Abstract
Worldwide infection and fatality by SARS-CoV-19 virus and its variants responsible for COVID 19 have impeded economic growth of developing nations beyond repair, General public in several nations have lost their livelihood, it has left severely impacted international relations and most importantly health infrastructures across the world have been tormented. This pandemic has already left footprints on human psychology, traits, and priorities and is certainly going to lead towards new world order in time to come. As always, science and technology come to rescue the human race. The prevention of infection by instant and repeated cleaning of surfaces which are most likely to be touched in daily life and sanitization drives using medically prescribed sanitizers and UV exposure of textiles are the first steps to break the chain. However, the real challenge is to develop and uplift medical infrastructure such as diagnostic tools capable of prompt diagnosis, instant and economic medical treatment available to masses. Two dimensional (2D) materials such as graphene are atomic sheets which have been in news from quite some time due to unprecedented electronic mobilities, high thermal conductivity, appreciable thermal stability, excellent anchoring capabilities, their optical transparency, mechanical flexibility and their unique capability to integrate to arbitrary surfaces. These attributes of 2D materials make them lucrative for their use as active materials platform for authentic and prompt (within minutes) disease diagnosis via electrical or optical diagnostic tools or via electrochemical diagnosis. We present the opportunities provided by 2D materials as a platform for SARS-CoV-2 diagnosis.
Graphical Abstract
This article reviews the use of two-dimensional materials as diagnostic platforms for the detection and sensing of the SARS-CoV-19 virus.
Introduction to COVID 19 infection:
The World Health Organization (WHO) received an alarming call from China Health Authority (CHA) on 31st December 2019 about an unknown infection spreading at Wuhan City in central China whose initial symptoms seems to be like pneumonia. However, little details were known about the virus origination and transmission rate. Acknowledging the call from CHA, WHO team visited Wuhan and Hubei provinces and reported an unknown strain of virus (a genetically modified form of Severe Acute Respiratory Syndrome human coronavirus (SARS-CoV)) on January 7th 2020 and named it as “2019-nCoV”[1]. Later, on further investigation, it was concluded that exposure to 2019-nCoV leads to the acute respiratory syndrome. Moreover, the amino acid sequence of the S-protein of the virus was 76.47% matched to that of the SARS-CoV virus and therefore the virus was renamed as SARS-CoV-2 [2]. The process of investigation meant that it took some time to understand and realize how deadly this virus is for humans and within a short time the number of infected people rapidly increased worldwide, reaching 1,013,606 by the 3rd April 2020. By the end of January 2020, there were 9826 confirmed cases of SARS-CoV-2 and 213 deaths. However, this was just the beginning of the wide spread of SARS-CoV-2; 19 countries reported confirmed cases of SARSCoV-2 and thus the WHO declared this outbreak of SARS-CoV-2 as a Public Health Emergency of International Concern (PHEIC) on 30th January 2020 [3–4]. Further, this PHEIC has been recognized globally as pandemic on 11th March 2020 [3].
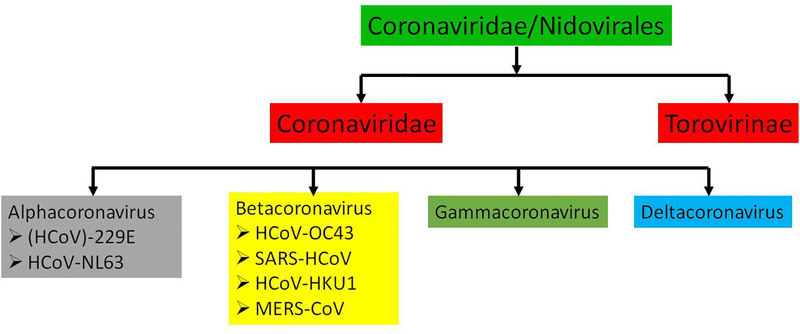
SARS-CoV-2 was found to be related to severe acute respiratory syndrome human coronavirus (SARS-CoV) and thus is in the family of Coronaviridae and other similar Nidovirale viruses [5]. Figure 1 represents the family and sub-categorized family members of the COVID-19 virus.
Figure 1.
Family of Coronaviridae virus
In the coronavirus family, alpha- and beta-coronaviruses are contagious to humans, while gamma- and delta-coronavirus are contagious to animals like pigs, birds, and whales [5–8]. It was found that the origination of these viruses is from a bat, however, this was just mere a speculation as only 79% similarity of the genome has matched with the genome of strain collected from the bat which has SARS-CoV or MERS-CoV virus[7–8]. Moreover, homology modelling discloses that although there is a variation in amino acid of COVID-19 virus in comparison to SARS-CoV the receptor-binding domain structures are the same[9]. However, it is early to say and it’s yet not specified that the origination of this virus is from a bat or some sea-foods. The question and understanding of origination, mutation, and transmission of COVID-19 virus warrant urgent investigation.
Fatality and transmission rate
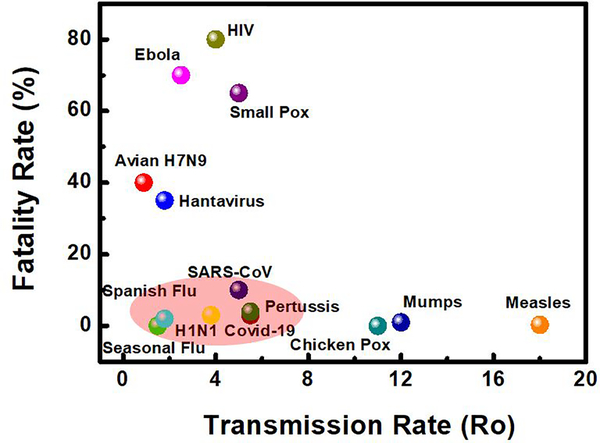
In order, to understand the transmission rate of COVID-19 (SARS-CoV- 2), we have considered some viruses which belong to the Coronaviridae virus family and some widely known viruses. At the time of writing this article, we found that the COVID-19 virus has a fatality rate of ~3%, while viruses such as Avian H7N9 (2013), Seasonal Flu, Hantavirus, Spanish Flu (1918), Ebola Virus, H1N1 (1918), HIV, SARS-CoV, Small Pox, Pertussis/whooping cough, Chicken Pox, Mumps, Measles Virus have a fatality rate of 40 %, 0%, 35%, 2%, 70%, 80%, 10%, 65%, 3%, 4%, 0%, 1% and 0.3% respectively[10–18]. While it is clear from Fig. 2 that COVID-19 has less fatality rate compared to other widely known viruses from his and another family, it does not mean that it will die out very soon and is harmless compared to others. Untreated humans can go to a ventilator in 7 days, as this virus attack severely to lungs and if not went to ventilator can damage the lungs and lead to death in 10 to 14 days.
Figure 2.
Fatality and Transmission rate of viruses.
Moreover, with a growing population, the density of people living in an area increases, therefore it will be misleading to compare the transmission rate of other known virus existing and COVID-19. As a result, the graph (Fig. 2) is meant to develop a perception to the reader, how viruses’ transmitted rate varies depending on the type of virus.
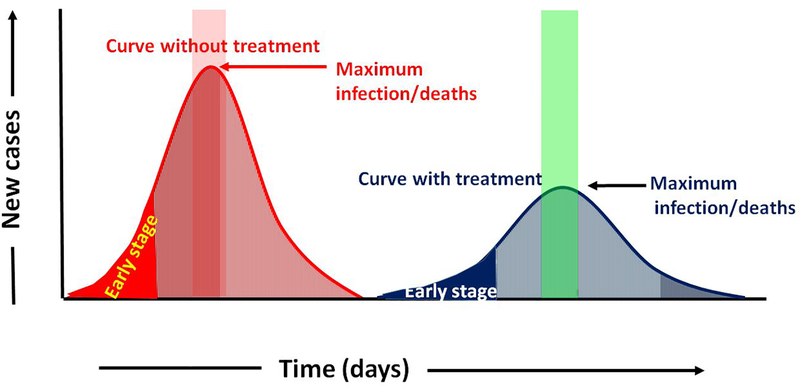
The mortality/fatality rate of these deadly viruses needs to detect at an early stage (see Fig. 3). However, presently relies on conventional techniques which although being reliable need time for getting test result. Therefore, worldwide there is a huge demand for the development of more realistic and reliable technology for the detection of specific biospecies/microorganism using very accurate diagnosis for monitoring the health of the patient. Fluorescence-based polymerase chain reaction (PCR), sequencing and immuno/affinity reaction biosensing are some of the ways to detect the COVID-19 virus (see Fig 4.) [19–21].
