Abstract
Background
Guillain–Barré syndrome (GBS) is an acute inflammatory neuropathy with a heterogeneous presentation. Although some evidences support the role of autoantibodies in its pathogenesis, the target antigens remain unknown in a substantial proportion of GBS patients. The objective of this study is to screen for autoantibodies targeting peripheral nerve components in Guillain–Barré syndrome.
Methods
Autoantibody screening was performed in serum samples from all GBS patients included in the International GBS Outcome study by 11 different Spanish centres. The screening included testing for anti-ganglioside antibodies, anti-nodo/paranodal antibodies, immunocytochemistry on neuroblastoma-derived human motor neurons and murine dorsal root ganglia (DRG) neurons, and immunohistochemistry on monkey peripheral nerve sections. We analysed the staining patterns of patients and controls. The prognostic value of anti-ganglioside antibodies was also analysed.
Results
None of the GBS patients (n = 100) reacted against the nodo/paranodal proteins tested, and 61 (61%) were positive for, at least, one anti-ganglioside antibody. GBS sera reacted strongly against DRG neurons more frequently than controls both with IgG (6% vs 0%; p = 0.03) and IgM (11% vs 2.2%; p = 0.02) immunodetection. No differences were observed in the proportion of patients reacting against neuroblastoma-derived human motor neurons. Reactivity against monkey nerve tissue was frequently detected both in patients and controls, but specific patterns were only detected in GBS patients: IgG from 13 (13%) patients reacted strongly against Schwann cells. Finally, we confirmed that IgG anti-GM1 antibodies are associated with poorer outcomes independently of other known prognostic factors.
Conclusion
Our study confirms that (1) GBS patients display a heterogeneous repertoire of autoantibodies targeting nerve cells and structures; (2) gangliosides are the most frequent antigens in GBS patients and have a prognostic value; (3) further antigen-discovery experiments may elucidate other potential antigens in GBS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12974-021-02301-0.
Keywords: Guillain–Barré syndrome (GBS), Autoantibodies, Anti-ganglioside, Neurons, Prognosis
Background
Guillain–Barré syndrome (GBS) is an acute inflammatory neuropathy with a heterogeneous presentation that includes diverse clinical variants [1–3]. Diagnosis is based on clinical criteria; diagnostic biomarkers are not available for most patients [4]. The exact immunopathogenic mechanisms of GBS are relatively unknown, but it is considered a paradigmatic post-infectious autoimmune disease [5]. Diverse mechanisms, including humoral and cellular immune responses, autoantibodies and complement, activated macrophages and lymphocytes, have been implicated in GBS pathogenesis [6, 7].
Anti-ganglioside antibodies are detected in up to half of GBS patients. These autoantibodies arise via microbial molecular mimicry [8] and the association of specific anti-ganglioside antibody reactivities and specific disease variants is well-established in the literature [9, 10], particularly the association of anti-GM1 [11] and GQ1b [12] antibodies with the pure motor and Miller Fisher syndrome (MFS) variants of GBS, respectively. In addition, the presence of antibodies targeting the GM1 [11] or GD1a [13, 14] gangliosides has also been associated with GBS prognosis. Antibodies against nodal and paranodal proteins (neurofascin 140/186 [15] -NF140/186-, neurofascin 155 [16, 17] -NF155-, contactin-associated protein 1 [18, 19] -CASPR1-, and contactin 1 [19] -CNTN1-) have also been described in patients diagnosed of GBS. However, the target antigens remain unknown in a substantial proportion of GBS patients, particularly of the acute inflammatory demyelinating polyradiculoneuropathy (AIDP) variant, the most frequent in patients of European ancestry.
Considering the broad clinical and epidemiological spectrum of GBS, the diverse infectious triggers and the T-cell independent nature of the immune reaction leading to the appearance of autoantibodies [20], we hypothesized that a broad repertoire of autoantibodies targeting diverse nerve components may be causing nerve pathology in GBS. This study aims to (1) screen for autoantibodies against known antigens; (2) screen for antibodies against human and rodent nerve cells and monkey nerve tissue; (3) describe the diversity of staining patterns and (4) perform clinical–immunological correlations, in a well-characterized GBS cohort.
Materials and methods
Patients and controls
Serum samples from 100 GBS patients included in the Spanish cohort of the International GBS Outcome study (IGOS) [21] were used in this screening. The IGOS is a multicentre, prospective, observational cohort study that investigates factors that determine and predict the clinical course, subtype and outcome of GBS [22]; including patients fulfilling diagnostic criteria of the National Institute of Neurological Disorders and Stroke (NINDS) [1] or Miller Fisher syndrome (MFS) and other variants of GBS [3, 23]. Patients from the Spanish cohort were enrolled between February 2013 and January 2020. All patients fulfilled diagnostic criteria for GBS and were included within 2 weeks from onset of weakness. Serum samples were aliquoted and stored at − 80 °C until needed. In this study, we used serum samples extracted at baseline. Sixty-two (62%) of the baseline samples analysed were collected before starting treatment.
Clinical variants were defined as sensorimotor, pure motor, pure sensory, Miller Fisher syndrome (MFS) and ataxic. Nerve conduction studies results were classified as acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor-sensory axonal neuropathy (AMSAN), equivocal or normal. The outcome of all patients with GBS at 6 months and 1 year from disease onset were assessed using the GBS disability score (GDS), a widely accepted system for evaluating the functional ability of patients [24]. Patients unable to walk independently (≥ 3) at 6 months were defined as having a poor outcome in this study.
Additionally, serum samples from a control group (n = 90) including 45 healthy controls and 45 patients with other neuromuscular disorders [23 amyotrophic lateral sclerosis (ALS), 22 Charcot–Marie–Tooth (CMT)] were included.
Autoantibody screening protocol
Autoantibody screening experiments included anti-ganglioside antibody detection with ELISA, nodo/paranodal (NF155, NF140, NF186, CNTN1 and CNTN1/CASPR1 complex) antibody detection by ELISA and cell-based assays, immunocytochemistry using patient sera on murine dorsal root ganglia (DRG) neurons and neuroblastoma-derived human motor neurons (IgG and IgM) and reactivity pattern assessment by immunohistochemistry on monkey sciatic nerve sections (IgG and IgM).
Testing for nodo/paranodal antibodies
Autoantibodies against NF140, NF186, NF155, CNTN1 and CASPR1 were tested by ELISA.
Maxisorb 96-well ELISA plates (Thermo Fisher Scientific, NUNC, Denmark) were coated with 1 μg/ml human recombinant CNTN1 protein (Sino Biological Inc., Georgia, USA), 1 μg/ml NF155 protein (Origene, Maryland, USA), 1 μg/ml NF140 protein (Sino Biological), 1 μg/ml NF186 protein (Origene) or 5 μg/ml CASPR1 protein (R&Dsystems, MI, USA) overnight at 4 °C. Wells were blocked with 5% non-fat milk in PBS 0.1% Tween 20 for 1 h, incubated with sera diluted 1/100 in blocking buffer for 1 h, and then incubated with peroxidase conjugated rabbit anti-human IgG secondary antibody (Invitrogen, CA, USA) for 1 h at room temperature. ELISA was developed with tetramethylbenzidine solution (Biolegend, California, USA), and the reaction was stopped with 25% sulfuric acid. Optical density (OD) was measured at 450 nm in a Multiscan ELISA reader. Samples were considered positive by ELISA when they had a ΔOD higher than mean healthy control ΔOD plus two standard deviations.
