Abstract
Intraoperative cancer imaging and fluorescence-guided surgery have attracted considerable interest because fluorescence signals can provide real-time guidance to assist a surgeon in differentiating cancerous and normal tissues. Recent advances have led to the clinical use of a natural fluorophore called protoporphyrin IX (PpIX) for image-guided surgical resection of high-grade brain tumors (glioblastomas). However, traditional fluorescence imaging methods have only limited detection sensitivity and identification accuracy, and are unable to detect low-grade or diffuse infiltrating gliomas (DIGs). Here we report a low-cost handheld spectroscopic device that is capable of ultrasensitive detection of protoporphyrin IX fluorescence in vivo, together with intraoperative spectroscopic data obtained from both animal xenografts and human brain tumor specimens. The results indicate that intraoperative spectroscopy is at least 3 orders of magnitude more sensitive than the current surgical microscopes, allowing ultrasensitive detection of as few as 1000 tumor cells. For detection specificity, intraoperative spectroscopy allows the differentiation of brain tumor cells from normal brain cells with a contrast signal ratio over 100. In-vivo animal studies reveal that protoporphyrin IX fluorescence is strongly correlated with both MRI and histological staining, confirming that the fluorescence signals are highly specific to tumor cells. Furthermore, ex-vivo spectroscopic studies of excised brain tissues demonstrate that the handheld spectroscopic device is capable of detecting diffuse tumor margins with low fluorescence contrast that are not detectable with current systems in the operating room. These results open new opportunities for intraoperative detection and fluorescence-guided resection of microscopic and low-grade glioma brain tumors with invasive or diffusive margins.
Graphical Abstract
Introduction
The development of advanced devices and contrast agents for image-guided interventions (such as image-guided microsurgery, stereotactic biopsy, and focused radiation therapy) is an area of considerable current interest.1–5 Recent advances in computed tomography (CT), positron emission tomography (PET), and hybrid techniques (such as CT/PET) have greatly improved tumor detection and surgical planning6,7, but these modalities do not provide real-time intraoperative assistance. Intraoperative magnetic resonance imaging (MRI) can assist in surgical resection of tumors, but it substantially lengthens the anesthesia and operation times, and is financially prohibitive.8 Intraoperative ultrasound has also shown promise for tumor detection, but it does not have sufficient sensitivity to detect tumor nodules smaller than 5 mm in size.9 For clinical studies, one of the most important advances is the use of contrast-enhanced fluorescence signals for image-guided surgical resection of malignant brain tumors.10,11 The basic principle is that malignant tumor cells are found to preferentially produce and accumulate a natural fluorophore called protoporphyrin IX (PpIX) after oral administration of a low-molecular-weight contrast agent called 5-aminolevulinic acid (5-ALA).12 The intracellular PpIX emits bright red fluorescence when illuminated with blue light, allowing the surgeon to visualize the tumor margins during surgery in real time.13 Pioneering work by Stummer and coworkers14,15 have shown a significant increase in the 6-month progression-free survival of patients with high-grade brain tumors (glioblastoma or GBM), in whom fluorescence-guided resection was performed as compared to surgical controls.
However, the current surgical microscopes are unable to detect low-grade brain tumors (gliomas) because of their limited detection sensitivity and identification accuracy.16 Low-grade gliomas have been found to accumulate PpIX but at a lower concentration that is difficult to detect by the operative microscope used for fluorescence-guided surgery.17 To overcome this problem, several groups have explored the use of intraoperative confocal microscopy,17,18 optical coherent tomography,19 native tissue fluorescence,20 quantitative spectroscopic measurement,21–24 or Raman spectroscopy,25 but none of these approaches has gained much traction in clinical applications. Recent work in our group26 has also developed handheld spectroscopic devices for intraoperative measurement of both near-infrared fluorescence and Raman scattering signals. However, the tumor detection sensitivity and specificity of intraoperative spectroscopy have not been determined, and it is not clear how much improvement could be expected in comparison with traditional fluorescence imaging.
In this work, we have developed a new handheld spectroscopic device that is capable of ultrasensitive detection of protoporphyrin IX fluorescence in vivo, and have evaluated its sensitivity and specificity for intraoperative tumor detection by using both animal models and human brain tumor specimens. In particular, we have compared its performance with that of current surgical microscopes under similar experimental conditions. The results show that the intraoperative spectroscopy is dramatically more sensitive (by 3–4 orders of magnitude) than traditional fluorescence imaging, allowing detection of microscopic tumor deposits with only 1000 cells. For detection specificity, we find that intraoperative spectroscopy allows the differentiation of brain tumor cells from normal brain cells with a contrast signal ratio over 100, a significant improvement over the previously reported contrast ratios of 5–10.27,28 We have further carried out detailed in-vivo animal studies of malignant brain tumors, which show that the PpIX fluorescence is strongly correlated with both MRI and histological staining, confirming that the ALA/PpIX contrast mechanism is indeed highly specific to tumor cells. Furthermore, ex-vivo spectroscopic studies of excised brain tissues indicate that the handheld spectroscopic device is capable of detecting diffuse tumor margins with low fluorescence contrast that are not detectable with current systems. With improved sensitivity and specificity, we believe that handheld spectroscopic devices are well suited for intraoperative detection and fluorescence-guided resection of low-grade brain tumors.
Experimental:
Reagents.
Ultrapure water (18.2 Ω) was used throughout this work. 5-aminolevulinic acid (5-ALA) and protoporphyrin IX (PpIX) were purchased from Sigma-Aldrich. RPMI-1640 cell culture media, fetal bovine serum (FBS), antibiotic/antimycotic solution, and phosphate buffered saline (PBS) were purchased from Corning (Cellgro). All reagents were used as purchased without further purification.
Handheld Spectrometer.
A handheld spectroscopic system was constructed with modular components. A bifurcated fiber optic probe was coupled with a violet LED excitation light source (405 nm) and 16-bit spectrometer (Ocean Optics QE65000). The probe consists of a single fiber for emission collection surrounded by 6 fibers for the excitation light source.
In Vitro Cell Staining.
Normal human astrocytes and a glioblastoma cell line transfected with a plasmid for overexpression of epidermal growth factor receptor deletion mutant (U87ΔEGFRvIII) were cultured in 8-well LabTek slides at 37°C and 5% CO2. At approximately 50% confluency, the cells were incubated with 2 mM 5-ALA to induce the production of protoporphyrin IX. After 24 hours, the cells were washed with 1x PBS and imaged with a fluorescence microscope (Olympus IX71, FITC fluorescence cube) and analyzed with ImageJ to determine the presence or absence of PpIX staining.
Sensitivity Measurement.
Glioblastoma cells (U87ΔEGFRvIII) were cultured in a T-25 flask at 37°C and 5% CO2. After reaching approximately 75% confluency, the cells were incubated with 2 mM 5-ALA for 24 hours to induce PpIX fluorescence. The cells were then washed with 1x PBS and detached from the culture plate using 0.05% trypsin and immediately washed with 1x PBS. The cells were counted using a hemacytometer and transferred to microfuge tubes in specific numbers. The microfuge tubes were centrifuged at 500 rpm for 5 mins to concentrate the cells and fluorescence measurements were taken using the handheld spectroscopic device.
In Vivo Animal Studies.
The human glioblastoma (GBM) cell line U87-MG (American Type Culture Collection- ATCC, Bethesda, MD) was transfected with a plasmid for overexpression of the epidermal growth factor receptor (EGFR) deletion mutant (EGFRvIII) present on human GBM cells (U87ΔEGFRvIII). Six-to-eight weeks old athymic nude (nu/nu) mice were used and the performed procedures were approved by the Institutional Animal Care Use Committee (IACUC) of Emory University. Athymic nude mice were anesthetized by intraperitoneal (IP) injection of an anesthetic mixture consisting of ketamine (80 mg/kg), xylazine (10 mg/kg) and acepromazine (3 mg/kg). Adequate depth of anesthesia was confirmed by performing toe pinch. Anesthetized animals were then temporarily restrained in a small animal stereotaxic instrument (David Kopf Instruments, Tujunga, CA). An incision was made on the scalp to expose the underlying cranium followed by a small cranial opening with a 26 gauge needle tip. The opening on the skull was made immediately posterior to the coronal suture, 2–3 mm to the right of the midline. A Hamilton syringe attached to the stereotaxic frame and fitted with a 30-gauge removable needle (Hamilton Co., Reno, Nevada) was used to stereotactically inoculate 5 × 105 U87ΔEGFRvIII cells in a total volume of 5 microliters, into the right striatum of each mouse brain. Immediately after the inoculation of cells, the cranial opening was sealed with bone wax to avoid backflow of cells outside of the brain. The scalp was then reapproximated by placement of interrupted 4–0 Vicryl sutures.
Maintenance of anesthesia of sedated animals during surgery was achieved by IP injection of ketamine (20mg/kg). Analgesics were also used both during surgery to control pain and maintain sedation and the first day after the surgical procedure to ensure proper pain control. For this purpose buprenorphine (0.1mg/kg) and meloxicam (1mg/kg) were used. After completion of the surgery the animals were monitored in frequent time intervals until full recovered and were kept warm by using heating pads to ensure fast and proper recovery. After 14 days, 5-ALA was administered orally or intraperitoneally to the mice using a dose of 200 mg/kg and the mice brains were removed 2 hours after 5-ALA administration. Fluorescence measurements were taken using the spectroscopic devive and the brains were the frozen for sectioning and H&E staining.
MRI Imaging.
