Abstract
Since colonizing land, plants have developed mechanisms to tolerate a broad range of abiotic stresses that include flooding, drought, high salinity, and nutrient limitation. Roots play a key role acclimating plants to these as their developmental plasticity enables them to grow toward more favorable conditions and away from limiting or harmful stresses. The phytohormone auxin plays a key role translating these environmental signals into developmental outputs. This is achieved by modulating auxin levels and/or signaling, often through cross talk with other hormone signals like abscisic acid (ABA) or ethylene. In our review, we discuss how auxin controls root responses to water, osmotic and nutrient-related stresses, and describe how the synthesis, degradation, transport, and response of this key signaling hormone helps optimize root architecture to maximize resource acquisition while limiting the impact of abiotic stresses.
Plant roots explore soil to acquire often scarce resources such as water and nutrients (Kiba and Krapp 2016). Soil constitutes a highly heterogenous environment (Morris et al. 2017) where resources can be locally limiting or replete and therefore act as stresses that restrict plant growth. Plants have developed myriad mechanisms to locally sense resources and translate them into optimizing root architecture, allowing growth toward more favorable conditions (Koevoets et al. 2016). Plants also rapidly inhibit their growth in response to stress, termed an “acute response,” followed by recovery and acclimation to the new conditions on a physiological and morphological level (Skirycz and Inzé 2010).
The phytohormone auxin is an important regulator of almost all aspects of plant growth and development (Salehin et al. 2015) and also plays a key role controlling acute growth arrest and mediating long-term morphological changes (Skirycz and Inzé 2010). These plant responses are initiated by modifying auxin synthesis, transport, signaling, and/or degradation (Teale et al. 2006). Modifying these regulatory pathways results in differences in auxin distribution at the organ and cellular level, which in turn alter growth rates, growth directions (tropisms), and organ initiation (Vanneste and Friml 2009). Several articles have reviewed auxin regulation of plant growth (Paque and Weijers 2016), tropisms (Muday 2001), and organ patterning (Banda et al. 2019). In this review, we discuss current understanding of how abiotic stresses modulate root growth and development by targeting the auxin synthesis, degradation, transport, and response machinery.
AUXIN-REGULATED ROOT BRANCHING IS CONTROLLED BY SOIL WATER AVAILABILITY
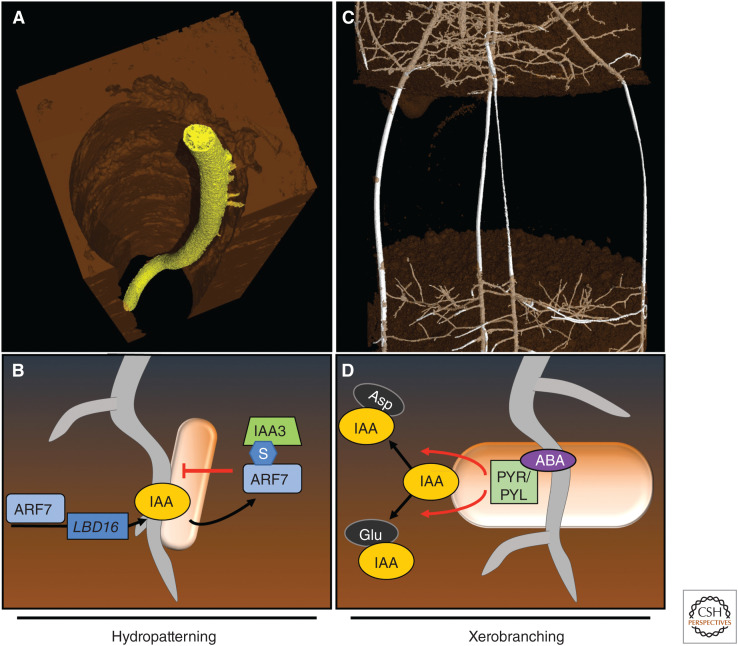
The highly heterogeneous nature of soil has a marked impact on root systems architecture (Morris et al. 2017). The ability of a plant to efficiently absorb water and nutrients from the complex soil environment is dependent on its root being able to sense these resources, and then optimizing its architecture to the spatial availability of these resources (Osmont et al. 2007; Morris et al. 2017; Banda et al. 2019). For example, roots have developed the ability to distinguish wet and dry microenvironments in the soil and modify the positioning of lateral roots (LRs) accordingly. This adaptive mechanism, termed LR hydropatterning, positions newly emerged lateral branches toward the soil water source (Bao et al. 2014). Noninvasive X-ray microCT imaging revealed that when maize roots grew down a large soil macropore, LRs preferentially emerged where roots were in direct contact with soil (Fig. 1A). This response can also be observed when growing seedlings on vertical agar plates, where LRs preferentially grow into or parallel to, rather away from, the agar surface (Bao et al. 2014; von Wangenheim et al. 2020). The importance of this response is highlighted by its conservation in both monocot and eudicot species (Bao et al. 2014).
Figure 1.
Auxin-regulated root-adaptive responses to water availability. (A) X-ray microCT image of a maize root growing down a macropore reveals lateral branches only form on the side in contact with soil employing the hydropatterning response. (B) Hydropatterning is regulated via the posttranslational modification of ARF7 by SUMO (blue hexagon “S”) in cells on the side of the root exposed to the air-filled macropore (denoted by bubble). SUMOylated ARF7 recruits the repressor Aux/IAA3, blocking auxin-dependent transcription and lateral root development. On the wet side of the root, ARF7 is not SUMOylated and therefore can activate LBD16 gene expression, leading to lateral root initiation. (C) X-ray microCT image of a barley root growing through an air-filled space, causing branching to cease until later reentering soil due to the xerobranching response. (D) A transient reduction in water uptake causes abscisic acid (ABA) to accumulate in root tip tissues during growth through the air space (denoted by bubble). ABA triggers its receptor PYR/PYL, which results in an increase of indole-3-acetic acid (IAA) conjugation to aspartate (Asp) and glutamate (Glu) by GH3 enzymes. This leads to a drop in free IAA levels, negatively affecting lateral root initiation.
LR hydropatterning depends on the perception of moisture across the primary root (PR) circumference. A root's ability to sense external water distribution appears to be linked to its uptake during cell expansion in the root elongation zone (Robbins and Dinneny 2018). This hydrological information influences the specification of xylem pole pericycle (XPP) cells in the basal meristem/elongation zone to become new LR primordia (von Wangenheim et al. 2020). The selection of LR founder cells based on external water availability was first reported to be regulated via an auxin response gradient by Bao et al. (2014). When seedling roots were grown along vertical agar plates, auxin biosynthesis (wei8-1), and transport (pin2/3/7) mutants were observed to exhibit a higher proportion of LRs emerging toward the air than wild-type (WT). This suggests the auxin synthesis and transport machinery partially control the sites of hormone accumulation during an LR hydropatterning response (Bao et al. 2014).
LR hydropatterning is also dependent on the auxin response factor 7 (ARF7) since roots of arf7 mutant seedlings no longer show a bias for their LRs to emerge toward the agar surface (Orosa-Puente et al. 2018). ARF7 is an important regulatory gene during every phase of LR development (Okushima et al. 2005; Lavenus et al. 2013, 2015). The ARF7 transcription factor induces the asymmetric expression of downstream LR regulatory genes, including LBD16 in XPP cells in the elongation zone (Orosa-Puente et al. 2018). Asymmetric LBD16 expression (which is required for LR hydropatterning) is regulated by the posttranslational modification (PTM) of ARF7 by the small ubiquitin-like modifier (SUMO) machinery. Mutating all four ARF7 SUMOylation sites disrupts the transcription factor's ability to rescue the arf7 mutant's LR hydropatterning defect and restore LBD16 asymmetric expression (Orosa-Puente et al. 2018). ARF7 SUMOylation disrupts its DNA-binding activity and promotes recruitment of a subset of Aux/IAA repressor proteins including IAA3/SHY2 (Fig. 1B). ARF7 and IAA3 interact via their SUMO PTM and SUMO interaction motif (SIM), respectively. Mutating ARF7 SUMO and IAA3 SIM sites disrupts their ability to interact and, in the case of the dominant IAA3 mutant form shy2-2, blocks its ability to disrupt LR development (Orosa-Puente et al. 2018). A gradient of ARF7 SUMOylation is hypothesized to form in response to a hydropatterning stimulus, causing LRs to preferentially form and emerge on the side of the root exposed to water (Fig. 1B). However, it remains unclear how the ARF7 SUMOylation gradient is regulated by external water availability. Components of the SUMO machinery represent promising regulators for monitoring soil water availability that merit further investigation to address this important question.
