Abstract
Autoinflammatory diseases are monogenic and polygenic disorders due to dysregulation of the innate immune system. The inherited conditions have been clustered with primary immunodeficiencies in the latest practice parameters, however, these diseases have unique clinical presentations, genetics and available therapies. Given the presentation of fevers, rashes and mucosal symptoms observed in many of these syndromes, patients are likely to present to an allergist/immunologist. While there has been attention in the literature to diagnosis and treatment of rare, genetically-defined autoinflammatory disorders, physicians are challenged by increasing numbers of patients with intermittent or periodic fevers who face unnecessary morbidities due to a lack of a diagnosis. The broad differential of diseases presenting with fever includes autoinflammatory syndromes, infections associated with immunodeficiency and/or allergies complicated by infection, and less commonly, autoimmune disorders or malignancy. To address this challenge, we review the history of the medical approach to fever, current diagnostic paradigms, and controversies in management. We describe the spectrum of disorders referred to a recurrent fever disorders clinic established in an Allergy/Immunology division at a tertiary pediatric care center. Finally, we provide practical recommendations including historical features and initial laboratory investigations that can help clinicians appropriately manage these patients.
Keywords: pediatrics, recurrent fever, periodic fever, autoinflammation
INTRODUCTION
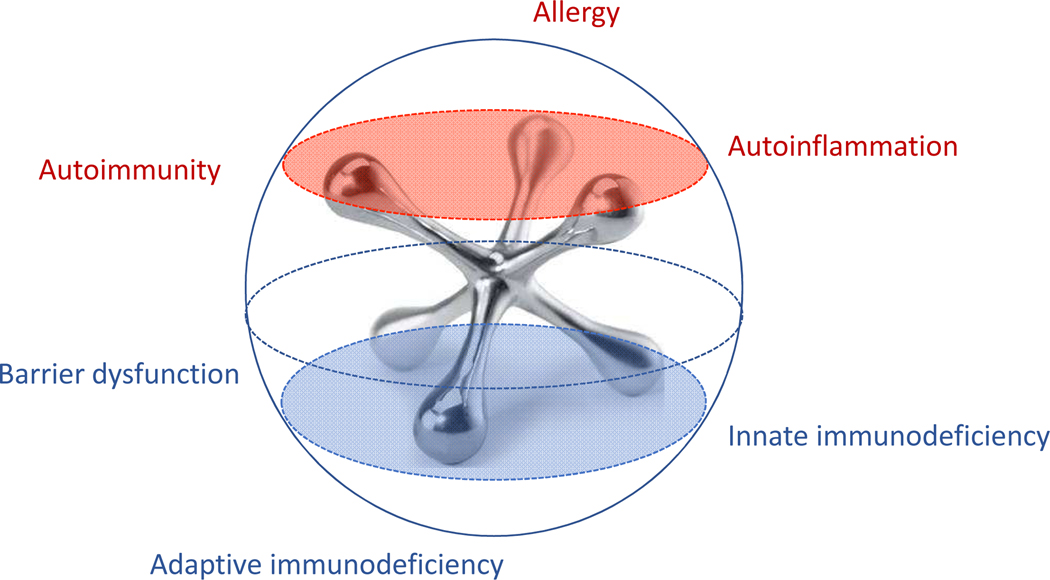
Fever is among the most common chief complaints reported to physicians, with estimates of 15% to 22% of all pediatric visits, and yet it remains a source of fear and uncertainty for many parents and clinicians.(1) While acute and chronic infections are frequently considered early in a differential diagnosis, prolonged or recurrent fevers may suggest a range of inflammatory disorders including chronic occult infections (i.e. endocarditis, chronic osteomyelitis, etc.) malignancy, autoimmunity, immunodeficiency, or the more recently described autoinflammatory syndromes. Therefore, these patients are often evaluated by multiple specialists including infectious disease, hematology/oncology, rheumatology as well as dermatology, gastroenterology, and otolaryngology based on specific associated symptoms. As such, these patients continue to experience delays in accurate diagnosis and initiation of appropriate treatment, (2) and each specialty takes a different approach. While allergists/immunologists are trained in recognition of classic primary immunodeficiencies, patients with chronic, multisystemic, or intermittent symptoms can present a particular challenge. Increased discussion and education in our field on the more well-known autoinflammatory diseases (familial Mediterranean fever, Hyper-IgD syndrome/mevalonic kinase deficiency, TNF receptor associated periodic syndrome, cryopyrin associated periodic syndrome, and Periodic Fever, Aphthous stomatitis, Pharyngitis and Adenitis (PFAPA) syndrome) suggests increased awareness of these disorders, (refer to review in this issue) however many patients present with chronic intermittent or periodic fevers that fail to fit into one of these diagnoses and often require further investigation. Our goal for this rostrum is to review the challenges of pediatric fever in the context of broad differential diagnoses. We further describe our prospective assessment of the variety of phenotypes presenting to a recurrent fever clinic in a children’s hospital from an allergist/immunologist perspective to represent our 10-year experience. We propose that as a specialty, we are uniquely qualified to evaluate and treat these patients (Figure 1).
Figure 1. The allergist/immunologist as a jack of all trades.
