In the setting of myocardial infarction (MI), modulation of the excessive postischemic inflammatory response has therapeutic potential for cardiac tissue recovery and prevention of subsequent progression to heart failure. This is supported by a large number of anti-inflammatory approaches that demonstrate cardioprotective effects in preclinical models of MI. However, translation of these strategies to achieve clinical benefit remains challenging, and no treatment is currently available for improving outcomes following MI. Recent preclinical evidence highlighted a novel and promising approach for cardiac recovery following MI using apolipoprotein A-I (apoA-I) nanoparticles as a short-term therapy (1,2). This indication is distinct from those being pursued in clinical trials investigating the efficacy of apoA-I/high-density lipoprotein therapies to regress and stabilize atherosclerotic plaques. Here, we highlight a potential new avenue for application of apoA-I therapies to support cardiac recovery and improve outcome after MI that, in contrast to atherosclerosis, involves rapid changes within the myocardium, including an acute and intense inflammatory response.
Previous studies have demonstrated that a single injection of reconstituted apoA-I (CSL-111) administered immediately after cardiac ischemia-reperfusion ameliorated the acute systemic and cardiac inflammatory response, reduced cardiac injury and improved long-term cardiac function (1,2). Furthermore, apoA-I interacted directly with neutrophils and monocytes and entered the infarcted cardiac tissue, particularly in the inflamed and apoptotic regions. These promising results are supported by clinical data demonstrating rapid anti-inflammatory effects, such as a reduction in circulating leukocyte number and activity, following apoA-I infusion in patients with type 2 diabetes mellitus (1,3). Importantly, the potential for apoA-I therapies to improve cardiac recovery is not limited to immunomodulatory effects, with apoA-I mediating multiple additional cardioprotective actions of mechanistic relevance to reducing the onset of heart failure following MI (Figure 1).
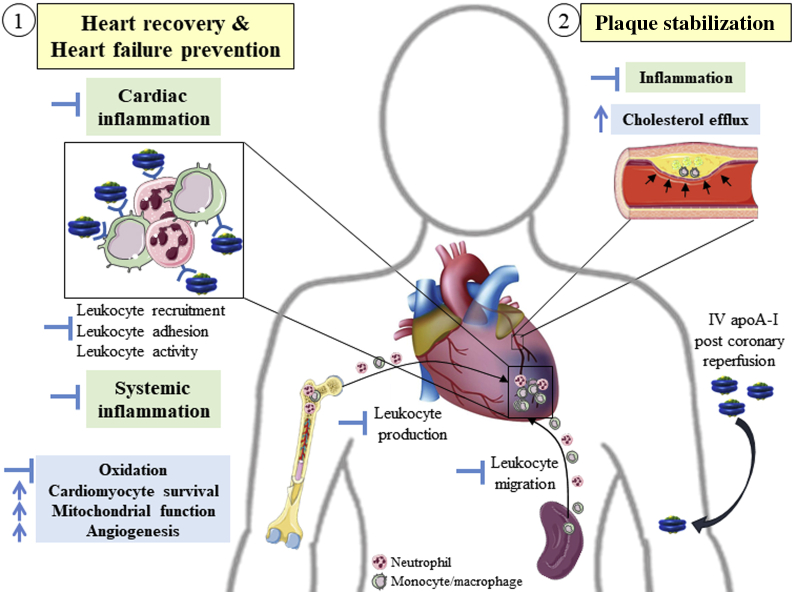
Figure 1.
Potential Pleiotropic Effects of ApoA-I Mediating Cardiac Protection Post–Myocardial Infarction
When delivered immediately following myocardial infarction, apolipoprotein A-I (apoA-I) may (1) improve cardiac recovery and preserve cardiac function via inhibition of excessive cardiac and systemic inflammation, as well as via immunoindependent cardioprotective effects; and (2) reduce the risk for recurrent events by stabilizing atherosclerotic plaque by increasing cholesterol efflux and limiting plaque inflammation. Green, anti-inflammatory mechanisms; blue, other mechanisms.
