Abstract
Convergent extension is a conserved mechanism for elongating tissues. In the Drosophila embryo, convergent extension is driven by planar polarized cell intercalation and is a paradigm for understanding the cellular, molecular, and biophysical mechanisms that establish tissue structure. Studies of convergent extension in Drosophila have provided key insights into the force-generating molecules that promote convergent extension in epithelial tissues, as well as the global systems of spatial information that systematically organize these cell behaviors. A general framework has emerged in which asymmetrically localized proteins involved in cytoskeletal tension and cell adhesion direct oriented cell movements, and spatial signals provided by the Toll, Tartan, and Teneurin receptor families break planar symmetry to establish and coordinate planar cell polarity throughout the tissue. In this chapter, we describe the cellular, molecular, and biophysical mechanisms that regulate cell intercalation in the Drosophila embryo, and discuss how research in this system has revealed conserved biological principles that control the organization of multicellular tissues and animal body plans.
1. Convergent extension: A conserved mechanism for shaping epithelia
A conserved feature of animal development is the formation of a body axis that is elongated from head to tail. This characteristic structure of the body plan is generated by dynamic cell movements that cause the embryo to narrow along one axis and elongate along a perpendicular axis, a process known as convergent extension. Convergent extension can occur through a wide range of cell behaviors, including polarized cell divisions, stereotyped changes in cell shape, and oriented cell movements. In particular, cell intercalation is an essential mechanism for convergent extension during body axis elongation in frogs, fish, flies, chicks, worms, and mice (Keller et al., 2000; Wallingford et al., 2002; Takeichi, 2014; Walck-Shannon and Hardin, 2014; Kong et al., 2017). First described in migratory mesenchymal cells in the Xenopus embryo (Keller et al., 2000; Wallingford et al., 2002), it is now appreciated that even highly adherent epithelial cells can dynamically remodel to drive tissue elongation. Studies of convergent extension in Drosophila have uncovered the force-generating machinery and tissue-wide spatial information systems that link axial patterning to polarized cell behavior. In this chapter, we describe the cellular, molecular, and biophysical mechanisms that regulate epithelial cell intercalation in the Drosophila germband, and we discuss outstanding questions in the field regarding the molecular basis of planar symmetry breaking and how biochemical and biophysical processes converge to control epithelial organization.
2. Cell rearrangements during convergent extension in the Drosophila embryo
A premier model for studying convergent extension is elongation of the Drosophila germband epithelium (Figure 1A). Pioneering mutagenesis screens carried out by Eric Wieschaus and Christiane Nüsslein-Volhard shed light on the upstream molecular cues that pattern the germband and surrounding tissues (Nüsslein-Volhard and Wieschaus, 1980; Wieschaus and Nüsslein-Volhard, 2016). Since then, elegant studies by many labs have revealed how networks of transcriptional enhancers and repressors generate complex patterns of gene expression from relatively simple starting inputs. However, a major challenge in the field has been to understand how these gene expression patterns induce changes in cell shape and behavior to yield proper embryo morphology.
Figure 1. Cell rearrangements during Drosophila germband extension.
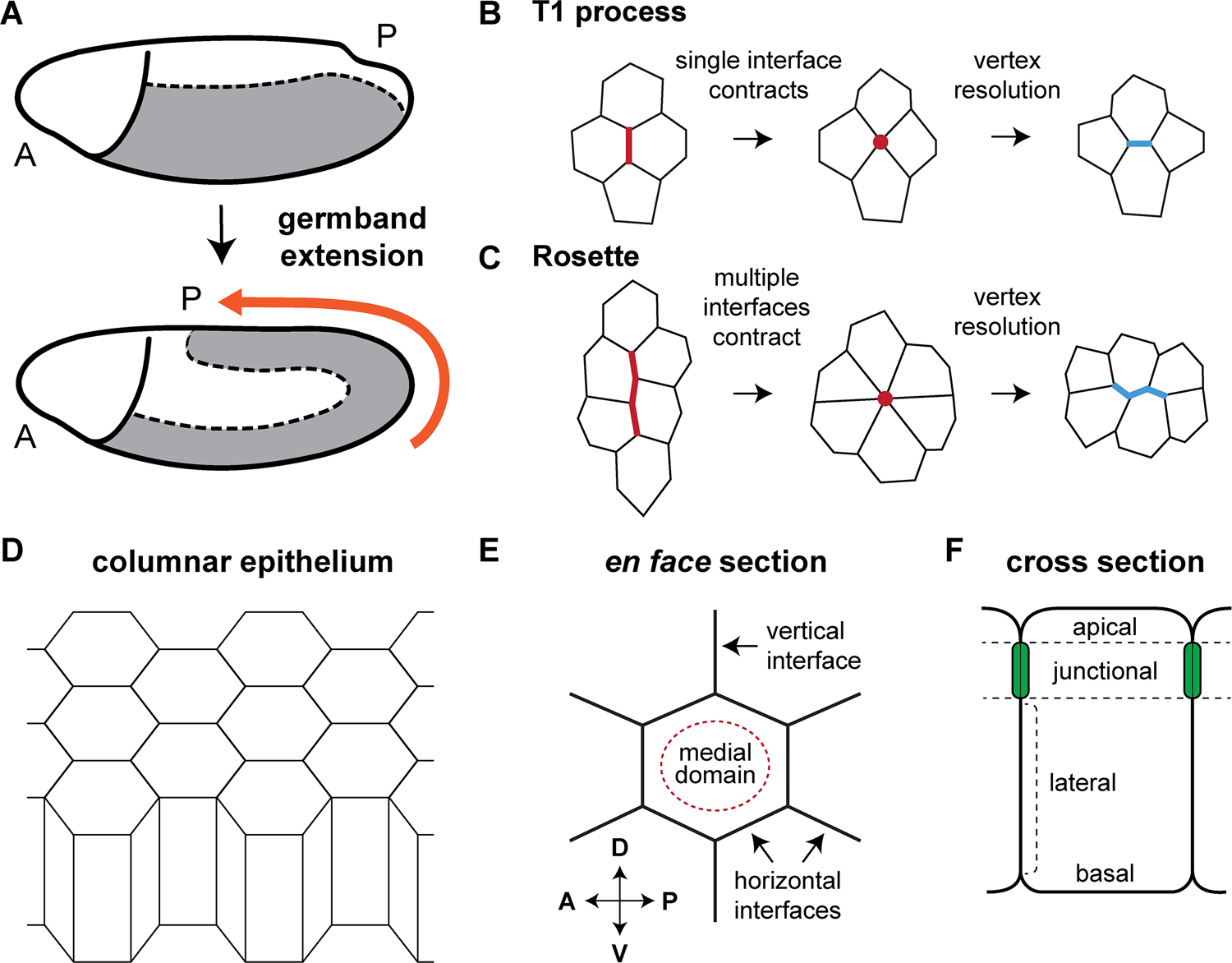
(A) Convergent extension in the germband ectoderm (gray) elongates the anterior-posterior (AP) body axis. (B) T1 processes occur through the contraction of a single vertical cell interface. (C) Multicellular rosettes form through the contraction of 2 interfaces (for a 5-cell rosette), 3 interfaces (for a 6-cell rosette), or 4 or more interfaces (for rosettes containing 7 or more cells). (D) The germband ectoderm is a simple columnar epithelium. (E) An en face view of showing one hexagonal cell and its neighbors. (F) Cross-sectional view showing different cellular domains along the apical-basal axis.
The germband ectoderm is a single-layered columnar epithelium on the ventrolateral surface of the developing embryo (Figure 1A and D–F) that differentiates into a wide range of tissues, including the nervous system, epidermis, airways, and imaginal discs. Several characteristics make the germband ectoderm an attractive system for studying the cellular basis of convergent extension, including 1) the relatively simple columnar organization of the epithelium, 2) the absence of cell division during most of this process, and 3) the ease with which cell behaviors can be visualized by microscopy. The Drosophila germband more than doubles in length along the AP axis over the course of approximately 2 hours (Figure 1A), with most of this elongation achieved during an initial, 30-minute fast phase. Time-lapse epi-illumination movies of ectodermal cells demonstrated that the fast phase of elongation occurs primarily through cell intercalation, in which cell movements oriented along the dorsal-ventral (DV) axis drive tissue elongation along the anterior-posterior (AP) axis (Irvine and Wieschaus, 1994). Importantly, mutations that eliminate mesodermal cell fates do not alter the rate or extent of elongation, indicating that axis elongation is driven solely by cell behaviors in the ectoderm (Irvine and Wieschaus, 1994).
Direct visualization of cell shapes during germband extension using fluorescent markers revealed several unexpected cell behaviors. Unlike mesenchymal cells, which intercalate largely by crawling on neighboring cells using spatially regulated protrusions (see chapters “Convergent extension in the amphibian, Xenopus laevis” by Keller and Sutherland; “Cellular and molecular mechanisms of convergence and extension in zebrafish” by Williams and Solnica-Krezel), intercalating epithelial cells in the germband rearrange by remodeling cell-cell junctions in a spatially regulated manner (Figure 1B and C). Cell-cell interfaces oriented perpendicular to the direction of tissue elongation (vertical interfaces) contract to form four-cell vertices, which resolve to produce a new cell-cell interface oriented parallel to the AP axis (horizontal interfaces) (Bertet et al., 2004) (Figure 1B). During each rearrangement, a pair of adjacent cells along the AP axis becomes separated, and two cells that were previously separated come into contact. These local cell rearrangements are referred to as neighbor exchange events or T1 processes, following the convention first introduced for two-dimensional foams (Weaire and Rivier, 1984).
Early models proposed that simple T1 processes within an ordered, hexagonal cell array are sufficient to account for tissue elongation (Bertet et al., 2004). However, it is now known that germband cells are not strictly hexagonal and display more complex behaviors (Zallen and Zallen, 2004). In particular, germband cells assemble into multicellular rosette structures that form and resolve directionally and are necessary for tissue elongation (Blankenship et al., 2006). During rosette formation, connected vertical interfaces contract to bring five or more cells into contact at a single point or vertex. This vertex then resolves in the perpendicular direction, transforming two columns of cells into two rows of cells (Figure 1C) (Blankenship et al., 2006). Mutants that assemble fewer rosettes, with no change in the number of T1 processes, display significantly reduced elongation, demonstrating that rosette behaviors are required for convergent extension (Tamada et al., 2012). Since the discovery of the rosette mechanism in Drosophila, rosette-based intercalation has now been shown to be a general mechanism for convergent extension in flies, worms, chicks, frogs, and mice (Nishimura and Takeichi, 2008; Chacon-Heszele et al., 2012; Lienkamp et al., 2012; Williams et al., 2014; Lau et al., 2015; McGreevy et al., 2015; Rozbicki et al., 2015; Shah et al., 2017; Sun et al., 2017).