In contrast to hydropatterning, when a root grows through an air space it temporarily loses contact with soil and is therefore deprived of an external water source. When barley and maize roots were grown through an air-filled space in a soil column, their roots transiently repressed root branching until roots grew into the next soil profile (Orman-Ligeza et al. 2018). MicroCT imaging noninvasively visualized this adaptive response in soil, highlighting the plasticity of root branching (Fig. 1C; Orman-Ligeza et al. 2018). Imaging also revealed the close relationship between moisture and LR formation, since growing roots through a water (rather than air)-filled space resulted in branching along the entire root radius (von Wangenheim et al. 2020). An earlier study, using aeroponically grown barley roots transiently deprived of a water source, observed LR development was blocked at the very first stage of organ initiation (Babé et al. 2012). This novel water-related root-adaptive response has been termed xerobranching (Orman-Ligeza et al. 2018).
The xerobranching response takes place in the elongation zone (Orman-Ligeza et al. 2018). The elongation zone is vital for both LR hydropatterning and xerobranching responses (Bao et al. 2014; Orman-Ligeza et al. 2018). Whereas both water-related responses superficially appear similar, mutant experiments have revealed distinct signals regulate each process. LR hydropatterning is regulated by auxin and not the water-stress-related signal abscisic acid (ABA), as mutants disrupting its synthesis exhibit a normal hydropatterning response (Bao et al. 2014). In contrast, levels of ABA were shown to rapidly increase in root tips exposed to a xerobranching stimulus (Orman-Ligeza et al. 2018). It is well known that ABA (and its signaling pathway) is induced if water availability is limited in roots (Babé et al. 2012; Vishwakarma et al. 2017; Dietrich 2018). Moreover, exogenous addition of ABA in the aeroponic spray could mimic water deficiency and cause a significant reduction in LR formation (Orman-Ligeza et al. 2018). Finally, the ABA-insensitive receptor mutant pyr/pyl 112458 did not form an LR repression zone, suggesting a key role of ABA signaling in xerobranching. Intriguingly, pyr/pyl 112458 mutants exhibited no LR hydropatterning defect, suggesting these two water-related adaptive responses employ distinct hormone signals and response pathways.
Although ABA appears to regulate xerobranching, also auxin plays a significant role. Repression of LR branching could be partially blocked by exogenous addition of the auxin analog indole-3-butyric acid (IBA) pre-drought stress (Babé et al. 2012). Interestingly, the plasma-membrane permeable auxin NAA was able to fully restore organ initiation in the LR repression zone, while treatment with actively transported IAA could only partially restore LR number (Orman-Ligeza et al. 2018). Additionally, profiling of auxin precursors and conjugates in excised root tips revealed a decrease in free IAA levels and an increase in auxin conjugates. These results imply a role for active auxin conjugation that impair auxin accumulation and create the LR repression zone (Orman-Ligeza et al. 2018). Collectively, these results suggest that, in contrast to LR hydropatterning, cross talk exists between ABA signaling and auxin accumulation necessary for repression of LR initiation during a xerobranching response (Fig. 1D).
FLOODING-INDUCED ADVENTITIOUS ROOT FORMATION RELIES ON AUXIN AND ETHYLENE
Water is crucial for root growth and development; however, an excess of water due to flooding, can severely limit this. Flooding can be subdivided into two main conditions: fully submerged or waterlogged (whereby only the root is submerged). Both conditions cause a drop in the rate of oxygen diffusion, about 10,000 times compared to that in air (Armstrong 1980), which can severely affect plant performance (Voesenek and Bailey-Serres 2015). Plant roots have evolved numerous adaptive responses to survive flooding such as formation of shallower adventitious roots (ARs) and air-filled gaps between cells termed aerenchyma, together with induction of shoot hyponasty and elongation (Visser et al. 2003). The gaseous hormone ethylene quickly builds up when plants are flooded, and this signal is thought to be the main regulator of this adaptive response (Jackson 1985). Auxin has also been shown to perform a crucial function through cross talk with ethylene to control multiple flooding-related developmental adaptations in roots (Fig. 2A; Visser and Voesenek 2005).
Figure 2.
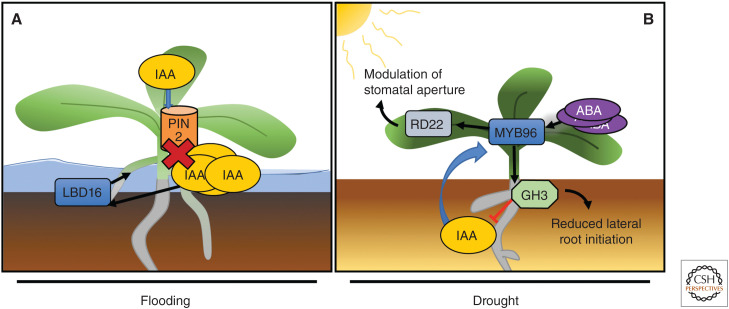
Systematic adaptive responses caused by flooding and drought stress. (A) During prolonged drought, exposure plants increase the initiation of adventitious roots as indicated in this simplified model. This initiation is induced by the inhibition of PIN2, decreasing auxin transport from shoot to root. The accumulation of indole-3-acetic acid (IAA) in the stem causes the induction of LBD16 (through auxin response factors [ARFs]) and the subsequent initiation of adventitious roots. (B) During long drought periods, plants accumulate abscisic acid (ABA) in leaf tissue. This accumulation leads to regulation of multiple genes of which one is the transcription factor MYB96. Through MYB96 induction, ABA regulates gene expression of RD22 and GH3. RD22 is important for drought responses in the shoot by modulating stomatal aperture. GH3 promotes conjugation of IAA in the root, thereby limiting auxin levels and restricting the initiation and development of lateral roots.
During prolonged floods, several plant species including rice (Oryza sativa), maize (Zea mays), sunflower (Helianthus annuus), barley, wheat, and Arabidopsis exchange their dying PR system for ARs. ARs contain more aerenchyma than PRs, thereby greatly increasing gas exchange capability (Visser and Voesenek 2005). ARs emerge from the stem or hypocotyl and although they do not necessarily originate from the pericycle, their development follows a similar auxin-controlled pathway to LR formation (Bellini et al. 2014). Polar auxin transport is crucial for flooding-induced AR formation, as the auxin transport inhibitor N-1-naphthylphalamic acid (NPA) inhibits ARs formation upon flooding (Visser et al. 1996). Additionally, mutants that affect expression and polar localization of the auxin efflux carrier PIN-formed 2 (PIN2) exhibit delays in AR emergence (Fig. 2B; Vidoz et al. 2010; Ivanchenko et al. 2015; Dawood et al. 2016).
In addition to polar transport, auxin signaling is also a target for regulation during flooding in multiple plant species. In Rumex palustris, while auxin levels were unaltered during flooding, auxin sensitivity increased due to ethylene accumulation, triggering increased AR formation (Visser et al. 1996). This observation reveals cross talk between ethylene and auxin signaling components. In the tomato sp. Solanum dulcamara, flooding induced expression of LBD16, LBD18, LBD29, and PUCHI orthologs in AR primordia (Dawood et al. 2016), revealing a very similar auxin-regulated response pathway to de novo LR formation is induced under flooding during AR development (Goh et al. 2019). Recent work has revealed a role for auxin coreceptor TIR1/AFB F-box protein AFB2 in AR formation, suggesting auxin signaling might play a more prominent role than previously thought (da Costa et al. 2020).
A role for auxin signaling during flooding-induced aerenchyma formation is less well understood. Aerenchyma, which are longitudinal interconnected air spaces formed through programmed cell death, become increasingly important under long-term flooding, contributing to gaseous exchange between aerial and submerged root tissues (Jackson and Armstrong 1999). Oxygen-deficient conditions, simulating water logging in rice, increases the level of induced lysigenous aerenchyma created by programmed cell death (Colmer and Voesenek 2009). Treatment with auxin transport inhibitor NPA strongly reduced lysigenous aerenchyma formation (Yamauchi et al. 2019). Furthermore, the dominant-negative iaa13 mutant, which strongly reduced auxin signaling, showed a steep decline in the number of aerenchyma, which could partially be restored by ethylene treatment (Yamauchi et al. 2020). Further studies are necessary to dissect cross talk between auxin and ethylene pathways in flooding adaptive mechanisms.
