Abstract
Dopamine‐replacing therapies are an effective treatment for the motor aspects of Parkinson's disease. However, its precise effect over the cognitive resting‐state networks is not clear; whether dopaminergic treatment normalizes their functional connectivity‐as in other networks‐ and the links with cognitive decline are presently unknown. We recruited 35 nondemented PD patients and 16 age‐matched controls. Clinical and neuropsychological assessments were performed at baseline, and conversion to dementia was assessed in a 10 year follow‐up. Structural and functional brain imaging were acquired in both the ON and practical OFF conditions. We assessed functional connectivity in both medication states compared to healthy controls, connectivity differences within participants related to the ON/OFF condition, and baseline connectivity of PD participants that converted to dementia compared to those who did not convert. PD participants showed and increased frontoparietal connectivity compared to controls: a pattern of higher connectivity between salience (SN) and default‐mode (DMN) networks both in the ON and OFF states. Within PD patients, this higher SN‐DMN connectivity characterized the participants in the ON state, while within‐DMN connectivity prevailed in the OFF state. Interestingly, participants who converted to dementia also showed higher SN‐DMN connectivity in their baseline ON scans compared to nonconverters. To conclude, PD patients showed higher frontoparietal connectivity in cognitive networks compared to healthy controls, irrespective of medication status, but dopaminergic treatment specifically promoted SN‐DM hyperconnectivity.
Keywords: cognitive networks, dopamine, functional MRI, Parkinson's disease
In this work, Aracil‐Bolaños et al. investigate the effects of dopaminergic treatment over the large‐scale cognitive networks of nondemented Parkinson's disease patients and healthy controls. Baseline frontoparietal connectivity was higher in Parkinson's disease than in controls irrespective of medication, but dopaminergic treatment within patients specifically promoted salience network connectivity.
1. INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative disease characterized by the progressive loss of nigrostriatal dopaminergic neurons. Its clinical hallmarks are the onset of tremor, rigidity, and bradykinesia (Postuma et al., 2015). Given the progressive loss of dopaminergic terminals and the efficacy of dopamine‐replacing therapies (DRTs) on motor symptoms, levodopa has been the mainstay of PD treatment over the past 50 years. However, some nonmotor aspects are not as well addressed by dopaminergic treatments. Chief among them is progressive cognitive impairment and dementia, which affects a majority of PD patients over the disease course (Hely, Reid, Adena, Halliday, & Morris, 2008). PD patients show dysexecutive symptoms from the onset of the disease, but the progressive emergence of posterior cortical disruptions heralds the slide into cognitive impairment (González‐Redondo et al., 2014; Mak et al., 2015; Pagonabarraga & Kulisevsky, 2012). The neural correlates of these early stages have been defined in structural terms: frontal areas exhibit reduced cortical thickness in PD patients compared to healthy controls (HCs), with patients with mild cognitive impairment (PD‐MCI) showing additional losses of gray matter in temporo‐parietal regions (Pagonabarraga, Soriano‐mas, & Llebaria, 2014; Segura et al., 2014). However, we still lack patterns of cognitive network disruption that predate cognitive decline, and structural imaging is not likely to provide them (Lanskey et al., 2018).
Using functional MRI, PD‐MCI has been linked to altered functional connectivity and graph theoretical metrics in diverse studies (Baggio et al., 2015; Sala‐llonch, Baggio, Valldeoriola, & Compta, 2015), and distinct subtypes of cognitive impairment can be outlined using these parameters (Lopes et al., 2016). More recently, metrics of dynamic functional connectivity have been adopted to study changes in time‐dependent components of brain networks, identifying distinct connectivity states related to cognition (Fiorenzato et al., 2019), and links of network dynamics to visuospatial memory (Engels, Vlaar, McCoy, Scherder, & Douw, 2018). Interestingly, the relative increase of frontoparietal functional connectivity in nondemented PD patients, compared to HCs, might point toward compensation or vulnerability features in at‐risk patients (Gorges et al., 2015). These features are distinct at a network level in normal cognition (PD‐NC), and seem to wane in the PD‐MCI patients (Aracil‐Bolaños et al., 2019), disrupting the functional integrity of frontoparietal networks in the absence of gray matter loss (Amboni et al., 2015). Thus, the investigation of early disruptions could provide network signatures of future decline in patients who show no overt clinical signs of cognitive impairment.
These mechanisms might be affected by dopaminergic treatment and therefore the role of DRT has to be accounted for. Studies that jointly analyze functional MRI and quantitative levels of dopamine via FP‐CIT have shown that dopamine‐dependent functional networks comprise motor and cerebellar regions, but also frontoparietal cognitive networks have links to cortical hubs such as the posterior cingulate cortex (Baik et al., 2014). However, while many excellent studies have approached the effects of dopamine depletion on brain topology (Shine et al., 2019), few have employed ON–OFF paradigms to explore resting‐state cognitive networks. A comprehensive review on the role of dopaminergic treatment over functional MRI concluded that the DRT tends to normalize the aberrant connectivity present in the OFF state (Tahmasian et al., 2015), but its effect on cognitive large‐scale networks is not well‐known. While older studies have focused on the acute effects of dopaminergic treatment over the dorsal and ventral connections of the striatum (Macdonald & Monchi, 2011), a perspective that encompasses the diversity of cognitive networks, beyond frontostriatal loops, is still lacking.
To sum up, no study to date has assessed the differences which levodopa causes on large‐scale cognitive networks, such as the default, salience or central executive networks. Furthermore, it is unclear whether these changes might constitute an adaptive mechanism or, on the contrary, could represent a vulnerability trait regarding long term conversion to dementia. Thus, in the present study we explored the role of dopaminergic treatment over cognitive networks by recruiting a group of nondemented PD participants and age‐matched HCs, taking into account dopaminergic treatment by acquiring functional MRI (fMRI) images both in the ON and the OFF conditions. We followed‐up a subgroup of participants and evaluated long‐term conversion to dementia in order to assess the clinical relevance of network connectivity differences.
2. METHODS
2.1. Participants
Forty participants with idiopathic PD regularly attending our Movement Disorders Outparticipant Unit and 16 age‐matched HCs who were willing to participate in this study were prospectively recruited. Inclusion criteria was the diagnosis of PD according to the United Kingdom PD Society Brain Bank (Hughes, Daniel, Kilford, & Lees, 1992) and exclusion criteria were: (a) presence of dementia according to MDS‐PDD Criteria (Emre et al., 2007) and Parkinson's Disease Cognitive Rating Scale (PD‐CRS) total score <64 (de Bobadilla et al., 2013); Hoehn & Yahr scale >III; (b) presence of any other significant psychiatric, neurological, or unstable systemic comorbidities; (c) pathological MRI findings beyond mild white matter hyperintensities; (d) presence of head motion or other MRI artifacts; and (e) inability to tolerate MRI acquisition in the OFF state. Four participants were excluded due to image quality‐checking and one participant because of dementia. All participants provided written informed consent according to the Declaration of Helsinki. The study was approved by the Ethics Committee for Clinical Research at the Hospital de la Santa Creu i Sant Pau, Barcelona.
