Highlights
-
•
The prevalence of heart failure with preserved ejection fraction among patients who present with symptoms of heart failure is approximately 50%.
-
•
Pharmacologic therapy has not conclusively shown benefits in morbidity and mortality in clinical trials.
-
•
Although it represents an active area of research, no device-based therapy has received regulatory approval for the treatment of heart failure with preserved ejection fraction.
-
•
Approaches such as atrial shunts, left ventricular expanders, mechanical circulatory support devices, and neurostimulators are at various stages of development.
Key Words: atrial shunt devices, electrostimulation, heart failure devices, heart failure with preserved ejection fraction, HFpEF, left ventricular expanders, mechanical circulatory support, neuromodulation
Abbreviations and Acronyms: BAT, baroreceptor activation therapy; CCM, cardiac contractility modulation; CRT, cardiac resynchronization therapy; HF, heart failure; HFmEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IASD, Interatrial Shunt Device; LAAD, left atrial assist device; LAP, left atrial pressure; PCWP, pulmonary capillary wedge pressure; LV, left ventricular; LVEF, left ventricular ejection fraction; MCS, mechanical circulatory support; NYHA, New York Heart Association; QoL, quality of life; TAA, transapical approach
Central Illustration
Summary
Characterized by a rapidly increasing prevalence, elevated mortality and rehospitalization rates, and inadequacy of pharmaceutical therapies, heart failure with preserved ejection fraction (HFpEF) has motivated the widespread development of device-based solutions. HFpEF is a multifactorial disease of various etiologies and phenotypes, distinguished by diminished ventricular compliance, diastolic dysfunction, and symptoms of heart failure despite a normal ejection performance; these symptoms include pulmonary hypertension, limited cardiac reserve, autonomic imbalance, and exercise intolerance. Several types of atrial shunts, left ventricular expanders, stimulation-based therapies, and mechanical circulatory support devices are currently under development aiming to target one or more of these symptoms by addressing the associated mechanical or hemodynamic hallmarks. Although the majority of these solutions have shown promising results in clinical or preclinical studies, no device-based therapy has yet been approved for the treatment of patients with HFpEF. The purpose of this review is to discuss the rationale behind each of these devices and the findings from the initial testing phases, as well as the limitations and challenges associated with their clinical translation.
Heart failure (HF), which occurs when the heart is unable to pump a sufficient amount of blood or fill adequately to keep up with the metabolic demands of the body, is a leading cause of death worldwide. It has diverse etiologies, including disorders of cardiovascular origin, systemic morbidity, and hereditary defects, and it is clinically recognized by a multitude of complex symptoms that arise due to molecular, structural, and functional cardiac abnormalities (1,2).
Traditionally, 2 main phenotypes of HF are distinguished based on the left ventricular ejection fraction (LVEF): 1) HF with reduced ejection fraction (HFrEF); and 2) HF with preserved ejection fraction (HFpEF). They are characterized by LVEF ≤40% and LVEF ≥50%, respectively (3, 4, 5, 6). The European Society of Cardiology HF guidelines were revised to introduce a third phenotype of HF, characterized by an LVEF of 40% to 49% and referred to as HF with mid-range ejection fraction (HFmEF) (7). Because these patients were historically either excluded from the vast majority of clinical trials for HF or combined with categories with LVEF <40% or LVEF >49%, patients with HFmEF have emerged as a borderline population (8). Recent studies have highlighted that patients with HFmEF have a similar risk of developing diabetes and atrial fibrillation as patients with HFpEF, which is higher than those with HFrEF. Conversely, the burden of ischemic heart disease is more common in patients with HFrEF and HFmEF compared with patients with HFpEF (9).
Although it has been established that each type of HF is characterized by distinct demographic characteristics, comorbidities, and response to therapies, research efforts have long focused on HFrEF (5,10). Nevertheless, the prevalence of HFpEF has been rapidly increasing in the last few decades, mainly in response to the rise in life expectancy, the growing prevalence of metabolic disorders often associated with this disease, and the lack of adequate therapies (5,11, 12, 13, 14). Consequently, it is estimated that >3 million people in the United States are currently affected by HFpEF, which has become the dominant type of HF and a major public health problem (5,15). Although it is difficult to accurately predict the economic burden of HFpEF, in 2030, the total medical cost of HF in the United States is estimated to reach approximately $53.1 billion (16). Because HFpEF currently accounts for approximately 50% of all cases of HF (12), and assuming this is still the case by 2030, it would translate to a total medical expenditure of $26.55 billion, $21.24 billion of which is expected to be spent on hospitalization.
It is known that hypertension (80%-90%) and obesity (60%-75%) are major risk factors for HFpEF (5), as they both induce a systemic pro-inflammatory state, ultimately driving cardiac remodeling and ventricular hypertrophy (17). Furthermore, the former is also responsible for causing a state of pressure overload, which alters the left ventricular (LV) biomechanics (as reviewed in the following section) (18), whereas the latter is associated with defects in fuel utilization and efficiency, lipotoxicity, and loss of cytoprotective signaling (19). All these mechanisms are believed to further promote myocardial fibrosis in HFpEF. Other comorbidities playing a critical role in disease onset and progression include aging, coronary artery disease, diabetes mellitus, chronic kidney disease, pulmonary hypertension, chronic obstructive pulmonary disease, and anemia (10,17,20).
Our current understanding of HFpEF pathophysiology has been hindered by the heterogeneity in disease phenotypes, the lack of consensus on diagnostic guidelines, and the absence of a robust animal model (21). As a result, patients with HFpEF typically have a survival rate comparable to that associated with HFrEF and lower than that of most cancers (10,12). To date, most of the pharmacologic agents investigated in clinical trials have generated inconclusive results for the treatment of HFpEF, which remains largely directed toward exercise and the management of symptoms of congestion and associated comorbidities (5) through diuretics and mineralocorticoid antagonists.
Atrial shunts, LV expanders, electrical and neurostimulators, and mechanical circulatory support (MCS) devices constitute the 4 main categories of device-based solutions for the treatment of HFpEF. Although none of these devices has yet been approved for clinical use in the United States, data from randomized clinical trials and preclinical testing are encouraging, instilling hope that they may eventually revolutionize the current paradigm of HFpEF management and improve the survival and quality of life (QoL) of patients with HFpEF.
Structural and Hemodynamic Abnormalities of HFpEF
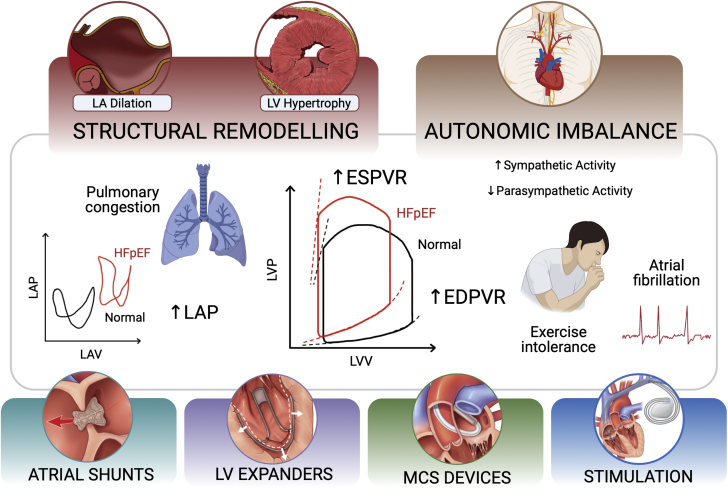
LV diastolic dysfunction is a key contributor to HFpEF pathophysiology and is often mediated by structural alterations. Diminished relaxation can be due to a variety of causes, including secondary LV hypertrophy and hypertrophic or restrictive cardiomyopathies (22), and causes the hemodynamics and symptoms of HFpEF. Figure 1 presents an overview of the pathophysiology of HFpEF and the 4 principal categories of device-based solutions currently under investigation.
Figure 1.
HFpEF Pathophysiology Overview
Overview of the structural, functional, and hemodynamic derangements associated with heart failure with preserved ejection fraction (HFpEF) as targeted by device-based solutions currently under development. The 4 main categories of medical devices for HFpEF are atrial shunts, left ventricular (LV) expanders, mechanical circulatory support (MCS) devices, and electrical and neurostimulators. EDPVR = end-diastolic pressure-volume relationship; ESPVR = end-systolic pressure-volume relationship; LA = left atrial; LAP = left atrial pressure; LAV = left atrial volume; LVP = left ventricular pressure; LVV = left ventricular volume.
In two thirds of these patients, matrix metalloproteinases and their inhibitors are down-regulated and up-regulated, respectively, causing a net increase in the amount of interstitial collagen (23,24). Changes in cardiomyocyte stiffness have been observed and mainly attributed to the cytoskeletal protein titin through a number of possible pathways, including isoform shifts (25,26), alterations in its phosphorylation state (27), and oxidative stress–induced disulfide bridge formation (28). Among others, these changes mediate thickening and stiffening of the LV wall, or concentric remodeling, characteristic of HFpEF. In patients with hypertension or aortic stenosis, these remodeling processes are believed to be partly induced by a state of LV pressure overload. Specifically, wall stress increases to maintain ejection performance under an elevated load that the heart pumps against during systole (ie, the afterload). In this context, concentric remodeling occurs to minimize the changes in wall stress, as predicted by the law of Laplace (18,29, 30, 31).
These structural alterations lead to diminished filling performance and elevated diastolic pressure, and hence the characteristic upward shift of the end-diastolic pressure-volume relationship (29). Analogously, the inability of the left ventricle to fill adequately causes a reduction in the LV end-diastolic volume, and thus a drop in stroke volume (32). Elevated LV end-diastolic pressures can be transmitted retrogradely to the left atrium, driving atrial remodeling and causing the symptoms of pulmonary congestion, exertional dyspnea, atrial fibrillation, rhythm abnormalities, and mechanical desynchrony often associated with HFpEF. Furthermore, autonomic imbalance with up-regulated sympathetic activity and withdrawal of the vagal tone has a profound effect on cardiac function and structure, as it is implicated in the pathogenesis of atrial fibrillation and contributes to chronotropic incompetence (33,34). Altogether, these changes are largely responsible for several of the chronic symptoms in HFpEF, including exercise intolerance, which is a strong determinant of prognosis and QoL (29,35, 36, 37).
In addition to impaired relaxation, several studies suggest that systolic dysfunction ensues (13). Regional measures of systolic function, including long-axis shortening velocity and longitudinal and radial strains, are depressed in HFpEF despite a normal LVEF (38,39), whereas global parameters of contractility, such as end-systolic elastance or the end-systolic pressure-volume relationship, seem to improve (40,41), likely due to the effects of concentric remodeling and LV geometry on these metrics (13). Although evidence of systolic dysfunction is not required for the diagnosis of HFpEF, it plays an important role in the setting of exercise as it may contribute to limited inotropic reserve and thus to symptoms of exercise intolerance and reduced aerobic capacity.
Due to the variety in the structural and hemodynamic aberrations seen in HFpEF, arising from the various etiologies associated with the disease and corresponding to a broad symptomatology, a number of classifications of HFpEF have been proposed (42). For example, Burkhoff et al (22) identified 4 categories of HFpEF, each corresponding to distinct hemodynamic characteristics and based on the underlying disease. According to this classification, type 1 HFpEF is due to hypertrophic cardiomyopathies of genetic etiology and is characterized by diastolic dysfunction and blunted cardiac output. Type 2 is caused by infiltrative cardiomyopathies (eg, amyloidosis, sarcoidosis, endomyocardial fibrosis) and is associated with some degree of LV remodeling. Type 3 HFpEF identifies patients with HFpEF with diastolic dysfunction, without significant hypertrophy or underlying cardiovascular diseases. Finally, type 4 occurs in patients with one or more cardiovascular conditions, of vascular (eg, coronary artery disease), valvular (eg, aortic stenosis), systemic (eg, hypertension), or electrical (eg, atrial fibrillation) origin, or other comorbidities (eg, diabetes mellitus, obesity). This category is associated with the most aberrant hemodynamic phenotype, due to severe diastolic dysfunction, with markedly elevated LV diastolic and left atrial pressures (LAPs) (22).
Management of Patients with HFpEF
The absence of effective treatment options for HFpEF is a major contributor to the challenges associated with the management of these patients, particularly in the outpatient setting (5,43). To date, the majority of large-scale clinical trials aiming to evaluate the efficacy of medical therapies for HFpEF have had neutral results (5,44), with a few exceptions. These exceptions include exercise training, which was shown to increase functional capacity and QoL (45); mineralocorticoid receptor antagonists, which were shown to reduce hospitalization rates (46); and a combination of a neprilysin inhibitor (sacubitril) and an angiotensin-receptor blocker (valsartan), which was recently shown to decrease hospitalization and cardiovascular death rates and is approved for use in patients with HFpEF (47).
Current guidelines for the management of HFpEF recommend treatment of the associated symptoms and comorbidities. For example, beta-blockers, angiotensin-converting enzyme inhibitors, or angiotensin receptor blockers should be used in patients with hypertension (48,49), and coronary revascularization is suggested in patients with coronary artery disease, which exacerbates symptoms of HFpEF (3). Treatment of patients with cardiac amyloidosis generally aims to stabilize the involved protein or suppress the production thereof, while limiting the consequences of the disease and avoiding iatrogenic effects (50,51). Because of the vast abundance of precursor proteins identified in humans and the diverse pathogenesis, management of these patients is remarkably heterogeneous. In patients with cardiac amyloidosis and evidence of diastolic dysfunction (ie, type 2 HFpEF), angiotensin receptor blockers should be used with caution to mitigate the risk of severe hypotension (especially in the presence of autonomic system involvement), beta-blockers should be avoided to prevent negative chronotropic effects, and diuretics should be administered carefully to avoid excessive depletion that may severely affect cardiac output (51). Conversely, for patients with HFpEF with atrial fibrillation, maintaining sinus rhythm via various rhythm control therapies (eg, antiarrhythmic drugs, ablation, cardioversion) is a key strategy to lowering the risk of cardiovascular death and hospitalization (52,53).
Although each of these therapies was shown to ameliorate HFpEF symptoms, they failed to conclusively show reductions in mortality (5,13). The broad spectrum of phenotypes and comorbidities of HFpEF has potentially confounded findings from randomized clinical trials and, in some cases, led to contrasting evidence that has been difficult to reconcile. Although various classifications have been proposed to support patient stratifications (20,22,42), these have not yet been adopted in clinical trials, which typically use broad inclusion criteria and fail to categorize patients based on their comorbidities, symptoms, or structural or hemodynamic abnormalities. Furthermore, the scarcity of preclinical testing due to the lack of a reliable animal model of HFpEF has been a crucial contributing factor to the evaluation of traditional therapeutics and the development of novel approaches for the treatment of this condition.
Advancements in Therapeutic Options for HFpEF
The aim of the current work was to provide a comprehensive review of the rationale and progress in device-based solutions that improve the physiology and hemodynamics of HFpEF by targeting one or more of the abnormal biomechanical pathways occurring in the disease or associated symptomatology. We briefly summarize here the insights from the trials of some of the most promising pharmacologic therapies and other interventions or approaches.