DROUGHT RESPONSES RELY ON CROSS TALK BETWEEN AUXIN AND OTHER HORMONES
Under water-deficient conditions, roots play a crucial adaptive role by perceiving differences in water availability in the first instance and subsequently modifying root growth rate, direction, and branching toward available water while avoiding dry areas (Seo et al. 2009; Robbins and Dinneny 2015). Drought stress also activates several signaling pathways involving ABA and ethylene (Tiwari et al. 2017). Cross talk between auxin and these pathways mediates a plethora of stress-adapted responses to regulate plant growth (Seo et al. 2009; Verma et al. 2016).
Auxin plays an important part in the plant's morphological response to drought. Levels of auxin decrease significantly under water deficit, although, at a higher auxin concentration, drought tolerance can be improved (Du et al. 2012). Hormonal cross talk between ABA and auxin under drought stress conditions optimizes root growth and branching toward moist soil, while limiting growth in dry soil (Fig. 2B). In Arabidopsis, ABA is able to induce the MYB96 transcription factor that in turn induces RD22 (RESPONSIVE TO DEHYDRATION 22) in the shoot (Seo et al. 2009, 2011). RD22 then modulates stomatal aperture during drought stress (Seo et al. 2011). Auxin can also induce MYB96, which then regulates a subset of the auxin-responsive GH3 (GRETCHEN HAGEN 3) genes under drought conditions in the root (Seo et al. 2009). The GH3 IAA-amido synthetases conjugate auxin to amino acids, thereby removing IAA from the active pool (Casanova-Sáez and Voß 2019). The addition of exogenous IAA leads to an accumulation of MYB96 transcript in the pericycle (Seo et al. 2009), indicating MYB96 regulates LR meristem activation. MYB96 OX mutants exhibit a reduction in LR number that was also seen in GH3 OX mutant lines (Seo et al. 2009). Essentially, MYB96 has been shown to mediate ABA-auxin cross talk that modulates auxin homeostasis during LR meristem activation with the aid of GH3 (Fig. 2B).
In O. sativa, OsGH3.13 is induced under drought stress (Du et al. 2012; Tiwari et al. 2017). The activation of OsGH3.13 leads to a reduction in endogenous free IAA but an increase in ABA levels, resulting in marked changes in plant architecture. This also enhanced expression of LEA (late embryogenesis abundant) genes leading to increased drought tolerance (Zhang et al. 2009; Du et al. 2012). Overexpressing OsGH3-2 was found to reduce IAA and ABA levels but led to increased water loss and thereby drought sensitivity, suggesting only precise tissue-specific GH3 induction increases drought tolerance (Zhang et al. 2009; Du et al. 2012). OsGH3-2 overexpressing lines display a clear reduction in LR number that is also observed in other auxin-deficient mutants, suggesting a key role in regulating LR development (Kong et al. 2019). In addition, active auxin levels can be further increased by modulating its synthesis in response to drought stress. Auxin is a tryptophan (Trp)-derived molecule synthesized by parallel routes, the most abundant being the IPyA pathway employing TAA1/TAR and YUCCA (YUC) enzymes (Casanova-Sáez and Voß 2019). It was determined in Arabidopsis and previously in potato that overexpressing YUC6 results in improved drought tolerance (Kim et al. 2013; Park et al. 2013; Cha et al. 2015). Collectively, these results suggest that changes in auxin homeostasis can influence synthesis of other signals such as ABA and thereby confer drought tolerance.
OSMOTIC STRESS RESPONSES RELY ON TISSUE-SPECIFIC CHANGES IN AUXIN METABOLISM
Osmotic stresses, including high salinity, leads to reduced water availability through changes in osmotic pressure and ionic toxicity (Chaves and Oliveira 2004; Munns and Tester 2008). Soil salinization is having an increasingly negative impact on global agriculture. Six percent of irrigated and 20% of the world's total cultivable land is affected by high salinity (FAO 2009). Salt stress tolerance varies significantly between crops. Barley (Hordeum vulgare), the most salt-tolerant cereal, can endure up to 250 mM NaCl (equivalent to 50% seawater), beyond which survival rates drop drastically. Other economically important cereals, such as durum wheat (Triticum turgidum ssp.), maize (Z. mays), and sorghum (Sorghum bicolor), are less tolerant to salinity (Maas and Hoffman 1977). Generally, leaf and shoot development are more sensitive to salinity than root growth. High salinity-specific growth adaptations in the root include inhibition of PR growth, halotropism, increased LR density, and reduced root hair length (West et al. 2004; Wang et al. 2009; Galvan-Ampudia and Testerink 2011). Salt-induced root growth inhibition is correlated with reduced auxin response (Wang et al. 2009; Zolla et al. 2010; Liu et al. 2015). Measurements of endogenous IAA in leaves and roots revealed IAA levels are significantly lower in plants grown in high salt conditions (Du et al. 2013; Liu et al. 2015). Generally, lower auxin levels and auxin signaling enhances salt/osmotic stress tolerance (Wang et al. 2009; Zolla et al. 2010; Liu et al. 2015).
Modulating auxin synthesis, storage, transport, degradation, and signaling all appear to be involved in translating osmotic and salt stress signals into developmental adaptations. Consistently, auxin inactivation genes in Arabidopsis (Park et al. 2007), cotton (Kirungu et al. 2019), or rice (Du et al. 2012) are generally up-regulated in response to osmotic stress. Auxin can be inactivated either by DAO-encoded IAA oxidases and conjugation to amino acids by GH3s or to sugars by UGT enzymes (Casanova-Sáez and Voß 2019). A remarkable proportion of Arabidopsis root-specific auxin inactivation genes are up-regulated upon salt treatment resulting in higher auxin turnover rates and lower root auxin levels (Korver et al. 2018). This suggests that auxin homeostasis plays an important role in regulating root growth in response to stress and that auxin-mediated growth mechanisms are important for adapting to challenging environments. For example, high salinity induces overexpression of the IPyA auxin synthesis pathway, leading to increased IAA synthesis and salinity tolerance in several species including Arabidopsis (Lee et al. 2012; Shi et al. 2014; Cha et al. 2015), poplar (Ke et al. 2015), cucumber (Yan et al. 2016), and potato (Kim et al. 2013). Furthermore, tissue-specific microarray data during salt stress from Arabidopsis roots indicate a tissue-specific shift of YUCCA expression rather than a general change in expression rates. For example, YUCCAs are strongly expressed in the columella under control conditions but are strongly expressed in the epidermis and cortex during salt stress. Similarly, the IAOx IAA synthesis pathway regulates salt stress-induced root branching. IAOx pathway genes CYP79B2/3 are mainly expressed in the QC, the differentiation zone (Korver et al. 2018), and specifically in cells underlying newly developing LRs and LR primordia (Ljung et al. 2005). cyp79b2;cyp79b3 double-knockout mutants exhibit decreased LR growth during salt stress (Julkowska et al. 2017). Salt-regulated transcriptional changes of the IAOx auxin synthesis machinery likely lead to a reduction in root tip auxin levels and therefore a reduction in root meristem growth, while higher auxin levels toward the differentiation zone promote root branching (Kilian et al. 2007; Dinneny et al. 2008; Korver et al. 2018).
Osmotic stress also impacts auxin distribution through differential regulation of PIN protein levels and localization during halotropism, for example. This adaptive growth response allows directional root growth away from high salt concentrations by reducing the gravitropic response (Dinneny et al. 2008; Sun et al. 2008; Rosquete and Kleine-Vehn 2013). PIN2, which is a key regulator of root gravitropism, plays a critical role during halotropism (Abas et al. 2006). PIN2 is internalized on the side of the root exposed to high salt, leading to reduced shootward auxin transport. Therefore, shootward auxin transport and cellular auxin concentrations are higher on the side exposed to low salt, leading to reduced cell elongation. Consequently, the root bends away from the local high salt environment. Interestingly this adaptive response does not occur when exposed to mannitol gradients (a nonanionic osmotic stress) but only when exposed to anionic osmotic gradients (Sun et al. 2008). In contrast, osmotic treatments with NaCl or mannitol lead to a quick internalization of PIN1 and PIN2 in root meristems (Nakayama et al. 2012; Galvan-Ampudia et al. 2013; Zwiewka et al. 2015). This raises the interesting question why some effects on PIN localization are salt specific, such as PIN2 internalization during halotropism, while others are generic to osmotic stress.
