CASE PRESENTATION
The patient is a 19-year-old woman who was originally diagnosed with ulcerative colitis (UC) at age 17. At the time of her initial presentation, the patient reported increased stool frequency, abdominal pain, and then developed hematochezia for approximately two months. She underwent a colonoscopy which demonstrated moderately severe, left-sided ulcerative colitis, with normal appearing mucosa in the transverse and ascending colon and cecum. Her terminal ileum was also normal on the initial colonoscopy. She was started on oral mesalamine 4.8 g daily and rectal mesalamine 1 g per rectum nightly with an initial improvement in symptoms. Nevertheless, within 1 month, the patient reported worsening of her symptoms including increased frequency and hematochezia. She had minimal response to multiple prednisone courses, and was therefore initiated on adalimumab monotherapy (40 mg subcutaneously every 2 weeks).
Though adalimumab led to clinical remission for 4–5 months, she subsequently experienced secondary loss of response with increased bowel frequency, abdominal pain, and recurrent rectal bleeding. The patient reported compliance with all therapies, with negative infectious stool studies, including a Clostridium difficile assay. At that time, an adalimumab trough level was assessed (Adalimumab + Ab [Serial Monitor], LabCorp, Burlington, NC, USA), which was 6 μg/mL (detectable) with an adalimumab antibody titer of 261 ng/mL (intermediate).
The patient was restarted on prednisone and continued on adalimumab 40 mg subcutaneously every other week. She was referred to our center for further management. On presentation to the University of North Carolina (UNC) Multidisciplinary Center for Inflammatory Bowel Diseases, the patient reported approximately six bowel movements per day, the majority containing blood. She denied urgency, abdominal pain, and extraintestinal manifestations of UC. In addition to adalimumab, her medications at that time included prednisone 20 mg daily and oral mesalamine 4.8 g daily. Given the presence of detectable adalimumab levels and intermediate antibody titers, the decision was made to initiate thiopurine therapy and increase adalimumab to weekly dosing in order to lower the antibodies to adalimumab. Along with thiopurine methyltransferase (TPMT) testing, HLA-DQA1*05 testing (McLendon Clinical Laboratories, University of North Carolina Medical Center, Chapel Hill, NC) was performed to assess this patient’s likelihood for progressive immunogenicity to adalimumab therapy.
Though the patient’s HLA-DQA1*05 testing indicated presence of this allele, with the changes in adalimumab dosing (40 mg subcutaneously weekly) and the addition of azathioprine 150 mg daily, the patient was able to taper off prednisone within 4 weeks. Her bowel frequency decreased with resolution of bleeding, ultimately achieving steroid-free clinical remission. Eight weeks following these therapeutic changes, the patient was determined to have an adalimumab level of 14 μg/mL and an anti-adalimumab level of <25 ng/mL.
KEY IMAGES:
Figure 1.
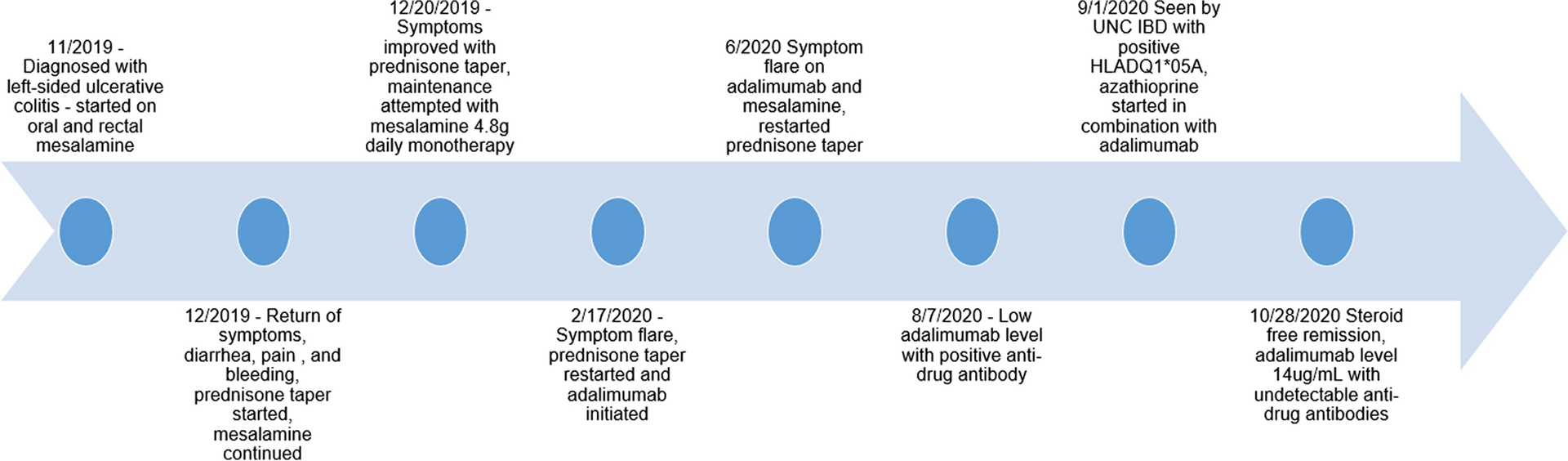
Timeline of Decision Making and Results
DISCUSSION:
Although many patients with UC can experience clinical remission with mesalamine-based regimens, a significant proportion of patients will require escalation of therapy to the biologic class of medications.1 For patients with moderately - severely active UC, currently available therapies employ multiple strategies, including inhibiting tumor necrosis factor-α (anti-TNF therapies), targeting integrins in the gastrointestinal tract (vedolizumab), blocking interleukin 12 and 23 (ustekinumab), and inhibiting Janus kinases (tofacitinib). Among these medications, anti-TNF medications are the most prone to anti-drug antibody formation.2
