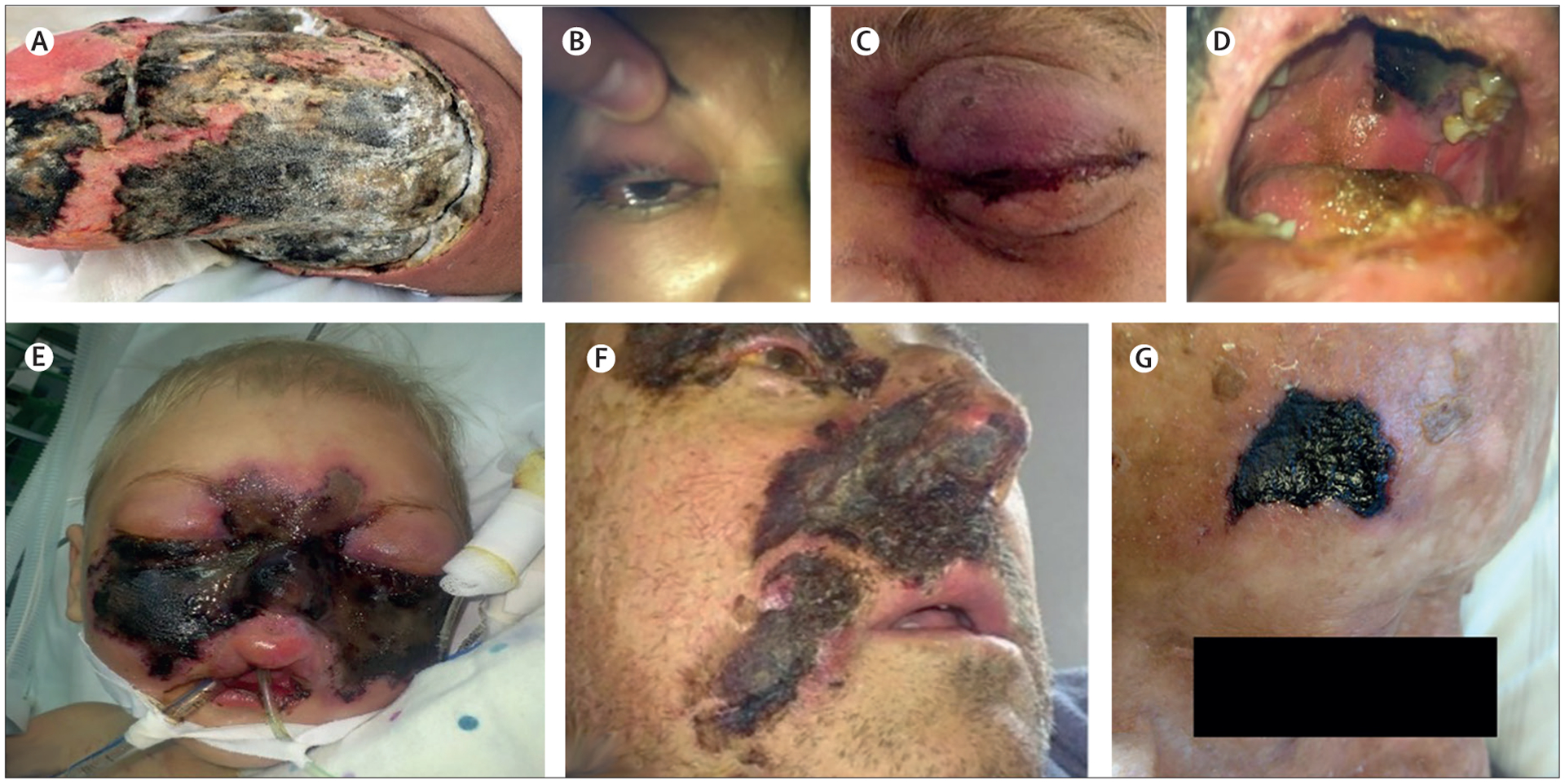
