Abstract
The biomechanics of the shoulder relies on careful balancing between stability and mobility. A thorough understanding of normal and degenerative shoulder anatomy is necessary, as the goal of anatomic total shoulder arthroplasty is to reproduce premorbid shoulder kinematics.
With reported joint reaction forces up to 2.4 times bodyweight, failure to restore anatomy and therefore provide a stable fulcrum will result in early implant failure secondary to glenoid loosening.
The high variability of proximal humeral anatomy can be addressed with modular stems or stemless humeral components. The development of three-dimensional planning has led to a better understanding of the complex nature of glenoid bone deformity in eccentric osteoarthritis.
The treatment of cuff tear arthropathy patients was revolutionized by the arrival of Grammont’s reverse shoulder arthroplasty. The initial design medialized the centre of rotation and distalized the humerus, allowing up to a 42% increase in the deltoid moment arm.
More modern reverse designs have maintained the element of restored stability but sought a more anatomic postoperative position to minimize complications and maximize rotational range of motion.
Cite this article: EFORT Open Rev 2021;6:918-931. DOI: 10.1302/2058-5241.6.210014
Keywords: complication, distalization, eccentricity, glenohumeral arthritis, glenosphere size, humeral and glenoid morphology, inclination, inlay, mismatch, neck shaft angle, onlay, polyethylene, prosthesis design, replacement, shoulder pathology
Introduction
The glenohumeral joint is a complex biomechanical entity. In the physiologic state, the shoulder relies on bony anatomy, as well as on static (labrum and ligaments) and dynamic structures (rotator cuff) to adequately balance the force couples applied to the humeral head.1 The goal of anatomic total shoulder arthroplasty (ATSA) is, therefore, to restore the premorbid state by recreating normal shoulder kinematics. This simple objective can, however, be challenging to achieve, as anatomy is subject to premorbid variations, in addition to distortion secondary to degenerative or traumatic changes.2 On the contrary, reverse shoulder arthroplasty (RSA) is a non-anatomic procedure that achieves stability through a semi-constrained design and relies on the deltoid and other remaining muscles to move the humerus around a fixed glenosphere. While originally intended to treat patients with cuff tear arthropathy, its indications are continually expanding. Since the initial Grammont design, much innovation has been proposed to optimize active and impingement-free range of motion.
We provide an overview of the current biomechanical understanding of ATSA and RSA. These principles should help surgeons to plan and perform shoulder replacement surgeries in daily practice.
Anatomic total shoulder arthroplasty (ATSA)
As mentioned, anatomy is key to successfully reproduce patients’ physiologic joint kinematics. By virtue of its mobility, the glenohumeral joint is predisposed to instability. One factor affecting stability is the radius of curvature mismatch between the humeral head and glenoid. Further, only 20 to 30% of the humeral head is in contact with the glenoid.3 The rotator cuff acts as an essential dynamic stabilizing force centring the humeral in the mid-portion of range of motion, and is crucial for an ATSA to be effective.4 The supraspinatus helps to centre the humeral head against the force of the deltoid in lower degrees of abduction, while the infraspinatus and teres minor help to clear the greater tuberosity under the coracoacromial arch when the arm is moved in abduction and external rotation.4,5 Lastly, even though the shoulder is not a weight-bearing joint, joint reaction forces as high as 2.4 times bodyweight have been reported during shoulder rehabilitation.6
Humeral head
Proximal humerus anatomy is subject to great variability, which is further significantly modified by arthritic changes.7,8 As ATSA can restore physiologic shoulder kinetics, a thorough knowledge of normal anatomy appears mandatory, as one cannot simply rely on perioperative measures (Fig. 1).9 The non-arthritic humeral head has a mean three-dimensional measured diameter of 46.2 ± 5.4 mm (range, 37.1 to 56.9 mm) and a humeral height of approximately 19 mm (Fig. 2).10–14
Fig. 1.
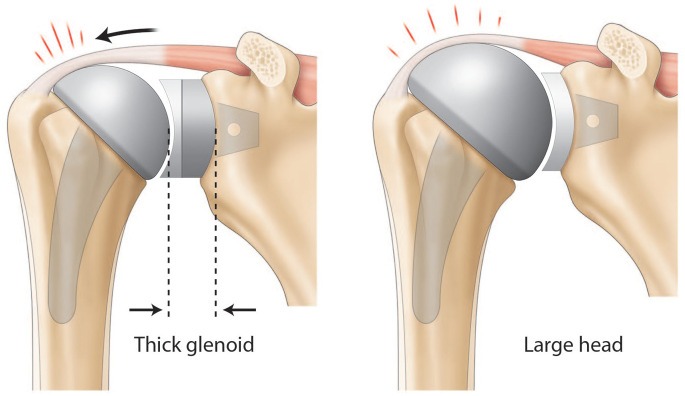
The goal of anatomic total shoulder arthroplasty (ATSA) is to restore physiologic shoulder kinetics. Glenoid lateralization or increased humeral component sizing (‘overstuffing’) will stress the rotator cuff.
Fig. 2.
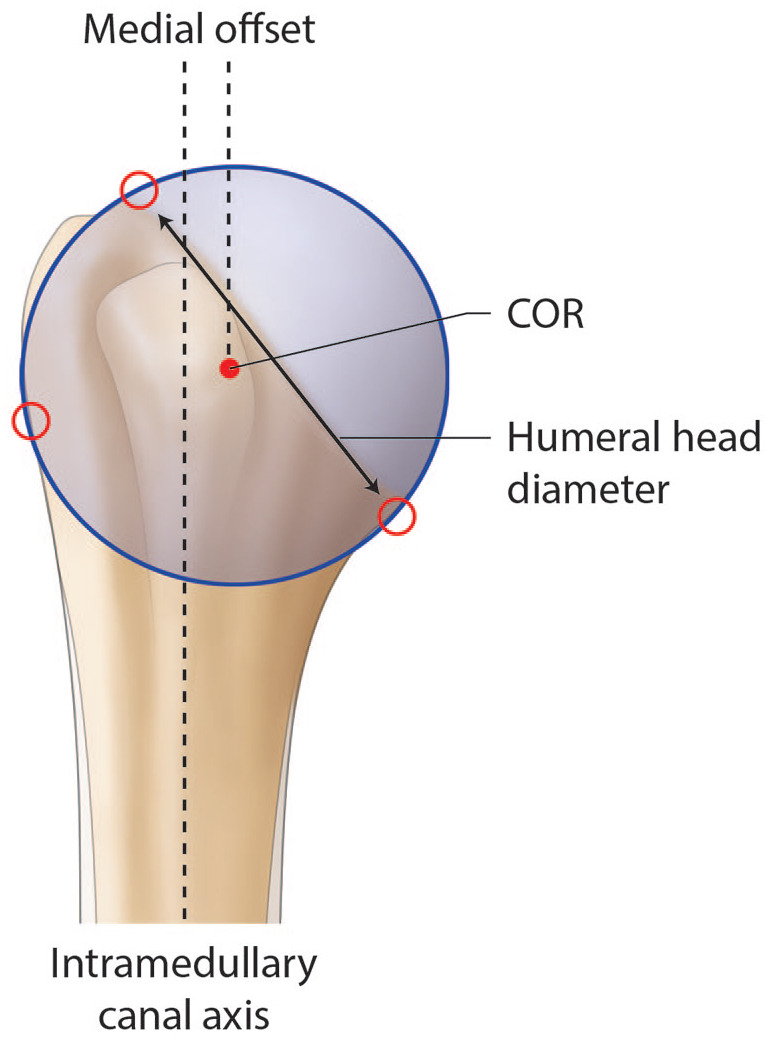
Illustration of a right non-arthritic humeral head. The humeral head diameter, the centre of rotation (COR), the intramedullary canal axis and the medial offset (distance between the intramedullary canal axis and the COR) are represented.
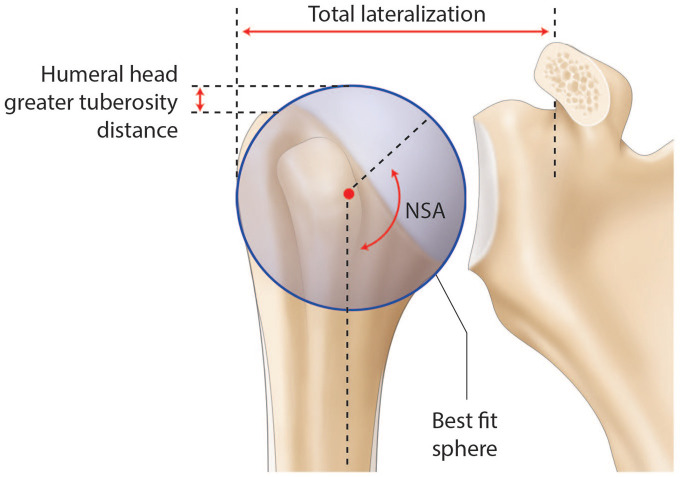
The osteoarthritic head is flattened and widened with a mean diameter of 59 ± 9 mm.7 The humeral head has the particularity to be elliptic in the periphery and become spherical in its central part, meaning that the cut surface will be about 2 mm larger from medial to lateral than from anterior to posterior.14 While spherical humeral head implants are mainly used in shoulder arthroplasty, elliptic implants have been proposed to reproduce anatomy and theoretically improve the rotational range of motion. The ratio between humeral head size and height is relatively constant.15 The highest point of the humeral head lies 8 ± 3.2 mm above the greater tuberosity (Fig. 3).14 Lastly, relative to the humeral canal, the head has a posterior and medial offset of 0.35 to 2.6 mm and 5.6 to 9.7 mm, respectively (Fig. 2 and Fig. 4).2,16
Fig. 3.
Illustration of a right non-arthritic humeral head. The humeral head–greater tuberosity distance, the neck-shaft angle (NSA), the best fit centre and the total lateralization are represented. The total lateralization reflects the glenohumeral offset, taking into account potential glenoid bone loss.
Fig. 4.
