Abstract
Background
Periodontitis is a chronic multifactorial inflammatory disease of the supportive tissues of the teeth. Pathophysiological evidence suggests a possible common inflammatory background between periodontitis and cardiovascular diseases (CVD). Pathological and epidemiological associations between these two diseases have been presented, but are still debated. This study aimed to investigate the association between the inflammatory burden of periodontitis and the presence and extent of coronary calcification. Secondary aims were to study other cardiovascular parameters and cardiovascular risk predictors in relation to periodontitis and dental health.
Methods
Healthy periodontitis or non-periodontitis patients 45–70 years of age were included in a prospective cross-sectional study. Full-mouth examinations were performed by a periodontist to determine their Periodontal Inflamed Surface Area (PISA) score and other dental parameters. To assess the cardiovascular conditions, Coronary Artery Calcium (CAC) scores, endothelial function assessments by the EndoPAT ™, and several physical and biochemical examinations were performed.
Results
Seventy-one patients were included. Elevated CAC scores and endothelial dysfunction were not significantly related to PISA or dental health. PISA was significantly related to the Framingham and Reynolds CVD risk predictors, but were no longer significant after correction for confounders. The same applied to the significant relations between tooth loss, dental plaque and bleeding scores and the CVD risk predictors.
Conclusions
Periodontitis is associated with increased CVD risk, but is not an independent risk factor. This link is still important to make to bridge the gap between dentistry and general medicine and to identify patients at risk for CVD in an earlier stage.
Keywords: Periodontitis, Dental health, Cardiovascular disease, Coronary artery calcium (CAC) score, CVD risk Prediction, CVD prevention
1. Introduction
Cardiovascular disease (CVD) is one of the leading causes of death and morbidity in the Western world [1]. The underlying pathology, atherosclerosis, is a progressive chronic inflammatory process. The observation that the atherosclerotic fibrofatty lesions are supplied with inflammatory cells was made in the late 1800s, but the contribution of immune cells to all stages of atherosclerosis began to be valued only in the last few decades [2,3]. Numerous studies have clarified the molecular mechanisms of inflammation in atherosclerosis, and it is widely accepted that both innate and adaptive immune responses play key roles in the initiation and progression of atherosclerosis, leading to clinical manifestations of CVD [4].
Periodontitis is a chronic multifactorial inflammatory disease of the supportive tissues of the teeth, with progressive destruction of alveolar bone and tooth attachment ending in tooth loss [5]. As a result of inflammation, the tissues surrounding the tooth are infiltrated by neutrophils, macrophages and activated lymphocytes, releasing cytokines and subsequently acute phase reactants (CRP, fibrinogen) [6]. Periodontitis is the most common oral disease, affecting 30–50% of adults and approximately 10% of the population in its most severe form [7].
Pathophysiological evidence points to a possible common inflammatory background between periodontitis and atherosclerosis [8]. The first study that found positive epidemiological evidence for the association between periodontitis and atherosclerosis was in 1989 by Mattila et al. [9]. In more recent years, remarkable pathological and epidemiological associations between these two diseases have been presented, though without any final conclusions [[10], [11], [12], [13]].
Since inflammation has emerged as an integrative factor for atherosclerosis, several inflammatory biomarkers, in particular high-sensitivity C-reactive protein (hsCRP), are used as surrogate biomarkers to investigate the association between periodontitis and CVD [14]. However, definitive randomized evidence for the role of hsCRP as a causative biomarker in atherosclerosis is lacking [15]. In addition to biochemical biomarkers, there are a number of non-invasive surrogate subclinical markers of cardiovascular disease, focused on the endothelial cell dysfunction and arterial stiffness, which are used to explore the association between periodontitis and CVD [16].
We designed a study focused on achieving a more definitive quantification of the association between periodontitis and coronary atherosclerosis by investigating the Coronary Artery Calcium (CAC) score. CAC scoring is a highly specific feature of coronary atherosclerosis, and has emerged as a widely available, consistent and reproducible means of assessing risk for major cardiovascular outcomes [17]. Compared with other surrogate biomarkers, the CAC score provides superior discrimination and risk reclassification of cardiovascular disease in individuals at intermediate risk [18].
The primary aim of this cross-sectional study was to determine if there is an association between the inflammatory burden of periodontitis (quantified by the Periodontal Inflamed Surface Area [PISA] score) and the presence and extent of coronary calcification (investigated by the CAC score) [19]. Secondary aims were to study other cardiovascular parameters and CVD risk predictors in relation to periodontitis and dental health.
2. Materials and methods
2.1. Study design
This prospective cross-sectional study was approved by the Medical Ethics Committee, Isala Academy, Zwolle, the Netherlands (NL43083.075.13) and has been registered in the ISRCTN trial registry with study ID ISRCTN55656827. All participants provided written consent for participation. This study was done in accordance with the Declaration of Helsinki guidelines for human research, 1964, and amended in 2013 (64th World Medical Association General Assembly, Fortaleza, Brazil). Data were collected, interpreted and analyzed by the authors.
2.2. Participants
We included patients, between 45 and 69 years of age, without known systemic diseases and with at least 10 teeth, who visited the Practice for Periodontology Zwolle (PPZ). Patients with diagnosed, untreated periodontitis and patients without (a history of) periodontitis were included.