Figure 3.
Prediction of the spread, cure, and death of the SARS-Cov-2 virus in terms of sensitivity and time of detection, with and without treatment.
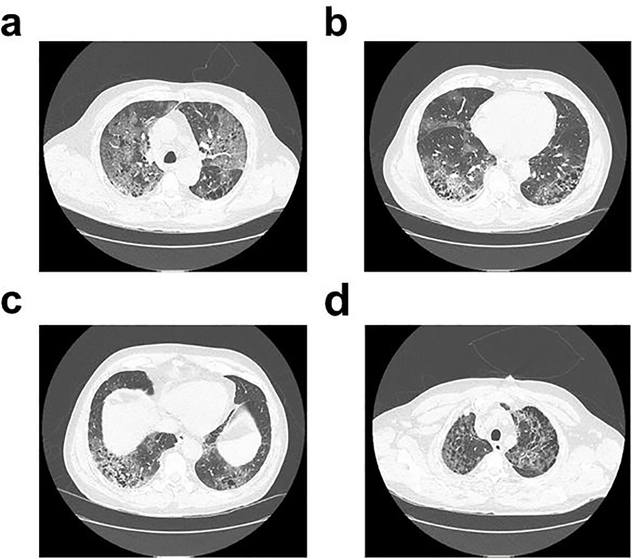
Figure 4.
CT scan of a 45 year-old COVID-19 patient demonstrating the spreading of the novel coronavirus (SARS-CoV-2) infection inside the lungs. Reproduced from Levine and Caputo [30] JACEP Open 2020, published by Wiley Periodicals LLC on behalf of the American College of Emergency Physicians, under Creative Commons Attribution License.
Covid 19 detection
Nasopharyngeal swabs sample collection is the initial step for the RT-PCR test. Contrary to the conventional PCR test, which needs an agarose gel and gives result after a complete scan, RT-PCR test used to detect and quantify nucleic acid presence during the succession of the reaction [22]. It can also detect the amount of DNA in the sample. Detection of SARS-CoV-2 involves a) identification of the envelope gene (E gene), the nucleocapsid protein gene (N gene) and the S gene [22–27]of the nucleic acid, b) opting any two identified gene and getting their fluorescent. This improves the sensitivity and specificity of the testing kit as it has been cross-verified two times in a single run. Chan et al. [28] further analyzed, designed and modified the RT-PCR assay by targeting the RdRp/Helicase. In comparison to RdRp-P2 assay, RdRp/Helicase assay does not get reacted with other member or family of coronaviruses. Thus, increases the sensitivity. Moreover, when the test sample is connected with PCR for the analysis, the signals for the virus are just being amplified only in the regions containing the virus sites [29]. In addition to this, a PCR test can be performed only by a skilled individual, thus, one can conclude that the use of PCR equipment for detecting COVID-19 virus makes it a labour-intensive, sophisticated, time consuming and expensive methodology, thus limiting their use in general laboratories [29].
Alongside laboratory testing, which often takes longer duration for detection, a prime detection technique which nowadays is very useful for the identification of COVID-19 is computed tomography imaging (CT-imaging). As COVID-19, attacks the lower respiratory tract infection such as pneumonia or bronchitis, it is easy to detect its presence through X-ray or Chest CT imaging.
CT imaging has shown a promising role in the detection of COVID, the CT imaging report of a COVID infected person shows a deterioration in his lung capacity as well as an increase in a bilateral multiple lobular over a while (see Fig. 4 (a–d)). This kind of report is found to be similar in case of diagnosis for pneumonia. However, the cases from the COVID infected person and pneumonia can be easily segregated if artificial intelligence (AI) can be implemented. For instance, AI-driven CT images can be used to understand the signature for COVID based on high resolution coloured image and signature of lobular obtained. This can be the key initial indicators for differentiating the results from pneumonia and can help to get control over the outbreak of the pandemic. These detection techniques are bound to reduce the fatality or the death rate as well as is expected to reduce hospitalization.
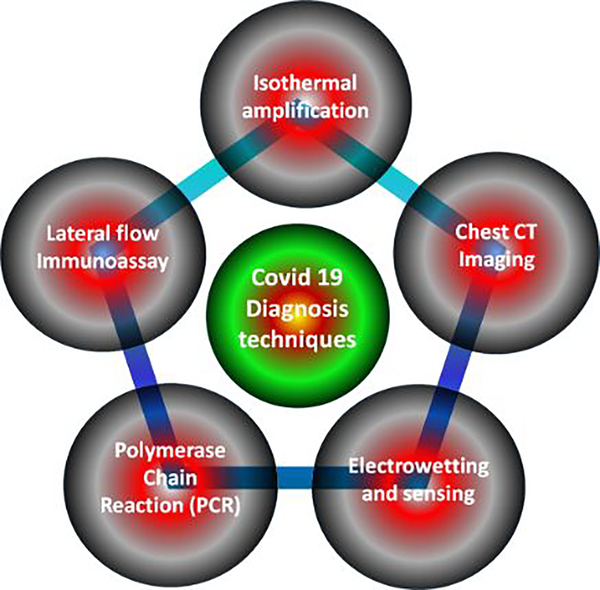
Out of several existing techniques (see Fig. 5) for detecting COVID rapidly and with high sensitivity, one of the promising ways is through Loop-mediated isothermal amplification (LAMP) test. Unlike, PCR test which is quite complex and is not economical, LAMP use to amplify nucleic acid signature under isothermal condition [31–33]. LAMP is a well-known and established a technique to detect a different kind of viruses. Le et al. used the LAMP technique for real-time monitoring of patients sample infected by influenza-like illness. The high sensitivity of > 88.8%, as well as specificity of ~100%, has been demonstrated by Takayama et. al in 2019 [34]. However, the techniques used by Takayama et al. involves the purification step of nucleic acid through the use of commercially available RNA isolation kit as well as preparation of the reaction mixture at very low temperature. Nakauchi et al. [35] proposed a much-optimized LAMP technique and is known as rRT-LAMP. It involves the preparation of reaction mixture in a lyophilized form, which eases the integration into the diagnostic kits, thereby enhancing transport and storage time during shipment. This technique is also known as the new direct rRT-LAMP assay is predicted to be a solution for rapid and simple molecular detection of viruses (such as COVID or influenza) as they have high sensitivity and specificity.
Figure 5.
COVID-19 detection techniques
Lateral flow (LF) immunoassay tests, is yet another technique widely adopted for the detection of COVID virus detection since introduced in the mid-1980s. It is also known as immune-chromatographic strip tests. As the name suggests “lateral flow” there is an involvement of fluid migration through paper or plastics. It is a very complex and more used technique instead involvement of many manuals as well as instrumental read and store test result for the targeted analytes[36]. LF is also been used in clinics, hospitals, and at home as it offers versatility to the manufacturers to tune its selectivity and specificity for any situation, which involves rapid detection. It also involves detection of disease-specific samples from urine, saliva, serum, plasma, blood, tissue or fluids. Another prominent reason for using more LF test is the cost and time involved in making the kit. In addition to all these technique SERS based detection technique is predicted to be most sensitive and accurate for detection of COVID (discussed in the later section of the draft).
A brief comparison to understand the different detection techniques for the SARS-CoV-2 virus can be summarized as follows: the RT-PCR and immunoassay methods have sensitivities of 95% and 20–80%, respectively, but at least 4 h detection time is required in both cases. It can be seen that RT-PCR and Immunoassays methods have a sensitivity of 95%, and 20% to 80% respectively, but a minimum of 2 h for detection time is required in both the cases. Interestingly, LAMP and Computed Tomography are the only two amongst various test, which has a sensitivity greater than 97% and 95% respectively. In comparison to RT-PCR and Immunoassays methods, LAMP and Computed Tomography requires only 30 min and >1 min respectively for detection of the COVID [34–42].