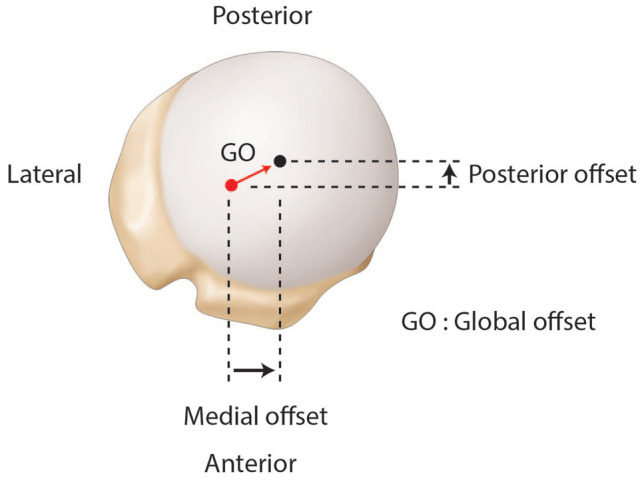
Superior view of a right shoulder. Representation of the medial, posterior and global (GO) offsets.
These parameters are helpful to select the appropriate humeral head implant, as this crucial step will ultimately determine the joint centre of rotation (COR). However, current biomechanical data do not support significant superiority of the elliptic design over the spherical one regarding the range of motion in internal and external rotation.17 Terrier et al illustrated in a numerical shoulder model that a 5 mm malposition of the humeral head implant resulted in impingement or subluxation for an inferior or superior shift, respectively. Both resulted in increased stress on the cement mantle.18 While joint COR can be determined three-dimensionally by a best-fit sphere using preserved non-articular landmarks, this technique has been translated to a two-dimensional process to allow intraoperative as well as postoperative radiographic evaluation (Fig. 2 and Fig. 3).8,19
However, there is no consensus on cut-off values for joint COR modification, as values as low as 2.5 mm can have been reported to impact impingement-free range of motion.20 Further, if the humeral head is implanted 5 mm too high in regard to the tuberosity, shoulder function will not solely be impaired by a 4 mm decrease in infraspinatus and subscapularis lever arms but also by the tight inferior capsule.21 Cadaveric studies have revealed that an increased humeral component sizing (commonly called ‘overstuffing’) would modify the COR and add stress to the rotator cuff (Fig. 1). Overstuffing not only decreases shoulder range of motion but also changes rotator cuff lever arm, exposing patients to the potential risk of secondary cuff failure.22,23 Restoring physiologic soft tissue tension will provide stability and prevent complications such as aseptic loosening and osteolysis induced by stress shielding.24Lastly, controversy exists regarding the superiority of resurfacing the humeral head over stemmed implants to reproduce physiological shoulder biomechanics.8,25
Neck-shaft angle
The mean neck-shaft angle (NSA) or inclination of the proximal humerus is approximately 135 degrees but variesbetween 115 and 148 degrees (Fig. 3). A study of 2058 humeri by Jeong et al notes that 22% are either < 130 degrees or > 140 degrees.26 Thus, fixed NSA humeral stems rely on surgeons to adapt their surgical techniques to accommodate patient anatomy. Modern modular systems provide centred and eccentric humeral heads as well as multiple NSA options.
Humeral torsion
Humeral head torsion is important in ATSA as it directly affects joint COR and thereby influences mobility in external rotation and shoulder stability.27–29 A cadaveric study by Pearl and Volk reported a mean humeral retrotorsion of 29.8 degrees with a 95% confidence interval of 7 to 52 degrees (Fig. 5).30 While they used the trochlear axis as reference, other reported values were based on the transepicondylar axis (which differs from 3 to 8 degrees). Furthermore, current systems use a jig aligned on the forearm as a reference, in this case, a 10 to 15 degree carrying angle must be added to the reported values (Fig. 5). When using a stem with lateral fins, another reliable landmark is to place it 12 ± 4 mm behind the bicipital groove.31 It should, however, be emphasized that the groove rotates about 16 ± 7 degrees and appears therefore as an unsuitable landmark in fracture or posttraumatic cases.32 Lastly, Raniga et al reported that in Walch B type glenoids, humeral retrotorsion is significantly lower compared to non-arthritic shoulders (14 ± 9 degrees vs. 36 ± 12 degrees, p < 0.001), suggesting a potential correlation between humeral retrotorsion and glenoid retroversion.33
Fig. 5.
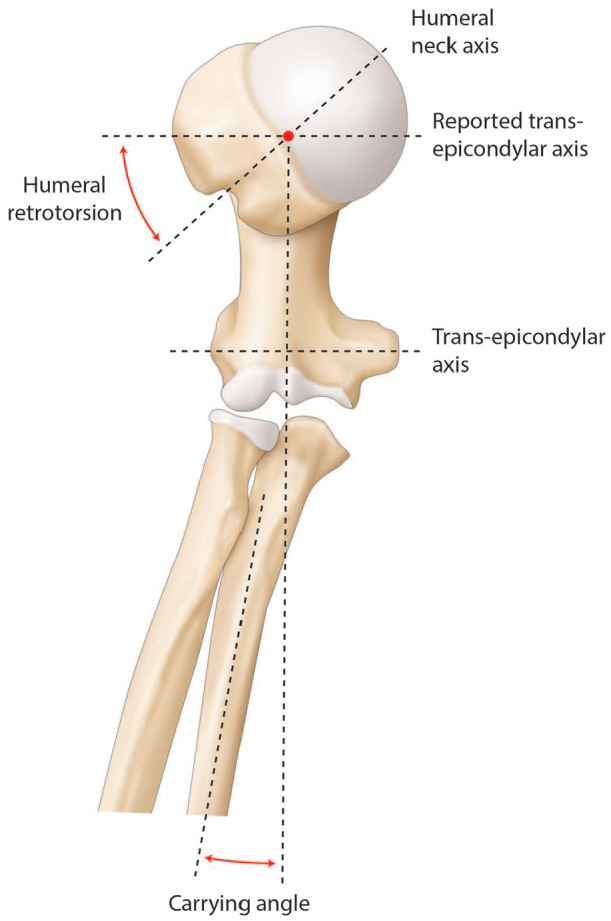
Illustration of right humerus and proximal radius and cubitus. The axes used to characterize the humeral retrotorsion and the carrying angle are the humeral neck, the diaphyseal, the trans-epicondylar, and the forearm axis. The humeral retrotorsion is defined by the angle between the humeral neck axis and the trans-epicondylar axis. The carrying angle is determined by the angle between the diaphyseal axis and the forearm axis. A humeral osteotomy guide relative to the forearm has to take into account the carrying angle.
Glenohumeral offset
Osteoarthritis results in loss of glenohumeral offset secondary to humeral and glenoid bone wear. While glenohumeral offset is subject to inter-person variability, a diminished glenohumeral offset implies altered deltoid and rotator cuff moment arms, as well as modified capsular tension (Fig. 2).10,14 This is thought to influence the postoperative range of motion by limiting active abduction as well as creating a tendency to inferiorly sublux the humeral head.28,34 Conversely, thick glenoid components create overstuffing (Fig. 1). Bodrogi et al recently described a reliable computerized tomography (CT)-based method to assess changes between pre- and post-arthroplasty glenohumeral offset measures.35 In the absence of humeral head sphericity (particularly in the setting of osteoarthritis), their method relied on the centre of the humeral shaft (rather than the centre of the humeral head) as described by Jacobsen and Friedman’s line to be independent of retroversion on the glenoid side.36
Medullary canal
Finally, the intramedullary canal not only becomes tighter but also increasingly retroverted from proximal to distal.13 Fixation of the humeral component is widely varied. Diaphyseal press-fit stems induce proximal stress shielding. Cementation is reliable at time zero but difficult in revision. The goals of reduced stress shielding, easier stem revision, and preservation of vascularity have led to a progressive shift towards short metaphyseal stem or stemless fixation.24 While a comparative cadaveric study revealed decreased micromotion and enhanced rotational stability in cemented stems,37 optimal stem fixation, length, and filling ratio to avoid stress shielding,38 subsidence,39 and misalignment remains controversial.40
Glenoid anatomy
Glenoid loosening remains the primary cause of ATSA failure.41 Similar to the humeral side, osteoarthritis appears to modify normal glenoid anatomy significantly. The glenoid seems relatively small and shallow compared to the humerus, with only 9 cm2 of articular surface.42 The glenoid is pear-shaped with a superior to an inferior dimension of 39 mm an inferior glenoid width averaging 29 mm.14 There is a radii mismatch between the glenoid and humeral head, while the radius of curvature is greater in the anteroposterior than the superoinferior direction (41 vs. 32 mm).3 Biomechanically, perfect conformity leads to a more stable joint but increased stress on the glenoid. On the other hand, an increased mismatch in radii will lead to increased translation of the humerus onto the glenoid with rim loading of the glenoid component causing a ‘rocking horse’ effect.43,44 Based on current techniques, the best compromise appears to be a mismatch ranging between 4 and 8 mm.45 However, it should be noted that these findings are based on a spherical humeral head. It has been proposed that conformed designs are better suited for elliptical heads.46
Glenoid version and inclination
Reported three-dimensional CT-derived measures report mean normal glenoid retroversion of 6 ± 4 degrees and inclination of 7 ± 5 degrees. Retroversion has been correlated (r = 0.7, P < 0.001) to posterior humeral head subluxation (59% ± 7%).47 The contralateral shoulder may be a reliable model; like side to side differences are limited to 5 degrees in 95% of cases.48 It is also important to assess the version in three dimensions, as in cases with > 10 degrees version, it is not solely direct posteriorly but also in superior, inferior, and anterior directions.49 A further important indicator when performing ATSA is that the version of the inferior part of the glenoid shows substantially less variability compared to the upper part and should therefore be used as the preferred intraoperative landmark in order to achieve adequate implant positioning.50
Concerning inclination, Moor et al proposed the critical shoulder angle (CSA) as a measure of scapular morphology with the benefit of combining measurements of glenoid inclination and lateral acromion coverage.51 They identified an angle inferior to 30 degrees as being associated with primary shoulder osteoarthritis. This finding is supported by subsequent biomechanical studies reporting increased joint reaction forces in case of a lower CSA.52,53 A CSA > 35 degrees is, on the other hand, related to an increased incidence of rotator cuff tears secondary to increased supraspinatus loading to compensate for increased joint instability as a consequence of increased glenohumeral joint shear forces.51,54,55 In the setting of ATSA, an increased CSA has been related to an increased incidence of glenoid radiolucencies.55
Humeral head subluxation