3. The molecular basis of epithelial cell intercalation
Intercalary behaviors must ultimately be propelled by proteins that are asymmetrically localized within the plane of the tissue, a property known as planar cell polarity, but the molecular nature of these asymmetries has long been mysterious. Key findings in the Drosophila germband demonstrate that cell intercalation is driven by a conserved set of planar polarized cytoskeletal and junctional proteins that modulate cortical tension and cell adhesion in distinct cellular domains (Figure 2), directly linking cellular force-generating machineries to polarized cell behavior.
Figure 2. Planar polarity in the Drosophila germband.
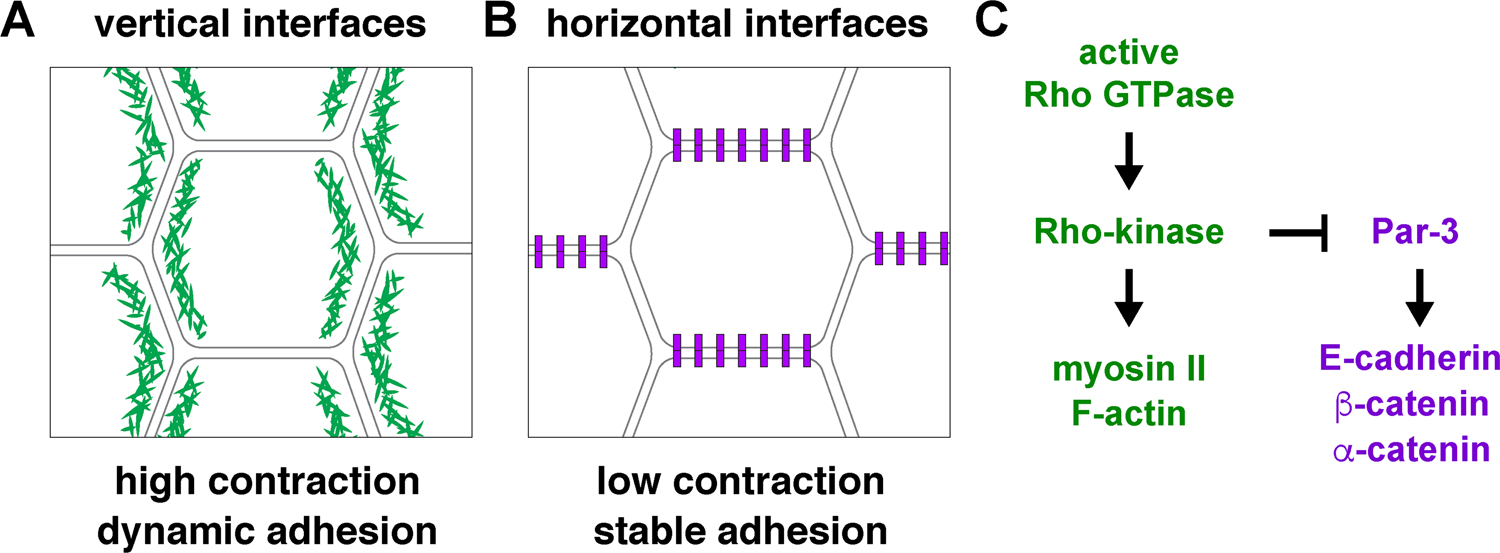
(A) Vertical cell interfaces in the germband display increased localization or activity of proteins involved in promoting cortical tension, adherens junction turnover, and endocytosis. (B) Horizontal interfaces are enriched for proteins involved in cell adhesion. (C) Rho-kinase activity at vertical interfaces directs multiple aspects of planar polarity.
The contractile domain of intercalating cells is established by the localized recruitment of nonmuscle myosin II (referred to here as myosin), a motor protein that binds to, crosslinks, and translocates along actin filaments (Bertet et al., 2004; Zallen and Wieschaus, 2004). In addition, a complementary adhesive domain is defined by Par-3, a PDZ protein that associates with and stabilizes adherens junctions (Zallen and Wieschaus, 2004; Simões et al., 2010). The loss of either protein leads to severe defects in axis elongation, demonstrating that these molecules are essential for polarized cell behavior (Bertet et al., 2004; Zallen and Wieschaus, 2004; Simões et al., 2010). Subsequent studies revealed that these patterns reflect a general segregation of the contractile and adhesive machinery within cells (Figure 2), as actin filaments colocalize with myosin at vertical interfaces (Blankenship et al., 2006), and the adherens junction proteins E-cadherin, α-catenin, and β-catenin are enriched at horizontal interfaces (Blankenship et al., 2006; Simoes et al., 2010; Levayer et al., 2011; Tamada et al., 2012; Levayer and Lecuit, 2013; Warrington et al., 2013). These results suggest a model in which cell intercalation is driven by complementary cellular domains specialized for contraction or adhesion, a molecular framework that has now been shown to be generalizable to many epithelial contexts.
The proof that planar polarized actomyosin contractility is necessary for cell intercalation came from the use of laser ablation methods to analyze cortical tension (Hutson et al., 2003; Farhadifar et al., 2007). Biophysical studies using this approach revealed that vertical interfaces are under higher tension than horizontal interfaces, proving that planar polarized myosin localization translates into planar polarized contractile forces (Rauzi et al., 2008; Fernandez-Gonzalez et al., 2009). Since its discovery in Drosophila, planar polarized actomyosin contractility is now recognized to drive convergent extension in many tissues, including the chick and mouse neural plate (Nishimura and Takeichi, 2008; Nishimura et al., 2012; Williams et al., 2014), the mouse cochlea and limb bud (Chacon-Heszele et al., 2012; Lau et al., 2015), the mouse and frog kidney (Lienkamp et al., 2012), the Xenopus and zebrafish notochord (Shindo and Wallingford, 2014; Williams et al., 2018), the chick primitive streak (Rozbicki et al., 2015), and the C. elegans nervous system (Shah et al., 2017).
The critical role of actomyosin contractility in driving cell intercalation raises the question of how planar polarized patterns of myosin localization and activity are established in the tissue. The complementary distributions of contractile and adhesive proteins in the germband are generated by a single serine-threonine protein kinase, Rho-kinase, which is asymmetrically localized to vertical interfaces and is essential for intercalary behavior (Simões et al., 2010). Rho-kinase regulates planar polarity in two ways. First, Rho-kinase phosphorylates the myosin regulatory light chain, enhancing myosin localization and activity at vertical edges (Simões et al., 2010; Kasza et al., 2014). In addition, Rho-kinase phosphorylates Par-3 and inhibits Par-3 association with the cell cortex at vertical edges (Simões et al., 2010), likely by preventing its interaction with phosphoinositide membrane lipids (Krahn et al., 2010). Par-3 in turn enhances cell-cell adhesion at horizontal interfaces (Simões et al., 2010). Thus, Rho-kinase is a critical regulator of planar polarity that has direct, opposing effects on contraction and adhesion at vertical interfaces.
The upstream signals that regulate Rho-kinase localization during cell intercalation are less well understood, but evidence points to an essential role for the small GTPase Rho, a conserved activator of Rho-kinase activity and actomyosin contraction in many contexts (Jaffe and Hall, 2005). The effects of Rho are difficult to test genetically, due to its pleiotropic requirements in many aspects of embryogenesis, but dominant-negative Rho abolishes planar polarity in the germband (Simões et al., 2014) and activity probes designed to detect active Rho are enriched at contracting cell interfaces during germband elongation (Simões et al., 2014; Munjal et al., 2015; Garcia De Las Bayonas et al., 2019). Elucidating the upstream mechanisms that direct localized Rho-kinase activity to establish planar cell polarity in the germband remains an important question in the field.
Several other mechanisms contribute to the planar polarized distribution of adhesion complexes during cell intercalation. The Abl tyrosine kinase localizes to vertical interfaces and enhances junctional turnover by phosphorylating β-catenin (Tamada et al., 2012). Proper localization of E-cadherin requires the glucosyltransferase Xiantuan (Zhang et al., 2014). Finally, components of the membrane trafficking machinery, including clathrin and dynamin (Levayer et al., 2011), and the Rab GTPase Rab35 (Jewett et al., 2017), promote the localized endocytosis of membrane and adherens junction complexes at vertical edges, resulting in edge shortening and cell separation.
Once planar polarized contraction and adhesion are established, positive and negative interactions dynamically reinforce planar polarity. For example, the F-actin-binding protein Shroom is required to maintain Rho-kinase planar polarity at later stages of intercalation (Simões et al., 2014), and Par-3 is required for the planar polarized localization of myosin (Simões et al., 2010). In addition, dynamic myosin flows along the medial (apical) cell cortex are oriented by fluctuations in E-cadherin levels (Levayer and Lecuit, 2013) and are associated with changes in apical cell area (Fernandez-Gonzalez and Zallen, 2011; Sawyer et al., 2011), edge contraction (Rauzi et al., 2010; Levayer and Lecuit, 2013; Munjal et al., 2015), and new edge formation (Collinet et al., 2015; Yu and Fernandez-Gonzalez, 2016). Finally, cells that undergo changes in angular orientation as they rearrange can reorganize Par-3 and myosin polarity to match their new orientation, revealing that planar polarity mechanisms display an unexpected plasticity (Farrell et al., 2017). In addition, vertex sliding (Vanderleest et al., 2018) and basolateral cellular protrusions (Sun et al., 2017) also contribute to intercalation and may be controlled by distinct molecular mechanisms. How these diverse cell biological processes are dynamically and coordinately controlled to establish and maintain planar polarized behaviors in the apical and basal domains of the cell is an important area for future study.
4. Biophysical control of epithelial cell intercalation
Epithelial cells are connected by adherens junctions that facilitate the transmission of mechanical forces from cell to cell. Therefore, a unique feature of cell intercalation in epithelia is that actomyosin forces generated in one cell can propagate to neighboring cells and influence their behavior. Indeed, laser ablation studies demonstrate that germband cells can respond to physical changes that occur several cell diameters away (Fernandez-Gonzalez et al., 2009; Wang et al., 2017), and genetic and biophysical experiments show that cells respond to mechanical forces generated in neighboring tissues (Collinet et al., 2015; Lye et al., 2015). An emerging picture is that physical forces provide an additional tier of mechanical regulation that influences cell behavior during intercalation.