2.3. Measures of dental health
All patients underwent a full-mouth periodontal examination performed by two trained periodontists at the Practice for Periodontology Zwolle (PPZ). These periodontists were calibrated by comparing individual measurements of ten random patients. The statistically determined intraclass correlation coefficient of 0,81 represented an excellent reliability according to Fleiss [20]. Periodontitis was initially diagnosed and staged according to the consensus report of the World Workshop on the classification of periodontal and peri-implant diseases and conditions [21]. The Periodontal Inflamed Surface Area (PISA) score was applied. This scoring tool calculated the amount of inflamed periodontal tissue in square millimeters and quantified the total infectious burden resulting from periodontitis [19]. The PISA score was calculated after extensive periodontal examination, including periodontal probing pocket depth (PD), plaque score and bleeding on probing (BOP). All measurements were performed on all teeth, on six sites per tooth using a manual periodontal standard probe.
2.4. Measures of general health
All patients filled out questionnaires to gather data on their medical history, perceived health, parental history, lifestyle, socio-economic status and oral hygiene.
At least two weeks after the periodontal examination, patients were examined by a trained nurse at the Department of Cardiology of the Isala hospital, Zwolle. Physical examinations were performed, and blood pressure (BP), heart rate (HR), body mass index (BMI), waist-to-hip ratio (WHR), and electrocardiogram measurements (ECG) were obtained. Venous blood was collected to determine levels of high sensitive C-reactive Protein (hsCRP [mg/L]), total cholesterol (mmol/L), HDL-cholesterol (mmol/L), LDL-cholesterol (mmol/L), triglycerides (mmol/L), estimated Glomerular Filtration Rate (eGFR ml/min/1,73 m2) and glycosylated hemoglobin (HbA1c [%]).
2.5. Measures of cardiovascular conditions
The presence and extent of coronary artery calcification were investigated by an ultrafast CT scan (LightSpeed VCT XT; GE Healthcare). The CT scan of the heart was rapidly acquired, prospectively electrocardiogram-triggered and without contrast. The CAC score was quantified using the Agatston method, in which the area of calcified atherosclerosis (defined as an area of at least 1 mm2 with a CT density >130 Hounsfield units [HU]) is multiplied by a density weighting factor and summed for the entire coronary artery tree using a 2.5–3.0 mm slice thickness CT dataset [22].
As secondary outcome we performed an endothelial function assessment by the EndoPAT ™ (Itmar Medical, Israel), based on noninvasive Peripheral Arterial Tone (PAT) signal technology measuring endothelium-mediated changes in vascular tone using bio-sensors placed on the fingertips [23]. The final result of the EndoPAT ™ is the Reactive Hyperemia Index (RHI), which is a ratio of the post-to-pre occlusion PAT amplitude of the tested arm, divided by the post-to pre-occlusion ratio of the control arm. A RHI score of 1.67 and below correlates to endothelial dysfunction [24,25].
2.6. Cardiovascular risk prediction
The commonly used Framingham risk score (including age, gender, total cholesterol, high-density lipoprotein cholesterol, smoking status, and systolic blood pressure) and Reynolds risk score (including age, current smoking, parental history of a cardiovascular event, <age 60 years, blood pressure, hs-CRP and total and HDL cholesterol) were calculated to predict the risk of a patient having a cardiovascular event in the next 10 years [26].
The Systemic Coronary Risk Evaluation (SCORE) algorithm, recommended by the European Society of Cardiology (ESC) for CVD risk stratification in asymptomatic individuals, also estimates the individual 10-year risk of death from CVD. SCORE is based on sex, smoking, systolic blood pressure, total cholesterol (mmol/L) and HDL cholesterol (mmol/L) [27]. In this study, SCORE was calculated using the online calculating tool HeartScore (https://www.heartscore.org).
The MESA (Multi-Ethnic Study of Atherosclerosis), a prospective community-based cohort study of 6814 participants age 45–84 years, who were free of clinical heart disease at baseline and followed for 10 years, created an algorithm for 10-year CVD risk estimation. An accurate estimate of 10-year CVD risk was obtained using the coronary artery calcium score (Agatston units) and traditional risk factors: age, sex, race/ethnicity, diabetes (yes/no), current smoker (yes/no), total and HDL cholesterol, use of lipid lowering medication (yes/no), systolic blood pressure (mmHg), use of anti-hypertensive medication (yes/no) and any family history of heart attack in a first-degree relative (yes/no) [28]. The MESA risk score was calculated using the online calculator (https://www.mesa-nhlbi.org).
2.7. Statistics
Descriptive statistics (mean ± standard deviations (SD), Median [IQR] or numbers (%) of subjects) were used to present patient characteristics, behavior and dental and cardiovascular findings. Group differences were tested by one-way analysis of variance (ANOVA), independent T-Tests for quantitative variables or Chi-square analysis for categorical variables. Univariate binary logistic regression analyses (for the dichotomized dependent variable) and univariate linear regression analyses (for the continuous dependent variables) were performed to assess the association between each independent variable and the dependent variables. Multivariate linear regression analysis was performed afterwards for the independent variables that were significant in the univariate analyses. In multivariate regression analysis, the independent variables were adjusted by the most relevant confounders (gender, age, BMI, waist-to-hip ratio, education, alcohol and smoking). If an independent variable did not receive a significant p-value in the univariate analysis, the subsequent multivariate analysis was not presented. The significance level was set at a p-value of 0.05. All analyses were performed using SPSS 26.0 (IBM Corp, Armonk, NY, USA).