Ahmadivand et al. [37] have proposed and detected (SARS)-CoV-2 virus protein at low level using femtomolar (fM) by a very effective and efficient mode of detection using plasmonic meta sensor technology. They fabricated a miniaturized plasmonic immunosensor based on toroidal electrodynamics concept, which works in terahertz (THz) frequencies. It enables SARS-CoV-2 spike proteins to detect at Femto-mole-level concentrations with high precision and can detect SARS-CoV-2 protein within ~ 80 min. In yet another seminal work reported by Kaushik et al. [43], Mujawar et al. [44] and Paliwal et al. [45] detection of SARS-CoV-2 virus using biosensing integrated with AI and IoT supported platform has been discussed to create a database for early-stage diagnosis, cognizance of the spread and its effect, type of strain and related disease in a sequential manner to overcome the problems associated with it. Moreover, the role of AI and IoT supported SARS-CoV-2 detection selectively at the low level desired for early-stage COVID-19 diagnostics point-of-care (POC) sensing by suitably opting nanotechnology-based device and its design, packaging, integration and sense has been discussed. POC was further used to generate crucial information in understanding the efficacy of therapy, progression of the disease, etc. Furthermore, Mujawar et al. have described the use of low-level targeted disease biomarker (pM level), detection which eventually is useful to correlate and study the progression of the disease and its therapy. Kaushik et al. have [46] reported an effective way to neutralize SARS-CoV-19 virus through manipulative magnetic nanomedicine (MMN) therapy technique which offers control over drug delivery and option to choose desired therapeutic medicine. In another seminal work by Vicky et al. [47] the authors have summarized, analyzed more than one thousand articles related to SARS-CoV-19 and justified why the Alzheimer and dementia-related illness person are more prone to risk. They reviewed nearly 28 vaccines, their treatment protocol, clinical trials, along with the diagnostic tools and therapeutics for SARS-CoV-19, for an effective and efficient way of handling the disease.
Suitability of 2D Materials in COVID 19 sensing
Two-dimensional (2D) materials such as graphene, borophene, transition metals dichalcogenides (TMDCs), MXenes, Plumbene, Hematene etc. have unprecedented physical and chemical properties. They have a high surface area, weak inter-layer bonding and strong covalent in-plane bonding open up a plethora of application in the sensitive, selective and specific platform which can rapidly detect and paves the path for early detection of analyte or molecules or microbes at parts per million/billion level [48]. Amongst the family of 2D materials, graphene, borophene and phosphorene have shown potential to address global societal challenges including healthcare-related problems. The role of 2D material (owing to their large surface-to-volume ratio, anchoring capability, thermal stability) is crucial in the detection of the analyte as they provide aggrandize electrical, optical, mechanical and chemical property for devices performance. Owing to their excellent optical and electronic properties, they have been considered as futuristic materials for imaging in photo-acoustic, photo-thermal and X-ray computed tomography. Moreover, due to their superior optoelectronic properties, they have been used in photodynamic and photothermal therapy. Transition metal dichalcogenides seem to be an obstacle for various biosensing applications due to their relatively lower carrier mobility in comparison to MXene (borophene, phosphorene and their cousins) and graphene. MXene family offers tunable bandgap which has the potentials to manipulate the interaction between electromagnetic waves and the Xenes, within the wavelength of NIR to UV region [49]. Surprisingly, these 2D materials and detection of molecules (such as germs or microbes) integrate many branches of science which includes nanotechnology, chemistry, physics, material science and collectively drives bioelectronics and bioengineering.
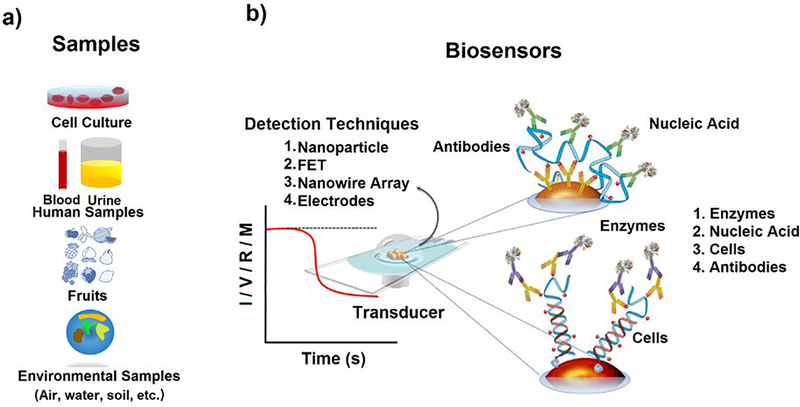
Sensing via SERS is a more rapid and effective detection technique for COVID virus and is an integral part of bioelectronics. It saves time and money. Bioelectronics plays a crucial role in the detection of various biological microorganism. It has opened a vibrant and vivid research field in the field of electronics, bioengineering and biotechnology. Detection of functional bioactive molecules such as DNA, RNA, protein, microorganism, antibodies [48–56] using techniques such as field-effect transistors (FETs), nanowire array, optical resonators, Surface-enhanced Raman scattering (SERS) and electrochemical sensing etc. (See Fig. 6) have been evolved over the past few decades[57–69]. Bioelectronic devices generate signals on interaction with bio-species. Typically, the signal generated is in the form of impulse or current, voltage, resistance, conductance, or frequency.
Figure 6.
(a) Target receptors for biosensing, (b) Schematic display for biosensors[30]Copyright 2018, Springer Nature.
Field-effect transistor based sensing
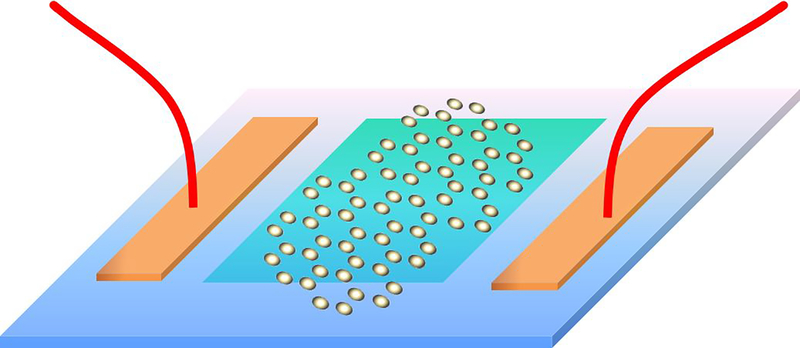
In the 1970s, ion-sensitive field-effect transistors (ISFET) was invented by Bergveld et al. [77]. It was realized that the ISFET can be used for detection of numerous biosensor targets. The upcoming time-based evolution of technology has changed the designed and fabrication of FETs. Typically, a FET consists of a semiconductor path called sensing channels, which has its two sides connected to the source (S) and drain (D) electrodes. The backside of the sensing channel is generally connected to the third electrode and is called a gate (G) (see Fig. 7). It is used for biasing and enhancing the sensitivity as well as selectivity of the device. The channel conductivity determines the presence of targets and thus gets recorded in an electrical signal. The FETs are divided into two based on the detection techniques, i.e n-type (it uses electrons as carrier charge) and p-type (it uses holes as carrier charge). For a typical, n-type FET, if the target molecules have a positive charge and get attached to the channel then it leads to increase in channel conductivity, while if the target molecules have negative charge then it will lead to decrease in channel-conductivity and vice-versa for p-type FET[78–79]. It can be inferred that all the anchoring, detection, and signal transmission part has been controlled by the channel path.
Figure 7.
