Abstract
Objective:
A non-synonymous single nucleotide polymorphism (rs61742642; C to T, P386S) in the ligand-binding domain of human estrogen-related receptor beta (ESRRβ) showed possible association to noise-induced hearing loss (NIHL) in our previous study.
Design:
This study was conducted to examine the effect of the ESRRβ rs61742642 T variant on temporary threshold shift (TTS). TTS was induced by 10minutes of exposure to audiometric narrow-band noise centered at 2000Hz. Hearing thresholds and distortion product otoacoustic emissions input output function (DP IO) at 2000, 3000, and 4000Hz were measured before and after the noise exposure.
Study sample:
Nineteen participants with rs61742642 CT genotype and 40 participants with rs61742642CC genotype were recruited for the study.
Results:
Participants with the CT genotype acquired a significantly greater TTS without convincing evidence of greater DP IO temporary level shift (DPTLS) compared to participants with the CC genotype.
Conclusion:
The results indicated that the ESRRβ polymorphism is associated with TTS. Future studies were recommended to explore molecular pathways leading to increased susceptibility to NIHL.
Keywords: Noise-induced hearing loss (NIHL), Estrogen-Related Receptor Beta (ESRRβ), Temporary Threshold Shift (TTS), Otoacoustic Emissions (OAE), Single Nucleotide Polymorphism (SNP)
Introduction
Noise-induced hearing loss (NIHL) remains a hearing health concern despite national standards for hearing protection and public health awareness campaigns. NIHL is defined as a bilateral audiometric hearing loss at the frequency range between 3000 to 6000Hz with recovery at 8000Hz. NIHL slowly develops as a function of continuous or intermittent acoustic exposure and duration (Kirchner et al, 2012). Historically, NIHL was recognized as an occupational hazard among individuals exposed to loud noise in the work place. Current research suggests that NIHL is no longer limited to industrial workers exposed to loud noise but it is found in adolescents, young adults (Niskar et al, 2001), and college-aged musicians (Phillips et al, 2009).
NIHL is a ‘complex disorder,’ generally defined as multiple factorial disorder, as their causes are associated with multiple genes in combination with lifestyle and environmental factors (Kowalski et al, 2014). Complex disorders do not show a clear-cut pattern of inheritance and almost always show family clustering; this makes it challenging to determine a person’s risk of inheriting or passing on these disorders (Craig, 2008). Therefore, it is important to study gene-environment interactions in complex disorders to identify susceptible individuals well before they acquire the disease (Hunter, 2005). A major environmental factor associated with NIHL is acoustic overexposure. NIHL can be caused by a single traumatic impulse sound but it is more typically caused by repeated loud sound exposures. Acoustic overexposure can cause direct mechanical trauma and indirect metabolic distress in the cochlea which can damage cochlear hair cells, stereocilia bundle, supporting cells, afferent synaptic junctions, and the stria vascularis (Henderson et al, 2006).
Variants of several cochlear genes have been associated with susceptibility to NIHL in factory workers exposed to loud industrial noise. Genetic variants in metabolic enzymes like glutathione S-transferase mu 1 (GSTM1), glutathione S-transferase theta 1 (GSTT1) and heat shock protein (HSP70), which are important genes in redox regulation following traumatic events have been associated with NIHL (Sliwinska-Kowalska & Pawelczyk, 2013). Variants in ion transport proteins (KCNE1 and KCNQ4), structural proteins (MYO14 and PCDH15) and gap junction proteins (GJB1 and GJB2) have also been associated with NIHL susceptibility (Van Laer et al, 2006; Pawelczyk et al, 2009; Konings et al, 2009a,b; Kowalski et al, 2014). Some of these studies have not been replicated in independent populations (Sliwinska-Kowalska & Pawelczyk, 2013). This replication failure may be attributed to (1) the NIHL phenotype definition which cannot differentiate between age-related hearing loss and NIHL, and (2) the study population of older factory workers who may have other confounding variables.
Phillips et al (2015) studied genetic links to NIHL in young college-aged musicians to control for age-related confounding variables. They defined high frequency ‘audiometric notch’ as a bilateral 4000–6000Hz reduction in hearing sensitivity of at least 15dB relative to the best threshold from 1000–3000Hz, with a recovery of at least 5 dB at 8000Hz. This NIHL phenotype was utilized to differentiate between age-related high frequency hearing loss and NIHL. Results of this study indicated that a single nucleotide polymorphism (SNP, rs61742642) in the estrogen-related receptor beta (ESRRβ), rs61742642 (C→T), which changes an evolutionarily conserved proline residue in the ligand-binding domain of the nuclear receptor to a serine (P386S), showed a promising association with NIHL. The minor allele frequency of the ESRRβ SNP is 7.01% in the general population (NCBI) resulting in expected population frequencies of CC, CT, and TT genotypes to be 86.47%, 13.03%, and 0.49% respectively. Musicians carrying the minor allele as the CT heterozygote accounted for almost 26% of those with the bilateral audiometric notch phenotype (Phillips et al, 2015).
ESRRβ is a transcription factor and a member of the nuclear-hormone-receptor family (Nuclear Receptor Nomenclature Committee, 1999). It has two main functional domains: (1) a ligand binding domain which contains 12 α-helices organized in a three-layered sandwich structure to attach with a ligand, and (2) a DNA binding domain which enables the ESRRβ protein to attach to a DNA molecule to regulate protein transcription (Wurtz et al, 1996). ESRRβ is critical for development, proper trophoblastic cell proliferation, and differentiation of cells (Chen & Nathans, 2007). ESRRβ is important to develop and maintain endolymphatic potential by regulating transcription of the potassium ion transporter proteins such as KCNE1, KCNQ1, and ATP1B2 (Chen & Nathans, 2007). It is expressed in cochlear structures such as the spiral limbus, supporting cells, Reissner’s membrane, stria vascularis, spiral ligament, spiral ganglion cells, and in the cochlear nerve fibers; ESRRβ is not expressed in the sensory cells (Collin et al, 2008). Mutations in the ligand binding domain of ESRRβ cause autosomal recessive non-syndromic deafness in the affected individuals (Collin et al, 2008; Ben Saïd et al, 2011; Lee et al, 2011; Brozkova et al, 2012). The evidence listed above suggests that ESRRβ is important for inner ear functioning.