ROOT RESPONSES TO LOW EXTERNAL PHOSPHATE LEVELS ARE REGULATED BY AUXIN
Phosphate (P) deficiency is considered a major abiotic stress significantly reducing global crop yields. P deficiency is exacerbated by its unequal distribution in top (versus sub)-soil and its tendency to form organic and inorganic complexes that limit its mobility in soil and availability to plants (Shen et al. 2011; Pandey et al. 2013). To tackle this, plants modify their root system architecture to enhance foraging for P in top soils (Williamson et al. 2001; Band et al. 2012) and reshuffle metabolic programs to reduce internal P shortages (Esfahani et al. 2016). For example, shallower root angles maximize topsoil foraging under P-deficient conditions (Lynch and Brown 2001; Ramaekers et al. 2010; Giri et al. 2018). Gravitropic sensing in columella cells at the root tip is regulated by the low P-responsive gene RICE MORPHOLOGY DETERMINANT 1 (RMD1) in rice. RMD1 is localized on the statolith surface in columella cells where it regulates the binding of actin filament and statoliths, thus controlling statolith sedimentation in response to gravitropic stimuli in the root tip (Huang et al. 2018). Under low P conditions, RMD levels are elevated, slowing gravity-sensing statolith sedimentation, which causes rice crown roots to grow at a shallower angle to improve P foraging in topsoil (Huang et al. 2018). Increased root hair elongation and LR branching are also considered a hallmark of P deficiency in rice, Arabidopsis, wheat, barley, and other crops (Lynch 2011; Péret et al. 2014). These root responses will serve to increase P uptake capacity by increasing root surface area to forage for this immobile soil nutrient. Root hair elongation under low P stress is regulated by increased auxin synthesis, transport, and response in Arabidopsis and rice (Bhosale et al. 2018; Giri et al. 2018).
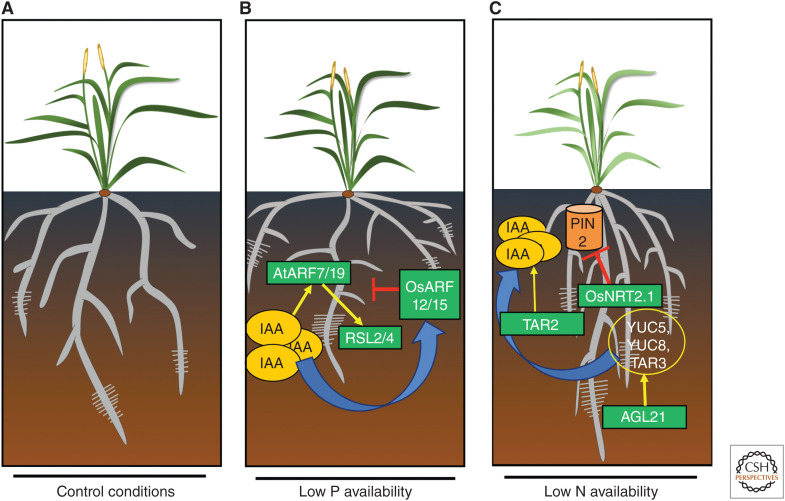
P deficiency appears to orchestrate root system architecture modifications in large part by regulation of the auxin response machinery. Levels of auxin in P-starved root tips do not change drastically, yet their auxin responses are hyperactivated (Bhosale et al. 2018). Auxin-inducible expression of ARF19 by ARF7 increases under low P, which then activates RSL2 and RSL4 TFs to enhance root hair elongation in Arabidopsis (Fig. 3A,B; Bhosale et al. 2018). Similarly, in rice, OsARF16 is induced under low P conditions and osarf16 mutants severely inhibit root hair elongation and LR branching capacity (Shen et al. 2013). On the other hand, OsARF12 and OsARF25 act as negative regulators of P homeostasis and regulate PR elongation and branching capacity (Wang et al. 2014). Moreover, Arabidopsis plants overexpressing the auxin signaling receptor TIR1 showed a hypersensitive response to low P conditions (Pérez-Torres et al. 2008). P deficiency thus promotes the auxin response pathway to trigger changes in root architecture. In contrast, auxin biosynthesis genes such as TAA1 exhibit limited transcriptional changes under low P conditions yet, rather puzzlingly, taa1 mutants disrupt the low P root hair elongation response (Bhosale et al. 2018). This may suggest that TAA1 activity is regulated by low P at the posttranscriptional level. Fascinatedly, Wang et al. (2020) recently reported the TAA1 activity is indeed controlled at the posttranscriptional level via phosphorylation. The authors also report TAA1 activity is controlled by an auxin feedback loop, in part, via the receptor TMK4. However, it is currently unclear whether external signals like low P also control TAA1 activity via this phosphorylation-based mechanism. Intriguingly, this TAA1-based mechanism appears to be highly conserved across plant evolution (Wang et al. 2020), revealing a fascinating new tier of regulation for auxin synthesis.
Figure 3.
Phosphorus (P) and nitrogen (N) availability steer root system architecture. (A) Representative cartoon of a cereal root architecture. (B) Auxin response and levels are enhanced in P-deficient roots. The elevated auxin response causes the activation of ARF7 and ARF19 regulating the expression of RSL2 and RSL4 transcription factors, ultimately regulating the root hair elongation. OsARF12 and OsARF15 act as negative regulators of P homeostasis and regulate the branching density and root hair elongation in rice. (C) Root architecture of a low N (nitrogen) stressed plants showing enhanced forging capacity by elongating root hairs, longer branching, and steep root angle. Low N stress increases the IAA level via up-regulating expression of auxin biosynthetic genes. For example, TAR2 expression is increased under low N and results in higher IAA levels, which in turn reshapes the root architecture to favor maximum N uptake. AGL21 also positively regulates the expression YUC5, YUC8, and TAR3, which increases IAA synthesis rate under low N conditions. OsNRT2.1, which acts as an auxin influx facilitator, regulates lateral branching in rice by controlling PIN2 expression.
AUXIN DYNAMICS SHAPE ROOT ARCHITECTURE UNDER LOW N REGIMES
Like P deficiency, N (nitrogen) deficiency in agricultural soils is a pervasive global issue and its deficiency causes significant drops in crop yields (Billen et al. 2013). To counter the impact of N deficiency in soils and to boost crop yield, farmers apply an excess of N fertilizers, which can be just as harmful as excess P for aquatic systems. Unlike the immobile nature of P, which accumulates in topsoils, highly soluble N forms like nitrate are prone to leach down into deeper soil layers with water (Meisinger and Delgado 2002). To access N from deeper soil layers, crops of several species increase their rooting depth to maximize N foraging, including rice, barley, and maize (Bonser et al. 1996; Trachsel et al. 2013; Ogawa et al. 2014; Yu et al. 2015b). Increased root branching length and steepness of growth angle are considered useful traits under N-limited conditions (Kiba and Krapp 2016), providing greater exposure to mobile N sources. Therefore, selection of long and steep branching genotypes with high yielding capacity could be useful to ameliorate N deficiency.
Each step of LR development: initiation, patterning, emergence, elongation, and maturation is tightly regulated by auxin (Casimiro et al. 2001; De Smet et al. 2007; Lavenus et al. 2013). Therefore, it comes as no surprise that auxin plays a pivotal role in inducing branching under N-limited conditions. NRT1.1, a nitrate transporter, has been elegantly shown to also function as an auxin influx facilitator during nitrate (NO3)-limiting conditions (Krouk et al. 2010). In addition, NRT1.1 regulates AUXIN SIGNALING F-BOX 3 (AFB3) expression in a dependent manner, directly connecting downstream NRT1.1 signaling with auxin signaling (Vidal et al. 2014). Its homolog in rice, OsNRT2.1, positively regulates LR development under low conditions. However, unlike Arabidopsis, osnrt2.1 suppresses OsPIN2 expression and forms fewer LRs (Huang et al. 2015).
