Abstract
Background & aims
Severe COVID-19 infection is characterized by an inflammatory response and lung injury that can evolve into an acute respiratory distress syndrome that needs support treatment in intensive care unit. Nutritional treatment is an important component of the management of critically ill patients and should be started in the first 48 h of ICU admission to avoid malnutrition. This study describes the characteristics of the patients treated in a tertiary hospital in Madrid during the months of March–May 2020 (first wave), the medical nutrition treatment employed and its influence in the clinical outcome of these patients.
Methods
This is a retrospective study including COVID-19 patients admitted in ICU that needed medical nutrition treatment (MNT). Collected variables included sex, age, BMI, underlying diseases, time from hospitalisation to ICU admission, type of respiratory support (invasive mechanical ventilation (IMV) or high flow nasal cannula (HFNC) or non-invasive ventilation (non-IMV)), caloric and protein requirements (25 kcal/kg adjusted body weight (ABW), 1.3 g/kg ABW/day), MNT type (enteral nutrition (EN), parenteral nutrition (PN), mixed EN + PN), total calories (including propofol) and proteins administered, percentage of caloric and protein goal in ICU day 4th and 7th, metabolic complications, acute kidney failure (AKF), length of stay (LOS) and mortality. Data are expressed as mean ± SD, median (IQR) or frequencies. Statistical analysis was performed with the IBM SPSS Statistics for Windows, Version 25.0. p < 0.05 were considered statistically significant.
Results
A total of 176 patients were included (72.7% male), 60.1 ± 13.5 years, BMI 29.9 ± 5.4 kg/m2. Underlying diseases included 47.4% overweight, 39.8% obesity, 49.1% hypertension, 41.4% dyslipidaemia. 88.6% of patients needed IMV, 89.1% prone position, 2.9% ECMO. Time to ICU admission: 2 (4.75) days. Estimated caloric and protein requirements were 1775 ± 202 kcal and 92.4 ± 10.3 g. Calories and proteins administered at days 4th and 7th were 1425 ± 577 kcal and 66 ± 26 g and 1574 ± 555 and 74 ± 37, respectively. Most of the patients received PN (alone or complementary to EN) to cover nutritional requirements (82.4% at day 4th and 77.9% at day 7th). IVM patients received more calories and proteins during the first week of ICU admission. Complications included 77.8% hyperglycaemia, 13.2% hypoglycaemia, 83.8% hypertriglyceridemia, and 35.1% AKF. ICU LOS was 20.5 (26) days. The mortality rate was 36.4%.
Conclusions
In our series, the majority of patients reached energy and protein requirements in the first week of ICU admission due to the use of PN (total or complementary to EN). Patients with HFNC or non-IMV may be at risk of malnutrition if total or complementary PN to oral diet/ONS/tube feeding is not used to cover nutritional requirements. Therefore, if EN is not possible or insufficient, PN can be safely used in critically ill patients with COVID-19 with a close monitoring of metabolic complications.
Keywords: COVID-19, Critically ill patients, Nutritional medical therapy, Outcome
1. Introduction
The spectrum of the COVID-19 disease is very broad with most cases classified as mild, but up to 5% of patients will require admission to critical care units [1]. These patients develop an inflammatory response with lung injury and viral pneumonia with severe hypoxemia and characteristic pulmonary infiltrates that can evolve into an acute respiratory distress syndrome (ARDS) that in the most severe forms will require urgent respiratory and hemodynamic support in the intensive care unit (ICU) [2,3].
Spain has been one of the most affected countries in the world during this pandemic, and areas as Madrid were specially hit during the first wave of the disease during the months of March–May 2020 [3,4]. In this scenery, the Spanish National Health System was overburdened in many areas, being forced to increase hospital beds in wards and intensive care units to care for these patients.
In the series published in the literature in different countries the most important risk factors for mortality in these patients are chronic diseases such as diabetes [[5], [6], [7]], hypertension, obesity [[8], [9], [10], [11], [12], [13]], cardiovascular disease, metabolic syndrome [14] and elderly patients [3,4,[15], [16], [17], [18]]. Malnutrition in both extremes, overweight and obesity and undernutrition, has been also associated with a worse outcome [13,[19], [20], [21], [22], [23]]. Nutritional treatment is an important component of the management of hospitalized patients and recent guidelines of the European Society for Clinical Nutrition and Metabolism (ESPEN) have established recommendations for the treatment of patients with polymorbidity and critical illness [5,6,24]. Furthermore, individualized nutritional support has proved to reduce mortality in a recent randomized controlled trial in hospitalized patients [25].
Critical illness is associated with a hypercatabolic response in the early phase with breakdown of muscle mass in order to deliver amino acids for liver gluconeogenesis, to produce inflammatory mediators and for tissue repair. This loss of muscle mass may reach 15–30% after 10 days of ICU stay, depending on the severity of the injury and the age of the patient [26]. As a consequence, ICU-acquired weakness syndrome appears in around 40% of critically ill patients and is associated with worse outcome [27].
According to clinical guidelines, nutritional support should be started in the first 48 h of ICU admission with enteral nutrition (EN) in a progressive way to avoid overfeeding in this early phase that could supress autophagy. The caloric and protein target should be reached at the end of the first week of ICU admission. Whenever EN is not fully tolerated, parenteral nutrition (PN) can be started after day 3rd-7th of ICU admission [24,28].