Cell-based assays were used as previously described [25] as a second confirmatory technique for questionable cases. Briefly, mammalian expression vectors encoding human NF140, NF186, NF155, CNTN1 or CASPR1 were transfected into HEK293 cells using Lipofectamine 2000 (Invitrogen). Cells were then fixed with 4% paraformaldehyde and blocked. ICC experiments were performed using patient’s sera and appropriate primary and secondary antibodies.
Testing for anti-ganglioside antibodies
Patients’ sera were screened for the presence of anti-ganglioside antibodies using a previously validated ELISA protocol [26] as the general detection method, and thin layer chromatography [27] for confirmatory experiments. Anti-ganglioside antibodies were considered positive at a 1/1000 titre.
Rat dorsal root ganglia neurons immunocytochemistry
DRG were dissected from E16 rat embryos, dissociated and plated in glass coverslips coated with laminin (Invitrogen) and poly-d-lysine (Sigma, MO, USA). Cells were grown in neurobasal medium (Gibco BRL, NY, USA) supplemented with B27 (Gibco), Glutamax (Gibco) and nerve growth factor (NGF) (Invitrogen). After 24 h, cytosine arabinoside (ARA-C) (Sigma) and fluorouracil (5-FU) (Sigma) were added to the medium to remove fibroblasts. Then, medium was replaced every other day until reaching complete growth and differentiation of DRG neurons.
Live DRG neurons were incubated for 1 h with patients’ sera diluted 1/100 (for IgG experiments) or 1/40 (for IgM experiments) in culture medium at 37 °C. Cells were then fixed for 10 min with 4% PFA and incubated with secondary antibodies. Goat anti-human IgG or IgM AF488 (Molecular probes, Oregon, USA) were used as secondary antibodies at 1/1000 concentration.
Coverslips were mounted with Vectashield with DAPI and fluorescence signal intensity was scored in a 0–3 scale by two independent researchers. Images were obtained with an Olympus BX51 Fluorescence Microscope (Olympus Corporation, Tokyo, Japan).
Human neuroblastoma-derived neurons immunocytochemistry
SH-SY5Y cells were plated in glass coverslips coated with laminin at 2.5 µg/ml (Invitrogen). Cells were grown in proliferation medium containing DMEM/F12 (1:1), fetal bovine serum (10%), l-glutamine (1%) and Sodium pyruvate (1%). After 24 h, proliferation medium was replaced by differentiation medium containing Neurobasal (Gibco) supplemented with B27 (Gibco), Glutamax (Gibco), nerve growth factor (Invitrogen) and retinoic acid at 10 µM (Sigma). Then, medium was replaced every other day until full differentiation was achieved. On days 5 or 6 of differentiation, cells were fixed for 15 min with paraformaldehyde 4%; and blocked with 5% normal goat serum in PBS; followed by incubation with patients’ sera at 1/40 (for IgM) or 1/100 (for IgG). To observe the correct differentiation of the cells we also incubated them with chicken anti-panNeurofascin mAb (R&Dsystems) at 1/200. Goat anti-chicken IgG AF594 and goat anti-human IgG AF488 or goat anti-human IgM AF488 (Molecular Probes) were used as secondary antibodies at 1/1000 concentration.
Coverslips were mounted with Vectashield with DAPI and fluorescence signal intensity was scored in a 0–3 scale by two independent researchers. Images were obtained with an Olympus BX51 Fluorescence Microscope.
Peripheral nerve immunohistochemistry
Macaque peripheral nerve tissue slides (Inova Diagnostics, Inc., San Diego, CA) were blocked with 5% normal goat serum in PBS; followed by incubation with patients’ sera at 1:10 (for IgM) or 1:20 (for IgG). Monkey-adsorbed goat anti-human IgG AF488 (Southern Biotech, Alabama, US) or goat anti-human IgM AF488 (Molecular Probes) were used as secondary antibodies at 1/500 concentration. Finally, slides were mounted with Fluoromount medium (Sigma) and examined by two independent observers. Immunostaining patterns were analysed scoring fluorescence signal intensity of each nerve structure in a 0–3 scale. The nerve structures analysed were: nodes or paranodes, myelin from small myelinated fibres, myelin from large myelinated fibres, Schwann cells from unmyelinated fibres, large-fibre axons, and small-fibre axons. Reactivity against other non-nerve structures (fibroblasts, connective tissue, vessels) was not considered in the analysis.
To further study the staining patterns, peripheral nerve tissue slides were coated with mouse anti-human CD56 antibody (Becton Dickinson, New Jersey, USA) at 1:50 to stain non-myelinating Schwann cells (Remak bundles); or with rabbit anti-human S100 antibody (Abcam, Cambridge, UK) at 1:50 to stain myelinating Schwann cells. Goat anti-mouse IgG AF594 (for CD56), and goat anti-rabbit IgG AF594 (for S100) were used as secondary antibodies at 1/500 concentration.
Images were acquired using Leica TSC SP5 confocal microscope.
Statistical analysis
Results were analysed by GraphPad Prism v8.0 (GraphPad Software). Statistical comparison of proportions among groups was performed using contingency analysis with the application of Chi-square and a two-tailed Fisher’s exact test, accepting an alpha-level < 0.05 for statistical significance. To represent the results and perform hierarchical clustering of the results heatmap diagrams using the Clustvis web tool were performed [28].
To investigate the association between anti-ganglioside antibodies and prognosis a multivariable logistic regression analysis to predict the inability to walk at 6 months and at 1 year of follow-up (GDS ≥ 3) was performed using the STATA software. A stepwise backward regression modelling to select variables independently associated with the outcome was performed first. The variables introduced in our initial multivariable models were selected based on known prognostic factors: age, initial GDS, diarrhoea, AMAN, serum NfL levels (analysed in a previous study with the same cohort) [29], serum anti-GM1 IgG antibodies and serum anti-GD1a IgG antibodies [29–32]. To perform the multivariable analysis patients with MFS were excluded, because our aim was to predict GBS prognosis and MFS is considered a different disease, including different pathophysiology, clinical presentation (it does not present with weakness), treatment (often untreated) and outcome (considered self-limiting and benign). Finally, the ability of the variable “presence of anti-GM1 IgG antibodies” to predict the inability to run at 1 year of follow-up (GDS ≥ 2) was evaluated in our previously reported multivariable logistic regression analysis [29].
Odds-ratios (OR) for the logistic regression analysis were reported with 95% confidence intervals and p values.
Results
Baseline characteristics
We included 100 participants from 11 Spanish centres participating in the IGOS study. GBS patients had an average of 57.4 years and were predominantly men (57%). 65% of patients presented with the sensorimotor variant, 19% presented with the pure motor GBS variant, 10% with MFS, 5% with the pure sensory variant and 1 patient with the ataxic variant. Regarding nerve conduction studies, 59% of patients were classified as AIDP, 12% as AMAN, 7% as AMSAN, 8% as normal, and 14% as equivocal. Detailed epidemiological features of the cohort were described elsewhere [29].
Screening for known autoantibodies
None of the GBS patients included in the study reacted against the paranodal and nodal proteins tested (NF155, NF140, NF186, CNTN1 and CASPR1).
Sixty-one patients tested positive for, at least, one anti-ganglioside antibody (GM1, GM2, GM3, GD1b, GD3, aGM1, GT1a, GT1b and GQ1b). Of these, 40 had IgG antibodies, 3 had IgM antibodies, and 18 had antibodies from both isotypes. Detailed anti-ganglioside reactivities are shown in Additional file 1.