Given the success of neurohormonal blockers for the treatment of HFrEF, significant efforts were made toward the investigation of these agents in HFpEF. Neurohormonal blockers have revolutionized the care of patients with HFrEF for several decades, as they reverse cardiac remodeling, thus increasing function and remarkably improving QoL and survival, and decreasing hospitalization rates in this patient population (54). However, several trials of the renin-angiotensin-aldosterone system blockers in patients with HFpEF failed to conclusively show benefits of this approach, causing it to be largely abandoned (19). Another approach that has been widely investigated is that of increasing cyclic guanosine monophosphate (cGMP)-protein kinase G signaling due to its role in the attenuation of pathological cardiac hypertrophy and remodeling (55). A multitude of trials have studied the effects of up-regulating cGMP synthesis through inorganic nitrates in HFpEF or of other agents such as soluble guanylate cyclase stimulators (eg, vericiguat, praliciguat) (56, 57, 58). In addition, the effects of phosphodiesterase type 5 and 9 inhibition have also been investigated (59,60). To date, however, none of these pathways has shown clinical benefit in patients with HFpEF.
Other studies have focused on the potential role of dopamine in improving diuresis and that of inotropic modulators, such as the phosphodiesterase type 3 inhibitor milrinone (61,62). As with to other trials, these failed to meet the primary endpoints that would support use of these agents in HFpEF (61,63). Among other pharmacologic therapies currently under investigation are: the interleukin-1 receptor antagonist anakinra, believed to improve cardiorespiratory fitness in patients with HFpEF (64); the sodium-glucose co-transporter 2 inhibitor empagliflozin, to alleviate symptoms of pulmonary congestion and exercise intolerance (65); and the beta-adrenergic agonist albuterol, which has recently been shown to promote pulmonary vasodilation during exercise and to enhance exercise reserve (66). Finally, a cardiosphere-derived cell-based therapy is currently under evaluation in a double-blind feasibility trial (67). This technique involves intracoronary infusion of allogenic cardiosphere-derived cells with anti-inflammatory and antifibrotic properties. In a rat model of HFPEF, this technique was shown to improve LV relaxation through hemodynamic assessment, decrease pulmonary congestion, and enhance survival (68).
To date, compelling evidence exists regarding the use of the mineralocorticoid antagonist spironolactone and the dual angiotensin-neprilysin inhibitor sacubitril-valsartan (69,70). Particularly, the TOPCAT (Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist) trial reported strongly reduced hospitalization rates in the spironolactone group compared with placebo (69,71). A subgroup analysis of this trial restricted to patients enrolled in North America and South America, with a more definitive diagnosis of HFpEF and greater compliance to the study, revealed that, in these groups, spironolactone also significantly reduced cardiovascular death rates and symptoms of pulmonary congestion (72,73). The PARAGON (Prospective Comparison of ARNI with ARB Global Outcomes in Heart Failure With Preserved Ejection Fraction) trial compared the effects of sacubitril-valsartan with those of valsartan alone in 4,822 patients with symptomatic HFpEF. The dual agent was found to improve hospitalization rates, New York Heart Association (NYHA) functional class, and QoL slightly more significantly than valsartan alone (70,74). In addition, compared with the valsartan-only cohort, absolute risk reduction in the dual-agent group was found to be more pronounced in patients enrolled early (≤30 days) after hospitalization than in patients who enrolled later or who were never hospitalized (47,70).
In addition to these pharmacologic therapies and device-based solutions that improve the pathophysiology and hemodynamics of HFpEF (which are discussed in the rest of this review), other interventions are currently being investigated in patients with HFpEF. Examples are whole-body vibration therapy to potentially improve overall fitness in patients with exercise intolerance (75) and renal nerve denervation (76) or splanchnic nerve resection (77) to reduce sympathetic stimulation. Resection of the splanchnic nerve was hypothesized to reduce venous return and thus central vascular congestion in HFpEF; however, a study in 25 patients showed no significant changes in either microvascular or macrovascular function using this approach (78). Finally, the CardioMEMS HF System (Abbott) is a device that allows remote monitoring of changes in pulmonary artery pressure, allowing for personalized patient management. The system comprises a pressure sensor permanently implanted in the distal pulmonary artery via a right heart catheterization procedure and a home unit for data acquisition and transmission. The CardioMEMS HF System was shown to reduce HF hospitalizations and mortality and to improve QoL and was therefore recently approved for HFpEF therapy as well as for HFrEF therapy (46,79, 80, 81, 82, 83, 84).
Device-Based Solutions for HFpEF
The inadequacy of medical therapies for HFpEF has spurred the development of device-based solutions that address the physiological or hemodynamic changes occurring in the disease or aim to ameliorate symptoms. In HFrEF, a variety of devices, including cardiac resynchronization therapy (CRT) devices and MCS devices, have completely revolutionized clinical management of these patients, dramatically improving morbidity and survival. Although no device has yet received approval by the U.S. Food and Drug Administration for HFpEF, several are currently under investigation and have shown promising results in a variety of preclinical and clinical studies (85).
Device-based solutions for HFpEF target one or more of the functional abnormalities described earlier. This section first focuses on atrial shunts, which aim to alleviate LAP, or pulmonary capillary wedge pressure (PCWP), and associated symptoms of pulmonary congestion. We describe LV expanders, which enhance ventricular filling by augmenting the elastic recoil of the left ventricle from the endocardium or epicardium. A description of electrical stimulators and neuromodulators to improve mechanical desynchrony and autonomic imbalance follows. Finally, advancements in the development of mechanical circulatory support devices aiming to decompress the left atrium and restore arterial pulsatility and cardiac output are discussed. A summary of the HFpEF devices currently under development and, where applicable, of their advancements in clinical trials is provided in Table 1 and Supplemental Table 1.
Table 1.
Devices Under Investigation for the Treatment of HFpEF and Clinical Trials (continued in Supplemental Table 1)
| Device Category | Physiological Target | Devices | Company | Description |
|---|---|---|---|---|
| Atrial shunts | Elevated left atrial pressure | IASD | Corvia Medical Inc | Bare-metal nitinol frame with an 8-mm shunting diameter |
| V-Wave Shunt | W-Wave Ltd | Hourglass-shaped self-expanding nitinol frame covered with porcine pericardial tissue | ||
| AFR | Occlutech | Self-expandable double-disk nitinol mesh braided into 2 flat discs | ||
| Transcatheter Atrial Shunt System | Edwards Lifesciences | Bare-nitinol implant flanked with an internal diameter of 7 mm for shunting from the LA to the coronary sinus | ||
| Left ventricular expanders | Diminished left ventricular compliance | ImCardia | CorAssist, Inc | Self-expanding device, which exerts outward and circumferential forces to the external left ventricular surface |
| CORolla TAA | CorAssist, Inc | Elastic, spring-like device implanted inside the LV | ||
| Stimulators | Low baroreflex sensitivity | BAROSTIM NEO | CVRx Inc | Implantable device that activates the baroreceptors in the wall of the carotid artery to restore autonomic balance |
| Diminished diastolic function | OPTIMIZER Smart System (CCM) | Impulse Dynamics | Minimally invasive implantable device that applies a nonexcitatory stimulation enhancing contractility | |
| Mechanical desynchrony | CRT devices | Various manufacturers (eg, Medtronic, Sorin Group, Abbott) | Implantable devices providing electrical stimulation to the cardiac tissue | |
| MCS | Diminished cardiac output | The Synergy System |
HeartWare International | Miniature continuous-flow circulatory support device, simulated in the LV-Ao and LA-Ao configurations |
| CoPulse | NA | Valveless, pulsatile pump for implantation at the apex made of an actuation and a blood chamber separated by a soft membrane | ||
| LAAD | NA | Continuous flow pump to be implanted between the LA and LV after mitral valve removal | ||
| VADovations cardiac assist system | VADovations | Ultra-miniature endovascular pump for endovascular delivery | ||
| PulseVAD | Northern Development | First-generation smart pump providing adaptive and pulsatile flow for HFrEF and HFpEF |
See Supplemental Table 1 for additional information on devices under investigation.
6MWT = 6-minute walk test; AFR = Atrial Flow Regulator; Ao = aorta; CCM = cardiac contractility modulation; CRT = cardiac resynchronization therapy; EQ-5D = EuroQoL 5-Dimension; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; KCCQ = Kansas City Cardiomyopathy Questionnaire; LA = left atrium; LAAD = left atrial assist device; LV = left ventricle; LVAD = left ventricular assist device; MCS = mechanical circulatory support; MLHFQ = Minnesota Living with Heart Failure Questionnaire; NA = not applicable; NT-proBNP = N-terminal pro–B-type natriuretic peptide; NR = not reported; PCWP = pulmonary capillary wedge pressure; RCT = randomized controlled trial.
Atrial shunt devices
Atrial shunt devices are designed to lower elevated LAP by creating a conduit from the left atrium to other chambers or structures. Based on the categorization of HFpEF phenotypes proposed by Burkhoff et al (22), atrial shunts could be suitable for all 4 types of HFpEF, and particularly for type 4, as this is associated with the greatest elevation in LAP compared with the other phenotypes. Although the majority of these devices rely on interatrial shunting (ie, from the left atrium to the right atrium), novel approaches have recently emerged that shunt blood from the left atrium to the coronary sinus, aiming to overcome some of the limitations associated with interatrial devices, such as the risks of atrial arrhythmias or paradoxical embolism.
Atrial shunts are currently being evaluated in large clinical trials and hold promise for improving symptoms of pulmonary congestion, exercise capacity, and QoL for patients with HFpEF. As a result, evidence of elevated PCWP at rest or exercise, of a positive left atrium to right atrium pressure gradient, and a history of exercise intolerance are required as inclusion criteria by most of these trials. Conversely, because of the risk of shunt occlusion or stenosis and the need for anticoagulation therapy, patients with a history of thromboembolic events or allergy to antiplatelet, anticoagulant, or antithrombotic agents are typically excluded. Atrial shunting may also impose extra stress on the right heart and significantly affect cardiac and pulmonary hemodynamics, making these devices not suitable for patients with right ventricular dysfunction, valvular defects, or severe restrictive or obstructive lung disease. In addition, patients with anatomic anomalies that preclude implantation, a history of atrial fibrillation, and allergy to nickel titanium–based materials are also typically excluded from these trials.
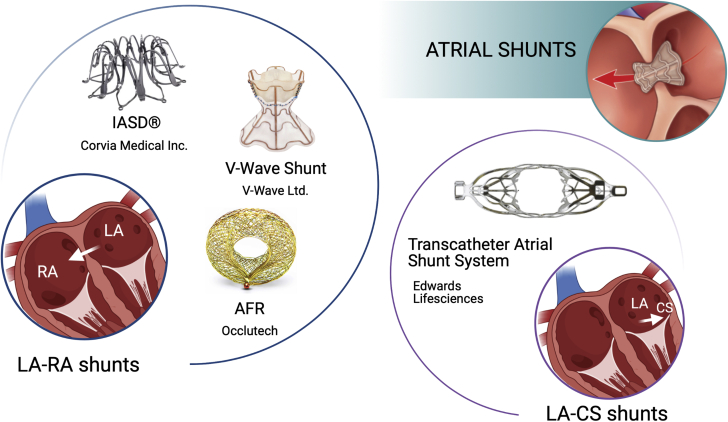
To date, 3 interatrial shunt devices are under clinical investigation (namely the Interatrial Shunt Device [IASD] [Corvia Medical, Inc], the V-Wave Shunt [V-Wave Ltd], and the Atrial Flow Regulator [AFR] [Occlutech]) and one left atrium–to–coronary sinus shunt, known as the Transcatheter Atrial Shunt System (Edwards Lifesciences) (Figure 2).
Figure 2.
Atrial Shunt Devices Under Development for the Treatment of HFpEF
These devices include the Interatrial Shunt Device (IASD), the V-Wave Shunt, and the Atrial Flow Regulator (AFR) as left atrium (LA) to right atrium (RA) shunts and the Transcatheter Atrial Shunt System as a LA to coronary sinus (CS) shunt. HFpEF = heart failure with preserved ejection fraction.
Images of the LA-RA shunts readapted with permission from Burlacu et al (85). Image of the Transcatheter Atrial Shunt System readapted with permission from Simard et al (115).
Interatrial shunt device
The IASD is a bare-metal nitinol frame device creating an 8-mm opening between the atria and allowing for blood to flow down the pressure gradient. The device is deployed percutaneously using a 16-F sheath via femoral venous access and under fluoroscopic and transesophageal echocardiographic guidance. After transseptal puncture at the mid fossa ovalis, the delivery catheter is positioned in the left atrium and stabilized across the septum (86).
The feasibility of the IASD was first reported in a pilot trial involving 11 patients (LVEF >45%, PWCP ≥15 mm Hg at rest, or PWCP ≥25 mm Hg during exercise), with one or more hospitalizations for HF within the 12 months before the beginning of the study or persistent NYHA functional class III/IV for at least 3 months (87). The device was successfully implanted in all patients and showed promising hemodynamic and functional results on 30-day follow-up, including a reduction in LV filling pressure (14.2 ± 2.7 mmHg compared with a baseline of 19.7 ± 3.4 mm Hg; P = 0.005), associated NYHA functional class of HF, and QoL improvements in the majority of the patients (87,88). These findings were corroborated with a 1-year follow-up study conducted on 64 patients (89), which showed stable reduction in the LV end-diastolic volume (P < 0.001) and work-indexed PWCP (P < 0.01), alongside sustained improvements in NYHA functional class (P < 0.001), QoL (P < 0.001), and 6-minute walking distance (P < 0.01). In addition, the study revealed no major peri-procedural or adverse events, with a 1-year survival of 95% and device patency with evidence of left-to-right shunting (90,91). Another study ongoing on a total of 44 patients (92), one half of whom underwent implantation of the IASD, has thus far confirmed significant reduction in PCWP during exercise at 1 month in the IASD cohort compared with the control group (P = 0.028) (93). Furthermore, a pooled analysis from these 2 trials (89,92) showed that implantation of an IASD in patients with LVEF ≥40% is associated with significant improvements in pulmonary vascular function, with increased pulmonary flow and pulmonary artery compliance during both rest and exercise, and that these changes are even more remarkable in patients with a history of atrial fibrillation (94). Other clinical trials are ongoing (95,96) or have been planned (97) with increasingly larger cohorts. Particularly, 608 participants are being recruited for a post-marketing trial after the device has received CE approval in the European Union to evaluate efficacy, safety, and QoL outcomes of patients with HFpEF 1 year after implantation of the IASD (95).
V-Wave shunt
The V-Wave Shunt allows unidirectional left atrium to right atrium flow when the pressure gradient exceeds 5 mm Hg. It consists of an hourglass-shaped nitinol frame encapsulated with a partially expanded polytetrafluoroethylene cover serving as an anchor for 3 porcine pericardial leaflets held together using a Prolene suture (Ethicon Inc) (98,99). The V-Wave Shunt is implanted percutaneously by using a similar procedure as that for IASD implantation; it involves transesophageal echocardiographic–guided femoral venous access and the creation of an aperture in the fossa ovalis as large as the inner diameter of the hourglass waist (5.1 mm).
