Abstract
Objective:
A major challenge that limits understanding and treatment of epileptic events from mesial temporal structures comes from our inability to detect and map interictal networks reproducibly using scalp electrodes. Here, we developed a novel approach to map interictal spike networks and demonstrate their relationships to seizure onset and lesions in patients with foramen ovale electrode implantations.
Methods:
We applied the direct Directed Transfer Function to reveal interictal spike propagation from bilateral foramen ovale electrodes on 10 consecutive patients and co-registered spatially with both seizure onset zones and temporal lobe lesions.
Results:
Highly reproducible, yet unique interictal spike networks were seen for each patient (correlation: 0.93±0.13). Interictal spikes spread in both anterior and posterior directions within each temporal lobe, often reverberating between sites. Spikes propagated to the opposite temporal lobe predominantly through posterior pathways. Patients with structural lesions (N=4), including tumors and sclerosis, developed reproducible spike networks adjacent to their lesions that were highly lateralized compared to patients without lesions. Only 5% of mesial temporal lobe spikes were time-locked with scalp electrode spikes. Our preliminary observation on two lesional patients suggested that along with lesion location, Interictal spike networks also partially co-registered with seizure onset zones suggesting interrelationship between seizure onset and a subset of spike networks.
Conclusions:
This is the first demonstration of patient-specific, reproducible interictal spike networks in mesial temporal structures that are closely linked to both temporal lobe lesions and seizure onset zones.
Significance:
Interictal spike connectivity is a novel approach to map epileptic networks that could help advance invasive and non-invasive epilepsy treatments.
Keywords: epilepsy, temporal lobe, interictal spikes, network, onset localization, dDTF, causality
1. Introduction:
Temporal lobe epilepsy (TLE) is often intractable and is one of the most common types of pharmacologically resistant epilepsy. Pathologically, hippocampal sclerosis (HS, mesial temporal sclerosis) is the most common substrate of TLE (Velasco et al., 2006), and is seen in about 70% of patients with drug-resistant TLE(Engel et al., 2012). In TLE, surgical removal of the epileptogenic zone can lead to seizure freedom in up to 89% of patients during the first 2–3 years of follow up (Salanova et al., 2002). To achieve a good surgical outcome, precise location of the epileptogenic zone is critical. In many patients, no definitive focus can be found with scalp electrodes (Bancaud et al., 1970), and hence invasive methods combined with imaging studies are preferred for accurate understanding of the epileptic onset.
Frequently used methods for intracranial recordings are grids (with subdural electrodes) or depth electrodes (stereo EEG-SEEG) both of which require invasive surgical procedures. Another less invasive approach is the insertion of foramen ovale (FO) electrodes to assess mesial temporal structures (Nilsson et al., 2009; Velasco et al., 2006). Intracranial FO electrodes can record epileptic interictal spikes (95% of spikes not observed on scalp EEG) and seizures which are often not observed on the scalp EEG (Fernández Torre et al., 1999; Lam et al., 2017). FO electrodes can also be useful in identifying focal seizure onset where scalp EEG is unable to detect these events (Velasco et al., 2006). Coupled with high resolution imaging including MRI, PET and SPECT, the FO electrode data can help guide surgical or other invasive treatments (Beleza et al., 2010).
Growing evidence suggests that epilepsy is not simply a disease of individual neurons, but of abnormal networks in the interictal state that predisposes the brain to seizures. To date, interictal networks have been measured mostly through fMRI based functional connectivity studies in temporal lobe epilepsy. Although limited, resting state functional MRI has shown disrupted connections in the temporal lobe (Waites et al., 2006), lateralization of hippocampal networks in relation to epileptic foci (Tracy and Doucet, 2015), and increased hippocampal functional network activity with neurocognitive function (Holmes et al., 2014). While some studies suggest that hippocampal functional connectivity increases in temporal lobe epilepsy (Haneef et al., 2014), contrasting studies estimate decreased hippocampal and amygdala connectivity(Pittau et al., 2012). While clearly demonstrating the existence of epileptic networks that can interfere with normal networks, functional imaging studies are mostly correlative and lack temporal resolution to capture network level changes during epileptic events. This comes from a lack sufficient time resolution of functional imaging studies compared to EEG studies(Maharathi et al., 2018).
Extensive EEG studies have been performed to characterize temporal lobe networks. Coita et. al revealed connectivity differences between left and right temporal lobes related to the laterality of the epilepsy using high density scalp EEG that correlated with neuropsychological impairments(Coito et al., 2015). Scalp EEG studies have compared TLE and non-TLE patients using spectral analysis and nonlinear correlations on EEG patterns in the absence of interictal abnormalities to understand associations between epileptic networks, duration of epilepsy, age, and seizure onset(Bettus et al., 2008)(Coito et al., 2016). Using SEEG, correlation-based measures of the total EEG signal suggest that patients with weak and homogenous temporal lobe connections were more likely to be seizure free after temporal lobectomy(Antony et al., 2013). Early work by Bancaud and Talairach have suggested that cortical regions with frequent interictal spiking (irritative zones) have a relationship with cortical regions that initiate seizures (epileptogenic zone) and suggested that, from a surgical perspective, these irritative zones should not be ignored (Kahane et al., 2006; Lüders et al., 2006).
While these earlier studies have advanced our understanding of temporal lobe networks, none have specifically examined the role of interictal spikes, which most clinical neurologists consider to be a hallmark of temporal lobe epilepsy. Specifically, we lack an understanding of how interictal spikes propagate within each temporal lobe, between the two temporal lobes, and between each temporal lobe and other neocortical regions, nor how interictal spikes relate to structural lesions and seizure onset zones.
Here, using a Granger causality based direct directed transfer function (dDTF) following interictal spike detection, we discovered highly reproducible interictal spike causal networks from long-term FO EEG recordings on 10 consecutive patients. Combined with high resolution MRI, we show strong relationships between an individual’s unique interictal spike network and both structural brain abnormalities and seizure onset zones. The identification of highly reproducible interictal spike networks could be of significant value for improving surgical and non-invasive treatments in epileptic patients.
2. Methods:
2.1. Patient Selection:
The retrospective study was approved by the Institutional Review Board at University of Illinois at Chicago. We studied 10 consecutive FO patients who had undergone FO electrode placement along with scalp electrode placement as a part of pre-surgical evaluation of drug resistant epilepsy. Before the FO placement, patients were evaluated with medical history, neurological and neuropsychological assessment, and magnetic resonance imaging (MRI) scans (T1 and T2). For surgical evaluation, scalp and FO electrodes were run simultaneous to the EEG recording using a digital video EEG system (Nihon Koden). Surface electrodes were placed on the scalp according to the international 10–20 system and placed sub temporally according to the 10–10 system. Six-contact AdTech FO electrodes (Ad-Tech Medical, Oak Creek, Wisc.) with 5-mm intercontact spacing were inserted percutaneously under general anesthesia with the aid of intraoperative fluoroscopy, positioned so contact 1 was at the end of the ambient cistern and contact 6 was above the foramen ovale. CT scans were performed after electrode implantation for coregistration of electrode positions. In all patients, anticonvulsant medications were withdrawn in various amounts after FO placement to try to elicit seizures.
2.2. EEG and MRI data:
EEG recording was done at 200Hz. An experienced electroencephalographer (AS) identified three 10-minute awake and three 10-minute asleep EEG segments totaling 1 hour of data, for each patient. These EEG segments were selected when the patient was resting with no rapid eye moment. Anti-epileptic medication was discontinued at least 24 hours before the sample time, and each sample was at least six hours away from any ictal activity. Ictal events were also isolated.
3D brain models for each patient were created from T1 MRI scans using Brainsuite(Shattuck and Leahy, 2002). The FO electrode locations were identified from CT scans and superimposed on the 3D brain model using a locally developed method. The structural lesion boundaries were marked manually on sequential MR image slices and the 3D surface was rendered using Brainsuite.