The Walch classification, with subsequent modifications, is the most common means of assessing glenoid changes secondary to primary osteoarthritis.56,57 Walch classified glenoid deformity based on posterior glenoid retroversion and humeral head subluxation. In opposition to type A glenoids (symmetrical bone loss), type B glenoids (asymmetrical bone loss) have been associated with progressive posterior glenoid bone loss over time.58 This factor is important when evaluating posterior humeral head subluxation; in type B3 glenoids, the head might be centred in regard to the glenoid but be posteriorly translated in relation to the scapula. Iannotti et al, by using three-dimensional standardized measures, reported a continuum of measures among the different type B and C glenoids rather than defined categories (B1, B2, B3, and C) in regard to glenoid retroversion and humeral head subluxation.59 Currently, it is still debated whether posterior humeral subluxation is the cause or consequence of increased retroversion.60 Static posterior humeral head subluxation and posterior glenoid wear have both been associated with premature osteoarthritis in young men and related to higher complication rates after ATSA.61–64 Recently, Beeler et al identified a flat acromion roof as a potential risk factor for posterior humeral head subluxation and posterior glenoid wear.65 This hypothesis was confirmed in a subsequent study by Meyer et al, reporting a median of 4 degrees more glenoid retroversion and a 5-degree less steep acromion in type B2 and C compared to type A and B1 glenoids (P ≤ 0.022).66
Instability
The rotator cuff and the horizontal force couple are critical to glenohumeral stability.1 By respecting cuff insertion and restoring bony anatomy, force couples should be adequately restored. Soft tissue balancing, by the combination of the anterior subscapularis tendon and capsule release sometimes associated with a capsulorraphy of the redundant posterior capsule, is indicated to reach Matsen’s criteria (40 degrees of external rotation, 60 degrees of internal rotation and a 50% posterior shift of the humeral head over the glenoid).67 If bony correction is necessary, one should carefully re-evaluate adequate humeral implant size as COR has likely changed secondary to the additional bone removal. When facing a retroverted glenoid, posterior instability can be compensated for by anteriorly offsetting the humeral head component, leading to a significant anterior humeral displacement on muscle activation as well as an anterior shift of the centre of pressure (p < 0.05).68,69 A major downside of this technique, however, is increased tension on the subscapularis with potentially higher rates of subscapularis failures. Chronic irreparable subscapularis deficiency is a contraindication to ATSA as it tends to destabilize the joint secondary to an upward migration of the humeral head and eccentric contact pressure onto the glenoid.70 While subscapularis preserving approaches have been described, most surgeons access the glenohumeral joint by subscapularis detachment with either a tenotomy, peel, or lesser tuberosity osteotomy. Effective subscapularis repair71 during surgery is therefore mandatory; a review of biomechanical cadaveric studies suggests superior load to failure for the osteotomy at time zero but no difference at cyclic loading.72,73 While de Wilde suggested that a C-block lesser tuberosity osteotomy might prevent postoperative subscapularis fatty infiltration, a recent systematic review reported no statistical difference in clinical and radiological outcomes between tenotomy, peel and osteotomy.74–76 In case of postoperative rupture, a prompt secondary repair can be considered to prevent instability but has been associated with variable results.77,78 The addition of anterior latissimus dorsi transfer seems biomechanically superior to the pectoralis major transfer in ATSA due to an improved internal rotation moment arm and more similar line of pull relative to the subscapularis.79
Glenoid bone loss
Correcting glenohumeral bone loss is an important step when implanting the glenoid component. Implanting the component in excessive retroversion will result in posterior translation of the humeral head and subsequent rim-loading known to cause early component loosening.80,81 According to a finite element model by Farron et al, 10 degrees of retroversion should be considered as the cut-off value.82 In their analysis, an implant with 20 degrees of retroversion resulted in a 326% increased stress within the cement mantel and a 706% increase of micromotion at the bone–cement interface. Recent work using statistical shape modelling allowed a computer reconstruction of the premorbid glenoid with a precision of about 1 mm and 2 degrees for version and inclination.83,84 Several techniques to correct retroversion were developed. If version is corrected alone by means of anterior glenoid reaming, it will lead to significant joint line medialization and central cortex perforation when correction exceeds 15 degrees.85 Consequently, posterior augmented glenoid implants were developed to avoid the medialization of the joint line, with encouraging early results.86 However, severe deformity has been associated with loosening of such components.87
Proper implantation technique avoiding superior inclination or retroversion is thought to be crucial to avoid edge-loading causing micromotion and subsequent breakdown at the bone–implant interface, ultimately leading to aseptic loosening.82,88 For the same reason, an intact cuff is also mandatory to conserve physiologic joint kinematics and therefore limit polyethylene wear.89 While most current ATSA heads are metallic, experimental studies suggest that a change to ceramic heads could reduce the polyethylene wear rate by up to 26.7%.90 A wide range of onlay all-polyethylene glenoid shapes (pear-shaped versus elliptic) and sizes are currently available on the market, with no current consensus on optimal designs regarding back surface (flat versus curved), anchorage (keel versus peg) or level of conformity.91 Further, a recent cadaveric study comparing inlay (implanted into the bone socket and therefore allowing for circumferential bone support) with onlay components revealed superior outcome regarding joint reaction forces and fatigue failure in favour of the inlay design.92 There is also renewed interest towards metal-back glenoids in response to the reported encouraging survival rates of modern designs.93 While the theoretical benefit of more stable fixation and easy conversion to RSA seems appealing, long-term outcomes are awaited based on the long list of retrieved pre-existing metal-back designs.94
Reverse shoulder arthroplasty
Historically, RSA was developed to address arthritis in cuff deficient shoulders as the loss of dynamic compression provided by the rotator cuff led to instability and early glenoid loosening, therefore resulting in unpredictable outcomes with large head hemiarthroplasty or ATSA.95,96 The reverse ball and socket ‘Grammont type’ RSA was introduced in 1985 and is based on the biomechanical principles of a medialized joint centre of rotation, distalized humerus, and a semi-constrained design with a constant joint COR.97 Contrary to ATSA, in which the humeral head rotates in a spinning motion around itself as the COR lies inside the humeral head, the constant COR in RSA lies inside the glenosphere and leads to a hinged motion of the humerus, making it prone to impingement thereby limiting range of motion (ROM).98
Modifications in muscle recruitment
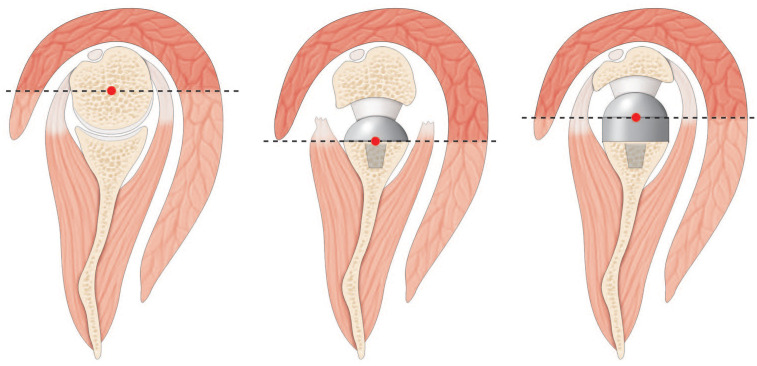
The aforementioned modifications to physiologic shoulder anatomy lead to a 42% increased deltoid lever arm, as well as an increased recruitment of anterior deltoid muscle fibres to perform abduction.99 The original design with a 155-degree non-anatomic stem further enhanced the deltoid lever arm by distalization of the humerus.100 The anterior deltoid becomes consecutively an important contributor to flexion and abduction moment arms.101 In case of a deficient anterior deltoid (i.e. revision surgery with detached or paretic anterior deltoid)102 compensation for abduction relies on significantly enhanced force of the subscapularis (195%) and middle portion of the deltoid (26%).103 There are, however, drawbacks to these anatomic modifications of physiologic moment arms. While the anterior and posterior deltoid as well as pectoralis major are recruited as additional flexors and abductors, the latissimus dorsi, teres major, and lower part of the pectoralis major have increased adductor and extensor moment arms, therefore directly limiting their participation in active internal and external rotation.104,105 As lever arms of the anterior and posterior cuff are already decreased secondary to humeral medialization, this adds to a further weakening of active internal and external rotation.106,107 This issue can either be addressed by the addition of a tendon transfer or by modifying the classic RSA design to a ‘lateralized’ one.108 This modification will preserve rotational moment arms of the subscapularis and teres minor and therefore enhance active range of motion in the axial plane (Fig. 6).109 Finally, while the postoperative range of motion takes place inside the prosthetic joint, scapulothoracic participation is significantly increased after RSA.110
Fig. 6.
(A) Native shoulder. The centre of rotation is in the humeral head, and the level of arm of deltoid does not allow consequent deltoid recruitment. (B) Reverse shoulder arthroplasty (RSA) with a medial glenoid/lateral humerus design in case of massive and irreparable rotator cuff lesion. Medialization of the centre of rotation and humeral lateralization allows important deltoid recruitment. (C) Lateral glenoid/medial humerus RSA. As in native shoulders, the bony lateralization of the centre of rotation decreases recruitment of the deltoid for rotation but allows for a retensioning of the rotator cuff.