Musicians with the ESRRβ rs61742642 CT genotype showed a high prevalence of an audiometric notch phenotype compared to musicians carrying the CC genotype in our previous study (Phillips et al, 2015). Clinical validation can be a useful tool in the examination of potential genetic associations. Therefore, the current study examined the audiometric responses of individuals with the ESRRβ CT genotype to a brief noise exposure. This strategy can be useful to the prevention of NIHL by individualized genetic risk profiling. Although the permanent threshold shift model is considered adequate to study the clinical relevance of NIHL prevention methods, many researchers have used a temporary threshold shift (TTS) model in humans (Marshall & Heller, 1998; Kramer et al, 2006; Lin et al, 2010; Le Prell et al, 2011a,b; 2012). The TTS model facilitates more control over experimental variables and requires a shorter time to complete data collection contrary to the permanent threshold shift model. It is cost-effective and provides better control over participants’ safety (Le Prell et al, 2012). Additionally, all genetic association studies of NIHL with human participants have used a cross-sectional research design (Sliwinska-Kowalska & Pawelczyk, 2013). A definitive permanent threshold shift model would require a longitudinal study of participants working in a noisy environment and carrying previously associated potential causal variants to NIHL. A TTS model does not require exposures that may cause participants to acquire permanent threshold shift. Therefore, we utilized a TTS model to study the relevance of ESRRβ rs61742642 CT genotype to noise exposure.
Materials and Methods
Participants
All data collection procedures were approved by the University of North Carolina at Greensboro (UNCG) Institutional Review Board. A genetic database of 321 music students, comprised of a subset of the participants in our previous study containing data of 271 SNPs in 52 cochlear genes, was available for the current project (Phillips et al, 2015). The subset included 45 musicians with ESRRβ rs61742642 CT genotype and 276 musicians with the rs61742642CC genotype. Details of the genetic data collection procedures and processing can be found in Phillips et al (2015). Multiple invitation emails were sent to 321 musicians using the Qualtrics online survey software (Qualtrics.com) and interested individuals were recruited on a first-come first-served basis. This software was set to send automatic emails. It sent two emails per day to individuals with the CT genotype and one email per week to individuals with the CC genotype. This strategy was used to improve participation of individuals carrying the rare genotype (i.e. CT). The study was performed in a double blind manner to control for experimental bias where neither the tester nor the participants knew their genotype at the time of testing, as scheduling was not managed by the tester. Subsequently the anonymous audiometric data was entered into a database and merged with the genetic data. At this point analyses could be performed with the sorted genetic grouping.
A total of 59 subjects were recruited for the current study of which 19 students were heterozygous (CT genotype: eight females and 11 males) and 40 subjects were homozygous (CC genotype; 17 females and 23 males) for ESRRβ rs61742642. Musicians with the ESRRβ CC genotype served as a control group and musicians with the ESRRβ CT genotype were considered to be the experimental group. During the recruitment process, we found two biological brothers, one with the ESRRβ CT genotype and the other with the ESRRβ CC genotype in our database. Biological brothers share half of their genome; therefore, comparing their audiometric results was potentially useful to strengthen the possibility of a genetic association to NIHL. All participants were UNCG music majors with daily exposure that included individual practice and ensemble practice. Participants were asked not to expose themselves to music and other TTS-inducing loud sounds for at least 12hours before data collection. All participants completed an online questionnaire just before the data collection session. We limited our investigation to individuals with self-reported European ancestry to control for confounding epidemiological variables (Tang et al, 2005).
Prerequisite testing
A normal otoscopic exam and immittance audiometry (Maico MI 24) were required for inclusion in the study. Participants considered for the study showed tympanograms with a compliance value ranging from 0.33 to 1.75cc, an ear canal volume ranging from 0.8 to 1.8cc, and middle-ear pressure ranging from −50 daPa to 25 daPa in both ears. Acoustic middle-ear muscle reflex (MEMR) thresholds were measured in both ears at 500, 1000 and 2000Hz. Audiometric thresholds were obtained at 1000, 2000, 3000, 4000, 6000, 8000Hz and audiometric narrow-band noise centered at 2000Hz (NBN2000), using the modified Hughson-Westlake procedure in 5-dB steps (Interacoustics AC-40 with insert receivers). Participants with pure-tone hearing thresholds higher than 25 dB HL from 1000 to 8000Hz, abnormal immittance findings, chronic tinnitus, neurological or immunological disorders were excluded from the study. Participants with a NBN 2000Hz threshold greater than 10dB HL were excluded from the study to ensure high noise-dose accuracy across the sample.
Transient otoacoustic emissions (TEOAEs) testing
TEOAEs were measured immediately after the prerequisite testing (ILO 292 USB–II0. They were recorded only before noise exposure. The probe calibration test as recommended by Otodynamics Ltd was run before testing. TEOAEs were recorded using an ILO ‘fast screening’ protocol at 84±3dB peSPL to minimize testing time. We calculated signal-to-noise ratios (SNRs) to compare TEOAE responses between the ESRRβ groups. SNRs were used in multivariate analysis as a previous study reported that TEOAE test performance identifying normal versus impaired ears can be improved when SNRs were used in multivariate analysis instead of absolute TEOAE amplitudes (Hussain et al, 1998).
Study procedure: Pre- and post-noise exposure measures
All testing procedures were performed in a sound treated booth (ANSI S3.1–1999). The left ear was chosen to perform the experimental procedure as it was associated with a higher susceptibility to NIHL (Nageris et al, 2007). Participants were instructed to remain quiet during the entire testing session. Pure-tone thresholds were measured at 2000, 3000, and 4000Hz using the modified Hughson-Westlake procedure. Once a threshold was obtained, the pure-tone intensity was increased to 5 dB SL and a 2dB up / 1dB down method was adapted to obtain a more accurate hearing threshold. The order of testing was standardized for the study. Hearing thresholds at 3000Hz were measured first, followed by 4000Hz and 2000Hz. This testing order was followed three times to calculate the average hearing threshold at each frequency. Frequency-specific TTS was calculated by subtracting average pre-exposure hearing threshold values from their respective average post-exposure values at 2000, 3000, and 4000Hz. TTS was calculated by averaging frequency-specific TTS values.