Myosin dynamics are sensitive to mechanical forces in single cells (Effler et al., 2006) and multicellular epithelia (Fernandez-Gonzalez et al., 2009; Pouille et al., 2009). During cell intercalation, cortical myosin turnover is selectively decreased in regions of high tension, resulting in the localized stabilization and accumulation of myosin (Fernandez-Gonzalez et al., 2009). Moreover, an applied force can recruit myosin to the cortex within tens of seconds (Fernandez-Gonzalez et al., 2009). These results demonstrate a positive feedback loop in which myosin generates cortical tension, which in turn recruits and stabilizes more myosin at the cortex. This mechanical feedback loop provides a mechanism by which tension from neighboring cells influences myosin contractility in a non-cell-autonomous manner, contributing to the formation of supracellular myosin cables that contract to form rosettes (Blankenship et al., 2006; Fernandez-Gonzalez et al., 2009).
In contrast to the planar polarized contractile structures that mediate cell interface contraction during intercalation, the mechanisms that control the direction of vertex resolution to promote productive cell rearrangement are less well understood. Pulsatile myosin flows along the medial cell surface are often observed in epithelial cells (Martin et al., 2009), and during cell intercalation, these flows are biased along the AP axis (Rauzi et al., 2010; Levayer and Lecuit, 2013). It has been proposed that medial myosin flows create local, nonautonomous AP-oriented pulling forces that influence the rate and direction of new interface formation (Collinet et al., 2015; Lan et al., 2015; Yu and Fernandez-Gonzalez, 2016). Consistent with this possibility, regulated actomyosin activity is necessary for directional vertex resolution (Kasza et al., 2014; Yu and Fernandez-Gonzalez, 2016), and laser ablation experiments demonstrate that nonautonomous forces can influence the orientation of new interface formation (Collinet et al., 2015; Yu and Fernandez-Gonzalez, 2016). These results raise the intriguing possibility that myosin-dependent mechanical forces directly regulate multiple steps of cell intercalation.
In addition to the transmission of mechanical forces between germband cells, forces that are transmitted between tissues can also influence cell intercalation. Notably, invagination of the posterior midgut induces a strong pulling force in the direction of tissue extension that contributes to elongation (Collinet et al., 2015; Lye et al., 2015), and mesoderm invagination imparts an orthogonal pulling force that causes transient cell-shape deformations (Butler et al., 2009; Farrell et al., 2017). Conversely, forces generated in the germband can affect cell behaviors in surrounding tissues, such as the anisotropic apical constriction of cells in the mesoderm (Martin et al., 2010) and the orientation of cell divisions in the mesectoderm (Wang et al., 2017). The ability to independently modulate forces in distinct tissues using genetic patterning mutations and laser ablation makes the Drosophila embryo a particularly attractive model for addressing the biomechanical relationships between cells and tissues in vivo.
5. Breaking planar symmetry
Despite significant strides in understanding the cellular, molecular, and biophysical processes that drive cell intercalation, the spatial cues that orient and coordinate planar polarity across the hundreds of cells of the germband had long been elusive. An important clue came from the discovery that germband extension requires inputs from the AP-patterning system (Irvine and Wieschaus, 1994). In particular, mutants lacking transcription factors encoded by gap or pair-rule genes exhibit a strong reduction in cell intercalation in discrete regions where these factors are normally present (Irvine and Wieschaus, 1994). By contrast, embryos with mutations that disrupt DV patterning, segment polarity genes (e.g., Wingless), or classical planar cell polarity signaling (Frizzled/Van Gogh) undergo robust cell intercalation and convergent extension (Irvine and Wieschaus, 1994, Zallen and Wieschaus, 2004). In addition, local differences in the expression of the pair-rule genes eve and runt are necessary and sufficient for planar cell polarity (Zallen and Wieschaus, 2004). These results indicate that striped patterns of gene expression along the AP axis orient cell intercalation (Wieschaus et al., 1991). However, it was two decades before the targets of the AP patterning system that break planar symmetry and align cell polarity with the body axes were identified.
Studies of striped proteins in the germband, combined with genome engineering approaches to knock out multiple genes simultaneously, revealed that two independent cell-surface receptor systems act downstream of the pair-rule genes to direct planar polarity (Figure 3) (Paré et al., 2014, 2019). The first system consists of three members of the Toll receptor family, Toll-2, Toll-6, and Toll-8, that direct actomyosin and adherens junction polarity and cell intercalation throughout the germband (Paré et al., 2014). The second system, involving the leucine-rich repeat (LRR) receptor Tartan and the teneurin Ten-m, controls planar polarity specifically at compartment (parasegment) boundaries (Paré et al., 2019). The discovery of these two systems has yielded a surprisingly complex answer to how patterned gene expression organizes cell behavior during convergent extension in the Drosophila germband.
Figure 3. Receptor systems mediating planar polarity in the germband.
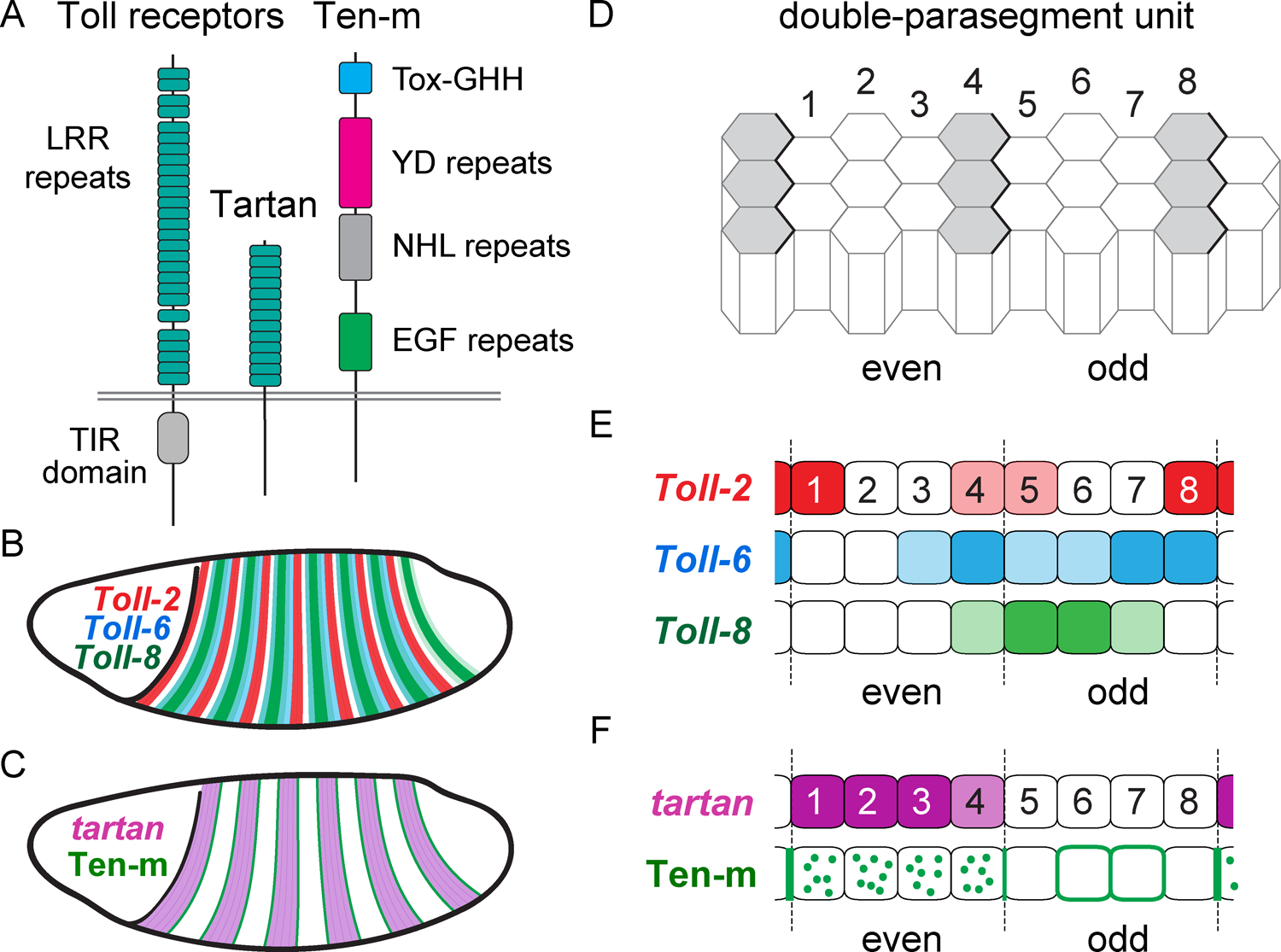
(A) Schematics of Toll receptors, Tartan, and Ten-m (not to scale). (B) Toll-2, Toll-6, and Toll-8 are expressed in staggered striped patterns in the germband. C) tartan (purple) is expressed in stripes and Ten-m protein (green) is enriched at the stripe borders. (D) The germband is organized into double-parasegment units, each made up of seven or eight columns of cells. Even parasegments (cells 1–4), odd parasegments (cells 5–8). Wingless-expressing cells are shown in gray. (E) Toll receptor expression within the double-parasegment unit. (F) Tartan expression and Ten-m localization within the double-parasegment unit. Ten-m is enriched at compartment boundaries and is absent from the membrane in Tartan-positive cells.
6. Toll receptors direct planar polarity and cell intercalation
The discovery that Toll-2, Toll-6, and Toll-8 are required for planar polarity and intercalary behavior during germband extension revealed the long-sought link between the transcriptional events that pattern the AP axis of the embryo and the morphogenetic processes that convert this pattern into changes in tissue structure. The absence of all three Toll-related receptors causes strong defects in planar polarity, cell intercalation, and axis elongation (Paré et al., 2014). This new morphogenetic role for Toll receptors in convergent extension was subsequently shown to be conserved in other arthropods (Benton et al., 2016). Toll family receptors are transmembrane proteins that contain extracellular LRRs and an intracellular TIR (Toll/interleukin-1 receptor) domain that signals to downstream effectors (Figure 3A). The founding member of this family, Toll, was originally discovered as a critical component of the DV patterning system in Drosophila (Anderson et al., 1985a; Anderson et al., 1985b; Hashimoto et al., 1998). This receptor family was subsequently shown to be essential for innate immunity in Drosophila and vertebrates (Anderson, 2000). The roles of Drosophila Toll and vertebrate Toll-like receptors in innate immunity have been the subject of intense study (Leulier and Lemaitre, 2008; Kawasaki and Kawai, 2014), but developmental roles of Toll family receptors, beyond DV patterning, have received comparably less attention.