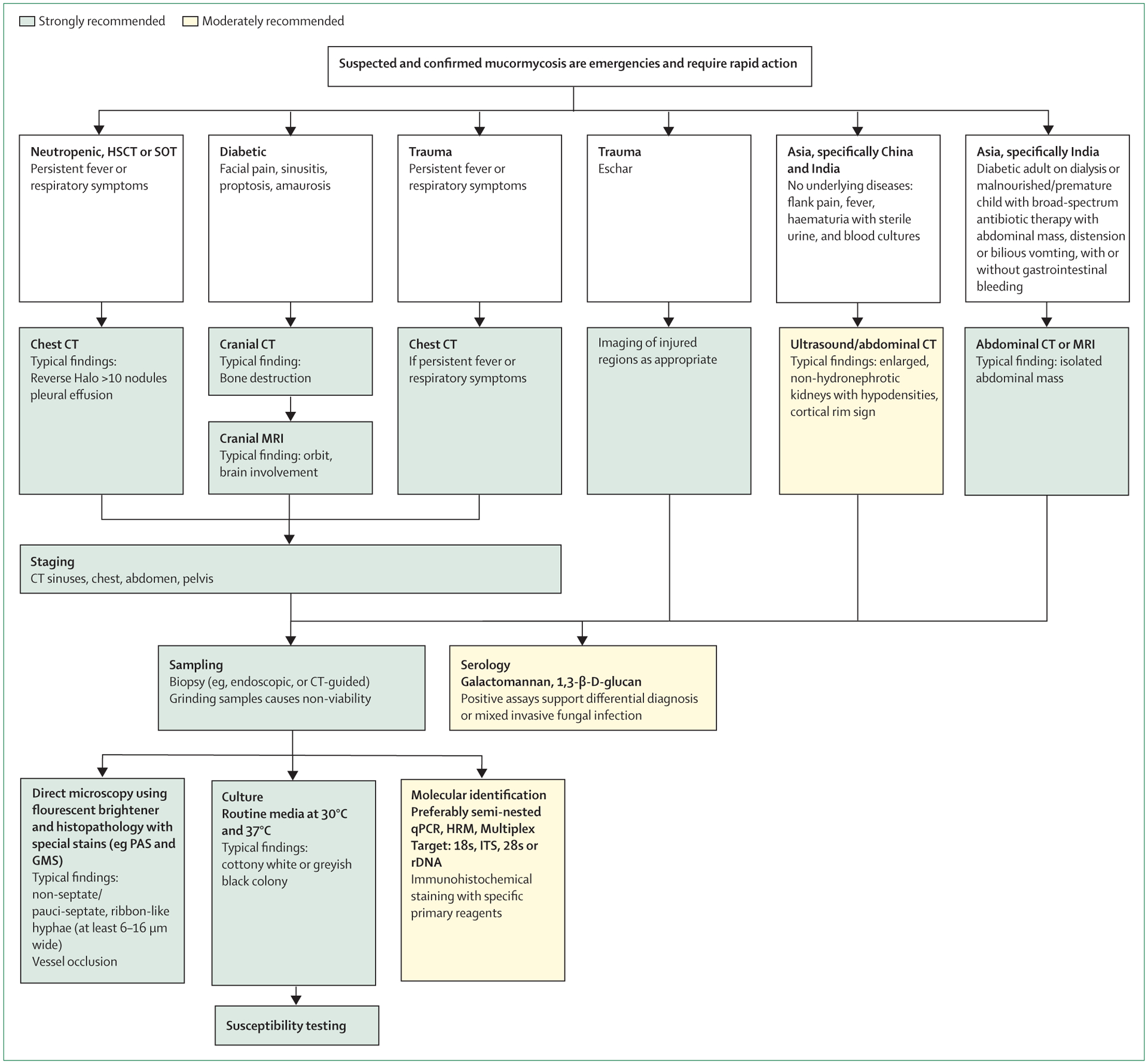
