Abstract
Depression and cognitive disorders are diseases with complex and not-fully understood etiology. Unfortunately, the COVID-19 pandemic dramatically increased the prevalence of both conditions. Since the current treatments are inadequate in many patients, there is a constant need for discovering new compounds, which will be more effective in ameliorating depressive symptoms and treating cognitive decline. Proteins attracting much attention as potential targets for drugs treating these conditions are sigma-1 receptors. Sigma-1 receptors are multi-functional proteins localized in endoplasmic reticulum membranes, which play a crucial role in cellular signal transduction by interacting with receptors, ion channels, lipids, and kinases. Changes in their functions and expression may lead to various diseases, including depression or memory impairments. Thus, sigma-1 receptor modulation might be useful in treating these central nervous system diseases. Importantly, two sigma-1 receptor ligands entered clinical trials, showing that this compound group possesses therapeutic potential. Therefore, based on preclinical studies, this review discusses whether the sigma-1 receptor could be a promising target for drugs treating affective and cognitive disorders.
Keywords: Sigma-1 receptors, Memory deficits, Depression, Animal models
1. Introduction
Sigma-1 receptors are proteins that attract much attention as potential targets for drugs treating many medical conditions. The substantial therapeutic potential of sigma-1 receptor ligands is due to the involvement of the sigma-1 receptor in the development of many disorders, such as retinal diseases (Cantarella et al., 2007; Ha et al., 2011; Martin et al., 2004; Smith et al., 2008), drug addiction (Hiranita et al., 2013; Romieu et al., 2002), alcohol addiction (Skuza, 2013), pain (Bravo-Caparrós et al., 2019; Bruna et al., 2018; Shin et al., 2020), ischemic stroke (Urfer et al., 2014), schizophrenia (Borison et al., 1991; Gewirtz et al., 1994), Alzheimer disease (Maurice et al., 1998), Parkinson disease (Francardo et al., 2019, 2014), amyotrophic lateral sclerosis (Mancuso et al., 2012; Ono et al., 2014), multiple sclerosis (Collina et al., 2017; Lisak et al., 2020), Huntington’s disease (Bol’shakova et al., 2017; Squitieri et al., 2015; Vetel et al., 2021), as well as cognitive and affective disorders (Albayrak and Hashimoto, 2012; Furuse and Hashimoto, 2010; Mandelli et al., 2017; Wang et al., 2019a). The prevalence of the latter two conditions has dramatically increased recently due to the COVID-19 pandemic (Ettman et al., 2020; Hampshire et al., 2021). Studies indicate that even three times as many adults report symptoms of depression or some form of cognitive decline (e.g., brain fog) now in comparison to last year (Ettman et al., 2020; Hampshire et al., 2021). Taking that into account, in this review, based on preclinical studies, we will discuss the therapeutic potential of sigma-1 receptor ligands as drugs improving mood and memory.
2. Basic information on sigma-1 receptor
2.1. Structure, distribution, and localization
Sigma-1 receptors are endoplasmic-reticulum-resident transmembrane proteins, acting as intracellular receptors with chaperone protein function. They are mainly expressed in the central nervous system (hippocampus, hypothalamus, mesencephalon, olfactory bulb). However, they can also be found in the liver, kidney, lungs, muscles, urinary bladder, as well as endocrine, immune and reproductive tissues (Gundlach et al., 1986; Kekuda et al., 1996; Mei and Pasternak, 2001). In the central nervous system, sigma-1 receptors are present on neurons and glial cells (astrocytes, oligodendrocytes, and ependymocytes).
The sigma-1 receptor is a single polypeptide containing 223 amino acids (26 kDa). The crystal structure revealed a trimeric organization with a single transmembrane domain for each protomer, and the carboxy-terminal domain included a cupin-like β-barrel with the ligand-binding site buried at its center (Schmidt et al., 2016). Sigma-1 receptors occur on the endoplasmic reticulum (ER)/mitochondrial associated membranes (MAM) and at very low levels in post-synaptic thickenings of the neuronendoplasmic reticulum (Alonso et al., 2000; Su et al., 2010). At MAM, they are sequestered with glucose-related protein 78/binding immunoglobulin protein (BiP), a major ER chaperone protein (Hayashi and Su, 2007). After the activation, the receptors separate from binding immunoglobulin protein and act either within the MAM or translocate to plasma membrane regulating calcium signaling (Hayashi and Su, 2007). Sigma-1 receptor may also transfer from the MAM to the nucleus membrane, interact with various nuclear factors and regulate gene transcription (Tsai et al., 2015).
2.2. Oligomerization
Studies demonstrated that sigma-1 receptors exist as monomers, dimers, and higher order oligomers (Pal et al., 2007). However, the ligand-binding properties occur only in an oligomeric state (Gromek et al., 2014). Interestingly, the agonist’s presence favors the monomeric and dimeric form of sigma-1 receptors, while the antagonist promotes higher order receptor oligomers (Mishra et al., 2015; Yano et al., 2018). It is an exciting feature of the sigma-1 receptor, considering its possible role in receptor functions. Furthermore, a GXXXG motif of the sigma-1 receptor takes part in its oligomerization – mutations within this region disrupt this process and result in an increased number of smaller oligomeric states (Gromek et al., 2014).
2.3. Functions of sigma-1 receptors
2.3.1. Interaction with other proteins
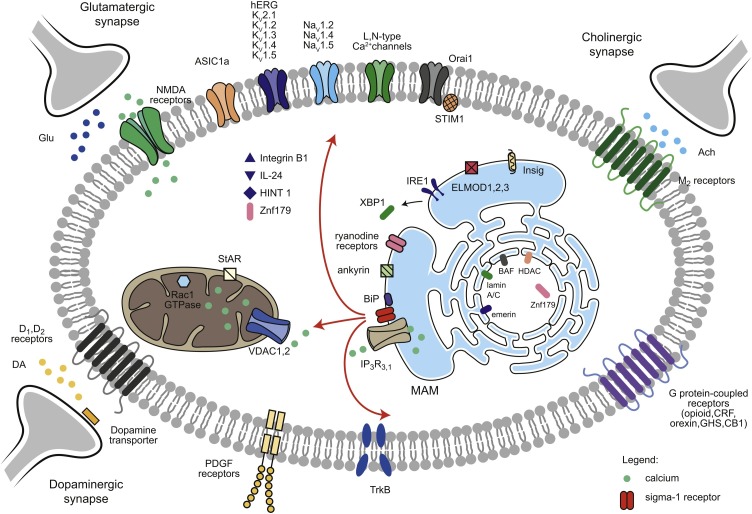
Sigma-1 receptors interact with ion channels, G-protein coupled receptors (GPCRs), and cell-signaling molecules (Fig. 1 ). Therefore, it is not surprising that the sigma-1 receptor participates in diverse physiological and pharmacological processes. Moreover, the sigma-1 receptor regulates the compartmentalization of lipids on the endoplasmic reticulum and their further transport to the plasma membrane and cytosol (Hayashi and Su, 2003).
Fig. 1.
A summary of the interaction of sigma-1 receptors with various proteins. After activating sigma-1 receptors, glucose-related protein 78/binding immunoglobulin protein (BiP) dissociates, and the sigma-1 receptor translocases into various cellular compartments, interacting with ion channels, G-protein coupled receptors, plasma proteins, mitochondria proteins, ER channels/proteins and nuclear proteins. BAF – barrier-to-autointegration factor, BiP – glucose-related protein 78/binding immunoglobulin protein, ELMOD – cell engulfment and motility domain, HDAC – histone deacetylase, HINT1 – histidine triad nucleotide-binding protein 1, IP3Rs – inositol 1,4,5-trisphosphate receptors, IRE1α – inositol requiring kinase 1α, MAM – mitochondrial associated membranes, NMDA – N-methyl-D-aspartate, Orai1 – calcium release-activated calcium channel protein 1, PDGF – platelet-derived growth factor, StAR – steroidogenic acute regulatory protein, STIM1 – stromal interaction molecule 1,TrkB – tropomyosin receptor kinase B, VDAC 1,2 – voltage-dependent anion channel 1,2, XBP1s – X-box-binding proteins, Znf179 – zinc finger protein 179.
2.3.1.1. Ion channels
Sigma-1 receptors regulate ion currents interacting with various channels, such as Kv1.2 channels, increasing their surface expression (Abraham et al., 2019; Kourrich et al., 2013). They also inhibit Kv1.3, Kv1.4, and Kv1.5 channels (Aydar et al., 2002; Kinoshita et al., 2012; Zhang and Cuevas, 2005), regulate Kv2.1 channels, inhibit L-type voltage-gated calcium channels (Church and Fletcher, 1995; Mueller et al., 2013; Sabeti et al., 2007; Tchedre et al., 2008; Zhang and Cuevas, 2002), inhibit N-type Ca2+ channels currents (Zhang and Cuevas, 2002; Zhang et al., 2017a, b, c), inhibit voltage-gated sodium ion channels: Nav1.2/1.4 (Gao et al., 2012) and Nav 1.5 (Johannessen et al., 2009; Koornneef et al., 2009), and regulate hERG channels (Balasuriya et al., 2014; Crottès et al., 2011). Moreover, sigma-1 receptors inhibit acid-sensing ion channels (ASIC1a) by activating a PTX-sensitive G-protein and stimulating AKAP150 bound calcineurin (Carnally et al., 2010; Mari et al., 2014). They also inhibit store-operated Ca2+ entry by diminishing coupling of stromal interaction molecule 1 (STIM1) to calcium release-activated calcium channel protein 1 (Orai1) (Srivats et al., 2016).
2.3.1.2. G-protein coupled receptors/transporters
As mentioned above, sigma-1 receptors also interact with many GPCRs, including opioid receptor (Kim et al., 2010), corticotropin-releasing factor (CRF) receptor (Navarro et al., 2015, 2019), orexin receptor (Navarro et al., 2015, 2019), growth hormone secretagogue receptor (GHS-R) (Aguinaga et al., 2019), cannabinoid receptor 1 (Sánchez-Blázquez et al., 2014), muscarinic M2 receptor (Hellström et al., 2003), and dopaminergic D1 and D2 receptors (Beggiato et al., 2017; Feltmann et al., 2018; Navarro et al., 2010, 2013). Interestingly, sigma-1 receptors modulate opioid transduction without influencing opioid receptor binding (Kim et al., 2010). Additionally, sigma-1 receptor agonists modulated conformation of dopamine transporter and enhanced binding as well as stimulant-evoked dopamine efflux via dopamine transporter and calcium signals (Hong et al., 2017; Sambo et al., 2017).
2.3.1.3. Plasma proteins
Sigma-1 receptors also interact with integrin beta-1 – SKF-10047, a sigma-1 receptor agonist, reduced cell adhesion and cholesterol binding (Palmer et al., 2007). Sigma-1 receptors are also crucial for cocaine-induced platelet-derived growth factor-BB (PDGF-BB) expression as sigma-1 receptor antagonists or sigma-1 receptor siRNA attenuated this process (Yao et al., 2011). Moreover, these receptors also form complexes with IL-24 (Do et al., 2013), tropomyosin receptor kinase B (TrkB) (Kimura et al., 2013), histidine triad nucleotide-binding protein 1 (HINT1) (Rodríguez-Muñoz et al., 2015), and zinc finger protein 179 (Znf179) (Su et al., 2016).
2.3.1.4. Mitochondria proteins
Sigma-1 receptors form a complex with steroidogenic acute regulatory protein (StAR) and voltage-dependent anion channel 2 (VDAC2), regulating in this way mitochondrial metabolism (Marriott et al., 2012). Moreover, via Rac1 signaling, sigma-1 receptors may induce mild oxidative stress affecting the neuroplasticity and prevent apoptosis and autophagy (Natsvlishvili et al., 2015).
2.3.1.5. ER channels/proteins
Sigma-1 receptors regulate inositol 1,4,5-trisphosphate (IP3) channels (Hayashi and Su, 2001) and interact with ryanodine receptors (Tagashira et al., 2013a). Pentazocine and fluvoxamine inhibit the ryanodine-induced calcium release from sarcoplasmic reticulum in cardiomyocytes (Tagashira et al., 2013a, 2014). Interestingly, activation of sigma-1 receptors provides different results in calcium release considering the type of IP3 receptors (IP3Rs). The sigma-1 receptor agonists enhance Ca2+ flux induced by IP3R3 (Delint-Ramirez et al., 2020; Hayashi et al., 2000; Hayashi and Su, 2007; Wu and Bowen, 2008). On the other hand, IP3R1 is negatively regulated by agonist-stimulated sigma-1 receptors in certain cell types like striatal medium spiny neurons (MSNs) (Ryskamp et al., 2017).
Sigma-1 receptors also regulate the Hsp70 binding immunoglobulin protein as they form together a stable complex, which is dissociated after binding sigma-1 receptor agonist (Hayashi and Su, 2007; Ortega-Roldan et al., 2013). The receptors also form a stable ternary complex with ankyrin and IP3R3 (Hayashi and Su, 2001). Moreover, sigma-1 receptors negatively regulate galactosylceramides synthesis by enhancing the degradation of ceramide galactosyltransferase (CgalT) through regulation of the dynamics of Insig (Hayashi et al., 2012). Importantly, sigma-1 receptors regulate the phosphorylation of inositol requiring kinase 1α (IRE1α) – the receptors’ overexpression led to an increased IRE1α phosphorylation and spliced X-box-binding proteins (XBP1s) expression as well as nuclear localization (Alam et al., 2017; Mori et al., 2013). On the other hand, the decreased IRE1α phosphorylation and XBP1s expression, as well as nuclear transport, are observed when the sigma-1 receptor is knocked down (Alam et al., 2017). However, lipopolysaccharide(LPS)-treated sigma-1 receptor knock-out mice had increased IRE1 activity (Rosen et al., 2019), which suggests that sigma-1 receptors modulate the phosphorylation of IRE1 depending on whether there is an inflammation or not. Additionally, sigma-1 receptors bind to cell engulfment and motility domain 1 and 2 (ELMOD), decreasing their GTPase-activating protein (GAP) activities (Ivanova et al., 2014).
2.3.1.6. Nuclear proteins
Sigma-1 receptors also interact with various proteins localized in the cell nucleus. For example, after the stimulation of sigma-1 receptors and their translocation from endoplasmic reticulum to the nuclear envelope, they bind emerin and form complex with lamin A/C, barrier-to-autointegration factor (BAF), and histone deacetylase (HDAC) and interact with the gene repressor specific protein 3 (Sp3) (Tsai et al., 2015). Interestingly, sigma-1 receptors also interfere with androgen receptor signaling – treatment with a sigma-1 inhibitor prevented 5α-dihydrotestosterone-mediated nuclear translocation of androgen receptors and induced their proteasomal degradation (Thomas et al., 2017). Moreover, sigma-1 receptors also regulate nuclear pore protein stability and interact with RNA – more specifically (G4C2)-RNA repeats observed in amyotrophic lateral sclerosis and frontotemporal dementia (Lee et al., 2020).
2.3.1.7. Neurotrophins
The sigma-1 receptors regulate the synthesis and secretion of various neurotrophic factors, including brain-derived neurotrophic factor (BDNF). Chronic treatment with E-5842, a sigma-1 receptor ligand, did not change the BDNF mRNA level in the rat hippocampus and prefrontal cortex; however, the acute administration decreased it (Ovalle et al., 2002). On the other hand, some studies demonstrated that chronic administration of sigma-1 receptor agonists boosted the BDNF level in the hippocampus (Kikuchi-Utsumi and Nakaki, 2008; Ring and Regan, 2013; Terada et al., 2014; Xu et al., 2015; Yagasaki et al., 2006). However, the receptor activation did not increase the total amount of BDNF but enhanced the post-translational modification of BDNF proteins related to the protein secretion (Fujimoto et al., 2012). It was probably due to the partial disassembly and remodeling of sigma-1 receptor microdomains in the ER, which eventually led to a rapid release of mature proteins (Zhemkov et al., 2021).
2.3.2. Neurotransmission
Activating sigma-1 receptor can enhance glutamatergic and cholinergic synaptic neurotransmissions. Low doses of sigma-1 receptor agonists potentiated the N-methyl-D-aspartate (NMDA)-induced activation of pyramidal neurons in the CA3 region of the hippocampus and monoamines release (Monnet et al., 1995, 1992). The possible mechanism of current enhancement through NMDA receptors involved preventing small conductance Ca2+-activated K+ current (SK) channel activation (Martina et al., 2007), which, in turn, increased Ca2+ influx through the NMDA receptors and potentiated receptor-mediated responses, including long-term potentiation (LTP). Interestingly, sigma-1 agonists increased the expression of GluN2A and GluN2B subunits and postsynaptic density protein 95 (PSD-95), proteins required for synaptic plasticity associated with NMDA receptor signaling. They also enhanced the interaction between GluN2 subunits and sigma-1 receptors but attenuated the coupling between GluN2B and PSD-95 (Pabba et al., 2014). Additionally, sigma-1 receptors stimulation increased the NMDA receptors levels on the cell surface (Pabba et al., 2014).
Moreover, the blockade of sigma-1 receptors modulated gamma-aminobutyric acid (GABA) and glutamate uptake, transport-mediated release, and exocytosis (Pozdnyakova et al., 2020). Interestingly, the sigma-1 receptor antagonist decreased the glutamate release but induced a biphasic response for GABA – low doses of NE-100 increased GABA uptake and with increasing dose, the uptake rate was decreasing (Pozdnyakova et al., 2020).
Sigma-1 receptors regulate the cholinergic neurotransmission either indirectly, through modulation of NMDA receptors in the brain, or directly. Stimulating sigma-1 receptors increased the extracellular acetylcholine level (Horan et al., 2002; Junien et al., 1991b; Matsuno et al., 1995, 1993a). Interestingly, this effect was region-specific as sigma-1 receptor agonists affected the neurotransmitter level in the hippocampus and prefrontal cortex, but not striatum (Horan et al., 2002; Kobayashi et al., 1996; Matsuno et al., 1992). The acetylcholine release is the effect of sigma-1 receptor-mediated modulation of Ca2+ mobilization via IP3 receptor-gated pools and voltage-gated K+ and Ca2+ channels (Hayashi et al., 2000).
2.3.3. Neurite outgrowth
Sigma-1 receptors influence neurite outgrowth. Scientists showed that sertraline inhibited nerve growth factor (NGF)-induced neurite outgrowth in PC12 cells, and this effect was blocked by both agonist and antagonist of sigma-1 receptors (Matsushima et al., 2019). The study suggested that the antidepressant acted as an inverse agonist of the sigma-1 receptor (Matsushima et al., 2019). On the other hand, fluvoxamine improved NGF-induced neurite outgrowth, which was inhibited by dexamethasone, acting via sigma-1 receptors and normalizing also the disrupted phosphorylation of a serine/threonine protein kinase (Akt) (Matsushima et al., 2018).
Similarly, dipentylammonium, a sigma-1 receptor agonist, enhanced neurite outgrowth (Brimson et al., 2018). Moreover, the compound protected from glutamate toxicity and prevented nuclear factor kappa B (NFκB) activation in HT-22 cells as well as dopamine-induced cell death in the APP/Swe expressing Neuro2A cells (Brimson et al., 2018). This stimulation of neurite-outgrowth might have involved increased production of adenosine triphosphate (ATP).
2.3.4. Neuroprotection
Sigma-1 receptors can promote neuroprotection. Studies demonstrated that sigma-1 receptor stimulation caused a neuroprotective effect, resulting from receptor translocation to the endoplasmic reticulum, stabilizing the IP3 receptor, reducing ER stress, and modulating calcium flux. Eventually, the normalization of the calcium flow to mitochondria prevented mitochondrial leakage and stimulated metabolism (Hayashi and Su, 2007). In addition, the sigma-1 receptor agonists are neuroprotective against glutamate-, rotenone-, oligomycin-, and NMDA-induced neurotoxicity in the neuronal cell lines (Brimson et al., 2018; Franchini et al., 2020; Nishikawa et al., 2000; Senda et al., 1998a, b; Shimazu et al., 2000).
N,N-Dimethyltryptamine, a sigma-1 receptor agonist, prevented cell apoptosis in the ischemic injury, suggesting that the compound counteracted ferroptotic cell death linked to oxidative stress (Szabó et al., 2021). Also, dehydroepiandrosterone (DHEA), neurosteroid stimulating sigma-1 receptors, showed anti-amnesic activity when it was administered between 3–48 h after ischemia. However, when given during ischemia or early reperfusion, it demonstrated neurotoxic effects by increasing Ca2+ influx through NMDA receptors (Li et al., 2009). Its neuroprotective mechanism may depend on sigma-1 receptor-mediated changes like inhibition of NMDA-induced nitric oxide synthase hyperactivity, reduction of nitric oxide production (Kurata et al., 2004), stimulation of phosphoinositide 3-kinase (PI3) activity (Raval et al., 2003), and antiapoptotic Bcl-2 proteins (Charalampopoulos et al., 2004), or upregulation of glutamate transporter-1 (GLT-1) (Sokabe et al., 2007) and extracellular signal-regulated kinase (ERK) phosphorylation (Shi et al., 2021). Interestingly, the optimal time window for the administration of sigma-1 receptor ligands is crucial for retinal neuroprotection – (+)-pentazocine given since post-natal day 14 (P14), but not P18, P21 or P24, to the Pde6βrd10/J (rd10) mice (mouse model of retinitis pigmentosa) preserved the visual acuity (Wang et al., 2021a, b). This neuroprotective mechanism of sigma-1 receptors may depend on modulation of miR-214-3p in retina, a microRNA involved in the oxidative stress (Wang and Smith, 2019).
Sigma-1 receptors are also a crucial node in pathways determining cellular survival – they enhance the IRE1 stability at MAMs under endoplasmic reticulum stress (Mori et al., 2013). Also, the overexpression of these receptors promotes the protection from ER stress, whilst their decrease leads to apoptosis (Hayashi and Su, 2007). The cytoprotective effect of sigma-1 receptor ligands can be observed in both ischemic hepatocytes and hypertrophic cardiocytes. In ischemic hepatocytes, the sigma-1 receptor-mediated cytoprotective effect results from reducing the leakage of hepatic enzymes, increasing the metabolic capacities, and increasing the ATP synthesis (Klouz et al., 2008). In hypertrophic cardiocytes, the same effect is due to restoring mitochondrial Ca2+ mobilization and ATP production (Tagashira et al., 2013b).
Moreover, the sigma-1 receptor also plays a significant role in mitochondria-targeted cell apoptosis – after activation, it increases a protective bcl-2 gene expression (Yang et al., 2007). In cultured cortical neurons co-incubated with Aβ25-35 peptide, sigma-1 receptor agonists prevented the cell death after exposure to β-amyloid toxicity (Marrazzo et al., 2005). Moreover, the sigma receptor antagonist diminished the neuroprotective effects of memantine against Aβ-induced neurotoxicity in SH-SY5Y cells (Keshavarz et al., 2020). Interestingly, an anxiolytic drug, afobazole, protected neurons regulating the intracellular calcium overload during ischemia and acidosis via activation of sigma-1 receptors (Cuevas et al., 2011a). Moreover, the compound also prevented the microglia activation and decreased their response to ATP during and after an ischemic episode in vitro (Cuevas et al., 2011b). That suggests the activation of sigma-1 receptors may exert neuroprotective properties modulating the microglia activation and functioning.
2.3.5. Oxidative stress
Sigma-1 receptor knock-out animals have increased oxidative stress levels (Pal et al., 2012). Similarly, in cells with overexpressed sigma-1 receptors, agonists decreased whilst antagonists increased oxidative stress levels (Pal et al., 2012). Interestingly, sigma-1 receptors activated the antioxidant response elements and upregulated NAD(P)H quinone oxidoreductase 1 (NQO1) and superoxide dismutase 1 (SOD1) mRNA expression (Pal et al., 2012). Moreover, reactive oxygen species (ROS) level was elevated in retinal Müller cells lacking sigma-1 receptors (Wang et al., 2015). On the other hand, the activation of sigma-1 receptors in retinal Müller cells attenuated the production of ROS by enhancing the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) signaling pathway (Wang et al., 2015). Furthermore, (+)-pentazocine, a sigma-1 receptor agonist, ameliorated the LPS-induced increase in ROS production (Zhao et al., 2014). Moreover, a novel sigma-1 modulator with acetylcholinesterase inhibiting properties decreased ROS production in SH-SY5Y human neuroblastoma cell lines (Rui et al., 2018). The sigma-1 receptor activation disrupts the complex between PSD-95 and neuronal nitric oxide synthase (nNOS) (Yang et al., 2010), leading to decreased nitric oxide production (Goyagi et al., 2001). On the other hand, in the spinal cord and brain mitochondria, activation of the sigma-1 receptor leads to increased ROS production (Choi et al., 2013; Natsvlishvili et al., 2015). In normal conditions, sigma-1 receptor agonists generate moderate oxidative stress in mitochondria through ROS production, involving complex I activity (Goguadze et al., 2019). In pathological conditions, such as Aβ-induced stress, sigma-1 receptor activation restores the mitochondrial physiology – normalizing the complex I and IV activity responsible for increased ROS production (Goguadze et al., 2019).
2.3.6. Neuroinflammation
Sigma-1 receptors, expressed in microglia, modulate microglial activation and attenuate neuroinflammation (for more information please see Jia et al., 2018). In the microglial cells treated with LPS, the expression of sigma-1 receptor was significantly decreased as a result of toll-like receptor 4 (TLR4) activation via the TLR4-TAK1-p38 MAPK pathway and the activation of histone deacetylase 6 (HDAC6) (Iwamoto et al., 2020). Another sigma-1 receptor agonist, (+)-pentazocine, reduced the release of tumor necrosis factor α (TNF-α), interleukin 10 (IL-10), monocyte chemoattractant protein-1 (MCP-1), and nitric oxide (NO) and inhibited LPS-induced activation of ERK and c-Jun N-terminal kinase (JNK) pathways (Zhao et al., 2014). Moreover, in murine microglial BV2 cells, the activation of the sigma-1 receptors by SKF83959 prevented M1 microglial activation induced by LPS and decreased the release of inflammatory cytokines like tumor TNF-α, IL-1β, and inducible nitric oxide synthase (iNOS) (Wu et al., 2015). Interestingly, in mouse model of motor neuron degeneration, sigma-1 receptors activation led to increased anti-inflammatory M2 microglial phenotypes (Peviani et al., 2014). The antioxidative and anti-inflammatory effect after the sigma-1 receptor activation was mediated by promoting Nrf2/HO-1 signaling and suppressing NF-κB activity (Wang and Zhao, 2019). SA4503 downregulated the expression of iNOS and TNF-α and upregulated glutathione (GSH) in cultured astrocytes after LPS-induced inflammation (Wang and Zhao, 2019). Moreover, activation of sigma-1 receptors is beneficial in mice after ischemic stroke, as it normalizes matrix metalloproteinase-9 overexpression and hyperreactive astrogliosis (Sánchez-Blázquez et al., 2018). Also, dexmedetomidine, a sigma-1 receptor agonist, alleviated the hypoxia/reoxygenation-induced neuroinflammation manifested by an increased level of inflammatory factors like TNF-α, IL-6 or MCP-1 (Zhao et al., 2021). What is worth mentioning, the activation of sigma-1 by afobazole regulated the microglial activation preventing the amyloid-induced toxicity (Behensky et al., 2013).