Clinical assessment by an allergist/immunologist can stratify the recurrent fever patient into one or a combination of disorders of immune dysregulation.
DEFINITION OF FEVER AND TEMPERATURE VARIATION
Body temperature is tightly regulated in part by temperature-sensitive neurons in the hypothalamus, with a homeostatic thermostat that is essential for survival.(3) Despite common colloquialisms of “low grade fever” and “running hot”, fever or pyrexia is clinically defined in pediatrics as a temperature above 100.4°F or 38°C, and represents a physiologic response due to exposure to pyrogens, such as IL-1β, TNF-α, and IL-6, as well as cyclooxygenase-2-induced prostaglandins.(4) In contrast, hyperthermia results from faulty hypothalamic regulation and a physiologically uncontrolled raise in body temperature due to increased heat absorption, heat production or an inability to lose heat.(5) Despite the importance of fever as a physiologic response mechanism, physicians have long recognized that nearly all parents believe that fever is physically harmful, establishing the notion of “fever phobia” in 1980. These early studies showed that 0% of parents can accurately define fever, and one survey also revealed that 93% of participants believe that high fever can cause brain damage, a notion which did not differ significantly when participants were stratified by site, race/ethnicity, language, or education.(6)
While infection is the leading cause of pediatric fever, other non-infectious causes including immune-mediated diseases, inflammatory conditions, drug-induced fever, and malignancy may generate similar increases in core temperature through the production of pyrogenic cytokines. Less frequently considered causes of abnormal temperature elevations in pediatrics include developmental changes such as teething, nutritional conditions, such as eating disorders and cachexia, cerebral vascular issues, or genetic disorders (Prader-Willi syndrome, Kallman syndrome, familial diabetes insipidus).(4) Furthermore, multiple physiologic factors result in temperature variation, including time of day (morning nadirs and late afternoon to evening peaks), age (younger children have higher temperatures than older children), and gender (during menstrual cycling). Consequently, the use of antipyretics is widespread in the community, despite lack of evidence that treating fever in the otherwise healthy child reduces morbidity and mortality, while concurrently promoting replication of viruses and bacteria. The extent of this misinformation has led the American Academy of Pediatrics to publish a policy statement stating that there is no evidence to recommend the use of antipyretics to reduce temperature in a febrile child, and rather the primary goal of antipyretic use should be to improve overall comfort.(6)
Recurrent fever
When fevers become recurrent, it is incumbent on the families and physicians to recognize, record, and begin to differentiate the repetitive pattern. This can be challenging as primary viral infections are frequent in young children, with a prevalence in toddlers of 7–10 independent viral infections per year, and as many as 14 per year if the child attends daycare. (7). Many respiratory viruses demonstrate seasonal variations in prevalence, leading to presumptive diagnoses, and delays in assessment of non-viral recurrent fevers.(7, 8) In spite of the large numbers of visits for fever, antibiotic use in pediatrics has decreased dramatically over the last two decades, due to physician and family target programs promoting judicious antibiotic use.(9, 10) Newer recommendations specifically emphasize reduced use for viral respiratory infections, the risks and benefits of antibiotic therapy, as well as increasing the knowledge of both physicians and families regarding the appropriate use of antibiotics.(9) While these measures have been successful in reducing antibiotic overuse in children, it also eliminates a potential diagnostic data point for therapeutic response in other causes of fever.
If patient-reported history, exam, or positive infectious cultures fail to identify an underlying etiology, there are limited specific diagnostic tests available for the evaluation of pediatric patients with recurrent fever. Typical investigations include attempts to distinguish the autoinflammatory syndromes from persistent infections including neutrophilic leukocytosis with a shift to the left in WBC numbers, and degrees of elevation of acute phase reactants such as CRP, ESR and procalcitonin.(11) Acute phase reactants are often used as a measure of following patients, but are by no means specific. Infection predictably remains the most common cause for these abnormal values, but a diagnosis still cannot be established in nearly 20% of cases of highly elevated inflammatory markers, (12) making pattern recognition of autoinflammatory features critical.
OVERVIEW OF AUTOINFLAMMATION
Historically, autoinflammation was practically defined as intermittent episodes of systemic inflammation and fever without evidence for infection, and no role for autoreactive T cells, B cells, or autoantibodies. The classic autoinflammatory disorders are characterized by recurrent episodes of systemic inflammation with fever, serosal inflammation, rash, lymphadenopathy and arthritis and elevated serum acute phase reactants. These disorders are largely driven by inborn errors of the innate immune system (see review in this issue) including hyperactivation of inflammasomes leading to dysregulated IL-1β or IL-18 signaling, abnormalities in type I interferon production, erroneous NF-κb signaling due to impaired ubiquitination, and errors in protein folding.(13) While critical to establishing the field of autoinflammation, the rise of clinical genetic sequencing and advances in biomolecular techniques has led to further characterization of autoinflammatory syndromes that may include elements of autoimmunity or immunodeficiency, making them truly disorders of immune dysregulation.