Medialization of the joint centre of rotation (COR)
The biomechanical benefit of a medialized joint COR is to convert torque forces into compressive forces across the bone–glenosphere interface and therefore provide stability and enhanced component integration.111 As the rotator cuff no longer provides its compressive forces, the fixed COR allows the deltoid to compensate and provide the needed compression to stabilize the joint.99 While in ATSA joint reaction forces can reach up to 90% of bodyweight at 90 degrees of abduction, RSA design reduces both compressive and shear stress and therefore joint reaction forces by up to 42%. This further allows active abduction with a 20% decreased deltoid activity in a cuff deficient shoulder.112–114
There is, however, a major drawback of COR medialization in the form of impingement between the scapular neck and humeral prosthetic component defined as scapular notching.115,116 Several technical factors improve impingement-free range of motion. One option is placing the glenosphere (not the baseplate) below the inferior glenoid rim or using an inferior eccentric glenosphere. De Wilde et al reported that a 5-mm overhang could improve impingement-free adduction by 39 degrees.117–119 Abduction is also positively correlated with acromiohumeral distance (r = 0.93; p < 0.001) which is increased with an eccentric glenosphere.120 The ideal amount of overhang relative to the glenoid appears to be about 2.5 mm based on clinical evidence.121 Alternatively, glenosphere diameter can be increased, therefore upsizing the diameter from 38 to 46 mm was reported to not only increase range of motion by 39% but also stability by a 36% increase in jump distance.122 According to a computer simulation of impingement-free range of motion, the single most effective modification in prosthetic design is the change of humeral neck-shaft angle from the classic 155 degrees towards a more anatomic angle.123,124
While joint COR needs to be medialized in regard to the native COR, slight lateralization of the glenosphere from the glenoid can further enhance compressive forces, which are thought to overcome the increased shear forces at the bone–component interface.111 Basic science studies show several benefits of lateralization. In both sawbone125 and computer models,123,126,127 lateralization improves ROM in all directions.127 There is an ongoing debate regarding the impact of lateralization on the risk of acromial stress fractures. Finite element analysis has suggested a 17.2% increased acromial stress secondary to 10 mm lateralization.128 Clinically, distalization has been implicated as more of a culprit than lateralization.129 Glenosphere lateralization has, further, a linear correlation with baseplate micromotion130 and therefore exposes patients to the risk of aseptic loosening.131 Giles et al tested the effect of glenoid and humeral lateralization on deltoid muscle load in vitro using a simulator. They reported that 10 mm of humeral lateralization was the only parameter that actually decreased deltoid force in abduction (65 ± 8%), however, they warned that this benefit may not compensate for the negative effects induced by glenosphere lateralization.132 Lastly, Boileau et al proposed a bony increased-offset reverse shoulder arthroplasty to lateralize the glenosphere, however, maintaining COR at the prosthesis–bone interface and thereby minimizing torque stress.133
Baseplate design
To allow bone ingrowth, baseplate micromotion must be inferior to 150 um.134 As baseplates are screwed down to the glenoid, research focused on the optimal configuration to enhance initial stability on polyurethane foam models. While increased screw length ( > 17 mm inside the glenoid) or screw diameter (3.5 vs. 5.0 mm) was shown to additionally reduce micromotion by up to 30%, inclining screws by 30 degrees (compared to 0 degrees) was the most effective, as it led to a 50% reduction in micromotion.111,135 With a central post design, the most important screw in the baseplate is thought to be the inferior one, as tensile forces are the highest at the inferior border secondary to humeral loading. A locking screw should therefore be favoured in this particular location, as a 7% enhanced load to failure was reported compared to standard cortical screws.136 Regarding the total number of screws, a cadaveric study comparing a two-peripheral-screw flat-backed baseplate construct (superior and inferior one) with a four-screw construct found no statistical difference regarding motion during cyclic loading.137 Regarding baseplate design, the central screw does not seem superior to the post regarding load to failure compared to the central post.138 Lastly, Gutiérrez et al investigated optimal baseplate position using a computer model. According to their work, which focused on uniform force distribution, a 15-degree inferior tilt is best suited for a concentric or lateral eccentric glenosphere, and for an inferior eccentric glenosphere a neutral inclination (0 degrees) is the preferred orientation.139,140 Superior tilt should always be avoided as stress at the bone interface increases. Boileau et al suggested that superior tilt is commonly underestimated during RSA planification.141 As the baseplate is implanted in the inferior part of the glenoid, they introduced the RSA angle, defined as the angle between the inferior part of the glenoid fossa and the perpendicular to the floor of the supraspinatus. Compared to the ATSA angle (β angle or global glenoid inclination angle), the RSA angle is 8 ± 4 degrees larger.
Stability
The stabilizing effect of the rotator cuff is inexistent in a cuff deficient shoulder, making it prone to instability.100 In the physiologic state, the glenoid serves as a pillar for the humeral head. During shoulder range of motion, combined physiologic glenohumeral and scapulohumeral motion keep this pillar beneath the humeral head. Altered muscle balance forces in cuff tear arthropathy shoulders disrupt this dynamic process and explain the eccentric wear pattern encountered in cuff tear arthropathy. The endpoint is reached when the humeral head migrates upward and creates an acetabularization of the acromion, allowing a neutralization of the dynamic instability.142
Instability is one of the most cited complications after RSA.143 A wide variety of actors potentially influence stability, including glenosphere (eccentricity, diameter, inclination), humeral socket depth, humeral implant version, as well as humeral lateralization and length, as well as remaining subscapularis.139,143–146 The arm position most prone to instability is 30 degrees of abduction with neutral or internal rotation.146 Increasing glenosphere diameter from 38 to 42 mm was reported to augment stability by 32% by increasing joint load and deltoid force.146,147 Glenopshere positioning will impact stability as a 2-mm inferior offset enhances stability by 17%.119,143 Biomechanical data also suggest that superior tilt exposes patients to a higher risk of instability.117,148 Glenosphere lateralization is effective to prevent scapular impingement with the arm in adduction and to increase the force needed for anterior dislocation, the biomechanical benefit of a reduced deltoid force to abduct the arm is unfortunately lost (with lateralization of 15 mm).149,150 Comparison of humeral neck-shaft angle (135 vs. 155 degrees) revealed only a minor benefit with higher dislocation forces required in 135-degree stems at 30 degrees of abduction; this effect was, however, negligible compared to a 6–9 mm glenoid lateralization.149 Avoiding excessive humeral retrotorsion ( > 10 degrees) seems to have a higher impact on stability than glenosphere retroversion ( > 20 degrees).151 Conformity in radii between the glenosphere and humeral socket present in RSA results in an enhanced joint-reaction force vector tolerance to up to 45 degrees (compared to 30 degrees in the setting of an ATSA).130 Lastly, humeral socket depth defined in ratio to glenosphere diameter will increase stability at the potential cost of a reduced range of motion.139,144,152
Distalization of the humerus
While distalization of the humerus is a central point in RSA with the primary goal of increasing the lever arm of the deltoid and improving functional outcomes, there are consequences to lengthening. Optimal lengthening is thought to be around 2 cm but is still debated.153 While insufficient lengthening (particularly in the revision setting) has been shown to be a critical factor regarding joint instability,153,154 downsides of excessive lengthening include increasing the risk of a neurological lesion (neurapraxia) and over-tensioning resulting in a decreased range of motion as well as increased joint reaction forces.155,156 Furthermore, lengthening via an onlay humeral component has been associated with an increased risk of acromial stress fracture compared to inlay components.129 While there is no current consensus regarding the optimal way to increase soft tissue tension while avoiding complications,144,157 recent biomechanical data suggest that humeral lateralization could potentially be a solution to improve joint and muscle loading.132,158,159 However, one must keep in mind that humeral lateralization also leads to distalization. In addition to the aforementioned consequences, distalization also changes the force vectors of the remaining rotator cuff. The latter may be particularly important in the use of RSA for diagnoses other than rotator cuff arthropathy in which much of the rotator cuff is still functional, such as primary glenohumeral arthritis with posterior subluxation and a biconcave glenoid. Thus, there are not only trade-offs to distalization, but the ideal amount may also vary by diagnosis.
Conclusion
As the number of primary and revision shoulder arthroplasties is projected to progress by up to 322% by 2050, a thorough understanding of the biomechanical principle seems mandatory. The key concepts between these two procedures are yet very different. Reproducing anatomy is at the centre of ATSA philosophy. Therefore, a thorough understanding of premorbid anatomy is crucial to success, as inadequate restoration of the joint centre of rotation will predispose patients to secondary cuff failure and glenoid implant loosening. Further, posterior glenoid bone loss and humeral head subluxation (typically seen in Walch B2 and C glenoids) should be corrected to avoid premature glenoid component failure. While posterior augmented anatomic glenoid implants might solve this issue in the near future, a shift towards RSA in this particular setting can already be observed. With its semi-constraint design, RSA was initially developed to treat cuff tear arthropathy patients. Original indications further expanded towards primary OA with glenoid dysplasia, irreparable rotator cuff tears, three- and four-part fractures as well as revision of failed ATSA. The main complication with the original Grammont design is scapular notching, which might lead to secondary glenoid loosening. Inferior baseplate positioning and therefore inferior glenosphere overhang, bony or metallic baseplate lateralization as well as avoiding superior inclination, all minimize the risk of scapular impingement. Lower humeral neck-shaft angles can further reduce the risk of scapular notching and might enhance deltoid muscle recruitment and cuff tension, thereby potentially improving active external rotation. Current research on optimal RSA design focuses on improved impingement-free ROM. However, increased ROM should not be made at the cost of decreased stability or scapular fractures. One should always keep in mind that the goal of every arthroplasty is to alleviate pain and restore the best possible function.
Footnotes
ICMJE Conflict of interest statement: Dr. Lädermann reports that he is a paid consultant for Arthrex, Wright and Medacta and receives royalties from Stryker. He is the founder of BeeMed.
Dr. Collin reports that he is a paid consultant for Wright, Smith and Nephew and ConMed and receives royalties from Wright, Stortz and Advanced Medical Applications.
Dr Patrick J. Denard reports that he is a paid consultant for Arthrex and receives royalties from Arthrex.
The other authors report no conflicts of interest.
Permissions: Not applicable
Social media: linkedin.com/in/alexandre-lädermann-25140831
Twitter: @Laedermann
OA licence text: This article is distributed under the terms of the Creative Commons Attribution-Non Commercial 4.0 International (CC BY-NC 4.0) licence (https://creativecommons.org/licenses/by-nc/4.0/) which permits non-commercial use, reproduction and distribution of the work without further permission provided the original work is attributed.
Funding statement
The author or one or more of the authors have received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article. In addition, benefits have been or will be directed to a research fund, foundation, educational institution, or other non-profit organization with which one or more of the authors are associated.
