In this issue of the Journal of Thrombosis and Haemostasis, Bar Barroeta and coworkers describe studies using hydrogen‐deuterium exchange mass spectrometry to investigate structural changes in factor XI (FXI) as it transitions to the protease factor XIa (FXIa).1 Their work is an important contribution to prior efforts to understand this process on the basis of enzyme kinetics and protein crystallography.
FXIa is a trypsinlike protease that contributes to thrombin generation primarily by catalyzing conversion of factor IX to factor IXaβ.2,3 Of the proteases required for normal thrombin formation during hemostasis, FXIa is functionally and structurally unique in several respects. The enzyme does not require vitamin K‐dependent posttranslational modification for normal function, and its activity is not influenced appreciably by phosphatidylserine‐containing lipids. The FXI polypeptide is organized into four 90‐amino‐acid to 91-amino‐acid repeats called apple domains (designated A1 to A4 from the N‐terminus) and a C‐terminal protease domain (Figure 1, top left image).2–5 The FXI homolog prekallikrein (PK), the precursor of the protease plasma kallikrein (PKa), is the only other protein known to share this particular configuration.6,7 In addition, FXIa is a dimer of two of the polypeptides just described,2–5,8 which is unusual for a trypsin‐like protease. These features make it difficult to extrapolate from work on the better‐studied vitamin K‐dependent coagulation proteases to gain insight into FXI and FXIa structure and enzymology. Compounding the situation, while there is a crystal structure for human FXI (PDB ID 6158),9 none is available for full length FXIa.
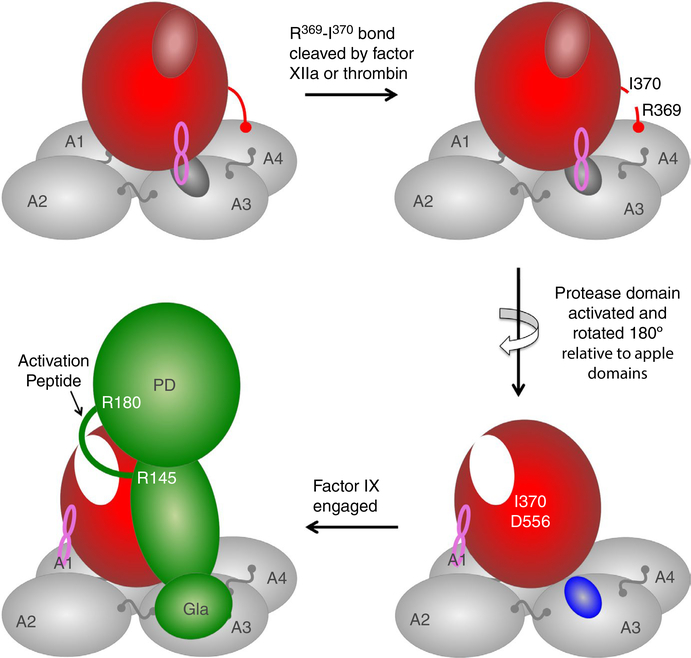
FIGURE 1.
A model for factor XI (FXI) activation. In each FXI subunit (upper left image) the protease domain (red oval) rests on a platform composed of four apple domains (A1 to A4). The active site is in a closed (inactive) conformation (small red oval). The protease domain covers an exosite for factor IX binding on the A3 domain (small gray oval) and is held in place by a set of interactions between the protease domain and the apple domains (latch loop, indicated in pink). The loop containing the activation cleavage site is indicated by a red line between the protease domain and A4. Cleavage of the peptide bond of Arg369‐Ile370 by factor XIIa or thrombin (upper right image) is followed by formation of a hydrogen bond (salt bridge) between the new N‐terminus of the protease domain (Ile370) and Asp556, resulting in the active site’s adopting an open (active) conformation (white, oval, lower right image). The original latch loop contact is broken, allowing the protease domain to rotate 180° relative to the apple domains, exposing the factor IX‐binding site on A3 (blue oval). New latch loop interactions form between the protease domain and the A1 domain stabilizing the FXIa structure. Initial engagement of FXIa by the substrate factor IX (green) involves binding of the factor IX gla‐domain to the exosite on the FXIa A3 domain. Engagement with A3 is required for proper positioning of the activation peptide (green loop) for sequential cleavage, first after Arg145 and then after Arg180 to form factor IXaβ. PD, protease domain of factor IX
FXIa is formed by cleavage of a FXI subunit after Arg369 (Figure 1, top right image).2–4 Factor XIIa and thrombin catalyze this reaction,2,3 which converts the 80 kDa FXI polypeptide into a 50 kDa heavy chain (apple domains) and 30 kDa light chain (protease domain) that remain connected by a disulfide bond.4,5 In addition to converting the protease domain to an active configuration, cleavage after Arg369 permits factor IX to bind to FXIa.10 Using recombinant FXIa molecules in which individual apple domains are replaced with the corresponding domain from PKa (a poor activator of factor IX), a putative factor IX‐binding exosite, was localized to the FXIa A3 domain.11 Subsequent scanning mutagenesis determined that areas of the N‐terminus and C‐terminus of A3 are most important for binding.12 The exosite is required for proper sequential catalysis of the factor IX peptide bonds after Arg145 and Arg180 during conversion to factor IXaβ, and disruption of the exosite reduces catalytic efficiency for cleavage of both bonds.13