Mice inoculated with U87ΔEGFRvIII cells (5 × 105) were followed by Magnetic Resonance Imaging (MRI) to confirm the presence of intracranial human GBM xenograft. For this purpose, animals were scanned on a 4.7-Tesla small animal MRI scanner using a mouse coil (Varian Unity). Animals were anesthetized using isoflurane vaporizer and were positioned into a molded plastic restraint providing animal support for imaging. Positioning of the required sensors for measuring physiologic parameters was also done before initiating imaging. Maintenance of anesthesia of sedated animals during the imaging was done using the information obtained from the sensors for heart rate, respiration rate and temperature which were connected to a monitoring system (SA, Instruments, Inc., Stony Brook, NY) and were displayed continuously at the scanner console on a PC screen. After imaging completion, the animals were removed from the scanner and were monitored until fully recovered from anesthesia.
Ex Vivo Analysis of Human Glioblastoma Specimens.
Human glioblastoma tumor specimens were collected from patients undergoing fluorescence-guided tumor resections in accordance with protocols approved by the Institutional Review Board (IRB) of Emory University. The patients were part of a Phase II clinical trial (IND #112246) at the Winship Cancer Institute of Emory University for 5-ALA fluorescence-guided surgery and received a 20 mg/kg oral dose of 5-ALA 3–5 hours prior to tumor resection. Tumor specimens removed from the patients were then analyzed ex vivo using the handheld spectroscopic device to determine its performance for detecting PpIX fluorescence in human tumor tissues.
Results:
Handheld Spectroscopy.
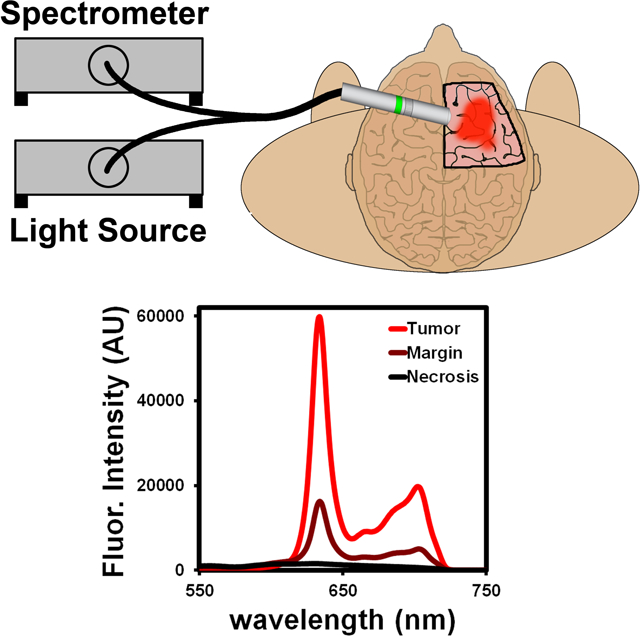
The general concept of intraoperative glioblastoma identification using handheld spectroscopy is illustrated in Fig. 1. An important component of this fluorescence-based surgical strategy is the use of 5-ALA and its metabolite, PpIX, as a contrast agent, which provides a high fluorescence signal in tumor regions to delineate the tumor and its boundaries while giving little or no signal in normal brain tissues. However, the growth pattern of glioblastoma tumors leads to diffuse protrusions that infiltrate into surrounding normal tissue, resulting in poorly defined margins that typically have a lower fluorescence signal compared with the bulk tumor. The fluorescence signals are typically observed in the operating room via an adapted surgical microscope, which is a Class I device that has 510(k) clearance for sale in the United States. These fluorescence microscopes include a combination of excitation and emission filters with slightly overlapping transmission integrated into the optical configuration. Due to this overlap, a small fraction of reflected excitation light generates a blue tone contrast from the healthy brain anatomy in contrast to bright red porphyrin florescence. The surgeon can view the surgical field with white light for normal anatomical visualization or a blue-violet excitation light for fluorescence using an electromagnetic filter switcher to introduce dielectrically-coated 440 nm short pass and long pass filters into the illumination and observation light paths. Filters were designed to transmit red porphyrin fluorescence as well as a fraction of backscattered blue excitation light necessary for distinguishing non-fluorescing tissue. A major limitation in the detection of the PpIX fluorescence signals is the long working distance (~30 cm) of currently used surgical microscopes (Fig. 1, right), which significantly reduces the illumination photon flux and may give the surgeon an incomplete or inaccurate visualization of the tumor boundaries due to the weak fluorescence signals.
Figure 1.
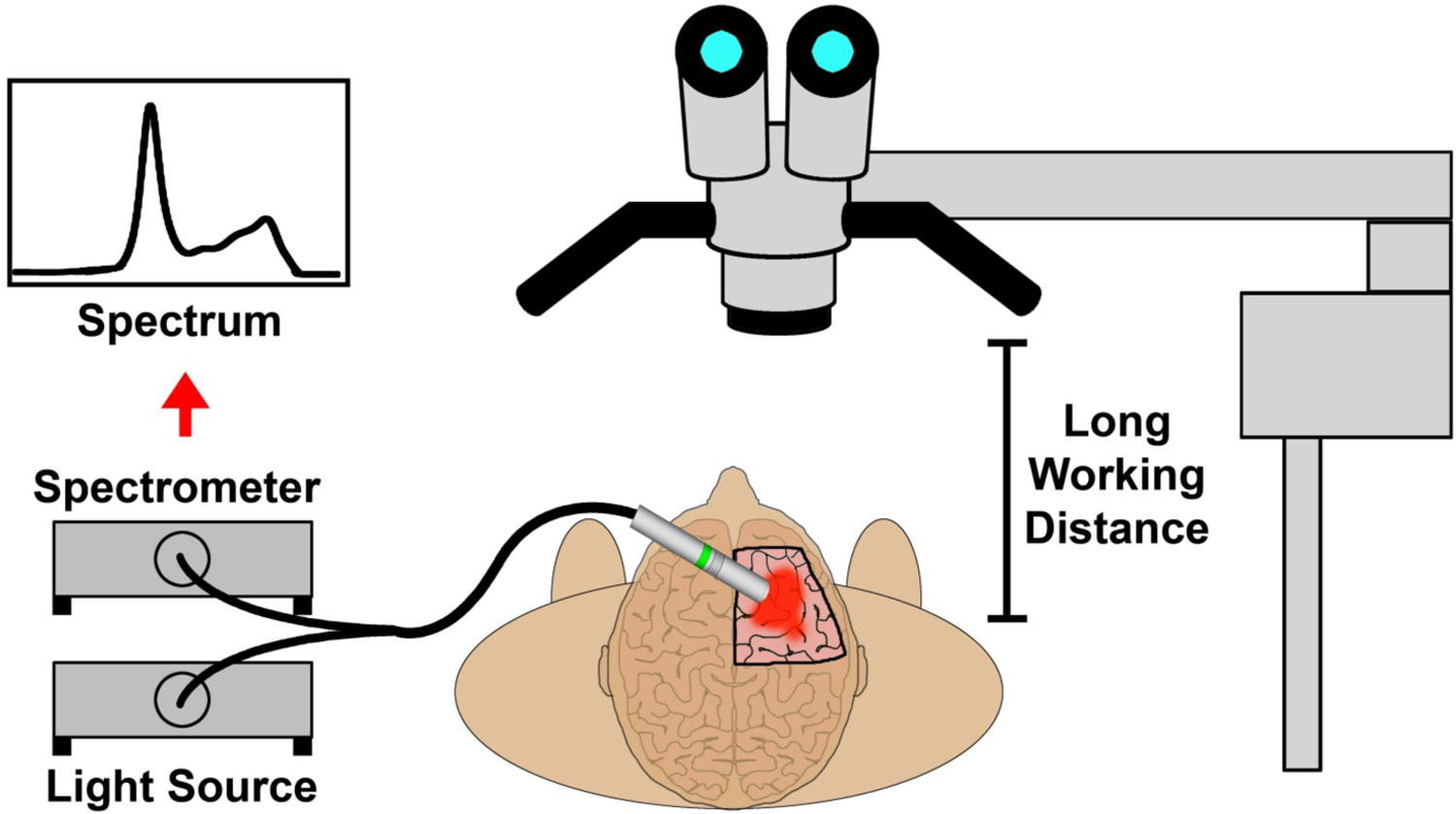
Schematic diagram showing handheld spectroscopic detection of human brain tumors with 5-aminolevulinic acid and protoporphyrin IX as contrast agents (left), in comparison with detection using a surgical fluorescence microscope (right).
To address this challenge, a handheld spectroscopic system consisting of a bifurcated fiber optic-coupled probe with a violet excitation light source (405 nm) and sensitive spectrometer was designed (Fig. 1, left). A fundamental advantage of this device is the ability to place the handheld probe directly onto the tissue of interest (<1 cm away), eliminating the problem of light diffusion at longer distances and allowing efficient light capturing and greatly increased detection sensitivity. The short working distance of the probe also allows the use of inexpensive, low power LED light sources for fluorescence excitation. Another key feature of the handheld system is the ability to accurately distinguish the PpIX fluorescence signal from background autofluorescence or other fluorescent compounds through spectroscopic analysis. The unique PpIX fluorescence spectrum is extracted from background signals by iterative least-squares curve fitting of the complex spectral data measured by the device, providing accurate signal measurements even at very low signal intensities. This is a significant improvement over the typical video camera systems used with fluorescent surgical microscopes which have difficulty distinguishing complex weak signals.
Detection Sensitivity and Specificity.