Specific recommendations for the nutritional treatment of critically ill patients with COVID-19 have been published in ESPEN and ASPEN (American Society of Parenteral and Enteral Nutrition) based in general guidelines of ICU patients [2].
This study describes the characteristics of the patients treated in a tertiary hospital in Madrid during the months of March–May 2020 (first wave), the medical nutrition treatment employed and its influence in the clinical outcome of these patients.
2. Materials and methods
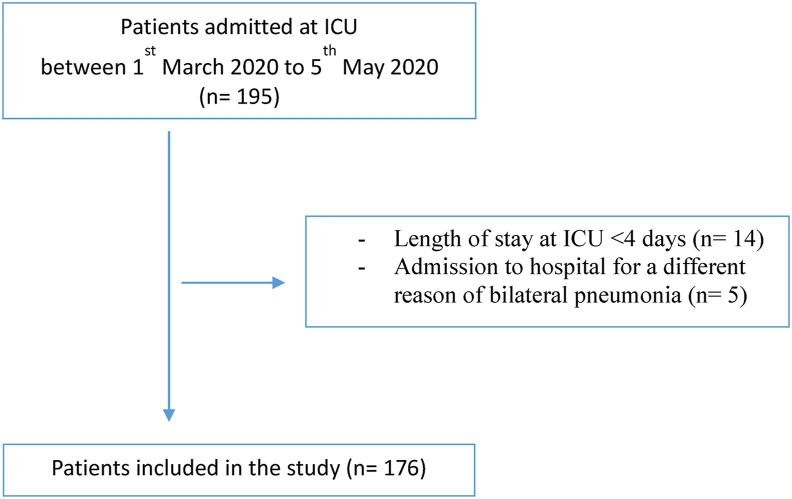
This is a retrospective observational study including all severely ill patients admitted to intensive care unit (ICU) of our hospital between 1st March 2020 to 5th May 2020. The inclusion criterion was confirmed SARS-CoV-2 infection. The exclusion criteria were admission to hospital for a different reason of bilateral pneumonia, length of stay at ICU <4 days and patients sent from the ICU of another hospital.
2.1. Data collection
The retrospective data collection was carried out by the Nutrition Unit staff following a structured form. The following data were recorded: age, sex, pre-existing comorbidities (obesity, hypertension, diabetes, dyslipidaemia, pulmonary disease, oncologic disease, immunological disease), admission hospital date and admission ICU date. We collected the ICU support therapies, including the type of respiratory support, use of extracorporeal membrane oxygenation (ECMO) and kidney replacement therapy (KRT).
Laboratory variables collected were the highest value during the first week of ICU hospitalization for D dimer, fibrinogen, protein C reactive and ferritin; the lowest value during the first week of ICU hospitalization for magnesium, phosphate, and potassium. AST, APT, GGT, bilirubin, hyperglycemia and triglyceride elevation (as qualitative variables: yes or no), the highest value of triglycerides, and the presence of folate and vitamin D deficiency (<3.1 μg/L and <30 μg/L, respectively).
Mortality, ICU and hospital length of stay (LOS) were included as outcome variables.
2.2. Nutritional support
Weight and height were estimated or collected from medical electronic record. Energy requirements were calculated by 25 kcal/kg body weight or adjusted weight (if BMI ≥25 kg/m2), and protein requirements by 1.3 kcal/kg body weight or adjusted weight (if BMI ≥25 kg/m2).
Energy and protein administered at 4th day and 7th day of ICU admission were calculated. Calories provided by propofol administration were taken into account.
The study was approved by the Local Ethics Committee of the hospital.
2.3. Statistical analysis
Continuous variables are represented by their mean and standard deviation. In categorical variables, the results are shown in frequencies and percentages. Numerical variables with non-normal distribution are described by their median and interquartile range (75th percentile-25th percentile). The study of the normality of variables was carried out with graphic tests and using the Kolmogorov–Smirnov or Shapiro–Wilk tests.
To study the differences in means between 2 or more groups, parametric tests (student's t or ANOVA) or non-parametric tests (Mann–Whitney or Kruskal–Wallis) were used, using the most appropriate in each case depending on the normality of the data and the total number of patients in each group. The association between qualitative variables was studied with Pearson's chi-square test or Fisher's exact test. The association between quantitative variables was studied using Spearman's correlation coefficient. The predictive capacity of nutritional treatment for mortality was evaluated through ROC curves. Multivariate Cox regression analysis was used to examine the relationships between nutritional treatment and mortality, ICU and hospital LOS with end points. Median survival was estimated using Kaplan–Meier survival curves and groups were compared using the log-rank test.
Statistical analysis was performed with the IBM SPSS Statistics for Windows, Version 25.0 program. Armonk, NY: IBM Corp. Two-tailed tests were used and results with p < 0.05 were considered statistically significant.
3. Results
3.1. Demographics and baseline characteristics
Of a total of 195 patients admitted to the ICU during the study period, 176 met the inclusion criteria (Fig. 1 ). Their mean age was 60.1 ± 13.5 years and 72.7% were male. The most common underlying comorbidities were 47.4% overweight, 39.8% obesity, 49.1% hypertension, 41.4% dyslipidaemia. Diabetes mellitus (20.3%), COPD (17.1%) and oncological disease (9.4%) were other frequently observed comorbidities.