The schematic diagram for the 2D material based channel FET biosensor
Change in the channel resistance of the FET device reveals the information about the analyte. This state of the art is currently being used for wearable devices and neural interfacing technology. Li et al. [80] demonstrated graphene-based FET (G-FET), in which the channel length was made up of micro mechanically exfoliated graphene sheet integrated with mercury ions. Graphene known for high mobility and conductivity has detected 0.1 parts per billion (ppb) of mercury. Surprisingly, the graphene-based channel length overpass the World Health Organization tolerance limit of 1 ppb and in comparison to commercially available ISEs devices for detection of mercury, the sensitivity has been increased by a factor or more than 1000 times [81–82]. Unlike graphene, other 2D materials such as MoS2, black phosphorus, and h-BN are also integrated on FET devices for channel length [83–85]. Lee et al. have demonstrated the use of MoS2 based channel length for detection of DNA hybridization [86]. In comparison to the PCR detection technique, which requires continuous amplification during the testing cycle, FET based devices give low detection of targeted nucleic acid, without pre-amplification [87]. Carbon nanotubes (CNTs) and Silicon (Si) nanowires have also been used as channel length in FET devices, however, the difficulty in fabrication of nanowires and nanotubes along with the reproducibility as well as the cost of production limits their use in FET devices [88–92]. Mannoor et al. demonstrated whole-cell bacterial detection using chemical vapour deposition (CVD) grown graphene as a channel for sensing [85]. They used CVD grown layers of graphene sheet anchored with antimicrobial peptides as well as coil micro antenna on biocompatible silk to detect a single E. coli bacterium [85]. More, such evidence of graphene and functionalized graphene for whole-cell detection has been reported by Guarnieri et al. [93] and Pandey et al. [94] for the detection of human intestinal epithelial cells and E. coli respectively.
Materials for channels are, therefore, the most crucial components which need to be evenly considered and designed. 2D materials, such as graphene, borophene, alpha lead oxide etc. not only provides high conductivity, mobility and enhanced mechanical strength but also helps in anchoring with the target molecules due to high surface to volume ratio and rapid, accurate, and early diagnosis of the molecules. FET based on 2D material platform have outnumbered all the conventional materials (as roughness in the morphology of the channel is bound to give errors and unrealizable outputs from the device due to scattering effect) used in FET. The most advantageous role of 2D materials in FETS are that they provide a rapid, economical, and ease of use because the real-time analysis is being monitored accompanied by low-cost meters which can eventually be calibrated or anchored for different end-user applications.
Although FET based biosensors have many advantages over other sensors, charge screening is the most common problem faced by FET devices for biological media detection. As charge screening is mainly associated with electrolytes and the Debye length (λD) and both factors are inversely related, one of the possible solutions is to avoid it, can be by diluting the sample into less concentration [94–96]. However, this may lead to surface functionalization of the targeted molecules or analyte. The next possible approach is to make targeted analyte within the size of the Debye length, but, seems to be less feasible considering the size of the target and functional groups. Many new innovative and upcoming way to avoid charge screening still need to be investigated and creates a new area of futuristic research based on biomolecules targeted FET devices [95–98].
Surface-Enhanced Raman Scattering (SERS) based sensing
SERS is amongst one of the techniques in bioelectronics that uses the change in frequency to provide a rapid, non-invasive way to detect the signature of biological samples with ultrahigh sensitivity. Moreover, it does not involve a complex process or skill hand to obtain the result. However, in earlier days for a SERS platform complex and complicated substrate are required. SERS techniques using nanostructured surfaces, use localized electromagnetic field (EMF) near to the surface zone and results in the enhancement of the signal by an order of typically 106 times. Usually, gold or silver nanoparticles having different sizes and shapes, composite/hybrid nanoparticles having core-shell structures or periodic structures are used for SERS platform. Lim et al. [99] utilize the SERS platform for detection of influenza virus. Moreover, a different strain of influenza virus was easily detected through SERS. Yeh et. al [100] detected avian influenza A viruses using the SERS platform. In addition, it was claimed that the virus such as rhinovirus, influenza virus, and parainfluenza viruses, can also be get detected with their unmodified structure and with new strains. It was also revealed that SERS can give selectivity when there is a presence of more than two viruses. More reports for active detection of influenza virus can see through the results by Park et al. and Kukushkin et al. [101,102].
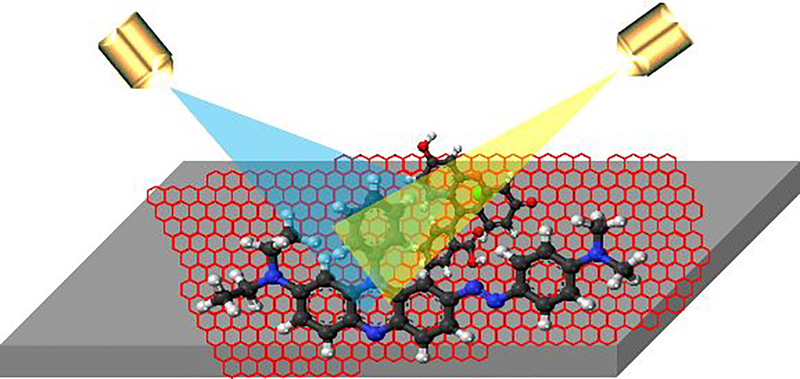
Graphene was the first amongst all 2D materials family which was used for SERS application and a variety of organic dyes (phthalocyanine (Pc), rhodamine 6G (R6G), PPP, and crystal violet (CV)) molecules have been used for detection using graphene [103]. Early-stage detection of viral infection is the key to cure and at a very early stage, SERS signal due to test molecules are very weak. To enhance the signal, therefore, one needs to enhance the laser intensity of the incident beam and at that intensity, unfortunately, test molecules get degraded. Interestingly, the high thermal conductivity of graphene comes to the rescue and helps remove interfacial heat generated upon laser exposure and thus acts as a laser shield for underlying plasmonic nanostructures on the one hand and prevents bond breaking and carbonization of test molecules on the surface on the other hand [104,105]. Atomic-scale integration of plasmonic nanostructure and graphene has been proposed and implemented earlier for effortless removal of heat during SERS measurement [106–109]. 2D materials hetero layers have designer materials behaviour such as mobility enhancement of graphene when placed on boron nitride (BN). Inter-layer coupling amongst atomic sheets can be exploited to manipulate out-of-plane tunnelling behaviour [110] in these heterolayer stacks and thus they can be implemented to attain functional fast biochips. The inception of 2D materials has completely revolutionized this field of research by providing a simple, scalable, low-cost platform. The absence of a dangling bond in (out-of-plane) 2D material makes them the most suitable platform to explore the chemical enhancement mechanism (see Fig. 8). However, 2D materials are yet to be used for the detection of viruses or microbes.
Figure 8.
The schematic diagram for the 2D material based SERS platform.
Challenges and Alternative Approaches
Control over the transmission of the contagious infection or the pandemic requires in-depth basic knowledge, skill and anti-viral research to overcome the outbreak. Material science broadens and opens up a plethora of opportunity to support antiviral research, treatment and investigation. In the present pandemic, the SARS-CoV-2 virus needs to be investigated from the structure, size, lifetime, transmission rate and its response to various antiviral drugs. These challenges need to be properly addressed to avoid any more upcoming pandemic in the future. The alternating approach to overcome any of the present pandemics is to use technology such as advance research machine and AI. To begin, with centrifuge machine powered with batteries would be an ideal solution to separate viral-strain from saliva or blood. The structure of the virus can be analyzed via environmental scanning electron microscopy or any confocal microscopy with high magnification. Time-Resolved Photoluminescence, Ultraviolet, Raman and Fourier Transform Infrared spectroscopy are few techniques which can be used to study the binding of the protein/shell of the virus as well the for the interaction between the drugs and virus. Alternatively, 3D printers can be used to produce a large number of masks, gloves, personal protective kit etc. without engaging labours. Electro-spinning is yet another technique which can help design and fabricate nanofibres. Artificial Intelligence will be a boon for the scientist in their research if data from the infected person can be monitored and tracked. These databases can be of utmost importance while designing and manufacturing anti-viral drugs or vaccines. Scalability of the drugs can be meet using the robotics or microfluidic technology and effectiveness can further be monitored by using AI. In short, multi-disciplinary research should be encouraged and collaboration between industries, research labs and scientist should be established.