Most frequent anti-ganglioside antibodies in our cohort were aGM1, GM1, GD1b and GQ1b. Overall, IgG anti-aGM1 antibodies were detected in 40% of patients; IgG and IgM anti-GM1 antibodies were detected in 27% and 15% of patients, respectively, IgG anti-GD1b antibodies in 30%, and IgG anti-GQ1b antibodies in 21% patients.
Antibodies targeting peripheral nerve neurons
ICC experiments with primary cultures of rat DRG neurons and human motor neurons derived from a neuroblastoma cell line were used to identify novel IgG and IgM reactivities against peripheral nerve neurons. The screening was performed in 100 serum samples from GBS patients and 90 serum samples from a control group (including healthy controls and patients with other neuromuscular diseases). ICC results were grouped in three separate categories: moderate-to-strong positives (including scores 2 and 3), all positives (including scores 1, 2 and 3), and negatives (score 0). Detailed results are shown in Additional file 2, Fig. 1 and Table 1.
Fig. 1.
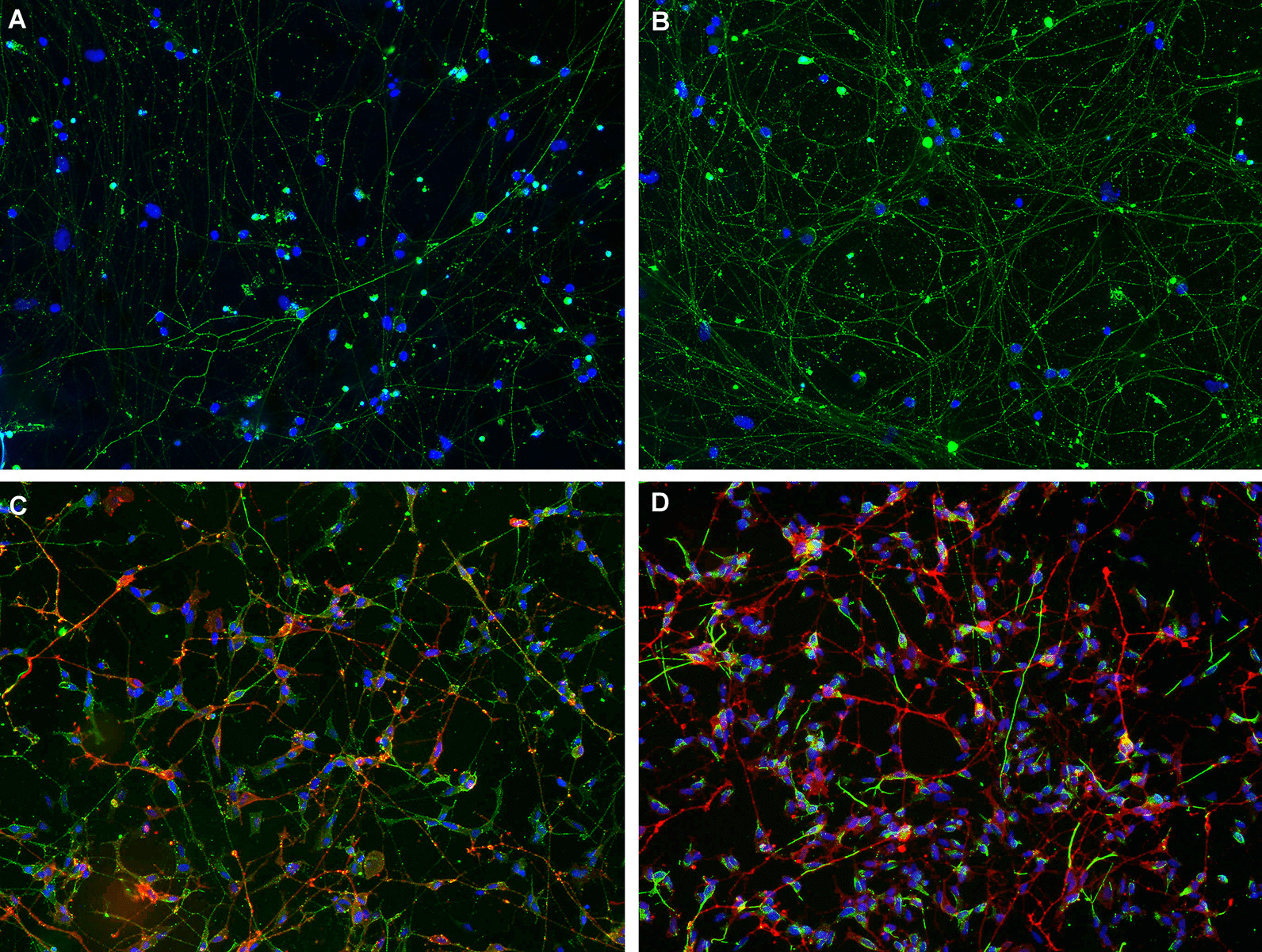
Reactivity against rat DRG neurons and human neuroblastoma-derived neurons. DRG neurons (A, B) stained with a GBS patient’s serum reacting moderately (score 2) in IgG (A), and a GBS patient’s serum reacting strongly (score 3) in IgM (B). Human neuroblastoma-derived neurons (C, D) stained in red with anti-panNeurofascin mAb, and in green with a GBS patient’s serum reacting moderately (score 2) in IgG (C) and a GBS patient’s serum reacting strongly (score 3) in IgM (D)
Table 1.
Statistical analysis of DRG and neuroblastoma neurons ICC, and monkey peripheral nerve IHC
| GBS patients (n = 100) | Controls (n = 90/56) | p value | ||||
|---|---|---|---|---|---|---|
| Any reactivity | Strong reactivity | Any reactivity | Strong reactivity | Any reactivity | Strong reactivity | |
| Neuroblastoma neurons IgG | 11 (11%) | 2 (2%) | 10/90 (11.1%) | 0/90 (0.0%) | > 0.999 (ns) | 0.499 (ns) |
| Neuroblastoma neurons IgM | 28 (28%) | 8 (8%) | 11/90 (12.2%) | 2/90 (2.2%) | 0.011 (*) | 0.105 (ns) |
| DRG neurons IgG | 32 (32%) | 6 (6%) | 6/90 (6.7%) | 0/90 (0.0%) | < 0.0001 (***) | 0.030 (*) |
| DRG neurons IgM | 34 (34%) | 11 (11%) | 22/90 (24.4%) | 2/90 (2.2%) | 0.156 (ns) | 0.020 (*) |
| Monkey peripheral nerve IgG | 56 (56%) | 17 (17%) | 30/56 (53.6%) | 3/56 (5.4%) | 0.8669 (ns) | 0.0455 (*) |
| Monkey peripheral nerve IgM | 44 (44%) | 10 (10%) | 20/56 (35.7%) | 6/56 (10.7%) | 0.3964 (ns) | > 0.999 (ns) |
Comparison between GBS patients and controls. Strong reactivity includes scores 2 and 3, and any reactivity includes scores 1, 2 and 3. Fluorescence intensity scores were analysed using contingency analysis with the application of a Fisher’s exact test, accepting an alpha-level of < 0.05 to determine significance
Overall, 22 (22%) GBS patients reacted moderately or strongly against DRG or neuroblastoma neurons, whereas 4 (4.4%) controls reacted only moderately. These differences were statistically significant (p = 0.0005).
Antibodies against DRG neurons appeared significantly more frequently in GBS patients than in controls (32% vs 6.7%, p < 0.0001) taking all positive tests in account; the same happened if only moderate and strong positives were considered, both in IgG (6% vs 0%, p = 0.03) and IgM experiments (11% vs 2.2%, p = 0.02).