In vitro cellular experiments were performed to characterize the specificity and sensitivity of the handheld spectroscopic device for the detection of glioblastoma cells. Initial studies were conducted to determine the specificity of the agent alone for cancerous cells compared with normal brain cells. Normal human astrocytes and a human GBM cell line that overexpresses the epidermal growth factor receptor deletion mutant EGFRvIII (U87-ΔEGFRvIII), were used.29 Both cell types were cultured and incubated with 5-ALA to induce the production of intracellular PpIX. After 24 hours, the cells were washed with PBS and imaged with a fluorescence microscope to determine the presence or absence of PpIX signals (Fig. 2). As expected, GBM cells (Fig. 2A and 2B) showed significant fluorescence after exposure to 5-ALA, indicating a high conversion and accumulation of PpIX. Conversely, normal brain cells (Fig. 2C and 2D) had no visible fluorescence signals, even when exposed to a large excess of 5-ALA. Detailed image analysis of the fluorescence intensity of the astrocytes showed no statistical difference between the normal brain cells and blank controls. These data are consistent with previous reports and demonstrate the high specificity of 5-ALA-induced fluorescence in high-grade glioblastomas compared with normal brain tissue.30–32 While the complete mechanism of PpIX accumulation in tumor cells is not fully understood, a primary factor influencing the intracellular PpIX concentration is the activity of ferrochelatase.33,34 This is an enzyme in the heme pathway responsible for catalyzing the insertion of ferrous iron (Fe2+) into PpIX to produce heme, which is no longer fluorescent. The expression of ferrochelatase is significantly reduced in many types of tumor cells34,35 and results in a buildup of fluorescent PpIX, while cells with normal ferrochelatase activity are able to complete the heme biosynthesis pathway and avoid an accumulation of PpIX. Another potential contributing factor is the increased metabolic activity required to fuel the growth of active cancer cells. Because heme is required for the production of cytochrome c, an essential protein in the electron transport chain, it is hypothesized that the heme biosynthesis pathway may be upregulated in cancer cells to meet this demand,36 thus resulting in an increased production of PpIX as well.
Figure 2.
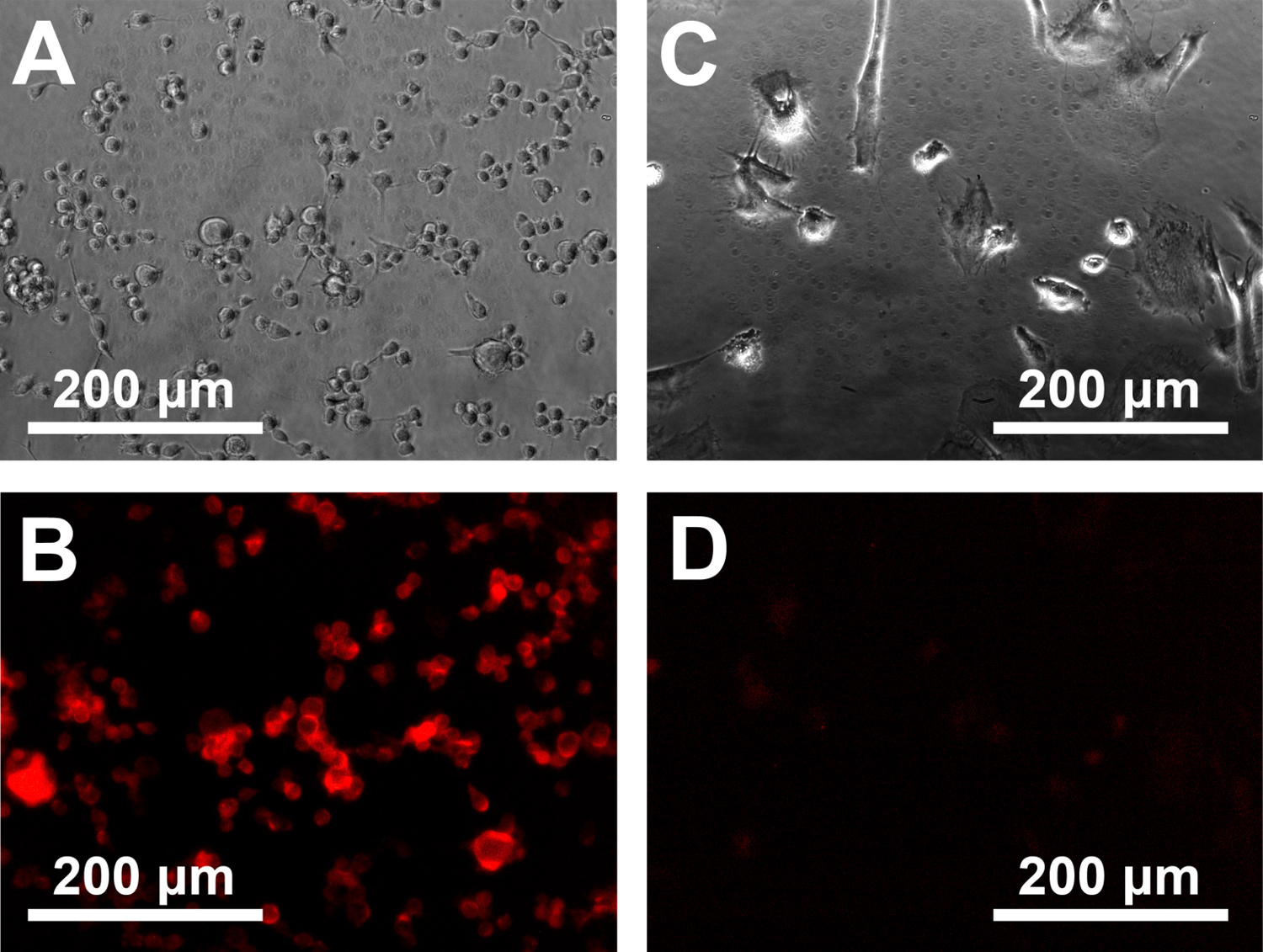
Comparison of cultured brain tumor cells (U87 EGFRvIII) and normal human brain cells before and after incubation with 5-ALA. (A, C) Phase-contrast photomicrographs showing the different morphologies of human glioblostoma tumor cells and normal human brain astrocytes. (B, D) Fluorescence images showing a strong buildup of fluorescent PpIX and the absence of fluorescence signals in normal brain cells.
After confirming the specificity of the fluorescence signal in GBM cells, separate in vitro studies were performed to determine the detection sensitivity of the handheld spectroscopic device. GBM cells were cultured and incubated with 5-ALA to induce PpIX fluorescence. The cells were then detached from the culture plate and transferred to microfuge tubes in decreasing numbers for fluorescence measurements with the handheld device. Spectroscopic measurements showed the characteristic shape for PpIX fluorescence (peaks at ~630 nm and ~700 nm) with decreasing intensity for lower numbers of cells (Fig. 3C). Analyzing the area under the curve of the measured spectra, fluorescence signal intensity was found to have a linear relationship with the number of cells in a range of 1,000 to 20,000 cells using the parameters detailed above (Fig. 3D). Further analysis revealed that the handheld spectroscopic device was able to detect as low as 1000 fluorescently active GBM cells under in vitro conditions using a 100 ms acquisition time. Assuming a cell size of approximately 10 microns, this corresponds to a cubic cluster of cells measuring approximately 0.1 millimeters in diameter (100 um microscopic tumors). The signal-to-noise ratio of the device can be even further improved by increasing the acquisition time as random shot noise decreases as the square root of the acquisition time.
Figure 3.
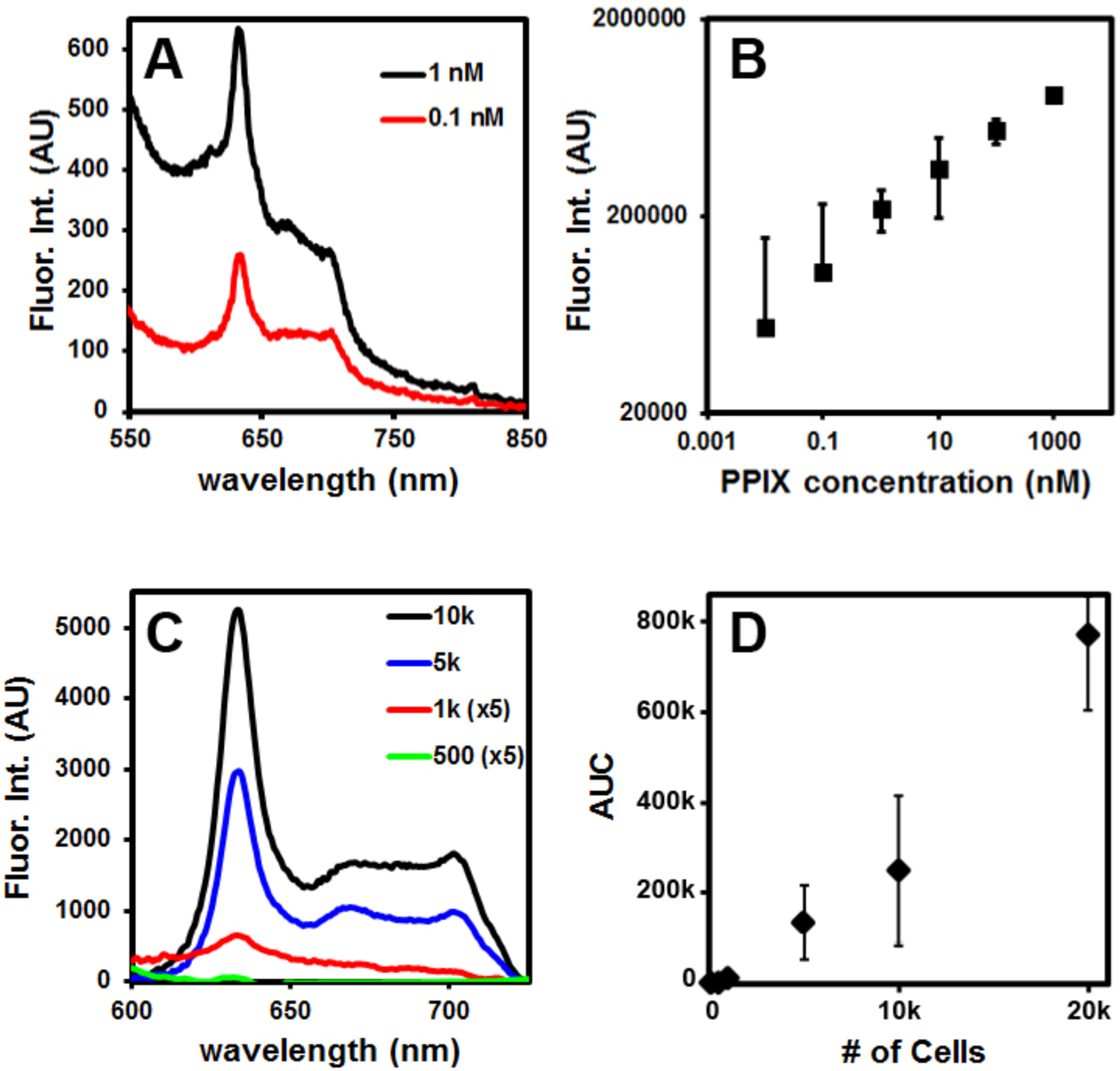
Detection sensitivity as evaluated by PpIX concentration in pure solution and by the number of cultured brain tumor cells after incubation with 5-ALA. (A, B) Fluorescence spectra of free PpIX and signal intensity as a function of concentration, showing detection sensitivity on the order of 10–100 pM PpIX. (C, D) Fluorescence spectra of metabolically generated PpIX in brain tumor cells (incubation with 5-ALA for 24 hours) and signal intensity dependence on the number of tumor cells.