To uncover the relationship between the auxin machinery and low N signaling, several genes have been identified. For example, the auxin synthesis gene TAR2 is up-regulated under low conditions, causing enhanced IAA levels in LR primordia. Additionally, tar2 mutants produced very few LRs, revealing a need for enhanced auxin biosynthesis under low conditions (Ma et al. 2014). AGL21, a MADS box gene induced under low N conditions, is also an auxin-responsive gene. Overexpressing of AGL21 exhibited longer lateral branches and, in contrast to overexpressing lines, agl21 mutant roots showed significantly fewer, smaller lateral branches under low conditions. Interestingly, auxin biosynthetic genes (YUC5, YUC8, and TAR3) were up-regulated in AGL21 overexpressing transgenics, explaining how higher IAA levels can help plants to elongate lateral branches in overexpressing roots especially under low conditions (Fig. 3C; Yu et al. 2014).
NUTRIENT AND HEAVY METAL STRESS AFFECT AUXIN HOMEOSTASIS AND ROOT ARCHITECTURE
Other nutrients and metals in soil have also been shown to signal via the root auxin machinery. For example, potassium (K) deficiency impacts root growth and development via inhibition of PR growth. Recently, AKT1, a low K sensor, was found to induce degradation of the PIN1 protein under low K conditions. akt1 mutants did not exhibit PR growth inhibition under low K conditions as compared to WT, suggesting AKT1 is a crucial component for root responses to K deficiency (Li et al. 2017).
Cadmium (Cd) is a toxic heavy metal and environmental pollutant. Cd is primarily stored in root xylem and transported to aerial tissues. However, excess Cd inhibits root growth and lateral branching. Cd causes increased expression of auxin reporters in poplar roots primarily in vascular tissues (Elobeid et al. 2012). This suggests that auxin-mediated root growth inhibition protects plants from the toxic effects of Cd. External application of Cd can perturb auxin biosynthesis and transport and has been shown to suppress LR formation in rice (Ronzan et al. 2018). Interestingly, the rice aux1 mutant is highly sensitive to external Cd stress and shows reduced LR branching, root hair elongation, and PR growth (Cha et al. 2015; Yu et al. 2015a).
Similar to Cd stress, arsenate V (As) is a toxic metalloid, which affects root systems architecture. As it is transported through Pi transporters and in contaminated soils, its accumulation in plant organs negatively affects both plant and human health (Meharg and Macnair 1992). External application of As to Arabidopsis roots exhibited a reduction in root branching and a slight increase in PR length (Piacentini et al. 2020). Interestingly, external application of As affected aux1, pin1, and pin2 mutants more than WT plants, revealing that auxin transport machinery plays an important role mitigating As stress (Krishnamurthy and Rathinasabapathi 2013).
CONCLUDING REMARKS
Our review highlights that auxin is a critical regulator of root responses to environmental signals in soil, enabling plants to maximize resource acquisition. Auxin synthesis, inactivation, transport, and signaling are modified by stress-related signaling pathways such as ABA or ethylene, allowing integration of local environmental conditions into growth outputs. Whereas our understanding of how stress signaling is transmitted to transcriptome changes has grown substantially over the past decade, we are only just starting to understand the role of PTMs like SUMO on auxin responses to abiotic stresses (Orosa-Puente et al. 2018). In addition, our knowledge on how changes at transcriptional and posttranslational levels are differentially regulated in individual cells, tissues, and organs in response to stress is very limited (Wang et al. 2020). Individual cells and tissues differentially regulate their auxin levels and subsequent growth responses. For example, salt stress reduces PR tip auxin levels and response but increases LR branching (high auxin). This suggests a need for spatiotemporally fine-tuning auxin metabolism, transport, and signaling to ensure optimal adaptation of growth responses to external conditions. In addition, where and how stress sensing is translated into local versus systemic changes in auxin distribution and signaling has not been addressed to date but would significantly add to understanding how auxin contributes to plant stress responses. Future research will shed light on how micro-environmental conditions shape specific plant stress responses and how these are regulated on gene and protein levels. As the need for new crop varieties adapted to specific environmental conditions will increase with global climate change, this knowledge will inform breeding programs and allow the development of new varieties with increased fitness for future adverse conditions.
ACKNOWLEDGMENTS
We thank the University of Nottingham (Nottingham Research Fellowship to U.V.); BBSRC Divining Root Grant (BB/T001437/1) to N.L. and M.B.; BBSRC Doctoral Training Partnering award to J.B. and M.B.; and Future Food Beacon PhD+ Fellowship and Royal Society Challenge Grant (CHG\R1\170040) to B.P. and M.B. We apologize to colleagues whose work could not be cited owing to space limitations.
Footnotes
Editors: Dolf Weijers, Karin Ljung, Mark Estelle, and Ottoline Leyser
Additional Perspectives on Auxin Signaling available at www.cshperspectives.org
REFERENCES
- Abas L, Benjamins R, Malenica N, Paciorek T, Wišniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C. 2006. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat Cell Biol 8: 249–256. 10.1038/ncb1369 [DOI] [PubMed] [Google Scholar]
- Armstrong W. 1980. Aeration in higher plants. Adv Bot Res 7: 225–332. 10.1016/S0065-2296(08)60089-0 [DOI] [Google Scholar]
- Babé A, Lavigne T, Séverin JP, Nagel KA, Walter A, Chaumont F, Batoko H, Beeckman T, Draye X. 2012. Repression of early lateral root initiation events by transient water deficit in barley and maize. Philos Trans R Soc B Biol Sci 367: 1534–1541. 10.1098/rstb.2011.0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Band LR, Wells DM, Larrieu A, Sun J, Middleton AM, French AP, Brunoud G, Sato EM, Wilson MH, Peŕet B, et al. 2012. Root gravitropism is regulated by a transient lateral auxin gradient controlled by a tipping-point mechanism. Proc Natl Acad Sci 109: 4668−4673. 10.1073/pnas.1201498109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda J, Bellande K, von Wangenheim D, Goh T, Guyomarc'h S, Laplaze L, Bennett MJ. 2019. Lateral root formation in Arabidopsis: a well-ordered LRexit. Trends Plant Sci 24: 826–839. 10.1016/j.tplants.2019.06.015 [DOI] [PubMed] [Google Scholar]
- Bao Y, Aggarwal P, Robbins NE, Sturrock CJ, Thompson MC, Tan HQ, Tham C, Duan L, Rodriguez PL, Vernoux T, et al. 2014. Plant roots use a patterning mechanism to position lateral root branches toward available water. Proc Natl Acad Sci 111: 9319–9324. 10.1073/pnas.1400966111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini C, Pacurar DI, Perrone I. 2014. Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol 65: 639–666. 10.1146/annurev-arplant-050213-035645 [DOI] [PubMed] [Google Scholar]
- Bhosale R, Giri J, Pandey BK, Giehl RFH, Hartmann A, Traini R, Truskina J, Leftley N, Hanlon M, Swarup K, et al. 2018. A mechanistic framework for auxin dependent Arabidopsis root hair elongation to low external phosphate. Nat Commun 9: 1409. 10.1038/s41467-018-03851-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billen G, Garnier J, Lassaletta L. 2013. The nitrogen cascade from agricultural soils to the sea: modelling nitrogen transfers at regional watershed and global scales. Philos Trans R Soc B Biol Sci 368: 20130123. 10.1098/rstb.2013.0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonser AM, Lynch J, Snapp S. 1996. Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytol 132: 281−288. 10.1111/j.1469-8137.1996.tb01847.x [DOI] [PubMed] [Google Scholar]