3. Sigma-1 receptor ligands
Even though the sigma-1 receptor is highly expressed in mammalian cells, no endogenous ligand has been clearly identified; however, both dimethyltryptamine (DMT) and neurosteroids activate the receptor and may be considered as putative candidates (Fontanilla et al., 2009; Maurice et al., 1999b). Moreover, the sigma-1 receptor binds various drugs and compounds with diverse chemical structures and different therapeutic and pharmacological profiles (Table 1 ) (Fallica et al., 2021; Weber and Wünsch, 2017).
Table 1.
Sigma-1 receptors ligands.
| Agonists | Antagonists | Modulators |
|---|---|---|
| (+)-pentazocine | NE-100 | SOMCL-668 |
| (+)-SKF10047 | Haloperidol | OZP002 |
| Fluvoxamine | Sertraline | ER1 |
| Igmesine (JO-1784) | BD1047 | |
| Pregnenolone (PRE) | BD1063 | |
| Dehydroepiandrosterone (DHEA) | PB212 | |
| Donepezil | AZ66 | |
| Cutamesine (SA4503) | BD1008 | |
| PRE-048 | ||
| Dipentylammonium | ||
| Quetiapine | ||
| ANAVEX2-73 | ||
| OPC-14523 | ||
| BB190 | ||
| Siramesine | ||
| Venlafaxine | ||
| Bupropion | ||
| Dextromethorphan | ||
| PPCC | ||
| Desipramine | ||
| Fluoxetine | ||
| R278995/CRA0450 | ||
| Berberine | ||
| Ropinirol | ||
| UMB23 | ||
| UMB82 | ||
| Ditolylguanidine (DTG) | ||
| 17β-estradiol | ||
| N-n-propyl-3-(3-hydroxyphenyl)piperidine) ((+)-3-PPP) | ||
| LS-1-137 | ||
| KT-95 | ||
| ANAVEX1-41 | ||
| Dimemorfan | ||
| (-)-MR22 | ||
| AF710B | ||
| Choline |
Unfortunately, most of the functional assays to identify and classify sigma-1 receptor ligands are not precise. Many studies described various in vitro, in vivo, and ex vivo systems responding in a specific manner to sigma-1 receptor agonists (for more information please see Colabufo et al., 2009). However, most of these methods are not appropriate for high throughput use as they rely on measuring downstream effects of sigma-1 receptor stimulation. For many years, by convention, we classify compounds as sigma-1 receptor agonists if they induce the typical sigma-1 receptor-mediated effects in animal studies or resemble the phenotypes of receptor overexpression, like increased translocation of sigma-1 receptors to the plasma membrane, interaction with various proteins, neuroprotection, but also increased proliferation of cancer cells (Alam et al., 2017; Hall et al., 2009; Hayashi and Su, 2007; Meunier and Hayashi, 2010; Wu et al., 2018). On the contrary, antagonists are compounds that do not exert pharmacological effects on their own but abolish the effects of sigma-1 receptors activation or mimic the phenotype of receptor knock-down.
On the other hand, since the sigma-1 receptors influence calcium signaling, some proposed methods measure calcium levels (Wu and Bowen, 2008). However, these assays lack the quantitative precision and dynamic range required to capture accurate dose-response curves adequately. Quantitative cell-based assays assuming that sigma-1 receptor agonists induce neurite outgrowth also depend on specific downstream effects, but a limited number of compounds demonstrate this effect (Takebayashi et al., 2002). Similar conclusions can be drawn from studies on striatal tissue from guinea pigs, where sigma-1 receptor activation inhibits the dopamine release from striatum – the assay is imperfect as a decrease in dopamine level may occur after stimulating or blocking other receptors (Gonzalez and Werling, 1997). Another proposed method is based on the use of novel receptor fluorescence resonance energy transfer (FRET) based biosensor – the sigma-1 receptor agonists decrease and antagonists increase the FRET signal (Gómez-Soler et al., 2014). This effect results from how sigma-1 receptor ligands affect the oligomerization process – antagonists promote the high order oligomers, while agonists – monomers and dimers (Mishra et al., 2015). However, the agonist-bound receptor crystallizes similarly to antagonist-bound sigma-1 receptors, and overall receptor conformation remains practically unchanged, except for a 1.8 Å shift of helix α4 found when compared the (+)-pentazocine-bound relative to the PD 144418- bound structure (Schmidt et al., 2018). Moreover, we cannot assume that all potential sigma-1 receptor agonists stabilize only the monomeric structure and antagonists – the high-molecular-weight oligomers. Therefore, current structural data are insufficient to differentiate the intrinsic activity of sigma-1 receptor ligands.
The most common and quite relevant method examines the sigma-1 receptor/BiP association in ELISA kits as activation of this receptor relies on BiP dissociation (Fujimoto et al., 2012). Additionally, the positive modulators of these receptors can be identified when they are co-administered with known sigma-1 receptor agonists, and they potentiated the efficacy of the sigma-1 receptor agonists (Martin et al., 2020).
What is worth mentioning is that agonists and antagonists affect the stability of sigma-1 receptors multimers, decreasing or increasing the number of receptor’s multimers, respectively (Hong, 2020). The bioluminescence resonance energy transfer (BRET) assays enable analyses of ligand-induced multimerization of sigma-1 receptors as well as its interaction with BiP (Yano et al., 2018). The antagonists enhanced sigma-1 receptor homomer BRET signals, whereas agonists do not. Moreover, BRET assays also help differentiate the agonists and antagonists based on the interaction between the sigma-1 receptor and BiP (Yano et al., 2018).
Most of the above methods are not ideal and do not give us the certainty of whether a compound is the agonist or antagonist of the sigma-1 receptor. However, multimodal compounds targeting several proteins that bind to sigma-1 receptors may be superior to compounds without the affinity towards these receptors. Thus, even determining whether a compound has sigma-1 receptor affinity alone can be useful. However, if we are looking for selective compounds and want to define their functional profile, the best way to do it is to apply the sigma-1/BiP dissociation assay, as it is relatively easy and provides clear-cut results. Therefore, it is often used in drug development programs.
4. Depressive-like state and sigma-1 receptors
Recent studies have shown that sigma-1 receptors play a significant role in the pathophysiology of neuropsychiatric disorders like depression, and their ligands may constitute a promising option for treating depression (reviewed in Fishback et al., 2010). In addition, we already know that many currently used antidepressants bind to sigma-1 receptors, and their pharmacological action may partially depend on these receptors (Hashimoto, 2009).
To assess the possible antidepressant-like effects of compounds targeting sigma-1 receptors, we can use either tests measuring the animals’ reaction to specific environmental conditions (like forced swim, tail suspension, and sucrose preference tests) (for more information on animal tests used in searching for novel antidepressants, please see Belovicova et al., 2017) or models mimicking not only behavioral but also cellular and molecular changes observed in depression (like unpredictable chronic mild stress procedure, olfactory bulbectomy, chronic corticosterone administration, maternal separation) (for more information on animal models of depression, please see Wang et al., 2017). In the following subchapters, we review the available literature on the role of the sigma-1 receptor in regulating depressive-like states in rodents.
4.1. Sigma-1 receptor knock-out animals
The sigma-1 receptor knockout mice show depressive-like behaviors observed in the forced swim and tail suspension tests (Akunne et al., 2001; Di et al., 2017; Sabino et al., 2009; Zhang et al., 2017a, b, c). Interestingly, the decrease in immobility and impaired neurogenesis was observed in male but not female knockout mice (Chevallier et al., 2011; Di et al., 2017). Only when sigma-1 knockout female mice were ovariectomized, these differences were not observed (Sha et al., 2015). Similarly, the same study reported that 17β-estradiol given to male mice demonstrated neuroprotective and antidepressant-like properties (Sha et al., 2015). The above studies suggest that estrogens can protect from depressive-like behavior and impaired neurogenesis induced by the absence of the sigma-1 receptor.
Moreover, sigma-1 receptor knockout mice had decreased expression of glucocorticoid receptors (GR) via reduced protein kinase C (PKC) phosphorylation (Di et al., 2017). The attenuated GR-mediated feedback inhibition of the hypothalamic–pituitary–adrenal (HPA) axis produced a long-lasting HPA axis hyperactivity and subsequent depressive-like phenotype (Di et al., 2017). Another research showed that the depressive-like phenotype observed in sigma-1 knockout mice developed due to decreased nNOS activity and nitric oxide level with a subsequent decrease in presynaptic glutamate and GABA release and impaired long-term depression (LTD) induction in the basolateral amygdala (Zhang et al., 2017a, b, c). However, the possible factors contributing to the specific change in behavior in sigma-1 knockout mice could be multifactorial.
4.2. The effect of sigma-1 receptor ligands on depressive-like behavior in rodents
Various sigma-1 receptor agonists showed antidepressant-like activity in tests assessing antidepressant-like effect (Kim et al., 2006; Matsuno et al., 1996; Phan et al., 2002; Reddy et al., 1998; Skuza and Rogóz, 2009; Ukai et al., 1998; Urani et al., 2001; Wang et al., 2007a, b). The ammonium salts are sigma-1 receptor ligands, among which the most promising is dipentylammonium. The compound binds not only to sigma-1 receptors but also to sigma-2 receptors, showing antidepressant-like activity in the forced swim and tail suspension tests (Brimson et al., 2020). Moreover, the administration of BD1047, a sigma-1 receptor antagonist, reversed the decrease in immobility in mice treated with dipentylammonium (Brimson et al., 2020). The results of this study suggest that sigma-1 receptor activation contributes to the antidepressant-like effect. The molecular mechanism responsible for the antidepressant-like activity of sigma-1 receptor agonists might involve Ca2+ mobilization (Urani et al., 2002a). The decrease in the immobility time in the forced swim test induced by igmesine (sigma-1 receptor agonist) was blocked by calcium chelator, which suggested that the antidepressant-like effect mediated by the sigma-1 receptor depended not only on extracellular calcium influx but also intracellular calcium mobilization (Urani et al., 2002a).
Not only sigma-1 receptor agonists improve the animals’ performance in the tests assessing depressive-like behaviors. Such properties also show allosteric modulators of the sigma-1 receptor. One of the examples is SOMCL-668, which decreased the immobility in the forced swim and tail suspension tests, and its effect was blocked after pretreatment with BD1047 (sigma-1 receptor antagonist) (Wang et al., 2016). OZP002, a positive modulator of sigma-1 receptors, showed antidepressant-like properties in the forced swim test and potentiated the effect of igmesine administered at sub-effective doses (Maurice et al., 2019). Given that the positive modulator led to the same behavioral effect as the classic sigma-1 receptor agonist, and the use of both sigma-1 receptors knock out mice or selective sigma-1 receptor antagonist prevented antidepressant-like effect, may suggest that OZP002 compound could potentiate the endogenous activation of the sigma-1 receptor.
Interestingly, scientists observed a depressive-like state assessed by the tail suspension, forced swim, and sucrose preference tests in mice with induced cardiac dysfunction. The depressive-like state was reversed by either intracerebroventricular infusion or per os administration of sigma-1 receptor agonists – PRE084 and SA4503, respectively (Ito et al., 2012; Shinoda et al., 2016). The observed changes in behavior were most likely due to the decreased sigma-1 receptor expression in the hippocampus caused by elevated corticosterone levels (Moriguchi et al., 2013). Moreover, chronic dexamethasone infusions resulted in behavioral, cellular, and molecular changes in animals – they were more immobile in the forced swim test (Terada et al., 2014). Dexamethasone-treated mice showed decreased sigma-1 receptors and BDNF expressions, and chronic administration of fluvoxamine reversed these changes. Considering that sigma-1 receptor activation increases BDNF expression via cAMP-response element binding protein (CREB) activation (Ji et al., 2017; Xu et al., 2015), the increased sigma-1 receptor expression after treatment with fluvoxamine may be related to the increased BDNF expression.
Importantly, the sigma-1 receptors are involved in the antidepressant-like properties of multimodal compounds (Bermack et al., 2004; Chaki et al., 2004; Dhir and Kulkarni, 2007a, 2008a; Khulbe et al., 2013; Kulkarni and Dhir, 2008; Nguyen et al., 2017; Villard et al., 2011b). For instance, sigma-1 receptors mediate the antidepressant-like activity of many antidepressants or antipsychotics. The quetiapine-dependent decrease in mice immobility in the forced swim test was potentiated by sigma-1 receptor agonists and abolished by pretreatment with sigma antagonists (Kotagale et al., 2013). Also, the anti-immobility effects of venlafaxine, bupropion, or fluvoxamine in the forced swim test may require interaction with sigma-1 receptors (Dhir and Kulkarni, 2007b, 2008c; Terada et al., 2014). Neurosteroids like dehydroepiandrosterone sulfate and pregnenolone sulfate also exhibited antidepressant-like effect in the tail suspension and the forced swim tests in mice after activating sigma-1 receptors (Dhir and Kulkarni, 2008b; Reddy et al., 1998; Urani et al., 2001). The synergic administration of inactive doses of sigma-1 receptor agonists with NMDA antagonist, 5-HT1A receptor agonist or selective serotonin reuptake inhibitors (SSRIs) decreased the immobility time of animals in the forced swim test, suggesting antidepressant-like effect (Rogóz and Skuza, 2006; Skuza and Rogóz, 2002, 2006, 2007).
Interestingly, we have recently discovered that HBK-15, a multimodal compound showing fast antidepressant-like effect and anti-amnesic properties (Pytka et al., 2015, 2017a, 2017b, 2018; Waszkielewicz et al., 2014), also binds to sigma-1 receptors (unpublished results). It is very likely that the unique pharmacological profile of HBK-15 may depend on sigma-1 receptor-mediated signaling. However, our hypothesis requires confirmation.
The possible molecular mechanism of the antidepressant-like effect of sigma-1 receptor agonists may involve various signaling pathways. Studies showed that sigma-1 receptor stimulation led to molecular, cellular, and biochemical changes, including an increase in NR2A, CREB, NGF, and BDNF (Fukunaga and Moriguchi, 2017; Ji et al., 2017; Matsushima et al., 2018; Yagasaki et al., 2006). Both sigma-1 agonist (PB190) and antagonist (PB212) decreased the corticosterone-induced catalase (CAT) activity, an enzyme involved in the oxidative stress responses (Skuza et al., 2011). That is an unexpected result, considering the activity of both agonist and antagonist of sigma-1 receptors. However, both compounds also possess a very high affinity towards sigma-2 receptors (Berardi et al., 2005), so these outcomes may be the effect of modulating the sigma-2 receptor’s activity. Fluvoxamine and dehydroepiandrosterone sulfate led to Akt-1 phosphorylation via sigma-1 receptors in PC12 cells, underlying their antidepressant-like activity (Nakano et al., 2010). Sigma-1 receptors also enhanced the glutamate release from presynaptic terminals caused by fluvoxamine in the stressed mice, which may be linked to the potential memory-improving effect of the drug in depressed individuals (Fu et al., 2012).
In animal models of depression, sigma-1 ligands also showed antidepressant-like activity. Rats, after chronic unpredictable mild stress procedure, demonstrated despair (increased immobility in the forced swim test), and anhedonic (decreased sucrose consumption) behaviors (Chen et al., 2020; Liu et al., 2019). Chronic treatment with SA4503 or fluvoxamine, sigma-1 receptor agonists, abolished the negative behavioral effects of chronic stress (Chen et al., 2020; Liu et al., 2018, 2019). Moreover, only one-week treatment with SOMCL-668 (an allosteric modulator of sigma-1 receptor) was enough to reverse the stress-induced decrease in the sucrose preference (Wang et al., 2016). Furthermore, the sigma-1 allosteric modulator inactivated glycogen synthase kinase 3 beta (GSK3β) and normalized the BDNF level in the stressed mice (Wang et al., 2016). Another study demonstrated that the stimulation of sigma-1 receptors by fluvoxamine ameliorated the depressive-like behaviors in streptozocin-induced diabetic rats, and the mechanism of this effect depended on the sigma-1 receptor-BDNF pathway (Lenart et al., 2016). In agreement, other findings indicated that the chronic stimulation of sigma-1 receptors increased the BDNF level in the hippocampus (Kikuchi-Utsumi and Nakaki, 2008). Moreover, several antidepressants increased the neurite outgrowth in PC12 cells via interaction with sigma-1 receptors (Ishima et al., 2014; Takebayashi et al., 2002). In addition, fluvoxamine increased the level of pro-BDNF, mature BDNF, and sigma-1 receptors which eventually led to cell protection in diabetic animals (Lenart et al., 2016). The sigma-1 receptors agonist also alleviated the symptoms of postpartum depression in mice by increasing the nNOS-NO-CREB signaling (Zhang et al., 2017a, b, c). In olfactory bulbectomized mice, the activation of sigma-1 receptors by chronic administration of dehydroepiandrosterone decreased the immobility time in the tail suspension and forced swim test (Moriguchi et al., 2013). The authors suspected that this effect occurred after increased neurogenesis in the hippocampus via activation of the Akt/GSK-3b/b-catenin pathway (Moriguchi et al., 2013). All above findings indicate that the antidepressant-like effect of sigma-1 agonists is mediated not by one but by several signaling pathways, including nNOS-NO-CREB, Akt/GSK-3b/b-catenin, or ERK.
In addition to neurons, astrocytes play a vital role in depression pathophysiology and treatment. Sigma-1 receptors modulate the activation of astrocytes by increased phosphorylation of ERK and GSK3β (Wang et al., 2019a, b). The activation of sigma-1 receptors also alleviated depressive-symptoms (in conditioned fear stress and forced swim test) in mice infused with β -amyloid protein (Urani et al., 2002b, 2004), proving that sigma-1 receptor agonists may exert antidepressant-like activity in various disorders often co-existing with depression.
A recent study shed new light on the role of sigma-1 receptors in depression. Plasma exosomes from depressed mice ameliorated depressive-like behaviors, deficiency of BDNF expression, and neuroinflammation in LPS-treated mice via sigma-1 receptor delivery (Wang et al., 2021a, b). The exosomes without sigma-1 receptors no longer showed the antidepressant-like effect, suggesting that sigma-1 receptors are crucial for antidepressant-like activity.
4.3. Sigma-1 receptor ligands in clinical trials
Up to date, only SA4503 (cutamesine) has completed phase II clinical trials for major depressive disorder (ClinicalTrials.gov Identifier: NCT00551109, Table 2 ) (M’s Science Corporation, 2008). Moreover, the compound was also tested for safety and motor function restoration in patients after acute ischemic stroke (ClinicalTrials.gov Identifier: NCT00639249) (M’s Science Corporation, 2009). Additionally, igmesine, another sigma-1 receptor agonist, was also tested in depressed patients; however, the compound failed to be effective in phase III clinical trials (Pande et al., 1998, 1999).
Table 2.
Sigma-1 receptor agonists in clinical studies.
| Sigma-1 receptor agonist | Conditions | Phase | Reference |
|---|---|---|---|
| SA4503 (cutamesine) | Major depressive disorder | 2 | (M’s Science Corporation, 2008) |
| Acute ischemic stroke | 2 | (M’s Science Corporation, 2009) | |
| ANAVEX2-73 (blarcamesine) | Alzheimer’s disease | 2b/3 | (Anavex Life Sciences Corp., 2021a, 2021b) |
| Parkinson’s disease dementia | 2 | (Anavex Life Sciences Corp, 2020) | |
| Rett syndrome | 2 | (Anavex Life Sciences Corp., 2021c, 2021d) |
4.4. Conclusion
To conclude, the lack of sigma-1 receptors contributes to the development of depressive-like phenotype in male rodents. Conversely, the activation of sigma-1 receptors mediates the antidepressant-like response in naïve and depressed animals (Fig. 2 ), probably via influence on BDNF and NGF expression. The pharmacological effects of sigma-1 receptor ligands in tests assessing antidepressant-like effect are summarized in Table 3 .
Fig. 2.
The role of sigma-1 receptors in the antidepressant-like effect. The stimulation of sigma-1 receptors is necessary for an antidepressant-like effect.
Table 3.
Antidepressant-like activity of sigma-1 receptor ligands.
| Potential modulator | Animal | Animal Models/Tests | Reference |
|---|---|---|---|
| Sigma-1 receptor agonists | |||
| SA4503, (+)-pentazocine, ditolylguanidine (DTG), JO-1784 (single administration) | Mice | Forced swim test | (Matsuno et al., 1996) |
| SA4503, (+)-pentazocine, DTG, desipramine, fluoxetine | Mice | Tail suspension test | (Ukai et al., 1998) |
| UMB23, UMB82 | Mice | Forced swim test | (Wang et al., 2007a, b) |
| Igmesine, (+)-SKF-10047, dehydroepiandrosterone sulfate | Naïve and adrenalectomized/castrated mice | Forced swim test | (Urani et al., 2001) |
| PRE-084 | Mice | Forced swim test | (Skuza and Rogóz, 2009) |
| Dehydroepiandrosterone sulfate (DHEAS), pregnenolone sulfate (PS) | Rats | Forced swim test | (Reddy et al., 1998) |
| Dipentylammonium (DPA) | Mice | Forced swim test, tail suspension test | (Brimson et al., 2020) |
| Igmesine | Mice | Forced swim test | (Urani et al., 2002a; Villard et al., 2011b) |
| PRE084 | Mice with accelerate cardiac dysfunction | Forced swim test | (Ito et al., 2012) |
| SA4503 | Mice after transverse aortic constriction | Forced swim test, tail suspension test, sucrose preference test | (Shinoda et al., 2016) |
| Dehydroepiandrosterone (DHEA) | Olfactory bulbectomized mice | Forced swim test, tail suspension test | (Moriguchi et al., 2013) |
| 17β-estradiol | Mice | Forced swim test, tail suspension test | (Dhir and Kulkarni, 2008a) |
| Deuterated (d6)-dextromethorphan | Mice | Forced swim test, tail suspension test | (Nguyen et al., 2017) |
| R278995/CRA0450 | Rats, mice | Forced swim test, tail suspension test | (Chaki et al., 2004) |
| Berberine | Naïve and reserpinized mice | Forced swim test, tail suspension test | (Kulkarni and Dhir, 2008) |
| Hydromethanolic extract of flowers of Tagetes erecta | Mice | Forced swim test | (Khulbe et al., 2013) |
| Ropinirol | Mice | Forced swim test, tail suspension test | (Dhir and Kulkarni, 2007a) |
| Quetiapine | Mice | Forced swim test | (Kotagale et al., 2013) |
| Venlafaxine | Mice | Forced swim test | (Dhir and Kulkarni, 2007b) |
| Bupropion | Mice | Forced swim test | (Dhir and Kulkarni, 2008c) |
| Dehydroepiandrosterone sulfate (DHEAS), pregnenolone sulfate (PS) | Mice | Tail suspension test | (Dhir and Kulkarni, 2008b) |
| DTG, SA4503 | Rats | Forced swim test | (Skuza and Rogóz, 2007) |
| SA4503, siramesine | Rats | Forced swim test | (Skuza and Rogóz, 2006, 2002) |
| Pramipexole + sertraline | Rats | Forced swim test | (Rogóz and Skuza, 2006) |
| SA4503 | Chronic unpredictable stressed rats | Sucrose preference test, forced swim test | (Liu et al., 2019, 2018) |
| Fluvoxamine | Chronic unpredictable stressed rats | Sucrose preference test, forced swim test | (Chen et al., 2020) |
| Fluvoxamine | Diabetic-induced depressed rats | Forced swim test | (Lenart et al., 2016) |
| PRE-084 | Ovariectomized mice | Forced swim test, tail suspension test | (Zhang et al., 2017a, b, c) |
| Igmesine, (+)-SKF-10,047, dehydroepiandrosterone sulfate | Aβ25-35 peptide-treated mice | Conditioned fear stress test | (Urani et al., 2004) |
| Igmesine, PRE-084 | Aβ25-35 peptide-treated mice | Forced swim test | (Urani et al., 2002b) |
| Sigma-1 receptor modulators | |||
| SOMCL-668 | Naïve mice, chronic unpredictable stressed mice | Forced swim test, tail suspension test, sucrose preference test | (Wang et al., 2016) |
| OZP002 | Mice | Forced swim test | (Maurice et al., 2019) |
5. Memory functioning and sigma-1 receptors
Sigma-1 receptor ligands, especially agonists, have been studied in various models of memory impairments in rodents, either assessing emotional/aversive memory (using passive avoidance test (Jarvik and Kopp, 1967) or fear conditioning (Anagnostaras et al., 1999)), spatial working and reference memory (using Morris water maze (Morris, 1984), Y-maze (Dennis and Sollenberger, 1934), or recognition/episodic-like memory (using object recognition test) (for more information on animal memory tests please see Beuzen and Belzung, 1995; Lueptow, 2017; Vorhees and Williams, 2014). Since compounds targeting sigma-1 receptors control calcium mobilization, neurotransmitters release, including glutamate and acetylcholine, and neurotrophic factors expression, it is not surprising that they often show anti-amnesic properties. Moreover, sigma-1 receptors may play a crucial role in neurodegenerative diseases like Alzheimer’s disease. Sigma-1 agonists not only reversed memory impairments in rodent Alzheimer’s disease models after a single injection before the test (Maurice et al., 1998) but also showed neuroprotective properties (Antonini et al., 2011; Marrazzo et al., 2005; Maurice et al., 2019).
5.1. Sigma-1 receptor knock-out animals
Sigma-1 knockout mice exhibited impaired NMDA-dependent neurogenesis, the formation of LTP and LTD (Sha et al., 2013; Snyder et al., 2016; Zhang et al., 2017a, b, c). The lack of sigma-1 receptors caused the overproliferation of progenitor cells, but newborn neuronal cells' survival was decreased in the dentate gyrus (Sha et al., 2013). Moreover, diminished expression of sigma receptors contributed to decreased NMDA NR2B phosphorylation in the basolateral amygdala and dentate gyrus, as well as the nNOS coupling to PSD-95 and NO level (Zhang et al., 2017a, b, c). Such an effect might be beneficial in neurodegenerative diseases because of the decrease in NMDA receptor-mediated neurotoxicity. The receptor knockdown also impaired the dendritic spine formation, and thus synaptic plasticity, crucial for memory and learning processes (Tsai et al., 2009).