FORE (Foundation for Research and Teaching in Orthopaedics, Sports Medicine, Trauma and Imaging in the Musculoskeletal System). Grant FORE 2021-53
References
- 1. Goetti P, Denard PJ, Collin P, Ibrahim M, Hoffmeyer P, Lädermann A. Shoulder biomechanics in normal and selected pathological conditions. EFORT Open Rev 2020;5:508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Getz CL, Ricchetti ET, Verborgt O, Brolin TJ. Normal and pathoanatomy of the arthritic shoulder: considerations for shoulder arthroplasty. J Am Acad Orthop Surg 2019;27:e1068–e1076. [DOI] [PubMed] [Google Scholar]
- 3. McPherson EJ, Friedman RJ, An YH, Chokesi R, Dooley RL. Anthropometric study of normal glenohumeral relationships. J Shoulder Elbow Surg 1997;6:105–112. [DOI] [PubMed] [Google Scholar]
- 4. Sharkey NA, Marder RA. The rotator cuff opposes superior translation of the humeral head. Am J Sports Med 1995;23:270–275. [DOI] [PubMed] [Google Scholar]
- 5. Lee SB, Kim KJ, O’Driscoll SW, Morrey BF, An KN. Dynamic glenohumeral stability provided by the rotator cuff muscles in the mid-range and end-range of motion: a study in cadavera. J Bone Joint Surg [Am] 2000;82-A:849–857. [DOI] [PubMed] [Google Scholar]
- 6. Bergmann G, Graichen F, Bender A, et al. In vivo gleno-humeral joint loads during forward flexion and abduction. J Biomech 2011;44:1543–1552. [DOI] [PubMed] [Google Scholar]
- 7. Knowles NK, Carroll MJ, Keener JD, Ferreira LM, Athwal GS. A comparison of normal and osteoarthritic humeral head size and morphology. J Shoulder Elbow Surg 2016;25:502–509. [DOI] [PubMed] [Google Scholar]
- 8. Alolabi B, Youderian AR, Napolitano L, et al. Radiographic assessment of prosthetic humeral head size after anatomic shoulder arthroplasty. J Shoulder Elbow Surg 2014;23:1740–1746. [DOI] [PubMed] [Google Scholar]
- 9. Büchler P, Farron A. Benefits of an anatomical reconstruction of the humeral head during shoulder arthroplasty: a finite element analysis. Clin Biomech (Bristol, Avon) 2004;19:16–23. [DOI] [PubMed] [Google Scholar]
- 10. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg [Br] 1997;79-B:857–865. [DOI] [PubMed] [Google Scholar]
- 11. Jun BJ, Iannotti JP, McGarry MH, Yoo JC, Quigley RJ, Lee TQ. The effects of prosthetic humeral head shape on glenohumeral joint kinematics: a comparison of non-spherical and spherical prosthetic heads to the native humeral head. J Shoulder Elbow Surg 2013;22:1423–1432. [DOI] [PubMed] [Google Scholar]
- 12. Berghs BM, Derveaux T, Speeckaert W, Vanslambrouck K, De Wilde LF. Three-dimensional analysis of the orientation and the inclination of the rotator cuff footprint. J Shoulder Elbow Surg 2011;20:637–645. [DOI] [PubMed] [Google Scholar]
- 13. Robertson DD, Yuan J, Bigliani LU, Flatow EL, Yamaguchi K. Three-dimensional analysis of the proximal part of the humerus: relevance to arthroplasty. J Bone Joint Surg [Am] 2000;82-A:1594–1602. [DOI] [PubMed] [Google Scholar]
- 14. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships: an anatomical study of one hundred and forty shoulders. J Bone Joint Surg [Am] 1992;74-A:491–500. [PubMed] [Google Scholar]
- 15. Hertel R, Knothe U, Ballmer FT. Geometry of the proximal humerus and implications for prosthetic design. J Shoulder Elbow Surg 2002;11:331–338. [DOI] [PubMed] [Google Scholar]
- 16. Barth J, Garret J, Boutsiadis A, et al. ; Shoulder Friends Institute. Is global humeral head offset related to intramedullary canal width? A computer tomography morphometric study. J Exp Orthop 2018;5:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muench LN, Otto A, Kia C, et al. Rotational range of motion of elliptical and spherical heads in shoulder arthroplasty: a dynamic biomechanical evaluation. Arch Orthop Trauma Surg 2020. 10.1007/s00402-020-03587-0 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 18. Terrier A, Ramondetti S, Merlini F, Pioletti DD, Farron A. Biomechanical consequences of humeral component malpositioning after anatomical total shoulder arthroplasty. J Shoulder Elbow Surg 2010;19:1184–1190. [DOI] [PubMed] [Google Scholar]
- 19. Youderian AR, Ricchetti ET, Drews M, Iannotti JP. Determination of humeral head size in anatomic shoulder replacement for glenohumeral osteoarthritis. J Shoulder Elbow Surg 2014;23:955–963. [DOI] [PubMed] [Google Scholar]
- 20. Favre P, Moor B, Snedeker JG, Gerber C. Influence of component positioning on impingement in conventional total shoulder arthroplasty. Clin Biomech (Bristol, Avon) 2008;23:175–183. [DOI] [PubMed] [Google Scholar]
- 21. Nyffeler RW, Sheikh R, Jacob HA, Gerber C. Influence of humeral prosthesis height on biomechanics of glenohumeral abduction: an in vitro study. J Bone Joint Surg [Am] 2004;86-A:575–580. [DOI] [PubMed] [Google Scholar]
- 22. Vaesel MT, Olsen BS, Søjbjerg JO, Helmig P, Sneppen O. Humeral head size in shoulder arthroplasty: a kinematic study. J Shoulder Elbow Surg 1997;6:549–555. [DOI] [PubMed] [Google Scholar]
- 23. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg 1996;5:320–326. [DOI] [PubMed] [Google Scholar]
- 24. Keener JD, Chalmers PN, Yamaguchi K. The humeral implant in shoulder arthroplasty. J Am Acad Orthop Surg 2017;25:427–438. [DOI] [PubMed] [Google Scholar]
- 25. Hammond G, Tibone JE, McGarry MH, Jun BJ, Lee TQ. Biomechanical comparison of anatomic humeral head resurfacing and hemiarthroplasty in functional glenohumeral positions. J Bone Joint Surg [Am] 2012;94-A:68–76. [DOI] [PubMed] [Google Scholar]
- 26. Jeong J, Bryan J, Iannotti JP. Effect of a variable prosthetic neck-shaft angle and the surgical technique on replication of normal humeral anatomy. J Bone Joint Surg [Am] 2009;91-A:1932–1941. [DOI] [PubMed] [Google Scholar]
- 27. Bryce CD, Davison AC, Okita N, Lewis GS, Sharkey NA, Armstrong AD. A biomechanical study of posterior glenoid bone loss and humeral head translation. J Shoulder Elbow Surg 2010;19:994–1002. [DOI] [PubMed] [Google Scholar]
- 28. Moeckel BH, Altchek DW, Warren RF, Wickiewicz TL, Dines DM. Instability of the shoulder after arthroplasty. J Bone Joint Surg [Am] 1993;75-A:492–497. [DOI] [PubMed] [Google Scholar]
- 29. Ovesen J, Nielsen S. Prosthesis position in shoulder arthroplasty: a cadaver study of the humeral component. Acta Orthop Scand 1985;56:330–331. [DOI] [PubMed] [Google Scholar]
- 30. Pearl ML, Volk AG. Retroversion of the proximal humerus in relationship to prosthetic replacement arthroplasty. J Shoulder Elbow Surg 1995;4:286–289. [DOI] [PubMed] [Google Scholar]
- 31. Doyle AJ, Burks RT. Comparison of humeral head retroversion with the humeral axis/biceps groove relationship: a study in live subjects and cadavers. J Shoulder Elbow Surg 1998;7:453–457. [DOI] [PubMed] [Google Scholar]
- 32. Itamura J, Dietrick T, Roidis N, Shean C, Chen F, Tibone J. Analysis of the bicipital groove as a landmark for humeral head replacement. J Shoulder Elbow Surg 2002;11:322–326. [DOI] [PubMed] [Google Scholar]
- 33. Raniga S, Knowles NK, West E, Ferreira LM, Athwal GS. The Walch type B humerus: glenoid retroversion is associated with torsional differences in the humerus. J Shoulder Elbow Surg 2019;28:1801–1808. [DOI] [PubMed] [Google Scholar]
- 34. Hsu HC, Wu JJ, Chen TH, Lo WH, Yang DJ. The influence of abductor lever-arm changes after shoulder arthroplasty. J Shoulder Elbow Surg 1993;2:134–140. [DOI] [PubMed] [Google Scholar]
- 35. Bodrogi A, Athwal GS, Howard L, Zhang T, Lapner P. A reliable method of determining glenohumeral offset in anatomic total shoulder arthroplasty. J Shoulder Elbow Surg 2019;28:1609–1616. [DOI] [PubMed] [Google Scholar]
- 36. Jacobson SR, Mallon WJ. The glenohumeral offset ratio: a radiographic study. J Shoulder Elbow Surg 1993;2:141–146. [DOI] [PubMed] [Google Scholar]
- 37. Harris TE, Jobe CM, Dai QG. Fixation of proximal humeral prostheses and rotational micromotion. J Shoulder Elbow Surg 2000;9:205–210. [PubMed] [Google Scholar]
- 38. Raiss P, Schnetzke M, Wittmann T, et al. Postoperative radiographic findings of an uncemented convertible short stem for anatomic and reverse shoulder arthroplasty. J Shoulder Elbow Surg 2019;28:715–723. [DOI] [PubMed] [Google Scholar]
- 39. Tross AK, Lädermann A, Wittmann T, et al. Subsidence of uncemented short stems in reverse shoulder arthroplasty: a multicenter study. J Clin Med 2020;9:E3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lädermann A, Chiu JC, Cunningham G, et al. Do short stems influence the cervico-diaphyseal angle and the medullary filling after reverse shoulder arthroplasties? Orthop Traumatol Surg Res 2020;106:241–246. [DOI] [PubMed] [Google Scholar]
- 41. Terrier A, Goetti P, Becce F, Farron A. Reduction of scapulohumeral subluxation with posterior augmented glenoid implants in anatomic total shoulder arthroplasty: short-term 3D comparison between pre- and post-operative CT. Orthop Traumatol Surg Res 2020;106:681–686. [DOI] [PubMed] [Google Scholar]