Fig. 1.
Flow chart of patients enrolled in the study.
The median time from hospitalization to ICU admission was 2 (4.75) days. Patients were admitted with a median PaFi 90 [42]. According to Berlin criteria for ARDS, 66.5% were severe (PaFi <100), 31.2% moderate (PaFi 100–200) and 2.3% mild (PaFi 200–300). Patients showed at admission a median Charlson index 1 [2], APACHE score 15 [7] and SOFA score 5 [3].
Invasive mechanical ventilation (IMV) was required in 88.6% patients and in 89.1% of cases prone positioning was needed. During ICU stay, 40.4% required tracheostomy and in 2.9% of the cases ECMO was used.
Hydroxychloroquine was prescribed in 99.4%, lopinavir/ritonavir in 96.6%, steroids in 87.4%, tocilizumab in 82.3%, IFN β in 58%, azitromicine in 58.6% and remdesivir in 24%.
The ICU length of stay (LOS) was 20.5 [26] days, and the hospital LOS was 35 (36.5). The ICU mortality was 36.4%. None of the patients died outside ICU.
3.2. Nutritional medical therapy
Estimated caloric and protein requirements were 1775 ± 202 kcal and 92.4 ± 10.3 g, respectively. Nutritional treatment was started at 48 h of ICU admission.
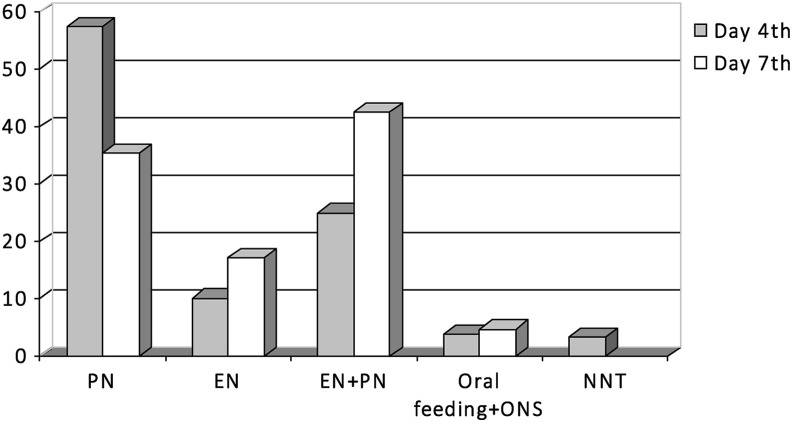
Nutritional treatment at day 4th and 7th is shown in Fig. 2 . Most of the patients received PN (alone or complementary to EN) to cover nutritional requirements (82.4% at day 4th and 77.9% at day 7th). Propofol was used in 66.4% patients. Prokinetics were used in 87.4% of the cases.
Fig. 2.
Nutritional treatment at days 4th and 7th of ICU admission. PN: parenteral nutrition, EN: enteral nutrition, ONS: oral nutritional supplements, NNT: no nutritional treatment.
Total calories, including those contributed by propofol, and proteins administered at day 4th and 7th of ICU admission and the percentage of estimated energy and protein requirements are shown in Table 1 .
Table 1.
Calories and proteins administered at days 4th and 7th (total and % of administered/estimated requirements).
| Calories (kcal) | Protein (g) | ||
|---|---|---|---|
| ICU:4th day | Total | 1425 ± 577 | 66 ± 26 |
| % | 81.5 ± 34 | 72.7 ± 30 | |
| ICU:7th day | Total | 1574 ± 555 | 74 ± 37 |
| % | 89.7 ± 32 | 81.4 ± 31 | |
Patients with IMV received more calories and protein at day 4th and 7th (Table 2 ). We did not observe statistically significant differences in calories and protein administered in patients in prone position.
Table 2.
Differences in calories and proteins administered by type of respiratory support.
| Invasive mechanical ventilation (IMV) | Non-IMV | p (Mann–Whitney) | |
|---|---|---|---|
| At day 4th | |||
| Calories (kcal) | 1450 ± 581.1 | 1149.3 ± 465,1 | 0.046∗ |
| % estimated calories | 83.2 ± 34.6 | 62.9 ± 25.9 | 0.025∗ |
| Protein (g) | 67.1 ± 26.1 | 53.3 ± 22.4 | 0.024∗ |
| % estimated protein | 74.3 ± 30.4 | 55.5 ± 22.3 | 0.007∗ |
| At day 7th | |||
| Calories (kcal) | 1607.7 ± 534 | 1186.3 ± 677.8 | 0.034∗ |
| % estimated calories | 91.9 ± 31.7 | 64.6 ± 36.6 | 0.017∗ |
| Protein (g) | 75.8 ± 27.3 | 56.4 ± 37.6 | 0.003∗ |
| % estimated protein | 83.3 ± 30.4 | 58.5 ± 38.1 | 0.020∗ |
∗p < 0.05.