The feasibility and performance of this device were first reported in several preclinical and clinical HFrEF studies. Implantation of the device in an ovine model of chronic ischemic HFrEF revealed LA unloading while preserving the right atrial or pulmonary artery pressures (100). The implanted V-Wave Shunt devices remained patent for the duration of the study (12-week follow-up) and improved LV filling pressure (14 ± 1 mm Hg vs 22 ± 2 mm Hg; P < 0.05), LVEF (42% ± 3% vs 25% ± 2%; P < 0.05), and 12-week survival (93% vs 57%) compared with controls (100).The first-in-human experience in a 70-year-old man with HFrEF (LVEF 35%, PCWP 19 mm Hg, and NYHA functional class III) showed significant improvements in functional and hemodynamic parameters and QoL at 90 days (98). A proof-of-principle cohort study in Canada enrolling 10 patients with HFrEF (NYHA functional class III, LVEF 25% ± 8%, and PCWP 23 ± 5 mm Hg) showed initial safety and clinical efficacy at the 3-month follow-up, including improvements in NYHA functional class (from class III to class II in 78% of patients and from class III to class I in 1 patient; P = 0.0004), QoL (24.8 ± 12.9 vs 13 ± 6.2; P = 0.016), and 6-minute walking distance (318 ± 134 m vs 244 ± 112 m; P = 0.016), as well as a reduction in PCWP (17 ± 8 mm Hg vs 23 ± 5 mm Hg; P = 0.035) with no increase in the right atrium or pulmonary artery pressures and no change in pulmonary resistance (99).
A prospective, open-label, multicenter study recruited 22 patients at 1 center in Canada and 16 patients at 5 centers in Israel and Spain (30 patients with HFrEF and 8 patients with HFpEF) to evaluate the safety, feasibility, and efficacy of the V-Wave Shunt at a median follow-up of 28 months (range: 18-48 months) (101,102). The shunt was successfully implanted in all 38 patients (100%) with 1 device- or procedure-related adverse event at 12 months (2.6%) and 3 all-cause adverse events at 12 months (7.8%), including 2 deaths. The device improved NYHA functional class (class I and II in 60% of the patients; P < 0.001), QoL (improvements ≥5 points in 73% of the patients; P < 0.001), and 6-minute walking distance (mean increase 28 ± 83 m; P = 0.012), whereas no significant changes were observed in laboratory parameters, echocardiographic variables, or hemodynamic status. Although shunt patency at 3 months was 100%, there was a 14% rate of shunt occlusion and a 36% rate of shunt stenosis at 12 months, likely due to pannus infiltration of the bioprosthetic leaflets (102).
Following enhancements in the device design to improve patency, the RELIEVE-PAH (Reducing Right Ventricular Failure in Pulmonary Arterial Hypertension) (103) and the RELIEVE-HF (Reducing Lung Congestion Symptoms using the V-Wave Shunt in Advanced Heart Failure) (104) trials were launched to evaluate safety and efficacy in patients with severe pulmonary arterial hypertension and HF regardless of LVEF, respectively. Preliminary results of the latter trial on a total of 10 patients undergoing implantation of the second-generation V-Wave Shunt showed patency and absence of stenosis in all cases at the 1-year follow-up. Significant improvements in NYHA functional class (16.7% vs 100% of patients in class III-IV; P = 0.013) and 6-minute walking distance (338 ± 104 m vs 274 ± 65; P = 0.169) were also observed, suggesting continued efficacy of the device (105).
Atrial flow regulator
The Occlutech atrial flow regulator (AFR) is a self-expandable nitinol mesh braided into 2 flat discs creating a 1- to 2-mm fenestrated neck. The opening can have various diameters (6, 8, and 10 mm) and is designed to allow interatrial bidirectional flow (106). The AFR is implanted in the interatrial septum through a femoral venous access after an interatrial septal puncture using a 10-F to 12-F introducing catheter (107).
The AFR device (Occlutechcal) was first implanted in a 54-year-old woman with severe irreversible pulmonary arterial hypertension (108), who showed improvements in energy level, resting oxygen saturation, 6-minute walking distance, and symptom relief from ascites and pedal edema at 6-week follow-up. A nonrandomized pilot study on 12 patients with severe pulmonary arterial hypertension undergoing AFR implantation reported analogous results, with improvements in 6-minute walking distance (432 ± 31.32 m vs 377.3 ± 33.2 m; P = 0.008), cardiac index (2.89 ± 0.56 L/min/m2 vs 2.36 ± 0.52 L/min/m2; P < 0.001), and systemic oxygen transport (428.0 ± 67.1 mL/min/m2 vs 367.5 ± 75.5 mL/min/m2; P = 0.04). Moreover, the device was shown to be patent in all patients at a median follow-up of 189 days (range: 10-296 days) (109). Results from the PRELIEVE (Pilot Study to Assess Safety and Efficacy of a Novel Atrial Flow Regulator [AFR] in Heart Failure Patients) trial (110), which evaluated device safety and feasibility in patients with both HFrEF and HFpEF, confirmed device patency in 92% of the cases at both 3 and 12 months, as well as a 5 mm Hg drop in PCWP (P = 0.0003) during rest at the 3-month follow-up (111). Improvements in PCWP were more significant in the HFpEF cohort than in the HFrEF cohort. However, changes in NYHA functional class, 6-minute walking distance, and QoL lacked significance, and 2 major adverse events were reported, namely device embolization and post-procedural bleeding and syncope.
Currently, a variety of trials are recruiting patients to test the safety and performance of the AFR. These include the Prophet (Pilot Study to Assess Safety and Efficacy of a Novel Atrial Flow Regulator [AFR] in Patients With Pulmonary Hypertension) trial (112), which seeks to evaluate feasibility in patients with pulmonary arterial hypertension; the AFteR registry (Follow-up Study to Monitor the Efficacy and Safety of the Occlutech AFR in Heart Failure Patients) (113), which targets a 3-year follow-up study in patients with HF; and FROST-HF (Flow Regulation by Opening the Septum in Patients With Heart Failure Trial) (114), which aims to investigate safety and performance of the AFR device specifically in patients with HFpEF.
Transcatheter atrial shunt system
To overcome some of the risks associated with interatrial shunts, including right-to-left shunting and systemic embolization, a novel approach was developed to reduce the PWCP through a shunt from the left atrium to the coronary sinus (115).
The Transcatheter Atrial Shunt System (Edwards Lifesciences) is a bare-nitinol implant with 4 arms and an internal shunting diameter of 7 mm. The device is deployed between the left atrium and the coronary sinus through a percutaneous atriotomy, a procedure involving coronary sinus cannulation from the right internal jugular vein, coronary sinus–to–LA puncture under coronary sinus angiography and balloon dilation of the LA wall (115).
The first study of this device was conducted on 11 patients (7 with HFpEF and 4 with HFrEF) with a median of 1 HF hospital admission in the year before implantation, NYHA functional class III or ambulatory class IV, and elevated PWCP. Of the 8 patients who underwent successful implantation, 87.5% improved their NYHA functional class to I or II, 42.9% experienced clinically significant improvements in the 6-minute walking distance (>32 m), and PCWP was reduced by 9 mmHg at a median follow-up of 100 days. In addition, follow-up echocardiography exhibited stable LVEF, LA volume index, and right ventricular function, whereas patency of all devices was confirmed by computed tomographic assessment (115). Based on these findings, a prospective early feasibility clinical trial was launched to evaluate safety, functionality, and efficacy of this device in a larger patient population (116). Despite circumventing the risk of paradoxical embolism, it remains to be assessed whether left atrium to coronary sinus shunting is effective in mitigating other risks associated with interatrial shunts, including that of atrial fibrillation and right HF, as well as other major adverse events or device-related complications.
LV expanders
LV expanders are spring-like devices that apply outward forces to the left ventricle to enhance filling performance, which is typically diminished in HFpEF. By storing elastic energy during cardiac contraction and transferring it to the LV wall in the diastolic phase, these devices seek to assist early diastolic recoil of the LV chamber (117,118). Analogously to atrial shunts, these devices could, in theory, augment filling in all 4 types of HFpEF. However, their performance could be largely affected by the degree of LV remodeling, which varies considerably across the HFpEF phenotypes. Moreover, diagnosis of hypertrophic cardiomyopathy or infiltrative heart disease was listed as exclusion criteria in clinical trials evaluating the safety and efficacy of LV expanders, effectively precluding investigations of these devices for types 1 and 2 HFpEF. In addition, patients with a history of pericardial disease, right HF, severe obstructive or restrictive lung disease, and who underwent cardiovascular interventions such as coronary artery bypass surgery and valve replacement or repair within 3 months before enrollment were also excluded from these trials. Diagnosis of HFpEF according to the 2016 European Society of Cardiology guidelines with a PCWP >15 mm Hg at rest was required for eligibility (7).
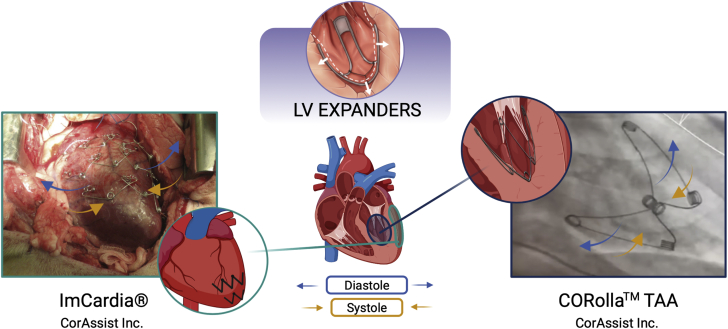
The ImCardia and the CORolla transapical approach (TAA) devices (CorAssist Inc) are the only LV expanders being developed and were designed for implantation on the pericardial and endocardial wall, respectively, as shown in Figure 3.
Figure 3.
LV Expander Devices Under Development for the Treatment of HFpEF
These devices include the ImCardia and CORolla transapical approach (TAA) for epicardial and endocardial implantation, respectively. Other abbreviations as in Figure 1.
Images readapted with permission from Feld et al (121).
ImCardia
The ImCardia is an elastic self-expanding device made of biocompatible Conichrome FWM 1058 to be implanted on the epicardial surface of the heart. The device is made of a series of springs, with free length ranging from 35 to 46 mm, which are connected to attachment elements and screwed into the epimyocardium of the LV free wall 17 to 28 mm apart (119).
In silico and in vitro investigations of this device predicted a reduction of the LV diastolic pressure curve with partial pressure-volume loop normalization. In addition, in vivo studies on 8 healthy sheep and 10 mini-pigs induced with diastolic dysfunction reported device safety and efficacy in improving filling dynamics. Particularly, the mini-pigs study showed an increase in the early apical reverse rotation rate as well as in the early diastolic to systolic strain-rate ratio at the mid-endocardium, indicative of enhanced cardiac relaxation (119).
Based on these findings, an open-label, parallel, nonrandomized clinical trial was launched to evaluate the safety and functionality of ImCardia in 19 patients with HFpEF undergoing aortic valve replacement up to a 36-month follow-up (120). Because improvements in cardiac function are anticipated in patients undergoing aortic valve replacement, efficacy was not set as an endpoint of this study. Nevertheless, all patients in the device group were shown to maintain systolic function, and improvements in LV mass (38% vs 21% decrease; P = 0.08) and LA area (17% decrease vs 7% increase; P = 0.02) were observed relative to control (121). However, the trial was terminated due to the invasive nature of the implantation procedure (120).
CORolla TAA
The CORolla TAA is a cone-like LV expander composed of 3 elastic arms for implantation on the endocardial wall. One of the main advantages of this device over the ImCardia LV expander is the minimally invasive implantation procedure it allows for, which only requires a small intercostal incision. For implantation, purse-string sutures are placed around the apex, and the transapical sheath is inserted to guide the delivery tool and thus device deployment in the appropriate orientation. During this procedure, the papillary muscle pointer guiding tool is used under echocardiography imaging to ensure that one of the device arms will be opened between the 2 papillary muscles. After release, the device is anchored to the apex through a fixation suture (121).
A report of preclinical testing on 76 Assaf breed healthy sheep indicated minimal adverse events, no weight reduction, and prompt recuperation of the animals at 24 months (122). In this study, only 1 sheep showed signs of reduced systolic function, with a significant reduction in LVEF (49% vs 65%) at the 6-month follow-up compared with baseline. In addition, 2 sheep developed mitral regurgitation; 1 due to chordae tearing at 4.5 months after implantation, and 1 due to mis-positioning of the device toward the ventricle base, which likely caused additional pressure below the mitral annulus. One sheep had signs of mitral valve endocardiosis without regurgitation. On histopathology, active thrombi were found in 7 sheep at the 3- to 6-month follow-up, whereas no active thrombi were present at 12 or 24 months in sheep given nonsteroidal anti-inflammatory (aspirin) and antiplatelet (Plavix) medications (121).
An ongoing first-in-human clinical trial aims to evaluate the safety, feasibility, and efficacy of the CORolla TAA in 10 patients during 24 months of follow-up (123). Preliminary results on 1 patient at a 12-month echocardiography follow-up showed reduction in the LV mass index (122 vs 142 g/m2), LA volume index (43 vs 58 mL/m2), and LV end-diastolic volume index (49 vs 84 mL/m2). In addition, functional results at 6 months showed an improvement in NYHA functional class (III to I), Minnesota Living with Heart Failure Questionnaire score (18 vs 60 points), and 6-minute walking test (420 vs 240 m) (122). Nevertheless, some of these changes became less significant at the 24-month follow-up, with LV mass index and LA volume index increasing to 130 g/m2 and 55 mL/m2, respectively. In addition, the Minnesota Living with Heart Failure Questionnaire score worsened to 44 points, and NYHA functional class was found to be the same as at baseline. Finally, a drop in LVEF (44% vs 48%) was also recorded, raising concerns about long-term progression to HFrEF (121).
However, it must be noted that these data were obtained on 1 patient only, and more extensive clinical testing of the CORolla TAA LV expander is required. Future clinical investigations of this device should evaluate the risks of impairing systolic function, damaging the mitral apparatus, or altering the electrical pathways in the heart due to endocardial adhesion of the device. Finally, the efficacy of the CORolla TAA in hearts with different degrees of hypertrophy has not been investigated or reported, raising concerns about the ability of LV expanders to provide patient-specific support, and thus about patient selection and eligibility.