- 42. Kwon YW, Powell KA, Yum JK, Brems JJ, Iannotti JP. Use of three-dimensional computed tomography for the analysis of the glenoid anatomy. J Shoulder Elbow Surg 2005;14:85–90. [DOI] [PubMed] [Google Scholar]
- 43. Anglin C, Wyss UP, Pichora DR. Shoulder prosthesis subluxation: theory and experiment. J Shoulder Elbow Surg 2000;9:104–114. [PubMed] [Google Scholar]
- 44. Franklin JL, Barrett WP, Jackins SE, Matsen FA, III. Glenoid loosening in total shoulder arthroplasty: association with rotator cuff deficiency. J Arthroplasty 1988;3:39–46. [DOI] [PubMed] [Google Scholar]
- 45. Schoch B, Abboud J, Namdari S, Lazarus M. Glenohumeral mismatch in anatomic total shoulder arthroplasty. JBJS Rev 2017;5:e1. [DOI] [PubMed] [Google Scholar]
- 46. Jun BJ, Lee TQ, McGarry MH, Quigley RJ, Shin SJ, Iannotti JP. The effects of prosthetic humeral head shape on glenohumeral joint kinematics during humeral axial rotation in total shoulder arthroplasty. J Shoulder Elbow Surg 2016;25:1084–1093. [DOI] [PubMed] [Google Scholar]
- 47. Gauci MO, Deransart P, Chaoui J, et al. Three-dimensional geometry of the normal shoulder: a software analysis. J Shoulder Elbow Surg 2020;29:e468–e477. [DOI] [PubMed] [Google Scholar]
- 48. Verhaegen F, Plessers K, Verborgt O, Scheys L, Debeer P. Can the contralateral scapula be used as a reliable template to reconstruct the eroded scapula during shoulder arthroplasty? J Shoulder Elbow Surg 2018;27:1133–1138. [DOI] [PubMed] [Google Scholar]
- 49. Terrier A, Ston J, Larrea X, Farron A. Measurements of three-dimensional glenoid erosion when planning the prosthetic replacement of osteoarthritic shoulders. J Bone Joint Surg [Br] 2014;96-B:513–518. [DOI] [PubMed] [Google Scholar]
- 50. De Wilde LF, Verstraeten T, Speeckaert W, Karelse A. Reliability of the glenoid plane. J Shoulder Elbow Surg 2010;19:414–422. [DOI] [PubMed] [Google Scholar]
- 51. Moor BK, Bouaicha S, Rothenfluh DA, Sukthankar A, Gerber C. Is there an association between the individual anatomy of the scapula and the development of rotator cuff tears or osteoarthritis of the glenohumeral joint? A radiological study of the critical shoulder angle. J Bone Joint Surg [Br] 2013;95-B:935–941. [DOI] [PubMed] [Google Scholar]
- 52. Engelhardt C, Farron A, Becce F, Place N, Pioletti DP, Terrier A. Effects of glenoid inclination and acromion index on humeral head translation and glenoid articular cartilage strain. J Shoulder Elbow Surg 2017;26:157–164. [DOI] [PubMed] [Google Scholar]
- 53. Viehöfer AF, Snedeker JG, Baumgartner D, Gerber C. Glenohumeral joint reaction forces increase with critical shoulder angles representative of osteoarthritis: a biomechanical analysis. J Orthop Res 2016;34:1047–1052. [DOI] [PubMed] [Google Scholar]
- 54. Gerber C, Snedeker JG, Baumgartner D, Viehöfer AF. Supraspinatus tendon load during abduction is dependent on the size of the critical shoulder angle: a biomechanical analysis. J Orthop Res 2014;32:952–957. [DOI] [PubMed] [Google Scholar]
- 55. Watling JP, Sanchez JE, Heilbroner SP, Levine WN, Bigliani LU, Jobin CM. Glenoid component loosening associated with increased critical shoulder angle at midterm follow-up. J Shoulder Elbow Surg 2018;27:449–454. [DOI] [PubMed] [Google Scholar]
- 56. Walch G, Badet R, Boulahia A, Khoury A. Morphologic study of the glenoid in primary glenohumeral osteoarthritis. J Arthroplasty 1999;14:756–760. [DOI] [PubMed] [Google Scholar]
- 57. Bercik MJ, Kruse K, II, Yalizis M, Gauci MO, Chaoui J, Walch G. A modification to the Walch classification of the glenoid in primary glenohumeral osteoarthritis using three-dimensional imaging. J Shoulder Elbow Surg 2016;25:1601–1606. [DOI] [PubMed] [Google Scholar]
- 58. Walker KE, Simcock XC, Jun BJ, Iannotti JP, Ricchetti ET. Progression of glenoid morphology in glenohumeral osteoarthritis. J Bone Joint Surg [Am] 2018;100-A:49–56. [DOI] [PubMed] [Google Scholar]
- 59. Iannotti JP, Jun BJ, Patterson TE, Ricchetti ET. Quantitative measurement of osseous pathology in advanced glenohumeral osteoarthritis. J Bone Joint Surg [Am] 2017;99-A:1460–1468. [DOI] [PubMed] [Google Scholar]
- 60. Domos P, Checchia CS, Walch G. Walch B0 glenoid: pre-osteoarthritic posterior subluxation of the humeral head. J Shoulder Elbow Surg 2018;27:181–188. [DOI] [PubMed] [Google Scholar]
- 61. Walch G, Ascani C, Boulahia A, Nové-Josserand L, Edwards TB. Static posterior subluxation of the humeral head: an unrecognized entity responsible for glenohumeral osteoarthritis in the young adult. J Shoulder Elbow Surg 2002;11:309–314. [DOI] [PubMed] [Google Scholar]
- 62. Walch G, Moraga C, Young A, Castellanos-Rosas J. Results of anatomic nonconstrained prosthesis in primary osteoarthritis with biconcave glenoid. J Shoulder Elbow Surg 2012;21:1526–1533. [DOI] [PubMed] [Google Scholar]
- 63. Sabesan VJ, Callanan M, Youderian A, Iannotti JP. 3D CT assessment of the relationship between humeral head alignment and glenoid retroversion in glenohumeral osteoarthritis. J Bone Joint Surg [Am] 2014;96-A:e64. [DOI] [PubMed] [Google Scholar]
- 64. Jacxsens M, Van Tongel A, Henninger HB, De Coninck B, Mueller AM, De Wilde L. A three-dimensional comparative study on the scapulohumeral relationship in normal and osteoarthritic shoulders. J Shoulder Elbow Surg 2016;25:1607–1615. [DOI] [PubMed] [Google Scholar]
- 65. Beeler S, Hasler A, Götschi T, Meyer DC, Gerber C. Different acromial roof morphology in concentric and eccentric osteoarthritis of the shoulder: a multiplane reconstruction analysis of 105 shoulder computed tomography scans. J Shoulder Elbow Surg 2018;27:e357–e366. [DOI] [PubMed] [Google Scholar]
- 66. Meyer DC, Riedo S, Eckers F, Carpeggiani G, Jentzsch T, Gerber C. Small anteroposterior inclination of the acromion is a predictor for posterior glenohumeral erosion (B2 or C). J Shoulder Elbow Surg 2019;28:22–27. [DOI] [PubMed] [Google Scholar]
- 67. Matsen F, Lippitt S, eds. Shoulder surgery: principles and procedures. Philadelphia, PA: Saunders, 2003. [Google Scholar]
- 68. Lewis GS, Conaway WK, Wee H, Kim HM. Effects of anterior offsetting of humeral head component in posteriorly unstable total shoulder arthroplasty: finite element modeling of cadaver specimens. J Biomech 2017;53:78–83. [DOI] [PubMed] [Google Scholar]
- 69. Kim HM, Chacon AC, Andrews SH, et al. Biomechanical benefits of anterior offsetting of humeral head component in posteriorly unstable total shoulder arthroplasty: a cadaveric study. J Orthop Res 2016;34:666–674. [DOI] [PubMed] [Google Scholar]
- 70. Terrier A, Larrea X, Malfroy Camine V, Pioletti DP, Farron A. Importance of the subscapularis muscle after total shoulder arthroplasty. Clin Biomech (Bristol, Avon) 2013;28:146–150. [DOI] [PubMed] [Google Scholar]
- 71. Denard PJ, Noyes MP, Lädermann A. A tensionable method for subscapularis repair after shoulder arthroplasty. JSES Open Access 2018;2:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Virk MS, Aiyash SS, Frank RM, et al. Biomechanical comparison of subscapularis peel and lesser tuberosity osteotomy for double-row subscapularis repair technique in a cadaveric arthroplasty model. J Orthop Surg Res 2019;14:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Van Thiel GS, Wang VM, Wang FC, et al. Biomechanical similarities among subscapularis repairs after shoulder arthroplasty. J Shoulder Elbow Surg 2010;19:657–663. [DOI] [PubMed] [Google Scholar]
- 74. Lapner PL, Sabri E, Rakhra K, Bell K, Athwal GS. Healing rates and subscapularis fatty infiltration after lesser tuberosity osteotomy versus subscapularis peel for exposure during shoulder arthroplasty. J Shoulder Elbow Surg 2013;22:396–402. [DOI] [PubMed] [Google Scholar]
- 75. Choate WS, Kwapisz A, Momaya AM, Hawkins RJ, Tokish JM. Outcomes for subscapularis management techniques in shoulder arthroplasty: a systematic review. J Shoulder Elbow Surg 2018;27:363–370. [DOI] [PubMed] [Google Scholar]
- 76. De Wilde LF, De Coninck T, De Neve F, Berghs BM. Subscapularis release in shoulder replacement determines structural muscular changes. Clin Orthop Relat Res 2012;470:2193–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Miller BS, Joseph TA, Noonan TJ, Horan MP, Hawkins RJ. Rupture of the subscapularis tendon after shoulder arthroplasty: diagnosis, treatment, and outcome. J Shoulder Elbow Surg 2005;14:492–496. [DOI] [PubMed] [Google Scholar]
- 78. Shi LL, Jiang JJ, Ek ET, Higgins LD. Failure of the lesser tuberosity osteotomy after total shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:203–209. [DOI] [PubMed] [Google Scholar]
- 79. Werthel JD, Schoch BS, Hooke A, et al. Biomechanical effectiveness of tendon transfers to restore active internal rotation in shoulder with deficient subscapularis with and without reverse shoulder arthroplasty. J Shoulder Elbow Surg 2021;30:1196–1206. [DOI] [PubMed] [Google Scholar]