3.3. Outcome and mortality
We observed low levels of P and Mg in 68.2% and 11.9% patients in the first week. Hyperbilirubinemia was presented in 56.3%, elevation of ALP, AST and GGT in 64.2%, 68.8% and 67.4% respectively. During ICU admission, patients developed metabolic complications, 77.8% hyperglycaemia, 13.2% hypoglycaemia, 83.8% hypertriglyceridemia (22% > 500 mg/dl), and 35.1% acute kidney failure and 16.4% needed KRT. Hyperglycaemia was more frequently observed in patients receiving PN in the first week, 81% vs 56% (p 0.005).
We observed a longer LOS in ICU among patients with higher APACHE scores (p 0.007), higher % protein administered at 7th (p 0.013), higher level of D dimer (p 0.021), lower level of P (p 0.004), higher level of triglycerides (p 0.020) and hyperglycaemia (p 0.003). A longer hospital LOS was observed in patients with lower level of P (p 0.002), higher level of triglycerides (p 0.016) and hyperglycaemia (p 0.05).
We did not observe any statistically significant difference between the nutritional therapy and ICU and hospital length of stay in the multivariate analysis performed.
In the mortality analysis, older age, higher Charlson index, APACHE and SOFA scores, and D dimer, and the presence of hypertension, diabetes mellitus, hyperglycaemia and hypertriglyceridemia showed a lower survival (Table 3 ). Obesity grade I was associated with lower ICU mortality (p 0.046). In the multivariate analysis only age, hypertension, Charlson index and SOFA score were associated with higher mortality.
Table 3.
Influence of baseline characteristics, nutritional treatment and analytical data on mortality.
| Variable | Overall | Survived | Died | P value |
|---|---|---|---|---|
| Number patients | 176 | 112 | 64 | |
| Age, (years) mean ± SD | 60.1 ± 13.5 | 57.3 ± 14.4 | 66.1 ± 10.2 | <0.001∗ |
| Male, n (%) | 128 (72.7) | 78 (69.6) | 50 (78.1) | 0.224 |
| BMI, mean ± SD | 29.9 ± 5.4 | 29.9 ± 5.3 | 29.8 ± 5.6 | 0.613 |
| Severity | ||||
| PaFi, median (IQR) | 90 (42) | 89.5 (43.5) | 90 (40) | 0.874 |
| Charlson, median (IQR) | 1 (2) | 1 (2) | 2 (3) | 0.001∗ |
| APACHE, median (IQR) | 15 (7) | 14 (6) | 16 (7.5) | 0.023∗ |
| SOFA at admission, median (IQR) | 5 (3) | 4 (3) | 6 (4) | 0.016∗ |
| Comorbidities | ||||
| HTA, n (%) | 85 (49.1) | 45 (40.9) | 40 (63.5) | 0.004∗ |
| Obesity grade I, n (%) | 40 (23.4) | 31 (28.2) | 9 (14.8) | 0.047∗ |
| Diabetes mellitus, n (%) | 35 (20.3) | 17 (15.6) | 18 (28.6) | 0.042∗ |
| Dyslipidaemia, n (%) | 71 (41.4) | 43 (39.1) | 29 (45.3) | 0.422 |
| COPD, n (%) | 29 (17.1) | 19 (17.6) | 10 (16.1) | 0.807 |
| Oncological disease, n (%) | 16 (9.4) | 10 (9.2) | 6 (9.7) | 0.914 |
| Inmunological disease, n (%) | 6 (3.5) | 2 (1.8) | 4 (6.5) | 0.115 |
| Nutritional medical therapy | ||||
| Time to start (hours), median (IQR) | 48 (48) | 48 (24) | 48 (48) | 0.268 |
| At day 4th | ||||
| % calories administered of estimated, mean ± SD | 81.5 ± 34.4 | 79.9 ± 34.1 | 84.1 ± 34.9 | 0.244 |
| % protein administered of estimated, mean ± SD | 72.7 ± 30.2 | 71.3 ± 30.6 | 75 ± 29.6 | 0.435 |
| At day 7th | ||||
| % calories administered of estimated, mean ± SD | 89.7 ± 32.8 | 88.5 ± 34.3 | 91.8 ± 30.3 | 0.326 |
| % protein administered of estimated, mean ± SD | 81.4 ± 31.6 | 80.1 ± 33.4 | 83.5 ± 28.5 | 0.553 |
| Laboratory data | ||||
| D dimer, median (IQR) | 3173 (7752) | 2552 (5102) | 5957 (9124) | <0.001∗ |
| Fibrinogen, median (IQR) | 845 (202) | 851 (212) | 832 (182) | 0.530 |
| CRP, median (IQR) | 23.3 (16.1) | 23.6 (15.6) | 23 (17.6) | 0.564 |
| Ferritin, median (IQR) | 1387 (1933) | 1409 (1997) | 1347 (1914) | 0.667 |
| Mg, median (IQR) | 2 (0.3) | 2 (0.3) | 1.9 (0.45) | 0.544 |
| P, median (IQR) | 2.1 (1) | 2.1 (1.1) | 2 (1) | 0.741 |
| Triglyceride, median (IQR) | 309 (250) | 304 (234) | 325 (264) | 0.590 |
| Hypertriglyceridemia, n (%) | 119 (83.8) | 74 (78.7) | 45 (93.8) | 0.022∗ |
| Hyperglycaemia, n (%) | 137 (77.8) | 77 (69) | 60 (94) | <0.001∗ |
| Folate deficiency, n (%) | 30 (27.3) | 18 (23.4) | 12 (36.4) | 0.161 |
| Vit D deficiency, n (%) | 67 (72) | 46 (67.6) | 21 (84) | 0.119 |
∗p < 0.05, HTA: hypertension, COPD: chronic obstructive pulmonary disease.