2.2. Clinical assessment
Participants were assessed using a battery of clinical and neuropsychological tests. All participants were clinically assessed at baseline, together with the MRI acquisition, and a subset of participants who completed follow up were assessed for conversion to dementia in a 10‐year follow‐up (2008–2018 period). Motor status was assessed using the Unified Parkinson's disease rating scale part III (UPDRS‐III). Anxiety and depression were evaluated using the Hospital Anxiety and Depression Scale (HADS). At time of inclusion, global cognitive status was addressed using the Parkinson's disease cognitive rating scale (PD‐CRS) and the Clinical Dementia Rating (CDR). The PD‐CRS comprises nine subtests that assess immediate verbal memory, naming, sustained attention, working memory, unprompted drawing of a clock, copy of a clock, delayed free recall, alternating verbal fluency, and action verbal fluency. The PD‐CRS provides a total score ranging from 0 to 134; cutoff scores <64 and <82 were previously proven to be reliable for the screening of dementia and PD‐MCI, respectively (de Bobadilla et al., 2013; Pagonabarraga et al., 2008; Pagonabarraga, Corcuera‐solano, Vives‐gilabert, Llebaria, & Garcı, 2013). The CDR was used as gold standard for cognitive status in several studies, including the validation study of the PD‐CRS (Pagonabarraga et al., 2008). This instrument assesses cognitive and functional performance in six areas: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care (Hughes, Berg, Danziger, Coben, & Martin, 1982). A CDR of 0 indicates no cognitive deficits, 0.5 indicates very mild cognitive impairment, and 1–3 indicate mild to severe cognitive impairment. As per inclusion criteria, all participants were free of cognitive impairment in the range of dementia and thus, had a PD‐CRS total score >64 and a CDR <1.
All participants were assessed in the practical OFF and ON conditions. For the ON acquisitions, and by virtue of being in a fluctuating stage of the disease, all participants were scanned after they took their levodopa dose in the OFF state in the morning and their best ON was achieved. For the OFF‐state acquisitions, participants observed a strict 12 hr practical OFF period, and they were examined by a trained neurologist prior to the acquisition to ensure that the scans were reflecting a true OFF state.
At follow‐up, the CDR was chosen to determine the global cognitive status of participants. Those with a CDR <1 were considered nondemented, whereas those with CDR equal or above 1 were considered as with major cognitive impairment in the range of dementia.
2.3. MRI acquisition and preprocessing
MRI acquisition was performed on a 1.5 Tesla Signa Excite system (General Electric, Milwaukee, WI) station scanner. A high‐resolution T1‐weighted anatomical image was obtained for each participant using a three‐dimensional fast spoiled gradient inversion‐recovery prepared sequence with 130 contiguous slices (TR, 11.8 ms; TE, 4.2 ms; flip angle, 15°; field of view, 30 cm; 256 × 256 pixel matrix; slice thickness, 1.2 mm).
Resting‐state BOLD images during 6 min were obtained using a functional MRI sequence consisting of gradient recalled acquisition in the steady state (TR = 2,000 ms, TE = 50 ms, flip angle 90°, 64 × 64 pixel matrix, FOV 240 mm, in‐plane voxel size 3.75 × 3.75 mm, slice thickness 4 mm), both in the ON and practical OFF conditions (12 hr withdrawal of dopaminergic medications prior to MRI acquisition).
2.4. Cortical thickness analysis
A standard cortical thickness pipeline was applied. Cortical thickness analysis was performed using the FreeSurfer 6.0 software package (https://surfer.nmr.mgh.harvard.edu/). The specific methods used for cortical reconstruction of T1‐MRI brain images have been described in detail elsewhere (Fischl & Dale, 2000). Briefly, optimized surface deformation models following intensity gradients accurately identify white matter and gray matter boundaries in the cerebral cortex, from which cortical thickness is computed at each vertex of the resulting surface. Finally, the resulting cortical surfaces were normalized to average space and smoothed using a Gaussian kernel of 10 mm FWHH.
2.5. Functional connectivity analysis
Functional imaging preprocessing was performed using CONN v20 software and its standard processing pipeline, described in depth in (Whitfield‐Gabrieli & Nieto‐Castanon, 2012). Briefly, functional scans were first slice‐timing corrected, realigned, and spatially normalized to the Montreal Neurological Institute (MNI) space using co‐registration with the associated anatomical data. Then, resting‐state images were submitted to CONN's standard denoising pipeline, which combines two steps: linear regression of potential confounding effects and temporal band‐pass filtering. For the first step, factors that are identified as potential confounding effects to the estimated BOLD signal are estimated and removed separately for each voxel and for each participant and functional run/session using Ordinary Least Squares regression to project each BOLD signal timeseries to the sub‐space orthogonal to all potential confounding effects. Potential confounding effects used in CONN's default denoising pipeline implement an anatomical component‐based noise correction procedure (aCompCor), and include: (a) Noise components from cerebral white matter and cerebrospinal áreas: potential confounding effects are defined from the observed BOLD signal within each of two anatomically‐defined noise areas computed by applying a one‐voxel binary erosion step to the masks of voxels with values above 50% in white matter and CSF posterior probability maps. Within each area five potential noise components are estimated: (a) the first computed as the average BOLD signal, and the next four computed as the first components in a Principal Component Analysis of the covariance within the subspace orthogonal to the average BOLD signal and all other potential confounding effects. (b) Estimated participant‐motion parameters: a total of 12 potential noise components are defined from the estimated participant‐motion parameters in order to minimize motion related BOLD variability: three translation and three rotation parameters plus their associated first‐order derivatives. (c) Identified outlier scans or scrubbing: a variable number of noise components (one for each identified outlier scan during the outlier identification preprocessing step) are used as potential confounding effects to remove any influence of these outlier scans on the BOLD signal. Potential outlier scans are identified from the observed global BOLD signal and the amount of participant‐motion in the scanner. Acquisitions with framewise displacement above 0.9 mm or global BOLD signal changes above 5 SD are flagged as potential outliers. Framewise displacement is computed at each timepoint by considering a 140 × 180 × 115 mm bounding box around the brain and estimating the largest displacement among six control points placed at the center of this bounding‐box faces. Global BOLD signal change is computed at each timepoint as the change in average BOLD signal within SPM's global‐mean mask scaled to standard deviation units. (d) Constant and first‐order linear session effects, and constant task effects, if applicable.
Then, the obtained data were filtered using a band‐pass filter in the range of 0.01–0.1 Hz in order to focus on slow‐frequency fluctuations while minimizing the influence of physiological, head‐motion, and other noise sources. Filtering is implemented using a discrete cosine transform windowing operation to minimize border effects, and performed after regression to avoid any frequency mismatch in the nuisance regression procedure.