Neuromodulation and electrostimulation therapy
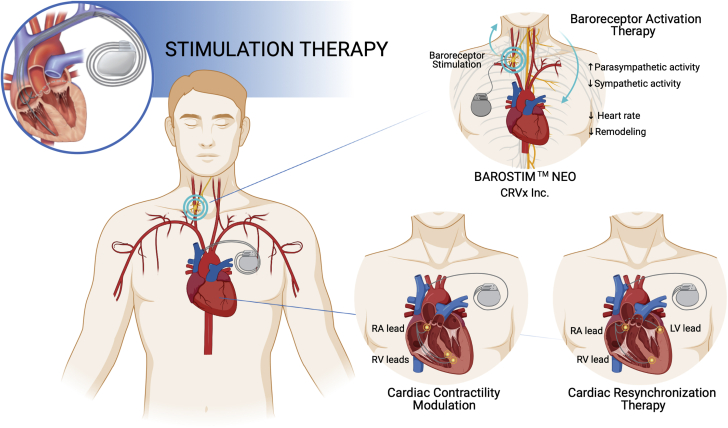
A variety of device-based solutions traditionally used for the treatment of conditions involving autonomic imbalance and abnormalities in the cardiac electrical activity are currently under investigation in patients with HFpEF. Broadly, these approaches include baroreceptor activation therapy (BAT) devices, such as the BAROSTIM NEO system (CVRx, Inc), cardiac contractility modulation (CCM), and CRT, as illustrated in Figure 4.
Figure 4.
Stimulation Therapy Devices Under Development for the Treatment of HFpEF
These devices include the BAROSTIM NEO for baroreceptor activation therapy and devices for cardiac contractility modulation and cardiac resynchronization therapy. RA = right atrial; RV = right ventricular; other abbreviations as in Figure 1.
Crucially, the adoption of neuromodulation and electrostimulation therapy in HFpEF emphasizes the importance of adequate patient stratification, due to the kaleidoscopic variety of underlying conditions and symptomatology associated with this disease. Although these devices have not been approved for HFpEF, use of BAT, CCM, and CRT is currently being investigated primarily in patients with HFpEF and signs or symptoms of hypertension, systolic dysfunction, and compromised electrical activity of the heart, respectively, as reviewed in the following sections. In this context, these co-morbidities may play a more critical role in patient stratification compared with structural and hemodynamic abnormalities that other classifications are based on. To date, further evidence is required to show long-term efficacy of these therapies in HFpEF.
BAROSTIM NEO
BAROSTIM NEO is a neuromodulation system that targets the diminished baroreceptor sensitivity observed in patients with HFpEF, affecting chronotropic reserve and heart rate recovery (14). Through the activation of the baroreceptors in the wall of the carotid artery, this system aims to stimulate both the afferent and efferent pathways of the autonomic nervous system, increasing the parasympathetic tone and diminishing sympathetic drive.
The device represents second-generation BAT technology, with reported improvements in safety and adverse events compared with the first-generation Rheos system by the same manufacturer (CVRx, Inc) (124, 125, 126). In the European Union, the BAROSTIM NEO device has received the CE mark for the treatment of HFrEF. It consists of a lead connected to a pulse generator, which is similar to a defibrillator in shape and size and to a pacemaker in safety profile (127,128). The device is implanted in the pectoral region with the lead tunneled subcutaneously to the ipsilateral carotid bifurcation (128).
Initially designed for the treatment of resistant hypertension, BAT was shown to drastically lower both systolic (144 ± 28 mm Hg vs 179 ± 24 mm Hg; P < 0.0001) and diastolic (85 ± 18 mm Hg vs 103 ± 16 mm Hg; P < 0.0001) blood pressure in a 6-year follow-up study (126). In addition, the same investigation highlighted that the effects of BAT were more evident in patients with signs of HF compared with patients with isolated hypertension.
In a 6-month proof-of-concept study on 11 patients with HF (NYHA functional class III, LVEF <40%) on optimal medical therapy (128), BAT was found to be safe while also improving muscle sympathetic nerve activity (31.3 ± 8.3 bursts/min vs 45.1 ± 77 bursts/min; P < 0.05) and clinical measures of NYHA functional class, QoL, and functional capacity.
Currently, the BAROSTIM THERAPY in Heart Failure With Preserved Ejection Fraction (HFpEF) trial (129) is recruiting patients with HFpEF with hypertension who are resistant to maximally tolerated drug therapy with a diuretic and 2 other antihypertensive medications. In addition, patients with baroreflex failure, autonomic neuropathy, symptomatic cardiac bradyarrhythmia, and evidence of ulcerative plaques or atherosclerosis in the carotid artery are excluded from this trial. With a 6-month follow-up, this study will evaluate changes in systolic blood pressure, as well as LV and LA mass indices, NYHA functional class, and rehospitalization for HF.
CCM therapy
With the goal of augmenting native cardiac contractility, CCM therapy involves the application of a high-voltage (∼7.5 V), long-duration (∼20 milliseconds), biphasic stimulation to the right ventricular septum (130). By targeting the absolute refractory period phase of the action potential, these electrical signals do not trigger new cardiac muscle contraction. Such a nonexcitatory stimulation, however, enhances the influx of calcium ions into the cardiomyocytes, resulting in a sustained increase in contractility, without raising myocardial oxygen consumption (131).
The Optimizer Smart System (Impulse Dynamics) is the most widely investigated CCM device. It includes an implantable stimulation device connected externally to a charger. The device is designed for subcutaneous implantation in the upper chest with leads inserted in the heart’s right ventricular septum.
A variety of clinical trials have evaluated the effects of this device in HFrEF (132, 133, 134, 135, 136, 137), leading to CE approval in the European Union and other countries, while other studies are ongoing (138,139). This system, however, is contraindicated in patients with permanent or long-standing atrial fibrillation or flutter, mechanical tricuspid valve, no venous access, or with 100% ventricle-ventricle–inhibited pacing. Together, these investigations corroborated the theory that CCM treatment is safe and improves exercise tolerance, NYHA functional class, and QoL, and lowers hospitalization rates for up to 2 years of follow-up (140, 141, 142, 143). Notably, the FIX-HF-5 (Evaluation of the Safety and Effectiveness of the OPTIMIZER System in Subjects With Heart Failure) (132) study and the FIX-HF-5C (FIX-HF-5 confirmatory study) (135) showed that larger effects were seen for elevated LVEF (35%-45%) compared with the group with LVEF <25%, prompting the evaluation of CCM in patients with HFpEF. The single-arm open-label CCM-HFpEF (CCM in Heart Failure With Preserved Ejection Fraction) trial (144) is currently recruiting patients with HFpEF (LVEF ≥50%, NYHA functional class II or III) to evaluate safety and efficacy, primarily through the Kansas City Cardiomyopathy Questionnaire score over a 24-week period. Notably, this trial excludes patients with cardiomyopathy or infiltrative heart disease, severe lung disorders, mechanical tricuspid valve, systolic blood pressure >160 mm Hg, PR interval >375 milliseconds, and a heart rate >110 beats/min for patients with atrial fibrillation.
Cardiac resynchronization therapy
CRT devices have revolutionized the therapeutic approach to HF in patients with signs of compromised electrical activity (145,146). They include a pulse-generating device generally inserted subcutaneously in the chest wall and 3 wire leads to deliver simultaneous electrical impulses to the right atrium and both the right and left ventricles, often asynchronous in the failing heart (147). Apart from being contraindicated in patients with a QRS duration <130 milliseconds, these devices are generally recommended for HFrEF patients with NYHA functional class III to IV despite optimal medical therapy and evidence of LV dilation, with the pacing system being largely dictated by the patient’s age and medical condition. As with other types of electrical stimulation, this technique was initially developed for HFrEF, with several studies showing improved NYHA functional class, exercise tolerance, and QoL (148, 149, 150), as well as reduced HF-associated mortality and hospitalization rates (151,152).
A subgroup analysis of the PROSPECT (Predictors of Response to CRT) trial, initially designed to test the performance of CRT in patients with HFrEF (153,154), revealed similar improvements in clinical scores in patients with LVEF >35% compared with those with LVEF <35% at a 6-month follow-up (155). This finding led to further studies aiming to investigate the efficacy of CRT in HFpEF, particularly with signs of conduction delays, chronotropic incompetence, and mechanical dyssynchrony (156).
Six patients with severe HFpEF with interatrial conduction delay were enrolled in a randomized, double-blind pilot study, aiming to evaluate the potential benefits of bi-atrial resynchronization therapy (157), a stimulation technique developed to prevent atrial fibrillation (158). After 3 months of pacing, improvements were seen in the 6-minute walking test (240 ± 24 m vs 190 ±15 m; P < 0.05), A-wave duration (104 ± 8 milliseconds vs 158 ± 25 milliseconds; P = 0.002), and mitral flow dynamics (157). In 2012, the LEAD (Left Atrial Pacing in Diastolic Heart Failure) trial began to confirm these findings in a larger cohort (159). More recently, the PACE (Physiologic Accelerated Pacing as a Treatment in Patients with Heart Failure with Preserved Ejection fraction) trial (160) was launched to evaluate the effects of personalized pacing on atrial fibrillation and hospitalization rates, as well as LA and LV pressures. Patients with uncontrolled hypertension, severe valvular disease, long-standing persistent atrial fibrillation, and QRS >150 milliseconds were excluded from this trial. Although findings from these studies have not yet been published, results from another investigation conducted on 22 patients with HFpEF with atrial fibrillation showed that His bundle pacing post-atrioventricular node ablation significantly improved echocardiographic measurements and NYHA classification and reduced the use of diuretics (161).
The potential benefits of atrial pacing for patients with LVEF ≥40% without atrial fibrillation but with signs of chronotropic incompetence are currently being investigated in the RAPID-HF (Efficacy Study of Pacemakers to Treat Slow Heart Rate in Patients With Heart Failure) trial (162). Similarly, the PREFECTUS (Cardiac Resynchronization Therapy Versus Rate-responsive Pacing in Heart Failure With Preserved Ejection Fraction) trial is evaluating the effects of CRT on chronotropic incompetence in patients with HFpEF (163). Through measurements of cardiac reserve and functional outcomes in 10 patients, this trial seeks to shed light on the benefits of CRT over other techniques such as rate-responsive pacing.
In a large cohort of patients with LVEF <50% and atrioventricular block, the safety and efficacy of biventricular pacing were evaluated in the BLOCK HF (Biventricular Versus Right Ventricular Pacing in Heart Failure Patients With Atrioventricular Block) trial (164). This study showed a lower incidence of adverse events and superior functional outcome measures compared with right ventricular pacing (165). Furthermore, a case study reported by Penicka et al (166) showed that this technique may also be effective in HFpEF, as it reportedly improved signs of LV desynchrony and normalized LV end-diastolic pressure in 1 patient with an LVEF of 64%. After this study, new control algorithms were developed to further improve the performance of biventricular pacing devices. Biventricular fusion pacing, for example, enables the atrioventricular delay to be adjusted to continuously synchronize biventricular pacing with the intrinsic atrioventricular conduction (167, 168, 169). In patients with atrioventricular block, this technique is associated with superior clinical outcomes compared with standard biventricular pacing as well as with a lower incidence of atrial fibrillation (167,170). The functional benefits of fusion pacing are currently under evaluation in patients with HFpEF (171), as further evidence of its efficacy in this patient population has yet to be provided.
Mechanical circulatory support
MCS devices provide hemodynamic support to underperforming left and/or right ventricles, aiming to improve the QoL of patients with end-stage HFrEF. Due to the success of MCS in the management of HFrEF, the development of analogous devices in HFpEF has recently gained considerable momentum.
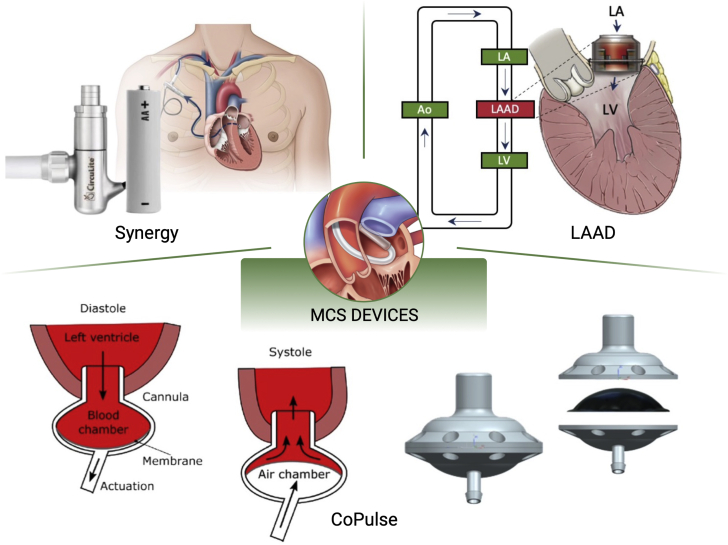
Figure 5 depicts the MCS solutions that have been investigated to date to improve HFpEF hemodynamics, namely the Synergy Pump (HeartWare) as an LA decompression pump, the CoPulse, and the left atrial assist device (LAAD). These devices aim to alleviate elevated LAP and associated symptoms while restoring adequate cardiac output. This is often blunted in HFpEF due to systolic dysfunction and autonomic imbalance, both contributing to reduced cardiac reserve and symptoms of exercise intolerance. As a result, these devices could be suitable for all types of HFpEF, as they are all characterized by high LAP, and particularly for type 1 HFpEF, due to the remarkably reduced cardiac output. The additional risks of LA collapse and pump obstruction imposed by the different levels of hypertrophy associated with each HFpEF phenotype require evaluation with respect to pump design and configuration. Although clinical studies have yet to be conducted, data from computational and ex vivo investigations strongly support the development of these MCS devices as a novel and promising therapy for HFpEF.
Figure 5.
MCS Devices Under Development for the Treatment of HFpEF
These devices include the Synergy pump, the left atrial assist device (LAAD), and the CoPulse valveless design, including schematics of their rationale. Ao = aorta; LA = left atrium; LV = left ventricle; MCS = mechanical circulatory support.
Images of the Synergy pump readapted with permission from Morgan and Naka (189). Images of the LAAD readapted with permission from Fukamachi et al (177). Images of the CoPulse readapted with permission from Granegger et al (175) and Escher et al (176).
In addition, other commercial options are currently under development. These include the VADovations cardiac assist system (VADovations), which allows for minimally invasive delivery, and the PulseVAD (Northern Development) device, which provides patient-specific adaptive circulatory support from the left atrium to the descending aorta for both HFrEF and HFpEF. However, no data have yet been made available regarding the safety or the feasibility of either of these commercial devices. This section thus primarily reviews the development and findings relative to the Synergy Pump, CoPulse, and LAAD MCS devices.
LA decompression pumps
Burkhoff et al (22) evaluated the feasibility of a partial support HFrEF device (Synergy Pump, HeartWare) as an LA decompression pump to address LA hypertension and dysfunction in HFpEF. Using a lumped-parameter model, the authors compared 2 different pump configurations: LA cannulation (ie, from left atrium to aorta) and LV cannulation (ie, from left ventricle to aorta). The authors concluded that the LA decompression pump yields an increase in cardiac output and a reduction in LAP in type 1 to 4 HFpEF regardless of the pumping source, albeit with a moderate increase in the systolic blood pressure.