In neuroblastoma-derived neuron ICC experiments 28 (28%) samples from the GBS group showed IgM autoantibodies; of these 8 (8%) showed moderate or strong reactivity. These proportions were significantly higher than in the control group (12.2% and 2.2%, respectively; p = 0.011). Differences in autoantibody proportions between GBS patients and controls were not observed in neuroblastoma-derived neuron experiments when assessing IgG antibodies.
Antibodies targeting peripheral nerve tissue
We analysed the full GBS cohort and 56 controls.
We analysed the staining intensity of six different structures within the nerve, including nodes or paranodes, myelin from small myelinated fibres, myelin from large myelinated fibres, Schwann cells from unmyelinated fibres, large-fibre axons, and small-fibre axons. Staining patterns can be found in Additional file 3.
IgG and IgM reactivity against nerve tissue was frequently detected in GBS patients and controls. Overall, about 70% of GBS patients and controls sera bound to nerve structures. IgG and IgM from GBS patients reacted moderately in 17 (17%) and strongly in 10 (10%) against monkey nerve structures. In the control group IgG and IgM reacted moderately in 8 (14.3%) and strongly in 1 (1.8%) against monkey nerve structures. The difference between the amount of GBS patients and controls reacting moderately or strongly against monkey peripheral nerve was statistically significant (p = 0.0455) only for IgG autoantibodies (Table 1).
Differences in IHC patterns of reactivity from GBS patients and controls were not statistically significant for any of the structures analysed (Additional file 4). Nonetheless, some specific reactivity patterns were only detected in GBS patients and not in controls (Fig. 2). Eight (8%) GBS patients’ IgG reacted strongly against myelin, whereas only 2 controls showed weak reactivity against this structure. Moreover, we observed that 13 (13%) GBS patients’ IgG had a strong reactivity against Schwann cells (myelinating and non-myelinating) while only one of the controls (1.8%) showed strong reactivity against Schwann cells (this difference is statistically significant; p = 0.0192).
Fig. 2.
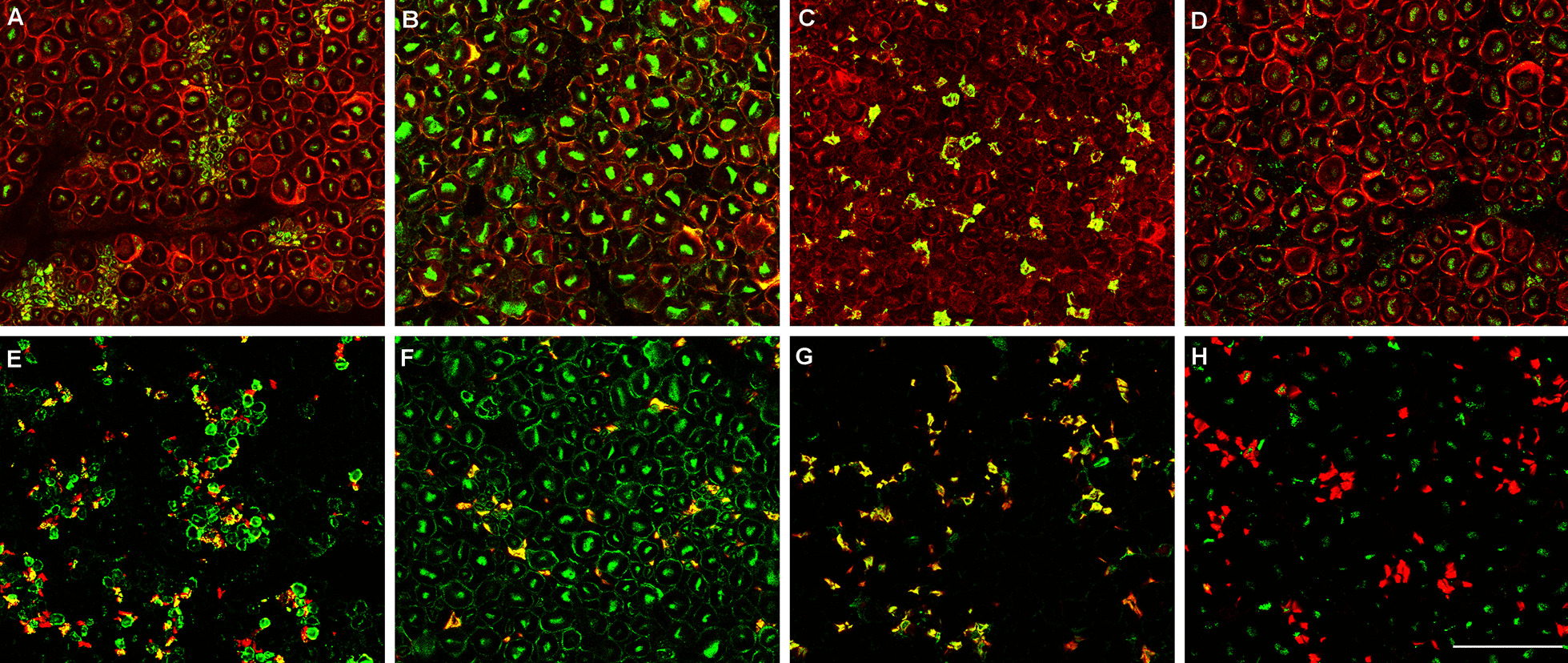
Reactivity against Schwann cells in peripheral nerve sections. Macaque peripheral nerve sections stained in red with S100 (A–D) or CD56 (NCAM) monoclonal antibody (E–H), and in green with GBS patient’s sera reacting against myelin from small myelinated fibres (A, E), myelin from large myelinated fibres (B, F), and non-myelinating Schwann cells (C, G). D and H are stained with sera from negative controls
Combined autoantibody screening analysis
We also analysed if GBS patients with or without anti-ganglioside antibodies differed in the reactivity patterns in the peripheral nerve cell and tissue autoantibody screening experiments. No differences were found between those two groups (Additional file 4), suggesting that the heterogeneity of the autoantibody repertoire appears even when a specific antigen is found.
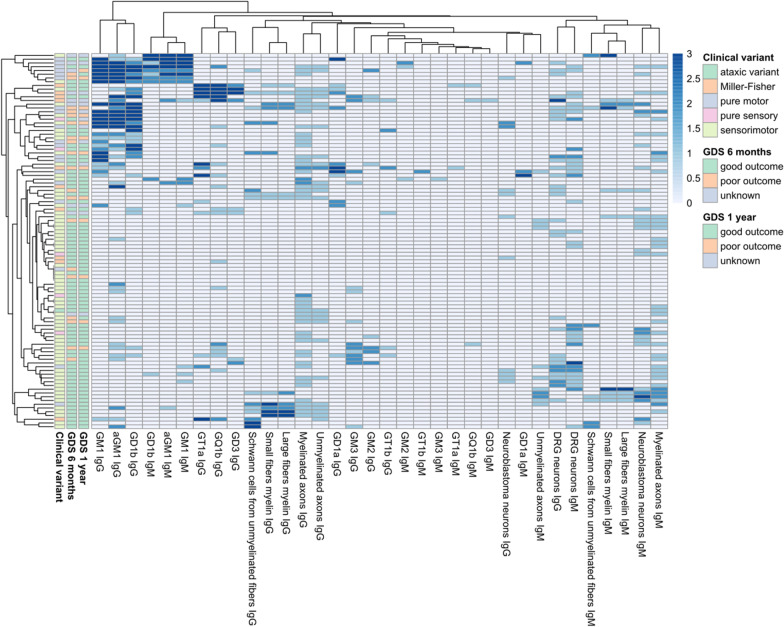
We used a heatmap graph to represent all the autoantibody screening results performed in our GBS cohort (Fig. 3) [28]. This graph provides visual representation of the heterogeneity of the autoantibody repertoire in GBS sera.