The ILO 292 USB-II was used to record distortion product otoacoustic emissions (DPOAEs). The OAE probe calibration test as recommended by Otodynamics Ltd was run before testing. Real ear probe calibration was performed using the ILO probe-fit check paradigm before running each OAE measurement. Pre-exposure DPOAE input-output function (DP IO) was measured at 2000, 3000, 4000, and 6000Hz. To facilitate comparison with audiometric thresholds, post-exposure DP IO function was measured at 2000, 3000, and 4000Hz. DP IO was measured using f2/ f1=1.22; intensities of the primary tones (L1 and L2) were varied using L1=(0.40) L2+39 from 75 to 30dB SPL in 5-dB steps (Kummer et al, 1998). L2 was varied manually from 75 to 30dB in 5-dB steps (10 point resolution) until the amplitude of DPOAEs became stable. Only those DP responses above the noise floor were used in further calculations. The cumulative SNR of the DP responses above the noise floor were multiplied by 10 and reported in dB SPL2 (area2). The square root of area2 (i.e. area in dB SPL) was used in the statistical analysis to relate area2 to the linear measure of the audiometric TTS. It was reasoned that the larger area values indicate robust DPs and the smaller values indicate diminished DPs, thereby providing a measure of overall strength of the cochlear amplifier (Gates et al, 2002). The frequency-specific DPOAE temporary level shift (DPTLS) was calculated by subtracting the post-exposure area (in dB SPL) values from their respective pre-exposure values (in dB SPL) at 2000, 3000, and 4000Hz. DPTLS was calculated (in dB SPL) by averaging frequency-specific DPTLS values. DP area was used for DPTLS calculations because the DP area was found to be a more robust measure of the systematic change in the DP IO function compared to the DP threshold and DP IO slope (Gates et al, 2002).
Testing sequence
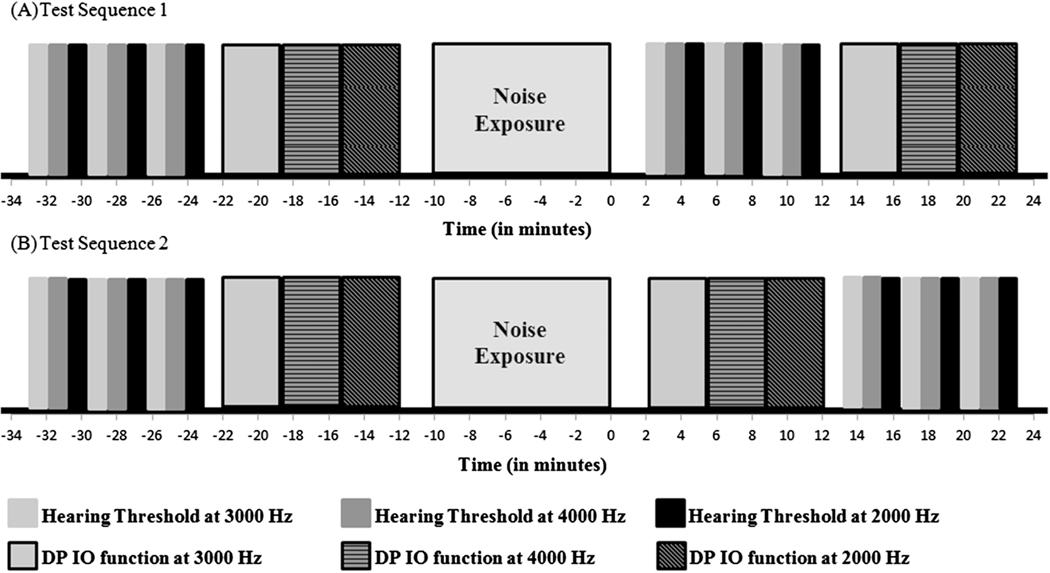
Pure-tone thresholds and DP IO functions were collected before and two minutes after noise exposure. Figure 1 shows the experimental paradigm and timeline for the study. Unknown to the volunteer participants and in the order they signed up for the data collection session, participants were assigned to the alternate experimental conditions of test sequence 1 or test sequence 2. In test sequence 1, the behavioral audiometry was performed two minutes after the noise exposure, followed by the DP IO test (Figure 1, A). This was reversed for test sequence 2 (Figure 1, B). Thirty individuals followed test sequence 1 (20 with CC genotype and 10 with CT genotype), and 29 individuals followed test sequence 2 (20 with CC genotype and 9 with CT genotype). This experimental paradigm was created to counterbalance the cochlear recovery effect on TTS and DPTLS.
Figure 1.
Experimental paradigm: (A) Test sequence 1: Post-exposure behavioral audiometry was followed by DP IO function test for 30 participants (NCC=20 and NCT=10), (B) Test sequence 2: Post-exposure DP IO function test was followed by behavioral audiometry for 29 participants (NCC=20, NCT=9).
Noise exposure
Audiometric narrowband noise centered at 2000Hz (NBN2000) was presented for 10minutes. The noise was presented at 90dB SL or 100dB SPL in the real ear, whichever value was less. A probe microphone (RE500, Audioscan) was inserted into the ear canal using the acoustic method described by Dirks et al (1996). After assuring that the probe microphone was near the tympanic membrane, a foam probe of the audiometric insert receiver was inserted into the ear canal. Audiometric threshold for the narrowband noise centered at 2000Hz was measured using a modified Hughson-Westlake procedure in 5-dB steps. This was followed by presentation of 90dB SL NBN2000. If the real ear SPL value exceeded 100dB SPL, then the noise was presented at the specific dial value equivalent to 100dB SPL in the real ear. The real ear SPL value was used in the statistical analysis to control for the noise exposure difference among participants.
Previous research indicates that the maximum amount of TTS is induced at the hearing frequency half octave above the center frequency of the noise (Ward et al, 1959), which suggests that NBN2000 should induce the maximum amount of TTS around 3000Hz. Our previous data indicated that almost 26% of musicians with the ESRRβ CT genotype showed audiometric notches, and that most of them showed elevated hearing thresholds around 4000 to 6000Hz (Phillips et al, 2015). Therefore, we utilized NBN2000 which is known to induce more TTS around 3000Hz and less TTS above 4000Hz and below 2000Hz. NBN2000 was chosen specifically to minimize the effects of pre-exposure threshold variation around 6000Hz on the TTS measurement.