In the FXI crystal structure the N‐terminus and C‐terminus of the A3 domain contribute to a hydrophobic pocket on the domain surface that is covered by the catalytic domain.10,14 This feature, which is not present on the PKa A3 domain, probably forms part of the factor IX recognition site.14 We postulated that FXI conversion to FXIa involves repositioning the catalytic domain relative to the A3 domain to expose this site. This notion is supported by a structure for human PKa (PDB ID 6144) reported earlier this year by Li et al15 A striking feature of the PKa structure is that the protease domain is rotated ~180° relative to its counterpart in the FXI structure, leaving the upper surface of the PKa A3 domain largely exposed to the solvent phase. Hydrogen‐deuterium exchange studies indicate the upper surface of the A3 domain is more exposed in PKa than in PK, consistent with movement of the protease domain relative to the apple domain disk upon activation.15 A similar rotation of the FXI protease domain upon conversion to FXIa might unmask the factor IX‐binding exosite, explaining why factor IX binds to FXIa but not FXI. The work by Bar Barroeta et al addresses this possibility.
They studied hydrogen‐deuterium exchange in FXI, FXIa, and FXIa in complex with factor IX, using recombinant FXI with the active site serine replaced to prevent autocatalysis or factor IX activation. Deuterium uptake in the N‐terminus of the A3 domain (Ala181‐Phe192) did not change appreciably upon conversion of FXI to FXIa, but was significantly reduced by factor IX binding. This could indicate the exosite is similarly accessible to deuterated water in FXI and FXIa, but less accessible in FXIa after factor IX binding. Deuterium uptake in the C‐terminus of A3 (Phe260‐Cys273) was reduced moderately after conversion to FXIa, and to a greater degree with factor IX bound to FXIa. Differences in uptake between FXI and FXIa were also noted in loops connecting the A2 and A3 domains (Lys173‐Leu180) and the A3 and A4 domains (Ser261‐Phe272). In addition, deuterium uptake was significantly reduced in FXIa in the loop connecting the A1 and A2 domains (Tyr80‐Ala91). The regions identified by hydrogen‐deuterium exchange were projected onto a model of FXIa based on the PKa crystal structure.15 Conversion of FXI to FXIa was predicted to reduce surface exposure of the A1 domain significantly as a result of rotation of the protease domain, and increase exposure of either end of the A3 domain (Ser171‐Leu177 and Phe260‐Leu277). Binding of factor IX to FXIa reduced deuterium exchange in the areas of A3 predicted to be exposed after conversion to FXIa, with a particularly large reduction noted for the Ala181‐Phe192 sequence thought to form part of the factor IX‐binding exosite.
The results from Bar Barroeta et al, combined with available structures for FXI 10 and PKa,15 support the model for FXI activation shown in Figure 1. In each FXI subunit the protease domain masks the factor IX binding exosite on the A3 domain, preventing substrate binding (Figure 1, top left image). The protease domain is held in place by “latch loops” connecting it to A3 and A4.15 Cleavage of the Arg369‐Ile370 bond creates a new N‐terminus for the protease domain (Ile370, Figure 1, top right image) that forms a hydrogen bond with Asp556, leading to an active open conformation of the protease active site.16 This is accompanied by release of the latch loops and rotation of the protease domain ~180° relative to its position in FXI (Figure 1, bottom right image). The protease domain now covers more of the surface of the A1 domain, while the surface of A3, including the factor IX binding exosite, is more exposed. Mutagenesis work suggests it is the phospholipid‐binding gla‐domain of factor IX that binds to the A3 exosite.10,14 In essence, the FXIa A3 domain substitutes for phospholipid surfaces as an anchoring site for factor IX, positioning the zymogen’s activation peptide properly for cleavage after Arg145 and Arg180 (Figure 1, bottom left image). This would explain why phospholipid is not required for FXIa activation of FIX. Proper configuration of the factor IX gla‐domain is required for binding to FXIa, consistent with the reaction’s requirement for Ca2+ ions.10,13
While structures for FXIa and the FXIa‐factor IX complex are required for verification, the work from Bar Barroeta and colleagues substantially strengthens the argument for the proposed model of FXI activation. A similar set of changes presumably occurs when PK is converted to PKa, and it will be important to determine whether the A3 domain of this protease also contains exosites for PKa substrates such as high‐molecular‐weight kininogen and factor XII.