Fig. 3.
Heatmap showing all the screening performed in GBS patients. Patients and reactivities against anti-ganglioside antibodies, neuroblastoma-derived human motor neurons, murine dorsal root ganglia neurons and monkey peripheral nerve tissue, are ordered according to Euclidean clustering. Each row represents one GBS patient. The score of the anti-ganglioside titre is indicated by the colour of the square (0 = < 1/1000, 1 = 1/1000–1/2500, 2 = 1/2500–1/12500, 3 = > 1/12500). The score of staining intensity in the other structures is indicated by the colour of the square (0 = negative, 1 = mild positive, 2 = moderate positive, 3 = strong positive). Columns in the left contain information related to the clinical variant and the outcome at 6 months and at 1 year of follow-up
Clinical correlations
Among patients with Miller Fisher syndrome, 8/10 (80%) had IgG anti-GQ1b antibodies, whereas in the rest of GBS patients only 14.4% (13/90) had these antibodies, usually in combination with other reactivities. IgG anti-GM1 antibodies were more frequently detected in patients with the pure motor variant than in those with other clinical variants [13/19 (68.4%) vs 14/81 (17.3%)]; and in patients classified electrophysiologically as AMAN than in the rest of GBS patients (83.3% vs 19.3%). All these differences were statistically significant (p < 0.0001).
When we analysed the general clinical characteristics of the subgroup of patients with strong IgG reactivity against Schwann cells (n = 13), we did not observe any specific pattern that could distinguish them from the rest of the cohort. Ten (76.9%) of these patients presented with the sensorimotor clinical variant, whereas in the general cohort 65% presented this variant; and the proportions of nerve conduction studies subgroups were similar to those found in the general cohort (53.8% vs 59% of AIDP electrophysiological variant). Regarding the outcome, the percentage of patients having a good outcome at 6 months and 1 year are similar in the two groups (about 75%).
In the subgroup of patients with IgG or IgM reactivity against DRG neurons (n = 14), we did not find clinical differences with the whole GBS cohort. Briefly, 71.4% of patients staining strongly DRG neurons were classified as AIDP, and 64.3% presented with the sensorimotor clinical variant.
We did not detect any difference in peripheral nerve cell and tissue reactivity patterns or frequencies between the samples collected before starting the treatment (62%) and those collected after the treatment (38%). We neither observed any correlation of any of the reactivity patterns with other clinical characteristics as pain, ataxia, or the presence of antecedent infection.
Prognostic value of anti-ganglioside antibodies
First, we conducted a univariate analysis to select variables that were associated with the outcome. Patients with serum IgG anti-GM1 antibodies presented poorer outcomes than patients without the antibodies at 6 months [38.1% vs 16.1% (p = 0.04)], and 1 year [35.3% vs 9.7% (p = 0.014)]. Anti-GD1a IgG antibodies were not associated with prognosis (Table 2). For the multivariate analysis, we included GM1 IgG, serum NfL levels, diarrhoea, age, and initial GDS.
Table 2.
Association between baseline anti-GM1 and anti-GD1a antibodies and prognostic
| Univariate and Multivariable logistic analysis for inability to walk independently at 6 months | |||||
|---|---|---|---|---|---|
| Variable | OR | 95% CI | p | ||
| Univariate analysis | |||||
| GM1 IgG | 3.2 | 1.05–9.71 | 0.04 | ||
| GD1a IgG | 0.48 | 0.05–4.24 | 0.515 | ||
| logNFL | 3.22 | 1.46–7.14 | 0.004 | ||
| AMAN | 2.97 | 0.73–12.07 | 0.127 | ||
| Diarrhoea | 3.64 | 1.21–10.91 | 0.021 | ||
| Age | 1.05 | 1.01–1.10 | 0.005 | ||
| Initial GDS | 3.15 | 1.38–7.18 | 0.006 | ||
| Multivariable logistic analysis | |||||
| logNfL | 3.13 | 1.27–7.67 | 0.012 | ||
| Age | 1.05 | 1.01–1.10 | 0.013 | ||
| Univariate and Multivariable logistic analysis for inability to walk independently at 1 year | |||
|---|---|---|---|
| Variable | OR | 95% CI | p |
| Univariate analysis | |||
| GM1 IgG | 5.09 | 1.38–18.73 | 0.014 |
| GD1a IgG | 0.92 | 0.10–8.44 | 0.944 |
| logNFL | 3.11 | 1.27–7.55 | 0.012 |
| AMAN | 1.475 | 0.27–7.97 | 0.652 |
| Diarrhoea | 2.7 | 0.74–9.82 | 0.131 |
| Age | 1.06 | 1.01–1.11 | 0.014 |
| Initial GDS | 2.79 | 1.12–6.93 | 0.027 |
| Multivariable logistic analysis | |||
| GM1 IgG | 6.98 | 1.60–30.36 | 0.01 |
| Age | 1.07 | 1.01–1.12 | 0.01 |
| Univariate and Multivariable logistic analysis for inability to run independently at 1 year | ||||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p | |||
| Univariate analysis | ||||||
| GM1 IgG | 4.16 | 1.39–12.49 | 0.011 | |||
| GD1a IgG | 0.72 | 0.13–3.99 | 0.710 | |||
| logNFL | 4.37 | 1.95–9.78 | < 0.0001 | |||
| AMAN | 6.53 | 1.57–27.17 | 0.01 | |||
| Diarrhoea | 2.33 | 0.83–6.57 | 0.109 | |||
| Age | 1.04 | 1.01–1.07 | 0.023 | |||
| Initial GDS | 1.81 | 1.08–3.04 | 0.025 | |||
| Multivariable logistic analysis | ||||||
| Age | 1.04 | 1.01–1.08 | 0.022 | |||
| AMAN | 6.19 | 1.01–38.02 | 0.049 | |||
| logNfL | 3.17 | 1.34–7.50 | 0.009 | |||
logNfL log-transformed neurofilament light chain; AMAN acute motor axonal neuropathy; GDS Guillain–Barré Syndrome Disability Score
We observed that having anti-GM1 IgG antibodies at baseline was independently associated with the inability to walk at 1 year of follow-up, after a backward stepwise selection modelling (OR 6.98, 95% CI 1.6–30.36; p = 0.01). However, the presence of anti-GM1 IgG antibodies was not independently associated with having a poor outcome at 6 months (Table 2).
To analyse if anti-GM1 titres were associated with the GBS disability score, we performed a linear regression. We did not observe a positive correlation between antibody titres and disability at 6 months and at 1 year.
Finally, when we included the presence of anti-GM1 antibodies in our previously reported prognostic study [29], we observed that having anti-GM1 IgG antibodies at baseline was associated with the inability to run at 1 year, but this association was not independent from the other known prognostic factors and sNfL, age and AMAN remained in the model as independent factors associated with residual disability at 1 year.
Discussion
Our work describes a comprehensive autoantibody screening that provides experimental evidence of the heterogeneity of the autoantibody repertoire in patients fulfilling GBS diagnostic criteria.