Future Work and Perspective
Immediate detection of the Covid-19 virus is the need of the hour. Moreover, lack of time and available technology, as well as the basic knowledge of this unknown new virus, is a threat and a bigger challenge to answer. As the virus is continuously modifying its strain, size, structure and spread rate, and the development of vaccination seems to be in its nascent stage, and out of the box, the solution needs to think of. Material science, especially 2D materials based devices are amongst one of the ways to detect and prevent the community from widespread of the virus. Also, early and rapid detection can prevent severe organ failure as well as the death rate. Though several portable, less expensive, invasive devices are present in the market for testing, they take time to give result for an infected person. Yet, after consuming time and delaying the treatment one infected person needs at that hour, these tests are not reliable everywhere as they need an expert hand and skilled labour.
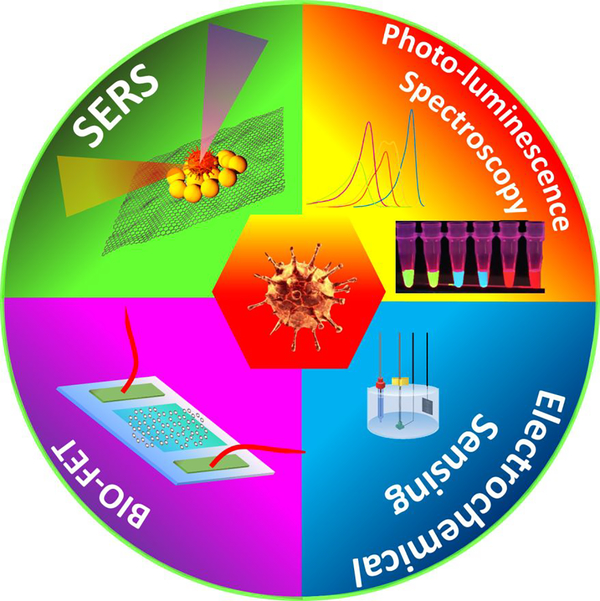
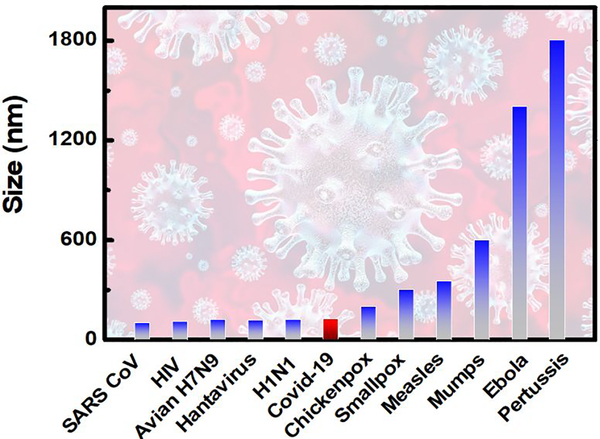
2D materials based detection techniques, such as SERS, Bio-FET, Electrochemical sensing, and Photo-Luminescence spectroscopy (see Fig. 9) is currently seems to be one of the upcoming technology for detection of Covid-19 virus. The absence of the dangling bond, high conductivity, mobility, luminescence (size dependent), weak van der Waals of attraction between layers as well as their anchoring capability (in case of graphene, borophene, phosphorene and transition metal dichalcogenides) makes them the desired futuristic material for detection of microbes. The unprecedented physical and chemical properties of 2D materials make them a platform for high sensitivity, specificity and selectivity for different virus detection and ensure the early detection, effectiveness, as well as rapid testing of microbes. Even in the short period, 2D materials have outperformed several existing and well-recognized methods for spreading of the Covid-19 virus. 2D materials (graphene) coated fabrics such as masks, PPE kit, gloves and shoes are currently being used at the commercial level. This is possible because the inter-atomic distance of two atoms in graphene is 0.14 nm, which is far small than almost each harmful microbes, apart from their mechanical flexibility. A size comparison of all harmful, fatal and mostly communicable viruses with Covid-19 has been depicted in Fig. 10. Graphene can even be embedded inside the polymer fabric and at filler level higher than a threshold; the fabric will be electrically conducting which then can be used for viral removal from its surface in an electrically controlled manner [111–123]. In case of rapid detection of any virus or analyte mobility and charge carriers plays a huge role. The electronic mobility of 2D material relies on size, shape, strain, valancy of an atom, crystalinity and chemical bonds. Therefore, the electronic mobility is an intrinsic characteristic of a material.
Figure 9.
2D material based devices for detection of Covid-19.
Figure 10.
Size of different viruses.
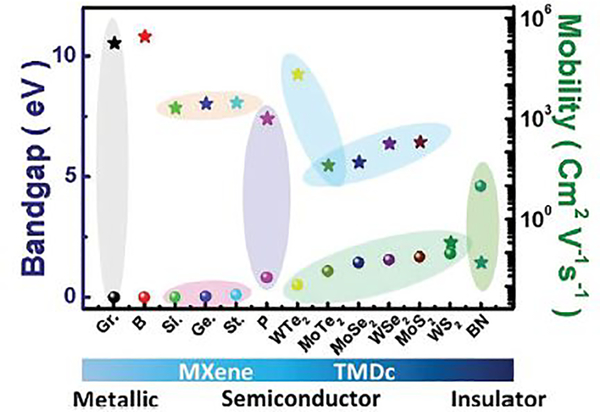
Amongst the Xene family, graphene and borophene have the electronic mobilities of 180000 and 280000 cm2/Vs respectively. These values are the highest amongst 2D materials. Cousins of graphene viz. silicene, germanene, stanene and phosphorene however have moderate mobilities 2100 cm2/Vs, 2800 cm2/Vs, 3000 cm2/Vs, 1000 cm2/Vs respectively [63, 69]. In general, semiconductor 2D materials from Transition Metal Dichalcogenides (TMDCs) family have low mobility, however compensated by carrier concentration [63, 69]. Thus, even though these 2D semiconductors may not be apt for ultrafast detection, electrical signal will be enhanced as conductivity is the product of mobility and carrier concentration.
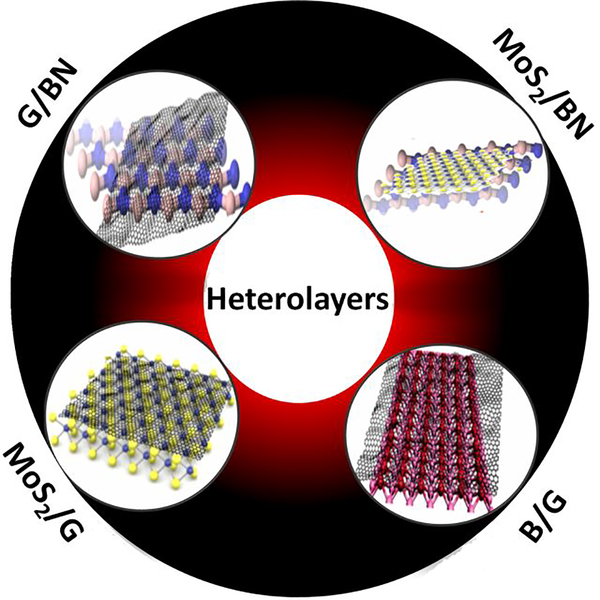
Even though Boron Nitride (BN) is an insulator and have lowest mobility 0.05 cm2/Vs Fig 11, 2D semi metals such as graphene, metals such as borophene and semiconductors such as MoS2 exhibit excellent mobility enhancement when placed on atomically smooth, dangling bond free and charge passivated BN layer. With the recent surge in 2D materials Moire’ super lattices as a tunable metamaterials, varieties of heterolayers composed of 2D materials are proposed as active surfaces for authentic COVID 19 detection via two probe, 4 probe, FET measurements as well as SERS based molecular sensing in liquid phase containing blood, tears, sweat or saliva. (see Fig. 12). Nanomaterials in general and atomic sheets in particular are thus proposed to have tremendous potential in prompt detection and prevention of COVID 19. Research in this direction has just commenced worldwide and as a materials platform, 2D materials are destined to bestow best performances in times to come. Even though recent literature on usage of nanotechnology motivates research along these directions and due implementation, the present review on employing two dimensional materials along with its doped and hybrid versions would inspire on-demand prompt diagnosis and treatments [123–133].
Figure 11.
Bandgap versus mobility of various 2D materials Reproduced with permission from Ranjan et al., [76] Adv Mater 2020, Copyright 2020, Wiley- VCH.