Toll-2, Toll-6, and Toll-8 are expressed in non-uniform patterns in the germband, placing them in a position to act as the cellular symmetry-breaking molecules that organize planar cell polarity. The Drosophila germband is transcriptionally subdivided into a series of repeating, double-parasegment units along the AP axis that each span approximately eight columns of cells (Figure 3D) (Akam, 1987; Clark, 2017). Toll-2, Toll-6, and Toll-8 are expressed in distinct patterns within this double-parasegment unit (Figure 3B,E) under the control of pair-rule genes (Chiang and Beachy, 1994; Eldon et al., 1994; Paré et al., 2014; Graham et al., 2019). Toll-2 is expressed in alternating major and minor stripes, whereas Toll-8 is expressed in fewer, broader stripes. Toll-6 has the most complex pattern, largely overlapping with Toll-8 but with differing regions of low and high expression. Thus, every column of cells within each double-parasegment unit has a distinct complement of Toll receptor types and expression levels (Paré et al., 2014; Paré et al., 2019). Notably, the loss of one or two Toll receptors disrupts planar polarity in different regions of the double-parasegment unit, and ectopic stripes of Toll receptor expression are sufficient to induce myosin planar polarity (Paré et al., 2014; Paré et al., 2019). These observations suggest that the juxtaposition of cells that express different levels or combinations of Toll receptors is a critical symmetry-breaking event in the establishment of planar polarity.
The expression of Toll receptors in striped patterns acts as a bridge between axial patterning information and polarized cell behavior. However, the mechanisms that convert local differences in receptor expression into planar polarity are not known. Here we consider two classes of models that could explain how striped Toll receptor expression leads to planar polarity, either through interactions between different types of Toll receptors (heterotypic models) or independent Toll receptor functions (single-component models). In a heterotypic activation model, trans interactions between different Toll receptors expressed in neighboring columns of cells could activate Toll receptors and myosin contractility at vertical cell interfaces (Figure 4A). In this model, Toll receptors serve as both ligand and receptor, eliminating the requirement for other patterned activators. Consistent with a heterotypic activation model, cells expressing Toll-2 interact heterotypically with cultured Drosophila cells expressing Toll-6 or Toll-8 (Paré et al., 2014). However, the heterotypic model does not explain why receptor activation does not occur at horizontal interfaces, as most cells in the germband express multiple receptor types and the subcelullar localization of Toll receptors has not been determined. In addition, there is a strong genetic argument against the heterotypic model. Namely, if heterotypic interactions are required for receptor activity, then a double mutant lacking two Toll receptors should be just as defective as a triple-mutant embryo lacking all three. However, triple mutants are generally more defective than double-mutant combinations (Paré et al., 2014). Thus, although heterotypic interactions may play some role in Toll receptor signaling during convergent extension, they cannot be strictly required for receptor function.
Figure 4. Models for how striped Toll receptors generate planar polarity.
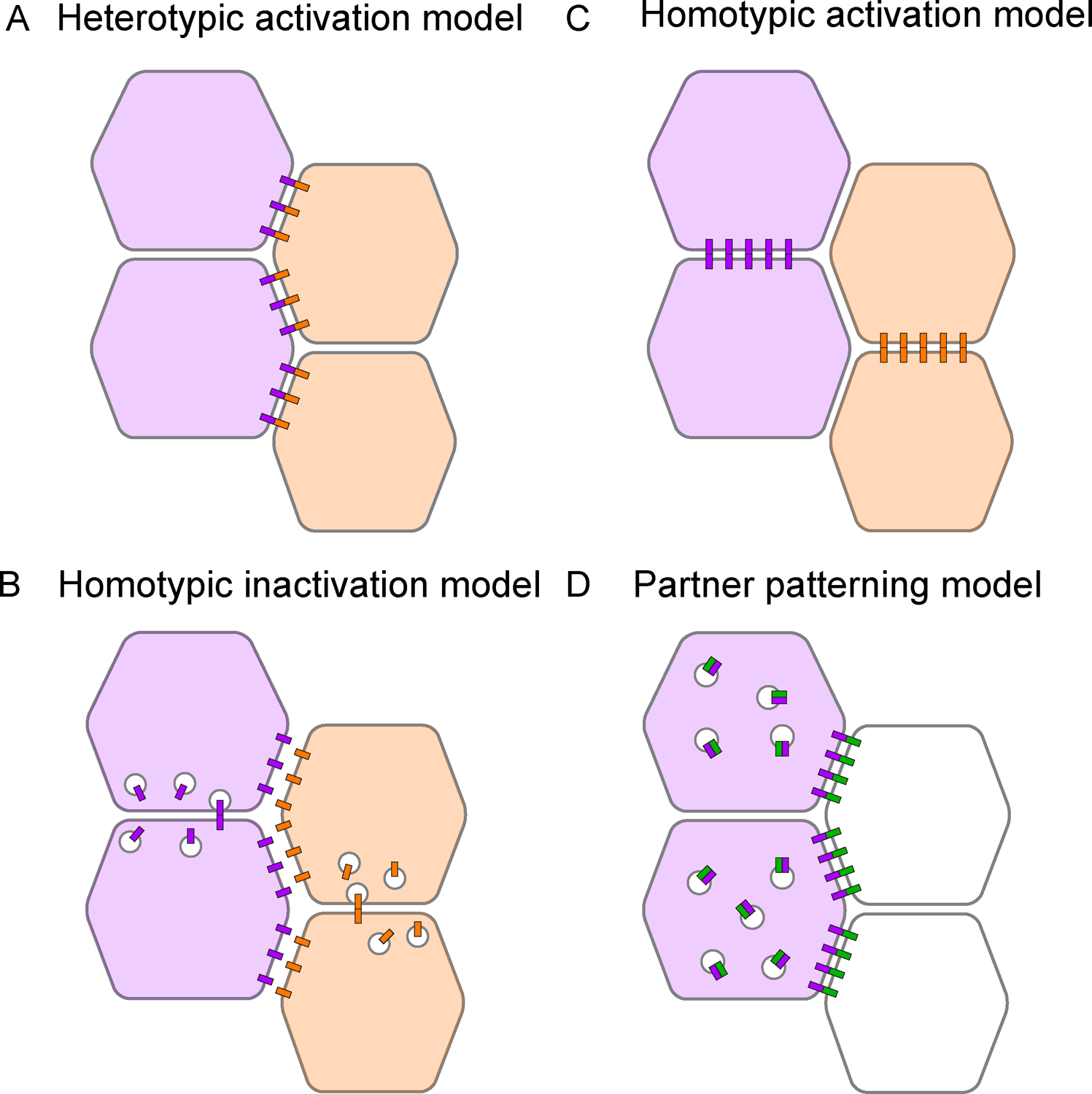
(A) In a heterotypic activation model, trans interactions between cells expressing different Toll receptors (purple and orange) in adjacent stripes could stabilize Toll receptors at vertical interfaces. (B) In a homotypic inactivation model, trans interactions between cells expressing the same Toll receptor type could destabilize Toll receptors at horizontal interfaces. (C) In a homotypic activation model, trans interactions between cells expressing the same Toll receptor type could stabilize receptors at horizontal interfaces. (D) In a partner patterning model, a patterned Toll receptor (purple) and an unpatterned interaction partner (green) could undergo inhibitory cis interactions and stabilizing trans interactions, promoting the enrichment of the partner at vertical interfaces along the Toll stripe border.
By contrast, single-component models propose that each Toll receptor type functions independently of the others. Single-component models could work in a variety of ways. In a homotypic inactivation model, inhibitory trans interactions between Toll receptors expressed in the same stripe could downregulate Toll receptors at horizontal cell interfaces (Figure 4B). Alternatively, in a homotypic activation model, activating trans interactions between Toll receptors in the same stripe could stabilize Toll receptors at horizontal cell interfaces (Figure 4C). In both models, Toll receptors would become planar polarized through homotypic interactions, and would go on to promote planar polarity by influencing either actomyosin contractility (Figure 4B) or cell adhesion (Figure 4C). Finally, in a partner-patterning model, Toll receptors could pattern the localization or activity of a ubiquitous interaction partner, causing it to become differentially localized in Toll-expressing and Toll-nonexpressing cells (Figure 4D). Such a mechanism was recently demonstrated for Tartan and Ten-m in the Drosophila germband extension (see below) (Paré et al., 2019). A significant advantage of single-component models is that they do not require pre-patterned interaction partners to trigger planar polarity. Instead, the capacity of Toll receptors to induce planar polarity is inherent in their striped expression patterns. Although there is currently no direct evidence for single-component models of Toll receptor activity, genetic evidence supports the idea of independent receptor functions (Paré et al., 2014). Insight into where Toll receptors are localized and active within cells will help to distinguish between these models.
Toll receptors display complex and nonuniform expression patterns in a variety of tissues throughout Drosophila development, including the embryonic head and germband (Chiang and Beachy, 1994; Eldon et al., 1994; Kambris et al., 2002; Paré et al., 2014) and the wing and leg imaginal discs (Kim et al., 2006; Yagi et al., 2010). These results raise the possibility that Toll receptors could influence cell polarity and behavior in other tissues. Consistent with their widespread expression, Toll receptor mutants have defects in the structure and organization of Drosophila appendages (Eldon et al., 1994; Yagi et al., 2010), follicle cells (Kleve et al., 2006), salivary glands (Kolesnikov and Beckendorf, 2007), and nervous system (Ballard et al., 2014; McIlroy et al., 2013; Ward et al., 2015; Foldi et al., 2017). In addition to their roles in development, Toll is required for wound repair in the late embryonic epidermis (Carvalho et al., 2014), and Toll-2, Toll-3, Toll-8, and Toll-9 regulate cell competition in wing imaginal discs (Meyer et al., 2014). A better understanding of how Toll receptors mediate cell polarity and behavior in the germband will provide insight into their roles in diverse biological processes involving cell and tissue remodeling.
7. Regulation of planar polarity at compartment boundaries
Compartment boundaries are physical structures that delineate distinct domains within epithelial sheets. Originally discovered in insects (Garcia-Bellido et al., 1973; Lawrence, 1973), compartment boundaries are now recognized to be important for tissue organization in both vertebrates and invertebrates (Dahmann and Basler, 1999; Dahmann et al., 2011; Batlle and Wilkinson, 2012; Fagotto, 2014). These boundaries not only prevent mixing between different cell lineages, they also provide natural, defined locations that position the source of diffusible morphogens (Lawrence and Struhl, 1996). As a consequence, compartment boundaries have profound effects on cell identity and tissue architecture. Compartment boundaries between parasegments in the germband are established early in embryogenesis (Vincent and O’Farrell, 1992), and they act as barriers to cell crossing throughout embryonic and larval development, as cells undergo dramatic displacements resulting from epithelial remodeling and tissue growh (Landsberg et al., 2009; Monier et al., 2010; Scarpa et al., 2018). While the transcriptional inputs that establish compartment boundaries have been well-characterized, the cellular mechanisms that create physical barriers between cell populations are less well understood.