2.3. Signal Analysis:
Seizure onset regions and automatically detected interictal spikes on FO electrodes were verified by experienced epileptologists (JAL, AS). The FO spike data were processed through our previously established multistep dDTF algorithm (Maharathi et al., 2018) that first requires accurate interictal spike detection(Barkmeier et al., 2012) and time-locked spike segments, followed by evaluating the causal propagation of spikes. Briefly described, a rule-based algorithm first identifies epochs of interictal spikes to separate active time segments from periods of non-spiking background activity. Then, these specific time blocks are inspected for temporally consecutive spikes within 50 milliseconds. Such spikes on multiple channels were further isolated and grouped into time blocks. Each spike block started 750 milliseconds before the first spike peak and ended 750 milliseconds after the last spike peak.
To determine network propagation activity, each time block was extracted and further processed through discrete short-time direct directed transfer function (dDTF). dDTF is a previously established Granger causality-based model that predicts the flow of information between signal pairs in the presence of all other signals(Franaszczuk et al., 1994). First, spike blocks tested for stationarity using the Phillips–Perron test (~96.6% of spikes were stationary with p<0.2 when tested on individual spikes). Spike blocks were then processed using methods described previously(Maharathi et al., 2018, 2016) where the entire signal was first filtered into specific frequency bands (20th order infinite impulse response filter), then fitted to a multivariate autoregressive model using modified covariance methods. Model order was determined by Akaike information criterion (AIC), corresponding to minimum AIC values (Model order: 13±12, minimum AIC value for each block: −0.0013±11, maximum model order: 50). The linear model was transformed to the frequency domain, and the resulting power spectra was used to identify partial coherence and full frequency-normalized direct transfer function (ffDTF). The dDTF is the linear product of the partial coherence and ffDTF and dDTF indicates the direction of information flow along with the strength of connection. Finally these values were statistically validated by exceeding the 95th percentile of 100 -fold phase-randomized surrogate data.(Maharathi et al., 2018, 2016)
To investigate the propagation in narrow as well as broad frequency bands, we evaluated dDTF in isolated narrow bands such as delta (1–4Hz), theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), gamma (30–50 Hz) and a broad band at 1–35Hz.
2.4. Statistics:
Pearson correlations were used for simple comparisons of propagation results. Multiple correlations used the correction , where R is the multiple correlation coefficient, c = (rx1y, rx2y, …, rxNy) T the correlations of rxny, between independent variables Xn and dependent variable y, and Rxx is correlation matrix, defined as
We used a threshold α=0.05 for significance on all comparisons.
3. Results:
3.1. Patient characteristics for interictal spike network analysis:
Based on the successful application of dDTF to map interictal spike networks in neocortical epilepsy from subdural recording electrodes(Maharathi et al., 2018), we asked whether interictal spike networks exist in patients being evaluated for temporal lobe epilepsy using bilateral FO recordings from 10 consecutive studies. Table 1 lists these patients with long-term, bilateral FO recordings of the mesial temporal surfaces as shown in Fig. 1A. All patients had simultaneous recordings from scalp electrodes. Four of these patients were lesional: two with unilateral hippocampal sclerosis, one with a teratoma tumor and one with focal dysplasia. Patients 5 and 10 were two separate recordings from the same patient with hippocampal asymmetry without sclerosis. The other 4 were either normal or showed diffuse atrophy on MRI. We captured seizures in all the lesional patients and in one of the non-lesional patients. Medication withdrawal was performed to try to elicit seizures in all patients.
Table 1.
Patient clinical information (note: 5 and 10 are the same patient with two separate recordings).
| Patient | Age (Years) | Sex | Age of Onset (Years) | MRI Image Findings | Seizure Onset |
|---|---|---|---|---|---|
| 1 | 39 | Female | 17 | Tumor (teratoma) in left hippocampus | Left anterior temporal |
| 2 | 44 | Male | 10 | Left hippocampal sclerosis | Left anterior temporal |
| 3 | 34 | Male | 20 | Left temporal dysplasia | Left posterior temporal |
| 4 | 45 | Female | 06 | Left hippocampal sclerosis | Bilateral independent temporal |
| 5 | 52 | Male | 27 | Diffuse volume loss | None captured |
| 6 | 55 | Male | 20 | Normal | None captured |
| 7 | 21 | Male | 14 | Normal | Extra temporal |
| 8 | 36 | Female | 0.5 | Normal | None captured |
| 9 | 48 | Male | 36 | Left hippocampal atrophy | None captured |
| 10 | 51 | Male | 27 | Diffuse volume loss | None captured |
Figure 1. Interictal spike propagation networks are measured using a direct directed transfer function (dDTF).
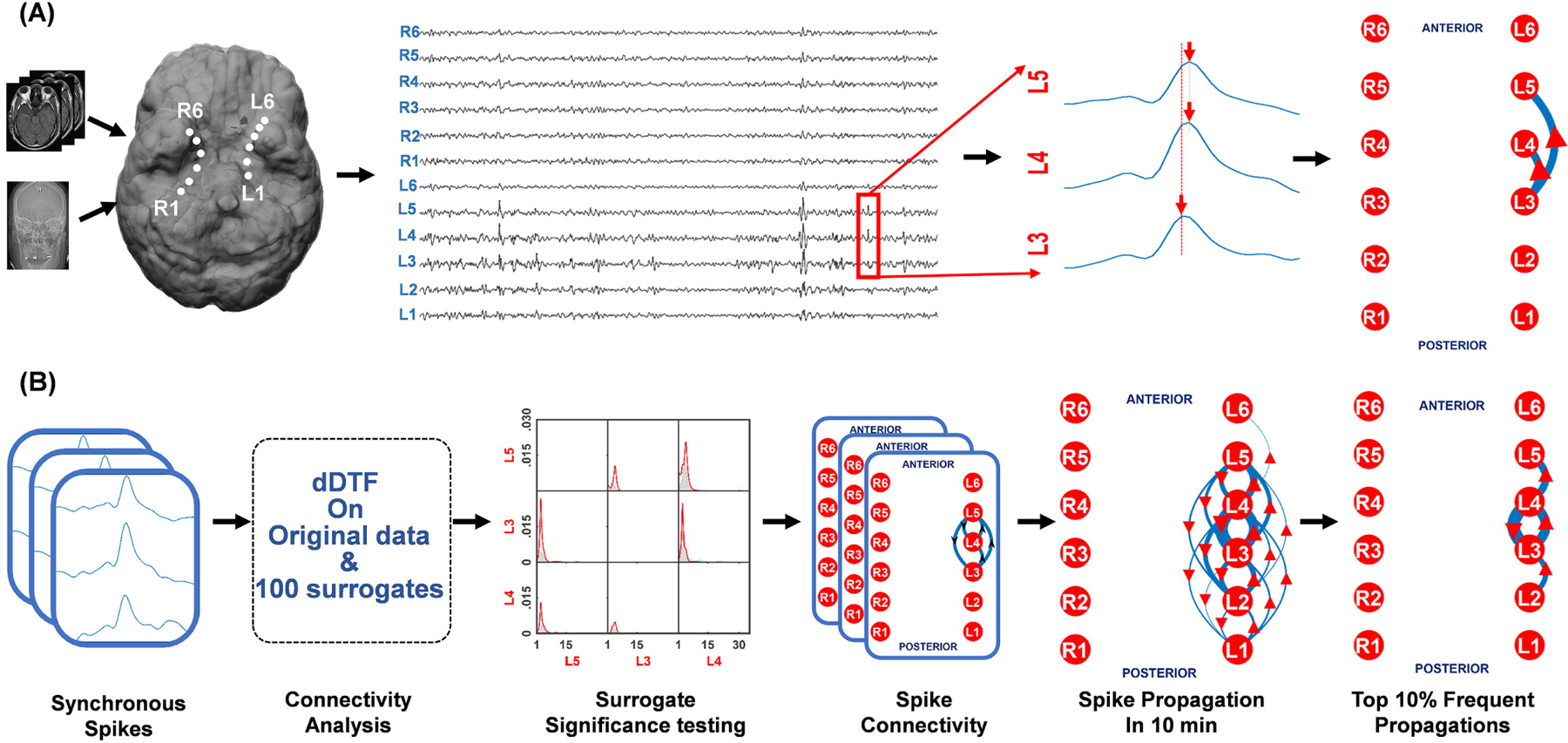
(A) Using the MRI and CT scan, the electrodes placed through the foramen ovale (FO) are co-registered showing their proximity to specific mesial temporal structures. Spikes detected from the FO electrodes appear synchronous, but when closely examined have clear time delays such as between electrodes L3 and L4/L5. (B) dDTF was applied to a given 10-minute EEG segments after spikes were first marked using an automated spike detection algorithm. The detected synchronous appearing spike blocks of spikes were then extracted and processed through the dDTF based connectivity analysis. This function, that combines surrogate data analysis and statistical validation, provides a spike propagation network for each block of spikes, which is then further consolidated for 10 minutes of EEG. The top 10% of spike propagations are shown in the far right to create a simplified network for further analysis. In each schematic the thickness of the arrow indicates the frequency of a particular propagation pattern and the arrowhead indicates the direction of spread.