We have also conducted additional experiments to compare the sensitivity of the handheld spectroscopic system with the conventional surgical microscope outfitted with a fluorescence filter (Zeiss Pentero 900 with blue 400 system). Pure PpIX was dissolved in saline, and serial dilutions were made to determine the minimum detectable concentration using each device. The fluorescence surgical microscope was capable of detecting 500 nM PpIX, whereas the handheld spectroscopic device was able to detect 10–100 pM PpIX with a data integration time of just 0.1 seconds) (Fig. 3). This represents an increase in detection sensitivity of 3–4 orders of magnitude (5–10×103 fold).
In Vivo Glioblastoma Detection by Intraoperative Spectroscopy.
To test the performance of the handheld spectroscopic device for intraoperative glioblastoma detection, we have conducted in vivo animal experiments using an intracranial human GBM xenograft mouse model. GBM cells (U87ΔEGFRvIII) transfected with a plasmid for the overexpression of EGFRvIII were stereotactically injected into the right cerebral hemisphere of athymic nude mice 14 days prior to the intraoperative spectroscopy procedures. The EGFRvIII mutant xenografts are an ideal model for assessing this device because they are highly tumorigenic in immunocompromised mice. Orthotopic GBM xenografts grow rapidly and mimic the aggressive and invasive nature of the disease in humans. 5-ALA was administered orally or intraperitoneally to the mice using a dose of 200 mg/kg, and the brains of the mice were removed 2 hours after 5-ALA administration (Fig. 4A). Upon 405 nm excitation with the handheld spectroscopic device, 5-ALA-induced PpIX fluorescence was visible in the region of the implanted tumor (Fig. 4B). Bright fluorescence was observed from the visible lesion on the surface of the right hemisphere while much weaker fluorescence was detected surrounding the lesion. Investigating the left hemisphere of the brain (assumed to be normal) showed no PpIX fluorescence. Spectroscopic glioblastoma identification was independently confirmed with preoperative MRI (Fig. 4C), which revealed the presence of a large tumor in the right hemisphere protruding to the brain surface (cyan arrow) and was highly correlated with the fluorescence measurements. Interestingly, the weak fluorescence surrounding the visible lesion that was detected using the spectroscopic device correlated well with the presence of tumor below the surface of the brain (1–2 mm). The emission of PpIX (between 630–700 nm, Fig. 3A) falls within a “clear” optical window for biological tissue and has an increased penetration depth compared with shorter wavelength dyes which emit in the blue or green.37 This demonstrates an important advantage of 5-ALA over fluorescent dyes that emit at shorter wavelengths (e.g., fluorescein) where tissue absorption and scattering are considerably higher.37, 38 Histological analysis also confirmed the correlation between the presence of tumor and the detection of fluorescence. Coronal sections of the whole mouse brain were prepared immediately following the spectroscopic measurements and H&E staining was used to visualize the GBM tumor (Fig. 4D). The coronal sections clearly showed the presence of tumor (stained blue) in the right hemisphere which protruded to the surface of the brain and correlated closely with both the MRI images and the spectroscopic data. Fluorescence imaging of the coronal sections further verified the tumor localization and the specificity of protoporphyrin IX fluorescence for tumor cells in vivo (Fig. 5).
Figure 4.
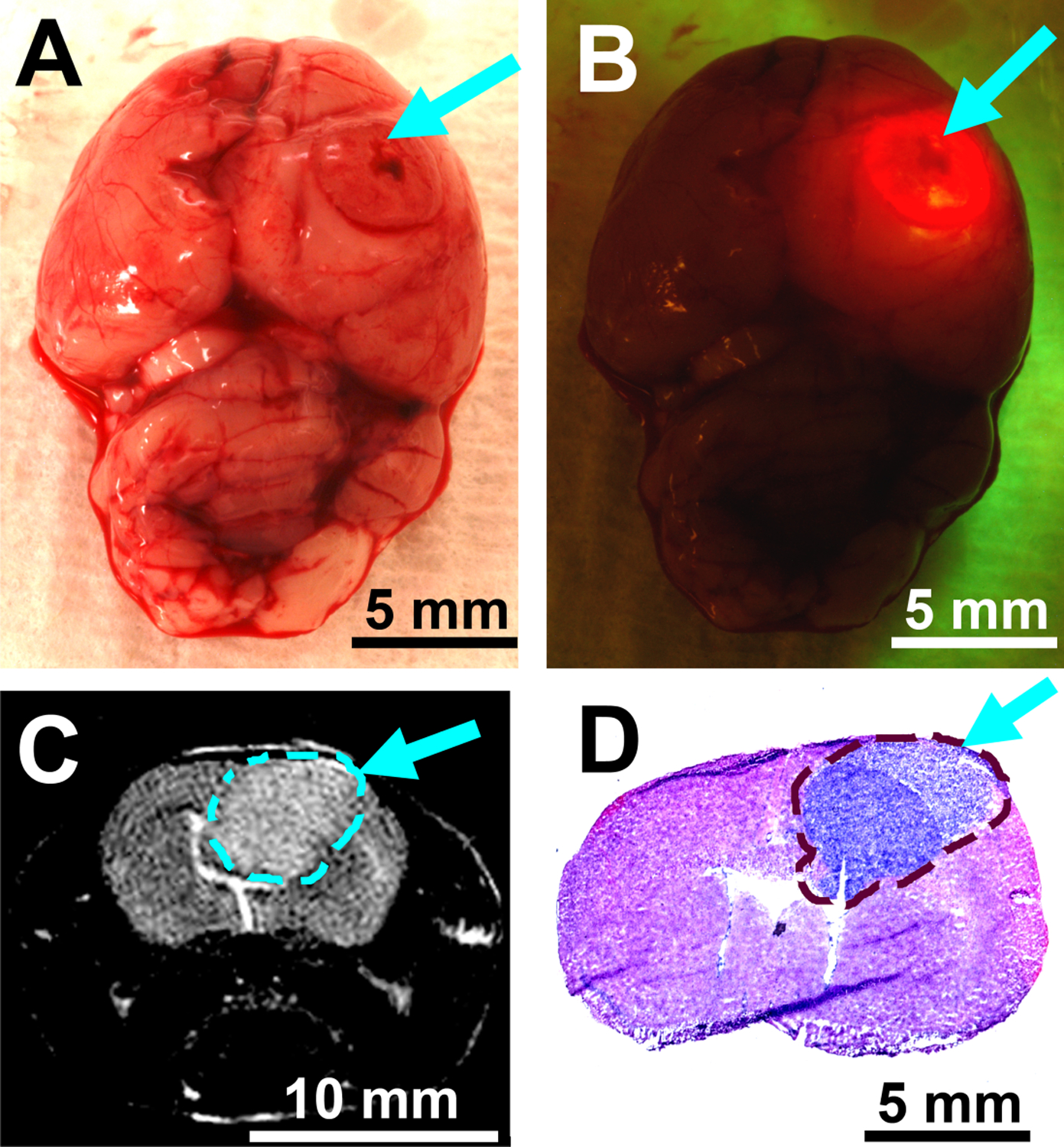
In-vivo fluorescence, magnetic resonance imaging (MRI), and histological staining studies of intracranial glioblastoma brain tumors grown in xenograft mouse models. (A) Color photograph of the mouse brain 14 days after tumor cell implantation. (B) Fluorescence photograph of the mouse brain 2 hours after 5-ALA administration. Bright PpIX fluorescence is visible from the bulk tumor upon excitation with the handheld spectroscopic device. Tumor localization was confirmed with MRI (C) prior to surgery and with coronal histological sections (D) immediately following spectroscopic investigations. Arrows and dashed lines denote the tumor location.
Figure 5.
