., Chen H.-D., Chen J., Luo Y., Guo H., Jiang R.-D., Liu M.-Q., Chen Y., Shen X.-R., Wang X.i., Zheng X.-S., Zhao K., Chen Q.-J., Deng F., Liu L.-L., Yan B., Zhan F.-X., Wang Y.-Y., Xiao G.-F., Shi Z.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jawaid Akhtar, COVID19 inhibitors: A prospective therapeutics, 10.1016/j.bioorg.2020.104027. [DOI] [PMC free article] [PubMed]
- 4.Fong Simon James, Dey Nilanjan, Chaki Jyotismita. An Introduction to COVID 19, Nature Public Health Emergency Collection PMCID: PMC7307707. Jumn. 2020;23:1–22. doi: 10.1007/978-981-15-5936-1. [DOI] [Google Scholar]
- 5.Thakur Uttam Singh, Subhashree Parida, Madhu Cholenahalli Lingaraju, Manickam Kesava, Dinesh Kumar, Raj Kumar Singh, Drug repurposing approach to fight COVID 19, 10.1007/2Fs43440-020-00155-6. [DOI] [PMC free article] [PubMed]
- 6.The Viral Life Cycle, Lumen Microbiology, https://courses.lumenlearning.com/microbiology/chapter/the-viral-life-cycle/.
- 7.The cycle of Infection, Encyclopaedia Britannica, https://www.Britannica.com/science/virus/The-cycle-of-infection.
- 8.Viral Infection, Teach me physiology, https://teachmephysiology.com/immune-system/immune-responses/viral-infection/.
- 9.Sommerstein R., Kochen M.M., Messerli F.H., Gräni C. Coronavirus disease 2019(COVID-19): Do angiotensin-converting enzyme inhibitors/angiotensin receptor blockers have a biphasic effect? J. Am. Heart. Assoc. 2020;9 doi: 10.1161/JAHA.120.016509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetesmellitus at increased risk for COVID-19 infection? Lancet. Resp. Med. 2020;8(4):e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan R., Zhang Y., Li Y., Xia L., Guo Y., Zhou Q. Structuralbasis for the recognition of the SARS-CoV-2 by full-length humanACE2. Science. 2020;367:1444–1448. doi: 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huentelman M.J., Zubcevic J., Hernández Prada J.A., Xiao X., Dimitrov D.S., Raizada M.K., Ostrov D.A. Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 2004;44(6):903–906. doi: 10.1161/01.HYP.0000146120.29648.36. [DOI] [PubMed] [Google Scholar]
- 14.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in Vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian X., Li C., Huang A., Xia S., Lu S., Shi Z., et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 2020;9:382–385. doi: 10.1080/22221751.2020.1729069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Rawling M., Savory E., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395(10223):e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coronavirus treatments: what drugs might work against COVID-19? April 16, 2020. https://theconversation.com/coronavirus-treatments-what-drugs-might-workagainst-covid-19-135352.
- 19.Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. Mechanism of inhibition of ebolavirus RNA-dependent RNA polymerase by remdesivir. Viruses. 2019;11:326. doi: 10.3390/v11040326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9:100128. doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao M.D.B., Wang M.D.Y., Wen M.D.D., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Eng. J. 2020 doi: 10.1056/NEJMoa2001282. https://publons.com/publon/30788006/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanofi doses first patient in global Kevzara COVID-19 trial, Published March 30th 2020. https://www.pmlive.com/pharma_news/sanofi_doses_first_patient_in_global_ kevzara_covid-19_trial_1331903.
- 23.A. Ghosh, U.S. stocks inched up Thu on coordinated global stimulus and hopes of animminent Trump 'magic drug' (Chloroquine) to treat COVID-19; Dow slips early Frion increasing U.S./global lockdown, Published 20th March 2020. https://www.iforex.in/news/us-stocks-inched-thu-coordinated-global-stimulus-and-hopes-imminent-trump-magic-drug-chloroquine-treat-covid-19-dow-slips-early-fri-increasingusglobal-lockdown-67589.
- 24.A Study to Evaluate the Safety, Pharmacokinetics and Antiviral Effects of Galidesivir in Yellow Fever or COVID-19, https://clinicaltrials.gov/ct2/show/study/NCT03891420.
- 25.Treatment of SARS Caused by COVID-19 With Ruxolitinib, https://clinicaltrials.gov/ct2/show/NCT04334044.
- 26.Ruxolitinib in Covid-19 Patients with Defined Hyper inflammation (RuxCoFlam), https://clinicaltrials.gov/ct2/show/NCT04338958.