Recent animal research found that the noise exposure causing 40–50dB TTS measured post 24hours caused permanent neural dysfunction (Kujawa & Liberman, 2009). In humans, Marshall and Heller (1998) showed that 10minutes of exposure to 105 dB SPL of noise centered at 1414Hz induced 10–15 dB TTS just following the exposure, but it resulted in virtually no TTS post 24hours (Marshall & Heller, 1998). Therefore, we chose noise exposure which was lower than the one utilized by Marshall & Heller (1998). Our exposure was significantly lower than the acoustic exposure used to induce TTS in a recent human study which reviewed current research and safety considerations while using a TTS model in humans (Le Prell et al, 2012; Kujawa & Liberman, 2009).
Questionnaire data
Appendices A and B provide descriptions of the Qualtrics survey and scoring procedure respectively. Music exposure profile, occupational noise exposure history, smoking history, gender, and eye color was derived from the survey. We reasoned that the effect of the ESRRβ variant may be masked by previously identified variables associated with NIHL. Therefore, the questionnaire data were collected to statistically control for the effects of these variables.
Statistical analysis
All statistical analyses were performed using the IBM SPSS version 20 statistics package. Inferential analyses of differences associated with the pre-exposure audiometric hearing thresholds were obtained using repeated measures ANOVA. In this model, the within subject factor was hearing thresholds at 1000, 2000, 3000, 4000, 6000, and 8000Hz, and the between subject factor was ESRRβ CC vs. CT genotypes. The MEMR thresholds, pre-exposure DP area and pre-exposure TEOAE one-third octave SNRs were analysed using repeated measures ANOVA where OAE amplitude at different frequencies served as a within subject factor and the ESRRβ genotypes served as the between subject factor.
A multiple linear regression model with a dependent variable, TTS, and seven predictors : ESRRβ polymorphism, music exposure profile, smoking history, gender, eye color, test sequence effect, and noise exposure level (in dB SPL), was used to evaluate the effects of the ESRRβ genotypes on TTS. We hypothesized that individuals with the CT genotype would exhibit greater TTS compared to individuals with the CC genotype. An unstandardized ESRRβ regression coefficient >2dB with a p value ≤0.05 was defined as statistical support for the hypothesis. Similarly, a multiple linear regression model with a dependent variable, DPTLS (in percentage), and seven predictors: ESRRβ polymorphism, music exposure profile, smoking history, gender, eye color, test sequence effect, and noise exposure level (in dB SPL), was used to evaluate the effects of the ESRRβ genotypes on DPTLS. A p value for unstandardized ESRRβ regression coefficient less than 0.05 was defined as statistical support for the difference between the ESRRβ groups.
Results
Overall results
Among the study sample, 89.8% showed no notch, 8.5% showed a unilateral notch and 1.7% showed bilateral notches (Appendix A). We found that 47.5%, 27.1%, 18.6%, 3.4%, and 1.7% of individuals reported woodwind, string, voice, percussion, and brass as their primary instrument respectively. We found no significant correlation between ESRRβ genotypes and music exposure [r(57)=0.066, p=0.62], suggesting that the study sample was not stratified for music exposure. Sixty-four percent of participants reported non-brown, and 36% reported brown eye color. Almost 6% reported that they smoked tobacco or tobacco-related products on a regular basis. None of the participants reported a history of occupational noise exposure. Therefore, this variable was not considered in statistical analyses.
Pre-exposure audiometric measurements
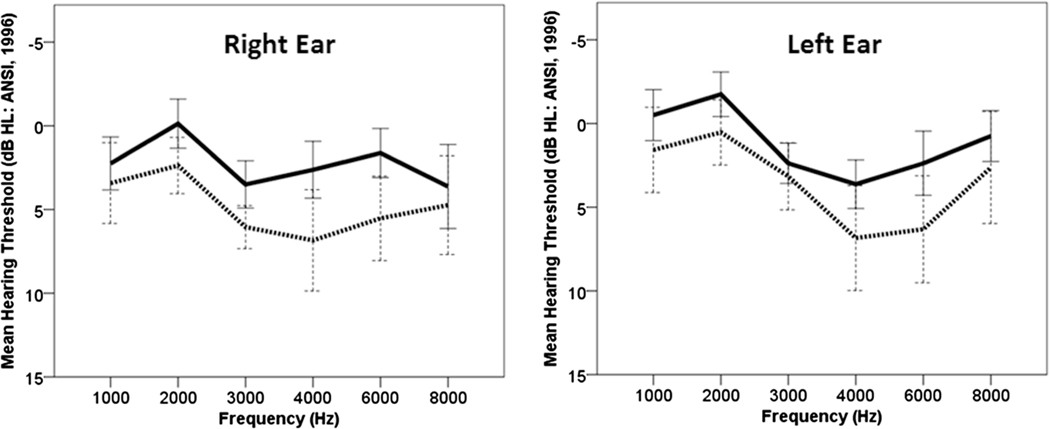
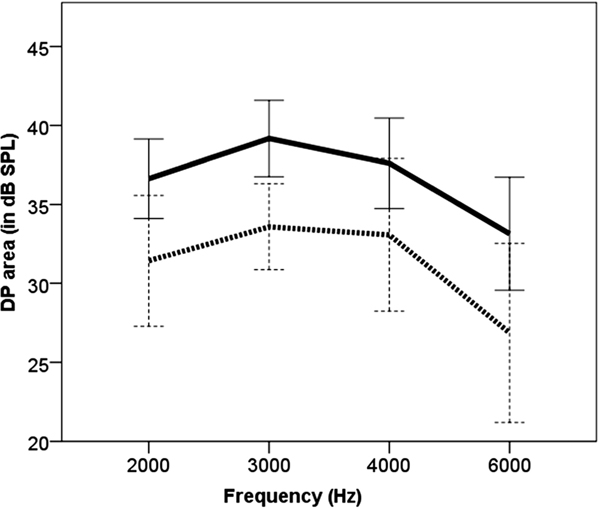
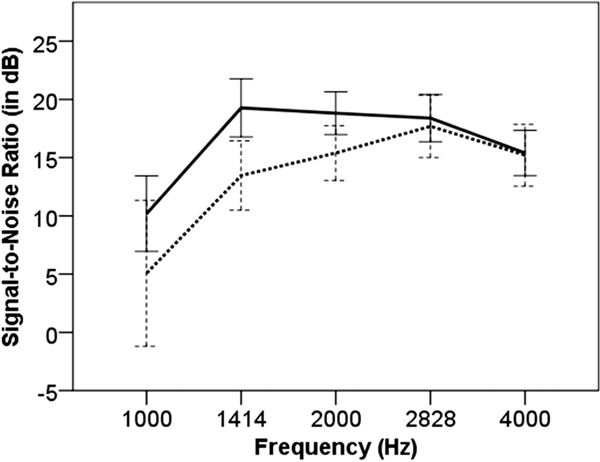
There was a significant main effect of the ESRRβ genotypes on pre-exposure audiometric thresholds in the right [F(1, 57)=8.078, p=0.006] and left ear [F(1, 57)=7.43, p=0.009]. The participants with the CT genotype showed elevated hearing thresholds from 3000 to 6000Hz in both ears (Figure 2). The main effect of the ESRRβ genotypes on MEMR thresholds was not significant. Pre-exposure DP areas (in dB SPL) were significant for the ESRRβ genotypes [F(1, 57)=9.135, p=0.004], as shown in Figure 3. A repeated measure ANOVA with five within-subject factors, TEOAE one-third octave SNRs at 1000, 1414, 2000, 2828, and 4000Hz bands, showed a significant main effect of ESRRβ genotypes [F(1, 57)=4.091, p=0.048, Figure 4). A repeated measure ANOVA revealed that TEOAE one-third octave noise levels were not significantly different between the ESRRβ groups [F(1, 57)=1.936, p=0.17].