3. Results
A total of 41 patients with diagnosed, untreated periodontitis and 30 patients without periodontitis were recruited. The patient characteristics of these 71 patients are presented in Table 1. The study population consisted of 43.7% (n = 31) male and 56.3% (n = 40) female patients. The mean age was 53.4 years (SD 6.5). All patients were Caucasian. The mean BMI and waist-to-hip ratio were 23.4 kg/m2 (SD 6.0) and 0.87 (SD 0.09), respectively. Most patients were tertiary-educated adults (63.4%) without a positive family history of chronic diseases (59.2%). Nine patients (12.7%) were current smokers and 36 patients (51.4%) had never smoked. The mean number of alcohol servings per week was 4.3 (SD 4.5). Most patients visited their dentist for a routine dental check-up twice a year (63.4%), never visited a dental hygienist (53.5%), brushed their teeth twice a day (73.2%) and performed interdental cleaning daily or more often (74.6%). The dental conditions (independent variables) and cardiovascular conditions (dependent variables) are similarly listed in Table 1. Due to technical problems with the EndoPAT ™, the Reactive Hyperemia Index (RHI) of 17 patients was unknown.
Table 1.
Study population.
|
Background characteristics n = 71 | |
| Gender | |
| Male | 31 (43.7) |
| Female | 40 (56.3) |
| Age (years) | 53.4 ± 6.5 |
| BMI | 23.4 ± 6.0 |
| Waist-to-hip ratio | 0.87 ± 0.09 |
| Education | |
| Primary | 3 (4.2) |
| Secondary | 23 (32.4) |
| Tertiary | 45 (63.4) |
| Positive family history | |
| None | 42 (59.2) |
| Hypertension | 20 (28.2) |
| Diabetes Type 1 | 3 (4.2) |
| Hypercholesterolemia | 12 (16.9) |
| Rheumatoid arthritis | 6 (8.5) |
| Smoking status | |
| Never smoked | 36 (51.4) |
| Past smoker | 34 (48.6) |
| Current smoker | 9 (12.7) |
| Pack-years | 4.9 ± 9.7 |
| Alcohol servings/week | 4.3 ± 4.5 |
| Routine dental check-up | |
| Never | 4 (5.6) |
| Once a year | 22 (31.0) |
| Twice a year | 45 (63.4) |
| Dental hygienist visit | |
| Never | 38 (53.5) |
| Once a year | 8 (11.3) |
| Twice a year | 16 (22.5) |
| ≥ three times a day | 9 (12.7) |
| Toothbrushing | |
| Once a day | 6 (8.5) |
| Twice a day | 52 (73.2) |
| ≥ three times a day | 13 (18.3) |
| Interdental cleaning | |
| Never | 8 (11.3) |
| 1–6 times a week | 10 (14.1) |
| ≥ daily |
53 (74.6) |
|
Dental conditions- Independent variables | |
| PISA | 1112.2 ± 797.3 |
| Tooth loss | 5.8 ± 3.4 |
| No periodontitis | 30 42.3) |
| Periodontitis (stage III/IV) | 41 (57.7) |
| Plaque score | 43.3 ± 25.3 |
| Bleeding score |
46.9 ± 27.0 |
|
Cardiovascular conditions - Dependent variables | |
| CAC | 0 [10] |
| CAC score | |
| CAC = 0 | 45 (63.4) |
| CAC ≥1 | 26 (36.6) |
| Endothelial dysfunction (RHI)* | 2.4 ± 0.8 |
| SCORE | 1.1 ± 1.4 |
| Reynolds Risk Score | 3.4 ± 3.9 |
| Framingham Risk Score | 4.3 ± 4.7 |
| MESA Risk Score | 3.0 ± 3.0 |
Values represent mean ± standard deviation, number of subjects (%) or median [IQR]. * RHI n = 17 unknown.
We observed that 63.4% of patients had a zero CAC score, while the remainder had CAC scores ranging from 1 to 320. The CAC scores were not normally distributed, which resulted in a median of 0 (IQR 10). This prompted us to stratify all individuals into two groups. One group contained 45 patients with a CAC score zero and the other group consisted of the patients with elevated CAC scores ≥1 (n = 26). The characteristics of this study population were arranged per CAC group, as shown in Table 2. The patients in the group with elevated CAC scores were significantly older (p = 0.001). There were no significant differences in the other characteristics, health-related behaviour and dental conditions between the zero CAC score and the elevated CAC score group. Endothelial dysfunction was not significantly related to elevated CAC scores (p = 0.498). We found significant relations between elevated CAC scores and all cardiovascular risk prediction scores: SCORE (p = 0.000), Reynolds risk score (p = 0.018), Framingham risk score (0.000) and MESA risk score (p = 0.000).
Table 2.
Characteristics of the study population in relation to elevated CAC scores.