The entire matrix of ROI‐to‐ROI functional connectivity values (using the bivariate correlation measure) was computed for each participant using the set of functional brain networks described in our previous work (Aracil‐Bolaños et al., 2019) and available at https://findlab.stanford.edu/functional_ROIs.html. In particular, we focused on 43 regions of interest (ROIs) located in the default‐mode (DMN), salience (SN), and Central Executive (CEN) networks, and also performed analyses on the 11 ROIs pertaining to the sensorimotor network. This parcellation scheme has been useful to delineate the network‐phenotype link in neurodegenerative disease (Seeley, Crawford, Zhou, Miller, & Greicius, 2009), allowing for a defined triple network psychopathological model (Menon, 2011) that is useful in diseases that show cognitive and affective symptoms, such as PD. Furthermore, this triple network model has been useful in the study of neurodegenerative conditions that span diverse clinical presentations, such as frontotemporal dementia (Chiong et al., 2013) or Alzheimer's disease (Zhou et al., 2010). In PD, dopaminergic dysfunction in the salience network has been linked to cognitive decline (Christopher et al., 2015), and altered connectivity between salience and default‐mode networks has also been associated with cognitive impairment in PD (Peraza et al., 2017) Therefore, this parcellation can be considered suitable for the study of the interaction between neurodegenerative disorders, large‐scale cognitive networks and clinical phenomena.
A ROI‐to‐ROI analysis between all the network components was performed from the Z‐score connectivity matrices of each participant. Both intragroup (PD‐ON vs. PD‐OFF) and intergroup (PD‐ON/OFF vs. Controls)) connectivity patterns were studied and compared. A full description of the cognitive ROI location is provided in Table S3.
2.6. Statistical analyses
For the cortical thickness analysis, a vertexwise generalized linear model was set to compare both nondemented PD participants with HC, and also to compare PD participants who converted to dementia with respect to those who did not. Only clusters surviving p‐value <.05 after multiple comparison correction by permutation‐testing with 10,000 permutations were considered significant.
For the functional connectivity comparisons, we performed two‐sample T‐tests (PD‐ON vs. controls, PD‐OFF vs. controls, PD‐converters vs. PD nonconverters) and a paired T‐test (PD‐ON vs. PD‐OFF) to compare the groups; age, sex, and other demographics were used as covariates of no interest whenever differences were found across groups: sex in PD versus control analyses, and age and PD‐CRS in dementia converters versus nonconverters (see Tables 1 and S1). Finally, in the subset of participants that completed the 10 year follow‐up and had both ON and OFF scans we performed a mixed ANOVA interaction analysis considering both within‐participant conditions (medication status) and between group conditions (converter vs. nonconverter).
TABLE 1.
Clinical and sociodemographic data of the study sample
| Group | Participants | Controls | p‐value |
|---|---|---|---|
| N | 35 | 16 | N/A |
| Age, years | 65 ± 9 | 66.8 ± 7.8 | .45 |
| Sex, % men | 74%, 26/35 | 50%, 8/16 | .06 |
| PD onset, years | 7.3 ± 4.5 | N/A | N/A |
| Education, years | 10 ± 5 | 10 ± 4.8 | .99 |
| MDS UPDRS III |
20 ± 7.1 (ON) 25 ± 10.2 (OFF) |
N/A | N/A |
| Hoehn & Yahr |
1.9 ± 0.3 (ON) 2.2 ± 0.6 (OFF) |
N/A | N/A |
| LEDD | 807 ± 447 | N/A | N/A |
| PD‐CRS score | 88 ± 15 | N/A | N/A |
| HADS score (ON) | 5 ± 3.3 | N/A | N/A |
| STAI score (ON) | 35 ± 14 | N/A | N/A |
| Fluctuating participants | 14/35 | N/A | N/A |
Abbreviations: HADS, Hospital Anxiety and depression scale; LEDD, Levodopa equivalent daily dose; PD, Parkinson disease; PD‐CRS, Parkinson's disease cognitive rating scale; STAI, State‐trait anxiety inventory; UPDRS, Unified Parkinson's Disease Rating Scale.
In ROI‐to‐ROI rsfMRI connectivity analyses there is a need to control Type I errors as the number of comparisons between ROI connections can exceed the thousands. Conservative approaches like the Holm–Bonferroni correction might however result in too conservative estimations, given that the number of tests grows quadratically with the number of nodes, resulting in p‐values lower than .00001 for a standard network of 90 nodes and a family‐wise data error of α = .05 (Zalesky, Cocchi, Fornito, Murray, & Bullmore, 2012). This has led to the development of techniques such as Network‐Based Statistics (NBS) or Spatial Pairwise Clustering (SPC) that aim to control Type I errors by clustering connections and applying permutation testing to ascribe a family‐wise error (FWE) corrected p‐value to each cluster. SPC shows lower sensibility but higher specificity and a finer resolution compared to NBS. Thus, we have used SPC to identify relevant clusters in each of the contrasts of interest; each cluster is characterized by its mass—sum of F‐ or T‐squared statistics over all connections within each cluster—and then compared to a distribution of expected cluster mass values under the null hypothesis using permutation testing (10.000 permutations) and a p‐value <.05. Finally, a multiple comparison correction using a stringent FWE correction with a p‐value <.05 is applied at the cluster level (unless specified as uncorrected).
3. RESULTS
3.1. Clinical and sociodemographic data
Thirty‐five participants in the mid stages of PD (age 65 ± 9 years; disease duration 7.3 ± 4.5 years, MDS‐UPDRS III 23.5 ± 9) and 16 HCs (age 66.8 ± 7.8 years) were included. PD participants showed no clinically relevant depression (HADS‐D 5 ± 3.3); anxiety as measured by the STAI showed that some participants cleared the commonly accepted threshold of 44 points (STAI 35 ± 14 in the ON state) but none showed HADS‐A scores above 11 points. All the participants had simple, predictable motor fluctuations according to their medical records, and upon evaluation, 14 participants were classified as fluctuating according to MDS‐UPDRS (Part 4.3 ≥1) (Table 1). No participants featured clinically meaningful dyskinesias. MRI scans were obtained for all controls and PD participants in the ON state, while 24 participants had scans acquired both in the ON and OFF states within a time period of 7 days; LEDD remained thus constant between ON and OFF acquisitions. Sociodemographic and clinical characteristics of participants with no MRI in the OFF state did not differ significantly from participants with MRI in the ON and OFF states. Regarding quality control of functional scans, PD‐ON, PD‐OFF, and controls had no significant differences in framewise displacement (controls 0.082, PD‐ON 0.106, PD‐OFF 0.108, One‐way ANOVA p = .44).