In the setting of MI, one of the major challenges for anti-inflammatory therapies is in the timing of delivery. Initiated immediately after ischemia, the inflammatory response is sustained during the first week and usually resolves about 2 weeks after the ischemic event. Reducing excessive or pathological inflammation during this time frame is crucial for cardiac tissue healing. Moreover, reducing the systemic inflammatory response to MI may also reduce the risk for further cardiovascular events, as inflammation also promotes atherosclerotic plaque destabilization. Most previous studies examining anti-inflammatory therapeutics in atherosclerotic cardiovascular disease have administered treatments weeks after the cardiac ischemic event, including the recent COLCOT (Colchicine Cardiovascular Outcomes Trial) (4) and CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcomes Study) (5) clinical trials, which have provided the first evidence for a reduction in recurrent cardiovascular events in individuals with previous MI. In the COLCOT and CANTOS trials, treatments were given approximately 2 weeks and more than 1 month post-MI, respectively, and thus their effects on the acute inflammatory response and cardiac function are unknown. The recent preclinical studies (1,2) demonstrating that apoA-I delivered immediately after cardiac ischemia-reperfusion reduced acute systemic and cardiac inflammation and improved postischemic heart function support the use of anti-inflammatory strategies shortly after MI to potentially reduce the onset of heart failure.
Another challenge for the application of anti-inflammatory therapies is to maintain an optimal level of inflammation consistent with myocardial healing. Inflammation, particularly the anti-inflammatory response, plays a key role in reparative pathways, and thus maintaining a balanced pro- and anti-inflammatory response is necessary for cardiac healing. This could be achieved by supporting the physiological transition from the pro- to the anti-inflammatory phase, possibly by promoting the differentiation of the proinflammatory monocyte subtype to an anti-inflammatory subtype. Indeed, in vitro studies demonstrated that apoA-I can stimulate such a transition from the proinflammatory toward the anti-inflammatory subtype. Moreover, in a mouse model of MI, it was suggested that apoA-I preferentially binds to the proinflammatory monocyte subtype, which was associated with an increase in the anti-inflammatory subtype and concomitant improvement of heart function (1,2).
Another key challenge of anti-inflammatory therapy is to inhibit pathological inflammation without compromising immune function and increasing the risk for serious infection. Indeed, although both the CANTOS and COLCOT trials provide positive support for long-term anti-inflammatory approaches to cardiovascular diseases, they also recorded a higher incidence of fatal infections and sepsis and an increased incidence of pneumonia, respectively (4,5). Short-term anti-inflammatory therapy as proposed with the novel apoA-I approach and its single dose delivered immediately after cardiac ischemia-reperfusion may represent a safer and more feasible strategy to limiting both cardiac and plaque inflammation during the vulnerable period post-MI (1,2).
Preclinical evidence supports the cardioprotective effects of targeted anti-inflammatory therapies including those directed against chemokines or integrins such as CCL2 or CD18. However, translation of these promising results to clinical benefit has been underwhelming and has raised concerns about safety. This may relate in part to a focus on specific cytokines or pathways directed only to the proinflammatory component of the postischemic cardiac response. Targeting multiple inflammatory pathways as observed using apoA-I may represent a superior alternative. Indeed, it has been shown that the immunomodulatory effects of apoA-I are not limited to immune cells. Endothelial cells also respond to apoA-I nanoparticles by lowering their expression of markers involved in the attraction and recruitment of immune cells, such as chemokines and integrins, as well as markers of inflammation. Following MI, the egress of leukocytes from the spleen to the circulation was reduced by apoA-I 24 hours postischemia (1). Overall, apoA-I has been shown to mediate a multifaceted immunomodulatory response, tempering immune cell production, activation, migration, and recruitment to damaged tissue.