| CAC = 0 n = 45 | CAC ≥1 n = 26 | P-value | |
|---|---|---|---|
| Gender* | 0.070 | ||
| Male | 16 (35.6%) | 15 (57.7%) | |
| Female | 29 (64.4%) | 11 (42.3%) | |
| Age (years)** | 51.6 ± 6.2 | 56.6 ± 5.9 | 0.001 |
| BMI** | 22.7 ± 7.2 | 24.6 ± 3.0 | 0.208 |
| Waist to hip** | 0.86 ± 0.06 | 0.90 ± 0.10 | 0.071 |
| Education* | 0.964 | ||
| Primary | 2 (4.5%) | 1 (3.8%) | |
| Secondary | 15 (33.3%) | 8 (30.8%) | |
| Tertiary | 28 (62.2%) | 17 (65.4%) | |
| Positive family history* | 0.664 | ||
| None | 26 (57.8%) | 16 (61.5%) | 0.756 |
| Hypertension | 12 (26.6%) | 8 (30.8%) | 0.711 |
| Diabetes type 1 | 3 (6.7%) | 0 (0.0%) | 0.179 |
| Hypercholesterolemia | 7 (15.6%) | 5 (19.2%) | 0.691 |
| Rheumatoid arthritis |
6 (13.3%) |
0 (0.0%) |
0.052 |
|
Health related behavior | |||
| Smoking status* | 0,602 | ||
| Never smoked | 25 (55.6) | 11 (44.0) | |
| Past smoker | 20 (44.4) | 14 (56.0) | |
| Current smoker | 5 (11.1%) | 4 (15.4%) | 0.602 |
| Pack-years** | 4.5 ± 9.9 | 5.6 ± 9.5 | 0.652 |
| Alcohol servings/week** | 3.7 ± 3.6 | 5.3 ± 5.7 | 0.171 |
| Routine dental check-up* | 0.285 | ||
| Never | 4 (8.9) | 0 (0.0) | |
| Once a year | 13 (28.9) | 9 (34.6) | |
| Twice a year | 28 (62.2) | 17 (65.4) | |
| Dental hygienist visit* | 0.921 | ||
| Never | 25 (55.6) | 13 (50.0) | |
| Once a year | 5 (11.1) | 3 (11.5) | |
| Twice a year | 9 (20.0) | 7 (26.9) | |
| ≥ three times a day | 6 (13.3) | 3 (11.5) | |
| Toothbrushing* | 0.778 | ||
| Once a day | 3 (6.7) | 3 (11.5) | |
| Twice a day | 33 (73.3) | 19 (73.1) | |
| ≥ three times a day | 9 (20.0) | 4 (15.4) | |
| Interdental cleaning* | 0.370 | ||
| Never | 6 (13.3) | 2 (7.7) | |
| 1–6 times a week | 2 (4.4) | 8 (30.8) | |
| ≥ daily |
37 (82.2) |
16 (61.5) |
|
|
Dental health | |||
| PISA score* | 1106.7 ± 805.1 | 1121.7 ± 799.4 | 0.940 |
| Tooth loss* | 5.3 ± 3.2 | 5.5 ± 3.6 | 0.177 |
| Periodontal Disease stage ≥ III** | 25 (55.6) | 16 (61.5) | 0.623 |
| Plaque score* | 41.9 ± 26.9 | 45.6 ± 22.6 | 0.558 |
| Bleeding score* |
45,5 ± 28.6 |
49.23 ± 24.4 |
0.583 |
|
Cardiovascular conditions and risk predictors | |||
| Endothelial dysfunction (RHI) * | 2.4 ± 0.8 | 2.3 ± 0.7 | 0.498 |
| SCORE* | 1.5 ± 0.7 | 5.7 ± 3.5 | 0.000 |
| Reynolds Risk Score * | 2.6 ± 3.1 | 4.9 ± 4.8 | 0.018 |
| Framingham Risk Score* | 2.9 ± 3.1 | 6.8 ± 5.9 | 0.000 |
| MESA Risk Score* | 1.5 ± 0.7 | 5.7 ± 3.5 | 0.000 |
Values represent number of subjects (%) or mean ± standard deviation. Group differences were tested by Chi-square analysis* or independent T-Test **. Statistically significant, P-value <0.05.
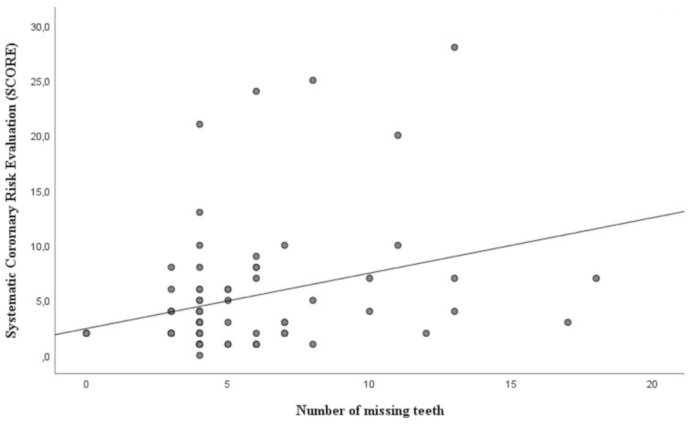
Table 3A shows the univariate regression analysis between the dental and the cardiovascular conditions. The Systematic Coronary Risk Evaluation (SCORE) algorithm showed a significant relation to tooth loss (p = 0.008), plaque score (p = 0.039) and bleeding score (p = 0.018). The Reynolds risk score was significantly associated to PISA (p = 0.05), plaque score (p = 0.017) and bleeding score (p = 0.007). The Framingham risk score displayed a significant relation to PISA (p = 0.005), plaque score (p = 0.027) and bleeding score (p = 0.003). In Fig. 1 we illustrate the association between tooth loss and the Systemic Coronary Risk Evaluation (SCORE) algorithm.
Table 3A.
Univariate regression analysis.