In addition, compared with LV cannulation, LA sourcing yielded a higher LV end-systolic volume, lowering the risk of atrial suction associated with pump-based devices. Although this analysis suggests that mechanical decompression support with LA sourcing could be a viable option for severe HFpEF (22,172), device-related problems, including pump thrombosis, remain to be addressed before clinical translation of this technology (172,173). Finally, continuous-flow pumps result in a dramatic drop in arterial pulsatility, which, in HFrEF, has been shown to be associated with increased gastrointestinal bleeding, degeneration of aortic wall tissue, and other complications (174). The use of an MCS that recreates pulsatile-flow physiology in HFpEF or that is capable of adjusting to patient-specific hemodynamics has yet to be investigated.
Valveless pulsatile pump (CoPulse)
The design and feasibility of a valveless pulsatile pump (CoPulse) for the treatment of HFpEF were recently described by Granegger et al (175) and Escher et al (176). Designed for implantation at the apex of the heart, the CoPulse device is composed of 1 blood chamber and 1 air chamber divided by a flexible polyurethane membrane. Blood enters the chamber during diastole and is ejected by the displacement of the separating membrane during systole, which occurs in synchrony with the native heartbeat. In an initial in silico study, the system was shown to be capable of improving diastolic hemodynamics in the HFpEF phenotypes previously described by Burkhoff et al (22). Specifically, 2 designs of the device were modeled, allowing for stroke volumes of 30 or 60 mL. Furthermore, the study showed that this device was successful in increasing the cardiac output, and both pump configurations reduced the LAP by approximately 30% and 15%, respectively (175).
Recently, Escher et al (176) developed a prototype of the CoPulse pump and tested its hydraulic characteristics by using computational fluid dynamics and 4-dimensional flow magnetic resonance imaging. The ex vivo results from the isolated porcine heart model revealed improved HFpEF hemodynamics by reducing LAP and increasing cardiac output. Furthermore, although concerns remain regarding the durability of the flexible membrane of this device for long-term support, the simulation showed the hemocompatibility of the pump as it results in only moderate shear stresses and has good pump washout performance.
LA assist device
Another MCS device for HFpEF, known as the LAAD, was most recently proposed by Fukamachi et al (177). The LAAD is a continuous pump with hybrid magnetic and hydrodynamic bearings designed for implantation at the mitral valve.
In vitro studies were performed to investigate the hemodynamic effects of this device on an experimental mock circulatory system, configured to simulate various degrees of diastolic dysfunction (177, 178, 179). The performance of the LAAD (ie, left atrium to left ventricle configuration) was compared with that of analogous pumps in 2 other configurations, namely left atrium to aorta and left ventricle to aorta. Comparison of the 3 configurations showed that the LAAD achieves the most significant hemodynamic improvements, restoring adequate cardiac output and aortic pressure, and lowering LAP independently of disease severity.
To date, the LAAD is the first MCS device for HFpEF undergoing animal testing. In a recent study of Miyagi et al (180), the LAAD was inserted in 4 healthy calves through a LA incision in the mitral valve position, showing improved hemodynamics in the normal calf heart. Particularly, by controlling the pump speed (3,600-4,400 rpm), increases in the cardiac output (6.0 L/min vs 5.3 L/min) and mean aortic pressure (77 mm Hg vs 69 mm Hg) were achieved, with a corresponding decrease in LAP (8.3 mm Hg vs 15.1 mmHg). In addition, atrial suction and other adverse events were rare and likely due to the relatively small size of the calf’s left atrium, whereas no evidence of LV outflow obstruction was reported. Miyagi et al (181) also explored the effect of alternative pump control options. Torque control of the LAAD was maintained in a mock circulatory loop, and the pump speed of the device was adjusted by using active torque control. The initial findings suggest appropriate cardiac output with reduced pump backflow and recovered aortic pressure levels under diastolic HF simulation conditions.
Although the LAAD has been shown to ameliorate the hemodynamics of HFpEF in a variety of studies (177, 178, 179, 180, 181), questions remain regarding the site of implantation of the device, as the implications associated with the removal of the mitral valve have yet to be assessed (182). In addition, although implantation at the supra-valvular level has been suggested to preserve the mitral valve, this configuration has not been studied thoroughly (181). Finally, the effects of this device on LV diastolic function should be investigated further in in vivo studies to more comprehensively evaluate the feasibility of the LAAD for the treatment of HFpEF.
Discussion
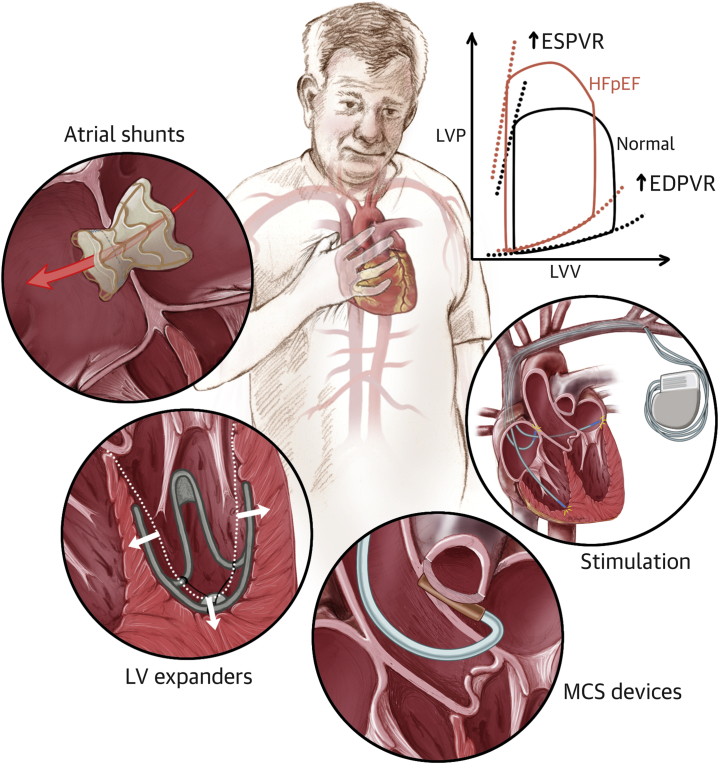
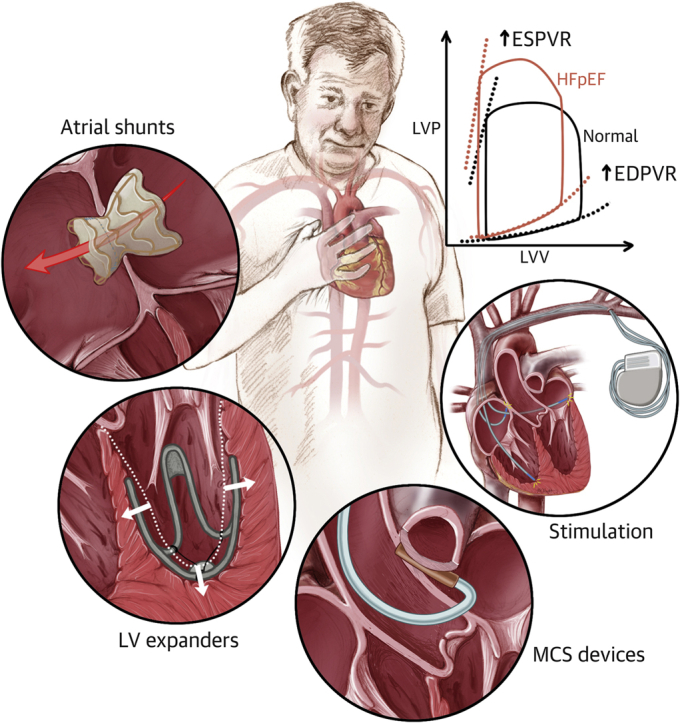
The development of device-based solutions for HFpEF seeks to address the lack of adequate medical therapies that can relieve symptoms and improve the QoL of these patients (Central Illustration). Stemming from the impact that devices have on the management of HFrEF, and supported by preliminary preclinical and clinical evidence, this approach holds great promise in addressing one of the major present challenges in the field of cardiovascular medicine. The complex and multifaceted nature of this condition, associated with a multitude of risk factors, symptoms, and complications, has made the development of suitable therapies particularly exacting. In addition, the absence of an animal model of HFpEF poses further complications in this context, potentially leading to inadequate preclinical testing and premature clinical trials.
Central Illustration.
Medical Devices for Heart Failure With Preserved Ejection Fraction
Atrial shunts, left ventricular (LV) expanders, mechanical circulatory support (MCS) devices, and stimulation therapy are currently under development to improve the hemodynamics and symptomatology of patients with heart failure with preserved ejection fraction (HFpEF). EDPVR = end-diastolic pressure-volume relationship; ESPVR = end-systolic pressure-volume relationship; LVP = left ventricular pressure; LVV = left ventricular volume.
Because device-based solutions of HFpEF are still in the development phase, to date, clinical trials have broadly focused on endpoints such as device safety, mortality, and incidence of post-implant adverse events, often combined into a composite primary endpoint to reduce study cost and time (183). These clinical trials have adopted broad recruitment criteria, with some having failed to make a distinction between HFmEF and HFpEF groups. Although this approach may increase the ability to recruit more patients, it may confound results (184). In addition, although it is critical to report changes in mortality and hospitalization rates, endpoints reflecting symptoms, functional capacity, and life quality metrics are often underemphasized. Given that, to date, very few trials have fully accomplished their endpoints, new complementary endpoints could be explored to assess more patient-centric outcomes (183,184), which could provide insight into the efficacy of these devices to mitigate the burden of the daily symptoms of HFpEF (184).
Crucially, the vast majority of the clinical investigations of devices for HFpEF have excluded patients with hypertrophic or restrictive cardiomyopathies, leading to unanswered questions regarding their safety and efficacy in these patient populations. Similarly, patients with comorbidities of cardiovascular, pulmonary, or systemic origin are underrepresented in clinical trials. Due to the multiorgan and systemic involvement of HFpEF, these groups represent a considerable portion of the HFpEF population and must therefore be included in future clinical investigations.
The current paper reviewed a variety of device types, each targeting a distinct pathophysiological mechanism of HFpEF. Atrial shunts, and particularly interatrial shunts, have been the most widely investigated in clinical trials and showed evidence of reducing LAP and improving functional outcome measures. However, the long-term safety of these devices still requires evaluation, due to concerns regarding the induced risks of atrial arrhythmias, paradoxical embolism, and right HF (106). Although novel approaches have been drawing increasing attention in the landscape of atrial shunts to address these potential risks, currently, left atrium to coronary sinus shunting represents the only alternative to interatrial devices. The findings reported on the first-in-human trial of the left atrium to coronary sinus shunt showed the device safety and efficacy, although studies with larger cohorts and longer follow-up are necessary to validate these results and advance this technology to the clinical stage.
LV expanders are designed to improve diastolic recoil in HFpEF. Currently, CorAssist Inc is the only medical company developing LV expanding technologies. Although there has been no report documenting the development of the ImCardia device after the termination of its first clinical trial, studies are ongoing to investigate the safety and feasibility of the CORolla TAA device, and encouraging first-in-human results have been reported (122). However, only 10 patients are enrolled in this single-arm, nonrandomized trial, and further evidence is required to support the initial findings. In addition, the degree of support that LV expanders are able to provide to patients with different HFpEF phenotypes, degrees of diastolic and systolic stiffening, and in the context of remodeling as a dynamic process have yet to be investigated.
The evaluation of a broad spectrum of stimulation therapy devices in HFpEF arose largely from their success shown in the treatment of other cardiovascular diseases, including hypertension, diminished contractility, and atrial fibrillation. Some of these conditions reportedly accelerate the onset of HFpEF, whereas others may often arise as a consequence of LV or LA remodeling (10). Despite lacking specificity to the HFpEF pathophysiology, therapies such as BAT, CCM, and CRT have shown overall improvements in symptoms, QoL, and rehospitalization rates in patients with HFpEF (126,132,152). Nevertheless, adequate stratification remains paramount to identify the patients who would benefit the most from any of these therapies. In the context of CRT in HFpEF, patients should be selected based on their underlying electrophysiological conduction disturbances to efficiently restore the cardiac electrical activity and improve diastolic function alongside clinical and functional outcomes.
In end-stage HFrEF, systolic function and adequate cardiac hemodynamics can be restored through MCS devices. Although the use of HFrEF MCS devices that support the pumping action of the left ventricle is not recommended in HFpEF, due to reported risks of atrial suction and intraventricular thrombosis (86,172,182,185, 186, 187, 188), analogous solutions that are specific for HFpEF are currently under development to reduce LAP, enhance LV filling, and restore cardiac output. In this context, a variety of in silico and in vitro studies have been conducted on a number of proposed approaches to investigate their feasibility (22,175, 176, 177, 178, 179). To date, however, animal experimentation for MCS for HFpEF remains minimal, and no clinical testing has yet been conducted. The safety of these devices for different HFpEF patient populations, as they are affected by various degrees of hypertrophy as well as of diastolic and systolic dysfunction, remains to be evaluated. Furthermore, each of these devices presents specific challenges that need to be addressed to support their clinical translation, including the durability of the flexible membrane of the CoPulse device and the implications associated with mitral valve removal required for implantation of the LAAD.
Conclusions
HFpEF constitutes a major clinical challenge that is profoundly affected by the lack of appropriate medical therapies. The need for alternative solutions has spurred the development of a wide spectrum of devices that aim to restore healthy hemodynamics and alleviate the symptoms of HFpEF. Devices for the treatment of HFpEF have advanced in 4 domains based on the targeted mechanism or symptom: atrial shunts to lower elevated LA pressure and symptoms of pulmonary congestion, LV expanders to augment LV filling performance, stimulation therapy to target autonomic imbalance and mechanical desynchrony, and MCS devices to enhance cardiac output.
To date, no device has obtained U.S. Food and Drug Administration approval for use in the United States, and only the IASD has received the CE mark specifically for the treatment of HFpEF. Although results from preclinical or clinical testing strongly support the continued development of each of these device categories, further clinical evidence is required to establish their long-term safety and efficacy in patients with HFpEF, and particularly in those with hypertrophic or restrictive cardiomyopathies or other comorbidities, who were broadly underrepresented in the majority of the clinical trials conducted to date. Undoubtedly, the development of HFpEF devices is an emerging endeavor of cardiovascular innovation that has been drawing increasing attention in the scientific community worldwide and holds remarkable promise for the future of HFpEF management and treatment.
Funding Support and Author Disclosures
Dr Hameed is leading a project to develop a novel device for HFpEF. The project is funded by the Enterprise Ireland through their Commercialization Fund (CF-2019-1136-P). This project is co-funded by the European Regional Development Fund under Ireland’s European Structural and Investment Funds Programme 2014-2020. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Contributor Information
Ellen T. Roche, Email: etr@mit.edu.
Aamir Hameed, Email: aamirhameed@rcsi.ie.
