Summary
EGFR-specific mAbs display limited therapeutic efficacy in EGFR-positive solid tumors. To overcome this limitation, the significant improvement of the ADCC mediated-anti-tumor activity of a novel EGFR-specific mAb is described. Its potential impact on the efficacy of immunotherapy of EGFR-positive solid tumors is discussed.
In this issue of Clinical Cancer Research, Gerdes and colleagues (1) describe the development and characterization of the functional properties of the novel Epidermal Growth Factor Receptor (EGFR)-specific monoclonal antibody (mAb) GA201. The latter IgG1 mAb was generated by humanization of the EGFR-specific rat mAb ICR62 and glycoengineering of its Fc portion to enhance its binding to FcγRIIIA expressed on effector cells.
EGFR has been shown to be expressed and activated in several epithelial malignancies including colorectal cancer (CRC), squamous cell carcinoma of the head and neck (SCCHN), and carcinoma of the pancreas, lung, cervix, renal cell, prostate, bladder and breast (2). Like other growth factor receptors, EGFR can mediate oncogenic signals involved in proliferation and survival of tumor cells. This background information has provided the rationale to develop EGFR-targeted therapies, with small molecule EGFR tyrosine kinase inhibitors (TKIs) and with EGFR-specific mAbs (3).
Several lines of evidence have convincingly shown that both TKIs and mAbs can blockade proliferative and/or anti-apoptotic pathways in tumor cells and that these mechanisms play a major role in their therapeutic activity. However the EGFR-TKIs and the EGFR-specific mAbs inhibit EGFR activation through different mechanisms; the latter block the EGF binding to EGFR (4), while the former inhibit its autophosphorylation (5).
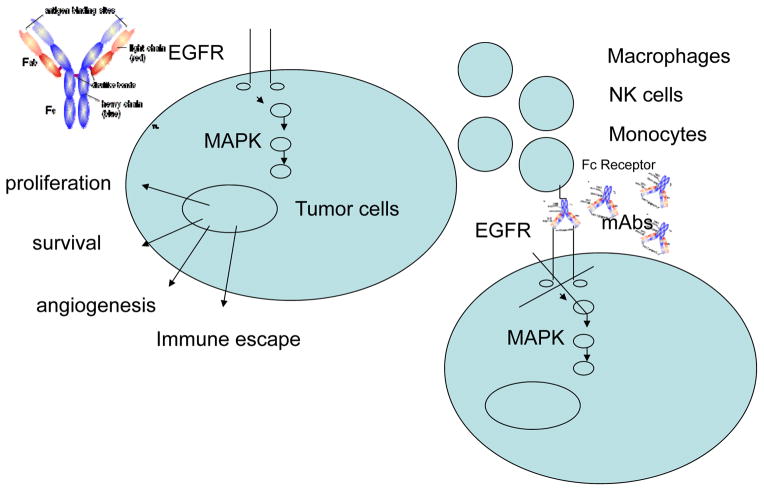
In addition to inhibiting EGFR activated signaling, IgG1 EGFR-specific mAbs may display anti-tumor activity through an antibody-dependent cell mediated cytotoxicity (ADCC) mechanism, i.e. by mediating the lysis of target cells by effector cells such as monocytes, macrophages and natural killer (NK) cells. This effect is influenced by the binding affinity of the mAb to the Fcγ receptors (FcγR) expressed by effector cells, as indicated by the association between polymorphism of FcγRIIIA and extent of lysis of target cells in ADCC (6) (Fig. 1).
Figure 1.
EGFR specific-mAb can mediate anti-tumor effect by inhibiting EGFR activation and mediating cell dependent lysis of tumor cells in ADCC
Some EGFR-TKIs (erlotinib and gefitinib) and EGFR-specific mAbs (chimeric IgG1 cetuximab and humanized IgG2 panitumumab) have received U.S. Food and Drug Administration (FDA) approval for treatment of various types of cancer either as single agents or in combination with chemotherapy or radiotherapy. In general EGFR-TKIs have been poorly effective in the treatment of malignancies with an EGFR pathogenesis, except for those which selectively target EGFR abnormalities responsible for the oncogenic signal. This is exemplified by the significant therapeutic efficacy of erlotinib and gefitinib in patients with lung adenocarcinoma harboring activating mutations in the EGFR TK domain (7). Modest clinical efficacy has also been reported for the FDA approved EGFR-specific mAbs cetuximab and panitumumab (8).
The anti-tumor activity mediated by EGFR-TKIs can be bypassed by mutations in molecules which activate oncogenic signals downstream EGFR blockade. These mutations appear to counteract also the immune mediated anti-tumor activity of the available EGFR-specific mAbs. This is exemplified by the poor therapeutic efficacy of the EGFR-specific mAbs, cetuximab and panitumumab, in patients with KRAS mutated CRC (8). These findings are surprising since no mechanism is readily available to explain why signaling activation downstream EGFR blockade can be associated, if not cause the resistance of CRC cells harboring KRAS mutations to the immune attack mediated by the IgG1 EGFR-specific mAbs used.