For each FO study, we selected six 10-minute EEG recordings with at least 100 interictal spikes (median spike count: 442±635) in each segment, marked using a validated spike detection algorithm(Barkmeier et al., 2012). Figure 1A shows that while spikes often appear synchronous, they are not. By measuring the delay between nearby spikes, it is possible to map the spike propagation pattern for each spike. In Fig. 1B, we used the dDTF algorithm to map the spike propagation network of all time-locked spikes from each 10 min EEG recording. The result produces a probability map and directionality for each pair of electrodes for the 12 FO electrodes. The algorithm uses surrogate data based statistical validation to determine whether a spike will propagate between any pair of electrodes more than would be expected by chance. The end result produces a spike network map shown on the right-hand part of Fig. 1B that quantitatively summarizes all of the spike propagations and their direction as arrows within a given 10 min period. The thicker the line, the greater the number of spike propagations. To simplify the ‘wiring diagram’ to indicate the most common spike propagations in the network, we also show the top 10 percentile spike propagations.
3.2. Interictal spike networks are highly consistent within each patient and are not state dependent:
Three of the six 10 min EEG segments were acquired during wakefulness and three in sleep. To measure the consistency of each patient’s network and the relationship of the awake versus sleep state, we compared the interictal spike networks across both sleep and awake time segments. When analyzed in 1–35Hz broad frequency band as shown in Fig. 2A, we found that each patient had a highly consistent networks across all wake and sleep time periods (0.93±0.13) with no significant difference between awake and sleep (p<0.05) (Fig. 2B). Note that the interictal spike networks from patients 1–5, that were both mostly unilateral and lesional, were the most consistent.
Figure 2. Interictal spike networks are highly reproducible and independent of sleep wake cycles.
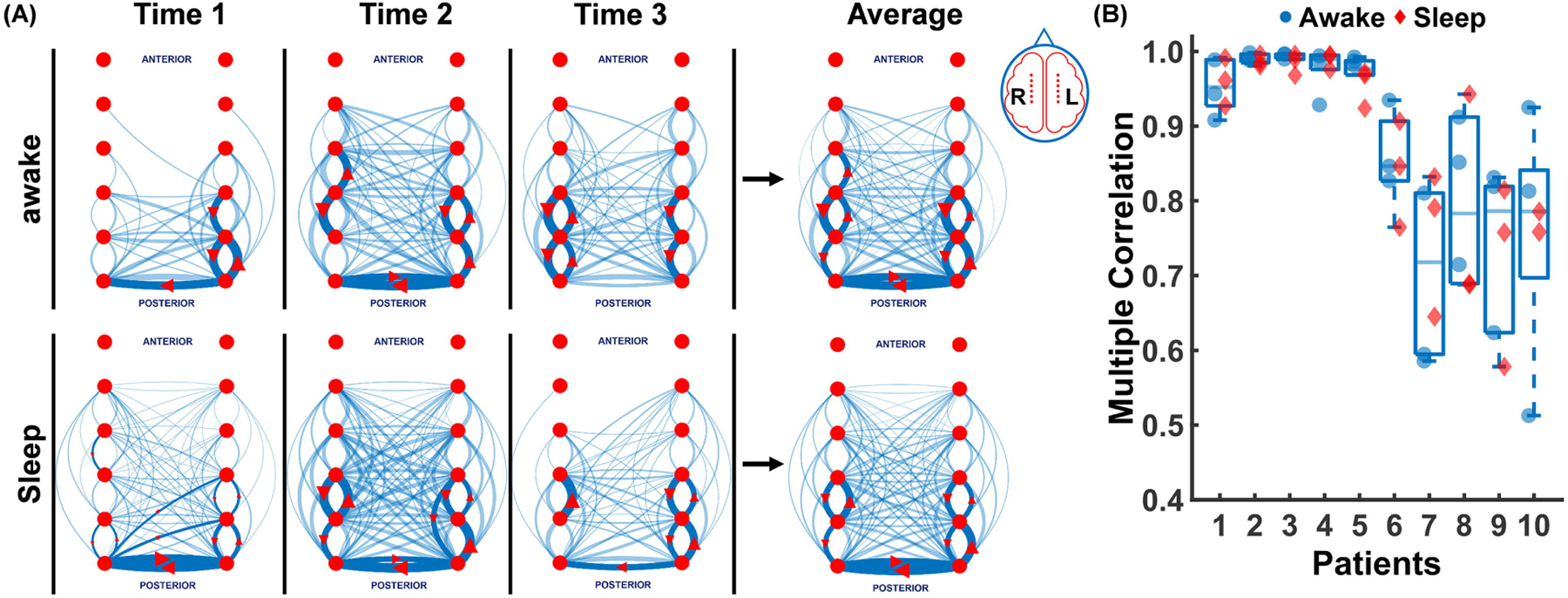
(A) The top 10% (solid blue lines with red arrow marks) and 90% remaining (faint blue lines) propagations are shown for patient 8 at three different awake and sleep 10 min intervals (Time1, Time2, Time3). Propagations here include all EEG activity between the broad band of 1–35 Hz. The average of the awake and sleep propagations are shown on the right panel which shows the high level of similarity for both wake and sleep spike propagations. (B) Multiple correlation coefficients across 3 sleep (red diamonds) and 3 awake (blue circles) 10 min EEG segments for each of 10 patients (0.93±0.13) is shown demonstrating the high reproducibility of the measured spike networks in both wake and sleep states.
3.3. Interictal spike networks are patient-specific, multidirectional, and cross over to the contralateral temporal lobe:
Figure 3A shows the interictal spike networks for each patient together with their top 10th percentile network (Fig. 3B). No two patients had the same networks. Within each network, spikes propagated both within and between temporal lobes. Spikes sometimes had a clear directionality of spread: for example, from anterior to posterior or posterior to anterior. For many brain regions spike networks were bidirectional and reverberated between two or more sites. In addition to spike propagations within a given temporal lobe, spikes also frequently propagated between temporal lobes. For each patient, the percentage of spike propagations within each temporal lobe (intra temporal) and between the two temporal lobes (cross temporal) are shown. Intra temporal propagations (67±13%) were significantly higher than cross temporal propagations (33±13%) in 9 out of 10 patients (p< 0.05) suggesting most of the propagations remain within the same temporal lobe. The patient with studies 5 and 10 is the same patient that was recorded twice (the earlier recording is 10, the recording done the following year is 5). Study 5 showed unilateral spike networks without cross temporal propagations, study 10 instead had bilateral spike networks with significant cross temporal propagations. It is possible that either medication differences (clobazam stopped the day prior to the recording) or the presence of a vagus nerve stimulation device placed prior to study 5, but not present for study 10, prevented these cross-temporal propagations and kept the spike network unilateral. In patient 7, although the intra temporal propagations were higher than the cross-temporal propagations, it was not significant (p< 0.1). Interestingly, most cross temporal lobe spikes utilized posterior rather than anterior anatomical connections. Of note, we did not see any significant effect of sleep or awake states on cross temporal propagations in any patient (data not shown).
Figure 3. Each patient has a unique spike propagation network that can be unilateral or bilateral.