Compartment boundaries act as barriers, at least in part, by locally increasing actomyosin contractility to create a contractile cable that resists cell crossing (Major and Irvine, 2005; Major and Irvine, 2006; Landsberg et al., 2009; Monier et al., 2010; Aliee et al., 2012; Umetsu et al., 2014; Tetley et al., 2016). Myosin is still enriched at compartment boundaries in Toll-deficient embryos, indicating that other signals regulate boundary formation in this tissue (Paré et al., 2019). A key insight into the mechanisms that establish compartment boundaries was the discovery that the segmentally expressed LRR protein Tartan and its binding partner Ten-m direct planar polarity specifically at compartment boundaries in the germband (Figure 3A,C,F) (Paré et al., 2019). Tartan influences cell shape in Drosophila epithelial tissues (Milán et al., 2001, 2005; Krause et al., 2006; Sakurai et al., 2007; Mao et al., 2008) and neurons (Kurusu et al., 2008), and teneurins regulate neural development in Drosophila (Hong et al., 2009; Mosca et al., 2012; Hong and Luo, 2014; Mosca, 2015; Baumgartner and Wides, 2019) and vertebrates (Leamey et al., 2008; Dharmaratne et al., 2012; Antinucci et al., 2013; Berns et al., 2018). Drosophila embryos defective for either Tartan or Ten-m display decreased myosin and increased Par-3 levels specifically at compartment boundaries, whereas non-boundary regions are not affected (Paré et al., 2019). These functions appear to involve direct receptor-receptor interactions, as Tartan and Ten-m can interact in trans when expressed in cultured cells in vitro, and Tartan recruits Ten-m protein to compartment boundaries, corresponding to the borders of the Tartan stripes, in vivo (Paré et al., 2019). Notably, either reducing Tartan or Ten-m expression or overexpressing either protein ubiquitously disrupts cell alignment at boundaries, indicating that local differences in Tartan and Ten-m are important for planar polarity and boundary structure (Paré et al., 2019).
These results identify a novel receptor interaction that regulates planar polarity at compartment boundaries during germband extension. Tartan is expressed in even parasegments, providing a clear source of differences in Tartan activity at compartment boundaries (Figure 3C,F) (Chang et al., 1993; Paré et al., 2019). By contrast, the Ten-m protein appears to be present in all cells, raising the question of how Ten-m is specifically enriched at compartment boundaries (Paré et al., 2019). Loss- and gain-of-function experiments show that Tartan inhibits Ten-m membrane localization when these proteins are present in the same cell (in cis), but stabilizes Ten-m when these proteins are present in neighboring cells (in trans). These dual interactions involving cis-inhibition and trans-activation result in the enrichment of Ten-m at the borders of Tartan stripes (Figure 3C,F) (Paré et al., 2019), and may represent a general mechanism for patterning the activity of uniformly expressed receptors (Figure 4D). Consistent with this possibility, there is evidence for similar mechanisms regulating Notch signaling (del Álamo et al., 2011) and the localized activities of proteins in the Frizzled/Dishevelled planar cell polarity (PCP) system (Peng and Axelrod, 2012; Yang and Mlodzik, 2015).
Together, the Tartan/Ten-m and Toll receptor systems represent spatially overlapping yet independent mechanisms for establishing planar polarity in distinct regions of the germband. However, how these signals influence actomyosin organization and cell polarity is not known. An intriguing idea is that the downstream effectors of Toll receptors may induce dynamic contractile structures that promote cell intercalation, whereas the effectors of Tartan and Ten-m may confer distinct contractile and adhesive properties at compartment boundaries that allow them to serve as stable barriers to cell crossing.
8. Control of junctional and medial myosin by G protein-coupled receptors
Apical constriction in the mesoderm, like cell intercalation in the ectoderm, relies on actomyosin contractility to produce spatially regulated and coordinated changes in cell shape (Figure 5A,C,D) (Sweeton et al., 1991; Dawes-Hoang et al., 2005; Gorfinkiel and Blanchard, 2011; Kasza and Zallen, 2011; Martin and Goldstein, 2014; Munjal and Lecuit, 2014). Recent studies demonstrate that these two cell behaviors also share common molecular regulators. In particular, signaling through distinct sets of upstream regulators in the G protein-coupled receptor (GPCR) and RhoGEF protein families plays a critical role in activating apical constriction in mesodermal cells and cell intercalation in the germband. These studies provide insight into how myosin activity is differentially activated in the ectoderm and mesoderm to mediate distinct cell behaviors in these neighboring tissues.
Figure 5. Region-specific cell behaviors in the Drosophila embryo.
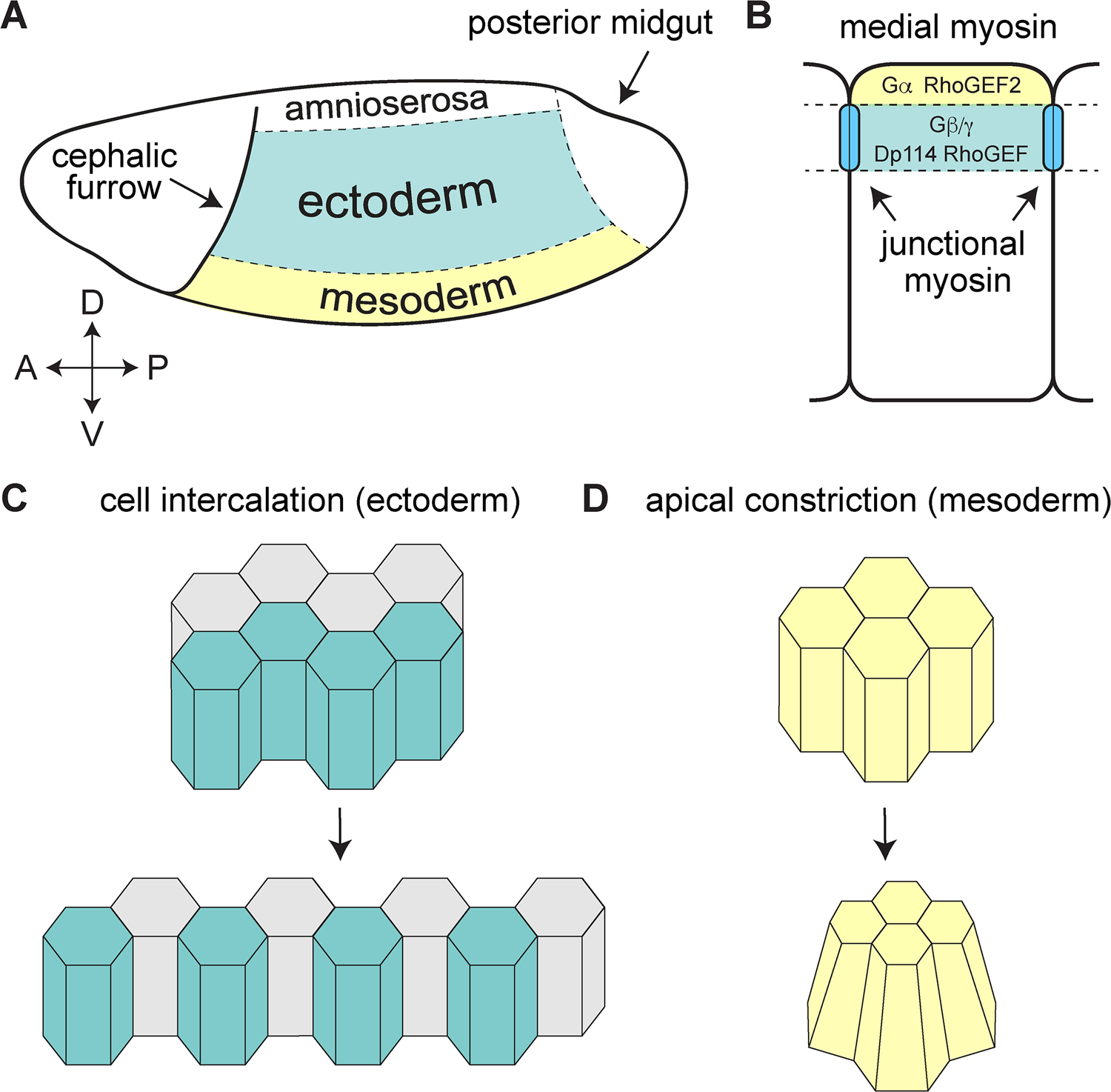
(A) Embryo schematic showing the germband ectoderm (blue) and mesoderm (yellow). (B) GPCR signaling through the Gα alpha subunit and RhoGEF2 activates myosin in the medial (apical) cellular domain. GPCR signaling through the Gβ/Gγ subunits and the RhoGEF Dp114/Cysts recruits myosin to adherens junctions in the ectoderm to promote cell intercalation. (C) Schematic of intercalating cells in the ectoderm. (D) Schematic of apically constricting cells in the mesoderm.
In the mesoderm, apical constriction is induced by the ventral expression of specific factors under the control of the DV-patterning system, including the secreted ligand Fog (Costa et al., 1994), the transmembrane protein T48 (Kölsch et al., 2007), and the GPCR Mist (Manning et al., 2013). Fog binds to Mist (Manning et al., 2013), and perhaps to the ubiquitously expressed GPCR Smog (Kerridge et al., 2016), leading to activation of the Gα protein Concertina (Parks and Wieschaus, 1991). T48 and Concertina localize to and activate RhoGEF2 at the apical cell cortex of mesodermal cells (Rogers et al., 2004; Kölsch et al., 2007), and RhoGEF2 in turn activates Rho GTPase by converting the inactive GDP-bound form to an active, GTP-bound form (Jaffe and Hall, 2005). Rho then activates Rho-kinase to trigger myosin contractility in the medial cell cortex, a process referred to as radial cell polarity (Mason et al., 2013; Martin and Goldstein, 2014). Myosin contractility decreases the apical surface area of mesodermal cells in a pulsatile fashion through physical linkages to the actin cytoskeleton and the cellular junctions, driving mesoderm invagination (Martin et al., 2009) (see chapter “The cellular and molecular mechanisms that establish the mechanics of Drosophila gastrulation” by Ko and Martin).