Twenty‐five PD patients were evaluated after 10 years of follow‐up. Ten participants were lost to follow‐up: five participants did not have enough clinical data to ascertain conversion to dementia, two chose to continue follow‐up in other hospitals, two had disabling neurological events (a debilitating stroke and an intracranial hemorrhage during a deep brain stimulation procedure) and one died shortly after study completion of non‐neurological causes. However, the excluded group did not differ significantly in age (68 vs. 64 years in excluded participants, p = .12), UPDRS (23 vs. 20 points in excluded participants, p = .29) or LEDD (743 vs. 832 mg in excluded participants, p = .60). Of the 25 participants that completed follow‐up, thirteen (52%) were classified as converters according to CDR. Both groups were comparable in disease duration, educational level, UPDRS‐III, and LEDD, but converters at baseline were older (p = .02) and had lower PD‐CRS scores (p = .03, Table S1). A subset of these participants (N = 17, with seven dementia converters) had MRI both in the ON and OFF conditions. Both groups were comparable in disease duration, educational level, UPDRS‐III, LEDD, and PD‐CRS, but converters were older at baseline (p = .049) (see Table S2).
3.2. Cortical thickness
In the structural imaging comparison, nondemented PD participants showed cortical thinning in regions of the right superior frontal cortex compared with controls (Figure S1). Controls did not show any reductions in cortical thickness compared to the PD group. Analyzing the baseline scans of the PD group that completed a 10‐year follow‐up, PD participant that developed dementia (PDD) did not show any cortical thinning compared to PD participants who did not develop dementia. Furthermore, using the cortical thickness of the right superior frontal gyrus as a covariate of no interest when comparing PD and control groups we found similar functional connectivity results (see Figure S1). A regression analysis between the cortical thickness of this cluster and the functional connectivity of the PD group showed a trend associating frontal cortical thickness with fronto‐parietal connectivity, but did not survive multiple comparison testing (not shown).
3.3. Functional connectivity differences between PD and HC in the ON state
PD patients showed higher connectivity than HCs in two clusters when using a parcellation that included both cognitive and sensorimotor ROIs (Figure 1).
FIGURE 1.
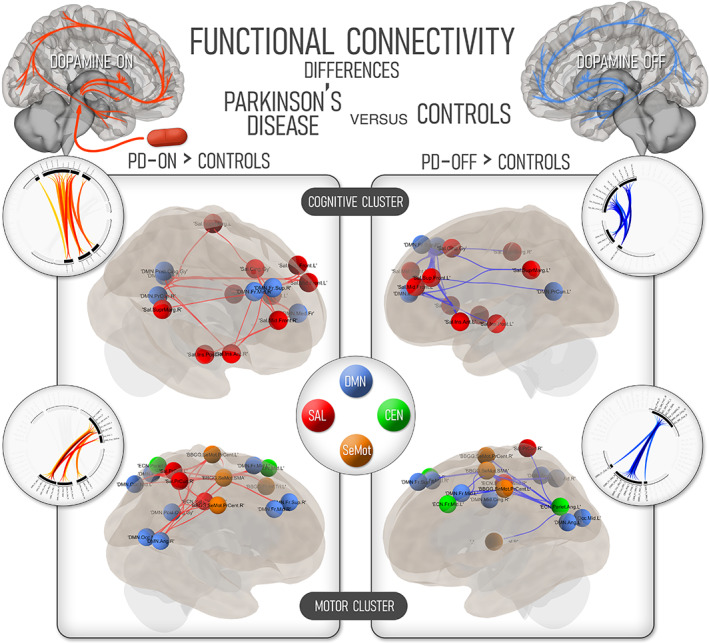
Differences in functional connectivity between nondemented Parkinson's disease participants and healthy, age‐matched controls. The three large‐scale cognitive networks are considered on the upper renders (labeled “cognitive cluster”), while the changes in the three cognitive networks plus the sensorimotor network are showcased in the lower renders. On the left part of the image, regions with higher functional connectivity in the Parkinson's disease participants in the ON state are highlighted. Regions with higher connectivity compared to controls in the OFF state are shown in the right part of the image. The color code corresponding to the four large‐scale networks is shown in the central ring. CEN, central executive network; DMN, default‐mode network; SAL, salience network; SeMot, sensorimotor network; PD, Parkinson's disease
The first cluster (mass = 410, pval = .006) connected the sensorimotor elements of the parcellation—including both precentral regions and the supplementary motor area—with both frontal and posterior regions of the DMN, including the precuneus, posterior cingulate cortex, and bilateral angular cortex. Some elements of the SN, including the mid cingulate gyrus and the SN regions of the precuneus, were also involved. These connections were not present in HCs, which furthermore showed no increased connectivity compared to PD patients.
The second cluster with higher connectivity in nondemented PD featured cognitive hubs of the SN and the DMN (mass = 379, p = .009). Higher connectivity was found among frontal nodes of both networks—featuring superior and mid frontal cortices both of SN and DMN—, but also spanning antero‐posterior connections of the bilateral precuneus and posterior cingulate cortex with both insular cortices and supramarginal gyrii. Again, there was no increased connectivity for HCs in any of these areas.
Finally, this increased connectivity of the frontoparietal nodes in the PD‐ON group was also correlated with higher PD‐CRS scores in a group of frontoparietal regions, headlined by SN nodes such as the cingulate, superior frontal and supramarginal gyrii, which showed higher connectivity with frontal and angular nodes of the DMN (mass 182, p = .02; see Figure S2).
3.4. Functional connectivity differences between PD and HC in the OFF state
In the OFF state, PD participants featured higher connectivity than HCs between sensory‐motor regions of the cortex and cognitive hubs of the DMN (mass = 333, p = .008), both containing similar components to the ON state. Assessing cognitive regions, PD participants also showed higher intrinsic connectivity between SN and DMN components without medication (mass = 252, pval = .02). This contrast showed a similar structure to the ON state but included fewer posterior cortical hubs of the DMN, with posterior cingulate and right precuneus not present in this comparison. No instances of higher connectivity were found in HCs (Figure 1).
3.5. Functional connectivity differences within nondemented PD: ON–OFF comparisons
Performing within‐participant comparisons in the ON and OFF states, PD participants showed a reconfiguration of cognitive networks under medication (Figure 2).
FIGURE 2.
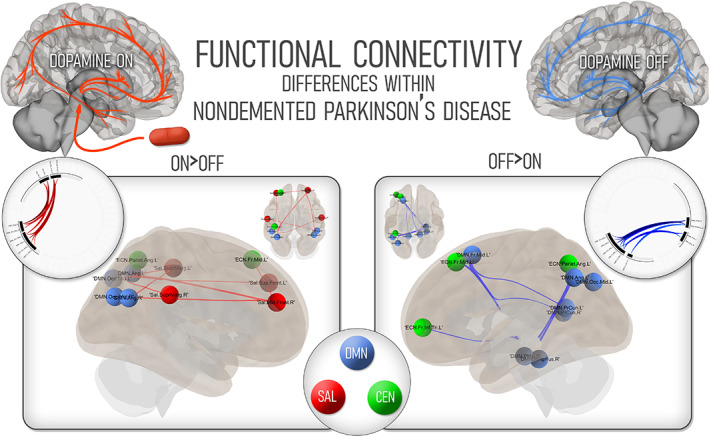
Changes within nondemented Parkinson's disease participants according to medication status. On the left, regions which showed higher connectivity in PD participants in the ON state; on the right, regions showing higher connectivity in the OFF state. The color code corresponding to the three large‐scale cognitive networks is shown in the central ring. CEN, central executive network; DMN, default‐mode network; PD, Parkinson's disease; SAL, salience network
In the ON state, PD participants featured a cluster of connections between SN nodes and predominantly posterior DMN nodes (mass = 141.46, p = .04). These connections included the bilateral supramarginal gyrii and frontal cortices of the SN and bilateral mid occipital and angular cortices on the DMN.