The first study on sigma-1 knockout mice showed no significant cognitive function changes (Langa et al., 2003). However, later experiments demonstrated that only female homozygous and heterozygous mice showed spatial and working memory impairments in the water and Y-maze tests, respectively (Chevallier et al., 2011; Xu et al., 2017). Moreover, these deficits were more pronounced with age, and they could be reversed by 17b-estradiol (Chevallier et al., 2011). Interestingly, the emotional memory deficits assessed by the passive avoidance test appeared only in 12-month-old homozygous females (Chevallier et al., 2011). On the other hand, sigma-1 knockout male and female mice had impaired long-term, but not short-term, recognition memory (Xu et al., 2017). Also, the Hamlet test, where animals are exposed to an enriched environment, showed that lack of sigma-1 receptors completely prevented the establishment of topographic memory and behavioral resilience, as well as the hippocampal neurogenesis (Crouzier et al., 2020). These differences between males and females suggest that sex hormones may affect sigma-1 receptors signaling and in consequence memory processes. However, not all types of memory are equally affected in knock-out animals proving that sigma-1 receptors may have a diverse effect on different types of memory and learning processes.
5.2. The role of sigma-1 receptor in the LTP formation
Long-term potentiation (LTP) is a process based on the long-lasting increase in the synaptic strength following a high-frequency stimulation, being a foundation of synaptic plasticity and, thus, learning and memory (for more information please see (Lynch, 2004)). The sigma-1 receptors are a critical node in the NMDA-independent LTP formation. Neurosteroids via sigma receptors transiently enhanced presynaptic function (Chen et al., 2006; Jafari-Sabet et al., 2019). The same effect was observed in the hippocampus of rats treated with alcohol, suggesting the significant role of sigma-1 receptors in synaptic plasticity (Sabeti and Gruol, 2008). Furthermore, impaired LTP formation in olfactory bulbectomized mice was improved after a chronic treatment with DHEA (Moriguchi et al., 2013), which increased calcium/calmodulin-dependent protein kinase II (CaMKII), PKC, and ERK activity in the hippocampus (Moriguchi et al., 2011). More specifically, DHEA led to increased CaMKII autophosphorylation and GluR1 phosphorylation in the dentate gyrus (Moriguchi et al., 2013). These molecular changes also affected animal behavior. DHEA reversed the spatial, recognition, and emotional memory deficits (Moriguchi et al., 2011). Moreover, the beneficial effect of DHEA was visible in the ischemic rats. The compound prevented the disruption of the LTP formation in CA1 by attenuating the reduction in tyrosine phosphorylation of NR2B (Li et al., 2006).
5.3. The effect of sigma-1 receptor ligands on various types of memory in rodents
Compounds with an affinity towards sigma receptors showed anti-amnesic properties in numerous studies, in which cognitive impairments were induced by different factors, such as drug (scopolamine, dizocilpine, phencyclidine, neurotransmission, cocaine, nimodipine) or toxin (β-amyloid(25–35)-peptide, CO, trimethyltin) administration, stress (post-traumatic stress disorder, prenatal restraint), or brain damage (brain ischemia, olfactory bulbectomy) (Maurice et al., 1994a, 1994b, 1998, 1999a, 2001a; Meunier et al., 2006c). All mentioned animal models cause memory deficits that differ in severity and memory type affected (for more information on animal models of memory deficits please see Gallagher, 1997; Götz and Götz, 2009; More et al., 2016; van der Staay et al., 2011). They may be a result of impaired cholinergic (scopolamine), glutamatergic (dizocilpine, phencyclidine) or serotonergic neurotransmission (para-chlorophenylalanine), neurons death (β-amyloid(25–35)-peptide, 192 IgG-saporin, trimethyltin, ibotenic acid, carbon monoxide (CO), brain ischemia, olfactory bulbectomy), oxidative stress (cocaine) or oxidative phosphorylation (prenatal stress) (for more information on animal models of cognitive impairments please see Beraki et al., 2009; Cheng et al., 2012; Deryabina et al., 2020; Li et al., 2020; Muriach et al., 2010; Nakajima et al., 2007; Pepeu, 2004; van der Staay et al., 2011).
Interestingly, these factors induce various changes in sigma-1 receptor density. For example, cholinergic lesion (caused by unilateral NMDA infusion to nucleus basalis) resulted in increased sigma-1 receptor expression in the parietal cortex, contrary to sleep deprivation that caused a decrease in the level of the receptor in the pons and midbrain (Ramakrishnan et al., 2015a). Mice during the alcohol withdrawal showed the increased sigma-1 receptor expression in the hippocampus (Meunier et al., 2006a), while phencyclidine administration led to a decrease in the sigma-1 receptor level in the same structure (Kunitachi et al., 2009). However, the density of sigma-1 receptors remained unchanged throughout life (Phan et al., 2003). These findings suggest that the level of the sigma-1 receptor may be affected by drug or toxin administration, but not age.
Results regarding whether the activation or blockade of sigma-receptors is beneficial for memory are ambiguous. Most studies demonstrated that administration of sigma-1 receptor agonists/antagonists did not affect normal memory function. However, they showed their pharmacological effects when learning was impaired. Interestingly, one study demonstrated a negative effect of the sigma-1 receptor agonist on emotional memory (assessed by the passive avoidance test) (Freeman and Young, 2001). The research was performed on a day-old chicks, in which sigma-1 receptor agonist administration 5 h after training induced learning and memory deficits (Freeman and Young, 2001). However, this effect occurred when specific dose of (+)-SKF10047 was given to chicks – higher or lower dose did not disrupt memory. This suggests that sigma-1 receptor activation might be crucial for emotional memory consolidation, possibly by modulating the NMDA, GABA, and acetylcholine neurotransmission. However, this study did not profoundly investigate the cause of the amnesic effect of the sigma-1 receptor agonist. Nevertheless, most studies concerning the effects of sigma-1 receptor ligands on memory were performed on mice or rats, not chicks, so these discrepancies could be due to species differences.
5.3.1. Emotional/aversive memory
Sigma-1 receptor agonists enhanced disrupted emotional memory in rodents (Earley et al., 1991; Matsuno et al., 1993b; Maurice et al., 1994a, 2000, 2001a). Sigma-1 receptor agonists reversed emotional memory deficits caused by either dizocilpine administration, probably via enhancing NMDA receptor-mediated neurotransmission (Espallergues et al., 2007; Maurice et al., 1994c, 1997; Maurice and Privat, 1997; Villard et al., 2011a) or scopolamine injection via increasing the acetylcholine level in the hippocampus (Espallergues et al., 2007; Malik et al., 2015; Matsuno et al., 1995, 1997; Senda et al., 1996, 1997; Tottori et al., 2002; Urani et al., 1998; Villard et al., 2011a). Moreover, the anti-amnesic effect of sigma-1 receptor agonists could be mediated via the increased release of BDNF from astrocytes (Malik et al., 2015). However, some compounds are not 100 % selective towards sigma-1 receptors and their anti-amnesic effect may depend on other biological targets such as the serotonin 5-HT1A receptor (OPC-14523) (Tottori et al., 2002) or muscarinic receptors (ANAVEX2-73) (Espallergues et al., 2007; Villard et al., 2011a). Similarly, sigma-1 receptor modulators (OZP002 or E1R) were effective in scopolamine-induced memory impairments and these compounds also enhanced the anti-amnesic effect of either PRE084 or igmesine (Maurice et al., 2019; Zvejniece et al., 2014). This suggests that sigma-1 receptors modulators affect the cholinergic neurotransmission, and they are an attractive therapeutic target for drugs treating memory deficits caused by a decrease in acetylcholine level.
Studies showed that sigma-1 receptor agonists might reverse memory deficits induced by sleep deprivation. Such example is cutamesine (SA4503), which prevented emotional memory impairment induced by sleep deprivation in the passive avoidance task (Ramakrishnan et al., 2015b). Interestingly, this anti-amnesic effect observed at higher doses may result from more prolonged exposure and/or drug binding to other receptors or channels. As sleep deprivation-induced memory impairments are caused by the increase in misfolded proteins’ level and expression of inflammatory cytokines (Esumi et al., 2011; Rothman et al., 2013), the fact that sigma-1 receptor agonist reversed the consolidation and memory deficits is promising. Considering that many diseases are associated with more or less pronounced sleep disturbances, which may worsen their clinical course and lead to memory deficits, treatment with sigma-1 receptor agonists might be a promising strategy.
Worth mentioning is that the anti-amnesic effect of memory-enhancing drugs such as donepezil or rivastigmine can be correlated with stimulation of NGF-mediated neurite outgrowth via sigma-1 receptors (Ishima et al., 2008; Terada et al., 2018). In mice with dizocilpine-induced emotional memory deficits, donepezil showed anti-amnesic activity, which was attenuated when the sigma-1 receptors were pharmacologically blocked (Maurice et al., 2006). The above findings suggest that sigma-1 is an essential target for anti-amnesic effect.
In trimethyltin-induced memory disruption, igmesine (JO-1784), PRE-084, and dextromethorphan, a nonselective serotonin reuptake inhibitor and a sigma-1 receptor agonist, improved emotional memory after sigma-1 receptors stimulation (Maurice et al., 1999a; O’Connell et al., 1996; Shin et al., 2007). Moreover, PRE-084 and DTG were effective to ameliorate the memory and learning deficits after chronic CO exposure (Maurice et al., 1994c, 1999a). However, the neuroprotective effect of another sigma-1 receptor agonist, DHEA in CO-induced emotional memory impairments did not depend on sigma-1 receptors as their pharmacological blockade did not abolish memory-enhancing properties of the neurosteroid (Maurice et al., 2000). The basal forebrain lesion, caused by ibotenic acid administration, caused memory deficit in the passive avoidance task and these impairments were reversed after sigma-1 receptor activation (Senda et al., 1996).
In p-chloroamphetamine-induced amnesia sigma-1 receptor agonists showed anti-amnesic effects in the passive avoidance test (Matsuno et al., 1994). Moreover, stimulating these receptors reversed memory impairments during the encoding, consolidation, and recall phase, thus all stages of learning and memory processes (Matsuno et al., 1994, 1998; Senda et al., 1997).
The anti-amnesic effect of sigma-1 receptors activation may strongly depend on restoring the disrupted acetylcholinergic transmission. This hypothesis is based on the fact that similar to acetylcholinesterase inhibitors, sigma-1 receptor agonists showed anti-amnesic effect after serotonin depletion in the central nervous system, and sigma-receptor stimulation increased acetylcholine level in the frontal cortex and hippocampus, key structures for memory (Junien et al., 1991b; Matsuno et al., 1992, 1993a). Also, the memory-enhancing properties disappeared after pretreatment with either scopolamine, a muscarinic receptor antagonist, or hemicholinum-3, a Na+-dependent high-affinity choline uptake inhibitor. However, scopolamine itself causes memory deficits so it is difficult to state whether the anti-amnesic effect of the sigma-1 receptor agonist was antagonized, or the potentiated memory impairments were too potent to be reversed by sigma-1 receptor activation.
On the contrary, in the methamphetamine-induced memory disruption, the blockade of sigma-1 receptors improved emotional memory, assessed by the passive avoidance test (Seminerio et al., 2013). This suggests that sigma-1 receptor antagonists improve memory deficits caused by decreased dopamine levels. Moreover, other studies showed that sigma-1 receptor antagonists prevented attenuated methamphetamine-induced apoptosis, necrosis, ROS/RNS generation, and dopamine release both in vitro and in vivo studies (Kaushal et al., 2012; Matsumoto et al., 2008).
Importantly, memory deficits occur most often during senescence. In senescence-accelerated mice, sigma-1 receptor agonists showed anti-amnesic properties by improving emotional memory (Maurice et al., 1996). Moreover, OPC-14523 (a sigma-1 receptor agonist) exhibited anti-amnesic properties in age-associated memory impairments (Tottori et al., 2002). However, as mentioned earlier, the compound also acts as a 5-HT1A receptor agonist. Therefore, its effects might be related to the influence on serotonergic neurotransmission. Interestingly, only a single administration of OPC-14523 was enough to reverse behavioral changes in the scopolamine-induced memory deficits model (Tottori et al., 2002). However, the compound was unable to reverse age-related memory and learning impairments when it was administered once. On the hand, OPC-14523 ameliorated age-induced cognitive impairments, when it was administered chronically (Tottori et al., 2002).
Emotional memory deficits can also be caused by ischemia. Studies demonstrated that in mice after brain ischemia, sigma-1 receptor agonists restored proper memory functioning assessed by the passive avoidance test (Xu et al., 2015, 2017; Yabuki et al., 2015). These memory-enhancing properties might be mediated via NR2A-calcium/calmodulin-dependent protein kinase type IV (CaMKIV) - target of rapamycin complex 1 (TORC1) pathway as the sigma-1 receptor activation normalized the level of BDNF, NR2A, CaMKIV and TORC1 in the hippocampus (Xu et al., 2015). Other studies show that neuroprotective properties of sigma-1 receptor agonists in ischemic mice may be the result of increased ATP production, activation of Akt, CaMKII, and ERK as well as up-regulation of sigma-1 receptor expression in the hippocampus (Yabuki et al., 2015).
The anti-amnesic effect of the sigma-1 receptor agonist was also seen in prenatally stressed juvenile rats. Igmesine improved spatial and emotional memory by normalizing the impaired glutamatergic, cholinergic, and adrenergic neurotransmission as well as HPA-axis functioning (decreasing the corticosterone release through an anti-CRF effect (Derocq et al., 1995; Eaton et al., 1996; Junien et al., 1991a; Meunier et al., 2004). Interestingly, another sigma-1 receptor agonist, SA-4503, also improved olfactory-bulbectomized rats’ performance in the cued and contextual fear conditioning and this effect was abolished after NMDA blockade (Wang et al., 2007a, b). This is an interesting result considering that memory deficits observed in olfactory bulbectomy arise from the degeneration of the cholinergic system and the decrease in acetylcholine level (Yamamoto et al., 1997; Yoshimura et al., 1974). That confirms that sigma-1 receptors affect cholinergic neurotransmission, and this effect may also depend on NMDA receptors.
Memory impairments also occurred after addictive drug exposure. Both igmesine and DHEA by activating sigma-1 receptors alleviated memory impairment observed in the passive avoidance task in prenatally cocaine-exposed mice (Meunier and Maurice, 2004). Studies showed that cocaine exposure led to sigma-1 receptor-dependent upregulation of D-type K+ current in the nucleus accumbens with a subsequent decrease in neuronal activity and enhancing behavioral cocaine response (Kourrich et al., 2013). The sigma-1 receptor agonist may modulate neuronal and behavioral response to cocaine since the cocaine-induced complex formation between the sigma-1 receptor and Kv1.2 channels redistributes both proteins from intracellular compartments to the plasma membrane (Kourrich et al., 2013).
5.3.2. Spatial working and reference memory
Various sigma-1 receptor agonists improved impaired spatial working and reference memory (water maze, eight-arm radial maze, Y-maze test) after dizocilpine treatment, and their memory-enhancing effect was abolished after pretreatment with sigma-1 receptor antagonist (Maurice et al., 1994a, 1994b, 1994d, 1997; Ohno and Watanabe, 1995; Zou et al., 1998, 2000) or oligodeoxynucleotide antisense (Maurice et al., 2001a, 2001b). Sigma-1 receptor agonists like (+)-SKF10047 or SA4503, or positive receptor modulators (OZP002 and E1R) reversed scopolamine-induced spatial short-term memory impairments in the Y-maze alternation test and long-term memory deficits in the water maze (Hiramatsu et al., 2002; Malik et al., 2015; Maurice et al., 2001a, 2019; Urani et al., 1998; Wang et al., 2003; Zvejniece et al., 2014).
Interestingly, both isomers of pentazocine exerted anti-amnesic properties in scopolamine-treated animals via stimulating sigma receptors (Hiramatsu and Hoshino, 2005). However, only the (+)-pentazocine reversed the dizocilpine-induced memory deficits in the Y-maze task. Another sigma-1 receptor agonist – OPC-14523 – showed memory-enhancing properties not only in mice with scopolamine-induced memory impairment (Morris water maze), but also improved cognition in age-induced learning and memory deficits (Tottori et al., 2002). Even when the nonselective compound was tested in animals with scopolamine-induced memory decline (KT-95 or U-50,488H with affinity toward all opioid receptors), its anti-amnesic effect depended mainly on sigma receptors (Hiramatsu et al., 2006; Hiramatsu and Hoshino, 2004). Also, ANAVEX2-73, sigma-1 and muscarinic receptor agonist, reversed the scopolamine- and dizocilpine-induced spatial memory impairments, and this effect depended on sigma-1 and muscarinic receptors (Espallergues et al., 2007; Villard et al., 2011a). Thus, the above studies suggest that muscarinic receptors are more important for the short-term memory, while sigma receptors are more engaged in the long-term memory formation.
What is interesting, sigma-1 receptors also play a significant role in the memory impairment caused by neurotoxic organometal – trimethyltin. Igmesine (JO 1784), PRE-084, and dextromethorphan reversed behavioral changes in the spatial memory assessing tasks (Maurice et al., 1999a; O’Connell et al., 1996; Shin et al., 2007). Since the neurotoxicity of trimethyltin is the result of overstimulation of NMDA receptors and increased glutamate release, it seems that sigma-1 receptor activation normalizes the disrupted glutamatergic neurotransmission.
Mild or severe memory deficits may also develop after partial or almost complete cholinergic depletion. The sigma-1 receptor agonists (PPCC and SA4503) ameliorated the working and reference memory impairments acting via sigma-1 receptors (Antonini et al., 2009; Senda et al., 1998a, b). Interestingly, the administration of the sigma-1 receptor to animals with normal cognitive functions did not change any parameters (Antonini et al., 2009). This suggests that stimulating sigma-1 receptors exerts memory-enhancing properties only in situations when cognition is impaired. Moreover, when the level of endogenous steroids is diminished, their role in the CA1 activity and spatial learning is more visible, as castration increased the sigma-1 receptor mRNA expression level (Moradpour et al., 2016; Phan et al., 1999).
Diminished NO levels can also cause cognitive impairments. The reduced NO synthesis led to the short-term spatial memory impairment, which was reversed after sigma-1 receptor activation and a subsequent guanylate cyclase enhancing NO/cGMP pathway (Mamiya et al., 2000). In the nimodipine-induced memory impairments, PRE-084 attenuated impaired performance in the Y-maze and water maze tasks (Maurice et al., 1995). The study showed that sigma-1 receptor agonists were effective in various models of memory impairments. Moreover, the study proved that sigma-1 receptor agonists show anti-amnesic properties via facilitating the intracellular Ca2+ fluxes, which are involved in the memory processes.
Repeated exposure to carbon monoxide leads to the long-lasting but delayed memory deficits (Piantadosi et al., 1997). Like models of brain ischemia, the etiology of memory and learning impairments involves the neurotoxicity of excitatory amino acids, and subsequently disrupted hippocampal cholinergic system by hypoxic toxicity. As the sigma-1 receptor stimulation attenuated the CO-induced spatial memory and learning deficits (T. Maurice et al., 1994c, a; Meunier et al., 2006b), we can conclude that the anti-amnesic effect is the result of sigma-1 receptor-mediated neuroprotection and antioxidative properties. Interestingly, similar to emotional memory, sigma-1 receptor activation also ameliorated spatial learning and memory deficits in brain ischemia/reperfusion mice via increasing the level of BDNF (Xu et al., 2015, 2017; Yabuki et al., 2015).
Numerous stress models disrupt memory in animals. The memory impairments can be observed in the rat model of post-traumatic stress disorder, and the sigma-1 receptor activation prevented the spatial memory deficits as well as increased the phosphorylation of BDNF, TrkB, and ERK (Ji et al., 2016). Also, the prenatal restraint stress impaired the later cognitive abilities during adulthood (Wu et al., 2007). Rats showed impaired spatial performance in the Y-maze and Morris water maze due to the disrupted neurotransmission and the HPA axis functioning (Meunier et al., 2004). The administration of igmesine normalized animals’ behavior and disrupted the activity of the HPA axis (Meunier et al., 2004). This suggests that sigma-1 receptor activation thanks to neuromodulatory effects can alleviate memory impairments linked to developmental disorders.
Additionally, spatial memory deficits may also occur after drug exposure, especially during the prenatal period. The cocaine-treated dams exhibited the memory impairment, which was reversed after the sigma-1 receptor stimulation by igmesine or dehydroepiandrosterone (Meunier and Maurice, 2004). This neuroprotective effect of both compounds may result from restoring the normal functioning of several receptors, including NMDA, acetylcholine, dopaminergic or norepinephrine receptors and K+ channels, and normalizing the activity of the HPA axis and stress hormones’ levels.
One of the possible explanations why sigma-1 receptors play a cricial role in spatial memory may be the fact that in various models of memory deficits we observe decreased expression of sigma-1 receptors in the hippocampus or hypothalamus (Ito et al., 2013). For example, mice with myocardial infarction showed impaired spatial working memory (decrease in Y-maze spontaneous alternation) (Ito et al., 2013), which was normalized via intracerebroventricular infusion of the sigma-1 receptor agonist (Ito et al., 2013).
What is worth mentioning, the sigma-1 receptor activation is also beneficial for age-related spatial working and reference memory impairments in senescence-accelerated mice as well as normal aging rats (Maurice et al., 1996; Maurice, 2001). Moreover, a recent study showed that sigma-1 receptor agonists were also effective in α-thalassemia X-linked intellectual disability (ATR-X) syndrome. SA4503, sigma-1 receptor agonist, reversed the abnormal axonal development and dendritic spine formation (Yamaguchi et al., 2018). ATR-X model mice spatial learning and memory deficits, which were ameliorated by SA4503 via increasing BDNF level in the medial prefrontal cortex (Yamaguchi et al., 2018).
5.3.3. Recognition/episodic-like memory
The role of the sigma-1 receptor in the recognition memory is ambiguous. Dehydrocorybulbine, a sigma-1 receptor ligand obtained from Corydalis yanhusuo, attenuated MK-801-induced memory deficits in the object recognition test (Wang et al., 2019a, b). However, it is difficult to unambiguously state whether this memory-enhancing effect was mediated via sigma receptors, since the alkaloid binds to the various receptors (dopaminergic, serotonergic or adrenergic) (Wang et al., 2019a, b).
Among currently used drugs, many show significant affinity toward sigma receptors. The chronic administration of either fluvoxamine or donepezil, drugs with significant sigma-1 receptor affinity, ameliorated phencyclidine-induced impairments of the recognition memory (Hashimoto et al., 2007; Kunitachi et al., 2009). The pretreatment with NE-100, a sigma-1 receptor antagonist, abolished the drugs’ effects (Hashimoto et al., 2007; Kunitachi et al., 2009). Thus, the anti-amnesic activity of fluvoxamine and donepezil depended on sigma-1 receptor stimulation. Moreover, SA4503, a sigma-1 receptor agonist, reversed the recognition memory deficits as well as abnormal dendritic spine formation in the mouse model of ATR-X syndrome (Yamaguchi et al., 2018). Given these results, sigma-1 receptor agonists are a promising therapeutic option for alleviating various symptoms of intellectual disability, like those observed in ATR-X syndrome.
Interestingly, both sigma-1 receptor agonist and antagonist reversed the impaired recognition memory in alcohol-dependent mice during the withdrawal (Meunier et al., 2006a). These surprising results may be explained by the down-regulation of the increased level of sigma-1 receptors. The sigma-1 receptor agonists normalized the receptor overexpression but led to the accumulation of the protein in the terminal sites. On the other hand, sigma-1 receptor antagonists decreased the receptor binding in the microsomal fractions (Meunier et al., 2006a). Moreover, another study showed that the blockade of the sigma-1 receptor by AZ66 improved the recognition memory in methamphetamine-treated mice (Seminerio et al., 2013). Additionally, AZ66 significantly abolished striatal dopamine depletions and dopamine transporter reductions caused by methamphetamine (Seminerio et al., 2013). This suggests that stimulating sigma-1 receptor leads to anti-amnesic effect in the case of memory impairments caused by decreased dopamine levels. Considering all reports, in this specific type of memory, a balanced modulation of sigma-1 receptor activity seems to be crucial. Studies suggest that depending on the cause of memory deficit either stimulation or blockade of sigma-1 receptors is responsible for anti-amnesic effect.
5.4. Sigma-1 receptor and Alzheimer’s disease – preclinical studies
Sigma-1 receptor agonists showed their anti-amnesic properties in various behavioral tests assessing different types of memory impaired in Alzheimer’s disease. The first paper linking the sigma-1 receptor with Alzheimer’s disease was a work by Maurice and colleagues (Maurice et al., 1998). In this study, the selective sigma-1 receptor agonists ((+)-pentazocine, PRE-084, SA4503) attenuated the learning and memory deficits after Aβ25-35 peptide infusion in the spontaneous alternation performance and passive avoidance tests (Maurice et al., 1998). Subsequent papers confirmed these findings showing that the administration of various sigma-1 receptor agonists like PRE-084, donepezil or (-)-MR22 restored the proper memory functioning in the Y-maze and passive avoidance tasks in the Aβ25-35 peptide-infused mice (Antonini et al., 2011; Maurice, 2016; Meunier et al., 2006c). Their additive properties might have involved enhancing acetylcholine neurotransmission since donepezil acts as both acetylcholinesterase inhibitor and agonist of α7 subtype of nicotinic receptors as well as sigma-1 receptor agonist, increasing the acetylcholine release in the hippocampus and cortex. Moreover, the neuroprotective activity of pregnenolone sulfate (PREGS) might depend not only on the sigma-1 receptor but also on α7 nicotinic receptors. The compound improved the Aβ25-35-treated animals’ performance in the Morris water maze (Yang et al., 2012). ANAVEX2-73 also ameliorated the spatial and emotional learning deficits in mice injected with Aβ25-35 peptide, also showing neuroprotective properties by decreasing the level of lipid peroxidation and blocking tau hyperphosphorylation (Lahmy et al., 2013; Villard et al., 2011a). However, as mentioned earlier, this effect may be mediated not only via sigma-1 receptors, but also muscarinic receptors. The same results were observed for another aminotetrahydrofuran derivative, which stimulates sigma-1 receptors – ANAVEX1-41 (Villard et al., 2009). Moreover, a compound with a similar pharmacological profile, AF710B, improved recognition and spatial memory in aged McGill-R-Thy1-APP rats by reducing amyloid plaques, normalizing the microglia distribution and synaptophysin levels (Hall et al., 2018). Fluoxetine treatment also reversed memory and learning deficits in the active avoidance task in the nucleus-basalis-lesioned rats (experimental model of Alzheimer’s disease) (Ivković et al., 2004). Treatment of J20 amyloidogenic mice with another SSRI, fluvoxamine, improved memory in the object recognition task but did not affect other types of memory (assessed in the Y-maze and cheeseboard task) (Kim et al., 2018). Interestingly, the chronic administration of choline, which also binds to sigma receptors and potentiates IP3-evoked Ca2+ release (Brailoiu et al., 2019), improved the spatial memory in the Morris water maze in APP/PS1 mice and, like afobazole, reduced the microglial activation (Velazquez et al., 2019). This anti-amnesic effect of sigma-1 receptor activation may involve the inhibition of γ-secretase activity (enzyme responsible for the cleavage of β-amyloid protein precursor) and subsequently reducing the Aβ production (Kim et al., 2018). The sigma-1 receptor positive modulator (OZP002) was also active in two mouse models of Alzheimer’s disease – it prevented the amyloid toxicity and memory impairment (Maurice et al., 2019).