Precision medicine, and the tailoring of treatment to deliver optimal care to an individual patient, remains an essential goal in the management of patients with inflammatory bowel disease (IBD).3 Recent studies have embarked on the process of optimizing biologic therapies in the individual patient with CD and UC using a personalized approach. Within a multicenter, prospective observational cohort from the United Kingdom (the PANTS [Personalising Anti-TNF Therapy in Crohn’s Disease] study), Sazonovs and colleagues performed a genome wide association study, identifying a variant known as HLA-DQA1*05 that was associated with an increased risk for the development of antibodies against infliximab and adalimumab.4 In this analysis, HLA-DQA1*05 was specifically associated with shorter time to immunogenicity among patients treated with these two anti-TNF therapies. Additionally, carriage of HLA-DQA1*05 was associated with higher maximal anti-drug antibody titers. Given these findings, the authors of this study concluded that testing of HLA-DQA1*05 may guide personalized anti-TNF therapy specifically with regard to need for combination therapy with an immunomodulator among patients with CD. Other studies have reproduced the association of HLA-DQA1*05 with anti-drug antibody formation and therapeutic failure of anti-TNF therapy.5–7 Prospective studies are currently underway to determine the contribution of HLA genotyping to formulating management decisions at treatment onset (Preemptive HLA Genotyping for the Safe Use of Infliximab-combination Therapy in Inflammatory Bowel Disease [INHERIT]; ClinicalTrials.gov Identifier: NCT04109300).
Though there is evidence that treatment optimization of anti-TNF therapy in patients with UC may include combination therapy, the patient population who may most benefit from this therapy is not known. In the UC-SUCCESS study, Pannacione et al. demonstrated that patients receiving combination therapy with infliximab and azathioprine were significantly more likely to achieve corticosteroid-free remission at week 16 compared with patients receiving monotherapy with either infliximab or azathioprine,8 mirroring findings from the earlier landmark SONIC study in patients with Crohn’s disease (CD).9 Multiple potential explanations have been suggested for the benefits of combination therapy, including suppression of anti-drug antibody formation, higher biologic drug levels, and synergistic effects of an immunomodulator with a biologic.10 Nevertheless, these results have not been replicated with all anti-TNF therapies or other immunomodulators, as evaluations of combination therapy with adalimumab or using methotrexate as the immunomodulator did not demonstrate the same robust benefit seen in UC-SUCCESS and SONIC.11, 12 Perhaps due to these findings, or to concerns regarding the risks imposed by dual immunosuppression, there has not been widespread uptake in the use of combination therapy by practicing gastroenterologists.13 Though the variety of UC treatments is growing, there remains a limited number of medications available to treat patients with the most severe disease. It is thus imperative to optimize all available therapies and avoid unnecessary interruptions in order to prevent loss of response, formation of anti-drug antibodies, and ultimately drug failure.