Our study shows that GBS patients have a heterogeneous repertoire of autoantibodies targeting nerve cells and structures. Except for patients with anti-ganglioside antibodies and a minor subset of patients with antibodies targeting Schwann cells and the myelin sheath, this repertoire varies in frequency and intensity of staining, but it is not qualitatively different from controls. Antibodies targeting peripheral nerve cells of both IgG and IgM isotypes are significantly more frequent in patients than in controls, but no clear differences are seen when antibodies are tested using immunohistochemistry on monkey nerve preparations. Considering that whole nerve monkey preparations likely display protein antigens in a conformation that is phylogenetically closer to that of human nerves, this may imply that autoantibodies targeting nerve structures are present in normal human repertoire at lower titers, that they arise as a natural epiphenomenon of a T-cell mediated damage and are not pathogenic, or that other autoantibodies, targeting different types of molecules (such as lipids or glycans), for which our techniques are not optimized, are yet to be discovered.
Whether these autoantibodies arise from a process of molecular mimicry, or from an unspecific and polyclonal activation of pre-existing B cells, remains unclear. The general absence of common patterns suggests the latter, but the well-established molecular mimicry process described in anti-ganglioside-associated GBS supports the former. In anti-GM1-associated GBS the sequence of pathogenic events includes an immune response to an infection leading to the appearance of antibodies cross-reacting with peripheral nerve and nerve root gangliosides and triggering post-infectious inflammation [33]. Interestingly, in this screening we did not find clear differences in the reactivity patterns between GBS patients with or without anti-ganglioside antibodies, but we observed in both groups a higher amount of patients staining nerve structures than in controls. These findings suggest that the immune response in GBS is not restricted to the production of anti-ganglioside antibodies, but it is also targeting other peripheral nerve structures. This observation may either reflect the presence of a polyclonal, not antigen-driven, reactivation of a pre-existing repertoire that, in some patients, includes gangliosides, or the concomitant activation (by epitope spreading or bystander activation) of unspecific B cells in addition to the ganglioside-driven antigen-specific response.
Previous studies in other inflammatory neuropathies such as chronic inflammatory demyelinating polyneuropathy (CIDP), showed that frequencies of reactivity against DRG neurons in CIDP patients did not differ from healthy controls [34], in contrast with our results (shown in Table 2). GBS and CIDP are similar diseases both clinically and electrophysiologically, so this difference supports the idea that a heterogeneous autoantibody response against multiple nerve antigens arises in GBS while this does not happen in chronic inflammatory and demyelinating neuropathies in which a specific, antigen-driven autoantibody response arises, as the recent discovery of the nodo-paranodal antibodies supports [35]. Differences in severity of these two diseases may also account for this observation.
We observed that 13% of GBS patients showed strong IgG reactivity against Schwann cells of monkey peripheral nerve. This observation is in agreement with previous findings: Kwa et al. observed that 24% of GBS patients had IgG antibodies against non-myelinating human Schwann cells [36], and Vallat et al. also detected that a significant percentage of CIDP and GBS patients (about 25%) presented with IgG or IgM reactivity against myelin and that the staining patterns on Schwann cells were diverse, suggesting that diverse myelin antigens are being recognized by the autoantibodies [37].
Our study also confirms, in a well-characterized GBS cohort, that gangliosides are the most frequent specific antigens in GBS patients and that they associate to specific disease variants. The value of testing anti-ganglioside antibodies in the GBS routine clinical care is controversial, but it is clear that some antibodies are associated with specific clinical phenotypes [38]. IgG anti-GQ1b antibody is a diagnostic marker and a pathogenic antibody in MFS, and is often cross-reactive with GT1a [9]. Moreover, IgG anti-GM1 antibodies associate with the pure motor (clinical) and AMAN (electrophysiological) variants. Our results, with 80% of MFS patients having anti-GQ1b antibodies and 68.4% of pure motor patients having anti-GM1 antibodies at baseline, confirm these associations. However, our study lacks power to find other potential associations previously described (anti-GD1b with acute ataxic neuropathy, anti-GT1a and pharyngo-cervico-brachial variant) [39, 40] that will need to be confirmed in even larger cohorts. Likewise, the clinical relevance of antibodies targeting other structures (neurons, peripheral nerve tissue…) is unclear, since their association with GBS is not completely specific or the number of patients with each particular reactivity is too low to draw any conclusions.
Some studies have reported a correlation between IgG anti-GM1 and anti-GD1a antibodies with a poor outcome in GBS patients [13, 14, 33, 41]. In our cohort, IgG anti-GD1a antibodies did not associate to a poor outcome of the disease [13]. However, our data confirm that IgG anti-GM1 antibody is an independent prognostic factor that associates with poor prognosis at 1 year, supporting that it may be a marker for long-term axonal damage. Whether the presence of complement-fixing anti-GM1 antibodies is the driver of this long-term disability, an important therapeutic question (that would enable the use of complement inhibitors in these patients), remains to be elucidated.
Although in this study we analysed the prognostic value of anti-ganglioside antibodies using the traditional outcome measures: inability to walk (GDS ≥ 3) at 6 months and at 1 year, we have recently used the inability to run (GDS ≥ 2) as a measure of the presence of long-term residual disability. In this recent study we showed that high baseline sNfL were independently associated with inability to run at 1 year [29]. In agreement with these findings, we observed that including in the model the variable “presence of serum IgG anti-GM1 antibodies”, sNfL levels remained as an independent prognostic factor, whereas anti-GM1 antibodies did not. These results confirm that sNfL levels are a prognostic factor that informs better on axon status and, consequently, on long-lasting disability.
It is interesting to note that we did not find any patient with anti-nodal/paranodal antibodies (CNTN1, NF140, NF186, NF155 and CASPR1) in our GBS cohort. Although previous studies from other authors have found some GBS positive patients in their cohorts [14–18], and case-reports and series describe the association of anti-nodal/paranodal antibodies with aggressive inflammatory neuropathies frequently misdiagnosed as GBS, these antibodies are rare and we cannot rule out the possibility that they are present in other selected patients that our cohort failed to capture.
One of the limitations of our study is the number of patients and controls included. We have small groups of patients with similar staining patterns in which it is difficult to establish clear clinical-immunological correlations. Nevertheless, this is the first large prospective study assessing the autoantibody repertoire against peripheral nerve structures in GBS patients and antigen-identification experiments will follow in those patients showing specific staining patterns that are absent in controls.
The existence of clear subgroups associated with anti-ganglioside antibodies, in contrast with the diversity in the new reactivities analysed, suggests that this apparent heterogeneity may be also due to technical caveats, because our study protocol is optimized for proteins and not for lipids or glycans. Moreover, other, not properly controlled factors, could have influenced heterogeneity in staining patterns (treatment, comorbidities…), and will need to be assessed in larger cohorts.
Conclusions
In conclusion, our study highlights the heterogeneity of the profile of autoantibodies targeting peripheral nerve structures, confirms gangliosides as the most frequent target antigens in the GBS autoantibody repertoire and their prognostic value in long-term GBS prognosis, and identifies small subsets of GBS patients with specific staining patterns in which further antigen-identification experiments could demonstrate novel and clinically relevant autoantibody reactivities in the future.
Supplementary Information
Additional file 1: Figure 1. Heatmap showing anti-ganglioside antibodies in the GBS cohort. Patients and reactivities against anti-ganglioside antibodies are ordered according to Euclidean clustering. Each row represents one GBS patient. The score of the anti-ganglioside titre is indicated by the colour of the square (0 = < 1/1000, 1 = 1/1000–1/2500, 2 = 1/2500–1/12500, 3 = > 1/12500). Columns in the left contain information related to the clinical variant and the outcome at 6 months and at 1 year of follow-up.