Current challenges in the diagnosis and management of autoinflammatory disease
The field of autoinflammation has been driven by an emphasis of a bench-to-bedside and careful phenotyping of individual patients and cohorts.(14) Selected autoinflammatory diseases have recently developed clinical diagnostic criteria,(15) centered on the initial descriptions and classic presentations, which often feature more severe phenotypes. Not only has the number of diseases rapidly expanded as new patients are sequenced and described, but there is a greater recognition of disease spectrums.(16) Consequently, patients without a Mendelian condition continue to face delays in diagnosis and targeted therapy.
Over the last several decades, more than 30 novel disorders presenting with symptoms of autoinflammation have been genetically determined leading to more accurate diagnoses and targeted therapy.(13) Next generation sequencing via targeted gene panel, whole exome, or whole genome sequencing is increasingly available for the diagnosis of autoinflammatory disorders. However, as more patients are sequenced, new issues have arisen including the recognition of patients with somatic mosaicism or low penetrance variants.(17) Both of these subsets may present with classic features of disease, or may have additional atypical features such as gastrointestinal symptoms.(18) Oligogenic inheritance where one inherits several low frequency variants, which also may be present in asymptomatic first degree relatives, adds further challenges. Often described as variants of unknown significance, their presence known as the burden of variants, can further add to the burden of disease and misdiagnosis.(16)
There has been a similar increase in the number of patients with undefined fever disorders with estimates of up to 80% of patients failing to achieve any molecular or genetic diagnosis.(16, 19) While in pediatrics, the primary etiology for recurrent fever is recurrent infections, patients with recurrent fever and systemic inflammation and no evidence of pathogenic microbial involvement are increasingly identified. These patients have variably been called atypical PFAPA, (20) undifferentiated autoinflammatory disorder, (19) or syndrome of undifferentiated recurrent fever (SURF).(21) It is currently unknown whether these patients represent autoinflammatory gene variants of known syndromes, complex genetic autoinflammatory disorders, or independent entities, (19, 22) and the most effective therapy remains challenging.
DEFINING OUR CLINIC EXPERIENCE USING A PROSPECTIVE SURVEY APPROACH
In 2011, we developed a Recurrent Fever Disorders clinic as a subspecialty clinic in the Division of Allergy/Immunology of the Department of Pediatrics at the University of California, San Diego and Rady Children’s Hospital San Diego. The clinic consists of board-certified allergist/immunologists and provides consultation in the outpatient setting for patients who live in San Diego County and Southern California, with referrals from western Arizona, Oregon, Hawaii and Mexico. At its inception, we expected to provide a dedicated clinic for patients with autoinflammatory disorders, expecting PFAPA syndrome to be the most common diagnosis.(23) However, we were surprised to identify numerous other disorders presenting with recurrent fevers, including patients who failed to meet criteria for the classic disorders, or who had atypical phenotypes. To assess the variety of diagnoses referred to our clinic in a non-biased fashion, we used a prospective survey approach to address symptomatology related to febrile episodes in pediatric patients over a six month period. We found that autoinflammatory diseases including genetically defined syndromes or PFAPA could be diagnosed in only 30% of cases.
General characteristics of our cohort
In our current cohort, female patients outnumbered males, 61% to 39%. The patient reported disease onset averaged 2.78 years, with age of symptom onset comparable between the assigned groups (Table 1). Febrile episodes were self-reported with an average duration of 3.8–6.2 days (low range 1–21 days, high range 2–60, median 3 – 4.5 days) and temperatures over the course of each episode ranging from 102.4–103.6°F (range 99–107°F, median 102–104°F). Febrile episodes occurred with a frequency 30.1–42.9 days (range 10–60, median 28 days), with 20% of respondents just stating “monthly” and 25.3% of respondents reporting a variable frequency. These similarities suggest that other features of the history may be more useful for stratifying patients with recurrent fevers. Subsequently, we found that the cohort had episodic symptoms beyond fever with features of autoinflammatory, immunodeficiency and/or allergic disease, which could be helpful to narrow the differential diagnosis. Based on recognizable patterns, we were able to stratify subjects to one of the following groups: PFAPA, known monogenic autoinflammatory syndrome variant identified, recurrent infections with or without associated allergic disease, or undefined recurrent fever (SURF).
Table 1.