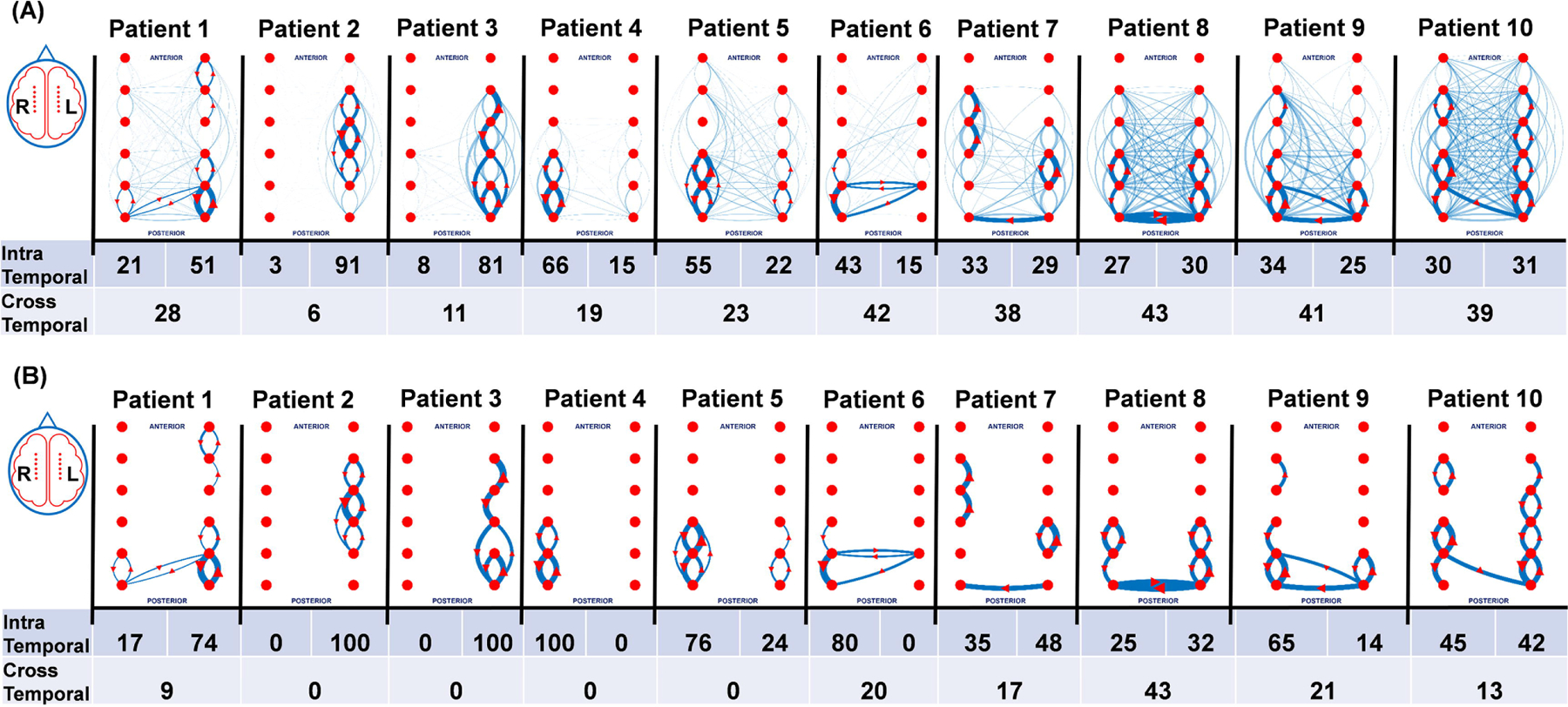
(A) The total spike propagation network and the percentage that occur within (intra temporal) and between (cross temporal) temporal lobes are shown in the top panel. The solid blue lines with red arrows represent top 10 percentile of the propagation, and the faint blue lines represent bottom 90 percentile of the propagation. (B) Data for only the top 10th percentile propagations from panel A show simplified network schemata for each patient as well as the intra and cross propagation percentages. Foramen Ovale implanted patient studies 1–5 were mostly unilateral, whereas 6–10 had a mix of intra and cross temporal propagations that may be related for some to the presence of ipsilateral lesions.
3.4. Interictal spike networks are strongly influenced by brain lesions:
In lesional patients, propagations were primarily ipsilateral to the lesion (patients 1–4). The patients without brain lesions (patients 6, 7, 8, and 10), had comparable propagations within each temporal lobe and had the most cross temporal propagations. Patient 9 had an MRI documented reduction in the volume of the anterior hippocampus with no sclerosis. While his spike propagations were bilateral and cross temporal, there were very few propagating spikes over the region with reduced hippocampal volume and most of his spike network involved the contralateral (right) side.
3.5. Interictal spike networks are consistent across frequency bands:
Given the growing interest in the predictive value of different frequency bands, we analyzed the interictal spike networks across delta, theta, alpha, beta, gamma, and broad bands (1–35 Hz). This is shown for patient 2 in Fig. 4A and for all patients in Fig. 4B. We did not find any significant differences in spike propagations on EEG data filtered for each of these frequency bands or as a function of specific awake and sleep cycles. While both low and high frequency bands had highly reproducible networks (0.97±0.006) within each patient, we failed to find any frequency-dependent differences. Although broad band and delta band appeared to have slightly higher connection density, no significant difference between other frequency bands were observed. We also noticed that the patient subgroup who had highly conserved networks in broad bands, did show higher network similarity across different frequency bands (median: 0.99) compared to the subgroup that had lower network similarity in broad band (median: 0.90). We did not specifically examine high frequency oscillations due to the limitations of our recordings, recorded at 200 Hz.
Figure 4. Interictal spike networks are highly reproducible across different frequency bands.
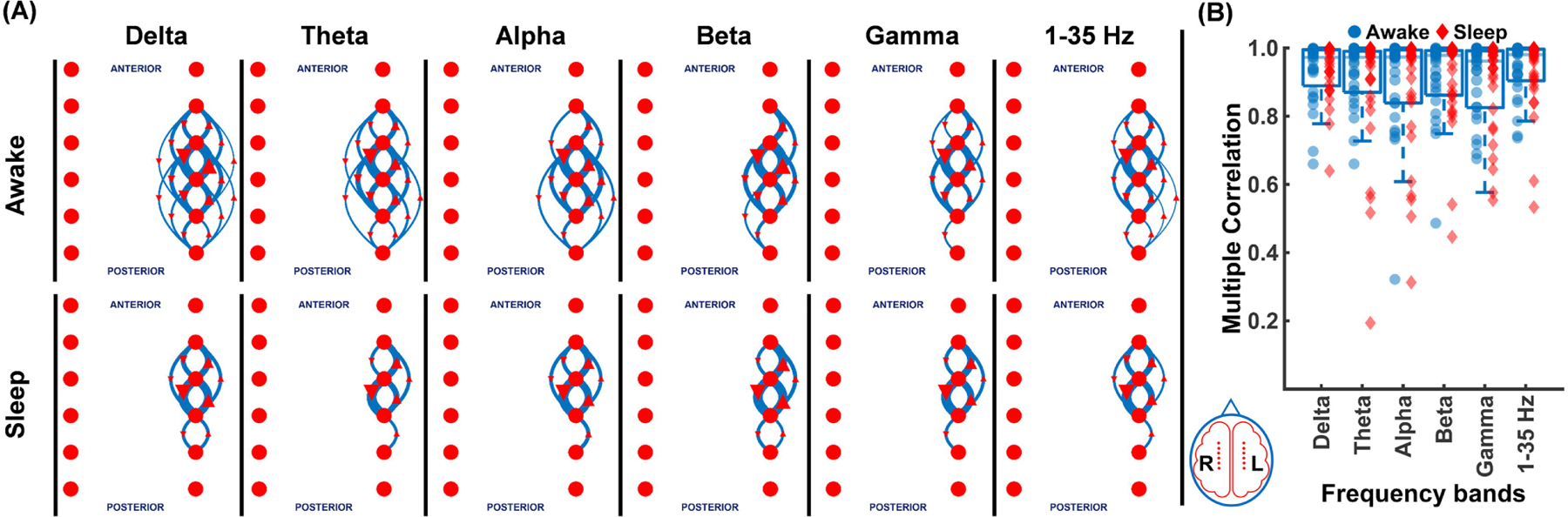
(A) The top 10 percentile spike propagation patterns at delta, theta, alpha, beta, gamma frequency bands, and 1–35 Hz broadband shown for patient 2 are remarkably similar. (B) Multiple correlation coefficients (0.97±0.006) for each frequency band across all patients during sleep (red diamonds) and awake (blue circles) are quite similar.