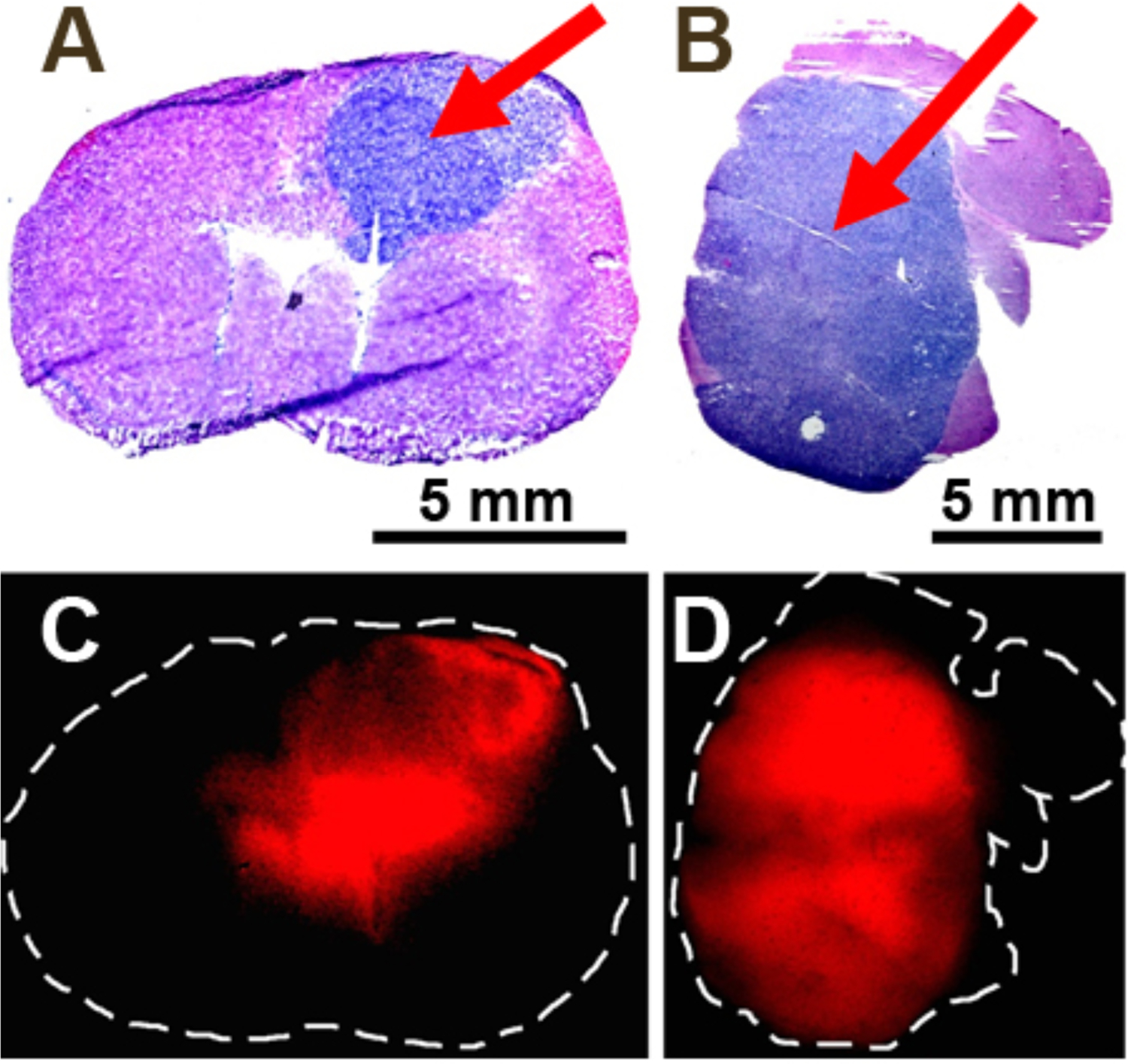
Correlation of brain tumor histopathology (A, B) and PpIX fluorescence (C, D) in intracranial xenograft models. Coronal (A) and axial (B) sections of whole mouse brains were taken and stained using H&E methods to visualize the tumors. Adjacent coronal (C) and axial (D) sections were imaged by fluorescence, showing a high degree of correlation between tumor pathology and PpIX fluorescence. Arrows denote the tumor location.
After the xenograft model was verified and 5-ALA-induced PpIX staining of the tumor was confirmed, additional in vivo studies were conducted to analyze the spectroscopic data of the tumors and quantitatively assess the specificity of the fluorescence signal for GBM tumors in comparison to normal brain tissue and non-target organs. Glioblastoma orthotopic xenograft models were prepared and 5-ALA was administered as described above. As expected, strong PpIX fluorescence was observed in the brain region where the tumor cells were implanted (right cerebral hemisphere) while no visible fluorescence was seen in other regions of the brain (Fig. 6A). Spectroscopic data from various points on the brain were then recorded and analyzed to quantitatively determine the signal intensity in the tumor and the signal-to-noise ratio of the handheld spectroscopic system (Fig. 6B). The measured fluorescence spectra were highly correlated with the visual observations, with tumor fluorescence signal over 100-fold higher than in normal brain tissue, consistent with data from the in vitro studies. Spectroscopic measurements were also taken to determine the nonspecific accumulation of PpIX in non-target organs. Little to no PpIX fluorescence was observed in the liver or spleen. This is not surprising as the administered agent (5-ALA) is not fluorescent and must be metabolically converted to PpIX intracellularly. Since most normal cells are able to efficiently complete the heme biosynthesis pathway, PpIX does not accumulate to a significant degree and the cells remain nonfluorescent. This is a key benefit over the administration of standard dyes such as ICG and fluorescein, which always emit fluorescence and can lead to false positive signals if they nonspecifically accumulate in normal tissue or organs.
Figure 6.
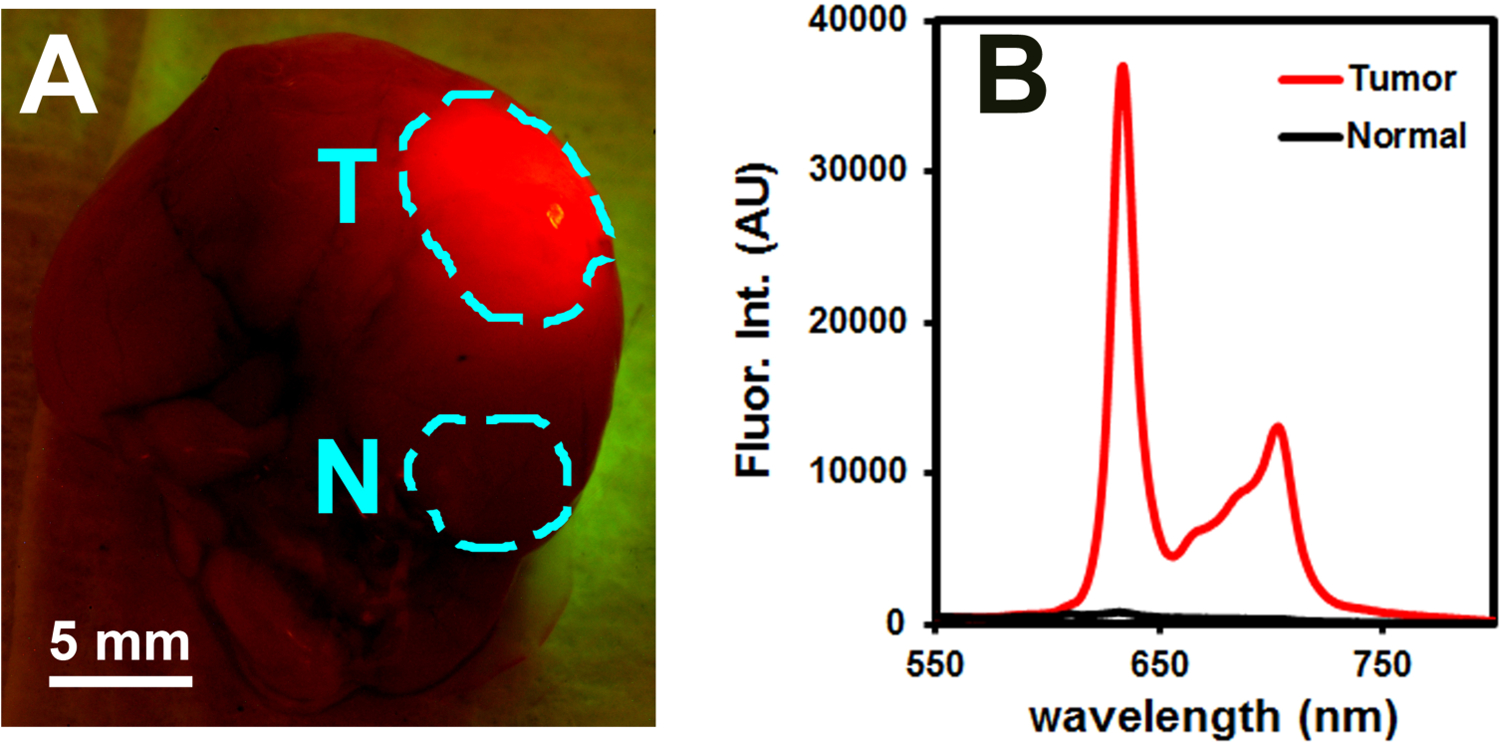
Fluorescence-guided detection of glioblastoma using a spectroscopic device. (A) Brain of a mouse with EGFRvIII glioblastoma tumor 2 hours after 5-ALA administration, showing strong fluorescence in the tumor (T) and no fluorescence in normal tissue (N). (B) Fluorescence spectra showing the unique spectral features of PpIX and dramatic intensity difference between the tumor and normal tissues.