Distinguishing clinical characteristics recurrent fever patients
| Recurrent Infections | Allergic rhinitis | PFAPA | SURF | Variant identified | p value* | |
|---|---|---|---|---|---|---|
| Patients, n (%) | 22 (34%) | 7 (11%) | 7 (11%) | 14 (22%) | 15 (23%) | |
| Age of onset (mean, range) | 2.2 years (0.5–7 years) | 3.7 years (0.25–9 years) | 1.78 years (1–2.5 years) | 1.2 years (birth – 6 years) | 3.4 years (birth to 16 years) | n.s. |
| Tmax (°F, average, range) | 102.3 (100–106) | 101.2 (99–104) | 104.1 (102–106) | 104.1 (102–107) | 103.6 (99.3–105.4) | n.s. |
| Duration (average, range) | 4.5 (1–13 days) | 9 (3–60 days) | 3.9 (3–6 days) | 4.4 (3–7 days) | 4.5 (1–10 days) | n.s. |
| interval (average, range) | 31.9 (10–120 days) | 30.1 (14–60 days) | 27.2 (21–35 days) | 29 (21–120) | 23.2 (7–60) | n.s. |
| Atopy (%) | 45 | 100 | 14.29 | 57.1 | 40 | <0.001 |
| FH fever (%) | 4.6 | 14.29 | 42.86 | 35.71 | 33.3 | <0.001 |
| FH autoimmune (%) | 22.8 | 42.86 | 42.86 | 35.71 | 46.67 | n.s. |
| Pharyngitis (%) | 40.9 | 42.9 | 71.4 | 35.7 | 33.3 | 0.038 |
| Cervical adenitis (%) | 31.8 | 14.3 | 42.9 | 21.4 | 20.0 | n.s. |
| mouth sores (%) | 22.7 | 28.6 | 57.1 | 21.4 | 33.3 | n.s. |
| Abdominal pain (%) | 50.0 | 42.9 | 28.6 | 50.0 | 53.3 | n.s. |
| Chest pain (%) | 13.6 | 28.6 | 0 | 0 | 26.7 | n.s. |
| Headache (%) | 40.9 | 42.9 | 42.9 | 28.6 | 53.3 | n.s. |
| Conjunctivitis (%) | 36.4 | 28.6 | 42.9 | 28.6 | 60.0 | n.s. |
| Eyelid swelling (%) | 22.7 | 28.6 | 14.3 | 14.3 | 40.0 | n.s. |
| Nausea (%) | 18.2 | 28.6 | 14.3 | 42.9 | 40.0 | n.s. |
| Vomiting (%) | 22.7 | 14.3 | 14.3 | 42.9 | 33.3 | n.s. |
| Arthralgia (%) | 40.9 | 28.6 | 28.6 | 14.3 | 66.7 | 0.038 |
| Rash (%) | 22.7 | 28.6 | 14.3 | 7.1 | 53.3 | 0.012 |
| Fatigue (%) | 18.2 | 0 | 28.6 | 0 | 20.0 | n.s. |
| Upper respiratory symptoms (%) | 18.2 | 42.9 | 0 | 14.3 | 6.7 | 0.02 |
| Irritability (%) | 9.1 | 14.3 | 14.3 | 7.1 | 13.3 | n.s. |
by Fisher’s exact test
bold font indicates features which distinguish among groups
Patients were referred largely from pediatricians, family practitioners, and other pediatric subspecialists including infectious diseases, rheumatology, hematology, oncology, otolaryngology and gastroenterology, as well as being self-referred for a chief complaint of recurrent fever. The average age for our assessment was 6.13 years, with patients experiencing symptoms for an average of more than 3 years prior to referral suggesting that significant delays in evaluation and diagnosis remain. While not as extreme as the delays in diagnosis for some immunodeficiencies, this serves to highlight that patients continue to go unrecognized and untreated. Only a subset of patients (21%) had a family history of recurrent fevers, and most of these relatives did not have a formal diagnosis, consistent with prior reports.(19)
Periodic fever, aphthous stomatitis, pharyngitis, adenitis syndrome (PFAPA)
Using a modified Marshall’s criteria, (23) we defined patients with PFAPA as patients who have regularly occurring fevers (without evidence of upper respiratory infection) with an onset prior to age 5 years. Patients must have at least one of the following associated with fevers: aphthous stomatitis, pharyngitis, cervical adenitis, and be asymptomatic between episodes with normal growth as assessed by WHO growth charts and an absence of laboratory evidence of inflammation between episodes (cardio/high sensitivity CRP <0.2 mg/L and ESR <15 mm/h).(23, 24) In addition, patients must have an absence of genetically defined autoinflammatory disorders (i.e. familial Mediterranean fever, Hyper-IgD syndrome/mevalonic kinase deficiency, TNF receptor associated periodic syndrome, cryopyrin associated periodic syndrome), cyclic neutropenia, immunodeficiency or autoimmunity (excluded clinically). Despite being a target population for our clinic, patients meeting these criteria represented only 9.3% of referrals in our cohort. Respondents included terms including “monthly”, “predictable” and “clockwork” in their questionnaires. Reported temperatures were uniformly markedly elevated with a median of 104.1°F and primarily associated with pharyngitis, aphthous stomatitis and cervical adenitis as previously described.(23, 24) Non-specific symptoms such as fatigue and generalized arthralgias were also reported. Forty-two percent of respondents reported a family history of recurrent fevers, but none with a formal diagnosis, and atopic disease was not frequently observed.
Prior to evaluation, PFAPA patients had treated febrile episodes with antipyretics (ibuprofen, acetaminophen, or both with alternating dosing) and a fraction had used other reported therapies, including on-demand, single-dose, oral corticosteroid.(23) Ultimately, 100% of patients who tried corticosteroid therapy were successful in aborting episodes within a few hours of treatment. No biologic therapies had been used in this cohort. Most patients had limited blood work performed during febrile episodes (namely CBC and CRP with or without ESR), but fewer than 50% of patients were assessed for subclinical inflammation prior to referral.