Targeting the inflammatory response alone does not address the other components of the cardiac response to ischemia, and it has been suggested that anti-inflammatory agents may need to be combined with other cardioprotective approaches to significantly improve outcomes post-MI. This highlights the potential of apoA-I therapies that, beyond limiting inflammation, mediate effects extending to the modulation of cardiomyocyte metabolism and survival, mitochondrial function, angiogenesis, vasodilation, platelet activation, and oxidation, which may further benefit postischemic heart recovery. Indeed, a single dose of apoA-I delivered immediately after cardiac ischemia-reperfusion stimulated cardiac glucose metabolism via direct and rapid effects on cardiac glucose uptake, glycolysis, and glucose oxidation, which are critical for myocyte survival in the setting of ischemia (2). Other studies have suggested a role for apoA-I in mitochondrial function, including via the regulation of the coenzyme Q10, a crucial component of the electron transport chain (6). Thus, apoA-I therapies may prevent postischemic mitochondrial injury, which would be expected to promote cardiomyocyte survival and limit cardiac injury in the context of MI. Furthermore, apoA-I may contribute to cardiac tissue preservation by increasing perfusion to the damaged myocardium via vasodilation and stimulation of angiogenesis at the periphery of the infarcted area (1). These multiple cardioprotective effects of apoA-I likely contribute to its short-term efficacy and provide strong support for further investigation of its use for the management of acute coronary syndromes in the setting of primary percutaneous coronary interventions.
Clinical translation of the pleiotropic effects of apoA-I remain to be validated, particularly in the setting of MI. The current AEGIS-II (Study to Investigate CSL-112 in Subjects With Acute Coronary Syndrome) phase 3 clinical trial (NCT03473223) will answer critical questions regarding the efficacy of apoA-I (CSL-112) infusions in patients presenting with acute coronary syndrome to reduce recurrent major adverse cardiovascular events and provide exploratory insight into potential effects on cardiac failure hospitalizations.
Funding Support and Author Disclosures
The main article under discussion (1) was supported by the National Health and Medical Research Council of Australia (grant APP103652 to Dr Kingwell, grant 1059454 to Dr Kingwell) and the Operational Infrastructure Support scheme of the Victorian State Government. CSL provided partial financial support as well as apoA-I nanoparticles (CSL-111) to the Baker Institute (Drs Kingwell and Richart as named investigators) under a nonrestrictive materials transfer agreement but had no role in the development of the study design, data acquisition, or interpretation of data. Since completing this work, Dr Kingwell has accepted an employment contract with CSL. Dr Richart currently receives financial support from CSL for research unrelated to the present study. Dr Calkin receives financial support from CSL and Cincera Therapeutics for research unrelated to those described in this paper.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Richart A.L., Reddy M., Khalaji M. Apo AI nanoparticles delivered post myocardial infarction moderate inflammation. Circ Res. 2020;127:1422–1436. doi: 10.1161/CIRCRESAHA.120.316848. [DOI] [PubMed] [Google Scholar]
- 2.Heywood S.E., Richart A.L., Henstridge D.C. High-density lipoprotein delivered after myocardial infarction increases cardiac glucose uptake and function in mice. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aam6084. [DOI] [PubMed] [Google Scholar]
- 3.Patel S., Drew B.G., Nakhla S. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol. 2009;53:962–971. doi: 10.1016/j.jacc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Tardif J.C., Kouz S., Waters D.D. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381(26):2497–2505. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 5.Everett B.M., Cornel J.H., Lainscak M. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139(10):1289–1299. doi: 10.1161/CIRCULATIONAHA.118.038010. [DOI] [PubMed] [Google Scholar]
- 6.Dadabayev A.R., Yin G., Latchoumycandane C., McIntyre T.M., Lesnefsky E.J., Penn M.S. Apolipoprotein A1 regulates coenzyme Q10 absorption, mitochondrial function, and infarct size in a mouse model of myocardial infarction. J Nutr. 2014;144:1030–1036. doi: 10.3945/jn.113.184291. [DOI] [PMC free article] [PubMed] [Google Scholar]