Figure 2.
Pre-exposure audiometric thresholds: Mean thresholds at 1000–8000Hz for individuals carrying ESRRβ rs61742642CC variant (solid line) vs. CT variant (dashed line). Error bars indicate 95% confidence interval.
Figure 3.
Pre-exposure DP area (left ear): DP area at 2000–6000Hz for individuals carrying ESRRβ rs61742642CC (solid line) vs. CT genotype (dashed line). Error bars indicate 95% confidence interval.
Figure 4.
Pre-exposure TEOAE one-third octave SNR (left ear): SNR values at 10004000Hz for individuals carrying ESRRβ rs61742642CC (solid line) vs. CT genotype (dashed line). Error bars indicate 95% confidence interval.
Pre-exposure NBN2000 threshold was not significantly different between the ESRRβ groups [MD=−0.82, t(50.4)= −1.19, p=0.23]. The 90dB SL noise level exceeded 100dB SPL in 22 participants with the CC genotype and 15 participants with the CT genotype. Noise level was adjusted to achieve a 100dB SPL reading from the probe microphone. The average reduction levels for the participants with the CC and CT genotypes were 3.18 and 2.6dB respectively. Statistical analysis suggested that there was no statistical difference between noise exposure level between the ESRRβ groups [MCC=98.62, SDCC=2.31; MCT=99.21, SDCT=2.12; t(57)=0.931, p=0.35].
Post-exposure audiometric measures
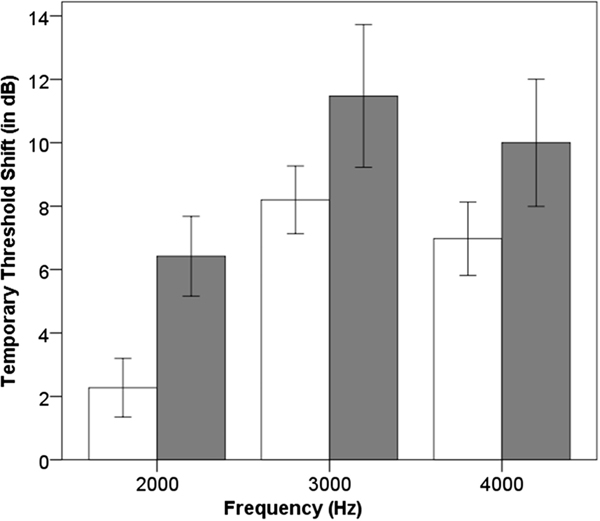
Multiple linear regression analyses were performed separately for TTS and DPTLS as dependent variables. The analyses were performed using an ‘enter’ method with seven predictors: ESRRβ genotypes, music exposure profile, gender, eye-color, history of tobacco smoking, test sequence effect, and noise exposure level (in dB SPL). ESRRβ genotypes with β=3.509, t(52)=5.002, p=0.000007; and the test sequence effect with β=−2.749, t(52)=−3.911, p=0.000273 predicted TTS. The effect of the ESRRβ genotypes on frequency-specific TTS is shown in Figure 5. The linear regression analysis signified that participants with the CT genotype acquired on an average 3.509dB more TTS compared to participants with the CC genotype. It further indicated that participants following test sequence 1 showed 2.749dB more TTS compared to participants following test sequence 2. The noise exposure level (in dB SPL) did not show a significant association with TTS (β=0.06, t(52)=0.441, p=0.661). This evidence indicated that noise exposure difference was not likely to contribute to the ESRRβ main effect on TTS. ESRRβ genotypes and the test sequence effect explained a significant proportion of variance in the TTS measure, with adjusted R2=0.394, F=6.391, p=0.000021. No other predictor showed a significant association with TTS. We used DPTLS in percentage to control for pre-exposure difference observed between the ESRRβ genotypes in DP area at 2000, 3000, and 4000Hz. The analysis showed that the test sequence effect was a significant predictor for DPTLS with β=7.438, t(52)=2.350, p=0.023. We found no other significant predictor for DPTLS. ESRRβ genotypes could not explain DPTLS, with β=−2.04%, t(52) β=−0.646, p=0.521.
Figure 5.
Post-exposure findings (left ear): Frequency-specific TTS values for individuals carrying ESRRβ rs61742642CC (blank bar) vs. CT genotype (gray bar). Error bars indicate 95% confidence interval.