The case for tonsillectomy in PFAPA has been both controversial and successful. In the early 1900s, tonsillectomy was the most frequently performed surgical procedure in the United States and Europe, driven by novel medical concepts, changes in health care, and a theory that they were “portals of infection.”(25) By the mid-twentieth century, criticisms over the procedure included questions of the lack of supporting evidence, politicalization of medicine, competing medical specialties and the role of the patient in medical decision making.(25) For those meeting criteria for the diagnosis of PFAPA, tonsillectomy has been shown to be a successful option as we described previously. (34)
Autoinflammatory diseases and related variants presenting with recurrent fever
Patients who underwent commercial panel genetic testing prior to our assessment had a range of heterozygous variants including NLRP3, NLRP12, MVK, ELANE and NOD2. As a group, this cohort, was much more variable. Initial symptoms were reported in early infancy, though others reported being well until their teenage years. Inflammatory episodes tended to be more variable in duration than the PFAPA cohort, and occurring more frequently. Similarly, interfebrile episodes were inconsistent with some patients reporting that symptoms occurred monthly, and others that episodes were sporadic, and without identifiable triggers. Maximum temperatures during episodes were more variable as well, ranging from 99.3 to 105.4°F. Patients with genetic variants identified were more likely to describe conjunctivitis, arthralgias, rash, and abdominal pain with their febrile episodes. In contrast to other reports, 40% of patients reported a history of atopic disease, primarily allergic rhinitis.(19) Only 4 patients had a known genetic variant in a family member suggesting these were largely de novo variants.
Treatment of these patients prior to referral was notably different. Besides antipyretics for their episodes, all of the patients had also been treated with at least one other medication including on demand corticosteroid, colchicine or montelukast with variable effects. Two patients obtained successful control of their inflammatory episodes with colchicine, whereas half were treated with either anakinra or canakinumab with positive response in controlling febrile episodes.
Non-autoinflammatory presentations of recurrent fever
Despite the initial goal of developing a subspecialty clinic for rare autoinflammatory patients, the vast majority of patients who presented with monthly fevers did not meet clinical or genetic criteria for PFAPA or genetically defined autoinflammatory disease. We identified several additional diseases and syndromes that present with a history of recurrent fever, including infectious and immunologic diseases, and attempted to further stratify these patients by episode characteristics.
Recurrent infections presenting as monthly fever.
We identified a subset of patients with more variable inflammatory episodes that presented primarily with recurrent infections. Respiratory tract infections were more common and included recurrent otitis media, recurrent sinusitis, pneumonia, while paronychia and recurrent urinary tract infections occurred less frequently. Not unexpectedly, of cultured infections, streptococcal and staphylococcal infections were most prevalent, and rarely Candida was identified. All infections were localized, and no instances of sepsis or meningitis were reported. As a group, these patients were younger in age and febrile temperatures occurred across a broader range from 100–106°F. While the duration of febrile episodes was similar to other patients, associated symptoms tended to be more variable, with non-predictable onsets.
In this group, 14% of patients had undergone adenoidectomy prior to evaluation, and 9% had at least one set of tympanostomy tubes placed. In contrast to the other patient subsets, greater than 95% of patients had no family history of recurrent fevers. Non-cyclic, intermittent neutropenia was observed in 9% of patients. A third of patients presenting with recurrent infections demonstrated poor responses to the initial pneumococcal immunization series with improvement in symptoms after immunization with pneumococcal polysaccharide vaccine (PPSV23), suggesting that a basic immunologic workup can be informative and therapeutic in this population of patients.
Recurrent infections associated with atopy.
We were surprised to identify a small cohort of patients with fevers that appeared to be partially attributed to allergic symptoms. The febrile episodes trended towards lower temperatures (average 101.2°F), and with a greater range in episode duration. Patients were noted to have seasonal or variable courses. Unlike the other subsets, the patients were found to have upper respiratory symptoms including cough, rhinorrhea and pharyngitis, as well as headache as prominent symptoms during febrile episodes. Atopic disease including asthma, eczema, and/or environmental allergen sensitization was uniformly present. While the precise inflammatory mechanism is unknown, in these cases, increased susceptibility to early symptoms of otitis media caused by obstruction of the eustachian canal, or subclinical sinusitis due to obstruction of sinus ostia or allergy-driven barrier dysfunction was hypothesized to be driving the recurring fever. Adequate treatment with intranasal corticosteroid spray led to improvement in symptoms in most of these patients.
Syndrome of Undifferentiated Recurrent Fevers (SURF)
A large subset of patients with undefined, non-infectious, recurrent fevers comprised the majority of referrals. These patients similarly experienced intermittent systemic inflammation with normalization between febrile episodes, and normal growth and development, but without meeting the strict clinical criteria proposed for PFAPA syndrome. Febrile episodes were similar to PFAPA, though tended to occur less frequently. In addition, respondents noted that inflammatory episodes did not have a characteristic symptom pattern and were difficult to predict. Respondents also had a greater presence of gastrointestinal symptoms including nausea, vomiting and abdominal pain with episodes, whereas arthralgias and fatigue were less commonly reported. Most had some history of infection requiring antibiotic therapy including streptococcal pharyngitis, otitis media, sinusitis, and/or pneumonia, which was uniformly absent in the PFAPA group. However, in contrast to the recurrent infection group, SURF patients also experienced febrile episodes in which no infection was identified and antibiotics were ineffective. Thirty-five percent had a family member with recurrent fevers of unknown origin that improved with age.