In the germband, actomyosin networks are organized into medial and junctional myosin populations (Rauzi et al., 2010; Levayer and Lecuit, 2013). Both populations of myosin are responsive to GPCR signaling, but they appear to be separately controlled by Gα and RhoGEF2 in the medial domain and by Gβ13F/Gγ1 and the RhoGEF Dp114/Cysts at junctions (Figure 5B) (Kerridge et al., 2016; Garcia De Las Bayonas et al., 2019). Disruption of RhoGEF2 or the Gα protein Concertina selectively affects medial myosin, without altering junctional myosin (Kerridge et al., 2016; Garcia De Las Bayonas et al., 2019). By contrast, mutations affecting Smog and Dp114/Cysts specifically reduce junctional myosin (Kerridge et al., 2016; Garcia de las Bayonas et al., 2019). Dp114/Cysts specifically localizes to and enhances Rho GTPase activity at adherens junctions in response to the apical polarity protein Crumbs (Silver et al., 2019), and Gβ13F/Gγ1 activity (Garcia De Las Bayonas et al., 2019). Importantly, junctional Dp114/Cysts localization is observed in the ectoderm, but not in the mesoderm, suggesting a mechanism for restricting junctional myosin localization to the germband (Garcia De Las Bayonas et al., 2019). The identification of an ectoderm-specific RhoGEF that promotes junctional myosin is a significant step forward in understanding how distinct cell behaviors are differentially activated in the ectoderm and mesoderm. It is interesting to speculate that cell intercalation may only occur in regions where Dp114/Cysts-mediated junctional myosin recruitment and Toll receptor-mediated planar polarity overlap. In this model, the ectoderm-specific junctional localization of Dp114/Cysts would render cells competent to support junctional myosin contractility, whereas patterned Toll receptors would be the triggering event that activates planar polarized actomyosin contractility to induce cell intercalation. Whether Toll receptors and GPCR signaling pathways function in parallel to mediate different aspects of cell polarity, or if these pathways converge at a molecular level to regulate Rho GTPase signaling, remains to be determined.
9. Current questions and future challenges
Significant advances have been made in understanding the cell behaviors, mechanical forces, and spatial cues that control convergent extension in epithelia. Four general principles of epithelial remodeling have emerged. First, the molecular basis of planar polarity during cell intercalation ultimately involves the spatially regulated localization and activities of contractile and adhesive proteins. Second, mechanical forces transmitted between cells and tissues provide important spatial inputs that modulate protein dynamics and cell behavior. Third, biochemical and mechanical signals are actively integrated to promote collective cell behaviors such as rosette assembly and boundary formation that profoundly influence tissue structure. Finally, cell polarity is oriented to the body axes by a high-resolution network of locally acting spatial cues that establish planar polarity, position compartment boundaries, and restrict intercalary behavior to the ectoderm. Together, these spatial cues provide the structural foundation for multicellular organization in the Drosophila embryo, linking intrinsic cell polarities to tissue-scale organization.
Despite significant progress, much remains to be discovered. First, the discovery that stripes of Toll receptor and Tartan expression are necessary for planar polarity suggest that neighboring epithelial cells compute axial patterning information by interpreting local differences in receptor levels. While heterotypic interactions between Tartan and Ten-m provide a plausible mechanism for demarcating compartment boundaries, the molecular logic of the Toll receptor code is less clear. Insight into how cells detect qualitative and quantitative receptor differences in neighboring cells will provide fundamental insights into how patterned gene expression is converted into tissue structure.
Second, although several cell-surface receptors that regulate planar polarity have now been identified, the molecular connections between these upstream inputs and the downstream effectors of polarity are not understood. Many receptors, in addition to the Toll receptor and Tartan/Ten-m systems, have been shown to induce myosin accumulation at interfaces between cells that express different levels or types of receptors (Bielmeier, 2016). The signaling pathways that communicate to the actomyosin cytoskeleton downstream of these diverse receptor families are not known. How molecularly distinct classes of receptors in the germband all converge on actomyosin contractility, yet elicit different cell behaviors in intercalating and boundary domains, will be a fascinating avenue of further investigation.
Finally, these studies of cells in dynamically reorganizing tissues reveal that mechanical forces play important roles in controlling contractile dynamics and cell behavior. However, the mechanosensors that detect these forces, as well as the downstream mechanotransduction pathways that convert mechanical forces into biochemical changes in protein localization and activity, largely remain to be discovered. A better understanding of how mechanical forces and biochemical signals are integrated during convergent extension will provide insight into how cell polarity and behavior are dynamic coordinated across multicellular tissues to produce robust and stereotyped tissue structures.
Acknowledgments
The authors thank all members of the Zallen and Paré labs, past and present, for helpful discussions, and Eric Brooks, Rodrigo Fernandez-Gonzalez, Karen Kasza, Sergio Simões, Masako Tamada, Athea Vichas, and Richard Zallen and for helpful comments on the manuscript. Research in ACP’s lab is funded by the Vice Provost for Research and Economic Development and the Department of Biological Sciences at University of Arkansas. Research in JAZ’s lab is funded by R01 GM079340. JAZ is an investigator of the Howard Hughes Medical Institute.
References
- Akam M, 1987. The molecular basis for metameric pattern in the Drosophila embryo. Development 101, 1–22. [PubMed] [Google Scholar]
- del Álamo D, Rouault H, Schweisguth F, 2011. Mechanism and significance of cis-inhibition in Notch signalling. Curr. Biol 21, R40–7. [DOI] [PubMed] [Google Scholar]
- Aliee M, Röper J-C, Landsberg KP, Pentzold C, Widmann TJ, Jülicher F, Dahmann C, 2012. Physical mechanisms shaping the Drosophila dorsoventral compartment boundary. Curr. Biol 22, 967–976. [DOI] [PubMed] [Google Scholar]
- Anderson KV, 2000. Toll signaling pathways in the innate immune response. Curr. Opin. Immunol 12, 13–19. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Bokla L, Nusslein-Volhard C, 1985a. Establishment of dorsal-ventral polarity in the Drosophila embryo: The induction of polarity by the Toll gene product. Cell 42, 791–798. [DOI] [PubMed] [Google Scholar]
- Anderson KV, Jürgens G, Nüsslein-Volhard C, 1985b. Establishment of dorsal-ventral polarity in the Drosophila embryo: Genetic studies on the role of the Toll gene product. Cell 42, 779–789. [DOI] [PubMed] [Google Scholar]
- Antinucci P, Nikolaou N, Meyer MP, Hindges R, 2013. Teneurin-3 specifies morphological and functional connectivity of retinal ganglion cells in the vertebrate visual system. Cell Rep 5, 582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard SL, Miller DL, Ganetzky B, 2014. Retrograde neurotrophin signaling through Tollo regulates synaptic growth in Drosophila. J. Cell Biol 204, 1157–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batlle E, Wilkinson DG, 2012. Molecular mechanisms of cell segregation and boundary formation in development and tumorigenesis. Cold Spring Harb. Perspect. Biol 4, a008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S, Wides R, 2019. Discovery of Teneurins. Front. Neurosci 13, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton MA, Pechmann M, Frey N, Stappert D, Conrads KH, Chen YT, Stamataki E, Pavlopoulos A, Roth S, 2016. Toll genes have an ancestral role in axis elongation. Curr. Biol 26, 1609–1605. [DOI] [PubMed] [Google Scholar]
- Berns DS, DeNardo LA, Pederick DT, Luo L, 2018. Teneurin-3 controls topographic circuit assembly in the hippocampus. Nature 554, 328–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T, 2004. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429, 667–671. [DOI] [PubMed] [Google Scholar]
- Bielmeier C, 2016. Interface Contractility Between Differently Fated Cells Drives Cell Elimination and Cyst Formation. Curr. Biol 26, 563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JSSP, Weitz O, Zallen JA, 2006. Multicellular Rosette Formation Links Planar Cell Polarity to Tissue Morphogenesis. Dev. Cell 11, 459–470. [DOI] [PubMed] [Google Scholar]
- Butler LC, Blanchard GB, Kabla AJ, Lawrence NJ, Welchman DP, Mahadevan L, Adams RJ, Sanson B, 2009. Cell shape changes indicate a role for extrinsic tensile forces in Drosophila germ-band extension. Nat. Cell Biol 11, 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho L, Jacinto A, Matova N, 2014. The Toll/NF-kappaB signaling pathway is required for epidermal wound repair in Drosophila. Proc. Natl. Acad. Sci. U. S. A 111, E5373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacon-Heszele MF, Ren D, Reynolds AB, Chi F, Chen P, 2012. Regulation of cochlear convergent extension by the vertebrate planar cell polarity pathway is dependent on p120-catenin. Development 139, 968–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Z, Price BD, Bockheim S, Boedigheimer MJ, Smith R, Laughon A, 1993. Molecular and genetic characterization of the Drosophila tartan gene. Dev. Biol 160, 315–332. [DOI] [PubMed] [Google Scholar]
- Chiang C, Beachy PA, 1994. Expression of a novel Toll-like gene spans the parasegment boundary and contributes to hedgehog function in the adult eye of Drosophila. Mech. Dev 47, 225–239. [DOI] [PubMed] [Google Scholar]
- Clark E, 2017. Dynamic patterning by the Drosophila pair-rule network reconciles long-germ and short-germ segmentation. PLoS Biol 15, e2002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinet C, Rauzi M, Lenne P-F, Lecuit T, 2015. Local and tissue-scale forces drive oriented junction growth during tissue extension. Nat. Cell Biol 17, 1247–1258. [DOI] [PubMed] [Google Scholar]
- Costa M, Wilson ET, Wieschaus E, 1994. A putative cell signal encoded by the folded gastrulation gene coordinates cell shape changes during Drosophila gastrulation. Cell 76, 1075–1089. [DOI] [PubMed] [Google Scholar]
- Dahmann C, Basler K, 1999. Compartment boundaries at the edge of development. Trends Genet 15, 320–326. [DOI] [PubMed] [Google Scholar]
- Dahmann C, Oates AC, Brand M, 2011. Boundary formation and maintenance in tissue development. Nat. Rev. Genet 12, 43–55. [DOI] [PubMed] [Google Scholar]
- Dawes-Hoang RE, Parmar KM, Chistiansen AE, Phelps CB, Brand AH, Wieschaus EF, 2005. folded gastrulation, cell shape change and the control of myosin localization. Development 132, 4165–4178. [DOI] [PubMed] [Google Scholar]