3.6. Mesial temporal spikes cannot be detected on surface EEG and do not clearly propagate to the cortical surface:
Previous studies using both intracranial and surface electrodes have shown the limitations of surface leads to detect EEG activities in deeper structures, such as the mesial temporal lobe(Lam et al., 2017; Velasco et al., 2006). An unanswered question is whether spikes that are detected by surface EEG in deep structures are detecting spikes generated from these deep structures via volume conduction or whether they are propagating to the cortical surface. We measured ~8000 time-locked spike blocks on FO electrodes and determined the percentage that coincided with scalp EEG spikes. On average, only 4% of mesial temporal spikes coincided with scalp EEG spikes (Fig. 5). This finding is in line with previously published results(Fernández Torre et al., 1999; Lam et al., 2017; Nayak et al., 2004). However, when we used the dDTF function to determine the directionality of propagation, we did not find evidence of mesial temporal spikes propagating to the scalp or vice versa (Fig. 5). While these findings confirm that surface EEG spikes are not generated from deeper temporal structures by volume conduction, they do not reveal clear direction of propagation and instead appear synchronous between mesial and lateral temporal lobe structures.
Figure 5. Medial temporal spikes rarely appear on scalp electrodes and are have no clear propagation pattern.
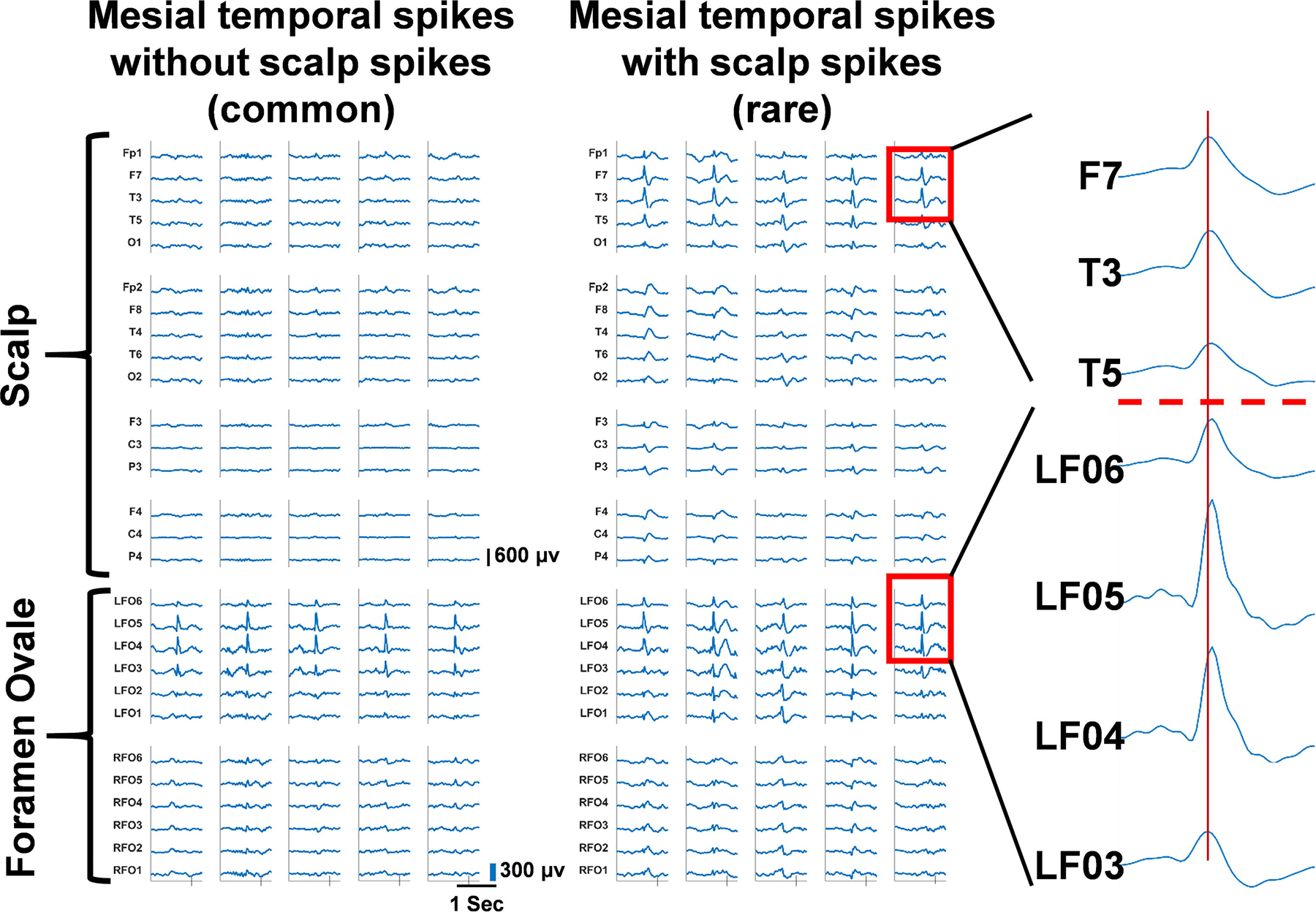
Medial temporal spikes recorded from Foramen Ovale (FO) electrodes (L3 – L6) show synchronous interictal spikes with the scalp leads only 4% of the time (left) at (F7, T3, T5) (right) for patient 2. A zoomed in visual of the spike events on FO electrodes and scalp electrodes show highly synchronous FO and scalp EEG spikes with no consistent time delays between FO and the scalp events, suggesting no clear pattern of propagation between these two recording sites.
3.7. Spike networks are closely related to seizure onset and lesion location:
While the present series is limited in the number and similarity of patients, in this retrospective analysis, we explored the relationships between interictal spike networks, seizures, and lesion locations in those with lesions in the hope that this type of analysis could in the future aid in surgical and non-surgical planning. Only 5 of 10 patients had seizures recorded and of these only two had well-defined lesions and focal seizures (patients 1 and 2, Table 1). The relationship between lesion, seizure onset, and spike networks are summarized in Fig. 6 for these two patients.
Figure 6. Relationships between interictal spike networks, structural lesions, and seizure onset zones.