Consistent with the variability in symptoms, patients had varied responses to therapy as well. Ibuprofen was helpful in the majority of patients, though colchicine, montelukast and prednisolone had also been prescribed with positive response in many patients. Beyond medical therapy, the similarities with PFAPA syndrome have led some families to pursue tonsillectomy with success in resolving febrile episodes. While the precise mechanisms are under investigation, our initial data suggest this is a viable option for pediatric patients with SURF as well (Broderick, unpublished).
Distinguishing features in the evaluation of recurrent fever episodes
Given the clinical similarities amongst the cohort, we asked whether any features or symptoms were more likely to distinguish one diagnosis from another. Neither the maximum daily temperature, average duration of fever symptoms, nor the frequency of episodes, were statistically significant amongst the diagnoses. Atopic disease was more likely to be present in the patients with infection associated fevers, and less likely to be reported in children with PFAPA syndrome. Patients with PFAPA syndrome, SURF, or positive genetic findings were more likely to have a positive family history of recurrent fevers, compared to the recurrent infections or allergic subsets. In contrast, there was no difference in the reporting of family history of autoimmune disease among any group. Beyond fever, several symptoms were noted to be most prominent in specific subsets, and may be clues to diagnosis: pharyngitis in PFAPA syndrome patients, upper respiratory symptoms in the allergic subset, and arthralgia and rash in those with an identified genetic variant (Table 1, bold).
Limitations of this study
Disease recognition remains imperative for the diagnosis of autoinflammatory disorders. The description of our experience is limited by the exit questionnaire strategy and recall bias by the parents. Further, it only captures patients and families that were referred specifically for an evaluation of recurrent fevers. Given the broad range of conditions that may present with fever, it is possible that similar patients were referred to other subspecialties such as rheumatology, infectious diseases or oncology. Many experienced delays in diagnosis of several years, which could contribute to other social and familial morbidity. The expanding spectrum of autoinflammatory diseases suggests that many more patients remain to be diagnosed, and should be considered in patients with recurrent fever.
PRACTICAL APPROACH TO PEDIATRIC RECURRENT FEVER
We and others appreciate the wide differential diagnosis for recurrent fevers in the pediatric population.(4, 19, 26) In our single center experience, we have made a variety of alternate diagnoses, each presenting as recurrent fever and/or presumed PFAPA syndrome, including infectious causes (dental/pharyngeal abscess, recurrent/chronic sinusitis with or without allergic sensitization), mild immunodeficiencies (weak response to pneumococcal vaccine, specific antibody deficiency, hypogammaglobulinemia, transient C4 deficiency), rheumatologic (progressive vesicoureteral reflux with uveitis, Takayasu’s arteritis, hematologic (malignancy, mast cell activation disorders) and recurrent fever associated with variants of unknown significance in autoinflammatory and immunodeficiency related genes. The variety of diagnoses suggests that a methodical approach should be taken to pediatric recurrent fever. (Figure 2).
Figure 2. Proposed algorithm for the evaluation of pediatric recurrent fever.
Initial considerations and diagnostic workup to begin to stratify the patient with recurrent fever. N.B., patients should be evaluated on an individual basis, and may require additional workup from that described here. CBC, complete blood count; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; Hib, Haemophilus influenzae type b; IBD, inflammatory bowel disease; SAA, serum amyloid A; S. pneumo, Streptococcus pneumoniae.
History remains the cornerstone of the allergist/immunologist evaluation. The recurrent nature of fever episodes may provide critical insights into the diagnosis. Duration of fever, fever-free days, seasonality, associated symptoms, similarity of episodes, presence of severe infections, and responses to therapies tried (i.e. anti-microbial, anti-inflammatory) should all be assessed. Family history or ethnicity may suggest a known genetic etiology. On physical exam, the presence of rash, non-infectious pharyngitis, aphthous stomatitis, lymphadenopathy, serositis, abdominal pain, or joint inflammation may suggest autoinflammation whereas presence of failure to thrive, various skin findings or absence of lymphoid tissue may be more suggestive of immunodeficiency as a cause of recurrent fever. In contrast, nasal examination may reveal findings consistent with allergic rhinitis, cystic fibrosis, or structural abnormalities predisposing to subclinical sinusitis (Figure 2).
We recommend a basic lab workup to begin to stratify these patients, to be measured during febrile episodes and the asymptomatic interval, including complete blood count and differential, high sensitivity CRP or serum amyloid A (SAA), ESR, and ferritin. A basic immune evaluation including immunoglobulin classes (IgA, IgG, IgM, IgD, IgE), as well as vaccine antibody titers, and lymphocyte subsets and mitogen testing, should also be considered. Percutaneous skin testing or serum evaluation for allergen sensitization may help complete the initial workup. Similar to a recently published retrospective study of patients referred to a pediatric infectious disease clinic, we were reassured that the overwhelming majority of patients did not have a serious underlying chronic infection.(26) However, depending on presenting symptoms and previous responses to therapy, additional workup may be warranted including imaging such as sinus CT for history consistent with chronic sinusitis, abdominal CT or endoscopy for inflammatory bowel disease, or bone marrow biopsy for malignancy.