Additional file 2: Figure 2. Staining paterns analized in ICC over rat DRG neurons and human neuroblastoma-derived neurons. DRG neurons (A–H) or neuroblastoma neurons (I–P) were stained with control or patient’s sera in green. Signal intensity of IgG (A–D, I–L) or IgM (E–H, M–P) reactivity was scored in a 0–3 scale (0 = negative, 1 = mild positive, 2 = moderate positive, 3 = strong positive). Neuroblastoma neurons were counterstained in red with anti-panNeurofascin mAb.
Additional file 3: Figure 3. Staining paterns analized in IHC over monkey peripheral nerve. Macaque peripheral nerve transverse sections stained with CNTN1 positive CIDP patient’s serum reacting against paranodes (A), small fiber axons (B), non-myelinating Schwann cells (C), myelin from small myelinated fibers (D), large fiber axons (E), and myelin from large myelinated fibers (F).
Additional file 4: Table 1. Statistical analysis of structures observed in IHC over monkey peripheral nerve. Table 2. Statistical comparison between GBS patients with and without anti-ganglioside antibodies.
Acknowledgements
The authors would like to acknowledge the Department of Medicine at the Universitat Autònoma de Barcelona and the IGOS consortium for their support. We also would like to thank all our patients for their patience and collaboration, and the animals that were killed to perform this study.
Abbreviations
- GBS
Guillain–Barré syndrome
- IGOS
International GBS Outcome Study
- DRG
Dorsal-root ganglia
- MFS
Miller Fisher syndrome
- AIDP
Acute inflammatory demyelinating polyneuropathy
- AMAN
Acute motor axonal neuropathy
- AMSAN
Acute motor–sensory axonal neuropathy
- GDS
GBS disability score
- ALS
Amyotrophic lateral sclerosis
- CMT
Charcot–Marie–Tooth
- NF155
Neurofascin-155
- NF140
Neurofascin-140
- NF186
Neurofascin-186
- CNTN1
Contactin-1
- CASPR1
Contactin-associated protein 1
- PFA
Paraformaldehyde
- NfL
Neurofilament light chain
- OR
Odds-ratio
- CIDP
Chronic inflammatory demyelinating polyneuropathy
Author’s contributions
CL acquired the data, performed the experiments, analysed the data and drafted the manuscript; TF performed anti-ganglioside antibodies experiments; LMA, EPG, JDM, RRG, ECV, JT, CC, CH, GGG, MCJM, JB, MJST, TGS, JPF, CMI, IRM, IJP, EMH, GMT and CDG acquired samples and data and revised the manuscript for intellectual content; MC, NL, EG, XSC, LMM, CJ and II revised the manuscript for intellectual content; LQ designed and conceptualized the study, interpreted the data and revised the manuscript for intellectual content. All authors read and approved the final manuscript.
Funding
This work is supported by Fondo de Investigaciones Sanitarias (FIS), Instituto de Salud Carlos III, Spain and FEDER under Grant FIS19/01407 and INT20/00080; by the GBS-CIDP Foundation International; and by the Gofundme campaign number 86547262 organized by Adela Gómez. LMA was supported by a personal Rio Hortega grant CM19/00042. XSC was supported by a “Sara Borrell” post-doctoral fellowship project “CD18/00195”, funded by Instituto de Salud Carlos III and co-funded by European Union (ERDF/ESF, “A way to make Europe”/“Investing in your future”). RR-G, NDL, EC-V, JT-S, EG, II and LQ are members of the ERN Euro-NMD.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Hospital de la Santa Creu i Sant Pau and the local institutional review boards of participating hospitals or universities. All patients gave written informed consent to participate in the study. Animal procedures were performed according to a protocol approved by our Institution’s Animal Ethics’ Committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cinta Lleixà and Lorena Martín-Aguilar have contributed equally to this work
References
- 1.Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain–Barré syndrome. Ann Neurol. 1990;27:S21–S24. doi: 10.1002/ana.410270707. [DOI] [PubMed] [Google Scholar]
- 2.Feasby TE, et al. An acute axonal form of Guillain–Barré polyneuropathy. Brain. 1986;109:1115–1126. doi: 10.1093/brain/109.6.1115. [DOI] [PubMed] [Google Scholar]
- 3.Wakerley BR, Uncini A, Yuki N. Guillain–Barré and Miller Fisher síndromes—new diagnostic classification. Nat Rev Neurol. 2014;10:537–544. doi: 10.1038/nrneurol.2014.138. [DOI] [PubMed] [Google Scholar]
- 4.Shahrizaila N, Lehmann HC, Kuwabara S. Guillain–Barré syndrome. Lancet (London, England) 2021;397:6736. doi: 10.1016/S0140-6736(21)00517-1. [DOI] [PubMed] [Google Scholar]
- 5.Van Den Berg B, et al. Guillain–Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10:469–482. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 6.Martín-Aguilar L, Pascual-Goñi E, Querol L. Autoantibodies in immune-mediated inflammatory neuropathies. Med Clín (English Ed) 2019;153:360–367. doi: 10.1016/j.medcli.2019.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Restrepo-Jiménez P, et al. The immunotherapy of Guillain–Barré syndrome. Expert Opin Biol Ther. 2018;18:619–631. doi: 10.1080/14712598.2018.1468885. [DOI] [PubMed] [Google Scholar]
- 8.Green DM. Advances in the management of Guillain–Barré syndrome. Curr Neurol Neurosci Rep. 2002;2:541–548. doi: 10.1007/s11910-002-0043-0. [DOI] [PubMed] [Google Scholar]
- 9.Kaida K, Kusunoki S. Antibodies to gangliosides and ganglioside complexes in Guillain–Barré syndrome and Fisher syndrome: mini-review. J Neuroimmunol. 2010;223:5–12. doi: 10.1016/j.jneuroim.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Emilien D, Hugh W. Diagnostic utility of auto antibodies in inflammatory nerve disorders. J Neuromuscul Dis. 2015;2:107–112. doi: 10.3233/JND-150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwabara S, et al. IgG anti-GM1 antibody is associated with reversible conduction failure and axonal degeneration in Guillain–Barre syndrome. Ann Neurol. 1998;44:202–208. doi: 10.1002/ana.410440210. [DOI] [PubMed] [Google Scholar]
- 12.Willison HJ, Veitch J, Paterson G, Kennedy PGE. Miller Fisher syndrome is associated with serum antibodies to GQ1b ganglioside. J Neurol Neurosurg Psychiatry. 1993;56:204–206. doi: 10.1136/jnnp.56.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamagishi Y, et al. Serum IgG anti-GD1a antibody and mEGOS predict outcome in Guillain–Barré syndrome. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323960. [DOI] [PubMed] [Google Scholar]
- 14.Rajabally YA, Uncini A. Outcome and its predictors in Guillain–Barré syndrome. J Neurol Neurosurg Psychiatry. 2012;83:711–718. doi: 10.1136/jnnp-2011-301882. [DOI] [PubMed] [Google Scholar]
- 15.Devaux JJ, Odaka M, Yuki N. Nodal proteins are target antigens in Guillain–Barré syndrome. J Peripher Nerv Syst. 2012;17:62–71. doi: 10.1111/j.1529-8027.2012.00372.x. [DOI] [PubMed] [Google Scholar]
- 16.Prüss H, Schwab JM, Derst C, Görtzen A, Veh RW. Neurofascin as target of autoantibodies in Guillain–Barré syndrome. Brain. 2011;134:1–2. doi: 10.1093/brain/awq372. [DOI] [PubMed] [Google Scholar]