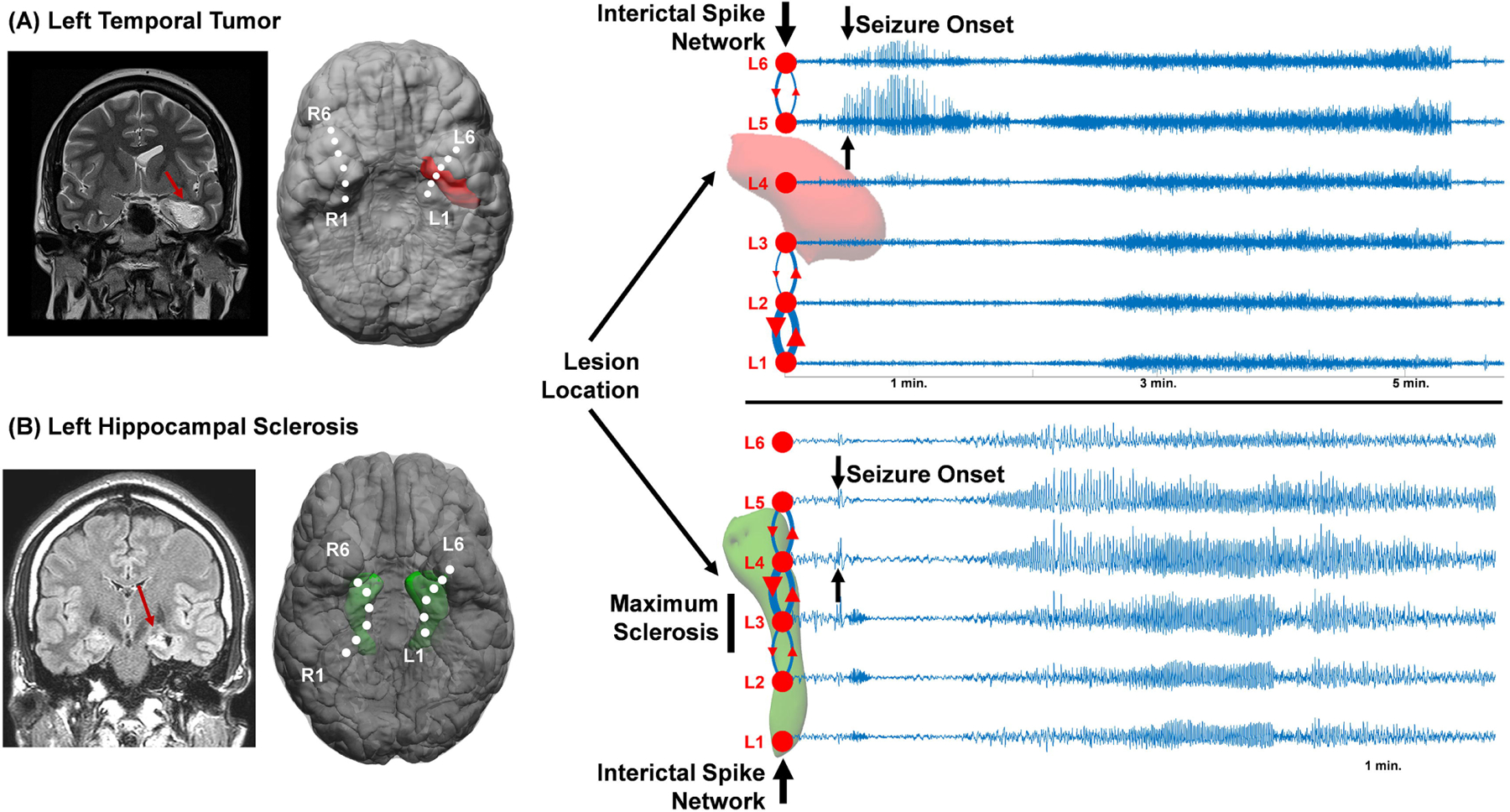
(A) Patient 1 had a teratoma tumor in the mesial temporal lobe that was co-registered to the Foramen Ovale electrodes. The top 10 percentile interictal spike network is shown in relation to the lesion and seizure onset zones. (B) Patient 2 had left hippocampal sclerosis with a smaller hippocampus and a bright T2 -FLAIR MRI signal. The interictal spike network and seizure onset zones were both present at the L4 and L5 electrodes which were on the edge of the sclerotic region.
Patient 1 had a teratoma tumor adjacent to the left hippocampus extending to the left temporal lobe. The L4 electrode was closest to the tumor, however, had very few spikes. The interictal spike network predominantly split into two regions: L1-L3, posterior to the tumor, and L5-L6, anterior to the tumor (Fig. 6A). Maximal spike reverberation occurred between L1 and L2. Three recorded seizures were generated anterior to the tumor at L5-L6 with subsequent spread to other electrodes. While the most active subnetwork involved the posterior electrodes (L1-L3), it was the anterior L5-L6 spike network that perfectly aligned with the seizure onset zone.
Patient 2 had left hippocampal sclerosis with maximal MRI FLAIR signal and volume loss seen at L3 (Fig. 6B). The spike network for this patient was broad from L2-L5 with maximum reverberations at L3-L4, just anterior of the region of maximum sclerosis. Three out of 4 seizures recorded had onset starting further anterior to the sclerosis that started with heralding spikes at L4-L5 followed by spread to L2-L3 posterior to the sclerosis and then more uniform ipsilateral involvement throughout the temporal lobe.
While the present series is limited, the use of dDTF for these 2 lesional patients reveals that both spikes and seizure networks were adjacent to the lesions, however a larger series will be required to generalize this limited finding to the role of lesions with respect to the spike and seizure networks. These examples also demonstrate that while a portion of the spike network overlaps with seizure onset, other portions of the network, often with the highest level of spiking occur at independent locations from the seizure network. The findings suggests that not all spike networks are related to seizures. Further research on a larger patient cohort will be required to develop stronger associations between interictal spikes, seizure, and brain lesions.
4. Discussion:
4.1. Highly Consistent Interictal Spike Networks in Foramen Ovale Patients:
Interictal spikes have been suggested to be a significant biomarker of epilepsy and are hypothesized to arise from the cumulative synchronous firing of millions of underlying neuronal cells. The concept of the epileptogenic focus has been recently revised to a broader definition of an “epileptogenic network” that includes different brain regions(Bartolomei et al., 2017), amongst which hippocampus is a key player. Understanding interictal networks is extremely important for predicting seizures networks and targeting therapies such as focal ablation and neuromodulation. While there have been many attempts to explore interictal network connectivity using both intracranial EEG and MRI studies, these have not produced highly reproducible networks nor has their relationship to structural lesions and seizure onset zones been clear. A previous study from our group showed that interictal spike propagation patterns in the neocortex are both unique, yet ‘hard-wired’ and highly consistent within a given patient(Maharathi et al., 2018). We also found that interictal spike networks are different from networks predicted based on the entire EEG dataset (similar to those described previously (Maharathi et al., 2019a)), suggesting the importance of first identifying spikes (Maharathi et al., 2019b; Maharathi et al., 2019a). Here we demonstrate this same hard wiring of interictal spike networks in patients being evaluated with foramen ovale electrodes, a less invasive form of intracranial EEG monitoring of the mesial temporal lobe.
An important limitation of previous functional connectivity approaches on the interictal state that use correlation, coherence and causality methodologies come from significant variability of the network and its dependence on other covariates. Similarly, studies that have looked only at the rate of occurrence (frequency) of interictal spikes in different brain regions are limited by their variability in occurrence, state dependence (awake/asleep) and in inconsistencies of this measure to predict seizure onset zones(Conrad et al., 2020). By focusing on spike propagation, we were able to uncover highly reproducible interictal networks, independent of the sleep-awake state. Interictal spike propagation was also independent of frequency bands, similar to what has been seen in the neocortex(Maharathi et al., 2018). This suggests that spike propagations have multiple frequencies in their signals that propagate together. While any cross frequency propagations are yet to be studied, the frequency spectrum of interictal spike propagation reflects a significant power content over wide frequencies which are not observed in the background interictal signal and hence would likely be missed using methods that do not first identify the spikes(Maharathi et al., 2019a). The reproducibility of interictal spike network that colocalizes with the active epileptic regions irrespective of state of the patient and the frequency band as observed in two patients might suggest that the spike network can be a reliable predictor of seizure onset zones, however more detailed studies in additional patients will be necessary to generalize this conclusion
4.2. Interictal Spike Networks are Bilateral but more often Unilateral in the Presence of Brain Lesions
Using the Granger causality based dDTF algorithm on intracranial recordings obtained with bilateral foramen ovale leads in 10 consecutive FO studies of epileptic patients, we found that patients with brain lesions had the most consistent spike networks and tended to be unilateral compared to non-lesional patients. Non-lesional patients had more bilateral propagations involving both temporal lobes along with cross-temporal propagations. Spikes propagated in both anterior and posterior directions along the mesial temporal surface and most cases, reverberated in both directions. This was particularly prominent in lesional patients where spike reverberations occurred near the lesion and did not cross to the other temporal lobe. Surprisingly, most cross-temporal lobe propagations occurred through the posterior electrode positions, suggesting usage of the hippocampal commissure within the fornix rather than the anterior commissure as the anatomical pathway for spike propagation.
The relationships between epileptic zones, irritative zones, and brain lesion location has been carefully studied over many years (Talairach and Bancaud, 1966). It is generally well-accepted that patients with temporal lobe lesions seen on MRI have a better surgical outcome than those without lesional forms of epilepsy(Clusmann et al., 2004). Lesional patients in our series had more restricted and unilateral interictal spike networks compared to non-lesional patients, who generally had bitemporal networks with more frequent spike crossings between the two temporal lobes. Non-lesional patients from our series either had no clear seizures or seizure onset zones outside of mesial temporal lobe structures. Lesional patients also had seizure onset zones partially overlapped with the interictal spike network as observed in two patients. Interestingly, these spike/seizure onset areas were not at regions with the highest frequency of interictal spiking. The remarkable reproducibility of spike networks adjacent to lesions, as observed in two patients, suggest that identifying and subsequently removing interictal spike networks at the time of lesion resection could improve patient outcome.