If no etiology for the recurrent fevers is identified, genetic sequencing for immune-related diseases should be considered, via targeted immune panel, whole exome, or whole genome sequencing approaches. We found only one quarter of our patients had any form of genetic sequencing prior to our evaluation, with the majority having signs of classic autoinflammation, including fever, rash, arthralgias and a family history of similar disease. Consistent with other reports, for many patients, the out-of-pocket cost of sequencing is a primary factor in the testing not being performed (27), but could be the defining diagnostic test leading to specific therapy. In some cases, despite these evaluations and genetic testing, a specific diagnosis may not be reached,(19) as new gene defects and broadening clinical phenotypes continue to be described.
SUMMARY
Given the rarity of autoinflammatory disorders, limited number of physician experts, and delays to diagnosis,(28) families report feeling uncertain about many aspects of their diagnosis and where to obtain care. In a German study, the overall state of care for patients with autoinflammatory diseases was rated as poor or non-sufficient by 40% of respondents, demonstrating a clear need to improve the diagnostic and treatment strategies, and holistic care for these patients.(29) These uncertainties lead many families to seek online support and a sense of community.(30) While some expert-driven patient resources exist, there is a wealth of misinformation and misinterpretation about fevers that can have detrimental consequences. Until genetic testing becomes more widely available, patients with undefined, non-infectious, recurrent fevers will continue to be a diagnostic challenge. The varied nature of possible diagnoses emphasizes the importance of disease recognition, not only for the diagnosis of autoinflammatory disorders, but also to avoid inappropriate testing and delayed therapy. The large presence of immunologically driven disease suggests that allergist/immunologists are appropriate healthcare providers for these patients, and ongoing education on disease recognition is critical for our specialty.
Acknowledgements:
The authors would like to acknowledge funding sources NIH 1K08HD075830-01A1 (L.B.), Thrasher Research Fund (L.B.), The Hartwell Foundation (L.B), and A.P. Giannini (L.B.), Dr. Alan Wanderer for thoughtful criticisms and discussion, and the patients and families who participated.
Abbreviations
- CAPS
cryopyrin associated periodic syndrome
- CBC
complete blood count
- CRP
C-reactive protein
- ESR
erythrocyte sedimentation rate
- FCAS
Familial cold autoinflammatory syndrome
- FMF
familial Mediterranean fever
- HIDS/MVK
Hyper-IgD syndrome/mevalonic kinase deficiency
- MWS
Muckle-Wells Syndrome
- NOMID
Neonatal onset multisystem inflammatory disease
- PFAPA
periodic fever, aphthous stomatitis, pharyngitis, and adenitis
- SAA
serum amyloid A
- SURF
Syndrome of undifferentiated recurrent fever
- TRAPS
TNF receptor associated periodic syndrome
- VUS
variant of unknown significance
Footnotes
Conflict Disclosures: L.B and H.H are speakers for Novartis, Inc. H.H. is a consultant for Novartis. L.B. has research collaborations with Regeneron, Inc. and IFM, Inc. H.H. has research collaborations with Regeneron, Inc.; Jecure, Inc., Zomagen, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Massin MM, Montesanti J, Gerard P, Lepage P. Spectrum and frequency of illness presenting to a pediatric emergency department. Acta Clin Belg. 2006;61(4):161–5. [DOI] [PubMed] [Google Scholar]
- 2.Erbis G, Schmidt K, Hansmann S, Sergiichuk T, Michler C, Kuemmerle-Deschner JB, et al. Living with autoinflammatory diseases: identifying unmet needs of children, adolescents and adults. Pediatr Rheumatol Online J. 2018;16(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao ZD, Yang WZ, Gao C, Fu X, Zhang W, Zhou Q, et al. A hypothalamic circuit that controls body temperature. Proc Natl Acad Sci U S A. 2017;114(8):2042–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbi E, Marzuillo P, Neri E, Naviglio S, Krauss BS. Fever in Children: Pearls and Pitfalls. Children (Basel). 2017;4(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis. 2000;31 Suppl 5:S157–61. [DOI] [PubMed] [Google Scholar]
- 6.Wallenstein MB, Schroeder AR, Hole MK, Ryan C, Fijalkowski N, Alvarez E, et al. Fever literacy and fever phobia. Clin Pediatr (Phila). 2013;52(3):254–9. [DOI] [PubMed] [Google Scholar]
- 7.Weintraub B. Upper Respiratory Tract Infections. Pediatr Rev. 2015;36(12):554–6. [DOI] [PubMed] [Google Scholar]
- 8.Colvin JM, Muenzer JT, Jaffe DM, Smason A, Deych E, Shannon WD, et al. Detection of viruses in young children with fever without an apparent source. Pediatrics. 2012;130(6):e1455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hersh AL, Jackson MA, Hicks LA, American Academy of Pediatrics Committee on Infectious D. Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics. 2013;132(6):1146–54. [DOI] [PubMed] [Google Scholar]