- 27.Investigational Treatments under FDA, https://www.drugs.com/condition/covid-19.html.
- 28.Hansen Johanna, Baum Alina, Pascal Kristen E., Russo Vincenzo, Giordano Stephanie, Wloga Elzbieta. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369(6506):1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jihad G. Youssef, Jonathan Javitt, Philip Lavin, Mukhtar Al-Saadi, Faisal Zahiruddin, Sarah Beshay, Mohammad Bitar, Joseph Kelley, Mohi Sayed, VIP in the Treatment of Critical COVID-19 With Respiratory Failure in Patients With Severe Comorbidity: A Prospective Externally-Controlled Trial (October 25, 2020). Available at SSRN: https://ssrn.com/abstract=3665228 or 10.2139/ssrn.3665228. [DOI]
- 30.Crop Biotech Update, Viable Vaccine Candidate for COVID-19 Developed Using Proprietary Plant-based Technology, 2020. http://www.isaaa.org/kc/cropbiotechupdate/article/default.asp?ID=18028.
- 31.Crop Biotech Update, Native Australian Plant Paves Way for Vaccine Development Against COVID-19, 2020. http://www.isaaa.org/kc/cropbiotechupdate/article/default.asp?ID=18054.
- 32.Koparde A.A., Doijad R.C., Magdum C.S. In: Pharmacognosy – Medicinal Plants. Doijad R.C, editor. IntechOpen; Rijeka: 2019. Natural products in drug discovery. p. 14. [DOI] [Google Scholar]
- 33.Thawabteh A., Juma S., Bader M., Karaman D., Scrano L., Bufo S., Karaman R. The biological activity of natural alkaloids against herbivores, cancerous cells and pathogens. Toxins. 2019;11(11):656. doi: 10.3390/toxins11110656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim D., Min J., Jang M., Lee J., Shin Y., Park C., Song J., Kim H., Kim S., Jin Y.-H., Kwon S. Natural bisbenzylisoquinolinealkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus oc43 infection of mrc-5 human lung cells. Biomolecules. 2019;9(11):696. doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.A. Hussein R., A. El-Anssary A. In: Herbal Medicine. F. Builders P., editor. IntechOpen; 2019. Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. [DOI] [Google Scholar]
- 36.Capell T., Twyman R.M., Armario N.V., Ma J.K.C., Schillberg S., Christou P. Potential applications of plant biotechnology against SARS-CoV- 2. Trends Plant Sci. 2020;25:635–643. doi: 10.1016/j.tplants.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C.-Y., Jan J.-T., Ma S.-H., Kuo C.-J., Juan H.-F., Cheng Y.-S.- E., Hsu H.-H., Huang H.-C., Wu D., Brik A., Liang F.-S., Liu R.-S., Fang J.-M., Chen S.-T., Liang P.-H., Wong C.-H. Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc. Natl. Acad. Sci. U. S. A. 2004;101(27):10012–10017. doi: 10.1073/pnas.0403596101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Q.-Y., Tian X.-Y., Fang W.-S. Bioactive coumarins from Boenninghausenia sessilicarpa. J. Asian Nat. Prod. Res. 2007;9(1):59–65. doi: 10.1080/10286020500382397. [DOI] [PubMed] [Google Scholar]
- 39.Li S.Y., Chen C., Zhang H.Q., Guo H.Y., Wang H., Wang L., et al. Identification of natural compounds with antiviral activities against SARS-associated coronavirus. Antiviral Res. 2005;67(1):18–23. doi: 10.1016/j.antiviral.2005.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J.Y., Kim Y.I., Park S.J., Kim I.K., Choi Y.K., Kim S.-H. Safe, high-throughput screening of natural compounds of MERS-CoV entry inhibitors using a pseudovirus expressing MERS-CoV spike protein. Int. J. Antimicrob. Agents. 2018;52(5):730–732. doi: 10.1016/j.ijantimicag.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng P.-W., Ng L.-T., Chiang L.-C., Lin C.-C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin. Exp. Pharmacol. Physiol. 2006;33(7):612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim D.E., Min J.S., Jang M.S., Lee J.Y., Shin Y.S., Song J.H., et al. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules. 2019;9:696. doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeong C.S., Hyun J.E., Kim Y.S., Lee E.-S. Ginsenoside Rb1: the anti-ulcer constituent from the head of Panax ginseng. Arch. Pharm. Res. 2003;26(11):906–911. doi: 10.1007/BF02980198. [DOI] [PubMed] [Google Scholar]