What is worth mentioning, when the activity of sigma-1 receptors was blocked (either genetically or pharmacologically), the memory impairments, lipid peroxidation and Bax expression, caused by Aβ25-35 infusion, were more pronounced compared with mice with normal sigma-1 receptor function (Maurice et al., 2018). Moreover, APPSwelnd/sigma-1 receptor knockout mice exhibited more deficits in learning and memory processes than APPSwelnd mice (Maurice et al., 2018). This suggests that abnormal levels of sigma-1 receptors determine more severe pathology of Alzheimer’s disease mouse models. However, Yin with co-workers showed that heterozygous sigma-1 receptors knockout mice injected with Aβ25-35 peptide did not exert such memory deficits compared with wild-type mice after Aβ25-35-treatment (Yin et al., 2015). Here, the study suggests that the decreased expression of sigma-1 receptors is neuroprotective in Alzheimer disease mouse model against Aβ25-35-induced increased NR2B phosphorylation and subsequent neuronal cell death and spatial memory deficits (Yin et al., 2015). Considering the previously described research, where the stimulation of sigma-1 receptors exerted neuroprotective and anti-amnesic effects in Aβ25-35-treated animals, this difference might lie in time-dependent modulation of sigma-1 receptor activity. Up to 48 h post- Aβ25-35 infusion, the genetic or pharmacological decrease in the activity of the sigma-1 receptors protects neurons from NMDA-dependent excitability. However, after that time, not the blockade but the stimulation of sigma-1 receptors is responsible for the neuroprotective effect, probably via ERK and PI3K-Akt pathways (Yang et al., 2012) or antioxidant properties (Hyrskyluoto et al., 2013; Pal et al., 2012). The neuroprotection and beneficial properties of sigma-1 receptor activation in the Alzheimer disease may also result from the formation and stabilization of mushroom spines (Ryskamp et al., 2019).
5.5. Sigma-1 receptor ligands in clinical trials
Only one sigma-1 receptor agonist has been investigated in clinical trials concerning various memory deficits to the best of our knowledge (Table 2). ANAVEX2-73 (blarcamesine), a mixed sigma-1/muscarinic receptor ligand, is currently in the phase 2b/3 clinical trial for Alzheimer’s disease (ClinicalTrials.gov Identifier: NCT04314934 and ClinicalTrials.gov Identifier: NCT03790709) (Anavex Life Sciences Corp., 2021a, 2021b) as well as in phase 2 clinical trial in Parkinson's disease dementia (ClinicalTrials.gov Identifier: NCT04575259) (Anavex Life Sciences Corp, 2020). Interestingly, ANAVEX2-73 also enters the phase 2 clinical trial in Rett syndrome patients (ClinicalTrials.gov Identifier: NCT04304482 and ClinicalTrials.gov Identifier: NCT03941444) (Anavex Life Sciences Corp., 2021c, 2021d).
5.6. Conclusion
Overall, most research indicates that sigma-1 receptor activation results in the anti-amnesic effect in various animal models of memory deficits, and sigma-1 receptor antagonists do not affect memory in rodents (Fig. 3 ). However, two studies show the opposite results, i.e., sigma-1 receptor antagonists reversed recognition and emotional memory deficits. These discrepancies can be explained by the different etiology of memory impairments and subsequent expression of sigma-1 receptors, as well as the administration of different compounds and different doses used. However, given that the vast majority of studies demonstrated that sigma-1 receptor antagonists are ineffective in rodent models of memory deficits, we can conclude that the sigma-1 receptor should be activated to achieve anti-amnestic effects. The anti-amnesic effect of sigma-1 receptor agonists may be the result of normalizing the disrupted glutamatergic and cholinergic neurotransmission and increasing BDNF and ERK levels. Moreover, only the sigma-1 receptor knock-out female mice had impaired memory. However, considering males, the research results were ambiguous, showing both impaired and normal memory functioning. The activity of sigma-1 receptor ligands in tests assessing the anti-amnesic effect is summarized in Table 4 .
Fig. 3.
The role of sigma-1 receptors in memory and learning. The activation of sigma-1 receptors improves various types of memory in general. However, a few studies showed that the blockade of these receptors might also mediate the anti-amnesic effect.
Table 4.
Anti-amnesic activity of sigma-1 receptor ligands.
| Potential modulator | Model of memory impairments | Animals | Animal Models/Tests | Reference |
|---|---|---|---|---|
| Sigma-1 receptor agonists | ||||
| (+)-SKF-10047 | Scopolamine | Rats | Passive avoidance | (Matsuno et al., 1995) |
| Igmesine, (+)-3-PPP, DTG |
Scopolamine | Rats | Passive avoidance | (Earley et al., 1991) |
| (+)-SKF-10047, (±)-pentazocine | Scopolamine | Mice | Passive avoidance | (Matsuno et al., 1993b) |
| SA4503 | Scopolamine, ibotenic acid forebrain lesion | Rats | Passive avoidance | (Senda et al., 1996) |
| SA4503 | Scopolamine | Rats | Passive avoidance | (Matsuno et al., 1997) |
| (+)-SKF-10047 | Scopolamine | Mice | Passive avoidance | (Senda et al., 1997) |
| SA4503 | Ibotenic acid forebrain lesion | Rats | Morris water maze | (Senda et al., 1998a, b) |
| DHEA-S, PREG-S | Scopolamine | Mice | Y-maze, water maze | (Urani et al., 1998) |
| PRE-084, SA4503 | Scopolamine | Mice | Y-maze, passive avoidance | (Maurice et al., 2001a) |
| OPC-14523 | Scopolamine | Rats | Morris water maze | (Tottori et al., 2002) |
| LS-1–137 | Scopolamine | Mice | Active avoidance, water maze | (Malik et al., 2015) |
| Fluoxetine | Nucleus basalis lesion | Rats | Active avoidance | (Ivković et al., 2004) |
| (+)-Pentazocine (+)-SKF-10047 | Scopolamine | Mice | Y-maze | (Hiramatsu et al., 2002; Hiramatsu and Hoshino, 2005, 2004) |
| KT-95 | Scopolamine | Mice | Y-maze, passive avoidance | (Hiramatsu et al., 2006) |
| ANAVEX1-41 | Scopolamine | Mice | Y-maze, passive avoidance, water maze | (Espallergues et al., 2007) |
| ANAVEX2-73 | Scopolamine | Mice | Y-maze, passive avoidance | (Villard et al., 2011a) |
| Dimemorfan | Scopolamine | Mice | Passive avoidance, water maze | (Wang et al., 2003) |
| (±)-PPCC | 192IgG-saporin induced lesions, atropine sulfate | Rats | Morris water maze | (Antonini et al., 2009) |
| (+)-SKF-10047, (+)-pentazocine | l-NAME, 7-nitroindazole | Mice | Y-maze | (Mamiya et al., 2000) |
| (+)-SKF-10047, (+)-pentazocine, DTG | Dizocilpine | Mice | Y-maze, passive avoidance, elevated plus maze | (Maurice et al., 1994a) |
| (+)-SKF-10047 | Dizocilpine | Rats | Three-panel runway task | (Ohno and Watanabe, 1995) |
| DHEA-S | Dizocilpine | Mice | Y-maze, passive avoidance | (Maurice et al., 1997) |
| SA4503 | Dizocilpine | Mice | Y-maze, passive avoidance | (Maurice and Privat, 1997) |
| (+)-SKF-10047, SA4503 | Dizocilpine | Rats | Radial arm maze | (Zou et al., 1998) |
| SA4503, DHEA-S, PREG-S | Dizocilpine | Rats | Radial arm maze | (Zou et al., 2000) |
| DTG, (+)-pentazocine, (+)-SKF-10047 | Dizocilpine | Mice | Y-maze | (Maurice et al., 1994b) |
| PRE-084, SA4503 | Dizocilpine | Mice | Y-maze, passive avoidance | (Maurice et al., 2001a) |
| PRE-084, DHEA-S, PREG-S | Dizocilpine | Mice | Y-maze, passive avoidance | (Maurice et al., 2001b) |
| SA4503, (+)-pentazocine, (+)-SKF- 10047 | Phencyclidine, dizocilpine | Mice | One-trial water-finding task | (Noda et al., 2001) |
| Donepezil, igmesine | Dizocilpine | Mice | Y-maze, passive avoidance | (Maurice et al., 2006) |
| ANAVEX2-73 | Dizocilpine | Mice | Y-maze, passive avoidance | (Villard et al., 2011a) |
| PRE-084 | Dizocilpine | Mice | Y-maze, passive avoidance | (Maurice et al., 1994d; Phan et al., 1999) |
| Fluvoxamine, SA4503, DHEA-S | Phencyclidine | Mice | Object recognition task | (Hashimoto et al., 2007) |
| Donepezil | Phencyclidine | Mice | Object recognition task | (Kunitachi et al., 2009) |
| (+)-SKF-10047, (±)-pentazocine | p-Chloroamphetamine | Mice | Passive avoidance | (Matsuno et al., 1993b) |
| (+)-SKF-10047, DTG, (+)-3-PPP | p-Chloroamphetamine | Mice | Passive avoidance | (Matsuno et al., 1994) |
| PRE-084 | Nimodipine | Mice | Y-maze, passive avoidance, water maze |
(Maurice et al., 1995) |
| (+)-SKF-10047, DTG, (+)-3-PPP | CDEP | Mice | Passive avoidance | (Matsuno et al., 1998) |
| (+)-SKF 10,047, DTG | Repeated CO exposure | Mice | Y-maze, passive avoidance | (Maurice et al., 1994c) |
| PRE-084, DTG | Repeated CO exposure | Mice | Passive avoidance | (Maurice et al., 1999a) |
| DHEA | Repeated CO exposure | Mice | Y-maze, passive avoidance | (Maurice et al., 2000) |
| Donepezil, igmesine | Repeated CO exposure | Mice | Y-maze, passive avoidance | (Meunier et al., 2006b) |
| Igmesine | Trimethyltin | Rats | Passive avoidance, radial arm maze | (O’Connell et al., 1996) |
| PRE-084, DTG | Trimethyltin | Mice | Passive avoidance | (Maurice et al., 1999a) |
| Dextromethorphan | Trimethyltin | Rats | Water maze, passive avoidance | (Shin et al., 2007) |
| Igmesine | Prenatal restraint | Rats | Y-maze, T-maze, water maze, passive avoidance | (Meunier et al., 2004) |
| Igmesine, DHEA | Prenatal cocaine treatment | Rats | T-maze, water maze, passive avoidance | (Meunier and Maurice, 2004) |
| Igmesine, PRE084 | Senescence-accelerated mouse | Mice | Y-maze, water maze, passive avoidance, open field | (Maurice et al., 1996) |
| PRE-084 | Normal aging | Rats | Morris water maze | (Maurice, 2001) |
| OPC-14523 | Normal aging | Rats | Morris water maze | (Tottori et al., 2002) |
| PRE-084 | Normal aging | Mice | Morris water maze | (Phan et al., 2003) |
| Cutamesine | Rapid eye movement sleep deprivation | Rats | Passive avoidance | (Ramakrishnan et al., 2015b) |
| PRE-084 | Post-traumatic stress disorder | Rats | Water maze | (Ji et al., 2016) |
| PRE-084 | Myocardial infarction | Mice | Y-maze | (Ito et al., 2013) |
| PRE-084, DTG | Brain ischemia | Mice | Y-maze, object recognition test, water maze | (Xu et al., 2015) |
| PRE-084 | Brain ischemia | Mice | Y-maze, object recognition test, water maze | (Xu et al., 2017) |
| DHEA | Brain ischemia | Mice | Y-maze, object recognition test, passive avoidance | (Yabuki et al., 2015) |
| SA4503 | Oflactory bulbectomy | Rats | Cued and contextual fear-conditioning test | (Wang et al., 2007a, b) |
| Igmesine | Alcohol withdrawal | Mice | Object recognition task | (Meunier et al., 2006a) |
| (+)-pentazocine, PRE-084, SA4503, PREG-S, DHEA-S | β-Amyloid(25–35) peptide | Mice | Y-maze, passive avoidance | (Maurice et al., 1998) |
| Donepezil, PRE084 | β-Amyloid(25–35) peptide | Mice | Y-maze, passive avoidance | (Meunier et al., 2006c) |
| ANAVEX1-41 | β-Amyloid(25–35) peptide | Mice | Y-maze, passive avoidance, radial arm maze | (Villard et al., 2009) |
| ANAVEX2-73 | β -Amyloid(25–35) peptide | Mice | Y-maze, passive avoidance, | (Villard et al., 2011a) |
| Dimemorfan | β-Amyloid(25–35) peptide | Mice | Passive avoidance, water maze | (Wang et al., 2003) |
| Fluvoxamine | J20 amyloidogenic mouse model of Alzheimer’s disease | Mice | Object recognition test | (Kim et al., 2018) |
| Choline | APP (amyloid precursor protein)/PS1 (presenilin1) mice | Mice | Morris water maze | (Velazquez et al., 2019) |
| (-)-MR22 | Cholinergic lesion and amyloid infusion | Rats | Morris water maze, radial arm water maze | (Antonini et al., 2011) |
| ANAVEX2-73 | Injection of Aβ25-35 peptide | Mice | spontaneous alternation in the Y-maze, passive avoidance test, object recognition task | (Lahmy et al., 2013) |
| AF710B | McGill-R-Thy1-APP transgenic (Tg) rats | Rats | Object recognition test, Morris water maze | (Hall et al., 2018) |
| ANAVEX2-73, PRE-084 (+donepezil) | Injection of Aβ25-35 peptide | Mice | spontaneous alternation in the Y-maze, passive avoidance test | (Maurice, 2016) |
| PREG-S | Injection of Aβ25-35 peptide | Mice | Morris water maze | (Yang et al., 2012) |
| SA4503 | α-thalassemia X-linked intellectual disability | Mice | Barnes maze, Y-maze, object recognition task | (Yamaguchi et al., 2018) |
| Sigma-1 receptor antagonists | ||||
| BD1047 | Alcohol withdrawal | Mice | Object recognition task | (Meunier et al., 2006a) |
| AZ66 | Methamphetamine | Mice | Object recognition task, passive avoidance task | (Seminerio et al., 2013) |
| Sigma-1 receptor modulators | ||||
| OZP002 | Scopolamine, Injection of Aβ25-35 peptide | Mice | T-maze, water-maze learning, passive avoidance, object recognition | (Maurice et al., 2019) |
| E1R | Scopolamine | Mice | Y-maze, passive avoidance | (Zvejniece et al., 2014) |
6. Discussion and conclusions
Sigma-1 receptors are unique and riveting therapeutic targets with exciting features. The last decades brought a new perspective on these proteins, first identified as a type of opioid receptors, and now classified as chaperone proteins. They outstand among other receptors and proteins as:
-
•
their sequence is quite different from other mammalian proteins, suggesting that their role in cells may be irreplaceable,
-
•
they are widely distributed in the organism, not only in the central nervous system but also in the peripheral tissues and organs; thus, they may be a pharmacological target in various diseases with different etiology and phenotypes,
-
•
they bind ligands with very diverse structures implicating that multiple compounds may modulate the activity of sigma-1 receptors, and therefore they can be used in the treatment of various disorders,
-
•
they can translocate from ER to different cell compartments, thus modulating the activity of ion channels, receptors, transporters, kinases, neurotrophic factors, and other proteins, rendering sigma-1 receptors as an important node in the inter-organelle communication in cells.
Moreover, we need to highlight that sigma-1 receptor agonists have a favorable safety profile – animal studies proved that stimulation of these receptors and their modulatory activity occurred only in the pathological conditions but not in the normal physiological state. Therefore, such compounds may have limited side effects, which is beneficial.
However, one of the problems we need to solve is establishing a standardized method to characterize the ligand’s intrinsic activity. Available methods and techniques are very diverse, most of them imperfect, often irreplicable, and do not give certainty whether a ligand is an agonist, antagonist, positive or negative allosteric modulator. So far, the function of sigma-1 receptor ligands is predominantly identified according to the specific in vitro or in vivo pharmacological effects compared to the phenotype of sigma-1 receptor knock-out animals or to the effects after administration of standard sigma-1 receptors ligands. Therefore, if we want to design novel sigma-1 receptor-targeted molecules with a possible therapeutic use, a standard functional assay is necessary. However, we need to raise the issue that since the sigma-1 receptor is a chaperone and not a classical receptor, it does not possess a specific transduction system. Nevertheless, by convention, we still name sigma-1 protein as a receptor. Therefore, we should consider whether using the terms: agonist or antagonist concerning sigma-1 receptor is appropriate and if we should not use the general description and classify compounds binding to the sigma-1 receptor just as ligands/modulators which affect the downstream pathways/proteins.
On the other hand, given that sigma-1 receptors affect numerous molecular proteins, a careful and detailed investigation of their role in the organism needs to be done as their upstream, and downstream intracellular signal cascades may differ depending on the cell types and organs, or systems. Moreover, scientists need to clarify the exact role of sigma-1 receptors in the pathophysiology of each disease, where the administration of sigma-1 receptor ligands may be beneficial. Importantly, we cannot exclude the possibility that broad possible indications for sigma-1 receptor ligands, reported so far, might be due to the activation or inhibition of other proteins.
As mentioned above, the currently known sigma-1 receptor ligands bind not only to sigma-1 receptors but also interact with other receptors or channels. Given that one of the intriguing features of sigma-1 receptors is their ability to interact with various molecules, which sometimes differ entirely from each other in terms of the chemical structure, it may be challenging to design highly selective sigma-1 receptor ligands. However, it does not necessarily need to be a disadvantage in treating complex CNS diseases such as depression or cognitive impairments. In such cases, multimodal compounds targeting the sigma-1 receptor might be superior to highly selective molecules. Thus, a multi-treatment approach may be optimal and the most beneficial if we want to treat complex neurodegenerative diseases with multi-dimensional etiologies and phenotypes.
As mentioned in the introduction COVID-19 pandemic increased the prevalence of depression and memory disorders . Interestingly, the sigma-1 receptor agonists may be attractive and putative therapeutic candidates for the treatment of COVID-19, not only considering their antidepressant-like and anti-amnesic properties, but also the role of sigma-1 receptors in the disease progression itself. The newest reports showed that the sigma-1 receptors are important in the replication of SARS-CoV-2 in cells and that the sigma-1 receptor is a promising therapeutic target for COVID-19 infection (reviewed in (Hashimoto, 2021)).
Nevertheless, growing evidence indicates that sigma-1 ligands can modulate multiple physiological and pathological processes, including excitotoxicity, calcium dysregulation, mitochondrial and endoplasmic reticulum dysfunction, oxidative stress, and inflammation. This feature may be associated with the significant involvement of the receptors in the pathomechanism of many CNS diseases, including affective and cognitive disorders. Thus, considering all published research, the sigma-1 receptor ligands, and predominantly agonists, might be the next generation of psychotherapeutic agents.
Funding
This study has been conducted as part of a research project financed by the National Science Centre, Poland (grant number 2019/34/E/NZ7/00454) and Jagiellonian University Medical College (grants number N42/DBS/000117, N42/DBS/000140).
References
- Abraham M.J., Fleming K.L., Raymond S., Wong A.Y.C., Bergeron R. The sigma-1 receptor behaves as an atypical auxiliary subunit to modulate the functional characteristics of Kv1. 2 channels expressed in HEK293 cells. Physiol. Rep. 2019;7:1–22. doi: 10.14814/phy2.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguinaga D., Medrano M., Cordomí A., Jiménez-Rosés M., Angelats E., Casanovas M., Vega-Quiroga I., Canela E.I., Petrovic M., Gysling K., Pardo L., Franco R., Navarro G. Cocaine blocks effects of hunger hormone, ghrelin, via interaction with neuronal sigma-1 receptors. Mol. Neurobiol. 2019;56:1196–1210. doi: 10.1007/s12035-018-1140-7. [DOI] [PubMed] [Google Scholar]
- Akunne H.C., Zoski K.T., Whetzel S.Z., Cordon J.J., Brandon R.M., Roman F., Pugsley T.A. Neuropharmacological profile of a selective sigma ligand, igmesine: a potential antidepressant. Neuropharmacology. 2001;41:138–149. doi: 10.1016/S0028-3908(01)00049-1. [DOI] [PubMed] [Google Scholar]
- Alam S., Abdullah C.S., Aishwarya R., Orr A.W., Traylor J., Miriyala S., Panchatcharam M., Pattillo C.B., Bhuiyan M.S. Sigmar1 regulates endoplasmic reticulum stress-induced C/EBP-homologous protein expression in cardiomyocytes. Biosci. Rep. 2017;37 doi: 10.1042/BSR20170898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albayrak Y., Hashimoto K. Beneficial effects of the sigma-1 agonist fluvoxamine for tardive dyskinesia in patients with postpsychotic depressive disorder of schizophrenia: report of 5 cases. Prim. Care Companion CNS Disord. 2012;14 doi: 10.4088/PCC.12br01401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G., Phan V.L., Guillemain I., Saunier M., Legrand A., Anoal M., Maurice T. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Anagnostaras S.G., Maren S., Sage J.R., Goodrich S., Fanselow M.S. Scopolamine and Pavlovian fear conditioning in rats: dose-effect analysis. Neuropsychopharmacology. 1999;21:731–744. doi: 10.1016/S0893-133X(99)00083-4. [DOI] [PubMed] [Google Scholar]
- Anavex Life Sciences Corp., 2020. ClinicalTrials.gov Identifier: NCT04575259 [WWW Document]. URL https://clinicaltrials.gov/ct2/show/NCT04575259?term=ANAVEX2-73&draw=2&rank=1 (Accessed 10 August 2021).
- Anavex Life Sciences Corp., 2021a. ClinicalTrials.gov Identifier: NCT03790709 [WWW Document]. URL https://clinicaltrials.gov/ct2/show/NCT03790709?term=ANAVEX2-73&draw=2&rank=8, (Accessed 10 August 2021).
- Anavex Life Sciences Corp 2021b. ClinicalTrials.gov Identifier: NCT04314934 [WWW Document]. URL https://clinicaltrials.gov/ct2/show/NCT04314934?term=ANAVEX2-73&draw=2&rank=2 (Accessed 10 August 2021).
- Anavex Life Sciences Corp., 2021c. ClinicalTrials.gov Identifier: NCT04304482 [WWW Document]. URL https://clinicaltrials.gov/ct2/show/NCT04304482?term=ANAVEX2-73&draw=2&rank=3 (Accessed 10 August 2021).
- Anavex Life Sciences Corp 2021d. ClinicalTrials.gov Identifier: NCT03941444 [WWW Document]. URL https://clinicaltrials.gov/ct2/show/NCT03941444?term=ANAVEX2-73&draw=2&rank=4 (Accessed 10 August 2021).