We did not find statistically significant differences in the total calories or protein administration at day 4th or 7th or in the percentage of estimated requirements administered and the mortality. Neither in the subgroup of patients with IMV.
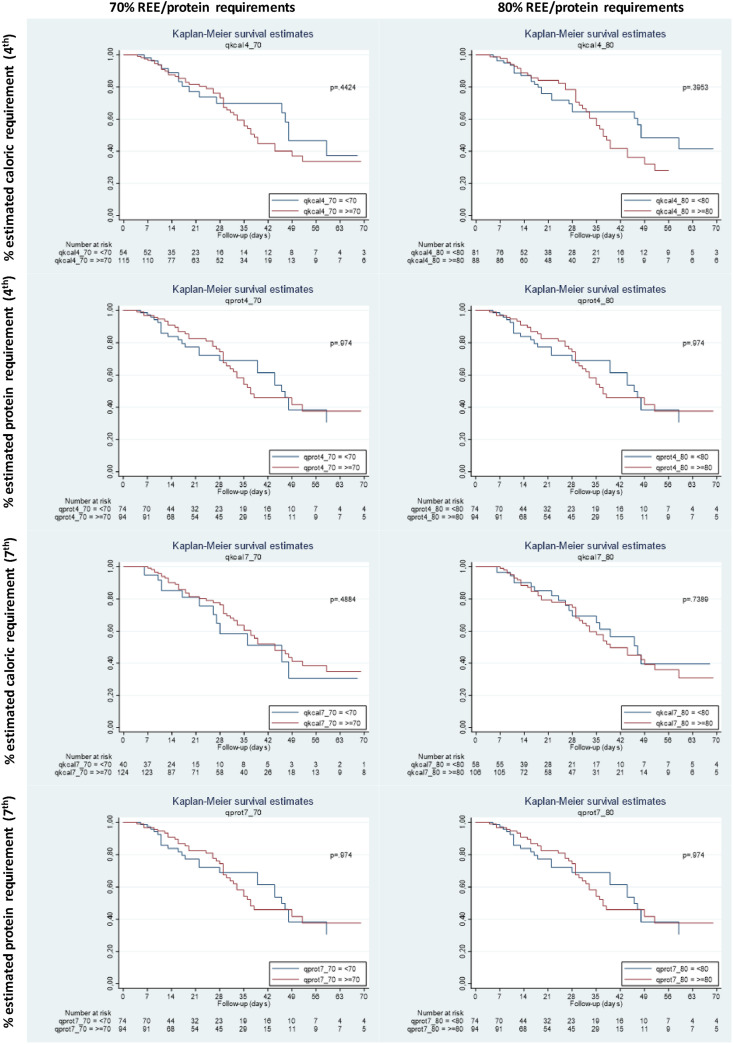
We did not find any differences in the survival analysis in patients who reached >70% or >80% of estimated caloric and protein requirements at day 4th and 7th (Fig. 3 ). We did not observe any statistical significance between nutritional therapy and mortality neither in the ROC curves nor in the multivariate analysis performed.
Fig. 3.
Kaplan–Meier survival analysis in patients who reach >70% or >80% of estimated caloric and protein requirement at days 4th and 7th of ICU admission.
4. Discussion
In this retrospective cohort study in 176 critically ill patients with COVID-19; overweight, obesity, hypertension and diabetes are the most frequent comorbidities observed, which is in line with previous reports [3,15]. The mean age of all the patients included was 60.1 ± 13.5 years, and 72.7% of the patients were male as same as previous reports [29,30]. Since admission in ICU, the patients had higher APACHE II (median 15) and SOFA scores (median 5).
Regarding critical support, 88.6% were treated with IMV, including 2.9% on ECMO at the same time and 16.4% were treated with continuous KRT. In comparison with previous studies, our sample required more IMV [3,29,31]. This can be explained by the fact that the patients with high flow nasal cannula (HFNC) or non-invasive ventilation (non-IMV) were treated outdoor ICU due to overburden in the hospital during the first wave.
We observed 36.4% rate mortality that was similar to other published series. A retrospective study of 1591 patients admitted in ICU in Milan, Italy, reported a mortality rate of 26% [31]. In a prospective study of 222 critically ill COVID-19 patients in Lombardy, Italy, reported a mortality rate of 32% [32]. In Spain, Ferrando et al. reported an ICU mortality of 31% from a prospective, multicentre, cohort study that enrolled 663 critically ill COVID-19 patients [3]. Most of the published studies lack of available data on nutritional support. An exception is the after mentioned study carried out in Lombardy where EN was provided to 209 patients, while exclusive PN and supplemental PN were administered to 4 and 33 patients, respectively. In this study, among patients still in the ICU and alive on day 4 (N 198), 129 (65.2%) and 72 (36.4%) reached a satisfactory caloric and protein intake (80–100% energy and protein requirements using predictive equations 25 kcal/kg/day, 1.3 g protein/kg/day), respectively. Among 174 patients alive on day 7, 134 (77.0%) and 81 (46.6%) were receiving an acceptable caloric and protein support, respectively.