In the current case, when the patient was first evaluated in our center with secondary loss of response, we demonstrated the presence of the HLA-DQA1*05 allele, which informed our decision to attempt optimization of adalimumab therapy and initiation of combination therapy with an immunomodulator. We believe that testing for HLA-DQA1*05 at baseline would have created a compelling rationale to use combination therapy from the onset and may have prevented the secondary loss of response. Nonetheless, we were fortunate in this case that antibodies were detected at an early phase in treatment and that we were able to recapture response for this patient, demonstrating the potential value of HLA-DQA1*05 testing in our quest to individualize therapy decisions. Despite the robust evidence demonstrated in SONIC3 and UC-SUCCESS2, many patients are initially treated with anti-TNF monotherapy. Yet, in the PANTS cohort, the highest rates of immunogenicity (92% at 1 year) were noted among patients who were carriers of HLA-DQA1*05 and treated with infliximab monotherapy.5
By implementing precision medicine principles in the treatment of this patient, we were able to not only recapture response with adalimumab, but also to effectively reduce her anti-drug antibody to undetectable levels. Although the loss of response secondary to the presence of anti-drug antibodies could potentially have been overcome by shortening the interval between adalimumab doses given that she was HLA-DQA1*05 positive, optimizing her adalimumab alone may not have been sufficient to improve clinical response. Furthermore, knowledge of her HLA-DQA1*05 status informed our decision not only to attempt optimization of adalimumab therapy with weekly dosing, but also to initiate long-term combination therapy to prevent recurrent antibody formation.
Although personalized medicine approaches have existed to some degree in the management of IBD (such as the use of TPMT testing and more recently nudix hydrolase 15 [NUDT-15] testing prior to thiopurine initiation),14 using precision medicine principles to predict drug response to newer therapies such as biologics and small molecules remains an important goal as the therapeutic armamentarium expands. Understanding the relationship between HLA-DQA1*05 and anti-TNF biologic immunogenicity may be among the first of several important genetic associations that translate to practice-changing advances in precision-medicine in IBD. The development of new precision medicine-based approaches to the treatment of IBD will likely be informed by both new analytic techniques such as the use of “big data” methodology, multi-omic analyses, and machine learning.15 These techniques will be combined with the existing heterogeneity and diversity among patients with CD and UC in the hopes of developing patient profiles that will allow for truly individualized care.
KEY POINTS.
HLA-DQA1*05 testing can identify patients at risk of immunogenicity to anti-TNF therapies requiring additional medications (or alternative approaches to treatment).
Intermediate anti-TNF antibodies may be overcome by adding immunomodulators in patients with inflammatory bowel disease.
New analytic “big data” techniques applied to the study of individual patient populations will continue to identify clinical tools to guide personalized treatment decisions in patients with IBD.
Grant Support:
This research was supported by grants from the National Institutes of Health [K23DK127157-01 ELB].
Conflicts of Interest:
Nannaya Jampala and Animesh Jain have no relevant disclosures for this work.
Hans H. Herfarth has served as a consultant for Alivio, AMAG, BMS, ExeGI, Finch, Janssen, Gilead, Lycera, Merck, Otsuka, Pfizer, PureTech, Seres and research support from Pfizer and Artizan Biosciences.
Millie D. Long has served as a consultant for AbbVie, UCB, Takeda, Janssen, Pfizer, Salix, Valeant, Target Pharmasolutions and has received research support from Pfizer and Takeda.
Kimberly N. Weaver has served as a consultant for AbbVie.