- Antonini V., Prezzavento O., Coradazzi M., Marrazzo A., Ronsisvalle S., Arena E., Leanza G. Anti-amnesic properties of (±)-PPCC, a novel sigma receptor ligand, on cognitive dysfunction induced by selective cholinergic lesion in rats. J. Neurochem. 2009;109:744–754. doi: 10.1111/j.1471-4159.2009.06000.x. [DOI] [PubMed] [Google Scholar]
- Antonini V., Marrazzo A., Kleiner G., Coradazzi M., Ronsisvalle S. Anti-amnesic and neuroprotective actions of the sigma-1 receptor agonist (-) -MR22 in rats with selective cholinergic lesion and amyloid infusion. J. Alzheimer’s Dis. 2011;24:569–586. doi: 10.3233/JAD-2011-101794. [DOI] [PubMed] [Google Scholar]
- Aydar E., Palmer C.P., Klyachko V.A., Jackson M.B. The sigma receptor as a ligand-regulated auxiliary potassium channel subunit. Neuron. 2002;34:399–410. doi: 10.1016/s0896-6273(02)00677-3. [DOI] [PubMed] [Google Scholar]
- Balasuriya D., D’Sa L., Talker R., Dupuis E., Maurin F., Martin P., Borgese F., Soriani O., Edwardson J.M. A direct interaction between the sigma-1 receptor and the hERG voltage-gated K+ channel revealed by atomic force microscopy and homogeneous time-resolved fluorescence (HTRF®) J. Biol. Chem. 2014;289:32353–32363. doi: 10.1074/jbc.M114.603506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggiato S., Borelli A.C., Borroto-Escuela D., Corbucci I., Tomasini M.C., Marti M., Antonelli T., Tanganelli S., Fuxe K., Ferraro L. Cocaine modulates allosteric D(2)-σ(1) receptor-receptor interactions on dopamine and glutamate nerve terminals from rat striatum. Cell. Signal. 2017;40:116–124. doi: 10.1016/j.cellsig.2017.09.007. [DOI] [PubMed] [Google Scholar]
- Behensky A.A., Yasny I.E., Shuster A.M., Seredenin S.B., Petrov A.V., Cuevas J. Stimulation of sigma receptors with afobazole blocks activation of Microglia and reduces toxicity caused by amyloid-β25-35. J. Pharmacol. Exp. Ther. 2013;347:458–467. doi: 10.1124/jpet.113.208348. [DOI] [PubMed] [Google Scholar]
- Belovicova K., Bogi E., Csatlosova K., Dubovicky M. Animal tests for anxiety-like and depression-like behavior in rats. Interdiscip. Toxicol. 2017;10:40–43. doi: 10.1515/intox-2017-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraki S., Diaz-Heijtz R., Tai F., Ögren S.O. Effects of repeated treatment of phencyclidine on cognition and gene expression in C57BL/6 mice. Int. J. Neuropsychopharmacol. 2009;12:243–255. doi: 10.1017/S1461145708009152. [DOI] [PubMed] [Google Scholar]
- Berardi F., Ferorelli S., Abate C., Pedone M.P., Colabufo N.A., Contino M., Perrone R. Methyl substitution on the piperidine ring of N-[omega-(6-methoxynaphthalen-1-yl)alkyl] derivatives as a probe for selective binding and activity at the sigma(1) receptor. J. Med. Chem. 2005;48:8237–8244. doi: 10.1021/jm050654o. [DOI] [PubMed] [Google Scholar]
- Bermack J.E., Haddjeri N., Debonnel G. Effects of the potential antidepressant OPC-14523 [1-[3-[4-(3-chlorophenyl) -1-piperazinyl]propyl]-5-methoxy-3,4-dihydro-2-quinolinone monomethanesulfonate] a combined σ and 5-HT1A ligand: modulation of neuronal activity in the dorsal raphe nucleus. J. Pharmacol. Exp. Ther. 2004;310:578–583. doi: 10.1124/jpet.104.066472. [DOI] [PubMed] [Google Scholar]
- Beuzen A., Belzung C. Link between emotional memory and anxiety states: a study by principal component analysis. Physiol. Behav. 1995;58:111–118. doi: 10.1016/0031-9384(95)00013-9. [DOI] [PubMed] [Google Scholar]
- Bol’shakova A.V., Kraskovskaya N.A., Gainullina A.N., Kukanova E.O., Vlasova O.L., Bezprozvanny I.B. Neuroprotective effect of σ1-receptors on the cell model of Huntington’s disease. Bull. Exp. Biol. Med. 2017;164:252–258. doi: 10.1007/s10517-017-3968-7. [DOI] [PubMed] [Google Scholar]
- Borison R.L., Diamond B.I., Dren A.T. Does sigma receptor antagonism predict clinical antipsychotic efficacy? Psychopharmacol. Bull. 1991;27:103–106. [PubMed] [Google Scholar]
- Brailoiu E., Chakraborty S., Brailoiu G.C., Unterwald E.M., Abood M.E., Taylor C.W., Brailoiu E., Chakraborty S., Brailoiu G.C., Zhao P., Barr J.L., Ilies M.A. Choline is an intracellular messenger linking extracellular stimuli to IP 3 -evoked Ca 2 + signals through sigma-1 receptors report choline is an intracellular messenger linking extracellular stimuli to IP 3 -evoked Ca 2 + signals through sigma-1 receptor. CellReports. 2019;26:330–337. doi: 10.1016/j.celrep.2018.12.051. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo-Caparrós I., Perazzoli G., Yeste S., Cikes D., Baeyens J.M., Cobos E.J., Nieto F.R. Sigma-1 receptor inhibition reduces neuropathic pain induced by partial sciatic nerve transection in mice by opioid-dependent and -Independent mechanisms. Front. Pharmacol. 2019;10:613. doi: 10.3389/fphar.2019.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimson J.M., Safrany S.T., Qassam H., Tencomnao T. Dipentylammonium binds to the sigma-1 receptor and protects against glutamate toxicity, attenuates dopamine toxicity and potentiates neurite outgrowth in various cultured cell lines. Neurotox. Res. 2018;34:263–272. doi: 10.1007/s12640-018-9883-5. [DOI] [PubMed] [Google Scholar]
- Brimson J.M., Akula K.K., Abbas H., Ferry D.R., Kulkarni S.K., Russell S.T., Tisdale M.J., Tencomnao T., Safrany S.T. Simple ammonium salts acting on sigma-1 receptors yield potential treatments for cancer and depression. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-65849-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruna J., Videla S., Argyriou A.A., Velasco R., Villoria J., Santos C., Nadal C., Cavaletti G., Alberti P., Briani C., Kalofonos H.P., Cortinovis D., Sust M., Vaqué A., Klein T., Plata-Salamán C. Efficacy of a novel sigma-1 receptor antagonist for oxaliplatin-induced neuropathy: a randomized, double-blind, placebo-controlled phase IIa clinical trial. Neurother. J. Am. Soc. Exp. Neurother. 2018;15:178–189. doi: 10.1007/s13311-017-0572-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarella G., Bucolo C., Di Benedetto G., Pezzino S., Lempereur L., Calvagna R., Clementi S., Pavone P., Fiore L., Bernardini R. Protective effects of the sigma agonist Pre-084 in the rat retina. Br. J. Ophthalmol. 2007;91:1382–1384. doi: 10.1136/bjo.2007.118570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnally S.M., Johannessen M., Henderson R.M., Jackson M.B., Edwardson J.M. Demonstration of a direct interaction between s -1 receptors and acid-sensing ion channels. BPJ. 2010;98:1182–1191. doi: 10.1016/j.bpj.2009.12.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S., Nakazato A., Kennis L., Nakamura M., MacKie C., Sugiura M., Vinken P., Ashton D., Langlois X., Steckler T. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur. J. Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Charalampopoulos I., Tsatsanis C., Dermitzaki E., Alexaki V.-I., Castanas E., Margioris A.N., Gravanis A. Dehydroepiandrosterone and allopregnanolone protect sympathoadrenal medulla cells against apoptosis via antiapoptotic Bcl-2 proteins. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8209–8214. doi: 10.1073/pnas.0306631101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Dai X.-N., Sokabe M. Chronic administration of dehydroepiandrosterone sulfate (DHEAS) primes for facilitated induction of long-term potentiation via sigma 1 (sigma1) receptor: optical imaging study in rat hippocampal slices. Neuropharmacology. 2006;50:380–392. doi: 10.1016/j.neuropharm.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Chen X., Zhang C., Guo Y., Liu X., Ye T., Fo Y., Qu C., Liang J., Shi S., Yang B. Chronic stimulation of the sigma-1 receptor ameliorates ventricular ionic and structural remodeling in a rodent model of depression. Life Sci. 2020;257 doi: 10.1016/j.lfs.2020.118047. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Thomas A., Mardini F., Bianchi S.L., Tang J.X., Peng J., Wei H., Eckenhoff M.F., Eckenhoff R.G., Levy R.J. Neurodevelopmental consequences of sub-clinical carbon monoxide exposure in newborn mice. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier N., Keller E., Maurice T. Behavioural phenotyping of knockout mice for the sigma-1 (σ1) chaperone protein revealed gender-related anxiety, depressive-like and memory alterations. J. Psychopharmacol. 2011;25:960–975. doi: 10.1177/0269881111400648. [DOI] [PubMed] [Google Scholar]
- Choi S.-R., Roh D.-H., Yoon S.-Y., Kang S.-Y., Moon J.-Y., Kwon S.-G., Choi H.-S., Han H.-J., Beitz A.J., Oh S.-B., Lee J.-H. Spinal sigma-1 receptors activate NADPH oxidase 2 leading to the induction of pain hypersensitivity in mice and mechanical allodynia in neuropathic rats. Pharmacol. Res. 2013;74:56–67. doi: 10.1016/j.phrs.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Church J., Fletcher E.J. Blockade by sigma site ligands of high voltage-activated Ca2+ channels in rat and mouse cultured hippocampal pyramidal neurones. Br. J. Pharmacol. 1995;116:2801–2810. doi: 10.1111/j.1476-5381.1995.tb15929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colabufo N.A., Contino M., Inglese C., Niso M., Perrone R., Roperto S., Roperto F. In vitro and ex vivo characterization of sigma-1 and sigma-2 receptors: agonists and antagonists in biological assays. Cent. Nerv. Syst. Agents Med. Chem. 2009;9:161–171. doi: 10.2174/1871524910909030161. [DOI] [PubMed] [Google Scholar]
- Collina S., Rui M., Stotani S., Bignardi E., Rossi D., Curti D., Giordanetto F., Malacrida A., Scuteri A., Cavaletti G. Are sigma receptor modulators a weapon against multiple sclerosis disease? Future Med. Chem. 2017;9:2029–2051. doi: 10.4155/fmc-2017-0122. [DOI] [PubMed] [Google Scholar]
- Crottès D., Martial S., Rapetti-Mauss R.L., Pisani D.F., Loriol C., Pellissier B., Martin P., Chevet E., Borgese F., Soriani O. Sig1R protein regulates hERG channel expression through a post-translational mechanism in leukemic cells. J. Biol. Chem. 2011;286:27947–27958. doi: 10.1074/jbc.M111.226738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzier L., Couly S., Roques C., Peter C., Belkhiter R., Arguel Jacquemin M., Bonetto A., Delprat B., Maurice T. Sigma-1 (σ1) receptor activity is necessary for physiological brain plasticity in mice. Eur. Neuropsychopharmacol. 2020;39:29–45. doi: 10.1016/j.euroneuro.2020.08.010. [DOI] [PubMed] [Google Scholar]
- Cuevas J., Behensky A., Deng W., Katnik C. Afobazole modulates neuronal response to ischemia and acidosis via activation of σ-1 receptors. J. Pharmacol. Exp. Ther. 2011;339:152–160. doi: 10.1124/jpet.111.182774. [DOI] [PubMed] [Google Scholar]
- Cuevas J., Rodriguez A., Behensky A., Katnik C. Afobazole modulates microglial function via activation of both σ-1 and σ-2 receptors. J. Pharmacol. Exp. Ther. 2011;339:161–172. doi: 10.1124/jpet.111.182816. [DOI] [PubMed] [Google Scholar]
- Delint-Ramirez I., Garcia-Oscos F., Segev A., Kourrich S. Cocaine engages a non-canonical, dopamine-independent, mechanism that controls neuronal excitability in the nucleus accumbens. Mol. Psychiatry. 2020;25:680–691. doi: 10.1038/s41380-018-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis W., Sollenberger R.T. Negative adaptation in the maze exploration of albino rats. J. Comp. Psychol. 1934;18:197–206. doi: 10.1037/h0073802. [DOI] [Google Scholar]
- Derocq J.M., Bourrié B., Ségui M., Le Fur G., Casellas P. In vivo inhibition of endotoxin-induced pro-inflammatory cytokines production by the sigma ligand SR 31747. J. Pharmacol. Exp. Ther. 1995;272:224–230. [PubMed] [Google Scholar]
- Deryabina I.B., Andrianov V.V., Muranova L.N., Bogodvid T.K., Gainutdinov K.L. Effects of thryptophan hydroxylase blockade by P-chlorophenylalanine on contextual memory reconsolidation after training of different intensity. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21062087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhir A., Kulkarni S.K. Involvement of dopamine (DA)/serotonin (5-HT)/sigma (σ) receptor modulation in mediating the antidepressant action of ropinirole hydrochloride, a D2/D3 dopamine receptor agonist. Brain Res. Bull. 2007;74:58–65. doi: 10.1016/j.brainresbull.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Dhir A., Kulkarni S.K. Involvement of sigma-1 receptor modulation in the antidepressant action of venlafaxine. Neurosci. Lett. 2007;420:204–208. doi: 10.1016/j.neulet.2007.04.055. [DOI] [PubMed] [Google Scholar]
- Dhir A., Kulkarni S.K. Antidepressant-like effect of 17β-estradiol: involvement of dopaminergic, serotonergic, and (or) sigma-1 receptor systems. Can. J. Physiol. Pharmacol. 2008;86:726–735. doi: 10.1139/Y08-077. [DOI] [PubMed] [Google Scholar]
- Dhir A., Kulkarni S.K. Involvement of sigma (σ1) receptors in modulating the anti-depressant effect of neurosteroids (dehydroepiandrosterone or pregnenolone) in mouse tail-suspension test. J. Psychopharmacol. 2008;22:691–696. doi: 10.1177/0269881107082771. [DOI] [PubMed] [Google Scholar]
- Dhir A., Kulkarni S.K. Possible involvement of sigma-1 receptors in the anti-immobility action of bupropion, a dopamine reuptake inhibitor. Fundam. Clin. Pharmacol. 2008;22:387–394. doi: 10.1111/j.1472-8206.2008.00605.x. [DOI] [PubMed] [Google Scholar]
- Di T., Zhang S., Hong J., Zhang T., Chen L. Hyperactivity of hypothalamic-pituitary-adrenal axis due to dysfunction of the hypothalamic glucocorticoid receptor in sigma-1 receptor knockout mice. Front. Mol. Neurosci. 2017;10:1–14. doi: 10.3389/fnmol.2017.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do W., Herrera C., Mighty J., Shumskaya M., Redenti S.M., Sauane M. Sigma 1 Receptor plays a prominent role in IL-24-induced cancer-specific apoptosis. Biochem. Biophys. Res. Commun. 2013;439:215–220. doi: 10.1016/j.bbrc.2013.08.057. [DOI] [PubMed] [Google Scholar]
- Earley B., Burke M., Leonard B.E., Gouret C.J., Junien J.L. Evidence for an anti-amnesic effect of JO 1784 in the rat: a potent and selective ligand for the sigma receptor. Brain Res. 1991;546:282–286. doi: 10.1016/0006-8993(91)91492-j. [DOI] [PubMed] [Google Scholar]
- Eaton M.J., Lookingland K.J., Moore K.E. The sigma ligand rimcazole activates noradrenergic neurons projecting to the paraventricular nucleus and increases corticosterone secretion in rats. Brain Res. 1996;733:162–166. doi: 10.1016/0006-8993(96)00290-9. [DOI] [PubMed] [Google Scholar]
- Espallergues J., Lapalud P., Christopoulos A., Avlani V.A., Sexton P.M., Vamvakides A., Maurice T. Involvement of the sigma1 (σ1) receptor in the anti-amnesic, but not antidepressant-like, effects of the aminotetrahydrofuran derivative ANAVEX1-41. Br. J. Pharmacol. 2007;152:267–279. doi: 10.1038/sj.bjp.0707386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi L.A., Palma B.D., Gomes V.L., Tufik S., Hipólide D.C. Inflammatory markers are associated with inhibitory avoidance memory deficit induced by sleep deprivation in rats. Behav. Brain Res. 2011;221:7–12. doi: 10.1016/j.bbr.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Ettman C.K., Abdalla S.M., Cohen G.H., Sampson L., Vivier P.M., Galea S. Prevalence of depression symptoms in US adults before and during the COVID-19 pandemic. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallica A.N., Pittalà V., Modica M.N., Salerno L., Romeo G., Marrazzo A., Helal M.A., Intagliata S. Recent advances in the development of sigma receptor ligands as cytotoxic agents: a medicinal chemistry perspective. J. Med. Chem. 2021;64:7926–7962. doi: 10.1021/acs.jmedchem.0c02265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltmann K., Borroto-Escuela D.O., Rüegg J., Pinton L., de Oliveira Sergio T., Narváez M., Jimenez-Beristain A., Ekström T.J., Fuxe K., Steensland P. Effects of long-term alcohol drinking on the dopamine D2 receptor: gene expression and heteroreceptor complexes in the striatum in rats. Alcohol. Clin. Exp. Res. 2018;42:338–351. doi: 10.1111/acer.13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishback J.A., Robson M.J., Xu Y.-T., Matsumoto R.R. Sigma receptors: potential targets for a new class of antidepressant drug. Pharmacol. Ther. 2010;127:271–282. doi: 10.1016/j.pharmthera.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanilla D., Johannessen M., Hajipour A.R., Cozzi N.V., Jackson M.B., Ruoho A.E. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science. 2009;323:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francardo V., Bez F., Wieloch T., Nissbrandt H., Ruscher K., Cenci M.A. Pharmacological stimulation of sigma-1 receptors has neurorestorative effects in experimental parkinsonism. Brain. 2014;137:1998–2014. doi: 10.1093/brain/awu107. [DOI] [PubMed] [Google Scholar]
- Francardo V., Geva M., Bez F., Denis Q., Steiner L., Hayden M.R., Cenci M.A. Pridopidine induces functional neurorestoration via the sigma-1 receptor in a mouse model of Parkinson’s disease. Neurother. J. Am. Soc. Exp. Neurother. 2019;16:465–479. doi: 10.1007/s13311-018-00699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini S., Linciano P., Puja G., Tait A., Borsari C., Denora N., Iacobazzi R.M., Brasili L., Sorbi C. Novel dithiolane-based ligands combining sigma and NMDA receptor interactions as potential neuroprotective agents. ACS Med. Chem. Lett. 2020;11:1028–1034. doi: 10.1021/acsmedchemlett.0c00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman F.M., Young I.G. Involvement of the σ- receptor in passive-avoidance learning in the day-old chick during the second wave of neuronal activity. Neurobiol. Learn. Mem. 2001;75:346–352. doi: 10.1006/nlme.2000.3985. [DOI] [PubMed] [Google Scholar]
- Fu Y., Yu S., Guo X., Li X., Li T., Li H., Dong Y. Fluvoxamine increased glutamate release by activating both 5-HT 3 and sigma-1 receptors in prelimbic cortex of chronic restraint stress C57BL/6 mice. Biochim. Biophys. Acta - Mol. Cell Res. 2012;1823:826–837. doi: 10.1016/j.bbamcr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Fujimoto M., Hayashi T., Urfer R., Mita S., Su T.P. Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor. Synapse. 2012;66:630–639. doi: 10.1002/syn.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K., Moriguchi S. Stimulation of the sigma-1 receptor and the effects on neurogenesis and depressive behaviors in mice. Adv. Exp. Med. Biol. 2017;964:201–211. doi: 10.1007/978-3-319-50174-1_14. [DOI] [PubMed] [Google Scholar]
- Furuse T., Hashimoto K. Fluvoxamine for aripiprazole-associated akathisia in patients with schizophrenia: a potential role of sigma-1 receptors. Ann. Gen. Psychiatry. 2010;9:11. doi: 10.1186/1744-859X-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher M. Animal models of memory impairment. Philos. Trans. R. Soc. Lond. Ser. B, Biol. Sci. 1997;352:1711–1717. doi: 10.1098/rstb.1997.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X.-F., Yao J.-J., He Y.-L., Hu C., Mei Y.-A. Sigma-1 receptor agonists directly inhibit Nav1.2/1.4 channels. PLoS One. 2012;7 doi: 10.1371/journal.pone.0049384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz G.R., Gorman J.M., Volavka J., Macaluso J., Gribkoff G., Taylor D.P., Borison R. BMY 14802, a sigma receptor ligand for the treatment of schizophrenia. Neuropsychopharmacology. 1994;10:37–40. doi: 10.1038/npp.1994.5. [DOI] [PubMed] [Google Scholar]
- Goguadze N., Zhuravliova E., Morin D., Mikeladze D., Maurice T. Sigma-1 receptor agonists induce oxidative stress in mitochondria and enhance complex I activity in physiological condition but protect against pathological oxidative stress. Neurotox. Res. 2019;35 doi: 10.1007/s12640-017-9838-2. [DOI] [PubMed] [Google Scholar]
- Gómez-Soler M., Fernández-Dueñas V., Portillo-Salido E., Pérez P., Zamanillo D., Vela J.M., Burgueño J., Ciruela F. Predicting the antinociceptive efficacy of σ(1) receptor ligands by a novel receptor fluorescence resonance energy transfer (FRET) based biosensor. J. Med. Chem. 2014;57:238–242. doi: 10.1021/jm401529t. [DOI] [PubMed] [Google Scholar]
- Gonzalez G.M., Werling L.L. Release of [3H]dopamine from guinea pig striatal slices is modulated by sigma1 receptor agonists. Naunyn. Schmiedebergs. Arch. Pharmacol. 1997;356:455–461. doi: 10.1007/pl00005076. [DOI] [PubMed] [Google Scholar]
- Götz J., Götz N.N. Animal models for Alzheimer’s disease and frontotemporal dementia: a perspective. ASN Neuro. 2009;1 doi: 10.1042/AN20090042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyagi T., Goto S., Bhardwaj A., Dawson V.L., Hurn P.D., Kirsch J.R. Neuroprotective effect of sigma(1)-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) is linked to reduced neuronal nitric oxide production. Stroke. 2001;32:1613–1620. doi: 10.1161/01.str.32.7.1613. [DOI] [PubMed] [Google Scholar]
- Gromek K.A., Suchy F.P., Meddaugh H.R., Wrobel R.L., LaPointe L.M., Chu U.B., Primm J.G., Ruoho A.E., Senes A., Fox B.G. The oligomeric states of the purified sigma-1 receptor are stabilized by ligands. J. Biol. Chem. 2014;289:20333–20344. doi: 10.1074/jbc.M113.537993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundlach A.L., Largent B.L., Snyder S.H. Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J. Neurosci. 1986;6:1757–1770. doi: 10.1523/JNEUROSCI.06-06-01757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Y., Saul A., Tawfik A., Williams C., Bollinger K., Smith R., Tachikawa M., Zorrilla E., Ganapathy V., Smith S.B. Late-onset inner retinal dysfunction in mice lacking sigma receptor 1 (σR1) Invest. Ophthalmol. Vis. Sci. 2011;52:7749–7760. doi: 10.1167/iovs.11-8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A.A., Herrera Y., Ajmo C.T.J., Cuevas J., Pennypacker K.R. Sigma receptors suppress multiple aspects of microglial activation. Glia. 2009;57:744–754. doi: 10.1002/glia.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H., Iulita M.F., Gubert P., Flores Aguilar L., Ducatenzeiler A., Fisher A., Cuello A.C. AF710B, an M1/sigma-1 receptor agonist with long-lasting disease-modifying properties in a transgenic rat model of Alzheimer’s disease. Alzheimer’s Dement. 2018;14:811–823. doi: 10.1016/j.jalz.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Hampshire A., Trender W., Chamberlain S.R., Jolly A.E., Grant J.E., Patrick F., Mazibuko N., Cr S., Barnby J.M., Hellyer P. Cognitive de fi cits in people who have recovered from COVID-19. EClinicalMedicine. 2021;000 doi: 10.1016/j.eclinm.2021.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Sigma-1 receptors and selective serotonin reuptake inhibitors: clinical implications of their relationship. Cent. Nerv. Syst. Agents Med. Chem. 2009;9:197–204. doi: 10.2174/1871524910909030197. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Repurposing of CNS drugs to treat COVID-19 infection: targeting the sigma-1 receptor. Eur. Arch. Psychiatry Clin. Neurosci. 2021;271:249–258. doi: 10.1007/s00406-020-01231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K., Fujita Y., Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of fluvoxamine: role of sigma-1 receptors. Neuropsychopharmacology. 2007;32:514–521. doi: 10.1038/sj.npp.1301047. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Su T.P. Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Su T.P. σ-1 receptors (σ1 binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J. Pharmacol. Exp. Ther. 2003;306:718–725. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Su T.P. Sigma-1 receptor chaperones at the ER- mitochondrion interface regulate Ca2+ signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Maurice T., Su T.-P. Ca(2+) signaling via sigma(1)-receptors: novel regulatory mechanism affecting intracellular Ca(2+) concentration. J. Pharmacol. Exp. Ther. 2000;293:788–798. [PubMed] [Google Scholar]
- Hayashi T., Hayashi E., Fujimoto M., Sprong H., Su T.-P. The lifetime of UDP-galactose:ceramide galactosyltransferase is controlled by a distinct endoplasmic reticulum-associated degradation (ERAD) regulated by sigma-1 receptor chaperones. J. Biol. Chem. 2012;287:43156–43169. doi: 10.1074/jbc.M112.380444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellström J., Oliveira A.L.R., Meister B., Cullheim S. Large cholinergic nerve terminals on subsets of motoneurons and their relation to muscarinic receptor type 2. J. Comp. Neurol. 2003;460:476–486. doi: 10.1002/cne.10648. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M., Hoshino T. Involvement of κ-opioid receptors and σ receptors in memory function demonstrated using an antisense strategy. Brain Res. 2004;1030:247–255. doi: 10.1016/j.brainres.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M., Hoshino T. Improvement of memory impairment by (+)- and (-)-pentazocine via sigma, but not kappa opioid receptors. Brain Res. 2005;1057:72–80. doi: 10.1016/j.brainres.2005.07.028. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M., Hoshino T., Kameyama T., Nabeshima T. Involvement of κ-opioid and σ receptors in short-term memory in mice. Eur. J. Pharmacol. 2002;453:91–98. doi: 10.1016/S0014-2999(02)02388-9. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M., Mizuno N., Kanematsu K. Pharmacological characterization of the ameliorating effect on learning and memory impairment and antinociceptive effect of KT-95 in mice. Behav. Brain Res. 2006;167:219–225. doi: 10.1016/j.bbr.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Hiranita T., Mereu M., Soto P.L., Tanda G., Katz J.L. Self-administration of cocaine induces dopamine-independent self-administration of sigma agonists. Neuropsychopharmacology. 2013;38:605–615. doi: 10.1038/npp.2012.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W.C. Distinct regulation of σ (1) receptor multimerization by its agonists and antagonists in transfected cells and rat liver membranes. J. Pharmacol. Exp. Ther. 2020;373:290–301. doi: 10.1124/jpet.119.262790. [DOI] [PubMed] [Google Scholar]