Figure 12.
2D material heterostructures as active materials in FET devices.
Conclusion
It is evident that the SARS-CoV-2 virus is a modified form of Severe Acute Respiratory Syndrome human coronavirus (SARS-CoV) and the alpha-coronavirus, and beta-coronavirus are contagious to humans. Moreover, the fatality rate of SARS-CoV-2 (nearly 3%) has been found be less than the HIV, Ebola, Small Pox and Avian H7N9. In addition, the transmission rate of SARS-CoV-2 is comparable with pertussis and is far less than mumps and measles. Detection of the SARS-CoV-2 virus is seems to be practically possible with various sensing technique such as PCR, Electro-wetting and sensing, Lamp test, etc. However, it was found that the RT-PCR test was the best amongst all existing technique considering time and sensitivity. Another way of detection of the SARS-CoV-2 virus is seems to be possible with the use of 2D materials as the sensing platform. In this aspect, different 2D materials such as graphene, borophene, etc. has been deemed to be effective in SERS and Biosensing (FET) for the detection of the SARS-CoV-2 virus. Biosensing using 2D materials can be used for rapid detection, analysis and research on the SARS-CoV-2 virus. Materials like graphene, MXene, borophene and their hybrids are the futuristic material for developing a testing kit to detect any alien virus.
Acknowledgments:
We acknowledge Indian Institute of Technology Patna for providing research facilities. VT acknowledges the financial support by P42-ES027723-01A1 from National Institute of Environmental Health Sciences (NIEHS) Superfund program grant. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of US National Institutes of Health (NIH).
Footnotes
Conflict of interest: Authors declare no conflict of interests.
References
- 1.Hui DS, E IA, Madani TA, Ntoumi F, Kock R, Dar O, Ippolito G, Mchugh TD, Memish ZA, Drosten C, Zumla A, Petersen E, Int J Infect Dis, 2020, 91, 264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, J Liu W, Wang D, Xu W, C Holmes E, F Gao G, Wu G, Chen W, Shi W, Tan W, Lancet 2020, 395, 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burki TK, The Lancet. Respiratory medicine, 2020, 8, 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO: coronavirus disease 2019 (COVID-19) situation report – 82, World Health Organization, Geneva, Switzerland, 2020 [Google Scholar]
- 5.Harapan H, Itoh N, Yufika A, Winardi W, Keam S, Te H, Megawati D, Hayati Z, Wagner AL, Mudatsir M, Journal of Infection and Public Health, 2020, 13, 667–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burrell C, Howard C, Murphy F, Fenner and White's medical virology, (5th ed.), Academic Press, United States, 2016 [Google Scholar]
- 7.Kramer A, Schwebke I, Kamp G, BMC Infect Dis, 2006, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pokhrel P, Hu C, Mao H, ACS Sens. 2020, 5, 2283–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasmi Y, Khataby K, Souiri A, Ennaji MM, Fundamental and Basic Virology Aspects of Human, Animal and Plant Pathogens, 2020, 127–149. [Google Scholar]
- 10.Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu Na, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, F Gao G, Wu G, Chen W, Shi W, Tan W, Lancet 2020, 395, 565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietz K, Statistical Methods in Medical Research, 1993, 2, 23–41. [DOI] [PubMed] [Google Scholar]
- 12.M Guerra F, Bolotin S, Lim G, Heffernan J, Deeks SL, Li Y, Crowcroft NS, Lancet Infect Dis 2017, 17, 420–28. [DOI] [PubMed] [Google Scholar]
- 13.Kretzschmar M, Teunis PFM, Pebody RG, PLOS Medicine, 2010, 7, 6,1000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferguson NM, Cummings DAT, Fraser C, Cajka JC, Cooley PC, Burke DS, Nature, 2006, 442, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Kerkhove MDV, Hollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, Jombart T, Hinsley WR, Grassly NC, Balloux F, Ghani AC, Ferguson NM, Rambaut A, Pybus OG, Gatell HL, Aranda CMA, Chapela IB, Zavala EP, Ma D. Guevara E, Checchi F, Garcia E, Hugonnet S, RothScience C, 2009, 324,1557–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kucharski AJ, Althaus CL, Surveill E, Euro Surveill, 2015, 20, 21167. [DOI] [PubMed] [Google Scholar]
- 17.Khan A, Naveed M, e-Ahmad MD, Imran M, Infectious Diseases of Poverty 2015, 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gani R, Leach S, Nature, 2001, 414, 748–751. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Microbes and Infection, 2020, 22, 69–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krejcova L, Richtera L, Hynek D, Labuda J, Adam V, Biosens. Bioelectron 2017, 97, 384–399. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Zhang X, Zhao K, J. Electroanal. Chem, 2016, 773, 63–68. [Google Scholar]
- 22.Kurita R, Niwa O, Anal. Chem. 2012, 84, 7533–7538. [DOI] [PubMed] [Google Scholar]
- 23.Dios L. T. d., Ibarra C, Velasquillo C, Investigación en discapacidad, 2013, 2, 70–78. [Google Scholar]
- 24.Lorussoa A, Calistria P, Mercantea MT, Monacoa F, Portanti O, Marcacci M et al. One Health. 2020, 10, 100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng D, Ying QM, Weng YS, Shen CB, Chu JG, Kong J et al. Clinica Chimica Acta. 2020, 506, 172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Zhou Y, Zong Z, Liang Z, Cao Y, Tang H et al. , Precision Clinical medicine, 2020, 3,14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao AT, Tong YX, Gao C, Zhu L, Zhang YJ, Zhang S, Journal of Clinical Virology, 2020, 127, 104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hogan C, Sahoo M, Huang CH, Garamani N, Stevens B, Journal of Clinical Virology, 2020, 127, 104383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan JFW, Yip CCY, To KKW, Tang THC, Wong SCY, Leung KH et al. , Journal of Clinical Microbiology, 2020, 58, 00310–20. [Google Scholar]
- 30.Levine R, Caputo N, JACEP OPEN, 2020, 1, 143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Povedano E, Vargas E, Montiel VR, et al. Sci Rep, 2018, 8, 6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagamine K, Hase T, Notomi T, Mol. Cell. Probes, 2002, 16, 223–229. [DOI] [PubMed] [Google Scholar]
- 33.Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T, Nucleic Acids Res, 2000, 28, E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takayama I, Nakauchi M, Takahashi H, Oba K, Semba S, Kaida A, Kubo H, Saito S, Nagata S, Odagiri T, Kageyama T, J. Virol. Methods, 2019, 267, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakauchi M, Yoshikawa T, Nakai H, Sugata K, Yoshikawa A, Asano Y, Ihira M, Tashiro M, Kageyama T, J. Med. Virol 2011, 83, 10–15. [DOI] [PubMed] [Google Scholar]
- 36.Yousif YA, Anderson J, Bergstrom CC, Kapil S, Clinical And Diagnostic Laboratory Immunology, 2002, 723–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmadivand A, Gerislioglu B, Ramezani Z, Kaushik A, Manickam P, Ghoreishi SA, Biosensors and Bioelectronics, 2021, 177, 112971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Cruz A RJ. Currier W, Sampson VB, ACS Cent. Sci 2020, 6, 591–605,32382657 [Google Scholar]
- 39.D’Cruz A RJ. Currier W, Sampson VB, Front. Cell Dev. Biol 2020, 8, 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter LJ, Garner LV, Smoot JW, Li Y, Zhou Q, Saveson CJ, Sasso JM, Gregg AC, Soares DJ, Beskid TR, Jervey SR, Liu C. ACS Cent Sci. 2020, 27, 591–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas.