| B (SE) | OR (95%CI) | P | |
|---|---|---|---|
| CAC score ≥ 1* | |||
| PISA | 0.000 (0.000) | 1.000 (0.999; 1.001) | 0.939 |
| Tooth loss | 0.098 (0.074) | 1.103 (0.955; 1.274) | 0.183 |
| PD stage ≥ III | 0.247 (0.502) | 0.781 (0.292; 2.092) | 0.623 |
| Plaque score | 0.006 (0.010) | 1.006 (0.987; 1.025) | 0.552 |
| Bleeding score | 0.005 (0.009) | 1.005 (0.987; 1.024) | 0.577 |
|
Unstandardized B (SE) |
95% CI of B |
P |
|
| RHI** | |||
| PISA | −4.796E-5 (0.000) | 0.000; 0.0000 | 0.710 |
| Tooth loss | 0.038 (0.029) | −0.020; 0.97 | 0.193 |
| PD stage ≥ III | 0.083 (0.211) | −0.339; 0.505 | 0.695 |
| Plaque score | −0.001 (.004) | −0.009; 0.008 | 0.893 |
| Bleeding score |
3.388E-5 (0.004) |
−0.007; 0.007 |
0.993 |
| SCORE** | |||
| PISA | 0.000 (0.000) | 0.000; 0.001 | 0.086 |
| Tooth loss | 0.131 (0.048) | 0.035; 0.227 | 0.008 |
| PD stage ≥ III | 0.559 (0.338) | −0.114; 1.233 | 0.102 |
| Plaque score | 0.014 (0.007) | 0.001; 0.027 | 0.039 |
| Bleeding score |
0.015 (0.006) |
0.003; 0.027 |
0.018 |
| Reynolds risk score** | |||
| PISA | 0.001 (0.001) | 0.000; 0.002 | 0.050 |
| Tooth loss | 0.237 (0.136) | −0.034; 0.508 | 0.086 |
| PD stage ≥ III | 1.367 (0.930) | −0.488; 3.221 | 0.146 |
| Plaque score | 0.044 (0.018) | 0.008; 0.079 | 0.017 |
| Bleeding score |
0.046 (0.016) |
0.013; 0.078 |
0.007 |
| Framingham risk score** | |||
| PISA | 0.002 (0.001) | 0.001; 0.003 | 0.005 |
| Tooth loss | 0.233 (0.164) | −0.095; 0.561 | 0.160 |
| PD stage ≥ III | 1.463 (1.118) | −0.795; 3.667 | 0.204 |
| Plaque score | 0.048 (0.021) | 0.006; 0.091 | 0.027 |
| Bleeding score |
0.060 (0.020) |
0.022; 0.099 |
0.003 |
| MESA risk score** | |||
| PISA | 0.001 (0.000) | 0.000; 0.002 | 0.157 |
| Tooth loss | 0.063 (0.107) | −0.151; 0.276 | 0.560 |
| PD stage ≥ III | 0.594 (0.725) | −0.852; 2.041 | 0.415 |
| Plaque score | 0.018 (0.014) | −0.010; 0.047 | 0.196 |
| Bleeding score | 0.021 (0.013) | −0.005; 0.047 | 0.113 |
Univariate binary logistic regression analysis* and univariate linear regression analyses** were performed to assess the association between each independent variable and the dependent variables. Statistically significant, P-value <0.05.
Fig. 1.
Association between tooth loss and SCORE.
In the multivariate regression analysis, we adjusted for the most relevant confounders: gender, age, BMI, waist-to-hip ratio, education, alcohol and smoking (Table 3B). None of the dental conditions were significantly related to the cardiovascular risk predictors after correcting for the confounders in the multivariate regression analysis.
Table 3B.
Multivariate regression analysis.
| Unstandardized B (SE) | 95% CI | P | |
|---|---|---|---|
| SCORE | |||
| Tooth loss | 0.032 (0.041) | −0.050; 0.114 | 0.437 |
| Plaque score | 0.004 (0.005) | −0.005; 0.014 | 0.375 |
| Bleeding score |
0.004 (0.005) |
−0.006; 0.014 |
0.396 |
| Reynolds risk score | |||
| PISA | −2.804E-5 (0.001) | −0.001; 0.001 | 0.963 |
| Plaque score | 0.023 (0.016) | −0.008; 0.055 | 0.144 |
| Bleeding score |
0.019 (0.016) |
−0.013; 0.051 |
0.241 |
| Framingham risk score | |||
| PISA | 0.000 (0.001) | −0.001; 0.001 | 0.676 |
| Plaque score | 0.019 (0.015) | −0.012; 0.50 | 0.231 |
| Bleeding score | 0.023 (0.016) | −0.009; 0.054 | 0.153 |
Multivariate regression analysis, adjusted by the most relevant confounders: gender, age, BMI, waist to hip ratio, education, alcohol and smoking. Statistically significant, P-value <0.05.
4. Discussion
In this prospective cross-sectional study, we found significant relations between tooth loss, dental plaque and bleeding scores and the CVD risk predictors: SCORE, Reynolds risk score and Framingham risk score. However, when adjusting for confounders (gender, age, BMI, waist-to-hip ratio, education, alcohol and smoking) this association was no longer significant. Similarly, the significant relations between the Periodontal Inflamed Surface Area (PISA) score and the Framingham and Reynolds CVD risk predictors were no longer significant after correcting for these confounders. We did not find significant associations between the presence and extent of coronary calcification, as investigated by the CAC score, and periodontitis or dental health. Nor did we find significant associations between endothelial dysfunction and periodontitis or poor dental health.
This study is the third to have explored the possible correlation between Coronary Artery Calcium (CAC) scores and dental health, especially periodontitis. The Atherosclerosis Risk in Communities (ARIC) study was the first study that used the CAC score to investigate the association with periodontitis. The ARIC study included healthy patients and patients with known (chronic) diseases (excepting clinically recognized CVD), more than 20 years ago. The mean interval between the dental examinations and CAC score was 2.4 years (range: 0.9–4.3 years). Its results suggested that periodontitis is not strongly associated with CAC [29]. The second study used the Coronary Artery Calcification in Type 1 diabetes patients (CACTI study), conducted in 2003. Periodontitis was self-reported by an unvalidated questionnaire and clinical dental examination was not performed. The researchers concluded that in patients with Type 1 diabetes, periodontal disease duration was significantly related to CAC progression, but this was not the case in subjects without diabetes [30]. Taking above mentioned into account, we are the first study that included exclusively, asymptomatic healthy patients from the dental practice. The CAC score was investigated approximately 2 weeks after the full-mouth examination, performed by a periodontist. Due to the explorative nature of this study, a proper power-analysis was not applicable and the Medical Ethics Committee approved this presented sample size. In retrospect, enlargement of the study population would have strengthened the current study.