In this issue of Clinical Cancer Research, Gerdes and colleagues (1) postulate that these surprising findings reflect the poor ADCC activity of the presently FDA approved EGFR-specific mAbs. This possibility is supported by the results Gerdes and colleagues (1) have obtained with their own newly developed mAb GA201. Comparison of the binding characteristics and of the functional proprieties of the latter mAb with the mAb cetuximab in in vitro assays and in animal model systems has shown that these two IgG1 mAbs recognize distinct and spatially distant EGFR epitopes. Furthermore mAb GA201 displays a lower affinity for EGFR than cetuximab. Nevertheless the two mAbs do not differ in their ability to inhibit tumor cell proliferation and to induce apoptosis in vitro. Both mAbs exert these effects by inhibiting EGFR/HER2 heterodimerization and downstream signaling. However mAb GA201 displays a significantly higher activity than cetuximab in ADCC assays performed with several types of effector cells and with target cells expressing different EGFR levels. Whether this difference reflects at least in part the distinct characteristics of the EGFR epitopes recognized by the two mAbs remains to be determined. Furthermore at variance with cetuximab, the ADCC activity of mAb GA201 is not influenced by its affinity for FcγRIIA and FcγRIIIA since the extent of lysis of target cells mediated by mAb GA201 is similar when FcγRIIIA high- and low-affinity human NK cells are used as effectors. It is noteworthy that differences in affinity of FcγRIIIA which reflect its polymorphism appear to have clinical significance since an association between FcγRIIIA polymorphism and clinical response to cetuximab in patients with CRC has been reported (9). This association is not unique of cetuximab since it has been described also in patients with follicular lymphoma and in patients with breast cancer treated with the CD20-specific mAb rituximab (10) and with the HER2-specific mAb trastuzumab (11), respectively. Lastly, at variance with cetuximab, mAb GA201 is not affected in its ADCC activity by the presence of KRAS mutation in target cells. mAb GA201 mediates lysis of target cells even when they express low EGFR level and the human NK cells used as effectors express a low-affinity FcγRIIIA.
The conclusions derived from the described in vitro experiments have been corroborated by those derived from in vivo experiments. Utilizing various types of human tumor cell lines grafted in immunodeficient mice Gerdes and colleagues (1) have convincingly shown that mAb GA201 is significantly more effective than cetuximab in controlling tumor growth, both as a single agent and in combination with chemotherapy. More importantly the in vivo anti-tumor activity of mAb GA201 does not appear to be affected by variables such as level of EGFR expression and/or presence of KRAS mutations which abrogate the cetuximab antitumor activity.
In view of the potential clinical relevance of Gerdes and colleagues’s results (1) it is noteworthy that the mAb GA201 broadens the patient population who may be treated with EGFR targeted immunotherapy. Specifically the patients to be treated with mAb GA201 will include also those with low affinity FcγRIIIA as well as those with KRAS mutated tumors.
The comparison of the properties of mAb GA201 and cetuximab would have benefited from the identification of the normal tissue(s) with an EGFR expression level sufficient to trigger an ADCC by mAb GA201. Are the likely side effects caused by this mechanism a major obstacle to the clinical use of mAb GA201? Furthermore does mAb GA201 like other tumor antigen –specific mAbs (12) trigger a tumor antigen-specific T cell response? Lastly, in view of the postulated role of cancer initiating cells in disease recurrence and metastatic spread, does mAb GA201 either as a single agent or in combination with chemotherapeutic agent(s) and/or inhibitor(s) of core stem cell pathways (Notch, Sonic Hedgehog, Wnt) target cancer initiating cells? Nevertheless Gerdes and colleagues’ compelling results emphasize the urgency to translate to a clinical setting the strategies developed with mAb GA201, once its potential toxicity has been better defined.
Acknowledgments
Financial Support
This work was supported by PHS grants RO1 CA110249 and RO1 CA138188 awarded by the National Cancer Institute to Soldano Ferrone.
Footnotes
Disclosure of Potential Conflicts of Interest: there are no conflicts of interest to disclose.
References
- 1.Gerdes CA, Nicolini V, Herter S, van Puijenbroek E, Lang S, Roemmele M, et al. GA201 (RG7160): a novel, humanised, glycoengineered anti-EGFR antibody with enhanced ADCC and superior in vivo efficacy compared with cetuximab. Clin Cancer Res. 2012;19 doi: 10.1158/1078-0432.CCR-12-0989. [DOI] [PubMed] [Google Scholar]
- 2.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 1995;19:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 3.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–65. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 4.Green MC, Murray JL, Hortobagyi GN. Monoclonal antibody therapy for solid tumors. Cancer Treat Rev. 2000;26:269–86. doi: 10.1053/ctrv.2000.0176. [DOI] [PubMed] [Google Scholar]
- 5.Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, et al. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;62:5749–54. [PubMed] [Google Scholar]
- 6.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda H, Kobayashi S, Costa DB. EGFR exon 20 insertion mutations in non-small-cell lung cancer: preclinical data and clinical implications. Lancet Oncol. 2012;13:e23–31. doi: 10.1016/S1470-2045(11)70129-2. [DOI] [PubMed] [Google Scholar]
- 8.Serrano C, Markman B, Tabernero J. Integration of anti-epidermal growth factor receptor therapies with cytotoxic chemotherapy. Cancer J. 2010;16:226–34. doi: 10.1097/PPO.0b013e3181e07670. [DOI] [PubMed] [Google Scholar]
- 9.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27:1122–9. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 10.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26:1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 11.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21:3940–7. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Lee SC, Srivastava RM, López-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res. 2011;50:248–54. doi: 10.1007/s12026-011-8231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]