Appendix
References
- 1.Yusuf S., Rangarajan S., Teo K. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371(9):818–827. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 2.Roger V.L. Epidemiology of heart failure. Circ Res. 2013;113(6):646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Yancy C.W., Jessup M., Bozkurt B. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinic. J Am Coll Cardiol. 2016;68(13):1476–1488. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Borlaug B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2020;17:559–573. doi: 10.1038/s41569-020-0363-2. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug B.A. Defining HFpEF: where do we draw the line? Eur Heart J. 2016;37(5):463–465. doi: 10.1093/eurheartj/ehv561. [DOI] [PubMed] [Google Scholar]
- 7.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution. Eur J Heart Fail. 2016;27(14):891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 8.Andronic A.A., Mihaila S., Cinteza M. Heart failure with mid-range ejection fraction—a new category of heart failure or still a gray zone. Maedica (Buchar) 2016;11(4):320–324. [PMC free article] [PubMed] [Google Scholar]
- 9.Albakri A. Heart failure with mid-range ejection fraction: a review of clinical status and meta-analysis of clinical management methods. Trends Res. 2018;1(4) [Google Scholar]
- 10.Lam C.S.P., Donal E., Kraigher-Krainer E., Vasan R.S. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13(1):18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owan T.E., Hodge D.O., Herges R.M., Jacobsen S.J., Roger V.L., Redfield M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 12.Oktay A.A., Rich J.D., Shah S.J. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10(4):401–410. doi: 10.1007/s11897-013-0155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borlaug B.A., Paulus W.J. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32(6):670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borlaug B.A. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11(9):507–515. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 15.Benjamin E.J., Muntner P., Alonso A. Heart Disease and Stroke Statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 16.Heidenreich P.A., Albert N.M., Allen L.A. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6(3):606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulus W.J., Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 18.Aurigemma G.P., Silver K.H., Priest M.A., Gaasch W.H. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol. 1995;26(1):195–202. doi: 10.1016/0735-1097(95)00153-q. [DOI] [PubMed] [Google Scholar]
- 19.Mishra S., Kass D.A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nature Reviews Cardiol. 2021:1–24. doi: 10.1038/s41569-020-00480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samson R., Jaiswal A., Ennezat P.V., Cassidy M., Jemtel T.H.L. Clinical phenotypes in heart failure with preserved ejection fraction. J Am Heart Assoc. 2016;5(1):1–15. doi: 10.1161/JAHA.115.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valero-Muñoz M., Backman W., Sam F. Murine models of heart failure with preserved ejection fraction: a “fishing expedition.”. J Am Coll Cardiol Basic Trans Science. 2017;2(6):770–789. doi: 10.1016/j.jacbts.2017.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burkhoff D., Maurer M.S., Joseph S.M. Left atrial decompression pump for severe heart failure with preserved ejection fraction: theoretical and clinical considerations. J Am Coll Cardiol HF. 2015;3(4):275–282. doi: 10.1016/j.jchf.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Borbély A., van der Velden J., Papp Z. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111(6):774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 24.Weber K.T., Brilla C.G., Janicki J.S. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27(3):341–348. doi: 10.1093/cvr/27.3.341. [DOI] [PubMed] [Google Scholar]
- 25.Nagueh S.F., Shah G., Wu Y. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110(2):155–162. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 26.Makarenko I., Opitz C.A., Leake M.C. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95(7):708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 27.Borbély A., Pires I.F., Heerebeek L.v. Hypophosphorylation of the stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104(6):780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 28.Grützner A., Manyes S.G., Kötter S., Badilla C.L., Fernandez J.M., Linke W.A. Modulation of titin-based stiffness by disulfide bonding in the cardiac titin N2-B unique sequence. Biophys J. 2009;97(3):825–834. doi: 10.1016/j.bpj.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borlaug B.A., Lam C.S.P., Roger V.L., Rodeheffer R.J., Redfield M.M. Contractility and ventricular systolic stiffening in hypertensive heart disease. Insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54(5):410–418. doi: 10.1016/j.jacc.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfeffer M.A. In: Atlas of Heart Failure. Colucci W.S., editor. Current Medicine Group; 2002. Cardiac remodeling and its prevention; pp. 87–101. [Google Scholar]
- 31.Carabello B.A., Paulus W.J. Aortic stenosis. Lancet. 2009;373(9667):956–966. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 32.Penicka M., Bartunek J., Trakalova H. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea. A pressure-volume loop analysis. J Am Coll Cardiol. 2010;55(16):1701–1710. doi: 10.1016/j.jacc.2009.11.076. [DOI] [PubMed] [Google Scholar]
- 33.Florea V.G., Cohn J.N. The autonomic nervous system and heart failure. Circ Res. 2014;114(11):1815–1826. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 34.Xi Y., Cheng J. Dysfunction of the autonomic nervous system in atrial fibrillation. J Thorac Dis. 2015;7(2):193–198. doi: 10.3978/j.issn.2072-1439.2015.01.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Upadhya B., Haykowsky M.J., Eggebeen J., Kitzman D.W. Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatric Cardiol. 2015;12(3):294–304. doi: 10.11909/j.issn.1671-5411.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawaguchi M., Hay I., Fetics B., Kass D.A. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107(5):714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 37.Borlaug B.A., Olson T.P., Lam C.S.P. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56(11):845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brucks S., Little W.C., Chao T. Contribution of left ventricular diastolic dysfunction to heart failure regardless of ejection fraction. Am J Cardiol. 2005;95(5):603–606. doi: 10.1016/j.amjcard.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 39.Wang J., Khoury D.S., Yue Y., Torre-Amione G., Nagueh S.F. Preserved left ventricular twist and circumferential deformation, but depressed longitudinal and radial deformation in patients with diastolic heart failure. Eur Heart J. 2008;29(10):1283–1289. doi: 10.1093/eurheartj/ehn141. [DOI] [PubMed] [Google Scholar]
- 40.Baicu C.F., Zile M.R., Aurigemma G.P., Gaasch W.H. Left ventricular systolic performance, function, and contractility in patients with diastolic heart failure. Circulation. 2005;111(18s):2306–2312. doi: 10.1161/01.CIR.0000164273.57823.26. [DOI] [PubMed] [Google Scholar]
- 41.Melenovsky V., Borlaug B.A., Rosen B. Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community. The role of atrial remodeling/dysfunction. J Am Coll Cardiol. 2007;49(2):198–207. doi: 10.1016/j.jacc.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 42.Lewis G.A., Schelbert E.B., Williams S.G. Biological phenotypes of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2017;70(17):2186–2200. doi: 10.1016/j.jacc.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Huusko J., Purmonen T., Toppila I., Lassenius M., Ukkonen H. Real-world clinical diagnostics of heart failure patients with reduced or preserved ejection fraction. ESC Heart Fail. 2020;7(3):1039–1048. doi: 10.1002/ehf2.12665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeffer M.A., Shah A.M., Borlaug B.A. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124(11):1598–1617. doi: 10.1161/CIRCRESAHA.119.313572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandey A., Parashar A., Kumbhani D. Exercise training in patients with heart failure and preserved ejection fraction meta-analysis of randomized control trials. Circ Heart Fail. 2015;8(1):33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adamson P.B., Abraham W.T., Bource R.C. Wireless pulmonary artery pressure monitoring guides management to reduce decompensation in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7(6):935–944. doi: 10.1161/CIRCHEARTFAILURE.113.001229. [DOI] [PubMed] [Google Scholar]
- 47.Vaduganathan M. Prior heart failure hospitalization, clinical outcomes, and response to sacubitril/valsartan compared with valsartan in HFpEF. J Am Coll Cardiol. 2020;75:245–254. doi: 10.1016/j.jacc.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanwar M., Walter C., Clarke M., Patarroyo-Aponte M. Targeting heart failure with preserved ejection fraction: current status and future prospects. Vasc Health Risk Manag. 2016;12:129–141. doi: 10.2147/VHRM.S83662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin N., Manoharan K., Thomas J., Davies C., Lumbers R.T. Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction. Cochrane Database Syst Rev. 2018;6(6):CD012721. doi: 10.1002/14651858.CD012721.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ihne S., Morbach C., Obici L., Palladini G., Störk S. Amyloidosis in heart failure. Curr Heart Fail Rep. 2019;16(6):285–303. doi: 10.1007/s11897-019-00446-x. [DOI] [PubMed] [Google Scholar]
- 51.Ternacle J., Krapf L., Mohty D. Aortic stenosis and cardiac amyloidosis: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;74(21):2638–2651. doi: 10.1016/j.jacc.2019.09.056. [DOI] [PubMed] [Google Scholar]
- 52.Machino-Ohtsuka T., Seo Y., Ishizu T. Relationships between maintenance of sinus rhythm and clinical outcomes in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Cardiol. 2019;74(3):235–244. doi: 10.1016/j.jjcc.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Kelly J.P., DeVore A.D., Wu J. Rhythm control versus rate control in patients with atrial fibrillation and heart failure with preserved ejection fraction: insights from Get With The Guidelines-Heart Failure. J Am Heart Assoc. 2019;8(24) doi: 10.1161/JAHA.118.011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.von Lueder T.G., Kotecha D., Atar D., Hopper I. Neurohormonal blockade in heart failure. Card Fail Rev. 2017;3(1):19. doi: 10.15420/cfr.2016:22:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Greene S.J., Gheorghiade M., Borlaug B.A. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J Am Heart Assoc. 2013;2(6) doi: 10.1161/JAHA.113.000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borlaug B.A., Anstrom K.J., Lewis G.D. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF Randomized Clinical Trial. JAMA. 2018;320(17):1764–1773. doi: 10.1001/jama.2018.14852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pieske B., Maggioni A.P., Lam C.S.P. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur Heart J. 2017;38(15):1119–1127. doi: 10.1093/eurheartj/ehw593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Udelson J.E., Lewis G.D., Shah S.V. Rationale and design for a multicenter, randomized, double-blind, placebo-controlled, phase 2 study evaluating the safety and efficacy of the soluble guanylate cyclase stimulator praliciguat over 12 weeks in patients with heart failure with preserved ejection fraction (CAPACITY HFpEF) Am Heart J. 2020;222:183–190. doi: 10.1016/j.ahj.2020.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Redfield M.M., Chen H.H., Borlaug B.A. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mishra S., Hahn V.S., Sadagopan N. PDE9 Inhibition Activates PPARα to Stimulate Mitochondrial Fat Metabolism and Reduce Cardiometabolic Syndrome. bioRxiv. 2021 02.02.429442 (2021) [Google Scholar]
- 61.Sharma K., Vaishnav J., Kalathiya R. Randomized evaluation of heart failure with preserved ejection fraction patients with acute heart failure and dopamine: the ROPA-DOP Trial. J Am Coll Cardiol HF. 2018;6(10):859–870. doi: 10.1016/j.jchf.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 62.Kaye D.M., Nanayakkara S., Vizi D., Byrne M., Mariani J.A. Effects of milrinone on rest and exercise hemodynamics in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;67:2554–2556. doi: 10.1016/j.jacc.2016.03.539. [DOI] [PubMed] [Google Scholar]
- 63.Nanayakkara S., Byrne M., Mak V., Carter K., Dean D., Kaye D.M. Extended-release oral milrinone for the treatment of heart failure with preserved ejection fraction. J Am Heart Assoc. 2020;9(13) doi: 10.1161/JAHA.119.015026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Tassell B.W., Trankle C.R., Canada J.M. IL-1 blockade in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2018;11(8) doi: 10.1161/CIRCHEARTFAILURE.118.005036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abraham W.T., Ponikowski P., Brueckmann M. Rationale and design of the EMPERIAL-Preserved and EMPERIAL-Reduced trials of empagliflozin in patients with chronic heart failure. Eur J Heart Fail. 2019;21(7):932–942. doi: 10.1002/ejhf.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reddy Y.N.V., Obokata M., Koepp K.E., Egbe A.C., Wiley B., Borlaug B.A. The β-Adrenergic agonist albuterol improves pulmonary vascular reserve in heart failure with preserved ejection fraction: a randomized controlled trial. Circ Res. 2019;124(2):306–314. doi: 10.1161/CIRCRESAHA.118.313832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Regression of Fibrosis & Reversal of Diastolic Dysfunction in HFPEF Patients Treated With Allogeneic CDCs (Regress-HFpEF). March 18, 2021. ClinicalTrials.gov identifier: NCT02941705. https://clinicaltrials.gov/ct2/show/NCT02941705 Accessed May 1, 2021.
- 68.Gallet R., de Couto G., Simsolo E. Cardiosphere-derived cells reverse heart failure with preserved ejection fraction in rats by decreasing fibrosis and inflammation. J Am Coll Cardiol Basic Trans Science. 2016;1(1-2):14–28. doi: 10.1016/j.jacbts.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pitt B., Pfeffer M.A., Assmann S.F. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–1392. doi: 10.1056/NEJMoa1313731. [DOI] [PubMed] [Google Scholar]
- 70.Solomon S.D., McMurray J.J.V., Anand I.S. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–1620. doi: 10.1056/NEJMoa1908655. [DOI] [PubMed] [Google Scholar]
- 71.Spironolactone Initiation Registry Randomized Interventional Trial in Heart Failure With Preserved Ejection Fraction (SPIRRIT). June 22, 2019. ClinicalTrials.gov identifier: NCT02901184. https://clinicaltrials.gov/ct2/show/NCT02901184 Accessed May 1, 2021. [DOI] [PMC free article] [PubMed]
- 72.Pfeffer M.A., Claggett B., Assmann S.F. Regional variation in patients and outcomes in the treatment of preserved cardiac function heart failure with an aldosterone antagonist (TOPCAT) trial. Circulation. 2015;131(1):34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 73.Selvaraj S., Claggett B., Shah S.V. Utility of the cardiovascular physical examination and impact of spironolactone in heart failure with preserved ejection fraction: TOPCAT. Circ Heart Fail. 2019;12(7) doi: 10.1161/CIRCHEARTFAILURE.119.006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction (PARAGON-HF). September 29, 2020. ClinicalTrials.gov identifier: NCT01920711. https://clinicaltrials.gov/ct2/show/NCT01920711 Accessed May 1, 2021.
- 75.3-Month Home-based Training With Whole Body Vibration (WBV) Device in Patients With Heart Failure and Preserved Ejection Fraction (GALILEO-HFpEF-HOME) (GALILEOHOME) (GALILEO-HFpEF). January 27, 2020. ClinicalTrials.gov identifier: NCT04239807. https://clinicaltrials.gov/ct2/show/NCT04239807 Accessed May 1, 2021.
- 76.Kresoja K.P., Rommel K.P., Fengler K. Renal sympathetic denervation in patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2021;14(3) doi: 10.1161/CIRCHEARTFAILURE.120.007421. [DOI] [PubMed] [Google Scholar]
- 77.Endovascular GSN Ablation in Subjects With HFpEF. March 20, 2020. ClinicalTrials.gov identifier: NCT04287946. https://clinicaltrials.gov/ct2/show/NCT04287946 Accessed May 1, 2021.