- 10.Vaz LE, Kleinman KP, Raebel MA, Nordin JD, Lakoma MD, Dutta-Linn MM, et al. Recent trends in outpatient antibiotic use in children. Pediatrics. 2014;133(3):375–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kraszewska-Glomba B, Szymanska-Toczek Z, Szenborn L. Procalcitonin and C-reactive protein-based decision tree model for distinguishing PFAPA flares from acute infections. Bosn J Basic Med Sci. 2016;16(2):157–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landry A, Docherty P, Ouellette S, Cartier LJ. Causes and outcomes of markedly elevated C-reactive protein levels. Can Fam Physician. 2017;63(6):e316–e23. [PMC free article] [PubMed] [Google Scholar]
- 13.Manthiram K, Zhou Q, Aksentijevich I, Kastner DL. The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat Immunol. 2017;18(8):832–42. [DOI] [PubMed] [Google Scholar]
- 14.Holzinger D, Kessel C, Omenetti A, Gattorno M. From bench to bedside and back again: translational research in autoinflammation. Nat Rev Rheumatol. 2015;11(10):573–85. [DOI] [PubMed] [Google Scholar]
- 15.Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, Kone-Paut I, Goldbach-Mansky R, Lachmann H, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. 2017;76(6):942–7. [DOI] [PubMed] [Google Scholar]
- 16.Schnappauf O, Aksentijevich I. Current and future advances in genetic testing in systemic autoinflammatory diseases. Rheumatology (Oxford). 2019;58(Suppl 6):vi44–vi55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka N, Izawa K, Saito MK, Sakuma M, Oshima K, Ohara O, et al. High incidence of NLRP3 somatic mosaicism in chronic infantile neurological cutaneous and articular syndrome patients: The results of an international multicenter collaborative study. Arthritis Rheum. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuemmerle-Deschner JB, Verma D, Endres T, Broderick L, de Jesus AA, Hofer F, et al. Clinical and Molecular Phenotypes of Low-Penetrance Variants of NLRP3: Diagnostic and Therapeutic Challenges. Arthritis Rheumatol. 2017;69(11):2233–40. [DOI] [PubMed] [Google Scholar]
- 19.Papa R, Rusmini M, Volpi S, Caorsi R, Picco P, Grossi A, et al. Next generation sequencing panel in undifferentiated autoinflammatory diseases identify patients with colchicines-responder recurrent fevers. Rheumatology (Oxford). 2019. [DOI] [PubMed] [Google Scholar]
- 20.Lantto U, Koivunen P, Tapiainen T, Renko M. Long-Term Outcome of Classic and Incomplete PFAPA (Periodic Fever, Aphthous Stomatitis, Pharyngitis, and Adenitis) Syndrome after Tonsillectomy. J Pediatr. 2016;179:172–7 e1. [DOI] [PubMed] [Google Scholar]
- 21.Broderick L, Kastner DL, Hoffman HM. Recurrent Fever Syndromes. In: Ochs HD, Smith CIE, Puck JM, editors. Primary Immunodeficiency Diseases: A Molecular and Genetic Approach. 3rd ed2013. [Google Scholar]
- 22.Manthiram K, Preite S, Dedeoglu F, Demir S, Ozen S, Edwards KM, et al. Common genetic susceptibility loci link PFAPA syndrome, Behcet’s disease, and recurrent aphthous stomatitis. Proc Natl Acad Sci U S A. 2020;117(25):14405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas KT, Feder HM Jr., Lawton AR, Edwards KM Periodic fever syndrome in children. J Pediatr. 1999;135(1):15–21. [DOI] [PubMed] [Google Scholar]
- 24.Luu I, Sharma A, Guaderrama M, Peru M, Nation J, Page N, et al. Immune Dysregulation in the Tonsillar Microenvironment of Periodic Fever, Aphthous Stomatitis, Pharyngitis, Adenitis (PFAPA) Syndrome. J Clin Immunol. 2020;40(1):179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grob GN. The rise and decline of tonsillectomy in twentieth-century America. J Hist Med Allied Sci. 2007;62(4):383–421. [DOI] [PubMed] [Google Scholar]
- 26.Statler VA, Marshall GS. Characteristics of Patients Referred to a Pediatric Infectious Diseases Clinic With Unexplained Fever. J Pediatric Infect Dis Soc. 2016;5(3):249–56. [DOI] [PubMed] [Google Scholar]
- 27.Heimall JR, Hagin D, Hajjar J, Henrickson SE, Hernandez-Trujillo HS, Tan Y, et al. Use of Genetic Testing for Primary Immunodeficiency Patients. J Clin Immunol. 2018;38(3):320–9. [DOI] [PubMed] [Google Scholar]
- 28.Federici S, Gattorno M. A practical approach to the diagnosis of autoinflammatory diseases in childhood. Best Pract Res Clin Rheumatol. 2014;28(2):263–76. [DOI] [PubMed] [Google Scholar]
- 29.Chuamanochan M, Weller K, Feist E, Kallinich T, Maurer M, Kummerle-Deschner J, et al. State of care for patients with systemic autoinflammatory diseases - Results of a tertiary care survey. World Allergy Organ J. 2019;12(3):100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hausmann JS, Lomax KG, Shapiro A, Durrant K. The patient journey to diagnosis and treatment of autoinflammatory diseases. Orphanet J Rare Dis. 2018;13(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]