If we analyze the nutritional support received, according to the ESPEN expert statements, in COVID-19 intubated patient EN should be started through a nasogastric tube. Meanwhile PN should be initiated in ICU patients who do not tolerate full dose EN during the first week in the ICU [33]. In the present study, at days 4th and 7th only 10.2% and 17.3% of patients were nourished exclusively by EN. Most of them (82.4% and 77.9%) received PN exclusively or complementing EN to reach their nutritional requirements.
ESPEN expert statements recommend that nutrition therapy should be started within 48 h and consider the prone position per se does not represent a limitation or contraindication for EN [28]. However, in clinical practice during this pandemic it was difficult to start EN early, due to multiple factors including overburden of the sanitary system with the need of unskilled healthcare professional not specialized in intensive care to monitor this treatment, the deep sedation of the patient and the use of protonation in the vast majority of the cases in the first days of ICU admission. In these cases, PN was used to secure the administration of the nutritional requirements, and EN was delayed after >48 h in many cases. Our mortality rate was similar to other studies, probably because we tried to avoid overfeeding the patients receiving mixed nutritional support [3,31,32] The effects of early parenteral nutrition in critically ill patients are controversial in the literature, with some studies finding a later recovery and increased infection rate [34] and others showing decreased days on mechanical ventilation and in the number of nosocomial infections [35,36].
It is known that both under and overfeeding during the first week appears to be harmful to mechanically ventilated critically ill patients. A progressive caloric intake is recommended in order to reach 70% of resting energy expenditure (REE) at 4th day in ICU [28,37]. Besides, the study of Lombardy in mechanically ventilated COVID-19 patients suggested that early caloric deficit may independently contribute to worsen survival [32]. In our study, patients received 81.5% and 89.7% of REE at 4th day and 7th day in the first week. We did not observe any statistical significance between nutritional therapy and mortality neither in the ROC curves nor in the multivariate analysis performed. However, it is difficult to analyze the nutritional support's impact on mortality, as the majority of our patients admitted to ICU received more than 70% of their requirements, in fact that may explain the absence of significant results in the mortality variable in our study in comparison with the results of the study of Lombardy [32].
Regarding protein requirements in critically ill patients there are conflicting data between the results of observational studies and randomized controlled trials on the amount of protein associated with a better outcome [28,38] ESPEN guidelines recommend giving 1.3 g protein/kg/day in a progressive way during the first week of hospitalization in the ICU [24] and higher protein goals can be considered in some patients (non-septic patients, those treated with CKRT or malnourished patients), or after the first week of hospitalization [37]. Our patients reached 72.7% and 81.4% of the protein goals at day 4th and 7th of ICU admission, and we did not find any association with the clinical outcome in the multivariate analysis.
Metabolic complications were frequent in our series, especially hyperglycemia and hypertriglyceridemia. Patients treated with PN had a higher incidence of hyperglycemia. However, in the multivariate analysis neither the use of PN during the first week nor the presence of hyperglycemia were associated with increased mortality. It has been reported that hyperglycaemia in patients with or without diabetes is a poor prognostic factor in hospitalized COVID-19 patients [39], [40]. In our series, out of 64 patients who died, 60 had hyperglycemia (93.8%) (p < 0.001). This agrees with the literature and reflects the effect of the intense inflammatory response, the secondary effects of the drugs employed in the treatment of these patients, the use of propofol and the medical nutritional therapy, on top of the baseline characteristic of these patients with frequent underlying metabolic diseases [41].
Obesity (mainly sarcopenic) and its comorbidities are important risk factors for severe COVID-19. As the ESPEN guidance paper on COVID-19 and obesity stand out, a correct diagnosis and nutritional management is essential in patients with obesity [42]. In our study energy and proteins requirements were calculated using adjusted weight if BMI ≥25kg/m2. In addition, we observed that those patients treated with IMV received more calories and protein than those treated HFNC or non-IMV, therefore special care should be taken in these cases to secure the delivery of nutrients and avoid underfeeding.
Even though this study includes a large number of patients mostly followed by a small number of Nutrition Unit staff, there are some important limitations. Firstly, we did not calculate the sample size, as we included all the patients admitted in ICU that fulfilled the inclusion criteria Secondly, it is a retrospective study, and some data were difficult to retrieve from the medical records. Thirdly, it is a unicentric study, so the results may not be generalizable to other centers. Fourthly, the caloric requirements were estimated as indirect calorimetry was not available. Fifthly, the start of EN was difficult in many cases because of the characteristics of this pandemic and the need to increase the ICU staff with unskilled heath care personnel. Lastly, the small difference in the provision of nutritional goals between patients that survived or not could be a limitation of the statistical analysis.
In conclusion, the majority of patients reached energy and protein requirements in the first week of ICU admission due to the use of PN (total or complementary to EN). Patients with HFNC or non-IMV may be at risk of malnutrition if total or complementary PN to oral diet/ONS/tube feeding is not used to cover nutritional requirements. Therefore, if EN is not possible or insufficient, PN can be safely used in critically ill patients with COVID-19 with a close monitoring of metabolic complications.