ACKNOWLEDGMENTS
The authors wish to acknowledge support from awards HL140025 from the National Heart, Lung and Blood Institute (D. Gailani) and Grant no. RG/12/9/29775 from the British Heart Foundation Programme (J. Emsley).
Funding information
National Heart, Lung and Blood Institute, Grant/Award Number: HL140025; British Heart Foundation Programme, Grant/Award Number: RG/12/9/29775
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
REFERENCES
- 1.Bar Barroeta A, van Galen J, Stroo I, Marquart JA, Meijer AB, Meijers JCM. Hydrogen‐deuterium exchange mass spectrometry highlights conformational changes induced by factor XI activation and binding of factor IX to factor XIa. (In press JTH) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammed BM, Matafonov A, Ivanov I, et al. An update on factor XI structure and function. Thromb Res. 2018;161:94–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emsley J, McEwan PA, Gailani D. Structure and function of factor XI. Blood. 2010;116:1185–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujikawa K, Chung DW, Hendrickson LE, Davie EW. Amino acid sequence of human factor XI, a blood coagulation factor with four tandem repeats that are highly homologous with plasma prekallikrein. Biochemistry. 1986;25:2417–2424. [DOI] [PubMed] [Google Scholar]
- 5.McMullen BA, Fujikawa K, Davie EW. Location of the disulfide bonds in human coagulation factor XI: the presence of tandem apple domains. Biochemistry. 1991;30:2056–2060. [DOI] [PubMed] [Google Scholar]
- 6.Chung DW, Fujikawa K, McMullen BA, Davie EW. Human plasma prekallikrein, a zymogen to a serine protease that contains four tandem repeats. Biochemistry. 1986;25:2410–2417. [DOI] [PubMed] [Google Scholar]
- 7.McMullen BA, Fujikawa K, Davie EW. Location of the disulfide bonds in human plasma prekallikrein: the presence of four novel apple domains in the amino‐terminal portion of the molecule. Biochemistry. 1991;30:2050–2056. [DOI] [PubMed] [Google Scholar]
- 8.Meijers JC, Mulvihill ER, Davie EW, Chung DW. Apple four in human blood coagulation factor XI mediates dimer formation. Biochemistry. 1992;31:4680–4684. [DOI] [PubMed] [Google Scholar]
- 9.Papagrigoriou E, McEwan PA, Walsh PN, Emsley J. Crystal structure of the factor XI zymogen reveals a pathway for transactivation. Nat Struct Mol Biol. 2006;13:557–558. [DOI] [PubMed] [Google Scholar]
- 10.Aktimur A, Gabriel MA, Gailani D, Toomey JR. The factor IX gamma-carboxyglutamic acid (Gla) domain is involved in interactions between factor IX and factor XIa. J Biol Chem. 2003;278:7981–7987. [DOI] [PubMed] [Google Scholar]
- 11.Sun Y, Gailani D. Identification of a factor IX binding site on the third apple domain of activated factor XI. J Biol Chem. 1996;271:29023–29028. [DOI] [PubMed] [Google Scholar]
- 12.Sun MF, Zhao M, Gailani D. Identification of amino acids in the factor XI apple 3 domain required for activation of factor IX. J Biol Chem. 1999;274:36373–36378. [DOI] [PubMed] [Google Scholar]
- 13.Geng Y, Verhamme IM, Messer A, et al. A sequential mechanism for exosite‐mediated factor IX activation by factor XIa. J Biol Chem. 2012;287:38200–38209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng Y, Verhamme IM, Sun MF, Bajaj SP, Emsley J, Gailani D. Analysis of the factor XI variant Arg184Gly suggests a structural basis for factor IX binding to factor XIa. J Thromb Haemost. 2013;11:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Voos KM, Pathak M, et al. Plasma kallikrein structure reveals apple domain disc rotated conformation compared to factor XI. J Thromb Haemost. 2019;17:759–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perona JJ, Craik CS. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;4:337–360. [DOI] [PMC free article] [PubMed] [Google Scholar]