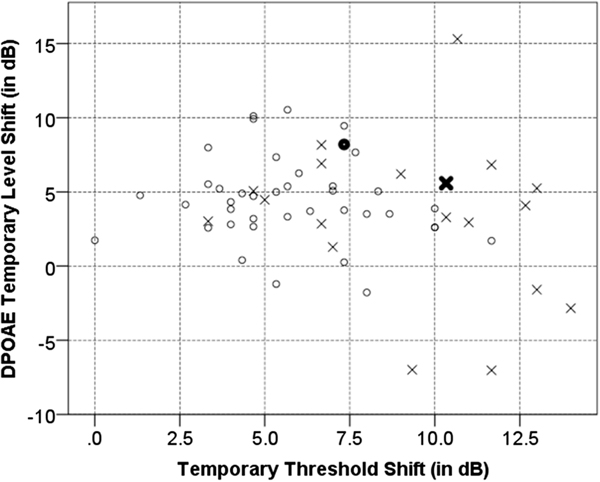
Of the two biological brothers in our sample, the one with the CT genotype had a history of professional music exposure for four years, and the brother with the CC genotype had a history of professional music exposure for more than 12 years. Both brothers showed 10dB HL or better hearing thresholds at all audiometric frequencies. The musician with the CT genotype showed a reduced average pre-exposure DP area (i.e. 26.74dB) compared to his brother with CC genotype (i.e. 32.92dB). The musician with the CT genotype showed higher TTS (i.e. 10.3dB) and lower DPTLS (i.e. 5.5 dB) compared to his brother who acquired 7.3dB TTS and 8.2dB DPTLS (Figure 6).
Figure 6.
Post-exposure findings (left ear): Individual values of TTS vs. DPTLS for musicians carrying ESRRβ rs61742642CC genotype (circle) vs. CT genotype (cross). Highlighted data points show results of the biological brothers. Musician brother with the ESRRβ CT genotype and four years of professional music exposure history acquired more TTS compared to his brother with ESRRβ CC genotype and 12 years of music exposure history.
Discussion
Results of the current study demonstrated that participants with the ERSSb CT genotype acquired significantly more TTS compared to the participants with the ESRRβ CC genotype following a brief noise exposure. The main effect of ESRRβ rs61742642 genotype was found in the test frequency region from 2000Hz to 4000Hz (Figure 5). This frequency region finding can be attributed to the use of NBN2000 to elicit TTS in this study (Ward et al, 1959). Our results may be valid for the entire hearing frequency range as there is no known report in the literature suggesting that the ESRRβ expression and functioning are different across the length of the cochlea. Previous research has shown that 48% of music students exceed the allowable sound level during daily practice sessions, and almost all of the music students are exposed to damaging sound levels on a regular basis (Phillips & Mace, 2008). Musicians with the CT genotype may acquire more TTS following their routine music exposure and/or recover slowly from TTS compared to musicians with the CC genotype, and subsequently may acquire audiometric notch phenotype.
Evidence of strial dysfunction
The major finding of this study is that individuals with the ESRRβ rs61742642 CT polymorphism showed significantly higher TTS without significantly different DPTLS compared to individuals with the ESRRβ rs61742642CC genotype. Greater decline of hearing thresholds compared to DPOAEs has been associated with strial dysfunction, and greater decline of DPOAEs compared to hearing threshold has been associated with hair cell dysfunction (Mills, 2001; Gates et al, 2002). The results of this study support the possibility that the individuals with the CT genotype experienced more strial dysfunction compared to individuals with the CC genotype. This finding is consistent with the role of ESRRβ protein in K+ion regulation in stria vascularis (Chen & Nathans, 2007), and with the absence of the ESRRβ protein in hair cells (Collin et al, 2008).
Little is known about the functioning of ESRRβ in the cochlea. Molecular pathways of ESRRβ activation and the cascade of physiological events following its ligand-dependent activation are largely unknown. Estrogen-related receptors, including ESRRβ, have been shown to regulate cellular redox states following traumatic events (Raghuram et al, 2007). Given that mutations in the ligand-binding domain of the ESRRβ protein lead to strial damage (Collin et al, 2008; Lee et al, 2011; Ben Saïd et al, 2011; Brozkova et al, 2012), it can be hypothesized that the rs61742642 T variant in the coding sequence of a ligand-binding domain of the ESRRβ gene, which changes an evolutionarily conserved proline residue to a serine (P386S), alters the conformation of the ligand-binding site resulting in an inefficient ESRRβ protein molecule. As a transcription factor that regulates other genes, the inefficient ESRRβ protein may be less responsive to a cellular redox state. This may result in inefficient regulation of KCNQ1, KCNE1, and ATP1B2 genes important in regulating K+ ion flow into the endolymph. Poorly regulated K+ ion influx into the endolymph following noise exposure may lead to a reduced endolymphatic potential, causing reduction in hearing sensitivity. As ESRRβ is expressed in the strial cells, but not in the hair cells (Collin et al, 2008), the immediate effect of the inefficient ESRRβ protein may not be evident on the cochlear hair cell physiology following brief noise exposure. Therefore, individuals with the inefficient ESRRβ protein would appear to experience greater reduction in audiometric thresholds than in DPOAEs compared to individuals with the efficient ESRRβ protein.
It is noteworthy that some individuals with the CT genotype did not show significantly higher TTS (Figure 6) compared to their counterparts. This observation illustrates the complex nature of multifactorial diseases where clinical manifestation of the disease depends upon multiple genes; which we have not included in this investigation. Additionally, lifestyle and environmental factors not included in these analyses might also influence the results.
Evidence of hair cell dysfunction
We found that individuals with the CT genotype showed poorer pre-exposure hearing thresholds in both ears (Figure 2). This finding is in accordance with our previous study where we observed that individuals carrying the CT genotype were more likely to acquire a bilateral audiometric notch phenotype compared to individuals with the CC genotype despite similar music exposure (Phillips et al, 2015). We found that individuals with the CT genotype exhibited poorer pre-exposure DPOAEs (Figure 3) and TEOAEs one-third octave SNRs (Figure 4) compared to individuals with CC genotype. Analysis of the data from two musician brothers supported the trend in audiological findings observed between the ESRRβ groups, suggesting that musicians with the CT genotype showed possible evidence of hair cell dysfunction.