- 80. Nyffeler RW, Sheikh R, Atkinson TS, Jacob HA, Favre P, Gerber C. Effects of glenoid component version on humeral head displacement and joint reaction forces: an experimental study. J Shoulder Elbow Surg 2006;15:625–629. [DOI] [PubMed] [Google Scholar]
- 81. Shapiro TA, McGarry MH, Gupta R, Lee YS, Lee TQ. Biomechanical effects of glenoid retroversion in total shoulder arthroplasty. J Shoulder Elbow Surg 2007;16:S90–S95. [DOI] [PubMed] [Google Scholar]
- 82. Farron A, Terrier A, Büchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg 2006;15:521–526. [DOI] [PubMed] [Google Scholar]
- 83. Abler D, Berger S, Terrier A, Becce F, Farron A, Büchler P. A statistical shape model to predict the premorbid glenoid cavity. J Shoulder Elbow Surg 2018;27:1800–1808. [DOI] [PubMed] [Google Scholar]
- 84. Plessers K, Vanden Berghe P, Van Dijck C, et al. Virtual reconstruction of glenoid bone defects using a statistical shape model. J Shoulder Elbow Surg 2018;27:160–166. [DOI] [PubMed] [Google Scholar]
- 85. Clavert P, Millett PJ, Warner JJ. Glenoid resurfacing: what are the limits to asymmetric reaming for posterior erosion? J Shoulder Elbow Surg 2007;16:843–848. [DOI] [PubMed] [Google Scholar]
- 86. Ghoraishian M, Abboud JA, Romeo AA, Williams GR, Namdari S. Augmented glenoid implants in anatomic total shoulder arthroplasty: review of available implants and current literature. J Shoulder Elbow Surg 2019;28:387–395. [DOI] [PubMed] [Google Scholar]
- 87. Ho JC, Amini MH, Entezari V, et al. Clinical and radiographic outcomes of a posteriorly augmented glenoid component in anatomic total shoulder arthroplasty for primary osteoarthritis with posterior glenoid bone loss. J Bone Joint Surg [Am] 2018;100-A:1934–1948. [DOI] [PubMed] [Google Scholar]
- 88. Karelse A, Van Tongel A, Verstraeten T, Poncet D, De Wilde LF. Rocking-horse phenomenon of the glenoid component: the importance of inclination. J Shoulder Elbow Surg 2015;24:1142–1148. [DOI] [PubMed] [Google Scholar]
- 89. Braun S, Schroeder S, Mueller U, Sonntag R, Buelhoff M, Kretzer JP. Influence of joint kinematics on polyethylene wear in anatomic shoulder joint arthroplasty. J Shoulder Elbow Surg 2018;27:1679–1685. [DOI] [PubMed] [Google Scholar]
- 90. Mueller U, Braun S, Schroeder S, et al. Influence of humeral head material on wear performance in anatomic shoulder joint arthroplasty. J Shoulder Elbow Surg 2017;26:1756–1764. [DOI] [PubMed] [Google Scholar]
- 91. Junaid S, Sanghavi S, Anglin C, et al. Treatment of the fixation surface improves glenoid prosthesis longevity in vitro. J Biomech 2017;61:81–87. [DOI] [PubMed] [Google Scholar]
- 92. Gagliano JR, Helms SM, Colbath GP, Przestrzelski BT, Hawkins RJ, DesJardins JD. A comparison of onlay versus inlay glenoid component loosening in total shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:1113–1120. [DOI] [PubMed] [Google Scholar]
- 93. Castagna A, Randelli M, Garofalo R, Maradei L, Giardella A, Borroni M. Mid-term results of a metal-backed glenoid component in total shoulder replacement. J Bone Joint Surg [Br] 2010;92-B:1410–1415. [DOI] [PubMed] [Google Scholar]
- 94. Castagna A, Garofalo R. Journey of the glenoid in anatomic total shoulder replacement. Shoulder Elbow 2019;11:140–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Somerson JS, Sander P, Bohsali K, Tibbetts R, Rockwood CA, Jr, Wirth MA. What factors are associated with clinically important improvement after shoulder hemiarthroplasty for cuff tear arthropathy? Clin Orthop Relat Res 2016;474:2682–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mahony GT, Werner BC, Chang B, et al. Risk factors for failing to achieve improvement after anatomic total shoulder arthroplasty for glenohumeral osteoarthritis. J Shoulder Elbow Surg 2018;27:968–975. [DOI] [PubMed] [Google Scholar]
- 97. Grammont PM, Trouilloud P, Latfay J, Deries X. Etude et réalisation d’une nouvelle prothèse d’épaule. Rhumatologie 1987;39:407–418. [Google Scholar]
- 98. Middernacht B, Van Tongel A, De Wilde L. A critical review on prosthetic features available for reversed total shoulder arthroplasty. BioMed Res Int 2016;2016:3256931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kontaxis A, Johnson GR. The biomechanics of reverse anatomy shoulder replacement: a modelling study. Clin Biomech (Bristol, Avon) 2009;24:254–260. [DOI] [PubMed] [Google Scholar]
- 100. Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. J Shoulder Elbow Surg 2005;14:147S–161S. [DOI] [PubMed] [Google Scholar]
- 101. Schwartz DG, Kang SH, Lynch TS, et al. The anterior deltoid’s importance in reverse shoulder arthroplasty: a cadaveric biomechanical study. J Shoulder Elbow Surg 2013;22:357–364. [DOI] [PubMed] [Google Scholar]
- 102. Lädermann A, Walch G, Denard PJ, et al. Reverse shoulder arthroplasty in patients with pre-operative impairment of the deltoid muscle. J Bone Joint Surg [Br] 2013;95-B:1106–1113. [DOI] [PubMed] [Google Scholar]
- 103. Gulotta LV, Choi D, Marinello P, et al. Anterior deltoid deficiency in reverse total shoulder replacement: a biomechanical study with cadavers. J Bone Joint Surg [Br] 2012;94-B:1666–1669. [DOI] [PubMed] [Google Scholar]
- 104. Ackland DC, Richardson M, Pandy MG. Axial rotation moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg [Am] 2012;94-A:1886–1895. [DOI] [PubMed] [Google Scholar]
- 105. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Moment arms of the shoulder musculature after reverse total shoulder arthroplasty. J Bone Joint Surg [Am] 2010;92-A:1221–1230. [DOI] [PubMed] [Google Scholar]
- 106. Herrmann S, König C, Heller M, Perka C, Greiner S. Reverse shoulder arthroplasty leads to significant biomechanical changes in the remaining rotator cuff. J Orthop Surg Res 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Simovitch RW, Helmy N, Zumstein MA, Gerber C. Impact of fatty infiltration of the teres minor muscle on the outcome of reverse total shoulder arthroplasty. J Bone Joint Surg [Am] 2007;89-A:934–939. [DOI] [PubMed] [Google Scholar]
- 108. Shi LL, Cahill KE, Ek ET, Tompson JD, Higgins LD, Warner JJ. Latissimus dorsi and teres major transfer with reverse shoulder arthroplasty restores active motion and reduces pain for posterosuperior cuff dysfunction. Clin Orthop Relat Res 2015;473:3212–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Greiner S, Schmidt C, König C, Perka C, Herrmann S. Lateralized reverse shoulder arthroplasty maintains rotational function of the remaining rotator cuff. Clin Orthop Relat Res 2013;471:940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kwon YW, Pinto VJ, Yoon J, Frankle MA, Dunning PE, Sheikhzadeh A. Kinematic analysis of dynamic shoulder motion in patients with reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:1184–1190. [DOI] [PubMed] [Google Scholar]
- 111. Harman M, Frankle M, Vasey M, Banks S. Initial glenoid component fixation in ‘reverse’ total shoulder arthroplasty: a biomechanical evaluation. J Shoulder Elbow Surg 2005;14:162S–167S. [DOI] [PubMed] [Google Scholar]
- 112. Terrier A, Reist A, Merlini F, Farron A. Simulated joint and muscle forces in reversed and anatomic shoulder prostheses. J Bone Joint Surg [Br] 2008;90-B:751–756. [DOI] [PubMed] [Google Scholar]
- 113. Ackland DC, Roshan-Zamir S, Richardson M, Pandy MG. Muscle and joint-contact loading at the glenohumeral joint after reverse total shoulder arthroplasty. J Orthop Res 2011;29:1850–1858. [DOI] [PubMed] [Google Scholar]
- 114. Rugg CM, Coughlan MJ, Lansdown DA. Reverse total shoulder arthroplasty: biomechanics and indications. Curr Rev Musculoskelet Med 2019;12:542–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Lädermann A, Gueorguiev B, Charbonnier C, et al. Scapular notching on kinematic simulated range of motion after reverse shoulder arthroplasty is not the result of impingement in adduction. Medicine (Baltimore) 2015;94:e1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. J Bone Joint Surg [Am] 2007;89-A:588–600. [DOI] [PubMed] [Google Scholar]
- 117. Nyffeler RW, Werner CM, Gerber C. Biomechanical relevance of glenoid component positioning in the reverse Delta III total shoulder prosthesis. J Shoulder Elbow Surg 2005;14:524–528. [DOI] [PubMed] [Google Scholar]
- 118. Chou J, Malak SF, Anderson IA, Astley T, Poon PC. Biomechanical evaluation of different designs of glenospheres in the SMR reverse total shoulder prosthesis: range of motion and risk of scapular notching. J Shoulder Elbow Surg 2009;18:354–359. [DOI] [PubMed] [Google Scholar]
- 119. de Wilde LF, Poncet D, Middernacht B, Ekelund A. Prosthetic overhang is the most effective way to prevent scapular conflict in a reverse total shoulder prosthesis. Acta Orthop 2010;81:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lädermann A, Denard PJ, Collin P, et al. Effect of humeral stem and glenosphere designs on range of motion and muscle length in reverse shoulder arthroplasty. Int Orthop 2020;44:519–530. [DOI] [PubMed] [Google Scholar]