- Hong W.C., Yano H., Hiranita T., Chin F.T., McCurdy C.R., Su T.-P., Amara S.G., Katz J.L. The sigma-1 receptor modulates dopamine transporter conformation and cocaine binding and may thereby potentiate cocaine self-administration in rats. J. Biol. Chem. 2017;292:11250–11261. doi: 10.1074/jbc.M116.774075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan B., Gifford A.N., Matsuno K., Mita S., Ashby C.R.J. Effect of SA4503 on the electrically evoked release of (3)H-acetylcholine from striatal and hippocampal rat brain slices. Synapse. 2002;46:1–3. doi: 10.1002/syn.10107. [DOI] [PubMed] [Google Scholar]
- Hyrskyluoto A., Pulli I., Törnqvist K., Huu Ho T., Korhonen L., Lindholm D. Sigma-1 receptor agonist PRE084 is protective against mutant huntingtin-induced cell degeneration: involvement of calpastatin and the NF-κB pathway. Cell Death Dis. 2013;4:1–9. doi: 10.1038/cddis.2013.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishima T., Nishimura T., Iyo M., Hashimoto K. Progress in Neuro-Psychopharmacology & Biological Psychiatry Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by donepezil: role of sigma-1 receptors and IP3 receptors. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:1656–1659. doi: 10.1016/j.pnpbp.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Ishima T., Fujita Y., Hashimoto K. Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. Eur. J. Pharmacol. 2014;727:167–173. doi: 10.1016/j.ejphar.2014.01.064. [DOI] [PubMed] [Google Scholar]
- Ito K., Hirooka Y., Matsukawa R., Nakano M., Sunagawa K. Decreased brain sigma-1 receptor contributes to the relationship between heart failure and depression. Cardiovasc. Res. 2012;93:33–40. doi: 10.1093/cvr/cvr255. [DOI] [PubMed] [Google Scholar]
- Ito K., Hirooka Y., Sunagawa K. Brain sigma-1 receptor stimulation improves mental disorder and cardiac function in mice with myocardial infarction. J. Cardiovasc. Pharmacol. 2013;62:222–228. doi: 10.1097/FJC.0b013e3182970b15. [DOI] [PubMed] [Google Scholar]
- Ivanova A.A., East M.P., Yi S.L., Kahn R.A. Characterization of recombinant ELMOD (cell engulfment and motility domain) proteins as GTPase-activating proteins (GAPs) for ARF family GTPases. J. Biol. Chem. 2014;289:11111–11121. doi: 10.1074/jbc.M114.548529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivković M., Damjanović A., Jasović-Gasić M., Paunović V.R. The effects of fluoxetine on cognitive functions in animal model of Alzheimer’s disease. Psychiatr. Danub. 2004;16:15–20. [PubMed] [Google Scholar]
- Iwamoto M., Nakamura Y., Takemura M., Hisaoka-nakashima K., Morioka N. TLR4-TAK1-p38 MAPK pathway and HDAC6 regulate the expression of sigma-1 receptors in rat primary cultured microglia. J. Pharmacol. Sci. 2020;144:23–29. doi: 10.1016/j.jphs.2020.06.007. [DOI] [PubMed] [Google Scholar]
- Jafari-Sabet M., Nemati S., Torab M. Cross state-dependency of learning between 5-HT1A and/or 5-HT7 receptor agonists and muscimol in the mouse dorsal hippocampus. J. Psychopharmacol. 2019;33:722–736. doi: 10.1177/0269881119826608. [DOI] [PubMed] [Google Scholar]
- Jarvik M.E., Kopp R. An improved one-trial passive avoidance learning situation. Psychol. Rep. 1967;21:221–224. doi: 10.2466/pr0.1967.21.1.221. [DOI] [PubMed] [Google Scholar]
- Ji L.L., Peng J.B., Fu C.H., Cao D., Li D., Tong L., Wang Z.Y. Activation of Sigma-1 receptor ameliorates anxiety-like behavior and cognitive impairments in a rat model of post-traumatic stress disorder. Behav. Brain Res. 2016;311:408–415. doi: 10.1016/j.bbr.2016.05.056. [DOI] [PubMed] [Google Scholar]
- Ji L.-L., Peng J.-B., Fu C.-H., Tong L., Wang Z.-Y. Sigma-1 receptor activation ameliorates anxiety-like behavior through NR2A-CREB-BDNF signaling pathway in a rat model submitted to single-prolonged stress. Mol. Med. Rep. 2017;16:4987–4993. doi: 10.3892/mmr.2017.7185. [DOI] [PubMed] [Google Scholar]
- Jia J., Cheng J., Wang C., Zhen X. Sigma-1 receptor-modulated neuroinflammation in neurological diseases. Front. Cell. Neurosci. 2018;12:314. doi: 10.3389/fncel.2018.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannessen M., Ramachandran S., Riemer L., Ramos-Serrano A., Ruoho A.E., Jackson M.B. Voltage-gated sodium channel modulation by σ-receptors in cardiac myocytes and heterologous systems. Am. J. Physiol. - Cell Physiol. 2009;296 doi: 10.1152/ajpcell.00431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junien J.L., Gue M., Bueno L. Neuropeptide Y and sigma ligand (JO 1784) act through a Gi protein to block the psychological stress and corticotropin-releasing factor-induced colonic motor activation in rats. Neuropharmacology. 1991;30:1119–1124. doi: 10.1016/0028-3908(91)90142-x. [DOI] [PubMed] [Google Scholar]
- Junien J.L., Roman F.J., Brunelle G., Pascaud X. JO1784, a novel sigma ligand, potentiates [3H]acetylcholine release from rat hippocampal slices. Eur. J. Pharmacol. 1991;200:343–345. doi: 10.1016/0014-2999(91)90593-f. [DOI] [PubMed] [Google Scholar]
- Kaushal N., Elliott M., Robson M.J., Iyer A.K.V., Rojanasakul Y., Coop A., Matsumoto R.R. AC927, a σ receptor ligand, blocks methamphetamine-induced release of dopamine and generation of reactive oxygen species in NG108-15 cells. Mol. Pharmacol. 2012;81:299–308. doi: 10.1124/mol.111.074120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kekuda R., Prasad P.D., Fei Y.J., Leibach F.H., Ganapathy V. Cloning and functional expression of the human type 1 sigma receptor (hSigmaR1) Biochem. Biophys. Res. Commun. 1996;229:553–558. doi: 10.1006/bbrc.1996.1842. [DOI] [PubMed] [Google Scholar]
- Keshavarz M., Farrokhi M.R., Amirinezhad Fard E., Mehdipour M. Contribution of lysosome and sigma receptors to neuroprotective effects of memantine against beta-amyloid in the SH-SY5Y cells. Adv. Pharm. Bull. 2020;10:452–457. doi: 10.34172/apb.2020.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khulbe A., Pandey S., Sah S. Antidepressant-like action of the hydromethanolic flower extract of Tagetes erecta L. in mice and its possible mechanism of action. Indian J. Pharmacol. 2013;45:386–390. doi: 10.4103/0253-7613.115026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi-Utsumi K., Nakaki T. Chronic treatment with a selective ligand for the sigma-1 receptor chaperone, SA4503, up-regulates BDNF protein levels in the rat hippocampus. Neurosci. Lett. 2008;440:19–22. doi: 10.1016/j.neulet.2008.05.055. [DOI] [PubMed] [Google Scholar]
- Kim Y., Seo S., Heo J., Park C., Lee E., Choi Y. Phenylalkyl 1,2-diamines as sigma-receptor ligands: the discovery of novel antidepressant agents. Methods Find. Exp. Clin. Pharmacol. 2006;28:7–11. doi: 10.1358/mf.2006.28.1.962771. [DOI] [PubMed] [Google Scholar]
- Kim F.J., Kovalyshyn I., Burgman M., Neilan C., Chien C.-C., Pasternak G.W. Sigma 1 receptor modulation of G-protein-coupled receptor signaling: potentiation of opioid transduction independent from receptor binding. Mol. Pharmacol. 2010;77:695–703. doi: 10.1124/mol.109.057083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W.S., Fu Y., Dobson-Stone C., Hsiao J.H.T., Shang K., Hallupp M., Schofield P.R., Garner B., Karl T., Kwok J.B.J. Effect of fluvoxamine on amyloid-β peptide generation and memory. J. Alzheimer’s Dis. 2018;62:1777–1787. doi: 10.3233/JAD-171001. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Fujita Y., Shibata K., Mori M., Yamashita T. Sigma-1 receptor enhances neurite elongation of cerebellar granule neurons via TrkB signaling. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M., Matsuoka Y., Suzuki T., Mirrielees J., Yang J. Sigma-1 receptor alters the kinetics of Kv1. 3 voltage gated potassium channels but not the sensitivity to receptor ligands. Brain Res. 2012;1452:1–9. doi: 10.1016/j.brainres.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klouz A., Ben Saïd D., Ferchichi H., Kourda N., Ouanes L., Lakhal M., Tillement J.P., Morin D. Protection of cellular and mitochondrial functions against liver ischemia by N-benzyl-N′-(2-hydroxy-3,4-dimethoxybenzyl)-piperazine (BHDP), a sigma1 ligand. Eur. J. Pharmacol. 2008;578:292–299. doi: 10.1016/j.ejphar.2007.09.038. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Matsuno K., Nakata K., Mita S. Enhancement of acetylcholine release by SA4503, a novel sigma 1 receptor agonist, in the rat brain. J. Pharmacol. Exp. Ther. 1996;279:106–113. [PubMed] [Google Scholar]
- Koornneef M., Hanhart C.J., Van Der Veen J.H., Josephs E., Lockwood A., Myllylä S., Oakley C., Palmer R., Fontanilla D., Johannessen M., Hajipour A.R., Cozzi N.V., Jackson M.B., Ruoho A.E. The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science (80-.) 2009:934–937. doi: 10.1126/science.1166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotagale N.R., Mendhi S.M., Aglawe M.M., Umekar M.J., Taksande B.G. Evidences for the involvement of sigma receptors in antidepressant like effect of quetiapine in mice. Eur. J. Pharmacol. 2013;702:180–186. doi: 10.1016/j.ejphar.2013.01.045. [DOI] [PubMed] [Google Scholar]
- Kourrich S., Hayashi T., Chuang J.Y., Tsai S.Y., Su T.P., Bonci A. Dynamic interaction between sigma-1 receptor and Kv1.2 shapes neuronal and behavioral responses to cocaine. Cell. 2013;152:236–247. doi: 10.1016/j.cell.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni S.K., Dhir A. On the mechanism of antidepressant-like action of berberine chloride. Eur. J. Pharmacol. 2008;589:163–172. doi: 10.1016/j.ejphar.2008.05.043. [DOI] [PubMed] [Google Scholar]
- Kunitachi S., Fujita Y., Ishima T., Kohno M., Horio M., Tanibuchi Y., Shirayama Y., Iyo M., Hashimoto K. Phencyclidine-induced cognitive deficits in mice are ameliorated by subsequent subchronic administration of donepezil: role of sigma-1 receptors. Brain Res. 2009;1279:189–196. doi: 10.1016/j.brainres.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Kurata K., Takebayashi M., Morinobu S., Yamawaki S. Beta-estradiol, dehydroepiandrosterone, and dehydroepiandrosterone sulfate protect against N-methyl-D-aspartate-induced neurotoxicity in rat hippocampal neurons by different mechanisms. J. Pharmacol. Exp. Ther. 2004;311:237–245. doi: 10.1124/jpet.104.067629. [DOI] [PubMed] [Google Scholar]
- Lahmy V., Meunier J., Malmström S., Naert G., Givalois L., Kim S.H., Villard V., Vamvakides A., Maurice T. Blockade of tau hyperphosphorylation and aβ 1-42 generation by the aminotetrahydrofuran derivative ANAVEX2-73, a mixed muscarinic and σ 1 receptor agonist, in a nontransgenic mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2013;38:1706–1723. doi: 10.1038/npp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa F., Codony X., Tovar V., Lavado A., Giménez E., Cozar P., Cantero M., Dordal A., Hernández E., Pérez R., Monroy X., Zamanillo D., Guitart X., Montoliu L. Generation and phenotypic analysis of sigma receptor type I (σ1) knockout mice. Eur. J. Neurosci. 2003;18:2188–2196. doi: 10.1046/j.1460-9568.2003.02950.x. [DOI] [PubMed] [Google Scholar]
- Lee P.T., Liévens J.C., Wang S.M., Chuang J.Y., Khalil B., Wu Hen, Chang W.C., Maurice T., Su T.P. Sigma-1 receptor chaperones rescue nucleocytoplasmic transport deficit seen in cellular and Drosophila ALS/FTD models. Nat. Commun. 2020;11:1–14. doi: 10.1038/s41467-020-19396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenart L., Hodrea J., Hosszu A., Koszegi S., Zelena D., Balogh D., Szkibinszkij E., Veres-Szekely A., Wagner L., Vannay A., Szabo A.J., Fekete A. The role of sigma-1 receptor and brain-derived neurotrophic factor in the development of diabetes and comorbid depression in streptozotocin-induced diabetic rats. Psychopharmacology (Berl.) 2016;233:1269–1278. doi: 10.1007/s00213-016-4209-x. [DOI] [PubMed] [Google Scholar]
- Li Z., Zhou R., Cui S., Xie G., Cai W., Sokabe M., Chen L. Dehydroepiandrosterone sulfate prevents ischemia-induced impairment of long-term potentiation in rat hippocampal CA1 by up-regulating tyrosine phosphorylation of NMDA receptor. Neuropharmacology. 2006;51:958–966. doi: 10.1016/j.neuropharm.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Li Z., Cui S., Zhang Z., Zhou R., Ge Y., Sokabe M. 2009. DHEA-Neuroprotection and -Neurotoxicity after Transient Cerebral Ischemia in Rats; pp. 287–296. [DOI] [PubMed] [Google Scholar]
- Li Y.-J., Yang L.-P., Hou J.-L., Li X.-M., Chen L., Zhu J.-H., Wang Q.-Y., Li G., Zhao P.-Y., Liu X.-H., Shi Z.-J. Prenatal stress impairs postnatal learning and memory development via disturbance of the cGMP-PKG pathway and oxidative phosphorylation in the Hippocampus of rats. Front. Mol. Neurosci. 2020;13:158. doi: 10.3389/fnmol.2020.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisak R.P., Nedelkoska L., Benjamins J.A. Sigma-1 receptor agonists as potential protective therapies in multiple sclerosis. J. Neuroimmunol. 2020;342 doi: 10.1016/j.jneuroim.2020.577188. [DOI] [PubMed] [Google Scholar]
- Liu X., Qu C., Yang H., Shi S., Zhang C., Zhang Y., Liang J., Yang B. Chronic stimulation of the sigma-1 receptor ameliorates autonomic nerve dysfunction and atrial fibrillation susceptibility in a rat model of depression. Am. J. Physiol. - Hear. Circ. Physiol. 2018;315:H1521–H1531. doi: 10.1152/ajpheart.00607.2017. [DOI] [PubMed] [Google Scholar]
- Liu X., Qu C., Shi S., Ye T., Wang L., Liu S., Zhang C., Liang J., Hu D., Yang B. The reversal effect of sigma-1 receptor (S1R) agonist, SA4503, on atrial fibrillation after depression and its underlying mechanism. Front. Physiol. 2019;10:1–12. doi: 10.3389/fphys.2019.01346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueptow L.M. Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 2017 doi: 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M.A. Long-term potentiation and memory. Physiol. Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- M’s Science Corporation, 2008. ClinicalTrials.gov Identifier: NCT00551109 [WWW Document]. URL https://clinicaltrials.gov/ct2/show/study/NCT00551109?term=cutamesine&draw=2&rank=1 (Accessed 10 August 2021).
- M’s Science Corporation, 2009. ClinicalTrials.gov Identifier: NCT00639249 [WWW Document]. URL https://clinicaltrials.gov/ct2/show/NCT00639249?term=cutamesine&draw=2&rank=2 (Accessed 10 August 2021).
- Malik M., Rangel-Barajas C., Sumien N., Su C., Singh M., Chen Z., Huang R.Q., Meunier J., Maurice T., Mach R.H., Luedtke R.R. The effects of sigma (σ1) receptor-selective ligands on muscarinic receptor antagonist-induced cognitive deficits in mice. Br. J. Pharmacol. 2015;172:2519–2531. doi: 10.1111/bph.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya T., Noda Y., Noda A., Hiramatsu M. Effects of sigma receptor agonists on the impairment of spontaneous alternation behavior and decrease of cyclic GMP level induced by nitric oxide synthase inhibitors in mice. Neuropharmacology. 2000;39:2391–2398. doi: 10.1016/s0028-3908(00)00078-2. [DOI] [PubMed] [Google Scholar]
- Mancuso R., Oliván S., Rando A., Casas C., Osta R., Navarro X. Sigma-1R agonist improves motor function and motoneuron survival in ALS mice. Neurother. J. Am. Soc. Exp. Neurother. 2012;9:814–826. doi: 10.1007/s13311-012-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelli L., Wang S.-M., Han C., Lee S.-J., Patkar A.A., Masand P.S., Pae C.-U., Serretti A. The impact of a single nucleotide polymorphism in SIGMAR1 on depressive symptoms in major depressive disorder and bipolar disorder. Adv. Ther. 2017;34:713–724. doi: 10.1007/s12325-017-0482-2. [DOI] [PubMed] [Google Scholar]
- Mari Y., Katnik C., Cuevas J. Σ-1 receptor inhibition of ASIC1a channels is dependent on a pertussis toxin-sensitive G-protein and an AKAP150/calcineurin complex. Neurochem. Res. 2014;40:2055–2067. doi: 10.1007/s11064-014-1324-0. [DOI] [PubMed] [Google Scholar]
- Marrazzo A., Caraci F., Salinaro E.T., Su T.P., Copani A., Ronsisvalle G. Neuroprotective effects of sigma-1 receptor agonists against beta-amyloid-induced toxicity. Neuroreport. 2005;16:1223–1226. doi: 10.1097/00001756-200508010-00018. [DOI] [PubMed] [Google Scholar]
- Marriott K.-S.C., Prasad M., Thapliyal V., Bose H.S. σ-1 receptor at the mitochondrial-associated endoplasmic reticulum membrane is responsible for mitochondrial metabolic regulation. J. Pharmacol. Exp. Ther. 2012;343:578–586. doi: 10.1124/jpet.112.198168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P.M., Ola M.S., Agarwal N., Ganapathy V., Smith S.B. The sigma receptor ligand (+)-pentazocine prevents apoptotic retinal ganglion cell death induced in vitro by homocysteine and glutamate. Brain Res. Mol. Brain Res. 2004;123:66–75. doi: 10.1016/j.molbrainres.2003.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., de Witte P.A.M., Maurice T., Gammaitoni A., Farfel G., Galer B. Fenfluramine acts as a positive modulator of sigma-1 receptors. Epilepsy Behav. 2020;105 doi: 10.1016/j.yebeh.2020.106989. [DOI] [PubMed] [Google Scholar]
- Martina M., Turcotte M.E.B., Halman S., Bergeron R. The sigma-1 receptor modulates NMDA receptor synaptic transmission and plasticity via SK channels in rat hippocampus. J. Physiol. 2007;578:143–157. doi: 10.1113/jphysiol.2006.116178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto R.R., Shaikh J., Wilson L.L., Vedam S., Coop A. Attenuation of methamphetamine-induced effects through the antagonism of sigma (sigma) receptors: evidence from in vivo and in vitro studies. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2008;18:871–881. doi: 10.1016/j.euroneuro.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K., Matsunaga K., Mita S. Increase of extracellular acetylcholine level in rat frontal cortex induced by (+)N-allylnormetazocine as measured by brain microdialysis. Brain Res. 1992;575:315–319. doi: 10.1016/0006-8993(92)90096-r. [DOI] [PubMed] [Google Scholar]
- Matsuno K., Matsunaga K., Senda T., Mita S. Increase in extracellular acetylcholine level by sigma ligands in rat frontal cortex. J. Pharmacol. Exp. Ther. 1993;265:851–859. [PubMed] [Google Scholar]
- Matsuno K., Senda T., Matsunaga K., Mita S., Kaneto H. Similar ameliorating effects of benzomorphans and 5-HT2 antagonists on drug-induced impairment of passive avoidance response in mice: comparison with acetylcholinesterase inhibitors. Psychopharmacology (Berl.) 1993;112:134–141. doi: 10.1007/BF02247374. [DOI] [PubMed] [Google Scholar]
- Matsuno K., Senda T., Matsunaga K., Mita S. Ameliorating effects of σ receptor ligands on the impairment of passive avoidance tasks in mice: involvement in the central acetylcholinergic system. Eur. J. Pharmacol. 1994;261:43–51. doi: 10.1016/0014-2999(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Matsuno K., Senda T., Kobayashi T., Mita S. Involvement of σ1 receptor in (+)-N-allylnormetazocine-stimulated hippocampal cholinergic functions in rats. Brain Res. 1995;690:200–206. doi: 10.1016/0006-8993(95)00618-Z. [DOI] [PubMed] [Google Scholar]
- Matsuno K., Kobayashi T., Tanaka M.K., Mita S. 1 Receptor subtype is involved in the relief of behavioral despair in the mouse forced swimming test. Eur. J. Pharmacol. 1996;312:267–271. doi: 10.1016/0014-2999(96)00497-9. [DOI] [PubMed] [Google Scholar]
- Matsuno K., Senda T., Kobayashi T., Okamoto K., Nakata K., Mita S. SA4503, a novel cognitive enhancer, with ol receptor agonistic properties. Behav. Brain Res. 1997;83:221–224. doi: 10.1016/s0166-4328(97)86074-3. [DOI] [PubMed] [Google Scholar]
- Matsuno K., Senda T., Kobayashi T., Murai M., Mita S. Reduction of 4-cyclohexyl-1-[(1R)-1,2-diphenylethyl]-piperazine-induced memory impairment of passive avoidance performance by sigma 1 receptor agonists in mice. Methods Find. Exp. Clin. Pharmacol. 1998;20:575–580. doi: 10.1358/mf.1998.20.7.485721. [DOI] [PubMed] [Google Scholar]
- Matsushima Y., Terada K., Takata J., Karube Y., Sugimoto Y. Effects of fluvoxamine on nerve growth factor- induced neurite outgrowth inhibition by dexamethasone in PC12 cells. Biosci. Biotechnol. Biochem. 2018;00:1–7. doi: 10.1080/09168451.2018.1553607. [DOI] [PubMed] [Google Scholar]
- Matsushima Y., Terada K., Kamei C., Sugimoto Y. Sertraline inhibits nerve growth factor-induced neurite outgrowth in PC12 cells via a mechanism involving the sigma-1 receptor. Eur. J. Pharmacol. 2019;853:129–135. doi: 10.1016/j.ejphar.2019.03.032. [DOI] [PubMed] [Google Scholar]
- Maurice T. Beneficial effect of the sigma(1) receptor agonist PRE-084 against the spatial learning deficits in aged rats. Eur. J. Pharmacol. 2001;431:223–227. doi: 10.1016/s0014-2999(01)01436-4. [DOI] [PubMed] [Google Scholar]
- Maurice T. Protection by sigma-1 receptor agonists is synergic with donepezil, but not with memantine, in a mouse model of amyloid-induced memory impairments. Behav. Brain Res. 2016;296:270–278. doi: 10.1016/j.bbr.2015.09.020. [DOI] [PubMed] [Google Scholar]
- Maurice T., Privat A. SA4503, a novel cognitive enhancer with σ1 receptor agonist properties, facilitates NMDA receptor-dependent learning in mice. Eur. J. Pharmacol. 1997;328:9–18. doi: 10.1016/S0014-2999(97)83020-8. [DOI] [PubMed] [Google Scholar]
- Maurice T., Hiramatsu M., Itoh J., Kameyama T., Hasegawa T., Nabeshima T. Behavioral evidence for a modulating role of σ ligands in memory processes. I. Attenuation of dizocilpine (MK-801)-induced amnesia. Brain Res. 1994;647:44–56. doi: 10.1016/0006-8993(94)91397-8. [DOI] [PubMed] [Google Scholar]
- Maurice T., Hiramatsu M., Itoh J., Kameyama T., Hasegawa T., Nabeshima T. Low dose of 1,3-di(2-tolyl)guanidine (DTG) attenuates MK-801-induced spatial working memory impairment in mice. Psychopharmacology (Berl.) 1994;114:520–522. doi: 10.1007/BF02249345. [DOI] [PubMed] [Google Scholar]
- Maurice T., Hiramatsu M., Kameyama T., Hasegawa T., Nabeshima T. Behavioral evidence for a modulating role of sigma ligands in memory processes. II. Reversion of carbon monoxide-induced amnesia. Brain Res. 1994;647:57–64. doi: 10.1016/0006-8993(94)91398-6. [DOI] [PubMed] [Google Scholar]
- Maurice T., Su T.-P., Parish D.W., Nabeshima T., Privat A. PRE-084, a sigma selective PCP derivative, attenuates MK-801-induced impairment of learning in mice. Pharmacol. Biochem. Behav. 1994;49:859–869. doi: 10.1016/0091-3057(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Maurice T., Su T.-P., Parish D.W., Privat A. Prevention of nimodipine-induced impairment of learning by the selective sigma ligand PRE-084. J. Neural Transm. Gen. Sect. 1995;102:1–18. doi: 10.1007/BF01276561. [DOI] [PubMed] [Google Scholar]
- Maurice T., Roman F.J., Su T.P., Privat A. Beneficial effects of sigma agonists on the age-related learning impairment in the senescence-accelerated mouse (SAM) Brain Res. 1996;733:219–230. doi: 10.1016/0006-8993(96)00565-3. [DOI] [PubMed] [Google Scholar]
- Maurice T., Junien J.L., Privat A. Dehydroepiandrosterone sulfate attenuates dizocilpine-induced learning impairment in mice via σ1-receptors. Behav. Brain Res. 1997;83:159–164. doi: 10.1016/S0166-4328(97)86061-5. [DOI] [PubMed] [Google Scholar]
- Maurice T., Su T.-P., Privat A. Sigma1 (sigma 1) receptor agonists and neurosteroids attenuate B25-35-amyloid peptide-induced amnesia in mice through a common mechanism. Neuroscience. 1998;83:413–428. doi: 10.1016/s0306-4522(97)00405-3. [DOI] [PubMed] [Google Scholar]
- Maurice T., Phan V.L., Noda Y., Yamada K., Privat A., Nabeshima T. The attenuation of learning impairments induced after exposure to CO or trimethyltin in mice by sigma (σ) receptor ligands involves both σ1 and σ2 sites. Br. J. Pharmacol. 1999;127:335–342. doi: 10.1038/sj.bjp.0702553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T., Phan V.L., Urani A., Kamei H., Noda Y., Nabeshima T. Neuroactive neurosteroids as endogenous effectors for the sigma1 (sigma1) receptor: pharmacological evidence and therapeutic opportunities. Jpn. J. Pharmacol. 1999;81:125–155. doi: 10.1254/jjp.81.125. [DOI] [PubMed] [Google Scholar]