The CAC score is proven as a strong biomarker for cardiovascular atherosclerotic diseases. The absence of CAC (CAC = 0) provides the strongest ‘negative risk factor’ compared to traditional end novel cardiovascular biomarkers, especially in asymptomatic patients [31]. All the patients in our study population were asymptomatic, and most of them had a zero CAC score. Instead, the presence of coronary calcification on a cardiac CT scan is a late phenomenon. Endothelial dysfunction has been recognized as an important indicator of more early-stage atherosclerosis. A possible clinical scenario could be to use the Reactive Hyperemia Index (RHI) as a first screening, and if this indicates vascular disease, CAC scores could be calculated to add one more prognostic indicator [24]. In this study, there was no significant relation between periodontitis or dental health and endothelial dysfunction. A limitation is that, due to technical problems with the EndoPAT™, the RHI of 17 patients was unknown.
Previous pathophysiological evidence points to the possible common inflammatory background between periodontitis and atherosclerosis [8]. Another dental pathological condition is apical periodontitis. In this situation, there is a chronic inflammation around the apex of a tooth, caused by bacterial invasion of the pulp and root canal, most often as a result of untreated dental carries. These peri-apical lesions contain bacteria that can be translocated throughout the body and lodge in various organs and atherosclerotic plaques [32]. Nevertheless, there are only a few studies that have suggested a link between CVD and chronic apical periodontitis [33,34]. In this study, the presence of peri-apical lesions was not taken into account, but we focused on the most common dental pathology: periodontitis.
Periodontitis and CVD are complex inflammatory diseases with genetic and epigenetic factors that interact with lifestyle and environmental factors such as smoking, nutrition and stress. Both diseases are considerably influenced by similar multilevel interactions between metabolic and immune systems. The relatively recent realization that obesity affects the immune system and promotes inflammation may provide a plausible mechanism for the observed overlap between periodontitis and cardiovascular diseases [35]. Like obesity, the other components of metabolic syndrome (dyslipidemia, diabetes/hyperglycemia, and hypertension) are also linked to periodontitis through a number of pathomechanisms [36]. Moreover, the shared genetic basis of periodontitis and CVDs has recently been demonstrated [37]. It seems that the overall profile of a typical periodontitis patients is similar to the profile of a CVD patient, and vice versa.
We included patients who visited a specialized dental clinic for periodontology. It must be taken into account that these patients are not fully representative of the general population. This selection bias is a limitation of this study.
To conclude, based on this study, periodontitis is associated to a higher risk for cardiovascular morbidity and mortality, but is not an independent risk factor. Considering the findings of this study and previous studies, it is still increasingly important to bridge the once-wide gap between dentistry and general medicine to identify patients at risk for cardiovascular diseases in an earlier stage.
Credit author statement
H.C.M. Donders: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, writing original draft, Writing – review & editing, Visualization, Project administration, Funding acquisition. E.O. Veth: Conceptualization, Investigation, Resources. A.W.J. van ‘t Hof: Conceptualization, Methodology, Formal analysis, writing original draft, Writing – review & editing, Supervision, Funding acquisition. J. de Lange: Conceptualization, Methodology, Formal analysis, writing original draft, Writing – review & editing, Supervision, Funding acquisition. B.G. Loos: Conceptualization, Methodology, Formal analysis, writing original draft, Writing – review & editing, Supervision.
Conflict of interest and sources of funding statement
The authors declare that there are no conflicts of interest in this study.
This work was supported by the I&W fund of the Isala Academy, Zwolle (INNO1310).
Registered in the ISRCTN trial registry
ISRCTN55656827 https://doi.org/10.1186/ISRCTN55656827.
The association between oral health and heart diseases.
The data underlying this article are available at https://doi.org/10.6084/m9.figshare.14984175.v1.
Acknowledgements
First of all, we acknowledge all the patients who voluntarily participated in this study. We thank all the staff of the Practice for Periodontology Zwolle (PPZ), especially Elinet Vader, for their generous support. We also acknowledge Heike Ruiterkamp (Isala Academy Zwolle) for her dedicated assistance. Furthermore, we thank Dr. Renske Thomas for her commitment during the design of this study and Dr. Naichuan Su for his statistical support.
This work was supported by the I&W fund of the Isala Academy, Zwolle (INNO1310).