In the OFF state, the relevant cluster showed enhanced connectivity of key hubs of the DMN with other DMN and CEN nodes (mass = 142, p = 0.04). Of note, the bilateral precuneus showed higher connectivity with occipital, frontal and angular cortices of the same network, with higher connectivity also present in the right fusiform and parahippocampal nodes of the DMN.
3.6. Functional connectivity differences in converters to dementia
Participants that converted to dementia in a 10‐year follow‐up showed distinct connectivity patterns compared to nonconverters in their baseline ON scans.
Dementia converters featured a cluster of connections that captured the higher connectivity between key SN and DMN hubs (mass = 268, p = .01) This higher connectivity was found specifically between the insular cortices and cingulate gyrus of the SN and the posterior cingulate cortex of the DMN, together with increased connectivity in other relevant regions such as the angular and occipital nodes of the DMN (Figure 3). Given the disparity in cognitive scores at baseline, we used the PD‐CRS score as a covariate of no interest in the analysis, and still found a cluster of increased connectivity across SN and DMN nodes (mass = 126, p = .009 uncorrected) No clusters featuring higher connectivity in nonconverters were found in this analysis.
FIGURE 3.
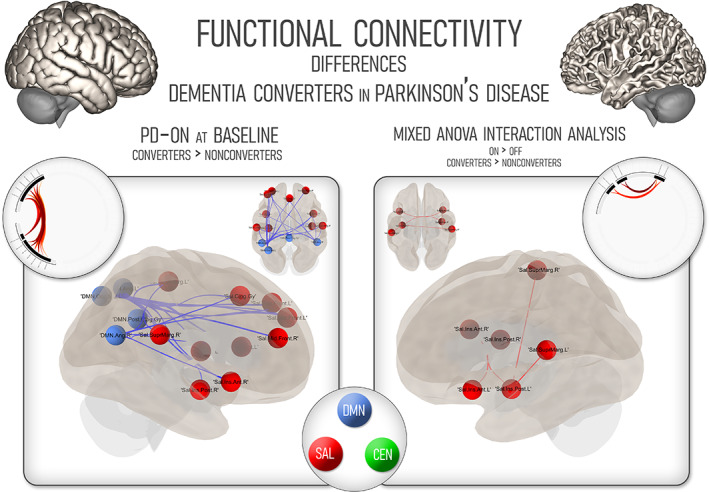
Cognitive network changes in Parkinson's disease participants in a 10‐year follow‐up. On the left, changes in functional connectivity comparing converters to nonconverters using baseline scans in the ON state. On the right, mixed interaction analysis according to both conversion to dementia and medication state. CEN, central executive network; DMN, default‐mode network; PD, Parkinson's disease; SAL, salience network
Finally, we performed an exploratory analysis—given the small sample size (seven participants) in the group of patients who converted to dementia—within the subset of participants that had both long‐term follow‐up and baseline scans in the ON and OFF conditions (Figure 3). The participants that converted to dementia showed, in the ON condition, higher connectivity between SN components, mainly within the insular cortex, also featuring the participation of the bilateral supramarginal gyri (mass = 98, p = .008 uncorrected). No differences were found in the OFF condition for any of the groups, nor were instances of higher connectivity found in the nonconverter group.
4. DISCUSSION
In this work, we have explored how cognitive networks in nondemented PD are shaped by dopaminergic treatment, both compared to age‐matched HCs and contrasting patients in the ON and OFF states.
We first conducted a cortical thickness study to frame our functional results. We found scarce differences between PD participants and age‐matched controls, mainly centered around the right superior frontal gyrus. While contrasting with previous studies (Mak et al., 2015), a recent longitudinal showed that the rate of cortical thinning of PD patients and healthy, age‐matched controls is indeed very similar after age 60 (Gorges et al., 2020). Therefore, structural changes in our cohort would be hardly sufficient to explain the differences in resting‐state cognitive networks, and using cortical thickness both as a covariate of no interest or as a regressor in functional connectivity analyses did not yield any significant results.
On the functional analysis, our first goal was to determine whether PD patients showed differences in cognitive network connectivity compared to HCs, and whether these differences were dependent on medication. In broad terms, our results showed that nondemented PD participants feature higher frontoparietal cognitive network connectivity, irrespective of medication status. These differences included two main sources of higher functional connectivity. The first was the higher connectivity compared to controls found between sensorimotor regions, such as the supplementary motor cortex and precentral gyrii, and a wide range of predominantly DMN nodes. The second was the higher connectivity between SN and DMN hubs in PD participants. These differences were similar overall in the ON and OFF conditions, although the ON state featured more widespread interactions between normally anticorrelated hubs, such as the insular cortices in the SN and the posterior cingulate/precuneus of the DMN. These differences could be interpreted as a decrease of normal anticorrelations between these large‐scale cognitive networks within the PD group, a feature that has been previously reported in PD (Aracil‐Bolaños et al., 2019; Peraza et al., 2017). With this knowledge, previous reports of higher frontoparietal connectivity in nondemented PD patients (Gorges et al., 2015)—which waned as patients slid into cognitive decline—, can be adscribed to cognitive networks and cannot be explained merely as an effect of dopaminergic medication.
Our second goal with this study was to ascertain which effect DRTs exerted over cognitive networks when comparing the ON and OFF states in PD participants. As previously mentioned, it is widely accepted that dopaminergic treatment normalizes functional connectivity in brain networks (Tahmasian et al., 2015). This could have implications when interpreting connectivity differences in nondemented PD, since the aforementioned higher frontoparietal connectivity could be an artifact introduced by the dopaminergic treatment. In our sample, PD participants in the ON state showed clear increases in SN‐DMN connectivity, whereas in the OFF state, a cluster of predominantly DMN connections was more prevalent. Consequently, DRT was associated with lower posterior‐DMN connectivity, at the same time that frontoparietal cognitive networks showed higher functional connectivity. Therefore, the effect of DRT on cognitive networks does not seem to merely “normalize” connectivity, especially in the systems that have a stronger dopaminergic component, such as the SN. Instead, DRT seems to promote network configurations that dampen anticorrelations and reduce within‐DMN connectivity The link between dopaminergic pathways and the SN has been explored in a recent study (McCutcheon et al., 2019), in which higher intrinsic SN functional connectivity was related to higher dopaminergic synthesis in the mesocorticolimbic pathway, measured using 18‐Fluorodopa. It is likely that DRT plays a similar role in PD patients, enhancing the connectivity of SN nodes with strong mesocorticolimbic input, such as the anterior insula, and depressing the functional connectivity of the anticorrelated posterior DMN hubs.