- Maurice T., Phan V.L., Sandillon F., Urani A. Differential effect of dehydroepiandrosterone and its steroid precursor pregnenolone against the behavioural deficits in CO-exposed mice. Eur. J. Pharmacol. 2000;390:145–155. doi: 10.1016/S0014-2999(00)00015-7. [DOI] [PubMed] [Google Scholar]
- Maurice T., Phan V.L., Privat A. The anti-amnesic effects of sigma1 (σ1) receptor agonists confirmed by in vivo antisense strategy in the mouse. Brain Res. 2001;898:113–121. doi: 10.1016/S0006-8993(01)02152-7. [DOI] [PubMed] [Google Scholar]
- Maurice T., Phan V.L., Urani A., Guillemain I. Differential involvement of the sigma1 (σ1 receptor in the anti-amnesic effect of neuroactive steroids, as demonstrated using an in vivo antisense strategy in the mouse. Br. J. Pharmacol. 2001;134:1731–1741. doi: 10.1038/sj.bjp.0704355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T., Meunier J., Feng B., Ieni J., Monaghan D.T. Interaction with σ1 protein, but not N-Methyl-D-aspartate receptor, is involved in the pharmacological activity of donepezil. J. Pharmacol. Exp. Ther. 2006;317:606–614. doi: 10.1124/jpet.105.097394. [DOI] [PubMed] [Google Scholar]
- Maurice T., Strehaiano M., Duhr F., Chevallier N. Amyloid toxicity is enhanced after pharmacological or genetic invalidation of the σ1 receptor. Behav. Brain Res. 2018;339:1–10. doi: 10.1016/j.bbr.2017.11.010. [DOI] [PubMed] [Google Scholar]
- Maurice T., Volle J.N., Strehaiano M., Crouzier L., Pereira C., Kaloyanov N., Virieux D., Pirat J.L. Neuroprotection in non-transgenic and transgenic mouse models of Alzheimer’s disease by positive modulation of σ 1 receptors. Pharmacol. Res. 2019;144:315–330. doi: 10.1016/j.phrs.2019.04.026. [DOI] [PubMed] [Google Scholar]
- Mei J., Pasternak G.W. Molecular cloning and pharmacological characterization of the rat sigma1 receptor. Biochem. Pharmacol. 2001;62:349–355. doi: 10.1016/s0006-2952(01)00666-9. [DOI] [PubMed] [Google Scholar]
- Meunier J., Hayashi T. Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kappaB. J. Pharmacol. Exp. Ther. 2010;332:388–397. doi: 10.1124/jpet.109.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J., Maurice T. Beneficial effects of the sigma1 receptor agonists igmesine and dehydroepiandrosterone against learning impairments in rats prenatally exposed to cocaine. Neurotoxicol. Teratol. 2004;26:783–797. doi: 10.1016/j.ntt.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Meunier J., Gué M., Récasens M., Maurice T. Attenuation by a sigma 1 (σ 1) receptor agonist of the learning and memory deficits induced by a prenatal restraint stress in juvenile rats. Br. J. Pharmacol. 2004;142:689–700. doi: 10.1038/sj.bjp.0705835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J., Demeilliers B., Célérier A., Maurice T. Compensatory effect by sigma1 (σ1) receptor stimulation during alcohol withdrawal in mice performing an object recognition task. Behav. Brain Res. 2006;166:166–176. doi: 10.1016/j.bbr.2005.07.019. [DOI] [PubMed] [Google Scholar]
- Meunier J., Ieni J., Maurice T. Antiamnesic and neuroprotective effects of donepezil against learning impairments induced in mice by exposure to carbon monoxide gas. J. Pharmacol. Exp. Ther. 2006;317:1307–1319. doi: 10.1124/jpet.106.101527. [DOI] [PubMed] [Google Scholar]
- Meunier J., leni J., Maurice T. The anti-amnesic and neuroprotective effects of donepezil against amyloid beta25-35 peptide-induced toxicity in mice involve an interaction with the sigma1 receptor. Br. J. Pharmacol. 2006;149:998–1012. doi: 10.1038/sj.bjp.0706927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A.K., Mavlyutov T., Singh D.R., Biener G., Yang J., Oliver J.A., Ruoho A., Raicu V. The sigma-1 receptors are present in monomeric and oligomeric forms in living cells in the presence and absence of ligands. Biochem. J. 2015;466:263–271. doi: 10.1042/BJ20141321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet F.P., Debonnel G., de Montigny C. In vivo electrophysiological evidence for a selective modulation of N-methyl-D-aspartate-induced neuronal activation in rat CA3 dorsal hippocampus by sigma ligands. J. Pharmacol. Exp. Ther. 1992;261:123–130. [PubMed] [Google Scholar]
- Monnet F.P., Mahé V., Robel P., Baulieu E.E. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc. Natl. Acad. Sci. U. S. A. 1995;92:3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradpour F., Fathollahi Y., Naghdi N., Hosseinmardi N., Javan M. Prepubertal castration-associated developmental changes in sigma-1 receptor gene expression levels regulate hippocampus area CA1 activity during adolescence. Hippocampus. 2016;26:933–946. doi: 10.1002/hipo.22576. [DOI] [PubMed] [Google Scholar]
- More S.V., Kumar H., Cho D.Y., Yun Y.S., Choi D.K. Toxin-induced experimental models of learning and memory impairment. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17091447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T., Hayashi T., Hayashi E., Su T.P. Sigma-1 receptor chaperone at the ER-mitochondrion interface mediates the mitochondrion-ER-nucleus signaling for cellular survival. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi S., Yamamoto Y., Ikuno T., Fukunaga K. Sigma-1 receptor stimulation by dehydroepiandrosterone ameliorates cognitive impairment through activation of CaM kinase II, protein kinase C and extracellular signal-regulated kinase in olfactory bulbectomized mice. J. Neurochem. 2011;117:879–891. doi: 10.1111/j.1471-4159.2011.07256.x. [DOI] [PubMed] [Google Scholar]
- Moriguchi S., Shinoda Y., Yamamoto Y., Sasaki Y., Miyajima K., Tagashira H., Fukunaga K. Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice. PLoS One. 2013;8:1–10. doi: 10.1371/journal.pone.0060863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Mueller B.H., 2nd, Park Y., Daudt D.R., 3rd, Ma H.-Y., Akopova I., Stankowska D.L., Clark A.F., Yorio T. Sigma-1 receptor stimulation attenuates calcium influx through activated L-type Voltage Gated Calcium Channels in purified retinal ganglion cells. Exp. Eye Res. 2013;107:21–31. doi: 10.1016/j.exer.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Muriach M., López-Pedrajas R., Barcia J.M., Sanchez-Villarejo M.V., Almansa I., Romero F.J. Cocaine causes memory and learning impairments in rats: involvement of nuclear factor kappa B and oxidative stress, and prevention by topiramate. J. Neurochem. 2010;114:675–684. doi: 10.1111/j.1471-4159.2010.06794.x. [DOI] [PubMed] [Google Scholar]
- Nakajima A., Yamakuni T., Haraguchi M., Omae N., Song S.Y., Kato C., Nakagawasai O., Tadano T., Yokosuka A., Mimaki Y., Sashida Y., Ohizumi Y. Nobiletin, a citrus flavonoid that improves memory impairment, rescues bulbectomy-induced cholinergic neurodegeneration in mice. J. Pharmacol. Sci. 2007;105:122–126. doi: 10.1254/jphs.SC0070155. [DOI] [PubMed] [Google Scholar]
- Nakano M., Osada K., Misonoo A., Fujiwara K., Takahashi M., Ogawa Y., Haga T., Kanai S., Tanaka D., Sasuga Y., Yanagida T., Asakura M., Yamaguchi N. Fluvoxamine and sigma-1 receptor agonists dehydroepiandrosterone (DHEA)-sulfate induces the Ser473-phosphorylation of Akt-1 in PC12 cells. Life Sci. 2010;86:309–314. doi: 10.1016/j.lfs.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Natsvlishvili N., Goguadze N., Zhuravliova E., Mikeladze D. Sigma-1 receptor directly interacts with Rac1-GTPase in the brain mitochondria. BMC Biochem. 2015;16:11. doi: 10.1186/s12858-015-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G., Moreno E., Aymerich M., Marcellino D., McCormick P.J., Mallol J., Cortés A., Casadó V., Canela E.I., Ortiz J., Fuxe K., Lluís C., Ferré S., Franco R. Direct involvement of sigma-1 receptors in the dopamine D1 receptor-mediated effects of cocaine. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18676–18681. doi: 10.1073/pnas.1008911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G., Moreno E., Bonaventura J., Brugarolas M., Farré D., Aguinaga D., Mallol J., Cortés A., Casadó V., Lluís C., Ferre S., Franco R., Canela E., McCormick P.J. Cocaine inhibits dopamine D2 receptor signaling via sigma-1-D2 receptor heteromers. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G., Quiroz C., Moreno-Delgado D., Sierakowiak A., McDowell K., Moreno E., Rea W., Cai N.-S., Aguinaga D., Howell L.A., Hausch F., Cortés A., Mallol J., Casadó V., Lluís C., Canela E.I., Ferré S., McCormick P.J. Orexin-corticotropin-releasing factor receptor heteromers in the ventral tegmental area as targets for cocaine. J. Neurosci. 2015;35:6639–6653. doi: 10.1523/JNEUROSCI.4364-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro G., Medrano M., Aguinaga D., Vega-Quiroga I., Lillo A., Jiménez J., Casanovas M., Canela E.I., Mallol J., Gysling K., Franco R. Differential effect of amphetamine over the corticotropin-releasing factor CRF(2) receptor, the orexin OX(1) receptor and the CRF(2)-OX(1) heteroreceptor complex. Neuropharmacology. 2019;152:102–111. doi: 10.1016/j.neuropharm.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Nguyen L., Scandinaro A.L., Matsumoto R.R. Deuterated (d6)-dextromethorphan elicits antidepressant-like effects in mice. Pharmacol. Biochem. Behav. 2017;161:30–37. doi: 10.1016/j.pbb.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Nishikawa H., Hashino A., Kume T., Katsuki H., Kaneko S., Akaike A. Involvement of direct inhibition of NMDA receptors in the effects of σ- receptor ligands on glutamate neurotoxicity in vitro. Eur. J. Pharmacol. 2000;404:41–48. doi: 10.1016/S0014-2999(00)00595-1. [DOI] [PubMed] [Google Scholar]
- Noda A., Noda Y., Kamei H., Ichihara K., Mamiya T., Nagai T., Sugiura S., Furukawa H., Nabeshima T. Phencyclidine impairs latent learning in mice: interaction between glutamatergic systems and sigma(1) receptors. Neuropsychopharmacology. 2001;24:451–460. doi: 10.1016/S0893-133X(00)00192-5. [DOI] [PubMed] [Google Scholar]
- O’Connell A.W., Earley B., Leonard B.E. The σ ligand JO 1784 prevents trimethyltin-induced behavioural and σ-receptor dysfunction in the rat. Pharmacol. Toxicol. 1996;78:296–302. doi: 10.1111/j.1600-0773.1996.tb01378.x. [DOI] [PubMed] [Google Scholar]
- Ohno M., Watanabe S. Intrahippocampal administration of (+)-SKF 10,047, a sigma ligand, reverses MK-801-induced impairment of working memory in rats. Brain Res. 1995;684:237–242. doi: 10.1016/0006-8993(95)00489-d. [DOI] [PubMed] [Google Scholar]
- Ono Y., Tanaka H., Takata M., Nagahara Y., Noda Y., Tsuruma K., Shimazawa M., Hozumi I., Hara H. SA4503, a sigma-1 receptor agonist, suppresses motor neuron damage in in vitro and in vivo amyotrophic lateral sclerosis models. Neurosci. Lett. 2014;559:174–178. doi: 10.1016/j.neulet.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Ortega-Roldan J.L., Ossa F., Schnell J.R. Characterization of the human sigma-1 receptor chaperone domain structure and binding immunoglobulin protein (BiP) interactions. J. Biol. Chem. 2013;288:21448–21457. doi: 10.1074/jbc.M113.450379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovalle S., Andreu F., Pérez M.P., Zamanillo D., Guitart X. Effect of the novel sigma1 receptor ligand and putative atypical antipsychotic E-5842 on BDNF mRNA expression in the rat brain. Neuroreport. 2002;13:2345–2348. doi: 10.1097/00001756-200212030-00035. [DOI] [PubMed] [Google Scholar]
- Pabba M., Wong A.Y.C., Ahlskog N., Hristova E., Biscaro D., Nassrallah W., Ngsee J.K., Snyder M., Beique J.C., Bergeron R. NMDA receptors are upregulated and trafficked to the plasma membrane after sigma-1 receptor activation in the rat hippocampus. J. Neurosci. 2014;34:11325–11338. doi: 10.1523/JNEUROSCI.0458-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A., Hajipour A.R., Fontanilla D., Ramachandran S., Chu U.B., Mavlyutov T., Ruoho A.E. Identification of regions of the α-1 receptor ligand binding site using a novel photoprobe. Mol. Pharmacol. 2007;72:921–933. doi: 10.1124/mol.107.038307. [DOI] [PubMed] [Google Scholar]
- Pal A., Fontanilla D., Gopalakrishnan A., Chae Y.K., Markley J.L., Ruoho A.E. The sigma-1 receptor protects against cellular oxidative stress and activates antioxidant response elements. Eur. J. Pharmacol. 2012;682:12–20. doi: 10.1016/j.ejphar.2012.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C.P., Mahen R., Schnell E., Djamgoz M.B.A., Aydar E. Sigma-1 receptors bind cholesterol and remodel lipid rafts in breast cancer cell lines. Cancer Res. 2007;67:11166–11175. doi: 10.1158/0008-5472.CAN-07-1771. [DOI] [PubMed] [Google Scholar]
- Pande A., Geneve J., Scherrer B. Igmesine, a novel sigma ligand, has antidepressant properties. Congr. Coll. Int. Neuro-Psychopharmacologicum. 1998 [Google Scholar]
- Pande A.C., Genève J., Scherrer B., Smith F., Leadbetter R.A., de Meynard C. A placebo-controlled trial of igmesine in the treatment of major depression. Eur. Neuropsychopharmacol. 1999;9:138. [Google Scholar]
- Pepeu G. Mild cognitive impairment: animal models. Dialogues Clin. Neurosci. 2004;6:369–377. doi: 10.31887/DCNS.2004.6.4/gpepeu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peviani M., Salvaneschi E., Bontempi L., Petese A., Manzo A., Rossi D., Salmona M., Collina S., Bigini P., Curti D. Neuroprotective effects of the Sigma-1 receptor (S1R) agonist PRE-084, in a mouse model of motor neuron disease not linked to SOD1 mutation. Neurobiol. Dis. 2014;62:218–232. doi: 10.1016/j.nbd.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Phan V.L., Su T.-P., Privat A., Maurice T. Modulation of steroidal levels by adrenalectomy/castration and inhibition of neurosteroid synthesis enzymes affect σ1 receptor-mediated behaviour in mice. Eur. J. Neurosci. 1999;11:2385–2396. doi: 10.1046/j.1460-9568.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- Phan V.L., Urani A., Romieu P., Maurice T. Strain differences in σ1 receptor-mediated behaviours are related to neurosteroid levels. Eur. J. Neurosci. 2002;15:1523–1534. doi: 10.1046/j.1460-9568.2002.01989.x. [DOI] [PubMed] [Google Scholar]
- Phan V.L., Urani A., Sandillon F., Privat A., Maurice T. Preserved sigma1 (σ1) receptor expression and behavioral efficacy in the aged C57BL/6 mouse. Neurobiol. Aging. 2003;24:865–881. doi: 10.1016/S0197-4580(02)00231-2. [DOI] [PubMed] [Google Scholar]
- Piantadosi C.A., Zhang J., Levin E.D., Folz R.J., Schmechel D.E. Apoptosis and delayed neuronal damage after carbon monoxide poisoning in the rat. Exp. Neurol. 1997;147:103–114. doi: 10.1006/exnr.1997.6584. [DOI] [PubMed] [Google Scholar]
- Pozdnyakova N., Krisanova N., Dudarenko M., Vavers E., Zvejniece L., Dambrova M., Borisova T. Inhibition of sigma-1 receptors substantially modulates GABA and glutamate transport in presynaptic nerve terminals. Exp. Neurol. 2020;333 doi: 10.1016/j.expneurol.2020.113434. [DOI] [PubMed] [Google Scholar]
- Pytka K., Partyka A., Jastrzębska-Więsek M., Siwek A., Głuch-Lutwin M., Mordyl B., Kazek G., Rapacz A., Olczyk A., Gałuszka A., Błachuta M., Waszkielewicz A., Marona H., Sapa J., Filipek B., Wesołowska A. Antidepressant- and anxiolytic-like effects of new dual 5-HT1A and 5-HT7 antagonists in animal models. PLoS One. 2015;10 doi: 10.1371/journal.pone.0142499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytka K., Gawlik K., Pawlica-Gosiewska D., Witalis J., Waszkielewicz A. HBK-14 and HBK-15 with antidepressant-like and/or memory-enhancing properties increase serotonin levels in the hippocampus after chronic treatment in mice. Metab. Brain Dis. 2017;32:547–556. doi: 10.1007/s11011-016-9932-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytka K., Głuch-Lutwin M., Kotańska M., Żmudzka E., Jakubczyk M., Waszkielewicz A., Janiszewska P., Walczak M. HBK-15 protects mice from stress-induced behavioral disturbances and changes in corticosterone, BDNF, and NGF levels. Behav. Brain Res. 2017;333:54–66. doi: 10.1016/j.bbr.2017.06.032. [DOI] [PubMed] [Google Scholar]
- Pytka K., Głuch-Lutwin M., Kotańska M., Waszkielewicz A., Kij A., Walczak M. Single administration of HBK-15—A triple 5-HT1A, 5-HT7, and 5-HT3 receptor antagonist—Reverses depressive-like behaviors in mouse model of depression induced by corticosterone. Mol. Neurobiol. 2018;55:3931–3945. doi: 10.1007/s12035-017-0605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan N.K., Marosi K., Nyakas C.J., Kwizera C., Elsinga P.H., Ishiwata K., Luiten P.G.M., Dierckx R.A.J.O., van Waarde A. Altered sigma-1 receptor expression in two animal models of cognitive impairment. Mol. Imaging Biol. 2015;17:231–238. doi: 10.1007/s11307-014-0780-x. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan N.K., Schepers M., Luurtsema G., Nyakas C.J., Elsinga P.H., Ishiwata K., Dierckx R.A.J.O., van Waarde A. Cutamesine overcomes REM sleep deprivation-induced memory loss: relationship to sigma-1 receptor occupancy. Mol. Imaging Biol. 2015;17:364–372. doi: 10.1007/s11307-014-0808-2. [DOI] [PubMed] [Google Scholar]
- Raval A.P., Dave K.R., Mochly-Rosen D., Sick T.J., Pérez-Pinzón M.A. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J. Neurosci. 2003;23:384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy D.S., Kaur G., Kulkarni S.K. Sigma (σ1) receptor mediated antidepressant-like effects of neurosteroids in the Porsolt forced swim test. Neuroreport. 1998;9:3069–3073. doi: 10.1097/00001756-199809140-00028. [DOI] [PubMed] [Google Scholar]
- Ring R.M., Regan C.M. Captodiamine, a putative antidepressant, enhances hypothalamic BDNF expression in vivo by synergistic 5-HT2c receptor antagonism and sigma-1 receptor agonism. J. Psychopharmacol. 2013;27:930–939. doi: 10.1177/0269881113497614. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Muñoz M., Cortés-Montero E., Pozo-Rodrigálvarez A., Sánchez-Blázquez P., Garzón-Niño J. The ON:OFF switch, σ1R-HINT1 protein, controls GPCR-NMDA receptor cross-regulation: implications in neurological disorders. Oncotarget. 2015;6:35458–35477. doi: 10.18632/oncotarget.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogóz Z., Skuza G. Mechanism of synergistic action following co-treatment with pramipexole and fluoxetine or sertraline in the forced swimming test in rats. Pharmacol. Rep. 2006;58:493–500. [PubMed] [Google Scholar]
- Romieu P., Phan V.L., Martin-Fardon R., Maurice T. Involvement of the sigma(1) receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology. 2002;26:444–455. doi: 10.1016/S0893-133X(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Rosen D.A., Seki S.M., Fernández-Castañeda A., Beiter R.M., Eccles J.D., Woodfolk J.A., Gaultier A. Modulation of the sigma-1 receptor-IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Sci. Transl. Med. 2019;11 doi: 10.1126/scitranslmed.aau5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman S.M., Herdener N., Frankola K.A., Mughal M.R., Mattson M.P. Chronic mild sleep restriction accentuates contextual memory impairments, and accumulations of cortical Aβ and pTau in a mouse model of Alzheimer’s disease. Brain Res. 2013;1529:200–208. doi: 10.1016/j.brainres.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui M., Rossino G., Coniglio S., Monteleone S., Scuteri A., Malacrida A., Rossi D., Catenacci L., Sorrenti M., Paolillo M., Curti D., Venturini L., Schepmann D., Wünsch B., Liedl K.R., Cavaletti G., Pace V., Urban E., Collina S. Identification of dual Sigma1 receptor modulators/acetylcholinesterase inhibitors with antioxidant and neurotrophic properties, as neuroprotective agents. Eur. J. Med. Chem. 2018;158:353–370. doi: 10.1016/j.ejmech.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Ryskamp D., Wu J., Geva M., Kusko R., Grossman I., Hayden M., Bezprozvanny I. The sigma-1 receptor mediates the beneficial effects of pridopidine in a mouse model of Huntington disease. Neurobiol. Dis. 2017;97:46–59. doi: 10.1016/j.nbd.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskamp D., Wu L., Wu J., Kim D., Rammes G., Geva M., Hayden M., Bezprozvanny I. Pridopidine stabilizes mushroom spines in mouse models of Alzheimer’s disease by acting on the sigma-1 receptor. Neurobiol. Dis. 2019;124:489–504. doi: 10.1016/j.nbd.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabeti J., Gruol D. Emergence of NMDAR-independent long-term potentiation at hippocampal CA1 synapses following early adolescent exposure to chronic intermittent ethanol: role for sigma-receptors. Hippocampus. 2008;18:148–168. doi: 10.1002/hipo. [DOI] [PubMed] [Google Scholar]
- Sabeti J., Nelson T.E., Purdy R.H., Gruol D.L. Steroid pregnenolone sulfate enhances NMDA-receptor-independent long-term potentiation at hippocampal CA1 synapses: role for L-type calcium channels and sigma-receptors. Hippocampus. 2007;17:349–369. doi: 10.1002/hipo.20273. [DOI] [PubMed] [Google Scholar]
- Sabino V., Cottone P., Parylak S.L., Steardo L., Zorrilla E.P. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav. Brain Res. 2009;198:472–476. doi: 10.1016/j.bbr.2008.11.036.Sigma-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambo D.O., Lin M., Owens A., Lebowitz J.J., Richardson B., Jagnarine D.A., Shetty M., Rodriquez M., Alonge T., Ali M., Katz J., Yan L., Febo M., Henry L.K., Bruijnzeel A.W., Daws L., Khoshbouei H. The sigma-1 receptor modulates methamphetamine dysregulation of dopamine neurotransmission. Nat. Commun. 2017;8:2228. doi: 10.1038/s41467-017-02087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Blázquez P., Rodríguez-Muñoz M., Herrero-Labrador R., Burgueño J., Zamanillo D., Garzón J. The calcium-sensitive sigma-1 receptor prevents cannabinoids from provoking glutamate NMDA receptor hypofunction: implications in antinociception and psychotic diseases. Int. J. Neuropsychopharmacol. 2014;17:1943–1955. doi: 10.1017/S1461145714000029. [DOI] [PubMed] [Google Scholar]
- Sánchez-Blázquez P., Pozo-Rodrigálvarez A., Merlos M., Garzón J. The sigma-1 receptor antagonist, S1RA, reduces stroke damage, ameliorates post-stroke neurological deficits and suppresses the overexpression of MMP-9. Mol. Neurobiol. 2018;55:4940–4951. doi: 10.1007/s12035-017-0697-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H.R., Zheng S., Gurpinar E., Koehl A., Manglik A., Kruse A.C. Crystal structure of the human σ1 receptor. Nature. 2016:1–16. doi: 10.1038/nature17391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H.R., Betz R.M., Dror R.O., Kruse A.C. Structural basis for σ1 receptor ligand recognition. Nat. Struct. Mol. Biol. 2018;25:981–987. doi: 10.1038/s41594-018-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminerio M.J., Hansen R., Kaushal N., Zhang H.T., McCurdy C.R., Matsumoto R.R. The evaluation of AZ66, an optimized sigma receptor antagonist, against methamphetamine-induced dopaminergic neurotoxicity and memory impairment in mice. Int. J. Neuropsychopharmacol. 2013;16:1033–1044. doi: 10.1017/S1461145712000831. [DOI] [PubMed] [Google Scholar]
- Senda T., Matsuno K., Okamoto K., Kobayashi T., Nakata K., Mita S. Ameliorating effect of SA4503, a novel σ1 receptor agonist, on memory impairments induced by cholinergic dysfunction in rats. Eur. J. Pharmacol. 1996;315:1–10. doi: 10.1016/S0014-2999(96)00572-9. [DOI] [PubMed] [Google Scholar]
- Senda T., Matsuno K., Kobayashi T., Mita S. Reduction of the scopolamine-induced impairment of passive-avoidance performance by σ receptor agonist in mice. Physiol. Behav. 1997;61:257–264. doi: 10.1016/S0031-9384(96)00447-7. [DOI] [PubMed] [Google Scholar]
- Senda T., Matsuno K., Kobayashi T., Nakazawa M., Nakata K., Mita S. Ameliorative effect of SA4503, a novel cognitive enhancer, on the basal forebrain lesion-induced impairment of the spatial learning performance in rats. Pharmacol. Biochem. Behav. 1998;59:129–134. doi: 10.1016/s0091-3057(97)00385-7. [DOI] [PubMed] [Google Scholar]
- Senda T., Mita S., Kaneda K., Kikuchi M., Akaike A. Effect of SA4503, a novel σ1 receptor agonist, against glutamate neurotoxicity in cultured rat retinal neurons. Eur. J. Pharmacol. 1998;342:105–111. doi: 10.1016/S0014-2999(97)01450-7. [DOI] [PubMed] [Google Scholar]
- Sha S., Qu W.J., Li L., Lu Z.H., Chen Lei, Yu W.F., Chen Ling. Sigma-1 receptor knockout impairs neurogenesis in dentate gyrus of adult hippocampus via down-regulation of nmda receptors. CNS Neurosci. Ther. 2013;19:705–713. doi: 10.1111/cns.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha S., Hong J., Qu W.J., Lu Z.H., Li L., Yu W.F., Chen L. Sex-related neurogenesis decrease in hippocampal dentate gyrus with depressive-like behaviors in sigma-1 receptor knockout mice. Eur. Neuropsychopharmacol. 2015;25:1275–1286. doi: 10.1016/j.euroneuro.2015.04.021. [DOI] [PubMed] [Google Scholar]