The diminished OAE amplitudes and evaluated hearing thresholds may be attributed to the inefficient ESRRβ protein. This protein may inefficiently regulate K+ ion transporter genes, and responds poorly to the cellular redox state. Inefficient cellular redox regulation can lead to temporary hair cell dysfunction, or permanent hair cell loss (Henderson et al, 2006). Noise-induced hair cell dysfunction and hair cell loss can cause a reduction in the OAE amplitude (Emmerich et al, 2000). Outer hair cell dysfunction and loss can further affect physical input to the inner hair cells especially at low sound levels, resulting into elevated hearing thresholds (Mills, 2001; Gates et al, 2002).
Recovery from noise exposure can be lengthier if the inefficient ESRRβ protein is slower in responding to the cellular redox state. Our participants were music students, and almost all of them were exposed to high sound intensities on a regular basis (Phillips & Mace, 2008). We instructed our participants to avoid exposure to loud sounds for at least 12hours before the data collection session. However, we did not collect music exposure data 48hours before testing, which potentially influenced our results. Our inclusion criterion was a practical compromise, considering the daily music exposure needs of the students. It is possible that the individuals with the CT genotype might not have recovered completely from their previous exposure. This may explain elevated pre-exposure audiometric thresholds and diminished OAE amplitudes. Future research will utilize noise dosimetry to quantify music exposure to study the genetic association of NIHL.
Limitations of the study
This was a volunteer sample which limits the generalizability of the results as the sample may not be representative of the target population of college-age musicians. This study did not evaluate participants with the rs61742642 TT genotype as it is rare. The results of this study do not fully reflect the complex genetic and environmental factors which can lead to NIHL. The survey tool devised for this study to quantify environmental exposure will also require further validation by supplementation with direct noise dosimetry.
Conclusion and future research directions
The results of this study suggest that the ESRRβ rs61742642 CT polymorphism is associated with increased susceptibility to TTS, and validates the usefulness of the audiometric notch phenotype to explore potential genetic risk factors for NIHL. Additionally, it suggests that a non-invasive audiological test battery may be useful to identify potential sub-phenotypes of NIHL associated with strial vs. hair cell dysfunction. Use of an animal model may lead to a verification of the predominant site of cochlear dysfunction due to the rs61742642 variant delineating molecular pathways leading to NIHL.
Supplementary Material
Acknowledgements
This work is supported by the Theodore and Loretta Williams Graduate Research Award Fund for Arts Health (2012). We also thank the Music Research Institute and the Center for Biotechnology, Genomics, and Health Research for their support. We also acknowledge the assistance of David Kemp for OAE methodology development, Sandra Teglas and Donald Hodges with data collection, Jenna Callendar and Renuka Shivaji for technical assistance with the preparation and processing of DNA samples.
Abbreviations
- DPIO
Distortion product otoacoustic emissions input output
- ESRRβ
Estrogen-related receptor beta
- NIHL
Noise-induced hearing loss
- SNP
Single nucleotide polymorphism
- TTS
Temporary threshold shift
Footnotes
Declaration of interest: The authors declare no conflicts of interest.
References
- American National Standards Institute (ANSI). 1999. Maximum permissible ambient noise level for audiometric test rooms. ANSI, S3.1–1999. [Google Scholar]
- Ben Saïd M, Ayedi L, Mnejja M, Hakim B, Khalfallah A et al. 2011. A novel missense mutation in the ESRRΒ gene causes DFNB35 hearing loss in a Tunisian family. Eur J Med Genet, 54(6), 535–541. [DOI] [PubMed] [Google Scholar]
- Brozkova DS, Lastuvkova J, Machalova E, Lisonova J, Trkova M et al. 2012. DFNB35 due to a novel mutation in the ESRRΒ gene in a Czech consanguineous family. Int J Ped Otorhinolaryngol, 76, 1681–1684. [DOI] [PubMed] [Google Scholar]
- Chen J & Nathans J 2007. Estrogen-related receptor beta/NR3B2 controls epithelial cell fate and endolymph production by the Stria Vascularis. Develop Cell, 13(3), 325–337. [DOI] [PubMed] [Google Scholar]
- Collet L 1993. Use of otoacoustic emissions to explore the medial olivocochlear system in humans. Br J Audiol, 27(2), 155–159. [DOI] [PubMed] [Google Scholar]
- Collin RW, Kalay E, Tariq M, Peters T, van der Zwaag B et al. 2008. Mutations of ESRRΒ encoding estrogen-related receptor beta cause autosomal-recessive nonsyndromic hearing impairment DFNB35. Am J Hum Genet, 82(1), 125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J 2008. Complex diseases: Research and applications. Nat Edu, 1(1), 184. [Google Scholar]
- Dirks DD, Ahlstrom JB & Eisenberg LS 1996. Comparison of probe insertion methods on estimates of ear canal SPL. J Am Acad Audiol, 7(1), 31–38. [PubMed] [Google Scholar]
- Emmerich E, Richter R, Reinhold U, Linss V & Linss W 2000. Effects of industrial noise exposure on distortion product otoacoustic emissions (DPOAEs) and hair cell loss of the cochlea-long term experiments in awake guinea pigs. Hear Res, 148(1–2), 9–17. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills D, Nam BH, D’Agostino R & Rubel EW 2002. Effects of age on the distortion product otoacoustic emission growth functions. Hear Res, 163(1–2), 53–60. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC & Hu BH 2006. The role of oxidative stress in noise-induced hearing loss. Ear Hear, 27, 1–19. [DOI] [PubMed] [Google Scholar]
- Hoffman H, Ko C, Themann C, Dillon C & Franks J 2006. Reducing noise-induced hearing loss (NIHL) to achieve us healthy people 2010 goals. Am J Epidemiol, S122. [Google Scholar]
- Hunter DJ 2005. Gene-environment interactions in human diseases. Nat Rev Genet, 6, 287–298. [DOI] [PubMed] [Google Scholar]