The extent to which these differences might contribute to cognitive decline is presently unknown. Previous studies show that the decline in dopamine receptor availability in the anterior insula has been linked to the onset of cognitive decline in PD (Christopher et al., 2015). The more immediate explanation would be that the increases in connectivity observed in SN nodes could constitute a compensation mechanism that delays cognitive decline. In this line, a recent study showed an association of preserved cognition with increased functional connectivity within subnetworks of the cingulate cortex—a key SN node—in PD patients, concurrent with deteriorated structural covariance in this regions (Zhou et al., 2020). We did indeed find that PD‐ON participants showed a positive correlation between higher frontoparietal functional connectivity and higher PD‐CRS scores at baseline. However, while hyperconnectivity can be considered a compensation mechanism (Hillary et al., 2015), studies in different neurological diseases show that this might not always be the case. In Alzheimer's disease, Apolipoprotein E4 carriers, who have an increased genetic risk of neurodegeneration, show increased coherence of the DMN during working memory tasks (Filippini et al., 2009). It has been shown that higher functional connectivity implies increasing metabolic costs as measured by 18‐FDG uptake (Tomasi, Wang, & Volkow, 2013). In our sample, the analysis of baseline scans in the ON state showed that the participants who converted to dementia featured higher functional connectivity between SN‐DMN nodes while under medication, compared to those who staved off cognitive decline. We also analyzed a subset of participants, which had data regarding conversion to dementia and both ON and OFF scans. Although these results should be interpreted with caution and considered as exploratory, when conversion to dementia and medication state were both factored in, higher connectivity within the SN was found to be a feature of PD patients that converted to dementia. These preliminary results suggest that rather than a compensation mechanism, this network reconfiguration under DRT could constitute a vulnerability trait in nondemented PD.
This study has some limitations. First, not all participants were scanned both in the ON and OFF states, given the intrinsic difficulties of dopamine withdrawal. Second, sample size limits the scope and statistical power of our findings, and we acknowledge that the control group was age but not sex‐matched. Third, cognitive assessment was not performed conforming to Level‐II criteria, though the administered Level‐I scale is a Movement Disorders Society endorsed tool (Skorvanek et al., 2018) and has shown very good accuracy compared to Gold‐Standard criteria (de Bobadilla et al., 2013). Strengths of this study include the multimodal approach, using both cortical thickness and functional connectivity, the standardized methodology and neuroimaging pipeline (Aracil‐Bolaños et al., 2019; Whitfield‐Gabrieli & Nieto‐Castanon, 2012) and the stringent statistical thresholds applied throughout the analysis.
To sum up, this study shows that dopaminergic therapies shape the functional connectivity of cognitive networks in nondemented PD patients, enhancing the link between normally‐anticorrelated SN and DMN. These differences in functional connectivity appear in regions with relatively preserved mesocorticolimbic input, such as the insular regions of the SN, and merit further investigations regarding their relevance in the progression of cognitive decline in PD.
DISCLOSURES
In the last 12 months, the work of Dr. Aracil has been supported by a research grant (CM19/00156G) from Instituto de Salud Carlos III (ISCIII). Jesus Pujol has received salary from IMI‐Hospital del Mar, Barcelona, Spain. Carles Soriano‐Mas has received salary from Instituto de Salud Carlos III (Miguel Servet grant). Frederic Sampedro is supported by a research grant from the Government of Spain (ISCIII). Dr. González de Echávarri is employed by Barcelonaβeta Brain Research Center (BBRC) and Pasqual Maragall Foundation and has received salary from Hospital de la Santa Creu I Sant Pau. Jaime Kulisevsky is employed by Hospital de la Santa Creu I Sant Pau and has received public research support from CIBERNED and Carlos III Institute, unrestrictive research support from Zambon and TEVA, and honoraria for lecturing and/or consulting from Zambon and TEVA. Dr. Javier Pagonabarraga is employed by Hospital de la Santa Creu I Sant Pau, has served on advisory or speakers' boards, and received honoraria from UCB, Zambon, AbbVie, Italfarmaco, Allergan, Ipsen, and Bial, and received grants from CIBERNED & FIS PI14/02058; Spanish Government grants) and Fundació La Marató de TV3 (20142910).
AUTHOR CONTRIBUTIONS
Ignacio Aracil‐Bolaños contributed to conception, organization, and execution of the research project; designed and executed the imaging and statistical analyses and wrote the first draft. Frederic Sampedro contributed to execution of the research project, assisted in statistical and imaging analyses, and provided review and critique of the manuscript. Jesus Pujol contributed to conception, organization, and execution of the research project and provided review and critique of the manuscript. Carles Soriano‐Mas contributed to conception, organization, and execution of the research project and provided review and critique of the manuscript. José María Gónzalez‐de‐Echávarri contributed to statistical and imaging analyses and provided review and critique of the manuscript. Jaime Kulisevsky contributed to conception, organization, and execution of the research project and provided review and critique of the manuscript. Javier Pagonabarraga contributed to conception, organization, and execution of the research project and provided review and critique of the manuscript.
Supporting information
Figure S1 Functional connectivity in the PD versus control contrast when accounting for the differences in the cortical thickness of the right superior frontal gyrus.
Figure S2. Regression analysis showing a positive correlation between PD‐CRS scores and functional connectivity of Salience Network and DMN nodes in the PD sample (cluster mass 182, p = .02 FWE corrected)
Table S1. Clinical and sociodemographic data according to conversion to dementia at long‐term follow up, using baseline ON scans. LEDD, Levodopa equivalent daily dose; PD, Parkinson's disease; PD‐CRS: Parkinson's disease cognitive rating scale; UPDRS, Unified Parkinson's Disease Rating Scale.
Table S2. Clinical and sociodemographic data according to conversion to dementia at long‐term follow up, in a subsample of patients with both ON and OFF scans. LEDD, Levodopa equivalent daily dose; PD, Parkinson's disease; PD‐CRS: Parkinson's disease cognitive rating scale; UPDRS, Unified Parkinson's Disease Rating Scale.
Table S3. Cluster data showing the magnitude of statistical effect (cluster mass), p‐value and Cohen's d value for the most statistically significant connection on the main clusters.
Table S4. Region of interest (ROI).