- 17.Stengel H, et al. Anti-pan-neurofascin IgG3 as a marker of fulminant autoimmune neuropathy. Neurol Neuroimmunol NeuroInflamm. 2019;6:1–11. doi: 10.1212/NXI.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doppler K, et al. Auto-antibodies to contactin-associated protein 1 (Caspr) in two patients with painful inflammatory neuropathy. Brain. 2016;139:2617–2630. doi: 10.1093/brain/aww189. [DOI] [PubMed] [Google Scholar]
- 19.Appeltshauser L. Anti-paranodal antibodies and IgG subclasses in acute autoimmune neuropathy. Neurol Neuroimmunol Neuroinflamm. 2020 doi: 10.1212/NXI.0000000000000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schafflick D, Kieseier BC, Wiendl H, Meyer zu Horste G. Novel pathomechanisms in inflammatory neuropathies. J Neuroinflamm. 2017;14:1–17. doi: 10.1186/s12974-017-1001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs BC, et al. International Guillain–Barré Syndrome Outcome Study: protocol of a prospective observational cohort study on clinical and biological predictors of disease course and outcome in Guillain–Barré syndrome. J Peripher Nerv Syst. 2017;22:68–76. doi: 10.1111/jns.12209. [DOI] [PubMed] [Google Scholar]
- 22.Doets AY, et al. Regional variation of Guillain–Barré syndrome. Brain. 2018;141:2866–2877. doi: 10.1093/brain/awy232. [DOI] [PubMed] [Google Scholar]
- 23.Sejvar JJ, et al. Guillain–Barré syndrome and Fisher syndrome: case definitions and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine. 2011;29:599–612. doi: 10.1016/j.vaccine.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Hughes RAC, Newsom-Davis J, Perkin G, Pierce J. Controlled trial of prednisolone in acute polyneuropathy. Lancet (London, England) 1978;312:750–753. doi: 10.1016/S0140-6736(78)92644-2. [DOI] [PubMed] [Google Scholar]
- 25.Siles AM, et al. Antibodies against cell adhesion molecules and neural structures in paraneoplastic neuropathies. Ann Clin Transl Neurol. 2018;5:559–569. doi: 10.1002/acn3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willison HJ, et al. Inter-laboratory validation of an ELISA for the determination of serum anti-ganglioside antibodies. Eur J Neurol. 1999;6:71–77. doi: 10.1046/j.1468-1331.1999.610071.x. [DOI] [PubMed] [Google Scholar]
- 27.Ohanlon GM, et al. Peripheral neuropathy associated with anti-GM2 ganglioside antibodies: clinical and immunopathological studies. Autoimmunity. 2000;32:133–144. doi: 10.3109/08916930008994083. [DOI] [PubMed] [Google Scholar]
- 28.Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martín-Aguilar L, et al. Serum neurofilament light chain predicts long-term prognosis in Guillain–Barré syndrome patients. J Neurol Neurosurg Psychiatry. 2020 doi: 10.1136/jnnp-2020-323899. [DOI] [PubMed] [Google Scholar]
- 30.Hiraga A, et al. Recovery patterns and long term prognosis for axonal Guillain–Barré syndrome. J Neurol Neurosurg Psychiatry. 2005;76:719–722. doi: 10.1136/jnnp.2004.051136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuwabara S, Mori M, Ogawara K, Hattori T, Yuki N. Indicators of rapid clinical recovery in Guillain–Barré syndrome. J Neurol Neurosurg Psychiatry. 2001;70:560–562. doi: 10.1136/jnnp.70.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadden RDM, et al. Preceding infections, immune factors, and outcome in Guillain–Barré syndrome. Neurology. 2001;56:758–765. doi: 10.1212/WNL.56.6.758. [DOI] [PubMed] [Google Scholar]
- 33.Van Koningsveld R, et al. Infections and course of disease in mild forms of Guillain–Barré syndrome. Neurology. 2002;58:610–614. doi: 10.1212/WNL.58.4.610. [DOI] [PubMed] [Google Scholar]
- 34.Querol L, et al. Antibodies against peripheral nerve antigens in chronic inflammatory demyelinating polyradiculoneuropathy. Sci Rep. 2017;7:1–9. doi: 10.1038/s41598-017-14853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Querol L, Devaux J, Rojas-Garcia R, Illa I. Autoantibodies in chronic inflammatory neuropathies: diagnostic and therapeutic implications. Nat Rev Neurol. 2017;13:533–547. doi: 10.1038/nrneurol.2017.84. [DOI] [PubMed] [Google Scholar]
- 36.Kwa MSG, et al. Autoimmunoreactivity to Schwann cells in patients with inflammatory neuropathies. Brain. 2003;126:361–375. doi: 10.1093/brain/awg030. [DOI] [PubMed] [Google Scholar]
- 37.Vallat JM, et al. Antibody- and macrophage-mediated segmental demyelination in chronic inflammatory demyelinating polyneuropathy: clinical, electrophysiological, immunological and pathological correlates. Eur J Neurol. 2020;27:692–701. doi: 10.1111/ene.14133. [DOI] [PubMed] [Google Scholar]
- 38.Leonhard SE, et al. Diagnosis and management of Guillain–Barré syndrome in ten steps. Nat Rev Neurol. 2019;15:671–683. doi: 10.1038/s41582-019-0250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pan CL, Yuki N, Koga M, Chiang MC, Hsieh ST. Acute sensory ataxic neuropathy associated with monospecific anti-GD1B IgG antibody. Neurology. 2001;57:1316–1318. doi: 10.1212/WNL.57.7.1316. [DOI] [PubMed] [Google Scholar]
- 40.Koga M, Yuki N, Ariga T, Morimatsu M, Hirata K. Is IgG anti-GT1a antibody associated with pharyngeal-cervical-brachial weakness or oropharyngeal palsy in Guillain–Barre syndrome? J Neuroimmunol. 1998;86:74–79. doi: 10.1016/S0165-5728(98)00016-2. [DOI] [PubMed] [Google Scholar]
- 41.Kuwabara S, et al. Intravenous immunoglobulin therapy for Guillain–Barré syndrome ith IgG anti-GM1 antibody. Muscle Nerve. 2001;24:54–58. doi: 10.1002/1097-4598(200101)24:1<54::AID-MUS6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure 1. Heatmap showing anti-ganglioside antibodies in the GBS cohort. Patients and reactivities against anti-ganglioside antibodies are ordered according to Euclidean clustering. Each row represents one GBS patient. The score of the anti-ganglioside titre is indicated by the colour of the square (0 = < 1/1000, 1 = 1/1000–1/2500, 2 = 1/2500–1/12500, 3 = > 1/12500). Columns in the left contain information related to the clinical variant and the outcome at 6 months and at 1 year of follow-up.
Additional file 2: Figure 2. Staining paterns analized in ICC over rat DRG neurons and human neuroblastoma-derived neurons. DRG neurons (A–H) or neuroblastoma neurons (I–P) were stained with control or patient’s sera in green. Signal intensity of IgG (A–D, I–L) or IgM (E–H, M–P) reactivity was scored in a 0–3 scale (0 = negative, 1 = mild positive, 2 = moderate positive, 3 = strong positive). Neuroblastoma neurons were counterstained in red with anti-panNeurofascin mAb.
Additional file 3: Figure 3. Staining paterns analized in IHC over monkey peripheral nerve. Macaque peripheral nerve transverse sections stained with CNTN1 positive CIDP patient’s serum reacting against paranodes (A), small fiber axons (B), non-myelinating Schwann cells (C), myelin from small myelinated fibers (D), large fiber axons (E), and myelin from large myelinated fibers (F).
Additional file 4: Table 1. Statistical analysis of structures observed in IHC over monkey peripheral nerve. Table 2. Statistical comparison between GBS patients with and without anti-ganglioside antibodies.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.