Ex Vivo Validation Studies with Human Clinical Specimens.
To further validate the animal model data, the handheld spectroscopic device was used to analyze specimens collected from human patients undergoing fluorescence-guided GBM resections. These patients were part of a Phase II clinical trial for 5-ALA guided surgery (IND #112246) and received a 20 mg/kg dose of 5-ALA 3–5 hours prior to GBM resection. Tumor specimens removed from the patients were analyzed ex vivo to determine the performance of the device for detecting PpIX fluorescence in human GBM tissue. H&E stained histological sections from the specimens showed nuclear pleomorphism and pseudopalisading cells surrounding necrotic foci (Fig. 7A), characteristic morphologic features of GBM. As expected, specimens identified by the primary surgeon (CH) as tumor exhibited strong fluorescence and resulted in extremely high signal intensity when measured with the spectroscopic system (Fig. 7B). Surprisingly, specimens intraoperatively identified as margin using the current surgical microscope also exhibited high PpIX signal when measured with the handheld device. In fact, there was no statistically significant difference in signal intensity between specimens labeled as tumor or margin (Fig. 7C), with both nearly saturating the spectrometer at an acquisition time of 0.1 seconds. This is likely due to the lower sensitivity of the fluorescence surgical microscope, which may be too low to detect diffuse margins with weak fluorescence signal.
Figure 7.
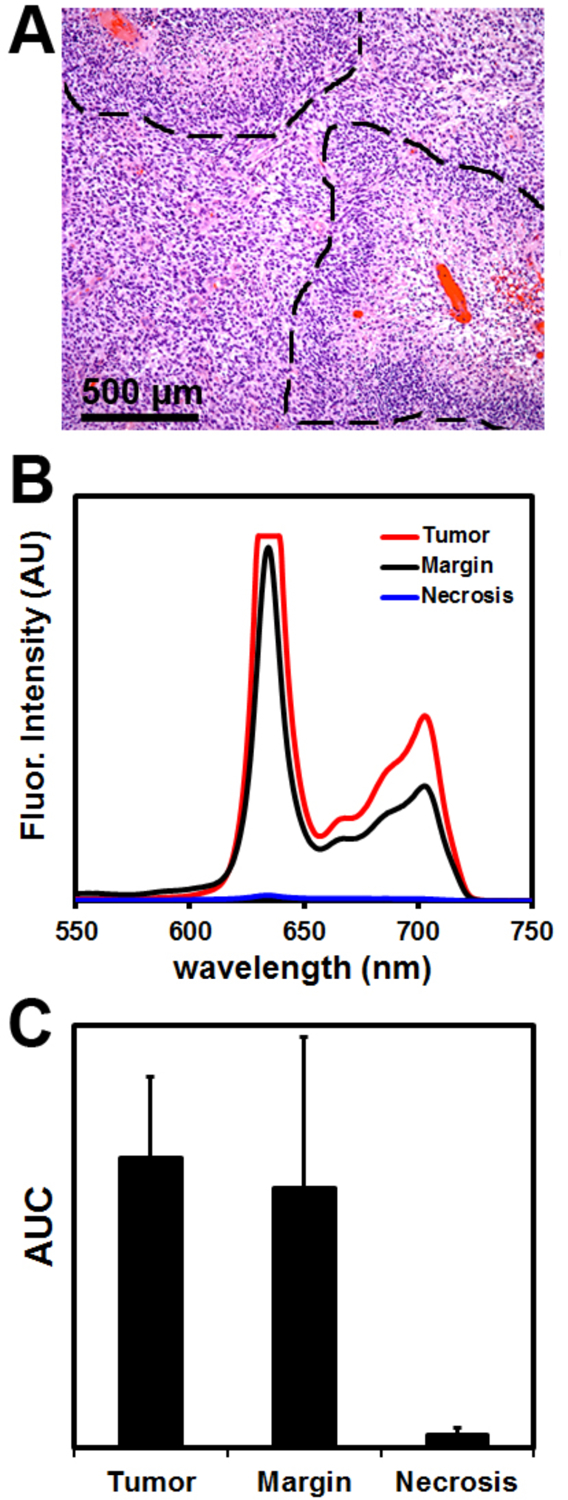
Ex vivo analysis of human tumor specimens from patients undergoing glioblastoma resection. (A) Micrograph of an H&E stained section of human glioblastoma, showing characteristic pseudopalisading (densely stacked nuclei; dotted lines) surrounding necrotic areas. (B) Measurements from the handheld spectroscopic device showed strong protoporphyrin IX signal in tissues identified as tumor or margin, while low signal was seen in necrotic regions. (C) Spectroscopic analysis shows tumor positive regions have a fluorescence signal as much as 150-fold higher than necrotic regions.
Analysis of fluorescence images taken intraoperatively with the surgical microscope confirms that PpIX signal was detectable in the bulk tumor (Fig. 8A) but could not be detected at the tumor margin (Fig. 8C). We hypothesize that these diffuse tumor margins are often left behind during GBM resections because their fluorescence signals are not strong enough for accurate identification. Also, specimens labeled as necrosis or necrotic core exhibited no visible fluorescence under the surgical microscope. This is expected, as necrotic regions consist primarily of dead cells which are unable to metabolize 5-ALA to produce fluorescent PpIX. However, areas of necrosis can contain a small population of living cells, which theoretically should result in a very weak PpIX fluorescence signal. While this PpIX signal intensity is very low, the fluorescence was still measureable with the spectroscopic system, demonstrating the dramatically enhanced sensitivity of the device compared with the fluorescence operative microscope. Analyzing the spectroscopic data from the human patient specimens, we estimate the fluorescence signal intensity of GBM tumors is over 100-fold higher than background signals, significantly higher than non-metabolic optical contrast agents such as fluorescein or ICG, which are only 5–10 fold higher in tumor in comparison with normal tissue.39, 40
Figure 8.
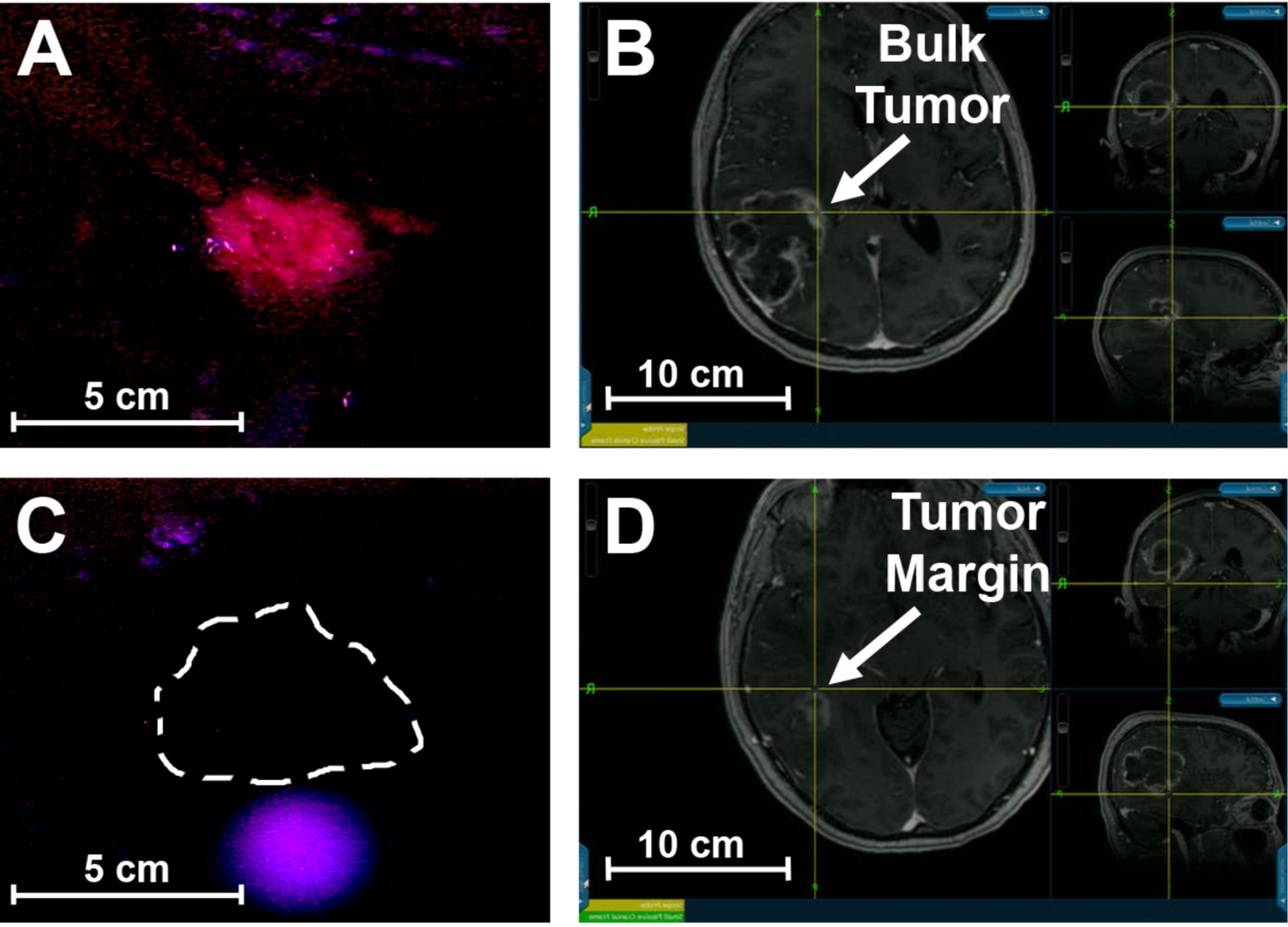
Intraoperative imaging of glioblastoma using a fluorescence surgical microscope and correlated mapping with preoperative MRI and neuronavigation. Red PpIX fluorescence signal is visible in the bulk tumor (A), located intraoperatively using neuronavigation (B). No detectable PpIX fluorescence was seen in the tumor margin (C), identified using neuronavigation and preoperative MRI (D).