Aracil‐Bolaños, I. , Sampedro, F. , Pujol, J. , Soriano‐Mas, C. , Gónzalez‐de‐Echávarri, J. M. , Kulisevsky, J. , & Pagonabarraga, J. (2021). The impact of dopaminergic treatment over cognitive networks in Parkinson's disease: Stemming the tide? Human Brain Mapping, 42(17), 5736–5746. 10.1002/hbm.25650
Funding information Agència de Gestió d'Ajuts Universitaris i de Recerca, Grant/Award Number: PERIS SLT008/18/00088; Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas; Fundació la Marató de TV3, Grant/Award Number: 20142910; Instituto de Salud Carlos III, Grant/Award Number: PI14/02058
Contributor Information
Jaime Kulisevsky, Email: jkulisevsky@santpau.cat.
Javier Pagonabarraga, Email: jpagonabarraga@santpau.cat.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Amboni, M. , Tessitore, A. , Esposito, F. , Santangelo, G. , Picillo, M. , Vitale, C. , … Barone, P. (2015). Resting‐state functional connectivity associated with mild cognitive impairment in Parkinson's disease. Journal of Neurology, 262, 425–434. [DOI] [PubMed] [Google Scholar]
- Aracil‐Bolaños, I. , Sampedro, F. , Marín‐Lahoz, J. , Horta‐Barba, A. , Martínez‐Horta, S. , Botí, M. , … Pagonabarraga, J. (2019). A divergent breakdown of neurocognitive networks in Parkinson's Disease mild cognitive impairment. Human Brain Mapping, 40, 3233–3242. 10.1002/hbm.24593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio, H. C. , Segura, B. , Garrido‐Millan, J. L. , Marti, M. J. , Compta, Y. , Valldeoriola, F. , … Junque, C. (2015). Resting‐state frontostriatal functional connectivity in Parkinson's disease‐related apathy. Movement Disorders, 30, 671–679. [DOI] [PubMed] [Google Scholar]
- Baik, K. , Cha, J. , Ham, J. H. , Baek, G. M. , Sunwoo, M. K. , Hong, J. Y. , … Lee, P. H. (2014). Dopaminergic modulation of resting‐state functional connectivity in de novo patients with Parkinson's disease. Human Brain Mapping, 35, 5431–5441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiong, W. , Wilson, S. M. , D'Esposito, M. , Kayser, A. S. , Grossman, S. N. , Poorzand, P. , … Rankin, K. P. (2013). The salience network causally influences default mode network activity during moral reasoning. Brain, 136, 1929–1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher, L. , Duff‐Canning, S. , Koshimori, Y. , Segura, B. , Boileau, I. , Chen, R. , … Strafella, A. P. (2015). Salience network and parahippocampal dopamine dysfunction in memory‐impaired Parkinson disease. Annals of Neurology, 77, 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bobadilla, R. F. , Pagonabarraga, J. , Martínez‐Horta, S. , Pascual‐Sedano, B. , Campolongo, A. , & Kulisevsky, J. (2013). Parkinson's disease‐cognitive rating scale: Psychometrics for mild cognitive impairment. Movement Disorders, 28, 1376–1383. [DOI] [PubMed] [Google Scholar]
- Emre, M. , Aarsland, D. , Brown, R. , Burn, D. J. , Duyckaerts, C. , Mizuno, Y. , … Dubois, B. (2007). Clinical diagnostic criteria for dementia associated with Parkinson's disease. Movement Disorders, 22, 1689–1707. [DOI] [PubMed] [Google Scholar]
- Engels, G. , Vlaar, A. , McCoy, B. , Scherder, E. , & Douw, L. (2018). Dynamic functional connectivity and symptoms of Parkinson's disease: A resting‐state fMRI study. Frontiers in Aging Neuroscience, 10, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini, N. , MacIntosh, B. J. , Hough, M. G. , Goodwin, G. M. , Frisoni, G. B. , Smith, S. M. , … Mackay, C. E. (2009). Distinct patterns of brain activity in young carriers of the APOE‐ε4 allele. Proceedings of the National Academy of Sciences of the United States of America, 106, 7209–7214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorenzato, E. , Strafella, A. P. , Kim, J. , Schifano, R. , Weis, L. , Antonini, A. , & Biundo, R. (2019). Dynamic functional connectivity changes associated with dementia in Parkinson's disease. Brain, 142, 2860–2872. 10.1093/brain/awz192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl, B. , & Dale, A. M. (2000). Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences, 97, 11050–11055. 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Redondo, R. , García‐García, D. , Clavero, P. , Gasca‐Salas, C. , García‐Eulate, R. , Zubieta, J. L. , … Rodríguez‐Oroz, M. C. (2014). Grey matter hypometabolism and atrophy in Parkinson's disease with cognitive impairment: A two‐step process. Brain, 137, 2356–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorges, M. , Kunz, M. S. , Müller, H. , Liepelt‐Scarfone, I. , Storch, A. , Dodel, R. , … Kassubek, J. (2020). Longitudinal brain atrophy distribution in advanced Parkinson's disease: What makes the difference in “ cognitive status ” converters ? Human Brain Mapping, 41, 1–19. 10.1002/hbm.24884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorges, M. , Müller, H. P. , Lulé, D. , LANDSCAPE Consortium , Pinkhardt, E. H. , Ludolph, A. C. , & Kassubek, J. (2015). Neurobiology of aging to rise and to fall: Functional connectivity in cognitively normal and cognitively impaired patients with Parkinson's disease. Neurobiology of Aging, 36, 1727–1735. [DOI] [PubMed] [Google Scholar]
- Hely, M. A. , Reid, W. G. J. , Adena, M. A. , Halliday, G. M. , & Morris, J. G. L. (2008). The Sydney Multicenter Study of Parkinson's disease: The inevitability of dementia at 20 years. Movement Disorders, 23, 837–844. [DOI] [PubMed] [Google Scholar]
- Hillary, F. G. , Roman, C. A. , Venkatesan, U. , Rajtmajer, S. M. , Bajo, R. , & Castellanos, N. D. (2015). Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology, 29, 59–75. [DOI] [PubMed] [Google Scholar]
- Hughes, C. P. , Berg, L. , Danziger, W. L. , Coben, L. A. , & Martin, R. L. (1982). A new clinical scale for the staging of dementia. The British Journal of Psychiatry, 140, 566–572. 10.1192/bjp.140.6.566 [DOI] [PubMed] [Google Scholar]