References
- 1.WHO, WHO . World Health Orginisation; 2015. Cardiovascular Diseases (CVDs) - Fact Sheet N°317.https://books.google.nl/books?hl=nl&lr=&id=SDYLDgAAQBAJ&oi=fnd&pg=PP1&dq=WHO+ 2015. 2015).+Cardiovascular+diseases+(CVDs)+-+Fact+sheet+N°317,+World+Health+Organization.&ots=SXa0LtGjiq&sig=Lmt_38Hv_H7OmL8Wd85I87ZVtKE#v=onepage&q&f=false. [Google Scholar]
- 2.Raggi P., Genest J., Giles J.T., Rayner K.J., Dwivedi G., Beanlands R.S., Gupta M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis. 2018;276:98–108. doi: 10.1016/j.atherosclerosis.2018.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Libby P. Inflammation in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2012;32:2045–2051. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriya J. Critical roles of inflammation in atherosclerosis. J. Cardiol. 2019;73:22–27. doi: 10.1016/j.jjcc.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet (London, England) 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 6.Loos B.G. Systemic markers of inflammation in periodontitis. J. Periodontol. 2005;76:2106–2115. doi: 10.1902/jop.2005.76.11-S.2106. [DOI] [PubMed] [Google Scholar]
- 7.Papapanou P.N., Susin C. Periodontitis epidemiology: is periodontitis under-recognized, over-diagnosed, or both? Periodontol. 2000;75:45–51. doi: 10.1111/prd.12200. 2017. [DOI] [PubMed] [Google Scholar]
- 8.Schenkein H.A., Loos B.G. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J. Clin. Periodontol. 2013;40(Suppl 1):S51–S69. doi: 10.1111/jcpe.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattila K.J., Nieminen M.S., Valtonen V.V., Rasi V.P., Kesäniemi Y.A., Syrjälä S.L., Jungell P.S., Isoluoma M., Hietaniemi K., Jokinen M.J. Association between dental health and acute myocardial infarction. BMJ. 1989;298:779–781. doi: 10.1136/bmj.298.6676.779. http://www.ncbi.nlm.nih.gov/pubmed/2496855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanz M., Marco del Castillo A., Jepsen S., Gonzalez-Juanatey J.R., D'Aiuto F., Bouchard P., Chapple I., Dietrich T., Gotsman I., Graziani F., Herrera D., Loos B., Madianos P., Michel J.B., Perel P., Pieske B., Shapira L., Shechter M., Tonetti M., Vlachopoulos C., Wimmer G. Periodontitis and cardiovascular diseases: consensus report. J. Clin. Periodontol. 2020;47:268–288. doi: 10.1111/jcpe.13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockhart P.B., Bolger A.F., Papapanou P.N., Osinbowale O., Trevisan M., Levison M.E., Taubert K.A., Newburger J.W., Gornik H.L., Gewitz M.H., Wilson W.R., Smith S.C.J., Baddour L.M. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association?: a scientific statement from the American Heart Association. Circulation. 2012;125:2520–2544. doi: 10.1161/CIR.0b013e31825719f3. [DOI] [PubMed] [Google Scholar]
- 12.Pietropaoli D., Del Pinto R., Ferri C., Wright J.T., Giannoni M., Ortu E., Monaco A. Poor oral health and blood pressure control among US hypertensive adults: results from the national health and nutrition examination survey 2009 to 2014. Hypertension. 2018;72:1365–1373. doi: 10.1161/HYPERTENSIONAHA.118.11528. [DOI] [PubMed] [Google Scholar]
- 13.Muñoz Aguilera E., Suvan J., Buti J., Czesnikiewicz-Guzik M., Barbosa Ribeiro A., Orlandi M., Guzik T.J., Hingorani A.D., Nart J., D'Aiuto F. Periodontitis is associated with hypertension: a systematic review and meta-analysis. Cardiovasc. Res. 2020;116:28–39. doi: 10.1093/cvr/cvz201. [DOI] [PubMed] [Google Scholar]
- 14.Ridker P.M., Silvertown J.D. Inflammation, C-reactive protein, and atherothrombosis. J. Periodontol. 2008;79:1544–1551. doi: 10.1902/jop.2008.080249. [DOI] [PubMed] [Google Scholar]
- 15.Yousuf O., Mohanty B.D., Martin S.S., Joshi P.H., Blaha M.J., Nasir K., Blumenthal R.S., Budoff M.J. High-sensitivity C-reactive protein and cardiovascular disease: a resolute belief or an elusive link? J. Am. Coll. Cardiol. 2013;62:397–408. doi: 10.1016/j.jacc.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Orlandi M., Suvan J., Petrie A., Donos N., Masi S., Hingorani A., Deanfield J., D'Aiuto F. Association between periodontal disease and its treatment, flow-mediated dilatation and carotid intima-media thickness: a systematic review and meta-analysis. Atherosclerosis. 2014;236:39–46. doi: 10.1016/j.atherosclerosis.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Greenland P., Blaha M.J., Budoff M.J., Erbel R., Watson K.E. Coronary calcium score and cardiovascular risk. J. Am. Coll. Cardiol. 2018;72:434–447. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeboah J., McClelland R.L., Polonsky T.S., Burke G.L., Sibley C.T., O'Leary D., Carr J.J., Goff D.C., Greenland P., Herrington D.M. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. J. Am. Med. Assoc. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nesse W., Abbas F., van der Ploeg I., Spijkervet F.K.L., Dijkstra P.U., Vissink A. Periodontal inflamed surface area: quantifying inflammatory burden. J. Clin. Periodontol. 2008;35:668–673. doi: 10.1111/j.1600-051X.2008.01249.x. [DOI] [PubMed] [Google Scholar]
- 20.Fleiss J.L. Wiley; 1999. The Design and Analysis of Clinical Experiments.https://www.wiley.com/en-us/Design+and+Analysis+of+Clinical+Experiments-p-9780471349914 [Google Scholar]
- 21.Papapanou P.N., Sanz M., Buduneli N., Dietrich T., Feres M., Fine D.H., Flemmig T.F., Garcia R., Giannobile W.V., Graziani F., Greenwell H., Herrera D., Kao R.T., Kebschull M., Kinane D.F., Kirkwood K.L., Kocher T., Kornman K.S., Kumar P.S., Loos B.G., Machtei E., Meng H., Mombelli A., Needleman I., Offenbacher S., Seymour G.J., Teles R., Tonetti M.S. Periodontitis: consensus report of workgroup 2 of the 2017 world Workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018;45:S162–S170. doi: 10.1111/jcpe.12946. [DOI] [PubMed] [Google Scholar]