Discussion:
Despite significant progress in radiotherapy and therapeutic agents,41–44 surgical resection is still the most common method for treating many human cancers.45 However, successful treatment requires removal of the bulk primary tumor as well as the tumor margin that contains tumor cells, which may result in tumor recurrence and progression if left behind. This can be difficult or impossible in some cancer types such as glioblastoma (GBM), which is highly infiltrative and has poorly defined margins.46 Due to this diffuse, infiltrating growth pattern and the need to avoid damage to critical brain areas important for function, surgical resection of GBM is typically incomplete. Newer technologies such as neuronavigation and intraoperative magnetic resonance imaging (iMRI), have expanded the surgeons’ toolkit for performing more radical tumor resections but have not dramatically improved their ability to accurately identify tumor-positive regions, particularly for infiltrative and diffusive tumors. As a result, tumor-positive margins may not be completely resected, leading to possible tumor recurrence and progression. A number of GBM studies have shown that a more complete resection (maximal surgical debulking) is correlated to a substantial improvement in overall patient survival compared with incomplete resection.15,47–49
Fluorescent contrast agents can address the challenges of intraoperative tumor identification by providing real-time intraoperative guidance to assist a surgeon in differentiating cancerous and normal tissue as well as identifying small nodules, tumor-positive margins, and metastatic lymph nodes that are undetectable using traditional surgical technologies.3,5,26,50–53 Fluorescence-guided surgery (FGS) has primarily focused on the use of fluorescein54–56 and indocyanine green (ICG)57,58 due to their FDA-approval status. ICG is particularly attractive because its excitation and emission are in the near-infrared window where tissue absorption and scattering are reduced, increasing the tissue penetration of the excitation light and fluorescent signal.37, 59 However, the near-infrared emission of ICG is invisible to the human eye and requires additional camera systems for visualization. ICG is also not specifically targeted to cancer cells, and it mainly binds to serum proteins and accumulates in solid tumors through the enhanced permeability and retention (EPR) effect.60,61
The use of 5-aminolevulinic acid (5-ALA) has shown considerable promise for delineating tumor-positive regions from normal brain tissue in the surgical resection of GBM.14,31,62 This molecule is naturally produced in cells as the first intermediate in the heme biosynthesis pathway and can be administered orally to patients 3–5 hours prior to surgery. 5-ALA is an ideal contrast agent for fluorescence-guided tumor resections because it is a non-fluorescent precursor that is taken up by malignant brain tumor cells and metabolized during heme biosynthesis to fluorescent protoporphyrin IX (PpIX), which accumulates intracellularly within tumor cells but is predominantly converted to non-fluorescent heme in normal tissues. Although the mechanism has not been completely elucidated, evidence suggests that the activity of ferrochelatase is significantly reduced in many tumor cells,33,34,63 preventing the conversion of PpIX to heme. This selective metabolic fluorescence activation/clearance pathway eliminates the nonspecific background signal in noncancerous normal tissue that is commonly seen when using traditional dyes such as ICG, and dramatically improves signal-to-noise for tumor cell detection.
In comparison with other spectroscopic studies, recent work by Jermyn et al25 has shown that Raman spectroscopy can accurately detect grade 2 to 4 gliomas in vivo during human brain cancer surgery, with the ability to distinguish cancer cell–invaded brain from normal brain, with sensitivity and specificity of >90%. This was accomplished using a handheld contact Raman spectroscopy probe illuminating a 0.5-mm-diameter tissue area with a depth sampling up to ~1 mm and a total acquisition time of 0.2 s. However, it is important to note that intrinsic Raman scattering measures the chemical composition of cancer tissues, not the genes or proteins that are directly involved in cancer development and progression. Previous studies have reported unacceptable false-positive rates for benign tissues and unacceptable false-negative rates for malignant tissues.64, 65 The underlying problem is that solid tumors are highly heterogeneous in molecular and cellular compositions,66 and the biochemical differences in malignant and benign tissues are subject to natural variations in patient physiology and pathology.67
Conclusion:
In conclusion, we have reported a handheld spectroscopic device for ultrasensitive intraoperative detection and image-guided surgery of malignant brain tumors. In comparison with the current fluorescence microscopes for brain tumor surgery, the handheld intraoperative system is dramatically more sensitive (by 3–4 orders of magnitude) and allows detection of as few as 1000 tumor cells. Also, the results indicate that intraoperative spectroscopy is capable of distinguishing brain tumor cells from normal brain cells with a contrast signal ratio over 100, much higher than the contrast ratios of 5–10 often reported with fluorescein and indocyanine green as contrast agents. This dramatic improvement arises from a unique contrast mechanism in which a small-molecule precursor (5-ALA) stimulates the biosynthesis and accumulation of a natural fluorophore (PpIX) inside metabolically active tumor cells. It is important to note that the metabolic uptake of 5-ALA is similar to that of 18F-fluorodeoxyglucose (FDG), a radioactive tracer widely used for PET (positron emission tomography) imaging of metabolic activity in solid tumors. However, the ALA/PpIX combination is much more specific because the biosynthesis of PpIX occurs in-situ and its accumulation depends on the impaired activity of ferrochelatase in most tumor cells. Indeed, in-vivo animal studies reveal that protoporphyrin IX fluorescence is strongly correlated with both MRI and histological staining, confirming that this fluorescence contrast mechanism is highly specific to tumor cells. Ex-vivo spectroscopic studies of excised human brain tumors further demonstrate that the handheld spectroscopic device is capable of detecting diffuse tumor margins with low fluorescence contrast that are not detectable with current systems. These results raise new possibilities for intraoperative detection and fluorescence-guided resection of microscopic and low-grade glioma brain tumors with invasive or diffusive margins.
Acknowledgments:
We acknowledge the National Institutes of Health for financial support (R01CA163256 and RC2CA148265 to S.N., R01CA176659 and R21CA186169 to C.G. H.) Dr. Costas Hadjipanayis also acknowledges grant support from NxDevelopment Corporation (Miami, Florida).
References:
- (1).Dip FD; Ishizawa T; Kokudo N; Rosenthal RJ Fluorescence Imaging for Surgeons: Concepts and Applications; Springer, 2015. [Google Scholar]
- (2).Mondal SB; Gao S; Zhu N; Liang R; Gruev V; Achilefu S Advances in Cancer Research 2014, 124, 171–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Nguyen QT; Tsien RY Nature Reviews Cancer 2013, 13, 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Singhal S; Nie S; Wang M Annual Review of Medicine 2010, 61, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Vahrmeijer AL; Hutteman M; van der Vorst JR; van de Velde CJ; Frangioni JV Nature Reviews Clinical Oncology 2013, 10, 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Gambhir SS Nature Reviews Cancer 2002, 2, 683–693. [DOI] [PubMed] [Google Scholar]
- (7).Townsend DW Journal of Nuclear Medicine 2001, 42, 533–534. [PubMed] [Google Scholar]
- (8).Ramina R; Neto MC; Giacomelli A; Barros E Jr; Vosgerau R; Nascimento A; Coelho G Acta Neurochirurgica 2010, 152, 27–33. [DOI] [PubMed] [Google Scholar]
- (9).Ngô C; Pollet AG; Laperrelle J; Ackerman G; Gomme S; Thibault F; Fourchotte V; Salmon RJ Annals of Surgical Oncology 2007, 14, 2485–2489. [DOI] [PubMed] [Google Scholar]
- (10).Liu JT; Meza D; Sanai N Neurosurgery 2014, 75, 61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Rosenthal EL; Warram JM; Bland KI; Zinn KR Annals of Surgery 2015, 261, 46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Stummer W; Stocker S; Wagner S; Stepp H; Fritsch C; Goetz C; Goetz AE; Kiefmann R; Reulen HJ Neurosurgery 1998, 42, 518–526. [DOI] [PubMed] [Google Scholar]