- 42.Islam KUI, Iqbal J, Front. Cell. Infect. Microbiology, 2020, 10, 560616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaushik K, Dhau JS, Gohel H, Mishra YK, Kateb B, Kim N-Y, Goswami DY, ACS Appl. Bio Mater 2020, 3, 7306–7325. [DOI] [PubMed] [Google Scholar]
- 44.Mujawar MA, Gohel H, Bhardwaj SK, Srinivasan S, Hickman N, Kaushik A, Materials Today Chemistry, 2020, 17, 100306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paliwal P, Sargolzaei S, Bhardwaj SK, Bhardwaj V, Dixit C, Kaushik A, Frontiers in Nanotechnology, 2020, 2, 517284. [Google Scholar]
- 46.Kaushik, Expert Opinion on Drug Delivery, 2020, 10.1080/17425247.2021.1860938. [DOI] [Google Scholar]
- 47.Vicky Y et al. , Journal of Alzheimer's Disease, 2020, 77, 459–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu C, Shin J, Son S, Choe Y, Farokhzad N, Tang Z, Xiao Y, Kong N, Xie T, Seung Kim J, Tao W, Chem. Soc. Rev, 2021, 50, 2260–2279. [DOI] [PubMed] [Google Scholar]
- 49.Tao W, ORCID, Kong N, Ji X, Zhang Y, Sharma A, Ouyang J, Qi B, Wang J, Xie N, Kang C, Zhang H, Farokhzad OC, Kim Jong Seung, Chem. Soc. Rev, 2019,48, 2891–2912. [DOI] [PubMed] [Google Scholar]
- 50.Sahu TK, Ranjan P, Kumar P, Emergent Mater. 2021, 1–10, 10.1007/s42247-021-00170-0. [DOI]
- 51.Tang Z, Kong N, Zhang X, Liu Y, Hu P, Mou S, Liljeström P, Shi J, Tan W, Kim JS, Cao Y, Langer R, Leong KW, Farokhzad OC, Tao W, Nature Reviews Materials, 2020, 5, 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Tang Z, Farokhzad N, Chen T, Tao W, Nano Today 2021, 36, 101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kurapati R, Kostarelos K, Prato M, Bianco A, Adv. Mater 2016, 28, 6052–6074. [DOI] [PubMed] [Google Scholar]
- 54.Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA, Science, 2004, 306, 666–669. [DOI] [PubMed] [Google Scholar]
- 55.Stine R, Mulvaney SP, Robinson JT, Tamanaha CR, Sheehan PE, Anal. Chem, 2013, 85, 509–521. [DOI] [PubMed] [Google Scholar]
- 56.Chen L, Xie J, Yoon H, Srivatsan M, Harbaugh RE, Varadan VK, Biohybrid Systems, 2011, 95–113. [Google Scholar]
- 57.Du HJ, Ye JS, Zhang JQ, Huang XD, Yu CZ, J. Electroanal. Chem, 2011, 650, 209–213. [Google Scholar]
- 58.Gan TA, Hu CG, Chen ZL, Hu SS, Sensors Actuators B Chem, 2010, 151, 8–14. [Google Scholar]
- 59.Garaj S, Hubbard W, Reina A, Kong J, Branton D, Golovchenko JA, Nature, 2010, 467, 190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gholivand MB, Khodadadian M, Biosens. Bioelectron, 2014, 53, 472–478. [DOI] [PubMed] [Google Scholar]
- 61.Ping J, Wu J, Wang Y, Ying Y, Biosens. Bioelectron, 2012, 34, 70–76. [DOI] [PubMed] [Google Scholar]
- 62.Keren LB, Hanein Y, Front Neural Circuit, 2012, 6, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Connolly P, Clark P, Curtis ASG, Dow JAT, Wilkinson CDW, Biosens. Bioelectron. 1990, 5, 223–234. [DOI] [PubMed] [Google Scholar]
- 64.Huys R, Braeken D, Jans D, Stassen A, Collaert N, Wouters J, Loo J, Severi S, Vleugels F, Callewaert G, Verstreken K, Bartic C, Eberle W, Lab Chip, 2012, 12, 1274–1280. [DOI] [PubMed] [Google Scholar]
- 65.Laha R, Ranjan P, AIP Conference Proceedings, 2016, 1731, 050150. [Google Scholar]
- 66.Li F, Xue M, Ma X, Zhang M, Cao T, Anal. Chem 2011, 83, 6426–6430. [DOI] [PubMed] [Google Scholar]
- 67.Ng AM, Kenry C, Lim T, Low HY, Loh KP, Biosens. Bioelectron, 2014, 65, 265–273. [DOI] [PubMed] [Google Scholar]
- 68.Park DW, Schendel AA, Mikael S, Brodnick SK, Richner TJ, Ness JP, Hayat MR, Atry F, Frye ST, Pashaie R, Thongpang S, Ma ZQ, Williams JC, Nat. Commun, 2014, 5, 5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spira ME, Hai A, Nat. Nanotechnol, 2013, 8, 83–94. [DOI] [PubMed] [Google Scholar]
- 70.Ranjan P, Sahu TK, Bhushan R, Yamijala SS, Late DJ, Kumar P, Vinu A, Advanced Materials 2019, 31, 1900353. [DOI] [PubMed] [Google Scholar]
- 71.Ranjan P, Agrawal S, Sinha A, Rao TR, Balakrishnan J, Thakur AD, Scientific reports, 2018, 8, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumar P, Liu J, Ranjan P, Hu Y, Yamijala SS, Pati SK, Irudayaraj J, J Cheng G, Small, 2018, 14, 1703346. [DOI] [PubMed] [Google Scholar]
- 73.Ranjan P, Tiwary P, Chakraborty AK, Mahapatra R, Thakur AD, Journal of Materials Science: Materials in Electronics, 2018, 29, 15946–15956 [Google Scholar]
- 74.Ranjan P, Laha R, Balakrishnan J, Journal of Raman Spectroscopy, 2017, 48, 15946–15956 [Google Scholar]
- 75.Ranjan P, Verma P, Agrawal S, Rao TR, Samanta SK, Thakur AD, Materials Chemistry and Physics, 2019, 226, 350–355. [Google Scholar]
- 76.Ranjan P, Lee JM, Kumar P, Vinu A, Advanced Materials, 2020, 32, 2000531. [DOI] [PubMed] [Google Scholar]
- 77.Bergveld P, IEEE Trans. Biomed. Eng 1970, 17, 70–71. [DOI] [PubMed] [Google Scholar]
- 78.Cui Y, Wei Q, Park H, Lieber CM, Science 2001, 293, 1289–1292. [DOI] [PubMed] [Google Scholar]
- 79.Zheng G, Patolsky F, Cui Y, Wang WU, Lieber CM, Nat. Biotechnol 2005, 23, 1294–1301. [DOI] [PubMed] [Google Scholar]
- 80.Li P, Liu B, Zhang D, Sun Y, Liu J, Appl. Phys. Lett 2016, 109, 153101. [Google Scholar]
- 81.Thomas Scientific. Thomas Brand Mercury Ion Electrodes; https://www.thomassci.com/Instruments/Electrodes-pH/_/THOMAS-BRAND-MERCURY-ION-ELECTRODES (accessed Apr 28, 2019).
- 82.NICO2000. Method for determining the concentration of MERCURY (Hg++) in Aqueous Solutions; http://www.nico2000.net/analytical/mercury.htm (accessed Apr 26, 2019).