- Dharmaratne N, Glendining KA, Young TR, Tran H, Sawatari A, Leamey CA, 2012. Ten-m3 is required for the development of topography in the ipsilateral retinocollicular pathway. PLoS One 7, e43083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effler JC, Kee Y-S, Berk JM, Tran MN, Iglesias PA, Robinson DN, 2006. Mitosis-specific mechanosensing and contractile-protein redistribution control cell shape. Curr. Biol 16, 1962–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldon E, Kooyer S, D’Evelyn D, Duman M, Lawinger P, Botas J, Bellen H, 1994. The Drosophila 18 wheeler is required for morphogenesis and has striking similarities to Toll. Development 120, 885–899. [DOI] [PubMed] [Google Scholar]
- Fagotto F, 2014. The cellular basis of tissue separation. Development 141, 3303–3318. [DOI] [PubMed] [Google Scholar]
- Farhadifar R, Röper J-C, Aigouy B, Eaton S, Jülicher F, 2007. The influence of cell mechanics, cell-cell interactions, and proliferation on epithelial packing. Curr. Biol 17, 2095–2104. [DOI] [PubMed] [Google Scholar]
- Farrell DL, Weitz O, Magnasco MO, Zallen JA, 2017. SEGGA: a toolset for rapid automated analysis of epithelial cell polarity and dynamics. Development 144, 1725–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Simões S. de M., Röper J-C, Eaton S, Zallen JA, 2009. Myosin II dynamics are regulated by tension in intercalating cells. Dev. Cell 17, 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Zallen JA, 2011. Oscillatory behaviors and hierarchical assembly of contractile structures in intercalating cells. Phys. Biol 8, 045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldi I, Anthoney N, Harrison N, Gangloff M, Verstak B, Nallasivan MP, AlAhmed S, Zhu B, Phizacklea M, Losada-Perez M, Moreira M, Gay NJ, Hidalgo A, 2017. Three-tier regulation of cell number plasticity by neurotrophins and Tolls in Drosophila. J. Cell Biol 216, 1421–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bellido A, Ripoll P, Morata G, 1973. Developmental Compartmentalisation of the Wing Disk of Drosophila. Nature New Biol 245, 251–253. [DOI] [PubMed] [Google Scholar]
- Garcia De Las Bayonas A, Philippe J-M, Lellouch AC, Lecuit T, 2019. Distinct RhoGEFs Activate Apical and Junctional Contractility under Control of G Proteins during Epithelial Morphogenesis. Curr. Biol 29, 3370–3385.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorfinkiel N, Blanchard GB, 2011. Dynamics of actomyosin contractile activity during epithelial morphogenesis. Curr. Opin. Cell Biol 23, 531–539. [DOI] [PubMed] [Google Scholar]
- Graham PL, Anderson WR, Brandt EA, Xiang J, Pick L, 2019. Dynamic expression of Drosophila segmental cell surface-encoding genes and their pair-rule regulators. Dev. Biol 447, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C, Hudson KL, Anderson KV, 1988. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52, 269–279. [DOI] [PubMed] [Google Scholar]
- Hong W, Luo L, 2014. Genetic control of wiring specificity in the fly olfactory system. Genetics 196, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Zhu H, Potter CJ, Barsh G, Kurusu M, Zinn K, Luo L, 2009. Leucine-rich repeat transmembrane proteins instruct discrete dendrite targeting in an olfactory map. Nat. Neurosci 12, 1542–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson MS, Tokutake Y, Chang MS, Bloor JW, Venakides S, Kiehart DP, Edwards GS, 2003. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science 300, 145–149. [DOI] [PubMed] [Google Scholar]
- Irvine KD, Wieschaus E, 1994. Cell intercalation during Drosophila germband extension and its regulaton by pair- rule segmentation genes. Development 120, 827–841. [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A, 2005. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol 21, 247–269. [DOI] [PubMed] [Google Scholar]
- Jewett CE, Vanderleest TE, Miao H, Xie Y, Madhu R, Loerke D, Blankenship JT, 2017. Planar polarized Rab35 functions as an oscillatory ratchet during cell intercalation in the Drosophila epithelium. Nat. Commun 8, 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambris Z, Hoffmann JA, Imler JL, Capovilla M, 2002. Tissue and stage-specific expression of the Tolls in Drosophila embryos. Gene Expr. Patterns 2, 311–317. [DOI] [PubMed] [Google Scholar]
- Kasza KE, Farrell DL, Zallen JA, 2014. Spatiotemporal control of epithelial remodeling by regulated myosin phosphorylation. Proc. Natl. Acad. Sci. U. S. A 111, 11732–11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza KE, Zallen JA, 2011. Dynamics and regulation of contractile actin-myosin networks in morphogenesis. Curr. Opin. Cell Biol 23, 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Kawai T, 2014. Toll-like receptor signaling pathways. Front. Immunol 5, 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller R, Davidson L, Edlund A, Elul T, Ezin M, Shook D, Skoglund P, 2000. Mechanisms of convergence and extension by cell intercalation. Philos. Trans. R. Soc. Lond. B Biol. Sci 355, 897–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerridge S, Munjal A, Philippe J-M, Jha A, de las Bayonas AG, Saurin AJ, Lecuit T, 2016. Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis. Nat. Cell Biol 18, 261–270. [DOI] [PubMed] [Google Scholar]
- Kim S, Chung S, Yoon J, Choi K-W, Yim J, 2006. Ectopic expression of Tollo/Toll-8 antagonizes Dpp signaling and induces cell sorting in the Drosophila wing Genesis. 44, 541–549. [DOI] [PubMed] [Google Scholar]
- Kleve CD, Siler DA, Syed SK, Eldon ED, 2006. Expression of 18-wheeler in the follicle cell epithelium affects cell migration and egg morphology in Drosophila. Dev. Dyn 235, 1953–1961. [DOI] [PubMed] [Google Scholar]
- Kolesnikov T, Beckendorf SK, 2007. 18 wheeler regulates apical constriction of salivary gland cells via the Rho-GTPase-signaling pathway. Dev. Biol 307, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölsch V, Seher T, Fernandez-Ballester GJ, Serrano L, Leptin M, 2007. Control of Drosophila gastrulation by apical localization of adherens junctions and RhoGEF2. Science 315, 384–386. [DOI] [PubMed] [Google Scholar]
- Kong D, Wolf F, Grosshans J Forces directing germ-band extension in Drosophila embryos. Mech. Dev 144, 11–22. [DOI] [PubMed] [Google Scholar]
- Krahn MP, Klopfenstein DR, Fischer N, Wodarz A, 2010. Membrane targeting of Bazooka/PAR-3 is mediated by direct binding to phosphoinositide lipids. Curr. Biol 20, 636–642. [DOI] [PubMed] [Google Scholar]
- Krause C, Wolf C, Hemphälä J, Samakovlis C, Schuh R, 2006. Distinct functions of the leucine-rich repeat transmembrane proteins capricious and tartan in the Drosophila tracheal morphogenesis. Dev. Biol 296, 253–264. [DOI] [PubMed] [Google Scholar]
- Kurusu M, Cording A, Taniguchi M, Menon K, Suzuki E, Zinn K, 2008. A screen of cell-surface molecules identifies leucine-rich repeat proteins as key mediators of synaptic target selection. Neuron 59, 972–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan H, Wang Q, Fernandez-Gonzalez R, Feng JJ, 2015. A biomechanical model for cell polarization and intercalation during Drosophila germband extension. Phys. Biol 12, 056011. [DOI] [PubMed] [Google Scholar]
- Landsberg KP, Farhadifar R, Ranft J, Umetsu D, Widmann TJ, Bittig T, Said A, Jülicher F, Dahmann C, 2009. Increased Cell Bond Tension Governs Cell Sorting at the Drosophila Anteroposterior Compartment Boundary. Curr. Biol 19, 1950–1955. [DOI] [PubMed] [Google Scholar]
- Lau K, Tao H, Liu H, Wen J, Sturgeon K, Sorfazlian N, Lazic S, Burrows JTA, Wong MD, Li D, Deimling S, Ciruna B, Scott I, Simmons C, Henkelman RM, Williams T, Hadjantonakis A-K, Fernandez-Gonzalez R, Sun Y, Hopyan S, 2015. Anisotropic stress orients remodelling of mammalian limb bud ectoderm. Nat. Cell Biol 17, 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence PA, 1973. A clonal analysis of segment development in Oncopeltus (Hemiptera). J. Embryol. Exp. Morphol 30, 681–699. [PubMed] [Google Scholar]
- Lawrence PA, Struhl G, 1996. Morphogens, compartments, and pattern: Lessons from Drosophila? Cell 85, 951–961. [DOI] [PubMed] [Google Scholar]
- Leamey CA, Glendining KA, Kreiman G, Kang N-D, Wang KH, Fassler R, Sawatari A, Tonegawa S, Sur M, 2008. Differential gene expression between sensory neocortical areas: potential roles for Ten_m3 and Bcl6 in patterning visual and somatosensory pathways. Cereb. Cortex 18, 53–66. [DOI] [PubMed] [Google Scholar]
- Leulier F, Lemaitre B, 2008. Toll-like receptors—taking an evolutionary approach. Nat. Rev. Genet 9, 165. [DOI] [PubMed] [Google Scholar]
- Levayer R, Lecuit T, 2013. Oscillation and Polarity of E-Cadherin Asymmetries Control Actomyosin Flow Patterns during Morphogenesis. Dev. Cell 26, 162–175. [DOI] [PubMed] [Google Scholar]
- Levayer R, Pelissier-Monier A, Lecuit T, 2011. Spatial regulation of Dia and Myosin-II by RhoGEF2 controls initiation of E-cadherin endocytosis during epithelial morphogenesis. Nat. Cell Biol 13, 529–542. [DOI] [PubMed] [Google Scholar]
- Lienkamp SS, Liu K, Karner CM, Carroll TJ, Ronneberger O, Wallingford JB, Walz G, 2012. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nat. Genet 44, 1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lye CM, Blanchard GB, Naylor HW, Muresan L, Huisken J, Adams RJ, Sanson B, 2015. Mechanical Coupling between Endoderm Invagination and Axis Extension in Drosophila. PLoS Biol 13, e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major RJ, Irvine KD, 2005. Influence of Notch on dorsoventral compartmentalization and actin organization in the Drosophila wing. Development 132, 3823–3833. [DOI] [PubMed] [Google Scholar]
- Major RJ, Irvine KD, 2006. Location and requirement for Myosin II at the dorsal-ventral compartment boundary of the Drosophila wing. Dev Dyn 235, 3051–3058. [DOI] [PubMed] [Google Scholar]
- Manning AJ, Peters KA, Peifer M, Rogers SL, 2013. Regulation of epithelial morphogenesis by the G protein-coupled receptor mist and its ligand fog. Sci. Signal 6, ra98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Kerr M, Freeman M, 2008. Modulation of Drosophila retinal epithelial integrity by the adhesion proteins capricious and tartan. PLoS One 3, e1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF, 2010. Integration of contractile forces during tissue invagination. J. Cell Biol 188, 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Goldstein B, 2014. Apical constriction: themes and variations on a cellular mechanism driving morphogenesis. Development 141, 1987–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF, 2009. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457, 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason FM, Tworoger M, Martin AC, 2013. Apical domain polarization localizes actin-myosin activity to drive ratchet-like apical constriction. Nat. Cell Biol 15, 926–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGreevy EM, Vijayraghavan D, Davidson LA, Hildebrand JD, 2015. Shroom3 functions downstream of planar cell polarity to regulate myosin II distribution and cellular organization during neural tube closure. Biology Open 4, 186–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy G, Foldi I, Aurikko J, Wentzell JS, Lim MA, Fenton JC, Gay NJ, Hidalgo A, 2013. Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila melanogaster CNS. Nat. Neurosci 16, 1248–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer SN, Amoyel M, Bergantinos C, de la Cova C, Schertel C, Basler K, Johnston LA, 2014. An ancient defense system eliminates unfit cells from developing tissues during cell competition. Science 346, 1258236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milán M, Pérez L, Cohen SM, 2005. Boundary formation in the Drosophila wing: functional dissection of Capricious and Tartan. Dev. Dyn 233, 804–810. [DOI] [PubMed] [Google Scholar]