- 78.Patel H.C., Hayward C., Keegan J. Effects of renal denervation on vascular remodelling in patients with heart failure and preserved ejection fraction: a randomised control trial. JRSM Cardiovasc Dis. 2017;6 doi: 10.1177/2048004017690988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heywood J.T., Jermyn R., Shavelle D. Impact of practice-based management of pulmonary artery pressures in 2000 patients implanted with the CardioMEMS Sensor. Circulation. 2017;135(16):1509–1517. doi: 10.1161/CIRCULATIONAHA.116.026184. [DOI] [PubMed] [Google Scholar]
- 80.Shavelle D.M., Desai A.S., Abraham W.T. Lower rates of heart failure and all-cause hospitalizations during pulmonary artery pressure-guided therapy for ambulatory heart failure: one-year outcomes from the CardioMEMS Post-Approval Study. Circ Heart Fail. 2020;13(8):229–238. doi: 10.1161/CIRCHEARTFAILURE.119.006863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abraham J., Bharmi R., Jonsson O. Association of ambulatory hemodynamic monitoring of heart failure with clinical outcomes in a concurrent matched cohort analysis. JAMA Cardiol. 2019;4(6):556–563. doi: 10.1001/jamacardio.2019.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abraham W.T., Stevenson L.W., Bourge R.C. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387(10017):453–461. doi: 10.1016/S0140-6736(15)00723-0. [DOI] [PubMed] [Google Scholar]
- 83.Angermann C.E., Assmus B., Anker S.D. Safety and feasibility of pulmonary artery pressure-guided heart failure therapy: rationale and design of the prospective CardioMEMS Monitoring Study for Heart Failure (MEMS-HF) Clin Res Cardiol. 2018;107(11):991–1002. doi: 10.1007/s00392-018-1281-8. [DOI] [PubMed] [Google Scholar]
- 84.Hemodynamic-GUIDEd Management of Heart Failure (GUIDE-HF). January 7, 2021. ClinicalTrials.gov identifier: NCT03387813. https://clinicaltrials.gov/ct2/show/NCT03387813 Accessed May 1, 2021.
- 85.Burlacu A., Simion P., Nistor I., Covic A., Tinica G. Novel percutaneous interventional therapies in heart failure with preserved ejection fraction: an integrative review. Heart Fail Rev. 2019;24(5):793–803. doi: 10.1007/s10741-019-09787-0. [DOI] [PubMed] [Google Scholar]
- 86.Miyagi C., Miyamoto T., Karimov J.H., Starling R.C., Fukamachi K. Device-based treatment options for heart failure with preserved ejection fraction. Heart Fail Rev. 2021;26:749–762. doi: 10.1007/s10741-020-10067-5. [DOI] [PubMed] [Google Scholar]
- 87.Feasibility Trial of the DC Devices Interatrial Septal Device (IASD) System. July 15, 2020. ClinicalTrials.gov identifier: NCT01570517. https://clinicaltrials.gov/ct2/show/NCT01570517 Accessed May 1, 2021.
- 88.Søndergaard L., Reddy V., Kaye D. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur J Heart Fail. 2014;16(7):796–801. doi: 10.1002/ejhf.111. [DOI] [PubMed] [Google Scholar]
- 89.REDUCE LAP-HF TRIAL (REDUCE LAP-HF) July 15, 2020. ClinicalTrials.gov identifier: NCT01913613. https://clinicaltrials.gov/ct2/show/NCT01913613 Accessed May 1, 2021.
- 90.Kaye D.M., Hasenfuß G., Neuzil P. One-year outcomes after transcatheter insertion of an interatrial shunt device for the management of heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9(12) doi: 10.1161/CIRCHEARTFAILURE.116.003662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hasenfuß G., Hayward C., Burkhoff D. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet. 2016;387(10025):1298–1304. doi: 10.1016/S0140-6736(16)00704-2. [DOI] [PubMed] [Google Scholar]
- 92.Reduce Lap-Hf Randomized Trial I November 13, 2020. ClinicalTrials.gov identifier: NCT02600234. https://clinicaltrials.gov/ct2/show/NCT02600234 Accessed May 1, 2021.
- 93.Feldman T., Mauri L., Kahwash R. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure with Preserved Ejection Fraction (REDUCE LAP-HF I): a phase 2, randomized, sham-controlled trial. Circulation. 2018;137(4):364–375. doi: 10.1161/CIRCULATIONAHA.117.032094. [DOI] [PubMed] [Google Scholar]
- 94.Obokata M., Reddy J.N.V., Shah S.J. Effects of interatrial shunt on pulmonary vascular function in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2019;74(21):2539–2550. doi: 10.1016/j.jacc.2019.08.1062. [DOI] [PubMed] [Google Scholar]
- 95.Reduce Lap-Hf Trial II 2017. ClinicalTrials.gov identifier: NCT03088033. https://clinicaltrials.gov/show/NCT03088033
- 96.REDUCE LAP-HF III Corvia Protocol 1701 (REDUCELAPHFIII). July 1, 2021. ClinicalTrials.gov identifier: NCT03191656. https://clinicaltrials.gov/ct2/show/NCT03191656 Accessed July 2, 2021.
- 97.Extended IASD Investigation: REDUCE LAP-HF IV November 17, 2020. ClinicalTrials.gov identifier: NCT04632160. https://clinicaltrials.gov/ct2/show/NCT04632160 Accessed May 1, 2021.
- 98.Amat-Santos I.J., Bergeron S., Bernier M. Left atrial decompression through unidirectional left-to-right interatrial shunt for the treatment of left heart failure: first-in-man experience with the V-Wave device. EuroIntervention. 2015;10(9):1127–1131. doi: 10.4244/EIJY14M05_07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Del Trigo M., Bergeron S., Bernier M. Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: a safety and proof-of-principle cohort study. Lancet. 2016;387(10025):1290–1297. doi: 10.1016/S0140-6736(16)00585-7. [DOI] [PubMed] [Google Scholar]
- 100.del Rio C.L., McConnell P.I., Niztan Y. Chronic pressure-dependent cardiac unloading with a novel intra-atrial shunt (v-wave device) in an ovine model of ischemic heart failure: evidence for shunt-mediated improvements in function and survivability. Circulation. 2013;128 [Google Scholar]
- 101.The V-Wave Shunt: FIM Safety and Feasibility Study (VW-SP-1). 2019. ClinicalTrials.gov identifier: NCT01965015. https://clinicaltrials.gov/ct2/show/NCT01965015
- 102.Rodés-Cabau J., Bernier M., Amat-Santos I.J. Interatrial shunting for heart failure: early and late results from the first-in-human experience with the V-Wave System. J Am Coll Cardiol Intv. 2018;11(22):2300–2310. doi: 10.1016/j.jcin.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 103.Reducing Right Ventricular Failure in Pulmonary Arterial Hypertension (RELIEVE-PAH) August 5, 2020. ClinicalTrials.gov identifier: NCT03838445. https://clinicaltrials.gov/ct2/show/NCT03838445 Accessed May 1, 2021.
- 104.Reducing Lung Congestion Symptoms in Advanced Heart Failure. June 3, 2018. ClinicalTrials.gov identifier: NCT03499236. https://clinicaltrials.gov/show/NCT03499236 Accessed July 2, 2021.
- 105.Guimarães L., Bergeron S., Bernier M. Interatrial shunt with the second-generation V-Wave system for patients with advanced chronic heart failure. EuroIntervention. 2002;15(16):1426–1428. doi: 10.4244/EIJ-D-19-00291. [DOI] [PubMed] [Google Scholar]
- 106.Gupta A., Bailey S.R. Update on devices for diastolic dysfunction: options for a no option condition? Curr Cardiol Rep. 2018;20(10):85. doi: 10.1007/s11886-018-1027-2. [DOI] [PubMed] [Google Scholar]
- 107.Lilly B.Y.S.M., Burkhoff D. Technologies for treating left atrial decompression in heart failure. Cardiac Interventions Today. 2018;12(1):59–62. https://www.occlutech.com/files/8026332_2018_AFR_Burkhoff_Technologies in LA decompression in HF.pdf [Google Scholar]
- 108.Patel M.B., Samuel B.P., Girgis R.E., Parlmer M.A., Vettukattil J.J. Implantable atrial flow regulator for severe, irreversible pulmonary arterial hypertension. EuroIntervention. 2015;11(6):706–709. doi: 10.4244/EIJY15M07_08. [DOI] [PubMed] [Google Scholar]
- 109.Rajeshkumar R., Pavithran S., Sivakumar K., Vettukattil J.J. Atrial septostomy with a predefined diameter using a novel Occlutech atrial flow regulator improves symptoms and cardiac index in patients with severe pulmonary arterial hypertension. Catheter Cardiovasc Interv. 2017;90(7):1145–1153. doi: 10.1002/ccd.27233. [DOI] [PubMed] [Google Scholar]
- 110.The Prelieve Trial—Pilot Study to Assess Safety and Efficacy of a Novel Atrial Flow Regulator (AFR) in Heart Failure Patients (PRELIEVE). June 12, 2020. ClinicalTrials.gov identifier: NCT03030274. https://clinicaltrials.gov/ct2/show/NCT03030274 Accessed May 1, 2021.
- 111.Paitazoglou C., Bergmann M.W., Özdemir R. One-year results of the first-in-man study investigating the Atrial-Flow-Regulator for left-atrial shunting in symptomatic heart failure patients: the PRELIEVE study. Eur J Heart Fail. 2021;23(5) doi: 10.1002/ejhf.2119. [DOI] [PubMed] [Google Scholar]
- 112.The Prophet Trial—Pilot Study to Assess Safety and Efficacy of a Novel Atrial Flow Regulator (AFR) in Patients With Pulmonary Hypertension. June 12, 2020. ClinicalTrials.gov identifier: NCT03022851. https://clinicaltrials.gov/ct2/show/NCT03022851 Accessed May 1, 2021.
- 113.The AFteR Registry—Follow-up Study to Monitor the Efficacy and Safety of the Occlutech AFR in Heart Failure Patients. December 4, 2020. ClinicalTrials.gov identifier: NCT04405583. https://clinicaltrials.gov/ct2/show/NCT04405583
- 114.Flow Regulation by Opening the Septum in Patients With Heart Failure Trial (FROST-HF). 2018. ClinicalTrials.gov identifier: NCT03751748. https://clinicaltrials.gov/ct2/show/NCT03751748
- 115.Simard T., Labinaz M., Zahr F. Percutaneous atriotomy for levoatrial–to–coronary sinus shunting in symptomatic heart failure: first-in-human experience. J Am Coll Cardiol Intv. 2020;13(10):1236–1247. doi: 10.1016/j.jcin.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 116.Early Feasibility Study—Transcatheter Atrial Shunt System (ALt FLOW US). April 28, 2020. ClinicalTrials.gov identifier: NCT03523416. https://clinicaltrials.gov/ct2/show/NCT03523416 Accessed May 1, 2021.
- 117.Feld Y., Dubi S., Reisner Y., Schwammenthal E., Elami A. Future strategies for the treatment of diastolic heart failure. Acute Cardiac Care. 2006;8(1) doi: 10.1080/14628840600548988. [DOI] [PubMed] [Google Scholar]
- 118.Nussinovitch U., Topaz G., Landesberg A., Feld Y. Emerging Technologies for Heart Diseases. Elsevier; 2020. Novel treatments for diastolic heart failure; pp. 95–127. [Google Scholar]
- 119.Feld Y., Dubi S., Reisner Y. Energy transfer from systole to diastole: a novel device-based approach for the treatment of diastolic heart failure. Acute Card Care. 2011;13(4) doi: 10.3109/17482941.2011.634012. [DOI] [PubMed] [Google Scholar]
- 120.ImCardiaTM for DHF to Treat Diastolic Heart Failure (DHF) Patient a Pilot Study (ImCardia). May 4, 2011. ClinicalTrials.gov identifier: NCT01347125. https://clinicaltrials.gov/ct2/show/NCT01347125 Accessed May 1, 2021.
- 121.Feld Y., Reisner Y., Meyer-Brodnitz G., Hoefler R. The CORolla device for energy transfer from systole to diastole: a novel treatment for heart failure with preserved ejection fraction. Heart Fail Rev. 2021;1:3. doi: 10.1007/s10741-021-10104-x. [DOI] [PubMed] [Google Scholar]
- 122.Feld Y. Energy transfer from systole to diastole, a novel device for Heart Failure with Preserved Ejection Fraction. in PCR innovators day (PCR, 2019). [DOI] [PubMed]
- 123.CORolla® TAA for Heart Failure With Preserved Ejection Fraction (HFpEF) and Diastolic Dysfunction (DD). October 8, 2020. ClinicalTrials.gov identifier: NCT02499601. https://clinicaltrials.gov/ct2/show/NCT02499601 Accessed May 1, 2021.
- 124.Bisognano J.D., de Leeuw P., Bach D.S., Lovett E.G., Kaufman C.L. Improved functional capacity and cardiovascular structure after Baroreflex Activation TherapyTM in resistant hypertension patients with symptomatic heart failure: results from European and United States Trials of the Rheos® System. J Card Fail. 2009;15(6) doi: 10.1016/j.cardfail.2009.06.191. [DOI] [Google Scholar]
- 125.Kroon A., Schmidli J., Scheffers I. Sustained blood pressure reduction by baroreflex activation therapy with a chronically implanted system: 4-year data of Rheos Debut-HT study in patients with resistant hypertension. J Hypertens. 2010;28 doi: 10.1097/01.hjh.0000379526.21089.39. [DOI] [Google Scholar]
- 126.de Leeuw W.P., Bisognano J.D., Bakris G. Sustained reduction of blood pressure with baroreceptor activation therapy. Hypertension. 2017;69(5):836–843. doi: 10.1161/HYPERTENSIONAHA.117.09086. [DOI] [PubMed] [Google Scholar]
- 127.Hoppe U.C., Brandt M.C., Wachter R. Minimally invasive system for baroreflex activation therapy chronically lowers blood pressure with pacemaker-like safety profile: results from the Barostim neo trial. J Am Soc Hypertens. 2012;6(4):270–276. doi: 10.1016/j.jash.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 128.Gronda E., Seravalle G., Brambilla G. Chronic baroreflex activation effects on sympathetic nerve traffic, baroreflex function, and cardiac haemodynamics in heart failure: a proof-of-concept study. Eur J Heart Fail. 2014;16(9):977–983. doi: 10.1002/ejhf.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.BAROSTIM THERAPYTM In Heart Failure With Preserved Ejection Fraction (HFpEF). Aug 4, 2020. ClinicalTrials.gov identifier: NCT02876042. https://clinicaltrials.gov/ct2/show/NCT02876042 Accessed May 1, 2021.
- 130.Borggrefe M., Mann D.L. Cardiac contractility modulation in 2018. Circulation. 2018;138(24):2738–2740. doi: 10.1161/CIRCULATIONAHA.118.036460. [DOI] [PubMed] [Google Scholar]
- 131.Imai M., Rastogi S., Gupta R.C. Therapy with cardiac contractility modulation electrical signals improves left ventricular function and remodeling in dogs with chronic heart failure. J Am Coll Cardiol. 2007;49(21):2120–2128. doi: 10.1016/j.jacc.2006.10.082. [DOI] [PubMed] [Google Scholar]
- 132.Evaluation of the Safety and Effectiveness of the OPTIMIZER System in Subjects With Heart Failure: FIX-HF-5. August 6, 2020. ClinicalTrials.gov identifier: NCT00112125. https://clinicaltrials.gov/ct2/show/NCT00112125 Accessed May 1, 2021.