- 121. Haidamous G, Lädermann A, Hartzler RU, et al. Radiographic parameters associated with excellent versus poor range of motion outcomes following reverse shoulder arthroplasty. Shoulder Elbow 2020;9:1758573220936234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Roche C, Flurin PH, Wright T, Crosby LA, Mauldin M, Zuckerman JD. An evaluation of the relationships between reverse shoulder design parameters and range of motion, impingement, and stability. J Shoulder Elbow Surg 2009;18:734–741. [DOI] [PubMed] [Google Scholar]
- 123. Gutiérrez S, Comiskey CA, IV, Luo ZP, Pupello DR, Frankle MA. Range of impingement-free abduction and adduction deficit after reverse shoulder arthroplasty: hierarchy of surgical and implant-design-related factors. J Bone Joint Surg [Am] 2008;90-A:2606–2615. [DOI] [PubMed] [Google Scholar]
- 124. Lädermann A, Denard PJ, Boileau P, et al. Effect of humeral stem design on humeral position and range of motion in reverse shoulder arthroplasty. Int Orthop 2015;39:2205–2213. [DOI] [PubMed] [Google Scholar]
- 125. Gutiérrez S, Levy JC, Frankle MA, et al. Evaluation of abduction range of motion and avoidance of inferior scapular impingement in a reverse shoulder model. J Shoulder Elbow Surg 2008;17:608–615. [DOI] [PubMed] [Google Scholar]
- 126. Kim SJ, Jang SW, Jung KH, Kim YS, Lee SJ, Yoo YS. Analysis of impingement-free range of motion of the glenohumeral joint after reverse total shoulder arthroplasty using three different implant models. J Orthop Sci 2019;24:87–94. [DOI] [PubMed] [Google Scholar]
- 127. Lädermann A, Tay E, Collin P, et al. Effect of critical shoulder angle, glenoid lateralization, and humeral inclination on range of movement in reverse shoulder arthroplasty. Bone Joint Res 2019;8:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wong MT, Langohr GDG, Athwal GS, Johnson JA. Implant positioning in reverse shoulder arthroplasty has an impact on acromial stresses. J Shoulder Elbow Surg 2016;25:1889–1895. [DOI] [PubMed] [Google Scholar]
- 129. Haidamous G, Lädermann A, Frankle MA, Gorman RA, II, Denard PJ. The risk of postoperative scapular spine fracture following reverse shoulder arthroplasty is increased with an onlay humeral stem. J Shoulder Elbow Surg 2020;29:2556–2563. [DOI] [PubMed] [Google Scholar]
- 130. Berliner JL, Regalado-Magdos A, Ma CB, Feeley BT. Biomechanics of reverse total shoulder arthroplasty. J Shoulder Elbow Surg 2015;24:150–160. [DOI] [PubMed] [Google Scholar]
- 131. Lädermann A, Schwitzguebel AJ, Edwards TB, et al. Glenoid loosening and migration in reverse shoulder arthroplasty. J Bone Joint Surg [Br] 2019;101-B:461–469. [DOI] [PubMed] [Google Scholar]
- 132. Giles JW, Langohr GD, Johnson JA, Athwal GS. Implant design variations in reverse total shoulder arthroplasty influence the required deltoid force and resultant joint load. Clin Orthop Relat Res 2015;473:3615–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Boileau P, Moineau G, Roussanne Y, O’Shea K. Bony increased-offset reversed shoulder arthroplasty: minimizing scapular impingement while maximizing glenoid fixation. Clin Orthop Relat Res 2011;469:2558–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Jasty M, Bragdon C, Burke D, O’Connor D, Lowenstein J, Harris WH. In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J Bone Joint Surg [Am] 1997;79-A:707–714. [DOI] [PubMed] [Google Scholar]
- 135. Hopkins AR, Hansen UN, Bull AM, Emery R, Amis AA. Fixation of the reversed shoulder prosthesis. J Shoulder Elbow Surg 2008;17:974–980. [DOI] [PubMed] [Google Scholar]
- 136. Chebli C, Huber P, Watling J, Bertelsen A, Bicknell RT, Matsen F, III. Factors affecting fixation of the glenoid component of a reverse total shoulder prothesis. J Shoulder Elbow Surg 2008;17:323–327. [DOI] [PubMed] [Google Scholar]
- 137. James J, Allison MA, Werner FW, et al. Reverse shoulder arthroplasty glenoid fixation: is there a benefit in using four instead of two screws? J Shoulder Elbow Surg 2013;22:1030–1036. [DOI] [PubMed] [Google Scholar]
- 138. Bonnevialle N, Geais L, Müller JH, Berhouet J, Berhouet J; Shoulder Friends Institute. Effect of RSA glenoid baseplate central fixation on micromotion and bone stress. JSES Int 2020;4:979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gutiérrez S, Keller TS, Levy JC, Lee WE, III, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clin Orthop Relat Res 2008;466:670–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Gutiérrez S, Walker M, Willis M, Pupello DR, Frankle MA. Effects of tilt and glenosphere eccentricity on baseplate/bone interface forces in a computational model, validated by a mechanical model, of reverse shoulder arthroplasty. J Shoulder Elbow Surg 2011;20:732–739. [DOI] [PubMed] [Google Scholar]
- 141. Boileau P, Gauci MO, Wagner ER, et al. The reverse shoulder arthroplasty angle: a new measurement of glenoid inclination for reverse shoulder arthroplasty. J Shoulder Elbow Surg 2019;28:1281–1290. [DOI] [PubMed] [Google Scholar]
- 142. Walker M, Brooks J, Willis M, Frankle M. How reverse shoulder arthroplasty works. Clin Orthop Relat Res 2011;469:2440–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Clouthier AL, Hetzler MA, Fedorak G, Bryant JT, Deluzio KJ, Bicknell RT. Factors affecting the stability of reverse shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg 2013;22:439–444. [DOI] [PubMed] [Google Scholar]
- 144. Chae J, Siljander M, Wiater JM. Instability in reverse total shoulder arthroplasty. J Am Acad Orthop Surg 2018;26:587–596. [DOI] [PubMed] [Google Scholar]
- 145. Ackland DC, Robinson DL, Wilkosz A, et al. The influence of rotator cuff tears on muscle and joint-contact loading after reverse total shoulder arthroplasty. J Orthop Res 2019;37:211–219. [DOI] [PubMed] [Google Scholar]
- 146. Pastor MF, Kraemer M, Wellmann M, Hurschler C, Smith T. Anterior stability of the reverse shoulder arthroplasty depending on implant configuration and rotator cuff condition. Arch Orthop Trauma Surg 2016;136:1513–1519. [DOI] [PubMed] [Google Scholar]
- 147. Langohr GD, Giles JW, Athwal GS, Johnson JA. The effect of glenosphere diameter in reverse shoulder arthroplasty on muscle force, joint load, and range of motion. J Shoulder Elbow Surg 2015;24:972–979. [DOI] [PubMed] [Google Scholar]
- 148. Gutiérrez S, Greiwe RM, Frankle MA, Siegal S, Lee WE, III. Biomechanical comparison of component position and hardware failure in the reverse shoulder prosthesis. J Shoulder Elbow Surg 2007;16:S9–S12. [DOI] [PubMed] [Google Scholar]
- 149. Ferle M, Pastor MF, Hagenah J, Hurschler C, Smith T. Effect of the humeral neck-shaft angle and glenosphere lateralization on stability of reverse shoulder arthroplasty: a cadaveric study. J Shoulder Elbow Surg 2019;28:966–973. [DOI] [PubMed] [Google Scholar]
- 150. Henninger HB, Barg A, Anderson AE, Bachus KN, Burks RT, Tashjian RZ. Effect of lateral offset center of rotation in reverse total shoulder arthroplasty: a biomechanical study. J Shoulder Elbow Surg 2012;21:1128–1135. [DOI] [PubMed] [Google Scholar]
- 151. Favre P, Sussmann PS, Gerber C. The effect of component positioning on intrinsic stability of the reverse shoulder arthroplasty. J Shoulder Elbow Surg 2010;19:550–556. [DOI] [PubMed] [Google Scholar]
- 152. Abdulla I, Langohr DG, Giles JW, Johnson JA, Athwal GS. The effect of humeral polyethylene insert constraint on reverse shoulder arthroplasty biomechanics. Shoulder Elbow 2018;10:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Lädermann A, Williams MD, Melis B, Hoffmeyer P, Walch G. Objective evaluation of lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2009;18:588–595. [DOI] [PubMed] [Google Scholar]
- 154. Lädermann A, Walch G, Lubbeke A, et al. Influence of arm lengthening in reverse shoulder arthroplasty. J Shoulder Elbow Surg 2012;21:336–341. [DOI] [PubMed] [Google Scholar]
- 155. Athwal GS, MacDermid JC, Reddy KM, Marsh JP, Faber KJ, Drosdowech D. Does bony increased-offset reverse shoulder arthroplasty decrease scapular notching? J Shoulder Elbow Surg 2015;24:468–473. [DOI] [PubMed] [Google Scholar]
- 156. Tashjian RZ, Burks RT, Zhang Y, Henninger HB. Reverse total shoulder arthroplasty: a biomechanical evaluation of humeral and glenosphere hardware configuration. J Shoulder Elbow Surg 2015;24:e68–e77. [DOI] [PubMed] [Google Scholar]
- 157. Pegreffi F, Pellegrini A, Paladini P, et al. Deltoid muscle activity in patients with reverse shoulder prosthesis at 2-year follow-up. Musculoskelet Surg 2017;101:129–135. [DOI] [PubMed] [Google Scholar]
- 158. Liou W, Yang Y, Petersen-Fitts GR, Lombardo DJ, Stine S, Sabesan VJ. Effect of lateralized design on muscle and joint reaction forces for reverse shoulder arthroplasty. J Shoulder Elbow Surg 2017;26:564–572. [DOI] [PubMed] [Google Scholar]
- 159. Hamilton MA, Diep P, Roche C, et al. Effect of reverse shoulder design philosophy on muscle moment arms. J Orthop Res 2015;33:605–613. [DOI] [PubMed] [Google Scholar]