Edward L. Barnes has served as a consultant for AbbVie, Takeda, and Target Pharmasolutions.
Footnotes
Publisher's Disclaimer: This AM is a PDF file of the manuscript accepted for publication after peer review, when applicable, but does not reflect post-acceptance improvements, or any corrections. Use of this AM is subject to the publisher’s embargo period and AM terms of use. Under no circumstances may this AM be shared or distributed under a Creative Commons or other form of open access license, nor may it be reformatted or enhanced, whether by the Author or third parties. See here for Springer Nature’s terms of use for AM versions of subscription articles: https://www.springernature.com/gp/open-research/policies/accepted-manuscript-terms
REFERENCES
- 1.Barnes EL, Jiang Y, Kappelman MD, et al. Decreasing Colectomy Rate for Ulcerative Colitis in the United States between 2007 and 2016: A Time Trend Analysis. Inflamm Bowel Dis 2020;26:1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Argollo M, Kotze PG, Kakkadasam P, et al. Optimizing biologic therapy in IBD: how essential is therapeutic drug monitoring? Nat Rev Gastroenterol Hepatol 2020;17:702–710. [DOI] [PubMed] [Google Scholar]
- 3.Denson LA, Curran M, McGovern DPB, et al. Challenges in IBD Research: Precision Medicine. Inflamm Bowel Dis 2019;25:S31–s39. [DOI] [PubMed] [Google Scholar]
- 4.Sazonovs A, Kennedy NA, Moutsianas L, et al. HLA-DQA1*05 Carriage Associated With Development of Anti-Drug Antibodies to Infliximab and Adalimumab in Patients With Crohn’s Disease. Gastroenterology 2020;158:189–199. [DOI] [PubMed] [Google Scholar]
- 5.Suris Marin G, Santacana E, Padullés N, et al. P264 Impact of the HLA-DQ1*05 alelle on the initial response to infliximab in patients with Inflammatory Bowel Disease. Journal of Crohn’s and Colitis 2021;15:S301–S302. [Google Scholar]
- 6.Wilson A, Peel C, Wang Q, et al. HLADQA1*05 genotype predicts anti-drug antibody formation and loss of response during infliximab therapy for inflammatory bowel disease. Aliment Pharmacol Ther 2020;51:356–363. [DOI] [PubMed] [Google Scholar]
- 7.Guardiola Capón J, SERRA K, Rodríguez-Alonso L, et al. P711 Carriage of the HLA-DQA1*05 allele is associated with a high risk of loss of response to adalimumab in patients with Crohn’s disease. Journal of Crohn’s and Colitis 2020;14:S574–S574. [Google Scholar]
- 8.Panaccione R, Ghosh S, Middleton S, et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 2014;146:392–400.e3. [DOI] [PubMed] [Google Scholar]
- 9.Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010;362:1383–95. [DOI] [PubMed] [Google Scholar]
- 10.Raine T, Kennedy NA. Immunomodulator and Biologic Combination Therapy in IBD: The Debate That Just Won’t Go Away? J Crohns Colitis 2020;14:1343–1344. [DOI] [PubMed] [Google Scholar]
- 11.Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology 2014;146:681–688 e1. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto T, Motoya S, Watanabe K, et al. Adalimumab Monotherapy and a Combination with Azathioprine for Crohn’s Disease: A Prospective, Randomized Trial. J Crohns Colitis 2016;10:1259–1266. [DOI] [PubMed] [Google Scholar]
- 13.Elias ED, Targownik LE, Singh H, et al. A Population-Based Study of Combination vs Monotherapy of Anti-TNF in Persons With IBD. Inflamm Bowel Dis 2020;26:150–157. [DOI] [PubMed] [Google Scholar]
- 14.Yang SK, Hong M, Baek J, et al. A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia. Nat Genet 2014;46:1017–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fiocchi C, Dragoni G, Iliopoulos D, et al. Results of the Seventh Scientific Workshop of ECCO: Precision medicine in IBD - what, why, and how. J Crohns Colitis 2021. epublished March 18, 2021. [DOI] [PubMed] [Google Scholar]