- Shi J.-J., Jiang Q.-H., Zhang T.-N., Sun H., Shi W.-W., Gunosewoyo H., Yang F., Tang J., Pang T., Yu L.-F. Sigma-1 receptor agonist TS-157 improves motor functional recovery by promoting neurite outgrowth and pERK in rats with focal cerebral ischemia. Molecules. 2021;26 doi: 10.3390/molecules26051212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu S., Katsuki H., Takenaka C., Tomita M., Kume T., Kaneko S., Akaike A. Sigma receptor ligands attenuate N-methyl-D-aspartate cytotoxicity in dopaminergic neurons of mesencephalic slice cultures. Eur. J. Pharmacol. 2000;388:139–146. doi: 10.1016/s0014-2999(99)00852-3. [DOI] [PubMed] [Google Scholar]
- Shin E.J., Nah S.Y., Chae J.S., Bing G., Shin S.W., Yen T.P.H., Baek I.H., Kim W.K., Maurice T., Nabeshima T., Kim H.C. Dextromethorphan attenuates trimethyltin-induced neurotoxicity via σ1 receptor activation in rats. Neurochem. Int. 2007;50:791–799. doi: 10.1016/j.neuint.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Shin S.M., Wang F., Qiu C., Itson-Zoske B., Hogan Q.H., Yu H. Sigma-1 receptor activity in primary sensory neurons is a critical driver of neuropathic pain. Gene Ther. 2020:20–27. doi: 10.1038/s41434-020-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y., Tagashira H., Bhuiyan M.S., Hasegawa H., Kanai H., Zhang C., Han F., Fukunaga K. Corticosteroids mediate heart failure-induced depression through reduced σ1-receptor expression. PLoS One. 2016;11:1–19. doi: 10.1371/journal.pone.0163992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuza G. Ethanol withdrawal-induced depressive symptoms in animals and therapeutic potential of sigma1 receptor ligands. Pharmacol. Rep. 2013;65:1681–1687. doi: 10.1016/s1734-1140(13)71530-5. [DOI] [PubMed] [Google Scholar]
- Skuza G., Rogóz Z. Effects of combined treatment with selective σ ligands and amantadine in the forced swimming test in rats. Pol. J. Pharmacol. 2002;54:699–702. [PubMed] [Google Scholar]
- Skuza G., Rogóz Z. The synergistic effect of selective sigma receptor agonists and uncompetitive NMDA receptor antagonists in the forced swim test in rats. J. Physiol. Pharmacol. 2006;57:217–229. [PubMed] [Google Scholar]
- Skuza G., Rogóz Z. Antidepressant-like effect of combined treatment with selective σ receptor agonists and 5-HT1A receptor agonist in the forced swimming test in rats. Pharmacol. Rep. 2007;59:773–777. [PubMed] [Google Scholar]
- Skuza G., Rogóz Z. Antidepressant-like effect of PRE-084, a selective σ1 receptor agonist, in Albino Swiss and C57BL/6J mice. Pharmacol. Rep. 2009;61:1179–1183. doi: 10.1016/S1734-1140(09)70181-1. [DOI] [PubMed] [Google Scholar]
- Skuza G., Szymańska M., Budziszewska B., Abate C., Berardi F. Effects of PB190 and PB212, new σ receptor ligands, on glucocorticoid receptor-mediated gene transcription in LMCAT cells. Pharmacol. Rep. 2011;63:1564–1568. doi: 10.1016/S1734-1140(11)70722-8. [DOI] [PubMed] [Google Scholar]
- Smith S.B., Duplantier J., Dun Y., Mysona B., Roon P., Martin P.M., Ganapathy V. In vivo protection against retinal neurodegeneration by sigma receptor 1 ligand (+)-pentazocine. Invest. Ophthalmol. Vis. Sci. 2008;49:4154–4161. doi: 10.1167/iovs.08-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M.A., Mccann K., Lalande M.J., Thivierge J., Bergeron R. 2016. Sigma Receptor Type 1-Knockout Mice Show a Mild Deficit in Plastici- ty but no Significant Change in Synaptic Transmission in the CA1 Region of the Hippocampus. [DOI] [PubMed] [Google Scholar]
- Sokabe M., Chen L., Furuya K. Potentiation of glutamate transporter GLT-1 by the neurosteroid DHEA in the rat hippocampal dentate gyrus. J. Physiol. Sci. 2007;57(Suppl) [Google Scholar]
- Squitieri F., Di Pardo A., Favellato M., Amico E., Maglione V., Frati L. Pridopidine, a dopamine stabilizer, improves motor performance and shows neuroprotective effects in Huntington disease R6/2 mouse model. J. Cell. Mol. Med. 2015;19:2540–2548. doi: 10.1111/jcmm.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivats S., Balasuriya D., Pasche M., Vistal G., Edwardson J.M., Taylor C.W., Murrell-Lagnado R.D. Sigma1 receptors inhibit store-operated Ca2+ entry by attenuating coupling of STIM1 to Orai1. J. Cell Biol. 2016;213:65–79. doi: 10.1083/jcb.201506022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T.-P., Hayashi T., Maurice T., Buch S., Ruoho A.E. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol. Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T.-C., Lin S.-H., Lee P.-T., Yeh S.-H., Hsieh T.-H., Chou S.-Y., Su T.-P., Hung J.-J., Chang W.-C., Lee Y.-C., Chuang J.-Y. The sigma-1 receptor-zinc finger protein 179 pathway protects against hydrogen peroxide-induced cell injury. Neuropharmacology. 2016;105:1–9. doi: 10.1016/j.neuropharm.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó Í., Varga V.É., Dvorácskó S., Farkas A.E., Körmöczi T., Berkecz R., Kecskés S., Menyhárt Á., Frank R., Hantosi D., Cozzi N.V., Frecska E., Tömböly C., Krizbai I.A., Bari F., Farkas E. N,N-Dimethyltryptamine attenuates spreading depolarization and restrains neurodegeneration by sigma-1 receptor activation in the ischemic rat brain. Neuropharmacology. 2021;192 doi: 10.1016/j.neuropharm.2021.108612. [DOI] [PubMed] [Google Scholar]
- Tagashira H., Bhuiyan M.S., Fukunaga K. Diverse regulation of IP3 and ryanodine receptors by pentazocine through σ1-receptor in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2013;305:H1201–12. doi: 10.1152/ajpheart.00300.2013. [DOI] [PubMed] [Google Scholar]
- Tagashira H., Zhang C., Lu Y.M., Hasegawa H., Kanai H., Han F., Fukunaga K. Stimulation of σ1-receptor restores abnormal mitochondrial Ca2 + mobilization and ATP production following cardiac hypertrophy. Biochim. Biophys. Acta - Gen. Subj. 2013;1830:3082–3094. doi: 10.1016/j.bbagen.2012.12.029. [DOI] [PubMed] [Google Scholar]
- Tagashira H., Bhuiyan M.S., Shioda N., Fukunaga K. Fluvoxamine rescues mitochondrial Ca2+ transport and ATP production through σ(1)-receptor in hypertrophic cardiomyocytes. Life Sci. 2014;95:89–100. doi: 10.1016/j.lfs.2013.12.019. [DOI] [PubMed] [Google Scholar]
- Takebayashi M., Hayashi T., Su T.P. Nerve growth factor-induced neurite sprouting in PC12 cells involves σ-1 receptors: implications for antidepressants. J. Pharmacol. Exp. Ther. 2002;303:1227–1237. doi: 10.1124/jpet.102.041970. [DOI] [PubMed] [Google Scholar]
- Tchedre K.T., Huang R.Q., Dibas A., Krishnamoorthy R.R., Dillon G.H., Yorio T. Sigma-1 receptor regulation of voltage-gated calcium channels involves a direct interaction. Investig. Ophthalmol. Vis. Sci. 2008;49:4993–5002. doi: 10.1167/iovs.08-1867. [DOI] [PubMed] [Google Scholar]
- Terada K., Izumo N., Suzuki B., Karube Y., Morikawa T., Ishibashi Y., Kameyama T., Chiba K., Sasaki N., Iwata K., Matsuzaki H., Manabe T. Fluvoxamine moderates reduced voluntary activity following chronic dexamethasone infusion in mice via recovery of BDNF signal cascades. Neurochem. Int. 2014;69:9–13. doi: 10.1016/j.neuint.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Terada K., Migita K., Matsushima Y., Sugimoto Y., Kamei C., Matsumoto T., Mori M., Matsunaga K., Takata J., Karube Y. Cholinesterase inhibitor rivastigmine enhances nerve growth factor-induced neurite outgrowth in PC12 cells via sigma-1 and sigma-2 receptors. PLoS One. 2018;13:1–16. doi: 10.1371/journal.pone.0209250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J.D., Longen C.G., Oyer H.M., Chen N., Maher C.M., Salvino J.M., Kania B., Anderson K.N., Ostrander W.F., Knudsen K.E., Kim F.J. Sigma1 targeting to suppress aberrant androgen receptor signaling in prostate cancer. Cancer Res. 2017;77:2439–2452. doi: 10.1158/0008-5472.CAN-16-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottori K., Nakai M., Uwahodo Y., Miwa T., Yamada S., Oshiro Y., Kikuchi T., Altar C. Attenuation of scopolamine-induced and age-associated memory impairments by the sigma and 5-hydroxytryptamine(1A) receptor agonist OPC-14523 (1-[3-[4-(3-chlorophenyl)-1-piperazinyl]propyl]-5-methoxy-3,4-dihydro-2[1H]-quinolinone monomethanesulfonate) J. Pharmacol. Exp. Ther. 2002;301:249–257. doi: 10.1124/jpet.301.1.249. [DOI] [PubMed] [Google Scholar]
- Tsai S.Y., Hayashi T., Harvey B.K., Wang Y., Wu W.W., Shen R.F., Zhang Y., Becker K.G., Hoffer B.J., Su T.P. Sigma-1 receptors regulate hippocampal dendritic spine formation via a free radical-sensitive mechanism involving Rac1·GTP pathway. Proc. Natl. Acad. Sci. U. S. A. 2009;106:22468–22473. doi: 10.1073/pnas.0909089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S.-Y.A., Chuang J.-Y., Tsai M.-S., Wang X.-F., Xi Z.-X., Hung J.-J., Chang W.-C., Bonci A., Su T.-P. Sigma-1 receptor mediates cocaine-induced transcriptional regulation by recruiting chromatin-remodeling factors at the nuclear envelope. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E6562–70. doi: 10.1073/pnas.1518894112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukai M., Maeda H., Nanya Y., Kameyama T., Matsuno K. Beneficial effects of acute and repeated administrations of σ receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol. Biochem. Behav. 1998;61:247–252. doi: 10.1016/S0091-3057(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Urani A., Privat A., Maurice T. The modulation by neurosteroids of the scopolamine-induced learning impairment in mice involves an interaction with sigma1 (σ1) receptors. Brain Res. 1998;799:64–77. doi: 10.1016/S0006-8993(98)00469-7. [DOI] [PubMed] [Google Scholar]
- Urani A., Roman F.J., Phan V.L., Su T.-P., Maurice T. The antidepressant-like effect induced by sigma(1)-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J. Pharmacol. Exp. Ther. 2001;298:1269–1279. [PubMed] [Google Scholar]
- Urani A., Romieu P., Portales-Casamar E., Roman F.J., Maurice T. The antidepressant-like effect induced by the sigma1 (σ1) receptor agonist igmesine involves modulation of intracellular calcium mobilization. Psychopharmacology (Berl). 2002;163:26–35. doi: 10.1007/s00213-002-1150-y. [DOI] [PubMed] [Google Scholar]
- Urani A., Romieu P., Roman F.J., Maurice T. Enhanced antidepressant effect of sigma1 (σ1) receptor agonists in β25-35-amyloid peptide-treated mice. Behav. Brain Res. 2002;134:239–247. doi: 10.1016/S0166-4328(02)00033-5. [DOI] [PubMed] [Google Scholar]
- Urani A., Romieu P., Roman F.J., Yamada K., Noda Y., Kamei H., Manh Tran H., Nagai T., Nabeshima T., Maurice T. Enhanced antidepressant efficacy of σ1 receptor agonists in rats after chronic intracerebroventricular infusion of β-amyloid-(1-40) protein. Eur. J. Pharmacol. 2004;486:151–161. doi: 10.1016/j.ejphar.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Urfer R., Moebius H.J., Skoloudik D., Santamarina E., Sato W., Mita S., Muir K.W. Phase II trial of the Sigma-1 receptor agonist cutamesine (SA4503) for recovery enhancement after acute ischemic stroke. Stroke. 2014;45:3304–3310. doi: 10.1161/STROKEAHA.114.005835. [DOI] [PubMed] [Google Scholar]
- van der Staay F.J., Rutten K., Erb C., Blokland A. Effects of the cognition impairer MK-801 on learning and memory in mice and rats. Behav. Brain Res. 2011;220:215–229. doi: 10.1016/j.bbr.2011.01.052. [DOI] [PubMed] [Google Scholar]
- Velazquez R., Ferreira E., Knowles S., Fux C., Rodin A., Winslow W., Oddo S. Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell. 2019;18:1–11. doi: 10.1111/acel.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetel S., Foucault-Fruchard L., Tronel C., Buron F., Vergote J., Bodard S., Routier S., Sérrière S., Chalon S. Neuroprotective and anti-inflammatory effects of a therapy combining agonists of nicotinic α7 and σ1 receptors in a rat model of Parkinson’s disease. Neural Regen. Res. 2021;16:1099–1104. doi: 10.4103/1673-5374.300451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villard V., Espallergues J., Keller E., Alkam T., Nitta A., Yamada K., Nabeshima T., Vamvakides A., Maurice T. Antiamnesic and neuroprotective effects of the aminotetrahydrofuran derivative ANAVEX1-41 against amyloid beta(25-35)-induced toxicity in mice. Neuropsychopharmacol. 2009;34:1552–1566. doi: 10.1038/npp.2008.212. [DOI] [PubMed] [Google Scholar]
- Villard V., Espallergues J., Keller E., Vamvakides A., Maurice T. Anti-amnesic and neuroprotective potentials of the mixed muscarinic receptor/sigma1 (σ1) ligand ANAVEX2-73, a novel aminotetrahydrofuran derivative. J. Psychopharmacol. 2011;25:1101–1117. doi: 10.1177/0269881110379286. [DOI] [PubMed] [Google Scholar]
- Villard V., Meunier J., Chevallier N., Maurice T. Pharmacological interaction with the sigma1 (σ1)-receptor in the acute behavioral effects of antidepressants. J. Pharmacol. Sci. 2011;115:279–292. doi: 10.1254/jphs.10191fp. [DOI] [PubMed] [Google Scholar]
- Vorhees C.V., Williams M.T. Assessing spatial learning and memory in rodents. ILAR J. 2014;55:310–332. doi: 10.1093/ilar/ilu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Smith S.B. A novel mechanism of sigma 1 receptor neuroprotection: modulation of miR-214-3p. Adv. Exp. Med. Biol. 2019;1185:463–467. doi: 10.1007/978-3-030-27378-1_76. [DOI] [PubMed] [Google Scholar]
- Wang H.-H., Chien J.-W., Chou Y.-C., Liao J.-F., Chen C.-F. Anti-amnesic effect of dimemorfan in mice. Br. J. Pharmacol. 2003;138:941–949. doi: 10.1038/sj.bjp.0705117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Noda Y., Tsunekawa H., Zhou Y., Miyazaki M., Senzaki K., Nitta A., Nabeshima T. Role of N-methyl-D-aspartate receptors in antidepressant-like effects of σ1 receptor agonist 1-(3,4-dimethoxyphenethyl)-4-(3- phenylpropyl)piperazine dihydrochloride (SA-4503) in olfactory bulbectomized rats. J. Pharmacol. Exp. Ther. 2007;322:1305–1314. doi: 10.1124/jpet.107.124685. [DOI] [PubMed] [Google Scholar]
- Wang J., Mack A.L., Coop A., Matsumotoa R.R. Novel sigma (σ) receptor agonists produce antidepressant-like effects in mice. Eur. Neuropsychopharmacol. 2007;17:708–716. doi: 10.1016/j.euroneuro.2007.02.007.Novel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Shanmugam A., Markand S., Zorrilla E., Ganapathy V., Smith S.B. Sigma 1 receptor regulates the oxidative stress response in primary retinal Müller glial cells via NRF2 signaling and system xc(-), the Na(+)-independent glutamate-cystine exchanger. Free Radic. Biol. Med. 2015;86:25–36. doi: 10.1016/j.freeradbiomed.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Guo L., Jiang H.F., Zheng L.T., Zhang A., Zhen X.C. Allosteric modulation of sigma-1 receptors elicits rapid antidepressant activity. CNS Neurosci. Ther. 2016;22:368–377. doi: 10.1111/cns.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Timberlake M.A., 2nd, Prall K., Dwivedi Y. The recent progress in animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;77:99–109. doi: 10.1016/j.pnpbp.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang Y., Wang C., Zhang X., Wang Z., Liang X., Alachkar A., Civelli O. A natural product with high affinity to sigma and 5-HT7 receptors as novel therapeutic drug for negative and cognitive symptoms of schizophrenia. Neurochem. Res. 2019;44:2536–2545. doi: 10.1007/s11064-019-02873-7. [DOI] [PubMed] [Google Scholar]
- Wang Y., Jiang Hfeng, Ni J., Guo L. Pharmacological stimulation of sigma-1 receptor promotes activation of astrocyte via ERK1/2 and GSK3β signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2019;392:801–812. doi: 10.1007/s00210-019-01632-3. [DOI] [PubMed] [Google Scholar]
- Wang J., Xiao H., Barwick S., Liu Y., Smith S.B. Optimal timing for activation of sigma 1 receptor in the Pde6b(rd10)/J (rd10) mouse model of retinitis pigmentosa. Exp. Eye Res. 2021;202 doi: 10.1016/j.exer.2020.108397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Gao C., Gao T., Zhao L., Zhu S., Guo L. Plasma exosomes from depression ameliorate inflammation-induced depressive-like behaviors via sigma-1 receptor delivery. Brain Behav. Immun. 2021;94:225–234. doi: 10.1016/j.bbi.2021.02.004. [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhao C.S. Sigma-1 receptor activation ameliorates LPS-induced NO production and ROS formation through the Nrf2/HO-1 signaling pathway in cultured astrocytes. Neurosci. Lett. 2019;711 doi: 10.1016/j.neulet.2019.134387. [DOI] [PubMed] [Google Scholar]
- Waszkielewicz A.M., Pytka K., Rapacz A., Wełna E., Jarzyna M., Satała G., Bojarski A., Sapa J., Zmudzki P., Filipek B., Marona H. Synthesis and evaluation of antidepressant-like activity of some 4-substituted 1-(2-methoxyphenyl) piperazine derivatives. Chem. Biol. Drug Des. 2014;85:326–335. doi: 10.1111/cbdd.12394. [DOI] [PubMed] [Google Scholar]
- Weber F., Wünsch B. Medicinal chemistry of σ(1) receptor ligands: pharmacophore models, synthesis, structure affinity relationships, and pharmacological applications. Handb. Exp. Pharmacol. 2017;244:51–79. doi: 10.1007/164_2017_33. [DOI] [PubMed] [Google Scholar]
- Wu Z., Bowen W.D. Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J. Biol. Chem. 2008;283:28198–28215. doi: 10.1074/jbc.M802099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Song T.-B., Li Y.-J., He K.-S., Ge L., Wang L.-R. Prenatal restraint stress impairs learning and memory and hippocampal PKCbeta1 expression and translocation in offspring rats. Brain Res. 2007;1141:205–213. doi: 10.1016/j.brainres.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Wu Z., Li L., Zheng L.-T., Xu Z., Guo L., Zhen X. Allosteric modulation of sigma-1 receptors by SKF83959 inhibits microglia-mediated inflammation. J. Neurochem. 2015;134:904–914. doi: 10.1111/jnc.13182. [DOI] [PubMed] [Google Scholar]
- Wu Y., Bai X., Li X., Zhu C., Wu Z.P. Overexpression of sigma-1 receptor in MCF-7 cells enhances proliferation via the classic protein kinase C subtype signaling pathway. Oncol. Lett. 2018;16:6763–6769. doi: 10.3892/ol.2018.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Ji X.F., Chi T.Y., Liu P., Jin G., Gu S.L., Zou L.B. Sigma 1 receptor activation regulates brain-derived neurotrophic factor through NR2A-CaMKIV-TORC1 pathway to rescue the impairment of learning and memory induced by brain ischaemia/reperfusion. Psychopharmacology (Berl.) 2015;232:1779–1791. doi: 10.1007/s00213-014-3809-6. [DOI] [PubMed] [Google Scholar]
- Xu Q., Ji X.F., Chi T.Y., Liu P., Jin G., Chen L., Zou L.B. Sigma-1 receptor in brain ischemia/reperfusion: possible role in the NR2A-induced pathway to regulate brain-derived neurotrophic factor. J. Neurol. Sci. 2017;376:166–175. doi: 10.1016/j.jns.2017.03.027. [DOI] [PubMed] [Google Scholar]
- Yabuki Y., Shinoda Y., Izumi H., Ikuno T., Shioda N., Fukunaga K. Dehydroepiandrosterone administration improves memory deficits following transient brain ischemia through sigma-1 receptor stimulation. Brain Res. 2015;1622:102–113. doi: 10.1016/j.brainres.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Yagasaki Y., Numakawa T., Kumamaru E., Hayashi T., Su T.P., Kunugi H. Chronic antidepressants potentiate via sigma-1 receptors the brain-derived neurotrophic factor-induced signaling for glutamate release. J. Biol. Chem. 2006;281:12941–12949. doi: 10.1074/jbc.M508157200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Shioda N., Yabuki Y., Zhang C., Han F., Fukunaga K. SA4503, A potent sigma-1 receptor ligand, ameliorates synaptic abnormalities and cognitive dysfunction in a mouse model of ATR-X syndrome. Int. J. Mol. Sci. 2018;19:1–15. doi: 10.3390/ijms19092811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Jin J., Watanabe S. Characteristics of memory dysfunction in olfactory bulbectomized rats and the effects of cholinergic drugs. Behav. Brain Res. 1997;83:57–62. doi: 10.1016/s0166-4328(97)86046-9. [DOI] [PubMed] [Google Scholar]
- Yang S., Bhardwaj A., Cheng J., Alkayed N.J., Hurn P.D., Kirsch J.R. Sigma receptor agonists provide neuroprotection in vitro by preserving bcl-2. Anesth. Analg. 2007;104:1179–1184. doi: 10.1213/01.ane.0000260267.71185.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z.J., Carter E.L., Torbey M.T., Martin L.J., Koehler R.C. Sigma receptor ligand 4-phenyl-1-(4-phenylbutyl)-piperidine modulates neuronal nitric oxide synthase/postsynaptic density-95 coupling mechanisms and protects against neonatal ischemic degeneration of striatal neurons. Exp. Neurol. 2010;221:166–174. doi: 10.1016/j.expneurol.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Chen Lei, Wang H., Xu B., Tomimoto H., Chen Ling. Anti-amnesic effect of neurosteroid PREGS in Aβ25-35- injected mice through σ1 receptor- and α7nAChR-mediated neuroprotection. Neuropharmacology. 2012;63:1042–1050. doi: 10.1016/j.neuropharm.2012.07.035. [DOI] [PubMed] [Google Scholar]
- Yano H., Bonifazi A., Xu M., Guthrie D.A., Schneck S.N., Abramyan A.M., Fant A.D., Hong W.C., Newman A.H., Shi L. Pharmacological profiling of sigma 1 receptor ligands by novel receptor homomer assays. Neuropharmacology. 2018;133:264–275. doi: 10.1016/j.neuropharm.2018.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H., Duan M., Buch S. Cocaine-mediated induction of platelet-derived growth factor: implication for increased vascular permeability. Blood. 2011;117:2538–2547. doi: 10.1182/blood-2010-10-313593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J., Sha S., Chen T., Wang C., Hong J., Jie P., Zhou R., Li L., Sokabe M., Chen L. Sigma-1 (σ1) receptor deficiency reduces β-amyloid25-35-induced hippocampal neuronal cell death and cognitive deficits through suppressing phosphorylation of the NMDA receptor NR2B. Neuropharmacology. 2015;89:215–224. doi: 10.1016/j.neuropharm.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Yoshimura H., Gomita Y., Ueki S. Changes in acetylcholine content in rat brain after bilateral olfactory bulbectomy in relation to mouse-killing behavior. Pharmacol. Biochem. Behav. 1974;2:703–705. doi: 10.1016/0091-3057(74)90041-0. [DOI] [PubMed] [Google Scholar]
- Zhang H., Cuevas J. Sigma receptors inhibit high-voltage-activated calcium channels in rat sympathetic and parasympathetic neurons. J. Neurophysiol. 2002;87:2867–2879. doi: 10.1152/jn.2002.87.6.2867. [DOI] [PubMed] [Google Scholar]
- Zhang H., Cuevas J. Σ receptor activation blocks potassium channels and depresses neuroexcitability in rat intracardiac neurons. J. Pharmacol. Exp. Ther. 2005;313:1387–1396. doi: 10.1124/jpet.105.084152. [DOI] [PubMed] [Google Scholar]
- Zhang B., Wang L., Chen T., Hong J., Sha S., Wang J., Xiao H., Chen L. Sigma-1 receptor deficiency reduces GABAergic inhibition in the basolateral amygdala leading to LTD impairment and depressive-like behaviors. Neuropharmacology. 2017;116:387–398. doi: 10.1016/j.neuropharm.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Zhang K., Zhao Z., Lan L., Wei X., Wang L., Liu X., Yan H., Zheng J. Sigma-1 receptor plays a negative modulation on N-type calcium channel. Front. Pharmacol. 2017;8:1–14. doi: 10.3389/fphar.2017.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Hong J., Zhang T., Wu J., Chen L. Activation of sigma-1 receptor alleviates postpartum estrogen withdrawal-induced “depression” through restoring hippocampal nNOS-NO-CREB activities in mice. Mol. Neurobiol. 2017;54:3017–3030. doi: 10.1007/s12035-016-9872-8. [DOI] [PubMed] [Google Scholar]
- Zhao J., Ha Y., Liou G.I., Gonsalvez G.B., Smith S.B., Bollinger K.E. Sigma receptor ligand, (+)-pentazocine, suppresses inflammatory responses of retinal microglia. Invest. Ophthalmol. Vis. Sci. 2014;55:3375–3384. doi: 10.1167/iovs.13-12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Yu S., Ling Y., Hao S., Liu J. The protective effects of dexmedetomidine against hypoxia/reoxygenation-induced inflammatory injury and permeability in brain endothelial cells mediated by sigma-1 receptor. ACS Chem. Neurosci. 2021;12:1940–1947. doi: 10.1021/acschemneuro.1c00032. [DOI] [PubMed] [Google Scholar]
- Zhemkov V., Geva M., Hayden M.R., Bezprozvanny I. 2021. Sigma-1 Receptor (S1R) Interaction with Cholesterol: Mechanisms of S1R Activation and its Role in Neurodegenerative Diseases; pp. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L.B., Yamada K., Nabeshima T. σ Receptor ligands (+)-SKF10,047 and SA4503 improve dizocilpine-induced spatial memory deficits in rats. Eur. J. Pharmacol. 1998;355:1–10. doi: 10.1016/S0014-2999(98)00464-6. [DOI] [PubMed] [Google Scholar]
- Zou L.B., Yamada K., Sasa M., Nakata Y., Nabeshima T. Effects of σ1 receptor agonist SA4503 and neuroactive steroids on performance in a radial arm maze task in rats. Neuropharmacology. 2000;39:1617–1627. doi: 10.1016/S0028-3908(99)00228-2. [DOI] [PubMed] [Google Scholar]
- Zvejniece L., Vavers E., Svalbe B., Vilskersts R., Domracheva I. 2014. The Cognition-Enhancing Activity of E1R, a Novel Positive Allosteric Modulator of Sigma-1 Receptors. [DOI] [PMC free article] [PubMed] [Google Scholar]