- Milán M, Weihe U, Pérez L, Cohen SM, 2001. The LRR proteins capricious and Tartan mediate cell interactions during DV boundary formation in the Drosophila wing. Cell 106, 785–794. [DOI] [PubMed] [Google Scholar]
- Monier B, Pélissier-Monier A, Brand AH, Sanson B, 2010. An actomyosin-based barrier inhibits cell mixing at compartmental boundaries in Drosophila embryos. Nat. Cell Biol 12, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca TJ, 2015. On the Teneurin track: a new synaptic organization molecule emerges. Front. Cell. Neurosci 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosca TJ, Hong W, Dani VS, Favaloro V, Luo L, 2012. Trans-synaptic Teneurin signalling in neuromuscular synapse organization and target choice. Nature 484, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munjal A, Lecuit T, 2014. Actomyosin networks and tissue morphogenesis. Development 141, 1789–1793. [DOI] [PubMed] [Google Scholar]
- Munjal A, Philippe J-M, Munro E, Lecuit T, 2015. A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 524, 351–355. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Honda H, Takeichi M, 2012. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 149, 1084–1097. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Takeichi M, 2008. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development 135, 1493–1502. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E, 1980. Mutations affecting segment number and polarity in Drosophila. Nature. 287, 795–801. [DOI] [PubMed] [Google Scholar]
- Paré AC, Naik P, Shi J, Mirman Z, Palmquist KH, Zallen JA, 2019. An LRR Receptor-Teneurin System Directs Planar Polarity at Compartment Boundaries. Dev. Cell. 51, 208–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré AC, Vichas A, Fincher CT, Mirman Z, Farrell DL, Mainieri A, Zallen JA, 2014. A positional Toll receptor code directs convergent extension in Drosophila. Nature 515, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks S, Wieschaus E, 1991. The Drosophila gastrulation gene concertina encodes a G alpha-like protein. Cell 64, 447–458. [DOI] [PubMed] [Google Scholar]
- Peng Y, Axelrod JD, 2012. Asymmetric protein localization in planar cell polarity: mechanisms, puzzles, and challenges. Curr. Top. Dev. Biol 101, 33–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille P-A, Ahmadi P, Brunet A-C, Farge E, 2009. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci. Signal 2, ra16. [DOI] [PubMed] [Google Scholar]
- Rauzi M, Lenne P-F, Lecuit T, 2010. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature 468, 1110–1114. [DOI] [PubMed] [Google Scholar]
- Rauzi M, Verant P, Lecuit T, Lenne P-F, 2008. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat. Cell Biol 10, 1401–1410. [DOI] [PubMed] [Google Scholar]
- Razzell W, Bustillo ME, Zallen JA, 2018. The force-sensitive protein Ajuba regulates cell adhesion during epithelial morphogenesis. J. Cell Biol 217, 3715–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Wiedemann U, Häcker U, Turck C, Vale RD, 2004. Drosophila RhoGEF2 associates with microtubule plus ends in an EB1-dependent manner. Curr. Biol 14, 1827–1833. [DOI] [PubMed] [Google Scholar]
- Rozbicki E, Chuai M, Karjalainen AI, Song F, Sang HM, Martin R, Knölker H-J, MacDonald MP, Weijer CJ, 2015. Myosin-II-mediated cell shape changes and cell intercalation contribute to primitive streak formation. Nat. Cell Biol 17, 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai KT, Kojima T, Aigaki T, Hayashi S, 2007. Differential control of cell affinity required for progression and refinement of cell boundary during Drosophila leg segmentation. Dev. Biol 309, 126–136. [DOI] [PubMed] [Google Scholar]
- Sawyer JK, Choi W, Jung KC, He L, Harris NJ, Peifer M, 2011. A contractile actomyosin network linked to adherens junctions by Canoe/afadin helps drive convergent extension. Mol. Biol. Cell 22, 2491–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa E, Finet C, Blanchard GB, Sanson B, 2018. Actomyosin-Driven Tension at Compartmental Boundaries Orients Cell Division Independently of Cell Geometry In Vivo. Dev. Cell 47, 727–740.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PK, Tanner MR, Kovacevic I, Rankin A, Marshall TE, Noblett N, Tran NN, Roenspies T, Hung J, Chen Z, Slatculescu C, Perkins TJ, Bao Z, Colavita A, 2017. PCP and SAX-3/Robo Pathways Cooperate to Regulate Convergent Extension-Based Nerve Cord Assembly in C. elegans. Dev. Cell 41, 195–203.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo A, Wallingford JB, 2014. PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science 343, 649–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JT, Wirtz-Peitz F, Simões S, Pellikka M, Yan D, Binari R, Nishimura T, Li Y, Harris TJC, Perrimon N, et al. (2019). Apical polarity proteins recruit the RhoGEF Cysts to promote junctional myosin assembly. J. Cell Biol 218, 3397–3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões S. de M., Blankenship JT, Weitz O, Farrell DL, Tamada M, Fernandez-Gonzalez R, Zallen JA, 2010. Rho-kinase directs bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev. Cell 19, 377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões S. de M., Mainieri A, Zallen JA, 2014. Rho GTPase and Shroom direct planar polarized actomyosin contractility during convergent extension. J. Cell Biol 204. 204, 575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Amourda C, Shagirov M, Hara Y, Saunders TE, Toyama Y, 2017. Basolateral protrusion and apical contraction cooperatively drive Drosophila germ-band extension. Nat. Cell Biol 19, 375–383. [DOI] [PubMed] [Google Scholar]
- Sweeton D, Parks S, Costa M, Wieschaus E, 1991. Gastrulation in Drosophila: the formation of the ventral furrow and posterior midgut invaginations. Development 112, 775–789. [DOI] [PubMed] [Google Scholar]
- Takeichi M, 2014. Dynamic contacts: rearranging adherens junctions to drive epithelial remodeling. Nat. Rev. Mol. Cell Biol 15, 397–410. [DOI] [PubMed] [Google Scholar]
- Tamada M, Farrell DL, Zallen JA, 2012. Abl Regulates Planar Polarized Junctional Dynamics through β-Catenin Tyrosine Phosphorylation. Dev. Cell 22, 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley RJ, Blanchard GB, Fletcher AG, Adams RJ, Sanson B, 2016. Unipolar distributions of junctional Myosin II identify cell stripe boundaries that drive cell intercalation throughout Drosophila axis extension. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu D, Dunst S, Dahmann C, 2014. An RNA interference screen for genes required to shape the anteroposterior compartment boundary in Drosophila identifies the Eph receptor. PLoS One 9, e114340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderleest TE, Smits CM, Xie Y, Jewett CE, Blankenship JT, Loerke D, 2018. Vertex sliding drives intercalation by radial coupling of adhesion and actomyosin networks during Drosophila germband extension. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JP, O’Farrell PH, 1992. The state of engrailed expression is not clonally transmitted during early Drosophila development. Cell 68, 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walck-Shannon E, Hardin J, 2014. Cell intercalation from top to bottom. Nat. Rev. Mol. Cell Biol 15, 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM, 2002. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev. Cell 2, 695–706. [DOI] [PubMed] [Google Scholar]
- Wang MFZ, Hunter MV, Wang G, McFaul C, Yip CM, Fernandez-Gonzalez R, 2017. Automated cell tracking identifies mechanically oriented cell divisions during Drosophila axis elongation. Development 144, 1350–1361. [DOI] [PubMed] [Google Scholar]
- Ward A, Hong W, Favaloro V, Luo L, 2015. Toll receptors instruct axon and dendrite targeting and participate in synaptic partner matching in a drosophila olfactory circuit. Neuron 85, 1013–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington SJ, Strutt H, Strutt D, 2013. The Frizzled-dependent planar polarity pathway locally promotes E-cadherin turnover via recruitment of RhoGEF2. J. Cell Sci 140, 1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaire D, Rivier N, 1984. Soap, cells and statistics—random patterns in two dimensions. Contemporary Physics 25, 59–99. [Google Scholar]
- Wieschaus E, Nüsslein-Volhard C, 2016. The Heidelberg screen for pattern mutants of Drosophila: A personal account. Annu. Rev. Cell Dev. Biol 32, 1–46. [DOI] [PubMed] [Google Scholar]
- Wieschaus E, Sweeton D, Costa M, 1991. Convergence and extension during germband elongation in Drosophila embryos. In: Keller R, editor. Gastrulation New York: Plenum Press; 1991, p. 213–223. [Google Scholar]
- Williams MLK, Sawada A, Budine T, Yin C, Gontarz P, Solnica-Krezel L, 2018. Gon4l regulates notochord boundary formation and cell polarity underlying axis extension by repressing adhesion genes. Nat. Commun 9, 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M, Yen W, Lu X, Sutherland A, 2014. Distinct apical and basolateral mechanisms drive planar cell polarity-dependent convergent extension of the mouse neural plate. Dev. Cell 29, 34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi Y, Nishida Y, Ip YT, 2010. Functional analysis of Toll-related genes in Drosophila. Dev. Growth Differ 52, 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Mlodzik. M, 2015. Wnt-Frizzled/planar cell polaritiy signaling: cellular orientation by facing the Wing (Wnt). Annu. Rev. Cell Dev. Biol 31, 623–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JC, Fernandez-Gonzalez R, 2016. Local mechanical forces promote polarized junctional assembly and axis elongation in Drosophila. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E, 2004. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell 6, 343–355. [DOI] [PubMed] [Google Scholar]
- Zallen JA, Zallen R, 2004. Cell-pattern disordering during convergent extension in Drosophila. J. Phys. Condens. Matter 16, S5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kong D, Reichl L, Vogt N, Wolf F, Grosshans J, 2014. The glucosyltransferase Xiantuan of the endoplasmic reticulum specifically affects E-Cadherin expression and is required for gastrulation movements in Drosophila. Dev. Biol 390, 208–220. [DOI] [PubMed] [Google Scholar]