- Casanova-Sáez R, Voß U. 2019. Auxin metabolism controls developmental decisions in land plants. Trends Plant Sci 24: 741–754. 10.1016/j.tplants.2019.05.006 [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, Graham N, Inzé D, Sandberg G, Casero PJ, et al. 2001. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell 13: 843−852. 10.1105/tpc.13.4.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha J-Y, Kim W-Y, Kang SB, Kim JI, Baek D, Jung IJ, Kim MR, Li N, Kim H-J, Nakajima M, et al. 2015. A novel thiol-reductase activity of Arabidopsis YUC6 confers drought tolerance independently of auxin biosynthesis. Nat Commun 6: 8041. 10.1038/ncomms9041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Oliveira MM. 2004. Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55: 2365–2384. 10.1093/jxb/erh269 [DOI] [PubMed] [Google Scholar]
- Colmer TD, Voesenek LACJ. 2009. Flooding tolerance: suites of plant traits in variable environments. Funct Plant Biol 36: 665–681. 10.1071/FP09144 [DOI] [PubMed] [Google Scholar]
- da Costa CT, Offringa R, Fett-Neto AG. 2020. The role of auxin transporters and receptors in adventitious rooting of Arabidopsis thaliana pre-etiolated flooded seedlings. Plant Sci 290: 110294. 10.1016/j.plantsci.2019.110294 [DOI] [PubMed] [Google Scholar]
- Dawood T, Yang X, Visser EJW, Te Beek TAH, Kensche PR, Cristescu SM, Lee S, Floková K, Nguyen D, Mariani C, et al. 2016. A co-opted hormonal cascade activates dormant adventitious root primordia upon flooding in Solanum dulcamara. Plant Physiol 170: 2351–2364. 10.1104/pp.15.00773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I, Tetsumura T, De Rybel B, dit Frey NF, Laplaze L, Casimiro I, Swarup R, Naudts M, Vanneste S, Audenaert D, et al. 2007. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 134: 681−690. 10.1242/dev.02753 [DOI] [PubMed] [Google Scholar]
- Dietrich D. 2018. Hydrotropism: how roots search for water. J Exp Bot 69: 2759–2771. 10.1093/jxb/ery034 [DOI] [PubMed] [Google Scholar]
- Dinneny JR, Long TA, Wang JY, Jung JW, Mace D, Pointer S, Barron C, Brady SM, Schiefelbein J, Benfey PN. 2008. Cell identity mediates the response of Arabidopsis roots to abiotic stress. Science 320: 942–945. 10.1126/science.1153795 [DOI] [PubMed] [Google Scholar]
- Du H, Wu N, Fu J, Wang S, Li X, Xiao J, Xiong L. 2012. A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J Exp Bot 63: 6467–6480. 10.1093/jxb/ers300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Liu H, Xiong L. 2013. Endogenous auxin and jasmonic acid levels are differentially modulated by abiotic stresses in rice. Front Plant Sci 4: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elobeid M, Göbel C, Feussner I, Polle A. 2012. Cadmium interferes with auxin physiology and lignification in poplar. J Exp Bot 63: 1413−1421. 10.1093/jxb/err384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani MN, Kusano M, Nguyen KH, Watanabe Y, Van Ha C, Saito K, Sulieman S, Herrera-Estrella L, Tran LSP. 2016. Adaptation of the symbiotic Mesorhizobium–chickpea relationship to phosphate deficiency relies on reprogramming of whole-plant metabolism. Proc Natl Acad Sci 113: E4610−E4619. 10.1073/pnas.1609440113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Agricultural Organization of the United Nations (FAO). 2009. High level expert forum—how to feed the world in 2050. Economic and Social Development, Rome.
- Galvan-Ampudia CS, Testerink C. 2011. Salt stress signals shape the plant root. Curr Opin Plant Biol 14: 296–302. 10.1016/j.pbi.2011.03.019 [DOI] [PubMed] [Google Scholar]
- Galvan-Ampudia CS, Julkowska MM, Darwish E, Gandullo J, Korver RA, Brunoud G, Haring MA, Munnik T, Vernoux T, Testerink C. 2013. Halotropism is a response of plant roots to avoid a saline environment. Curr Biol 23: 2044–2050. 10.1016/j.cub.2013.08.042 [DOI] [PubMed] [Google Scholar]
- Giri J, Bhosale R, Huang G, Pandey BK, Parker H, Zappala S, Yang J, Dievart A, Bureau C, Ljung K, et al. 2018. Rice auxin influx carrier OsAUX1 facilitates root hair elongation in response to low external phosphate. Nat Commun 9: 1810. 10.1038/s41467-018-04280-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh T, Toyokura K, Yamaguchi N, Okamoto Y, Uehara T, Kaneko S, Takebayashi Y, Kasahara H, Ikeyama Y, Okushima Y, et al. 2019. Lateral root initiation requires the sequential induction of transcription factors LBD16 and PUCHI in Arabidopsis thaliana. New Phytol 224: 749–760. 10.1111/nph.16065 [DOI] [PubMed] [Google Scholar]
- Huang S, Chen S, Liang Z, Zhang C, Yan M, Chen J, Xu G, Fan X, Zhang Y. 2015. Knockdown of the partner protein OsNAR2.1 for high-affinity nitrate transport represses lateral root formation in a nitrate-dependent manner. Sci Rep 5: 18192. 10.1038/srep18192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Liang W, Sturrock CJ, Pandey BK, Giri J, Mairhofer S, Wang D, Muller L, Tan H, York LM, et al. 2018. Rice actin binding protein RMD controls crown root angle in response to external phosphate. Nat Commun 9: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanchenko MG, Zhu J, Wang B, Medvecká E, Du Y, Azzarello E, Mancuso S, Megraw M, Filichkin S, Dubrovsky JG, et al. 2015. The cyclophilin a DIAGEOTROPICA gene affects auxin transport in both root and shoot to control lateral root formation. Development 142: 712–721. 10.1242/dev.113225 [DOI] [PubMed] [Google Scholar]
- Jackson MB. 1985. Ethylene and responses of plants to soil waterlogging and submergence. Annu Rev Phytopathol 36: 145–174. [Google Scholar]
- Jackson MB, Armstrong W. 1999. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biol 1: 274–287. 10.1111/j.1438-8677.1999.tb00253.x [DOI] [Google Scholar]
- Julkowska MM, Koevoets IT, Mol S, Hoefsloot H, Feron R, Tester MA, Keurentjes JJB, Korte A, Haring MA, de Boer GJ, et al. 2017. Genetic components of root architecture remodeling in response to salt stress. Plant Cell 29: 3198–3213. 10.1105/tpc.16.00680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Q, Wang Z, Ji CY, Jeong JC, Lee HS, Li H, Xu B, Deng X, Kwak SS. 2015. Transgenic poplar expressing Arabidopsis YUCCA6 exhibits auxin-overproduction phenotypes and increased tolerance to abiotic stress. Plant Physiol Biochem 94: 19–27. 10.1016/j.plaphy.2015.05.003 [DOI] [PubMed] [Google Scholar]
- Kiba T, Krapp A. 2016. Plant nitrogen acquisition under low availability: regulation of uptake and root architecture. Plant Cell Physiol 57: 707−714. 10.1093/pcp/pcw052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D'Angelo C, Bornberg-Bauer E, Kudla J, Harter K. 2007. The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50: 347–363. 10.1111/j.1365-313X.2007.03052.x [DOI] [PubMed] [Google Scholar]
- Kim JI, Baek D, Park HC, Chun HJ, Oh DH, Lee MK, Cha JY, Kim WY, Kim MC, Chung WS, et al. 2013. Overexpression of Arabidopsis YUCCA6 in potato results in high-auxin developmental phenotypes and enhanced resistance to water deficit. Mol Plant 6: 337–349. 10.1093/mp/sss100 [DOI] [PubMed] [Google Scholar]
- Kirungu JN, Magwanga RO, Lu P, Cai X, Zhou Z, Wang X, Peng R, Wang K, Liu F. 2019. Functional characterization of Gh_A08G1120 (GH3.5) gene reveal their significant role in enhancing drought and salt stress tolerance in cotton. BMC Genet 20: 62. 10.1186/s12863-019-0756-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koevoets IT, Venema JH, Elzenga JTM, Testerink C. 2016. Roots withstanding their environment: exploiting root system architecture responses to abiotic stress to improve crop tolerance. Front Plant Sci 7: 1335. 10.3389/fpls.2016.01335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Zhong H, Deng X, Gautam M, Gong Z, Zhang Y, Zhao G, Liu C, Li Y. 2019. Evolutionary analysis of GH3 genes in six Oryza species/subspecies and their expression under salinity stress in Oryza sativa ssp. Japonica. Plants (Basel) 8: 30. 10.3390/plants8020030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korver RA, Koevoets IT, Testerink C. 2018. Out of shape during stress: a key role for auxin. Trends Plant Sci 23: 783–793. 10.1016/j.tplants.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy A, Rathinasabapathi B. 2013. Auxin and its transport play a role in plant tolerance to arsenite-induced oxidative stress in Arabidopsis thaliana. Plant, Cell Environ 36: 1838−1849. 10.1111/pce.12093 [DOI] [PubMed] [Google Scholar]
- Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, et al. 2010. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell 18: 927–937. 10.1016/j.devcel.2010.05.008 [DOI] [PubMed] [Google Scholar]
- Lavenus J, Goh T, Roberts I, Guyomarc'h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L. 2013. Lateral root development in Arabidopsis: fifty shades of auxin. Trends Plant Sci 18: 450−458. 10.1016/j.tplants.2013.04.006 [DOI] [PubMed] [Google Scholar]
- Lavenus J, Goh T, Guyomarc'H S, Hill K, Lucas M, Voß U, Kenobi K, Wilson MH, Farcot E, Hagen G, et al. 2015. Inference of the Arabidopsis lateral root gene regulatory network suggests a bifurcation mechanism that defines primordia flanking and central zones. Plant Cell 27: 1368–1388. 10.1105/tpc.114.132993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Jung JH, Han DY, Seo PJ, Park WJ, Park CM. 2012. Activation of a flavin monooxygenase gene YUCCA7 enhances drought resistance in Arabidopsis. Planta 235: 923–938. 10.1007/s00425-011-1552-3 [DOI] [PubMed] [Google Scholar]
- Li J, Wu WH, Wang Y. 2017. Potassium channel AKT1 is involved in the auxin-mediated root growth inhibition in Arabidopsis response to low K+ stress. J Integr Plant Biol 59: 895−909. 10.1111/jipb.12575 [DOI] [PubMed] [Google Scholar]
- Liu W, Li RJ, Han TT, Cai W, Fu ZW, Lu YT. 2015. Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol 168: 343–356. 10.1104/pp.15.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G. 2005. Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17: 1090–1104. 10.1105/tpc.104.029272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2011. Root phenes for enhanced soil exploration and phosphorus acquisition: tools for future crops. Plant Physiol 156: 1041−1049. 10.1104/pp.111.175414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Brown KM. 2001. Topsoil foraging—an architectural adaptation of plants to low phosphorus availability. Plant Soil 237: 225−237. 10.1023/A:1013324727040 [DOI] [Google Scholar]
- Ma W, Li J, Qu B, He X, Zhao X, Li B, Fu X, Tong Y. 2014. Auxin biosynthetic gene TAR2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. Plant J 78: 70−79. 10.1111/tpj.12448 [DOI] [PubMed] [Google Scholar]
- Maas EV, Hoffman GJ. 1977. Crop salt tolerance—current assessment. J Irrig Drain Div 103: 115–134. 10.1061/JRCEA4.0001137 [DOI] [Google Scholar]
- Meharg AA, Macnair MR. 1992. Genetic correlation between arsenate tolerance and the rate of influx of arsenate and phosphate in Holcus lanatus L. Heredity (Edinb) 69: 336−341. 10.1038/hdy.1992.133 [DOI] [Google Scholar]
- Meisinger JJ, Delgado JA. 2002. Principles for managing nitrogen leaching. J Soil Water Conservation 57: 485−498. [Google Scholar]
- Morris EC, Griffiths M, Golebiowska A, Mairhofer S, Burr-Hersey J, Goh T, von Wangenheim D, Atkinson B, Sturrock CJ, Lynch JP, et al. 2017. Shaping 3D root system architecture. Curr Biol 27: R919–R930. 10.1016/j.cub.2017.06.043 [DOI] [PubMed] [Google Scholar]
- Muday GK. 2001. Auxins and tropisms. J Plant Growth Regul 20: 226–243. 10.1007/s003440010027 [DOI] [PubMed] [Google Scholar]
- Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681. 10.1146/annurev.arplant.59.032607.092911 [DOI] [PubMed] [Google Scholar]
- Nakayama N, Smith RS, Mandel T, Robinson S, Kimura S, Boudaoud A, Kuhlemeier C. 2012. Mechanical regulation of auxin-mediated growth. Curr Biol 22: 1468–1476. 10.1016/j.cub.2012.06.050 [DOI] [PubMed] [Google Scholar]
- Ogawa S, Valencia MO, Ishitani M, Selvaraj MG. 2014. Root system architecture variation in response to different NH4+ concentrations and its association with nitrogen-deficient tolerance traits in rice. Acta Physiol Plant 36: 2361−2372. 10.1007/s11738-014-1609-6 [DOI] [Google Scholar]
- Okushima Y, Overvoorde PJ, Arima K, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Lui A, Nguyen D, et al. 2005. Functional genomic analysis of the AUXIN RESPONSE FACTOR gene family members in Arabidopsis thaliana: unique and overlapping functions of ARF7 and ARF19. Plant Cell 17: 444–463. 10.1105/tpc.104.028316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orman-Ligeza B, Morris EC, Parizot B, Lavigne T, Babé A, Ligeza A, Klein S, Sturrock C, Xuan W, Novák O, et al. 2018. The xerobranching response represses lateral root formation when roots are not in contact with water. Curr Biol 28: 3165–3173.e5. 10.1016/j.cub.2018.07.074 [DOI] [PubMed] [Google Scholar]
- Orosa-Puente B, Leftley N, von Wangenheim D, Banda J, Srivastava AK, Hill K, Truskina J, Bhosale R, Morris E, Srivastava M, et al. 2018. Root branching toward water involves posttranslational modification of transcription factor ARF7. Science 362: 1407–1410. 10.1126/science.aau3956 [DOI] [PubMed] [Google Scholar]
- Osmont KS, Sibout R, Hardtke CS. 2007. Hidden branches: developments in root system architecture. Annu Rev Plant Biol 58: 93–113. 10.1146/annurev.arplant.58.032806.104006 [DOI] [PubMed] [Google Scholar]
- Pandey J, Singh AV, Singh A, Singh R. 2013. Impacts of changing atmospheric deposition chemistry on nitrogen and phosphorus loading to Ganga River (India). Bull Environ Contam Toxicol 91: 184–190. 10.1007/s00128-013-1016-5 [DOI] [PubMed] [Google Scholar]
- Paque S, Weijers D. 2016. Q&A: auxin: the plant molecule that influences almost anything. BMC Biol 14: 67. 10.1186/s12915-016-0291-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JE, Park JY, Kim YS, Staswick PE, Jeon J, Yun J, Kim SY, Kim J, Lee YH, Park CM. 2007. GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J Biol Chem 282: 10036–10046. 10.1074/jbc.M610524200 [DOI] [PubMed] [Google Scholar]
- Park HC, Cha JY, Yun DJ. 2013. Roles of YUCCAs in auxin biosynthesis and drought stress responses in plants. Plant Signal Behav 8: e24495. 10.4161/psb.24495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, Desnos T, Jost R, Kanno S, Berkowitz O, Nussaume L. 2014. Root architecture responses: in search of phosphate. Plant Physiol 166: 1713−1723. 10.1104/pp.114.244541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Torres CA, López-Bucio J, Cruz-Ramírez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, Herrera-Estrella L. 2008. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 20: 3258−3272. 10.1105/tpc.108.058719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini D, Corpas FJ, D'Angeli S, Altamura MM, Falasca G. 2020. Cadmium and arsenic-induced-stress differentially modulates Arabidopsis root architecture, peroxisome distribution, enzymatic activities and their nitric oxide content. Plant Physiol Biochem 148: 312−323. 10.1016/j.plaphy.2020.01.026 [DOI] [PubMed] [Google Scholar]
- Ramaekers L, Remans R, Rao IM, Blair MW, Vanderleyden J. 2010. Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crop Res 117: 169–176. 10.1016/j.fcr.2010.03.001 [DOI] [Google Scholar]
- Robbins NE, Dinneny JR. 2015. The divining root: moisture-driven responses of roots at the micro- and macro-scale. J Exp Bot 66: 2145–2154. 10.1093/jxb/eru496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins NE, Dinneny JR. 2018. Growth is required for perception of water availability to pattern root branches in plants. Proc Natl Acad Sci 115: E822–E831. 10.1073/pnas.1710709115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzan M, Piacentini D, Fattorini L, Della Rovere F, Eiche E, Riemann M, Altamura MM, Falasca G. 2018. Cadmium and arsenic affect root development in Oryza sativa L. negatively interacting with auxin. Environ Exp Bot 151: 64−75. 10.1016/j.envexpbot.2018.04.008 [DOI] [Google Scholar]
- Rosquete MR, Kleine-Vehn J. 2013. Halotropism: turning down the salty date. Curr Biol 23: R927–R929. 10.1016/j.cub.2013.08.020 [DOI] [PubMed] [Google Scholar]