- (13).Hadjipanayis CG; Widhalm G; Stummer W Neurosurgery 2015, 0, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Stummer W; Pichlmeier U; Meinel T; Wiestler OD; Zanella F; Reulen H-J The Lancet Oncology 2006, 7, 392–401. [DOI] [PubMed] [Google Scholar]
- (15).Stummer W; Reulen H-J; Meinel T; Pichlmeier U; Schumacher W; Tonn J-C; Rohde V; Oppel F; Turowski B; Woiciechowsky C; Franz K; Pietsch T Neurosurgery 2008, 62, 564–576. [DOI] [PubMed] [Google Scholar]
- (16).Valdés PA; Jacobs V; Harris BT; Wilson BC; Leblond F; Paulsen KD; Roberts DW Journal of Neurosurgery 2015, 123, 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Sanai N; Snyder LA; Honea NJ; Coons SW; Eschbacher JM; Smith KA; Spetzler RF Journal of Neurosurgery 2011, 115, 740–748. [DOI] [PubMed] [Google Scholar]
- (18).Mooney MA; Zehri AH; Georges JF; Nakaji P Neurosurgical Focus 2014, 36, E9. [DOI] [PubMed] [Google Scholar]
- (19).Böhringer H; Boller D; Leppert J; Knopp U; Lankenau E; Reusche E; Hüttmann G; Giese A Lasers in Surgery and Medicine 2006, 38, 588–597. [DOI] [PubMed] [Google Scholar]
- (20).Toms SA; Lin W-C; Weil RJ; Johnson MD; Jansen ED; Mahadevan-Jansen A Neurosurgery 2005, 57, 382–391. [DOI] [PubMed] [Google Scholar]
- (21).Haj-Hosseini N; Richter J; Andersson-Engels S; Wårdell K Lasers in Surgery and Medicine 2010, 42, 9–14. [DOI] [PubMed] [Google Scholar]
- (22).Valdés PA; Kim A; Brantsch M; Niu C; Moses ZB; Tosteson TD; Wilson BC; Paulsen KD; Roberts DW; Harris BT Neuro-oncology 2011, 13, 846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Valdés PA; Leblond F; Kim A; Harris BT; Wilson BC; Fan X; Tosteson TD; Hartov A; Ji S; Erkmen K Journal of Neurosurgery 2011, 115, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Haj-Hosseini N; Richter JC; Hallbeck M; Wårdell K Photodiagnosis and Photodynamic Therapy 2015, 2, 209–214. [DOI] [PubMed] [Google Scholar]
- (25).Jermyn M; Mok K; Mercier J; Desroches J; Pichette J; Saint-Arnaud K; Bernstein L; Guiot M-C; Petrecca K; Leblond F Science Translational Medicine 2015, 7, 274ra19. [DOI] [PubMed] [Google Scholar]
- (26).Mohs AM; Mancini MC; Singhal S; Provenzale JM; Leyland-Jones B; Wang MD; Nie S Analytical Chemistry 2010, 82, 9058–9065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Stummer W; Stocker S; Novotny A; Heimann A; Sauer O; Kempski O; Plesnila N; Wietzorrek J; Reulen H Journal of Photochemistry and Photobiology B: Biology 1998, 45, 160–169. [DOI] [PubMed] [Google Scholar]
- (28).Hefti M; Maximilian Mehdorn H; Albert I; Dorner L Current Medical Imaging Reviews 2010, 6, 254–258. [Google Scholar]
- (29).Hadjipanayis CG; Machaidze R; Kaluzova M; Wang L; Schuette AJ; Chen H; Wu X; Mao H Cancer Research 2010, 70, 6303–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Hefti M; Campe G. v.; Moschopulos M; Siegner A; Looser H; Landolt H Swiss Medical Weekly 2008, 138, 180–185. [DOI] [PubMed] [Google Scholar]
- (31).Stummer W; Novotny A; Stepp H; Goetz C; Bise K; Reulen HJ Journal of Neurosurgery 2000, 93, 1003–1013. [DOI] [PubMed] [Google Scholar]
- (32).Duffner F; Ritz R; Freudenstein D; Weller M; Dietz K; Wessels J Journal of Neuro-oncology 2005, 71, 107–111. [DOI] [PubMed] [Google Scholar]
- (33).El-Sharabasy M; El-Waseef A; Hafez M; Salim S British Journal of Cancer 1992, 65, 409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Krieg RC; Fickweiler S; Wolfbeis OS; Knuechel R Photochemistry and Photobiology 2000, 72, 226–233. [DOI] [PubMed] [Google Scholar]
- (35).Ohgari Y; Nakayasu Y; Kitajima S; Sawamoto M; Mori H; Shimokawa O; Matsui H; Taketani S Biochemical Pharmacology 2005, 71, 42–49. [DOI] [PubMed] [Google Scholar]
- (36).di Salvo ML; Contestabile R; Paiardini A; Maras B Medical Hypotheses 2013, 80, 633–636. [DOI] [PubMed] [Google Scholar]
- (37).Weissleder R Nature Biotechnology 2001, 19, 316–316. [DOI] [PubMed] [Google Scholar]
- (38).Acerbi F; Broggi M; Eoli M; Anghileri E; Cuppini L; Pollo B; Schiariti M; Visintini S; Orsi C; Franzini A Acta Neurochirurgica 2013, 155, 1277–1286. [DOI] [PubMed] [Google Scholar]
- (39).Mancini MC; Mohs AM; Provenzale JM; Saba CF; Cornell KK; Howerth EW; Nie S IEEE Trans. Biomed. Eng 2015, 62, 1416–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Okusanya OT; Madajewski B; Segal E; Judy BF; Venegas OG; Judy RP; Quatromoni JG; Wang MD; Nie S; Singhal S Annals of thoracic surgery 2014, 98, 1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Adams GP; Weiner LM Nature Biotechnology 2005, 23, 1147–1157. [DOI] [PubMed] [Google Scholar]
- (42).Laperriere N; Zuraw L; Cairncross G Radiotherapy and Oncology 2002, 64, 259–273. [DOI] [PubMed] [Google Scholar]
- (43).Khuntia D; Brown P; Li J; Mehta MP Journal of Clinical Oncology 2006, 24, 1295–1304. [DOI] [PubMed] [Google Scholar]
- (44).Stupp R; Hegi ME; Mason WP; van den Bent MJ; Taphoorn MJ et al. The Lancet Oncology 2009, 10, 459–466. [DOI] [PubMed] [Google Scholar]
- (45).NCI. Surveillance, Epidemiology, and End Results (SEER) Program 2009.
- (46).Claes A; Idema AJ; Wesseling P Acta Neuropathologica 2007, 114, 443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Lacroix M; Abi-Said D; Fourney DR; Gokaslan ZL; Shi W; DeMonte F; Lang FF; McCutcheon IE; Hassenbusch SJ; Holland E; Hess K; Michael C; Miller D; Sawaya R Journal of Neurosurgery 2001, 95, 190–198. [DOI] [PubMed] [Google Scholar]
- (48).Pichlmeier U; Bink A; Schackert G; Stummer W Neuro-oncology 2008, 10, 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Bristow RE; Tomacruz RS; Armstrong DK; Trimble EL; Montz F Journal of Clinical Oncology 2002, 20, 1248–1259. [DOI] [PubMed] [Google Scholar]
- (50).Troyan SL; Kianzad V; Gibbs-Strauss SL; Gioux S; Matsui A; Oketokoun R; Ngo L; Khamene A; Azar F; Frangioni JV Annals of Surgical Oncology 2009, 16, 2943–2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).van der Vorst JR; Schaafsma BE; Hutteman M; Verbeek FP; Liefers GJ; Hartgrink HH; Smit VT; Löwik CW; van de Velde CJ; Frangioni JV; Vahrmeijer AL Cancer 2013, 119, 3411–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Tanaka E; Choi HS; Hirofumi F; Bawendi MG; Frangioni JV Annals of Surgical Oncology 2006, 13, 1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Sevick-Muraca EM; Houston JP; Gurfinkel M Current Opinion in Chemical Biology 2002, 6, 642–650. [DOI] [PubMed] [Google Scholar]
- (54).Shinoda J; Yano H; Yoshimura S-I; Okumura A; Kaku Y; Iwama T; Sakai N Journal of Neurosurgery 2003, 99, 597–603. [DOI] [PubMed] [Google Scholar]
- (55).Kabuto M; Kubota T; Kobayashi H; Nakagawa T; Ishii H; Takeuchi H; Kitai R; Kodera T Neurological Research 1997, 19, 9. [DOI] [PubMed] [Google Scholar]
- (56).van Dam GM; Themelis G; Crane LM; Harlaar NJ; Pleijhuis RG; Kelder W; Sarantopoulos A; de Jong JS; Arts HJ; van der Zee AG; Bart J; Low PS; Ntziachristos V Nature Medicine 2011, 17, 1315–1319. [DOI] [PubMed] [Google Scholar]
- (57).Kitai T; Inomoto T; Miwa M; Shikayama T Breast Cancer 2005, 12, 211–215. [DOI] [PubMed] [Google Scholar]
- (58).Schaafsma BE; Mieog JSD; Hutteman M; Van der Vorst JR; Kuppen PJ; Löwik CW; Frangioni JV; Van de Velde CJ; Vahrmeijer AL Journal of Surgical Oncology 2011, 104, 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Smith AM; Mancini MC; Nie S Nature Nanotechnology 2009, 4, 710–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Maeda H; Wu J; Sawa T; Matsumura Y; Hori K Journal of Controlled Release 2000, 65, 271–284. [DOI] [PubMed] [Google Scholar]
- (61).Torchilin V Advanced Drug Delivery Reviews 2011, 63, 131–135. [DOI] [PubMed] [Google Scholar]
- (62).Hadjipanayis CG; Jiang H; Roberts DW; Yang L In Seminars in Oncology; Elsevier, 2011, pp 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Dailey HA; Smith A Biocheical Journal 1984, 223, 441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Ramanujam N; Mitchell MF; Mahadevan-Jansen A; Thomson SL; Staerkel G; Malpica A; Wright T; Atkinson N; Richards-Kortum R Photochemistry and Photobiology 1996, 64, 720–735. [DOI] [PubMed] [Google Scholar]
- (65).Schomacker KT; Frisoli JK; Compton CC; Flotte TJ; Richter JM; Nishioka NS; Deutsch TF Lasers in Surgery and Medicine 1992, 12, 63–78. [DOI] [PubMed] [Google Scholar]
- (66).Liu J; Lau S; Varma V; Moffitt R; Caldwell M; Liu T; Young A; Petros J; Osunkoya A; Krogstad T; Leyland-Jones B; Wang MD; Nie S ACS Nano 2010, 4, 2755–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Kanter EM; Majumder S; Kanter GJ; Woeste EM; Mahadevan-Jansen A American Journal of Obstetrics and Gynecology 2009, 200, 512. e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