- 133.Continued Access Protocol for the Evaluation of the OPTIMIZER Smart System (FIX-HF-5CA). January 14, 2020. ClinicalTrials.gov identifier: NCT03102437. https://clinicaltrials.gov/ct2/show/NCT03102437 Accessed May 1, 2021.
- 134.Evaluation of the Safety and Efficacy of the 2-Lead OPTIMIZER® Smart System (FIX-HF-5C2). January 14, 2020. ClinicalTrials.gov identifier: NCT03339310. https://clinicaltrials.gov/ct2/show/NCT03339310 Accessed May 1, 2021.
- 135.Evaluate Safety and Efficacy of the OPTIMIZER® System in Subjects With Moderate-to-Severe Heart Failure: FIX-HF-5C (FIX-HF-5C). Feb 6, 2020. ClinicalTrials.gov identifier: NCT01381172. https://clinicaltrials.gov/ct2/show/NCT01381172 Accessed May 1, 2021.
- 136.Cardiac Contractility Modulation (CCM) Therapy in Subjects With Medically Refractory Heart Failure (IMPULSE-HF). September 2, 2020. ClinicalTrials.gov identifier: NCT02857309. https://clinicaltrials.gov/ct2/show/NCT02857309 Accessed May 1, 2021.
- 137.Effects of CCM-Therapy in Patients With Heart Failure (CCM-HF). September 9, 2016. ClinicalTrials.gov identifier: NCT02895048. https://clinicaltrials.gov/ct2/show/NCT02895048 Accessed May 1, 2021.
- 138.Post Approval Study (PAS) of the OPTIMIZER Smart and CCM Therapy (PAS). 2021. ClinicalTrials.gov identifier: NCT03970343. https://clinicaltrials.gov/ct2/show/NCT03970343 Accessed May 1, 2021.
- 139.CCM Italian Registry. June 24, 2020. ClinicalTrials.gov identifier: NCT04327323. https://clinicaltrials.gov/ct2/show/NCT04327323 Accessed May 1, 2021.
- 140.Borggrefe M.M., Lawo T., Butter C. Randomized, double blind study of non-excitatory, cardiac contractility modulation electrical impulses for symptomatic heart failure. Eur Heart J. 2008;29(8):1019–1028. doi: 10.1093/eurheartj/ehn020. [DOI] [PubMed] [Google Scholar]
- 141.Kadish A., Nademanee K., Volosin K. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am Heart J. 2011;161(2) doi: 10.1016/j.ahj.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 142.Kuschyk J., Roeger S., Schneider R. Efficacy and survival in patients with cardiac contractility modulation: Long-term single center experience in 81 patients. Int J Cardiol. 2015;183:76–81. doi: 10.1016/j.ijcard.2014.12.178. [DOI] [PubMed] [Google Scholar]
- 143.Abraham W.T., Kuck K.-H., Goldsmith R.L. A randomized controlled trial to evaluate the safety and efficacy of cardiac contractility modulation. J Am Coll Cardiol HF. 2018;6(10):874–883. doi: 10.1016/j.jchf.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 144.CCM in Heart Failure With Preserved Ejection Fraction (CCM-HFpEF). April 26, 2020. ClinicalTrials.gov identifier: NCT03240237. https://clinicaltrials.gov/ct2/show/NCT03240237 Accessed May 1, 2021.
- 145.Friedman D.J., Emerek K., Søgaard P., Vejdani-Jahromi M., Kisslo J., Atwater B.D. The mechanical and hemodynamic effects of left ventricular pacing in heart failure with preserved ejection fraction and left bundle branch block. J Electrocardiol. 2018;51(5):859–862. doi: 10.1016/j.jelectrocard.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Abraham W.T. In: Heart Failure. Semirgran M., Shin J.T., editors. 2017. Cardiac resynchronization therapy. 2nd ed. CRC Press. 2012. [Google Scholar]
- 147.Leclercq C., Hare J.M. Ventricular resynchronization: current state of the art. Circulation. 2004;109(3):296–299. doi: 10.1161/01.CIR.0000113458.76455.03. [DOI] [PubMed] [Google Scholar]
- 148.Abraham W.T., Fisher W.G., Smith A.L. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 149.Cazeau S., Leclercq C., Lavergne T. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344(12):873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 150.Auricchio A., Stellbrink C., Sack S. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol. 2002;39(12):2026–2033. doi: 10.1016/s0735-1097(02)01895-8. [DOI] [PubMed] [Google Scholar]
- 151.Bradley D.J., Bradley E.A., Baughman K.L. Cardiac resynchronization and death from progressive heart failure: a meta-analysis of randomized controlled trials. J Am Med Assoc. 2003;289(6):730–740. doi: 10.1001/jama.289.6.730. [DOI] [PubMed] [Google Scholar]
- 152.Sharma E., Wu M., Sheikh W. Abstract 15354: CRT versus RV Pacing—can we reduce readmissions in HFpEF through better device selection? Circulation. 2019;(140) [Google Scholar]
- 153.Yu C.M., Abraham W.T., Bax J. Predictors of response to cardiac resynchronization therapy (PROSPECT)—study design. Am Heart J. 2005;149(4):600–605. doi: 10.1016/j.ahj.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 154.Chung E.S., Leon A.R., Tavazzi L. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 155.Chung E.S., Katra R.P., Ghio S. Cardiac resynchronization therapy may benefit patients with left ventricular ejection fraction >35: a PROSPECT trial substudy. Eur J Heart Fail. 2010;12(6):581–587. doi: 10.1093/eurjhf/hfq009. [DOI] [PubMed] [Google Scholar]
- 156.Lam C.S.P. Elsevier; 2014. Heart Failure with Preserved Ejection Fraction. 3rd ed, vol. 10. [Google Scholar]
- 157.Laurent G., Eicher J.C., Mathe A. Permanent left atrial pacing therapy may improve symptoms in heart failure patients with preserved ejection fraction and atrial dyssynchrony: a pilot study prior to a national clinical research programme. Eur J Heart Fail. 2013;15(1):85–93. doi: 10.1093/eurjhf/hfs150. [DOI] [PubMed] [Google Scholar]
- 158.Levy T., Walker S., Rex S., Paul V. A comparison between passive and active fixation leads in the coronary sinus for biatrial pacing: initial experience. Europace. 2000;2(3):228–232. doi: 10.1053/eupc.2000.0105. [DOI] [PubMed] [Google Scholar]
- 159.LEft Atrial Pacing in Diastolic Heart Failure (LEAD). December 27, 2018. ClinicalTrials.gov identifier: NCT01618981. https://clinicaltrials.gov/ct2/show/NCT01618981 Accessed May 1, 2021.
- 160.Physiologic Accelerated Pacing as a Treatment in Patients With Heart Failure With Preserved Ejection Fraction (PACE HFpEF). February 25, 2021. ClinicalTrials.gov identifier: NCT04546555. https://clinicaltrials.gov/ct2/show/NCT04546555 Accessed May 1, 2021.
- 161.Huang W., Su L., Wu S. Benefits of permanent His bundle pacing combined with atrioventricular node ablation in atrial fibrillation patients with heart failure with both preserved and reduced left ventricular ejection fraction. J Am Heart Assoc. 2017;6(4) doi: 10.1161/JAHA.116.005309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Efficacy Study of Pacemakers to Treat Slow Heart Rate in Patients With Heart Failure (RAPID-HF). January 28, 2021. ClinicalTrials.gov identifier: NCT02145351. https://clinicaltrials.gov/ct2/show/NCT02145351 Accessed May 1, 2021.
- 163.Cardiac Resynchronisation Therapy Versus Rate-responsive Pacing in Heart Failure With Preserved Ejection Fraction (PREFECTUS). July 24, 2020. ClinicalTrials.gov identifier: NCT03338374. https://clinicaltrials.gov/ct2/show/NCT03338374 Accessed May 1, 2021.
- 164.Biventricular Versus Right Ventricular Pacing in Heart Failure Patients With Atrioventricular Block (BLOCK HF). March 26, 2014. ClinicalTrials.gov identifier: NCT00267098. https://clinicaltrials.gov/ct2/show/NCT00267098 Accessed May 1, 2021.
- 165.Curtis A.B., Worley S.J., Adamson P.B. Biventricular Pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368(17):1585–1593. doi: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]
- 166.Penicka M., Kocka V., Herman D., Trakalova H., Herold M. Cardiac resynchronization therapy for the causal treatment of heart failure with preserved ejection fraction: insight from a pressure-volume loop analysis case report. Eur J Heart Fail. 2010;12(6):634–636. doi: 10.1093/eurjhf/hfq068. [DOI] [PubMed] [Google Scholar]
- 167.AlTurki A., Ylima P.Y., Vidal A. Fusion pacing in patients with right bundle branch block who undergo cardiac resynchronization therapy. J Electrocardiol. 2021;64:66–71. doi: 10.1016/j.jelectrocard.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 168.Varma N., O'Donnell D., Bassiouny M. Programming cardiac resynchronization therapy for electrical synchrony: reaching beyond left bundle branch block and left ventricular activation delay. J Am Heart Assoc. 2018;7(3) doi: 10.1161/JAHA.117.007489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Thibault B., Ritter P., Bode K. Dynamic programming of atrioventricular delay improves electrical synchrony in a multicenter cardiac resynchronization therapy study. Heart Rhythm. 2019;16(7):1047–1056. doi: 10.1016/j.hrthm.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 170.Hsu J.C., Birnie D., Stadler R.W., Cerkvenik J., Feld G.K., Birgersdotter-Green U. Adaptive cardiac resynchronization therapy is associated with decreased risk of incident atrial fibrillation compared to standard biventricular pacing: a real-world analysis of 37,450 patients followed by remote monitoring. Heart Rhythm. 2019;16(7):983–989. doi: 10.1016/j.hrthm.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 171.Pacing for Heart Failure With Preserved Ejection Fraction (HFpEF). May 30, 2013. ClinicalTrials.gov identifier: NCT01045291. https://clinicaltrials.gov/ct2/show/NCT01045291 Accessed May 1, 2021.
- 172.Burkhoff D. Commentary: pumps for pEF. J Thorac Cardiovasc Surg. 2020;162(1):127–128. doi: 10.1016/j.jtcvs.2020.01.070. [DOI] [PubMed] [Google Scholar]
- 173.Sheth S., Bandeali S., George J. Mechanical Circulatory Support for Advanced Heart Failure: A Texas Heart Institute/Baylor College of Medicine Approach. Springer International Publishing; 2017. Bridge-tobridge strategies with IABP, impella, and tandemHeart; pp. 57–67. [DOI] [Google Scholar]
- 174.Healy A.H., McKellar S.H., Drakos S.G., Koliopoulou A., Stehlik J., Selzman C.H. Physiologic effects of continuous-flow left ventricular assist devices. J Surg Res. 2016;202(2):363–371. doi: 10.1016/j.jss.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Granegger M., Dave H., Knirsch W., Thamsen B., Schweiger M., Hübler M. A valveless pulsatile pump for the treatment of heart failure with preserved ejection fraction: a simulation study. Cardiovasc Eng Technol. 2019;10(1):69–79. doi: 10.1007/s13239-018-00398-8. [DOI] [PubMed] [Google Scholar]
- 176.Escher A., Choi Y., Callaghan F. A valveless pulsatile pump for heart failure with preserved ejection fraction: hemo- and fluid dynamic feasibility. Ann Biomed Eng. 2020;48(6):1821–1836. doi: 10.1007/s10439-020-02492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fukamachi K., Horvath D.J., Karimov J.H. Left atrial assist device to treat patients with heart failure with preserved ejection fraction: initial in vitro study. J Thorac Cardiovasc Surg. 2020;162(1):120–126. doi: 10.1016/j.jtcvs.2019.12.110. [DOI] [PubMed] [Google Scholar]
- 178.Kado Y., Kowski A.R., Kuban B.D. Left atrial assist device function at various heart rates using a mock circulation loop. Int J Artif Organs. 2020 doi: 10.1177/0391398820977508. [DOI] [PubMed] [Google Scholar]
- 179.Kado Y., Polakowski A.R., Kuban B.D. The effects of preserving mitral valve function on a left atrial assist device: an in vitro mock circulation loop study. ASAIO J. 2021;67(5):567–572. doi: 10.1097/MAT.0000000000001257. [DOI] [PubMed] [Google Scholar]
- 180.Miyagi C., Karimov J.H., Kuban B.D. Development of the left atrial assist device for patients with heart failure with preserved ejection fraction: first in vivo results. J Heart Lung Transplant. 2021;40(4):S176–S177. [Google Scholar]
- 181.Miyagi C., Kuban B.D., Flick C.R. Left atrial assist device for heart failure with preserved ejection fraction: initial results with torque control mode in diastolic heart failure model. Heart Fail Rev. 2021 doi: 10.1007/s10741-021-10117-6. [DOI] [PubMed] [Google Scholar]
- 182.Moazami N., Smith D. Commentary: can we pump our way out of heart failure with preserved ejection fraction? Not so soon. J Thorac Cardiovasc Surg. 2020;162(1):129–130. doi: 10.1016/j.jtcvs.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 183.Butler J., Hamo C.E., Udelson J.E. Exploring new endpoints for patients with heart failure with preserved ejection fraction. Circ Heart Fail. 2016;9(11) doi: 10.1161/CIRCHEARTFAILURE.116.003358. [DOI] [PubMed] [Google Scholar]
- 184.Parikh K.S., Sharma K., Fiuzat M., Surks H.K., George J.T. Heart failure with preserved ejection fraction expert panel report: current controversies and implications for clinical trials. J Am Coll Cardiol HF. 2018;6(8):619–632. doi: 10.1016/j.jchf.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 185.Topilsky Y., Pereira N.L., Shah D.K. Left ventricular assist device therapy in patients with restrictive and hypertrophic cardiomyopathy. Circ Heart Fail. 2011;4(3):266–275. doi: 10.1161/CIRCHEARTFAILURE.110.959288. [DOI] [PubMed] [Google Scholar]
- 186.Wynne E., Bergin J.D., Ailawadi G., Kern J.A., Kennedy J.L.W. Use of a left ventricular assist device in hypertrophic cardiomyopathy. J Card Surg. 2011;26(6):663–665. doi: 10.1111/j.1540-8191.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- 187.Muthiah K., Phan J., Robson D. Centrifugal continuous-flow left ventricular assist device in patients with hypertrophic cardiomyopathy: a case series. ASAIO J. 2013;59(2):183–187. doi: 10.1097/MAT.0b013e318286018d. [DOI] [PubMed] [Google Scholar]
- 188.Lund L.H., Matthews J., Aaronson K. Patient selection for left ventricular assist devices. Eur J Heart Fail. 2010;12(5):434–443. doi: 10.1093/eurjhf/hfq006. [DOI] [PubMed] [Google Scholar]
- 189.Morgan J.A., Naka Y. In: Surgical Treatment for Advanced Heart Failure. Morgan J., Naka Y., editors. Springer; 2013. Mechanical circulatory support as a bridge to transplantation; pp. 121–136. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.