While our series is limited, both spike networks and seizure onset zones mostly involved lesion borders. The lesions themselves, including tumors and hippocampal sclerosis, had reduced spike networks and were not the clear sites of seizure onset. Spike propagations commonly reverberated at perilesional boundaries. Other studies highlight this observation. One study showed peritumoral spikes to be most frequent and sharper than other interictal spikes(Mittal et al., 2016). Similarly, studies in tuberous sclerosis suggest that epileptic spikes are often seen long before they have their first seizure and the spikes often localize with seizure onset zones that are peri-tuberal(Wu et al., 2016). Similar observations have also been reported for cortical dysplasias where interictal spikes were observed over the perilesional spaces (Tassi, 2002). This is not surprising given that many tumors, including the teratoma patient studied here, are not themselves capable of generating epileptic activity. In the case of hippocampal sclerosis, sub-regions of the hippocampus with the most gliosis and neuronal cell loss are also less likely to generate epileptic activities as easily as adjacent, more intact regions of the hippocampus.
It has been postulated that bilateral (bitemporal) discharges are the consequence of the progressive nature of epileptogenesis (Morrell, 1989). Based on this, it was thought that the longer the duration of epilepsy, the more likely it will spread to the contralateral side. Within our limited patient cohort, there was no correlation between age of onset or duration of epilepsy, but instead we found that the absence of temporal lobe lesions was the strongest indicator of bilaterality. Interestingly, the same patient recorded on two separate occasions (patient 5 and 10) was initially bilateral (10), but became unilateral (5) while taking the medication clobazam and having vagus nerve stimulation. This observation demonstrates the potential to explore the specific effects of medications and other treatments on interictal spike networks as a potential therapeutic approach. It also supports the effect of neuromodulation on spike networks.
4.3. A Small Fraction of Mesial Temporal Spikes are Associated with Cortical Spikes but without a Clear Pattern of Propagation:
The relationship between cortical and hippocampal electrical events is far from clear. Previous studies suggest that the ictal events originating from hippocampus are distinct from the ictal events with cortical onset observed on scalp EEG. This is further complicated by observations that events present in medial brain regions are often not detected by scalp recording electrodes (Ebersole and Pacia, 1996; Pacia and Ebersole, 1997). Consistent with other studies on interictal spikes(Lam et al., 2017), we found that only 4% of mesial temporal spikes seen with the FO electrodes were time-locked with spikes on scalp electrodes. Patients with lesional epilepsy had high amplitude scalp spikes that were closely time-locked with spikes seen with the FO electrodes, and were always lateralized to the side of the lesion. Non-lesional patients who had less well-formed spike networks on the FO electrodes, did not have synchronous scalp spikes. However, unlike spikes within or between the mesial temporal lobe structures, coincident spikes in the lateral temporal cortex (seen by FO and surface electrodes at the same time) had no clear directionality of spread. These results strongly suggest that temporal lobe spikes detected with scalp EEG are not being generated from mesial temporal structures and detected by volume conduction. Instead, they are generated in neocortical regions closest to the surface EEG electrode. Previous studies looking at neocortical spikes detected by subdural grids and surface EEG concluded that cortical sources of scalp EEG spikes require the synchronous activation of at a minimum of 6–10 cm2 of neocortex, with 20−30 cm2 needed to produce prominent scalp EEG spikes(Tao et al., 2005). The fact that there was no clear directionality of spread between FO and surface-detected spikes, suggests that the onset of the spike may be generated somewhere between medial and lateral structures. It is also possible that lesional patients recruit a larger neuronal network than non-lesional patients thus generating larger interictal spike fields that more readily propagate to the cortical surface.
4.4. Uniqueness and Potential Roles for Interictal Spike Networks in Epilepsy Therapeutics:
Several methods have been used to analyze interictal and ictal epileptic networks. These can be broadly classified into connectivity analysis, causality analysis and graph-theory based analysis (Bartolomei et al., 2017). Connectivity analysis that takes advantage of linear or non-linear correlation and regression techniques to establish the pairwise relationship between signals. Such connectivity analysis provides preliminary information on the interaction of different brain regions, however, does not delineate the specific directionality. Frequency domain specific connectivity analysis, such as coherence and non-linear information theory-based approaches, does reveal information about connectivity, but is often limited by the number of signals that can be analyzed at a given time. More advanced causality-based analyses provide a better understanding of the directionality of the information flow. Granger causality based multichannel methods such as dDTF and partial direct coherence provide a more comprehensive view of the causal network while normalizing the global effects (Astolfi et al., 2007, 2005; Bianchi et al., 2013). Other types of analysis have been based on graph-theory approaches such as the concept of “small world”, node strength, centrality measures (Guye et al., 2010; Watts and Strogatz, 1998). Here, we combined the multichannel causality analysis with graph theory (Maharathi et al., 2018; Maharathi et al., 2019a) to analyze the association between interictal spike networks and seizure onset in the presence or absence of lesions.
Taken together, while the current study has limitations in cohort size, electrode coverage, and diversity of patients, it provides evidence of highly reproducible interictal spike networks. These networks are closely linked to both lesions and seizure onset zones. Our results show that the most frequent and consistent spike networks come from perilesional tissues and correspond to seizure onset zones. Non-lesional patients have spike networks that are more diffuse, and more often bilateral. Interictal spike networks in patients with temporal lobe seizures had their spike network partially overlapped with their seizure onset zone, whereas the remaining spike network regions, often with the highest spike occurrence, were spatially distinct from the seizure onset zone. Further studies will be needed to delineate both spatial and temporal relationships between interictal spike networks and seizures to see if interictal spike networks can be effectively used to predict seizure onset zone location and timing. Understanding spike networks could help determine which spiking regions need to be removed for better postsurgical outcomes. Given the expanding use of closed loop and direct stimulation devices for the treatment of refractory epilepsy, a precise spatial and temporal understanding of interictal spike and seizure networks could vastly improve the utility of these and other devices.
Highlights.
Interictal spike network is highly reproducible across time and frequency bands in temporal lobe patients with foramen ovale electrodes.
Interictal spike networks are both bilateral and unilateral, where they are closely related to seizure onset zones and structural lesions.
A small fraction of mesial temporal Spikes are associated with cortical spikes but without a clear pattern of propagation.
Acknowledgements
This work was funded by NIH/NINDS Grants NIH NS109515, NIH NS083527 (JL), NIH UL1TR002003 (BM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Nothing to report.