Author contributions
CC, MM, VC had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis.
CC, MM, VC are chief investigators and act as guarantors for this work.
Concept and design: CC, MM, VC, CM, SC, BI, MM.
Acquisition, analysis, or interpretation of data: CC, MM, VC, SC, CJ, AL, CML, MA, BN.
Drafting of the manuscript: CC, MM, VC, AL.
Critical revision of the manuscript for important intellectual content: CC, CM, BI, CJ, SC, MM.
Statistical analysis: VC.
Funding
None.
Conflict of interest
None of the authors has any conflicts of interest to disclose.
References
- 1.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. J Am Med Assoc. 2020 Apr 7;323(13):1239. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Patel J.J., Martindale R.G., McClave S.A. Relevant nutrition therapy in COVID-19 and the constraints on its delivery by a unique disease process. Nutr Clin Pract. 2020;35(5):792–799. doi: 10.1002/ncp.10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrando C., Mellado-Artigas R., Gea A., Arruti E., Aldecoa C., Bordell A., et al. English Edition. Revista Española de Anestesiología y Reanimación; 2020 Jul. Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: a prospective, cohort, multicentre study. S2341192920300986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera-Izquierdo M., Valero-Ubierna M. del C., R-delAmo J.L., Fernández-García M.Á., Martínez-Diz S., Tahery-Mahmoud A., et al. Sociodemographic, clinical and laboratory factors on admission associated with COVID-19 mortality in hospitalized patients: a retrospective observational study. PLoS One. 2020 Jun 25;15(6) doi: 10.1371/journal.pone.0235107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roncon L., Zuin M., Rigatelli G., Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020:104354. doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Salameh A., Lanoix J.-P., Bennis Y., Andrejak C., Brochot E., Deschasse G., et al. Characteristics and outcomes of COVID-19 in hospitalized patients with and without diabetes. Diabetes Metab Res Rev. 2020 Jul 19:e3388. doi: 10.1002/dmrr.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maddaloni E., D'Onofrio L., Alessandri F., Mignogna C., Leto G., Pascarella G., et al. Cardiometabolic multimorbidity is associated with a worse Covid-19 prognosis than individual cardiometabolic risk factors: a multicentre retrospective study (CoViDiab II) Cardiovasc Diabetol. 2020 Oct 1;19(1):164. doi: 10.1186/s12933-020-01140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S., et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Busetto L., Bettini S., Fabris R., Serra R., Dal Pra C., Maffei P., et al. Obesity and COVID-19: an Italian snapshot. Obesity. 2020;28(9):1600–1605. doi: 10.1002/oby.22918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalligeros M., Shehadeh F., Mylona E.K., Benitez G., Beckwith C.G., Chan P.A., et al. Association of obesity with disease severity among patients with coronavirus disease 2019. Obesity. 2020;28(7):1200–1204. doi: 10.1002/oby.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T., Lu L., Zhang W., Tao Y., Wang L., Bao J., et al. Clinical characteristics of 312 hospitalized older patients with COVID-19 in Wuhan, China. Arch Gerontol Geriatr. 2020 Nov 1;91:104185. doi: 10.1016/j.archger.2020.104185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai S.-H., Liao W., Chen S.-W., Liu L.-L., Liu S.-Y., Zheng Z.-D. Association between obesity and clinical prognosis in patients infected with SARS-CoV-2. Infect Dis Poverty. 2020:80. doi: 10.1186/s40249-020-00703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang Y., Lu Y., Huang Y.-M., Wang M., Ling W., Sui Y., et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020 Dec;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie J., Zu Y., Alkhatib A., Pham T.T., Gill F., Jang A., et al. Metabolic syndrome and COVID-19 mortality among adult black patients in new orleans. Diabetes Care. 2020;44(1):188–193. doi: 10.2337/dc20-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi M., Chen L., Yang Y., Zhang J., Xu J., Xu G., et al. Analysis of clinical features and outcomes of 161 patients with severe and critical COVID-19: a multicenter descriptive study. J Clin Lab Anal. 2020 Sep;34(9) doi: 10.1002/jcla.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garg S. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020 doi: 10.15585/mmwr.mm6915e3. https://www.cdc.gov/mmwr/volumes/69/wr/mm6915e3.htm [cited 2020 Nov 1];69. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khamis F., Al-Zakwani I., Al Naamani H., Al Lawati S., Pandak N., Omar M.B., et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health. 2020 Jul;13(7):906–913. doi: 10.1016/j.jiph.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah P., Owens J., Franklin J., Mehta A., Heymann W., Sewell W., et al. Demographics, comorbidities and outcomes in hospitalized Covid-19 patients in rural southwest Georgia. Ann Med. 2020;52(7):354–360. doi: 10.1080/07853890.2020.1791356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allard L., Ouedraogo E., Molleville J., Bihan H., Giroux-Leprieur B., Sutton A., et al. Malnutrition: percentage and association with prognosis in patients hospitalized for coronavirus disease 2019. Nutrients. 