- Hughes, A. J. , Daniel, S. E. , Kilford, L. , & Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson's disease: A clinico‐pathological study of 100 cases. Journal of Neurology, Neurosurgery, and Psychiatry, 55, 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanskey, J. H. , McColgan, P. , Schrag, A. E. , Acosta‐Cabronero, J. , Rees, G. , Morris, H. R. , & Weil, R. S. (2018). Can neuroimaging predict dementia in Parkinson's disease? Brain, 141, 2545–2560. 10.1093/brain/awy211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, R. , Delmaire, C. , Defebvre, L. , Moonen, A. J. , Duits, A. A. , Hofman, P. , … Dujardin, K. (2016). Cognitive phenotypes in Parkinson's Disease differ in terms of brain‐network organization and connectivity. Human Brain Mapping, 38, 1604–1621. 10.1002/hbm.23474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald, P. A. , & Monchi, O. (2011). Differential effects of dopaminergic therapies on dorsal and ventral striatum in Parkinson's disease: Implications for cognitive function. Parkinson's Disease, 2011, 572743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, E. , Su, L. , Williams, G. B. , Firbank, M. J. , Lawson, R. A. , Yarnall, A. J. , … O'Brien, J. T. (2015). Baseline and longitudinal grey matter changes in newly diagnosed Parkinson's disease: ICICLE‐PD study. Brain, 138, 2974–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon, R. A. , Nour, M. M. , Dahoun, T. , Jauhar, S. , Pepper, F. , Expert, P. , … Howes, O. D. (2019). Mesolimbic dopamine function is related to salience network connectivity: An integrative positron emission tomography and magnetic resonance study. Biological Psychiatry, 85, 368–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. (2011). Large‐scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences, 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga, J. , Corcuera‐solano, I. , Vives‐gilabert, Y. , Llebaria, G. , & Garcı, C. (2013). Pattern of regional cortical thinning associated with cognitive deterioration in Parkinson's disease. PLoS One, 8, e54980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagonabarraga, J. , & Kulisevsky, J. (2012). Cognitive impairment and dementia in Parkinson's disease. Neurobiology of Disease, 46, 590–596. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga, J. , Kulisevsky, J. , Llebaria, G. , García‐Sánchez, C. , Pascual‐Sedano, B. , & Gironell, A. (2008). Parkinson's disease‐cognitive rating scale: A new cognitive scale specific for Parkinson's disease. Movement Disorders, 23, 998–1005. [DOI] [PubMed] [Google Scholar]
- Pagonabarraga, J. , Soriano‐mas, C. , & Llebaria, G. (2014). Parkinsonism and related disorders neural correlates of minor hallucinations in non‐demented patients with Parkinson's disease. Parkinsonism & Related Disorders, 20, 290–296. [DOI] [PubMed] [Google Scholar]
- Peraza, L. R. , Nesbitt, D. , Lawson, R. A. , Duncan, G. W. , Yarnall, A. J. , Khoo, T. K. , … Taylor, J. P. (2017). Intra‐and inter‐network functional alterations in Parkinson's disease with mild cognitive impairment. Human Brain Mapping, 38, 1702–1715. 10.1002/hbm.23499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma, R. B. , Berg, D. , Stern, M. , Poewe, W. , Olanow, C. W. , Oertel, W. , … Deuschl, G. (2015). MDS clinical diagnostic criteria for Parkinson's disease. Movement Disorders, 30, 1591–1601. 10.1002/mds.26424 [DOI] [PubMed] [Google Scholar]
- Sala‐llonch, R. , Baggio, H. , Valldeoriola, F. , & Compta, Y. (2015). Cognitive impairment and resting‐state network connectivity in Parkinson's Disease. Human Brain Mapping, 212, 199–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, W. W. , Crawford, R. K. , Zhou, J. , Miller, B. L. , & Greicius, M. D. (2009). Neurodegenerative diseases target large‐scale human brain networks. Neuron, 62, 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segura, B. , Baggio, H. C. , Marti, M. J. , Valldeoriola, F. , Compta, Y. , Garcia‐Diaz, A. I. , … Junque, C. (2014). Cortical thinning associated with mild cognitive impairment in Parkinson's disease. Movement Disorders, 29, 1495–1503. [DOI] [PubMed] [Google Scholar]
- Shine, J. M. , Bell, P. T. , Matar, E. , Poldrack, R. A. , Lewis, S. J. G. , Halliday, G. M. , & O'Callaghan, C. (2019). Dopamine depletion alters macroscopic network dynamics in Parkinson's disease. Brain, 142, 1024–1034. 10.1093/brain/awz034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skorvanek, M. , Goldman, J. G. , Jahanshahi, M. , Marras, C. , Rektorova, I. , Schmand, B. , … the members of the MDS Rating Scales Review Committee . (2018). Global scales for cognitive screening in Parkinson's disease: Critique and recommendations. Movement Disorders, 33, 208–218. [DOI] [PubMed] [Google Scholar]
- Tahmasian, M. , Bettray, L. M. , van Eimeren, T. , Drzezga, A. , Timmermann, L. , Eickhoff, C. R. , … Eggers, C. (2015). A systematic review on the applications of resting‐state fMRI in Parkinson's disease: Does dopamine replacement therapy play a role? Cortex, 73, 80–105. [DOI] [PubMed] [Google Scholar]
- Tomasi, D. , Wang, G. J. , & Volkow, N. D. (2013). Energetic cost of brain functional connectivity. Proceedings of the National Academy of Sciences of the United States of America, 110, 13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli, S. , & Nieto‐Castanon, A. (2012). Conn: A functional connectivity toolbox for correlated and Anticorrelated brain networks. Brain Connectivity, 2, 125–141. [DOI] [PubMed] [Google Scholar]
- Zalesky, A. , Cocchi, L. , Fornito, A. , Murray, M. M. , & Bullmore, E. (2012). Connectivity differences in brain networks. NeuroImage, 60, 1055–1062. [DOI] [PubMed] [Google Scholar]
- Zhou, C. , Gao, T. , Guo, T. , Wu, J. , Guan, X. , Zhou, W. , … Zhang, M. (2020). Structural covariance network disruption and functional compensation in Parkinson's disease. Frontiers in Aging Neuroscience, 12, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. , Greicius, M. D. , Gennatas, E. D. , Growdon, M. E. , Jang, J. Y. , Rabinovici, G. D. , … Seeley, W. W. (2010). Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain, 133, 1352–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Functional connectivity in the PD versus control contrast when accounting for the differences in the cortical thickness of the right superior frontal gyrus.
Figure S2. Regression analysis showing a positive correlation between PD‐CRS scores and functional connectivity of Salience Network and DMN nodes in the PD sample (cluster mass 182, p = .02 FWE corrected)
Table S1. Clinical and sociodemographic data according to conversion to dementia at long‐term follow up, using baseline ON scans. LEDD, Levodopa equivalent daily dose; PD, Parkinson's disease; PD‐CRS: Parkinson's disease cognitive rating scale; UPDRS, Unified Parkinson's Disease Rating Scale.
Table S2. Clinical and sociodemographic data according to conversion to dementia at long‐term follow up, in a subsample of patients with both ON and OFF scans. LEDD, Levodopa equivalent daily dose; PD, Parkinson's disease; PD‐CRS: Parkinson's disease cognitive rating scale; UPDRS, Unified Parkinson's Disease Rating Scale.
Table S3. Cluster data showing the magnitude of statistical effect (cluster mass), p‐value and Cohen's d value for the most statistically significant connection on the main clusters.
Table S4. Region of interest (ROI).
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