References
- Antony AR, Alexopoulos AV, González-Martínez JA, Mosher JC, Jehi L, Burgess RC, et al. Functional Connectivity Estimated from Intracranial EEG Predicts Surgical Outcome in Intractable Temporal Lobe Epilepsy. PLoS One 2013;8:e77916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, Marciani MG, Baccala LA, de Vico Fallani F, et al. Comparison of different cortical connectivity estimators for high-resolution EEG recordings. Hum Brain Mapp 2007;28:143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astolfi L, Cincotti F, Mattia D, de Vico Fallani F, Lai M, Baccala L, et al. Comparison of different multivariate methods for the estimation of cortical connectivity: simulations and applications to EEG data. Conf Proc. Annu Int Conf IEEE Eng Med Biol Soc IEEE Eng Med Biol Soc Annu Conf 2005;5:4484–7. [DOI] [PubMed] [Google Scholar]
- Bancaud J, Angelergues R, Bernouilli C, Bonis A, Bordas-Ferrer M, Bresson M, et al. Functional stereotaxic exploration (SEEG) of epilepsy. Electroencephalogr Clin Neurophysiol 1970;28:85–6. [PubMed] [Google Scholar]
- Barkmeier DT, Shah AK, Flanagan D, Atkinson MD, Agarwal R, Fuerst DR, et al. High inter-reviewer variability of spike detection on intracranial EEG addressed by an automated multichannel algorithm. Clin Neurophysiol 2012;123:1088–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolomei F, Lagarde S, Wendling F, McGonigal A, Jirsa V, Guye M, et al. Defining epileptogenic networks: Contribution of SEEG and signal analysis. Epilepsia 2017;58:1131–47. [DOI] [PubMed] [Google Scholar]
- Beleza P, Rémi J, Feddersen B, Peraud A, Noachtar S. Epidural and foramen-ovale electrodes in the diagnostic evaluation of patients considered for epilepsy surgery. Epileptic Disord 2010;12:48–53. [DOI] [PubMed] [Google Scholar]
- Bettus G, Wendling F, Guye M, Valton L, Régis J, Chauvel P, et al. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res 2008;81:58–68. [DOI] [PubMed] [Google Scholar]
- Bianchi AM, Marchetta E, Tana MG, Tettamanti M, Rizzo G. Frequency-based approach to the study of semantic brain networks connectivity. J Neurosci Methods 2013;212:181–9. [DOI] [PubMed] [Google Scholar]
- Clusmann H, Kral T, Fackeldey E, Blümcke I, Helmstaedter C, von Oertzen J, et al. Lesional mesial temporal lobe epilepsy and limited resections: prognostic factors and outcome. J Neurol Neurosurg Psychiatry 2004;75:1589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coito A, Genetti M, Pittau F, Iannotti GR, Thomschewski A, Höller Y, et al. Altered directed functional connectivity in temporal lobe epilepsy in the absence of interictal spikes: A high density EEG study. Epilepsia 2016;57:402–11. [DOI] [PubMed] [Google Scholar]
- Coito A, Plomp G, Genetti M, Abela E, Wiest R, Seeck M, et al. Dynamic directed interictal connectivity in left and right temporal lobe epilepsy. Epilepsia 2015;56:207–17. [DOI] [PubMed] [Google Scholar]
- Conrad EC, Tomlinson SB, Wong JN, Oechsel KF, Shinohara RT, Litt B, et al. Spatial distribution of interictal spikes fluctuates over time and localizes seizure onset. Brain 2020;143:554–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersole JS, Pacia SV. Localization of Temporal Lobe Foci by Ictal EEG Patterns. Epilepsia 1996;37:386–99. [DOI] [PubMed] [Google Scholar]
- Engel J, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 2012;307:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández Torre J, Alarcón G, Binnie C, Polkey C. Comparison of sphenoidal, foramen ovale and anterior temporal placements for detecting interictal epileptiform discharges in presurgical assessment for temporal lobe epilepsy. Clin Neurophysiol 1999;110:895–904. [DOI] [PubMed] [Google Scholar]
- Franaszczuk PJ, Bergey GK, Kamiński MJ. Analysis of mesial temporal seizure onset and propagation using the directed transfer function method. Electroencephalogr Clin Neurophysiol 1994;91:413–27. [DOI] [PubMed] [Google Scholar]
- Guye M, Bettus G, Bartolomei F, Cozzone PJ. Graph theoretical analysis of structural and functional connectivity MRI in normal and pathological brain networks. MAGMA 2010;23:409–21. [DOI] [PubMed] [Google Scholar]
- Haneef Z, Lenartowicz A, Yeh HJ, Levin HS, Engel J, Stern JM. Functional connectivity of hippocampal networks in temporal lobe epilepsy. Epilepsia 2014;55:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M, Folley BS, Sonmezturk HH, Gore JC, Kang H, Abou-Khalil B, et al. Resting state functional connectivity of the hippocampus associated with neurocognitive function in left temporal lobe epilepsy. Hum Brain Mapp 2014;35:735–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahane P, Landré E, Minotti L, Francione S, Ryvlin P. The Bancaud and Talairach view on the epileptogenic zone: a working hypothesis. Epileptic Disord 2006;8 Suppl 2:S16–26. [PubMed] [Google Scholar]
- Lam AD, Deck G, Goldman A, Eskandar EN, Noebels J, Cole AJ. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat Med 2017;23:678–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders HO, Najm I, Nair D, Widdess-Walsh P, Bingman W. The epileptogenic zone: general principles. Epileptic Disord 2006;8 Suppl 2:S1–9. [PubMed] [Google Scholar]
- Maharathi B, Loeb JA, Patton J. Epileptic spike functional networks best predict seizure onset zones. 2019 9th Int. IEEE/EMBS Conf. Neural Eng., IEEE; 2019a, p. 895–8. [Google Scholar]
- Maharathi B, Loeb JA, Patton J. Central sulcus is a barrier to causal propagation in epileptic networks. 2019 41st Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., IEEE; 2019b, p. 2555–9. [DOI] [PubMed] [Google Scholar]
- Maharathi B, Loeb JA, Patton J. Estimation of resting state effective connectivity in epilepsy using direct-directed transfer function. 2016 38th Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., vol. 2016-Octob, IEEE; 2016, p. 716–9. [DOI] [PubMed] [Google Scholar]
- Maharathi B, Wlodarski R, Bagla S, Asano E, Hua J, Patton J, et al. Interictal spike connectivity in human epileptic neocortex. Clin Neurophysiol 2018;130:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal S, Barkmeier D, Hua J, Pai DS, Fuerst D, Basha M, et al. Intracranial EEG analysis in tumor-related epilepsy: Evidence of distant epileptic abnormalities. Clin Neurophysiol 2016;127:238–44. [DOI] [PubMed] [Google Scholar]
- Morrell F. Varieties of human secondary epileptogenesis. J Clin Neurophysiol 1989;6:227–75. [DOI] [PubMed] [Google Scholar]
- Nayak D, Valentín A, Alarcón G, GarcíaSeoane JJ, Brunnhuber F, Juler J, et al. Characteristics of scalp electrical fields associated with deep medial temporal epileptiform discharges. Clin Neurophysiol 2004;115:1423–35. [DOI] [PubMed] [Google Scholar]
- Nilsson D, Fohlen M, Jalin C, Dorfmuller G, Bulteau C, Delalande O. Foramen ovale electrodes in the preoperative evaluation of temporal lobe epilepsy in children. Epilepsia 2009;50:2085–96. [DOI] [PubMed] [Google Scholar]
- Pacia SV, Ebersole JS. Intracranial EEG Substrates of Scalp Ictal Patterns from Temporal Lobe Foci. Epilepsia 1997;38:642–54. [DOI] [PubMed] [Google Scholar]
- Pittau F, Grova C, Moeller F, Dubeau F, Gotman J. Patterns of altered functional connectivity in mesial temporal lobe epilepsy. Epilepsia 2012;53:1013–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanova V, Markand O, Worth R. Temporal Lobe Epilepsy Surgery: Outcome, Complications, and Late Mortality Rate in 215 Patients. Epilepsia 2002;43:170–4. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal 2002;6:129–42. [DOI] [PubMed] [Google Scholar]
- Talairach J, Bancaud J. Lesion, “irritative” zone and epileptogenic focus. Confin Neurol 1966;27:91–4. [DOI] [PubMed] [Google Scholar]
- Tao JX, Ray A, Hawes-Ebersole S, Ebersole JS. Intracranial EEG Substrates of Scalp EEG Interictal Spikes. Epilepsia 2005;46:669–76. [DOI] [PubMed] [Google Scholar]
- Tassi L. Focal cortical dysplasia: neuropathological subtypes, EEG, neuroimaging and surgical outcome. Brain 2002;125:1719–32. [DOI] [PubMed] [Google Scholar]
- Tracy JI, Doucet GE. Resting-state functional connectivity in epilepsy: growing relevance for clinical decision making. Curr Opin Neurol 2015;28:158–65. [DOI] [PubMed] [Google Scholar]
- Velasco TR, Sakamoto AC, Alexandre V, Walz R, Dalmagro CL, Bianchin MM, et al. Foramen ovale electrodes can identify a focal seizure onset when surface EEG fails in mesial temporal lobe epilepsy. Epilepsia 2006;47:1300–7. [DOI] [PubMed] [Google Scholar]
- Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol 2006;59:335–43. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of “small-world” networks. Nature 1998;393:440–2. [DOI] [PubMed] [Google Scholar]
- Wu JY, Peters JM, Goyal M, Krueger D, Sahin M, Northrup H, et al. Clinical Electroencephalographic Biomarker for Impending Epilepsy in Asymptomatic Tuberous Sclerosis Complex Infants. Pediatr Neurol 2016;54:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]