- Hussain DM, Gorga MP, Neely ST, Keefe DH & Peters J 1998. Transient evoked otoacoustic emissions in patients with normal hearing and in patients with hearing loss. Ear Hear, 19(6), 434–449. [DOI] [PubMed] [Google Scholar]
- Kirchner DB, Evenson E, Dobie RA, Rabinowitz P, Crawford J et al. 2012. Occupational noise-induced hearing loss: ACOEM task force on occupational hearing loss. J Occup Environ Med, 54, 106–108. [DOI] [PubMed] [Google Scholar]
- Konings A, Van Laer L & Van Camp G 2009a. Genetic studies on noise-induced hearing loss: a review. Ear Hear, 30(2), 151–159. [DOI] [PubMed] [Google Scholar]
- Konings A, Van Laer L, Wiktorek-Smagur A, Rajkowska E, Pawelczyk M et al. 2009b. Candidate Gene Association Study for Noise-induced Hearing Loss in Two Independent Noise-exposed Populations. Ann Hum Genet, 73(2), 215–224. [DOI] [PubMed] [Google Scholar]
- Kowalski TJ, Pawelczyk M, Rajkowska E, Dudarewicz A & Sliwinska-Kowalska M 2014. Genetic variants of CDH23 associated with noise-induced hearing loss. Otol Neurotol, 35(2), 358–365. [DOI] [PubMed] [Google Scholar]
- Kramer S, Dreisbach L, Lockwood J, Baldwin K, Kopke R et al. 2006. Efficacy of the antioxidant N-acetylcysteine (NAC) in protecting ears exposed to loud music. J Am Acad Audiol, 17(4), 265–278. [DOI] [PubMed] [Google Scholar]
- Kujawa SG & Liberman MC 2009. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci, 29(45), 14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer P, Janssen T & Arnold W 1998. The level and growth behavior of the 2 f1-f2 distortion product otoacoustic emission and its relationship to auditory sensitivity in normal hearing and cochlear hearing loss. J Acoust Soc Am, 103(6), 3431–3444. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Dell S, Hensley B, Hall JW, Campbell KC et al. 2012. Digital music exposure reliably induces temporary threshold shift in normal-hearing human subjects. Ear Hearing, 33(6), e44–e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Johnson AC, Lindblad AC, Skjonsberg A, Ulfendahl M et al. 2011a. Increased vitamin plasma levels in Swedish military personnel treated with nutrients prior to automatic weapon training. Noise Health, 13(55), 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell CG, Yang Q & Harris JG 2011b. Modification of digital music files for use in human temporary threshold shift studies. J Acoust Soc Am, 130(4), EL142–EL146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Khan S, Ansar M, Santos-Cortez RL, Ahmad W et al. 2011. A novel ESRRΒ deletion is a rare cause of autosomal recessive nonsyndromic hearing impairment among Pakistani families. Genet Res Int, 2011, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Wu JL, Shih TS, Tsai PJ, Sun YM et al. 2010. N-Acetylcysteine against noise-induced temporary threshold shift in male workers. Hear Res, 269(1–2), 42–47. [DOI] [PubMed] [Google Scholar]
- Marshall L & Heller LM 1998. Transient-evoked otoacoustic emissions as a measure of noise-induced threshold shift. J Speech Lang Hear Res, 41(6), 1319–1334. [DOI] [PubMed] [Google Scholar]
- Mills DM 2001. Distortion product otoacoustic emissions and neural responses measure different things: Using the difference for differential diagnosis of cochlear dysfunction . Abstract. XVIIth Biennial Symposium of the International Evoked Response Audiometry Study Group [Google Scholar]
- Nageris BI, Raveh E, Zilberberg M & Attias J 2007. Asymmetry in noise-induced hearing loss: relevance of acoustic reflex and left or right handedness. Otol Neurotol, 28(4), 434–437. [DOI] [PubMed] [Google Scholar]
- Niskar AS, Kieszak SM, Holmes AE, Esteban E, Rubin C et al. 2001. Estimated prevalence of noise-induced hearing threshold shifts among children 6 to 19 years of age: The third national health and nutrition examination survey, 1988–1994, United States. Pediatrics, 108(1), 40–43. [DOI] [PubMed] [Google Scholar]
- Nuclear Receptors Nomenclature Committee. 1999. A unified nomenclature system for the nuclear receptor superfamily. Cell, 97(2), 161–163. [DOI] [PubMed] [Google Scholar]
- Pawelczyk M, Van Laer L, Fransen E, Rajkowska E, Konings A et al. 2009. Analysis of gene polymorphisms associated with K ion circulation in the inner ear of patients susceptible and resistant to noise-induced hearing loss. Ann Hum Genet, 73(Pt 4), 411–421. [DOI] [PubMed] [Google Scholar]
- Phillips SL & Mace S 2008. Sound level measurements in music practice rooms. Music Perform Res, 2(1), 36–47. [Google Scholar]
- Phillip SL, Henrich VC & Mace ST 2009. Prevalence of noise-induced hearing loss in student musicians. Int J Audiol, 49(4), 309–316. [DOI] [PubMed] [Google Scholar]
- Phillips SL, Richter SJ, Teglas SL, Bhatt IS, Morehouse RC et al. 2015. Feasibility of a bilateral 4000–6000Hz notch as a phenotype for genetic association analysis. Int J Audiol, 54(10), 645–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK et al. 2007. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol, 14(12), 1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan R 2000. Centrifugal pathways protect hearing sensitivity at the cochlea in noisy environments that exacerbate the damage induced by loud sound. J Neurosci, 20(17), 6684–6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwinska-Kowalska M & Pawelczyk M 2013. Contribution of genetic factors to noise-induced hearing loss: A human studies review. Mutat Res, 752(1), 61–65. [DOI] [PubMed] [Google Scholar]
- Tang H, Quertermous T, Rodriguez B, Kardia SL, Zhu X et al. 2005. Genetic structure, self-identified race/ethnicity, and confounding in case-control association studies. Am J Hum Genet, 76(2), 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laer L, Carlsson P, Ottschytsch N, Bondeson M, Konings A et al. 2006. The contribution of genes involved in potassium-recycling in the inner ear to noise-induced hearing loss. Hum Mutat, 27(8), 786–795. [DOI] [PubMed] [Google Scholar]
- Ward WD, Glorig A & Sklar DL 1959. Temporary threshold shift from octave-band noise: Applications to damage-risk criteria. J Occup Med, 1(7), 408. [Google Scholar]
- Wurtz JM, Bourguet W, Renaud JP, Vivat V, Chambon P et al. 1996. A canonical structure for the ligand-binding domain of nuclear receptors. Nat Struct Biol, 3(1), 87–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.