- 83.Li P, Zhang D, Jiang C, Zong X, Cao Y, Biosens. Bioelectron 2017, 98, 68–75. [DOI] [PubMed] [Google Scholar]
- 84.Hasan N, Hou B, Moore AL, Adv. Mater. Technol 2018, 3, 1800133. [Google Scholar]
- 85.Lee WD, Lee J, Sohn IY, Kim BY, Son YM, Bark H, Jung J, Choi M, Kim TH, Lee C, Lee NE, Nano Res. 2015, 8, 2340–2350. [Google Scholar]
- 86.Lee DW, Lee J, Sohn IY, Kim BY, Son YM, Bark H, Jung J, Choi M, Kim TH, Lee C, Lee NE, Nano Res. 2015, 8, 2340–2350. [Google Scholar]
- 87.Campos R, Borme J, Guerreiro JR, Machado G, Cerqueira MF, Petrovykh DY, Alpuim P, ACS Sens 2019, 4, 286–293. [DOI] [PubMed] [Google Scholar]
- 88.Rajan NK, Brower K, Duan X, Reed MA, Appl. Phys. Lett 2014, 104, 084106. [Google Scholar]
- 89.Rani D, Pachauri V, Ingebrandt S, Springer International Publishing, 2018, 27–57. [Google Scholar]
- 90.Namdari P, Daraee H, Eatemadi A, Nanoscale Res. Lett 2016, 11, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fadel T. Ed., National Science and Technology Council Committee on Technology: Washington, D.C., 2014. [Google Scholar]
- 92.Mannoor MS, Tao H, Clayton JD, Sengupta A, Kaplan DL, Naik RR, Verma N, Omenetto FG, McAlpine MC, Nat. Commun 2012, 3, 763. [DOI] [PubMed] [Google Scholar]
- 93.Guarnieri D, Moreno PS, Castillo AEDR, ´ Bonaccorso F, Gatto F, Bardi G, Martín C, Vazquez E, Catelani T, ´ Sabella S, Small 2018, 14, 1800227. [DOI] [PubMed] [Google Scholar]
- 94.Pandey A, Gurbuz Y, Ozguz V, Niazi JH, Biosens. Bioelectron 2017, 91, 225–231. [DOI] [PubMed] [Google Scholar]
- 95.Ono T, Kanai Y, Inoue K, Watanabe Y, Nakakita SI, Kawahara T, Suzuki Y, Matsumoto K, Electrical Nano Lett. 2019, 19, 4004–4009. [DOI] [PubMed] [Google Scholar]
- 96.Hinnemo M, Makaraviciute A, Ahlberg P, Olsson J, Zhang Z, Zhang SL, Zhang ZB, IEEE Sens. J. 2018, 18, 6497–6503. [Google Scholar]
- 97.Chu CH, Sarangadharan I, Regmi A, Chen YW, Hsu CP, Chang WH, Lee GY, Chyi JI, Chen CC, Shiesh SC, Lee GB, Wang YL, Sci. Rep 2017, 7, 5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao N, Zhou W, Jiang X, Hong G, Fu TM, Lieber CM, Nano Lett. 2015, 15, 2143–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lim JY, Nam JS, Yang S, Shin H, ha Jang Y, Un Bae G, Kang T, il Lim K, Choi Y, Anal. Chem 2015, 87, 23, 11652–11659. [DOI] [PubMed] [Google Scholar]
- 100.Yeha YT, Gulino K, Zhang YH, Sabestien A, Chou TW, Zhou B, Lin Z, Albert I, Lu H, Swaminathan V, Ghedin E, Terrones M, PNAS, 2020, 117, 895–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Park HJ, Yang SC, Choo J, Korean Chem. Soc 2016, 37, 2019–2024. [Google Scholar]
- 102.Kukushkin VI, Ivanov NM, Novoseltseva AA, Gambaryan AS, Yaminsky IV, Kopylov AM, Zavyalova EG, PLoS ONE, 2019, 14, e0216247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ling X, Xie L, Fang Y et al. , Nano Letters, 2010, 10, 553–561. [DOI] [PubMed] [Google Scholar]
- 104.Ling X, Wu J, Xie L, Zhang J, J. Phys. Chem. C 2013, 117, 2369. [Google Scholar]
- 105.Ling X, Xie L, Fang Y, Xu H, Zhang H, Kong J, Dresselhaus MS, Zhang J, Liu Z, Nano Lett. 2010, 10, 553. [DOI] [PubMed] [Google Scholar]
- 106.Das SR, Nian Q, Saei M, Jin S, Back D, Kumar P, Janes DB, Alam MA, Cheng GJ, ACS Nano 2015, 9, 11121–11133. [DOI] [PubMed] [Google Scholar]
- 107.Hu Y, Kumar P, Yi X, Deng B, Qi M, and Cheng GJ, Adv. Optical Mater 2016, 4, 1811–1823. [Google Scholar]
- 108.Hu Y, Lee S, Kumar P, Nian Q, Wang W, Irudayaraj J and Cheng GJ, Nanoscale, 2015, 7, 19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee S, Kumar P, Hu Y, Cheng GJ and Irudayaraj J, Chem. Commun, 2015, 51, 15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kumar P, Liu J, Motlag M, Tong L, Hu Y, Huang X, Bandopadhyay A, Pati SK, Ye L, Irudayaraj J, Cheng GJ, Nano Lett. 2019, 19, 283–291. [DOI] [PubMed] [Google Scholar]
- 111.Spackman E, Methods Mol Biol. 2008, 436, 1–6, [DOI] [PubMed] [Google Scholar]
- 112.Zerboni L, Sen N, Oliver SL, Arvin AM, Nat Rev Microbiol. 2014, 12, 197–210, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.shereen MA, Khan S, Kazmi A, Bashir N, Siddique R, Journal of Advanced Research 2020, 24, 91–98, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aleksandrowicz P, Marzi A, Biedenkopf N, Beimforde N, Becker S, Hoenen T, Feldmann H, and Schnittler H-J, Ebola Virus Entry, JID 2011, 204, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muyangwa M, Martynova EV, Khaiboullina S SF. Morzunov P, Rizvanov AA, Frontiers in Microbiology, 2013, 6, 1326, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gürtler L et al. Transfus Med Hemother 2016, 43, 203–222, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lund GA, Tyrrell DLJ, Bradley RD, Scraba DG, J. gen. Virol 1984, 65, 1535–1542, [DOI] [PubMed] [Google Scholar]
- 118.Rubin S, Eckhaus M, Rennick LJ, Bamford CGG, Duprex WP, J Pathol. 2015, 235, 242–252, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Institute of Medicine (US) Committee on the Assessment of Future Scientific Needs for Live Variola Virus. Assessment of Future Scientific Needs for Live Variola Virus. Washington (DC): National Academies Press (US); 1999. https://www.ncbi.nlm.nih.gov/books/NBK230919/ doi: 10.17226/6445, [DOI] [PubMed] [Google Scholar]
- 120.Bar-on YM, Flamholz A, Phillips R, Milo Ron, eLife 2020, 9, e57309, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jilani TN, Jamil RT, Siddiqui AH https://www.ncbi.nlm.nih.gov/books/NBK513241,
- 122.Boron S, https://www.ncbi.nlm.nih.gov/books/NBK7627/?report=reader [Google Scholar]
- 123.Rao CNR, Subrahmanyam KS, Matte HSSR, Abdulhakeem B, Govindaraj A, Das B, Kumar P, Ghosh A, Late DJ, Sci. Technol. Adv. Mater 2010, 11, 054502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Carriere M, Fusco L, Capua I, Nava JAR, Pasquali M, Scott JA, Vitale F, Unal MA, Mattevi C, Bedognetti D, Merkoci A,¸ Tasciotti E, Yilmazer A, Gogotsi Y, Stellacci F, Delogu LG, ACS Nano 2020, 14, 6383–6406. [DOI] [PubMed] [Google Scholar]
- 125.Lovato A, Filippis C. d., Ear Nose Throat J. 2020, No. 014556132092076. [Google Scholar]
- 126.Palmieri V, Papi M, Nano Today, 2020, 33, 100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Junior JACN, Santos AM, Júnior LJQ, Walke CIB, Borgesa LP, Serafini MR, Expert Opinion On Therapeutic Patents, 2020, 30, 567–579. [DOI] [PubMed] [Google Scholar]
- 128.Parihar A, Ranjan P, Sanghi SK, Srivastava AK, Khan R, ACS Appl. Bio Mater. 2020, 3, 7326–7343. [DOI] [PubMed] [Google Scholar]
- 129.Hitzky ER, Darder M, Wicklein B, Garcia CR, Sampedro RM, Aranda GRP, Adv. Healthcare Mater 2020, 9, 2000979. [DOI] [PubMed] [Google Scholar]
- 130.Sharma S, Saini S, Khangembam M, Singh V, IEEE Sensors Journal, 2020, 10.1109/JSEN.2020.3036748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Samson R, Navale GR, & Dharne MS Biosensors: frontiers in rapid detection of COVID-19. 3 Biotech, 2020, 10, 385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Antiochia R, Microchim Acta, 2020, 187, 639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Srivastava AK, Dwivedi N, Dhand C, Khan R, Sathish N, Gupta MK, Kumar R, Kumar S, Materials Today Chemistry, 2020, 18, 100385. [DOI] [PMC free article] [PubMed] [Google Scholar]