2020 Nov 28;12(12) doi: 10.3390/nu12123679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X., Deng H., Wang Y., Chen L., Gu X., Wang X. Predictive value of the prognostic nutritional index for the severity of coronavirus disease 2019. Nutrition. 2021 Apr;84:111123. doi: 10.1016/j.nut.2020.111123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li G., Zhou C., Ba Y., Wang Y., Song B., Cheng X., et al. Nutritional risk and therapy for severe and critical COVID-19 patients: a multicenter retrospective observational study. Clin Nutr. 2021;40(4):2154–2161. doi: 10.1016/j.clnu.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li T., Zhang Y., Gong C., Wang J., Liu B., Shi L., et al. Prevalence of malnutrition and analysis of related factors in elderly patients with COVID-19 in Wuhan, China. Eur J Clin Nutr. 2020 Jun;74(6):871–875. doi: 10.1038/s41430-020-0642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Földi M., Farkas N., Kiss S., Dembrovszky F., Szakács Z., Balaskó M., et al. Visceral adiposity elevates the risk of critical condition in COVID-19: a systematic review and meta-analysis. Obesity. 2021 Mar;29(3):521–528. doi: 10.1002/oby.23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer P., Blaser A.R., Berger M.M., Alhazzani W., Calder P.C., Casaer M.P., et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. doi: 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 25.Schuetz P., Fehr R., Baechli V., Geiser M., Deiss M., Gomes F., et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019;393(10188):2312–2321. doi: 10.1016/S0140-6736(18)32776-4. 08. [DOI] [PubMed] [Google Scholar]
- 26.Puthucheary Z.A., Rawal J., McPhail M., Connolly B., Ratnayake G., Chan P., et al. Acute skeletal muscle wasting in critical illness. J Am Med Assoc. 2013 Oct 16;310(15):1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 27.García-Martínez M.Á., Montejo González J.C., García-de-Lorenzo Y Mateos A., Teijeira S. Muscle weakness: understanding the principles of myopathy and neuropathy in the critically ill patient and the management options. Clin Nutr. 2020 May;39(5):1331–1344. doi: 10.1016/j.clnu.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 28.Zusman O., Theilla M., Cohen J., Kagan I., Bendavid I., Singer P. Resting energy expenditure, calorie and protein consumption in critically ill patients: a retrospective cohort study. Crit Care. 2016 Nov 10;20(1):367. doi: 10.1186/s13054-016-1538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020 Feb 15;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region. Italy. JAMA. 2020 Apr 28;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cereda E., Guzzardella A., Klersy C., Belliato M., Pellegrini A., Sciutti F., et al. Early caloric deficit is associated with a higher risk of death in invasive ventilated COVID-19 patients. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.02.020. Mar 2:S0261-5614(21)00094-7. Epub ahead of print. PMID: 33933297; PMCID: PMC7921717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020 Jun 1;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casaer M.P., Mesotten D., Hermans G., Wouters P.J., Schetz M., Meyfroidt G., et al. Early versus late parenteral nutrition in critically ill adults. N Engl J Med. 2011 Aug 11;365(6):506–517. doi: 10.1056/NEJMoa1102662. [DOI] [PubMed] [Google Scholar]
- 35.Doig G.S., Simpson F., Sweetman E.A., Finfer S.R., Cooper D.J., Heighes P.T., et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition: a randomized controlled trial. J Am Med Assoc. 2013 May 22;309(20):2130–2138. doi: 10.1001/jama.2013.5124. [DOI] [PubMed] [Google Scholar]
- 36.Heidegger C.P., Berger M.M., Graf S., Zingg W., Darmon P., Costanza M.C., et al. Optimisation of energy provision with supplemental parenteral nutrition in critically ill patients: a randomised controlled clinical trial. Lancet. 2013 Feb 2;381(9864):385–393. doi: 10.1016/S0140-6736(12)61351-8. [DOI] [PubMed] [Google Scholar]
- 37.van Zanten A.R.H., De Waele E., Wischmeyer P.E. Nutrition therapy and critical illness: practical guidance for the ICU, post-ICU, and long-term convalescence phases. Crit Care. 2019 21;23(1):368. doi: 10.1186/s13054-019-2657-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allingstrup M.J., Kondrup J., Wiis J., Claudius C., Pedersen U.G., Hein-Rasmussen R., et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017 Nov;43(11):1637–1647. doi: 10.1007/s00134-017-4880-3. [DOI] [PubMed] [Google Scholar]
- 39.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020 Jul;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao S., Ali K., Dennis J., Berdine G., Test V., Nugent K. Analysis of glucose levels in patients hospitalized with COVID-19 during the first phase of this pandemic in west Texas. J Prim Care Community Health. 2020 Dec;11 doi: 10.1177/2150132720958533. 2150132720958533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martindale R, Patel JJ, Taylor B, Warren M, Mc Clave SA. Nutrition therapy in the patient with COVID-19 disease requiring ICU care.
- 42.Barazzoni R., Bischoff S.C., Busetto L., Cederholm T., Chourdakis M., Cuerda C., et al. Nutritional management of individuals with obesity and COVID-19: ESPEN expert statements and practical guidance. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.05.006. May 11:S0261-5614(21)00248-X. Epub ahead of print. PMID: 34140163; PMCID: PMC8110326. [DOI] [PMC free article] [PubMed] [Google Scholar]