- 22.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. http://www.ncbi.nlm.nih.gov/pubmed/2407762 [DOI] [PubMed] [Google Scholar]
- 23.Rubinshtein R., Kuvin J.T., Soffler M., Lennon R.J., Lavi S., Nelson R.E., Pumper G.M., Lerman L.O., Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010;31:1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 24.Bonetti P.O., Pumper G.M., Higano S.T., Holmes D.R., Kuvin J.T., Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004;44:2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 25.Matsuzawa Y., Kwon T.-G., Lennon R.J., Lerman L.O., Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tattersall M.C., Gangnon R.E., Karmali K.N., Keevil J.G. Women up, men down: the clinical impact of replacing the Framingham Risk Score with the Reynolds Risk Score in the United States population. PLoS One. 2012;7 doi: 10.1371/journal.pone.0044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.-T., Corrà U., Cosyns B., Deaton C., Graham I., Hall M.S., Hobbs F.D.R., Løchen M.-L., Löllgen H., Marques-Vidal P., Perk J., Prescott E., Redon J., Richter D.J., Sattar N., Smulders Y., Tiberi M., van der Worp H.B., van Dis I., Verschuren W.M.M., De Backer G., Roffi M., Aboyans V., Bachl N., Bueno H., Carerj S., Cho L., Cox J., De Sutter J., Egidi G., Fisher M., Fitzsimons D., Franco O.H., Guenoun M., Jennings C., Jug B., Kirchhof P., Kotseva K., Lip G.Y.H., Mach F., Mancia G., Bermudo F.M., Mezzani A., Niessner A., Ponikowski P., Rauch B., Rydén L., Stauder A., Turc G., Wiklund O., Windecker S., Zamorano J.L. European guidelines on cardiovascular disease prevention in clinical practice: the sixth joint task force of the European society of Cardiology and other societies on cardiovascular disease prevention in clinical Practice (constituted by representat. Eur. J. Prev. Cardiol. 2016;23:NP1–NP96. doi: 10.1177/2047487316653709. 2016. [DOI] [PubMed] [Google Scholar]
- 28.McClelland R.L., Jorgensen N.W., Budoff M., Blaha M.J., Post W.S., Kronmal R.A., Bild D.E., Shea S., Liu K., Watson K.E., Folsom A.R., Khera A., Ayers C., Mahabadi A.-A., Lehmann N., Jöckel K.-H., Moebus S., Carr J.J., Erbel R., Burke G.L. 10-Year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic study of atherosclerosis) with validation in the HNR (heinz nixdorf recall) study and the DHS (dallas heart st. J. Am. Coll. Cardiol. 2015;66:1643–1653. doi: 10.1016/j.jacc.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakib S.A., Pankow J.S., Beck J.D., Offenbacher S., Evans G.W., Desvarieux M., Folsom A.R. Periodontitis and coronary artery calcification: the Atherosclerosis Risk in Communities (ARIC) study. J. Periodontol. 2004;75:505–510. doi: 10.1902/jop.2004.75.4.505. [DOI] [PubMed] [Google Scholar]
- 30.Groves D.W., Krantz M.J., Hokanson J.E., Johnson L.R., Eckel R.H., Kinney G.L., Rewers M., Snell-Bergeon J.K., Alman A.C. Comparison of frequency and duration of periodontal disease with progression of coronary artery calcium in patients with and without Type 1 diabetes mellitus. Am. J. Cardiol. 2015;116:833–837. doi: 10.1016/j.amjcard.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blaha M.J., Cainzos-Achirica M., Greenland P., McEvoy J.W., Blankstein R., Budoff M.J., Dardari Z., Sibley C.T., Burke G.L., Kronmal R.A., Szklo M., Blumenthal R.S., Nasir K. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the multi-ethnic study of atherosclerosis (MESA) Circulation. 2016;133:849–858. doi: 10.1161/CIRCULATIONAHA.115.018524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georgiou A.C., Crielaard W., Armenis I., de Vries R., V van der Waal S. Apical periodontitis is associated with elevated concentrations of inflammatory mediators in peripheral blood: a systematic review and meta-analysis. J. Endod. 2019;45:1279–1295. doi: 10.1016/j.joen.2019.07.017. e3. [DOI] [PubMed] [Google Scholar]
- 33.Berlin-Broner Y., Febbraio M., Levin L. Association between apical periodontitis and cardiovascular diseases: a systematic review of the literature. Int. Endod. J. 2017;50:847–859. doi: 10.1111/iej.12710. [DOI] [PubMed] [Google Scholar]
- 34.González-Navarro B., Segura-Egea J.J., Estrugo-Devesa A., Pintó-Sala X., Jane-Salas E., Jiménez-Sánchez M.C., Cabanillas-Balsera D., López-López J. Relationship between apical periodontitis and metabolic syndrome and cardiovascular events: a cross-sectional study. J. Clin. Med. 2020;9:3205. doi: 10.3390/jcm9103205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aarabi G., Zeller T., Seedorf H., Reissmann D.R., Heydecke G., Schaefer A.S., Seedorf U. Genetic susceptibility contributing to periodontal and cardiovascular disease. J. Dent. Res. 2017;96:610–617. doi: 10.1177/0022034517699786. [DOI] [PubMed] [Google Scholar]
- 36.Jepsen S., Suvan J., Deschner J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontol. 2000;83:125–153. doi: 10.1111/prd.12326. 2020. [DOI] [PubMed] [Google Scholar]
- 37.Munz M., Richter G.M., Loos B.G., Jepsen S., Divaris K., Offenbacher S., Teumer A., Holtfreter B., Kocher T., Bruckmann C., Jockel-Schneider Y., Graetz C., Munoz L., Bhandari A., Tennstedt S., Staufenbiel I., van der Velde N., Uitterlinden A.G., de Groot L.C.P.G.M., Wellmann J., Berger K., Krone B., Hoffmann P., Laudes M., Lieb W., Franke A., Dommisch H., Erdmann J., Schaefer A.S. Genome-wide association meta-analysis of coronary artery disease and periodontitis reveals a novel shared risk locus. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-31980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]