- Salehin M, Bagchi R, Estelle M. 2015. ScfTIR1/AFB-based auxin perception: mechanism and role in plant growth and development. Plant Cell 27: 9–19. 10.1105/tpc.114.133744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo PJ, Xiang F, Qiao M, Park JY, Lee YN, Kim SG, Lee YH, Park WJ, Park CM. 2009. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiol 151: 275–289. 10.1104/pp.109.144220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo PJ, Lee SB, Suh MC, Park MJ, Park CM. 2011. The MYB96 transcription factor regulates cuticular wax biosynthesis under drought conditions in Arabidopsis. Plant Cell 23: 1138–1152. 10.1105/tpc.111.083485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen X, Zhang W, Zhang F. 2011. Phosphorus dynamics: from soil to plant. Plant Physiol 156: 997−1005. 10.1104/pp.111.175232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Wang S, Zhang S, Xu Y, Qian Q, Qi Y, Jiang DA. 2013. OsARF16, a transcription factor, is required for auxin and phosphate starvation response in rice (Oryza sativa L.). Plant Cell Environ 36: 607−620. 10.1111/pce.12001 [DOI] [PubMed] [Google Scholar]
- Shi H, Chen L, Ye T, Liu X, Ding K, Chan Z. 2014. Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol Biochem PPB 82: 209–217. 10.1016/j.plaphy.2014.06.008 [DOI] [PubMed] [Google Scholar]
- Skirycz A, Inzé D. 2010. More from less: plant growth under limited water. Curr Opin Biotechnol 21: 197–203. 10.1016/j.copbio.2010.03.002 [DOI] [PubMed] [Google Scholar]
- Sun F, Zhang W, Hu H, Li B, Wang Y, Zhao Y, Li K, Liu M, Li X. 2008. Salt modulates gravity signaling pathway to regulate growth direction of primary roots in Arabidopsis. Plant Physiol 146: 178–188. 10.1104/pp.107.109413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K. 2006. Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 7: 847–859. 10.1038/nrm2020 [DOI] [PubMed] [Google Scholar]
- Tiwari S, Lata C, Chauhan PS, Prasad V, Prasad M. 2017. A functional genomic perspective on drought signalling and its crosstalk with phytohormone-mediated signalling pathways in plants. Curr Genomics 18: 469−482. 10.2174/1389202918666170605083319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel S, Kaeppler SM, Brown KM, Lynch JP. 2013. Maize root growth angles become steeper under low N conditions. Field Crop Res 140: 18−31. 10.1016/j.fcr.2012.09.010 [DOI] [Google Scholar]
- Vanneste S, Friml J. 2009. Auxin: a trigger for change in plant development. Cell 136: 1005–1016. 10.1016/j.cell.2009.03.001 [DOI] [PubMed] [Google Scholar]
- Verma V, Ravindran P, Kumar PP. 2016. Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16: 86. 10.1186/s12870-016-0771-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal EA, Álvarez JM, Gutiérrez RA. 2014. Nitrate regulation of AFB3 and NAC4 gene expression in Arabidopsis roots depends on NRT1.1 nitrate transport function. Plant Signal Behav 9: e28501. 10.4161/psb.28501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoz ML, Loreti E, Mensuali A, Alpi A, Perata P. 2010. Hormonal interplay during adventitious root formation in flooded tomato plants. Plant J 63: 551–562. 10.1111/j.1365-313X.2010.04262.x [DOI] [PubMed] [Google Scholar]
- Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, et al. 2017. Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci 8: 161. 10.3389/fpls.2017.00161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EJW, Voesenek LACJ. 2005. Acclimation to soil flooding-sensing and signal-transduction. Plant Soil 274: 197–214. 10.1007/s11104-004-1650-0 [DOI] [Google Scholar]
- Visser EJW, Cohen JD, Barendse GWM, Blom CWPM, Voesenek LACJ. 1996. An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded Rumex palustris Sm. Plant Physiol 112: 1687–1692. 10.1104/pp.112.4.1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser EJW, Voesenek LACJ, Vartapetian BB, Jackson MB. 2003. Flooding and plant growth. Ann Bot 91: 107–109. 10.1093/aob/mcg014 [DOI] [Google Scholar]
- Voesenek LACJ, Bailey-Serres J. 2015. Flood adaptive traits and processes: an overview. New Phytol 206: 57–73. 10.1111/nph.13209 [DOI] [PubMed] [Google Scholar]
- von Wangenheim D, Banda J, Schmitz A, Boland J, Bishopp A, Maizel A, Stelzer EHK, Bennett M. 2020. Early developmental plasticity of lateral roots in response to asymmetric water availability. Nat Plants 6: 73–77. 10.1038/s41477-019-0580-z [DOI] [PubMed] [Google Scholar]
- Wang Y, Li K, Li X. 2009. Auxin redistribution modulates plastic development of root system architecture under salt stress in Arabidopsis thaliana. J Plant Physiol 166: 1637–1645. 10.1016/j.jplph.2009.04.009 [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang S, Sun C, Xu Y, Chen Y, Yu C, Qian Q, Jiang DA, Qi Y. 2014. Auxin response factor (OsARF12), a novel regulator for phosphate homeostasis in rice (Oryza sativa). New Phytol 201: 91−103. 10.1111/nph.12499 [DOI] [PubMed] [Google Scholar]
- Wang Q, Qin G, Cao M, Chen R, He Y, Yang L, Zeng Z, Yu Y, Gu Y, Xing W, et al. 2020. A phosphorylation-based switch controls TAA1-mediated auxin biosynthesis in plants. Nat Commun 11: 679. 10.1038/s41467-020-14395-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- West G, Inzé D, Beemster GTS. 2004. Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol 135: 1050–1058. 10.1104/pp.104.040022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson LC, Ribrioux SPCP, Fitter AH, Ottoline Leyser HM. 2001. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol 126: 875−882. 10.1104/pp.126.2.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Tanaka A, Inahashi H, Nishizawa NK, Tsutsumi N, Inukai Y, Nakazono M. 2019. Fine control of aerenchyma and lateral root development through AUX/IAA- and ARF-dependent auxin signaling. Proc Natl Acad Sci 116: 20770–20775. 10.1073/pnas.1907181116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Tanaka A, Tsutsumi N, Inukai Y, Nakazono M. 2020. A role for auxin in ethylene-dependent inducible aerenchyma formation in rice roots. Plants (Basel) 9: 610. 10.3390/plants9050610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Che G, Ding L, Chen Z, Liu X, Wang H, Zhao W, Ning K, Zhao J, Tesfamichael K, et al. 2016. Different cucumber CsYUC genes regulate response to abiotic stresses and flower development. Sci Rep 6: 20760. 10.1038/srep20760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LH, Miao ZQ, Qi GF, Wu J, Cai XT, Mao JL, Xiang CB. 2014. MADS-Box transcription factor AGL21 regulates lateral root development and responds to multiple external and physiological signals. Mol Plant 7: 1653−1669. 10.1093/mp/ssu088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Sun C, Shen C, Wang S, Liu F, Liu Y, Chen Y, Li C, Qian Q, Aryal B, et al. 2015a. The auxin transporter, OsAUX1, is involved in primary root and root hair elongation and in Cd stress responses in rice (Oryza sativa L.). Plant J 83: 818−820. 10.1111/tpj.12929 [DOI] [PubMed] [Google Scholar]
- Yu P, Li X, White PJ, Li C. 2015b. A large and deep root system underlies high nitrogen-use efficiency in maize production. PLoS ONE 10: e0126293. 10.1371/journal.pone.0126293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SW, Li CH, Cao J, Zhang YC, Zhang SQ, Xia YF, Sun DY, Sun Y. 2009. Altered architecture and enhanced drought tolerance in rice via the down-regulation of indole-3-acetic acid by TLD1/OsGH3.13 activation. Plant Physiol 151: 1889–1901. 10.1104/pp.109.146803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla G, Heimer YM, Barak S. 2010. Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. J Exp Bot 61: 211–224. 10.1093/jxb/erp290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwiewka M, Nodzyński T, Robert S, Vanneste S, Friml J. 2015. Osmotic stress modulates the balance between exocytosis and clathrin-mediated endocytosis in Arabidopsis thaliana. Mol Plant 8: 1175–1187. 10.1016/j.molp.2015.03.007 [DOI] [PubMed] [Google Scholar]