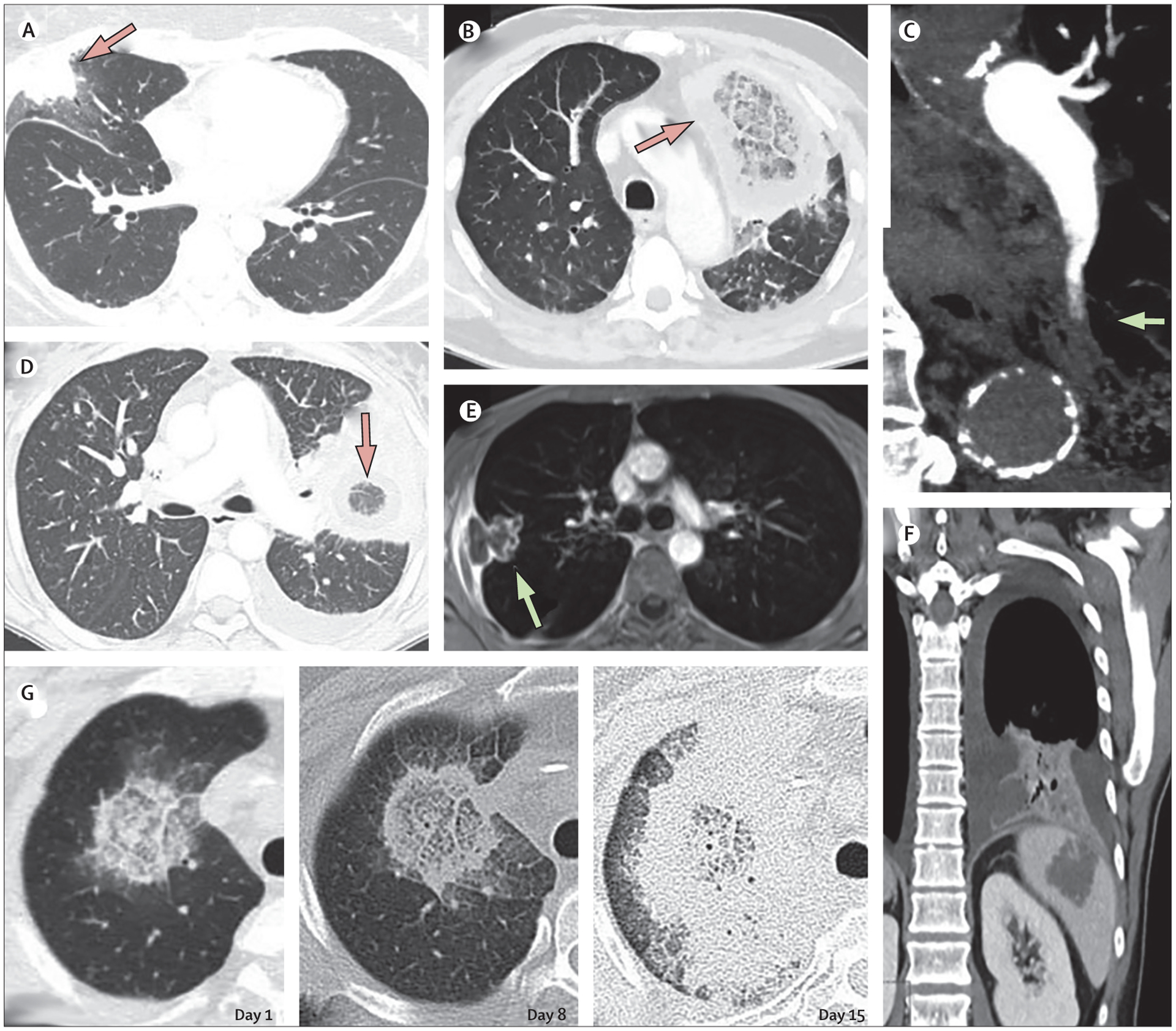
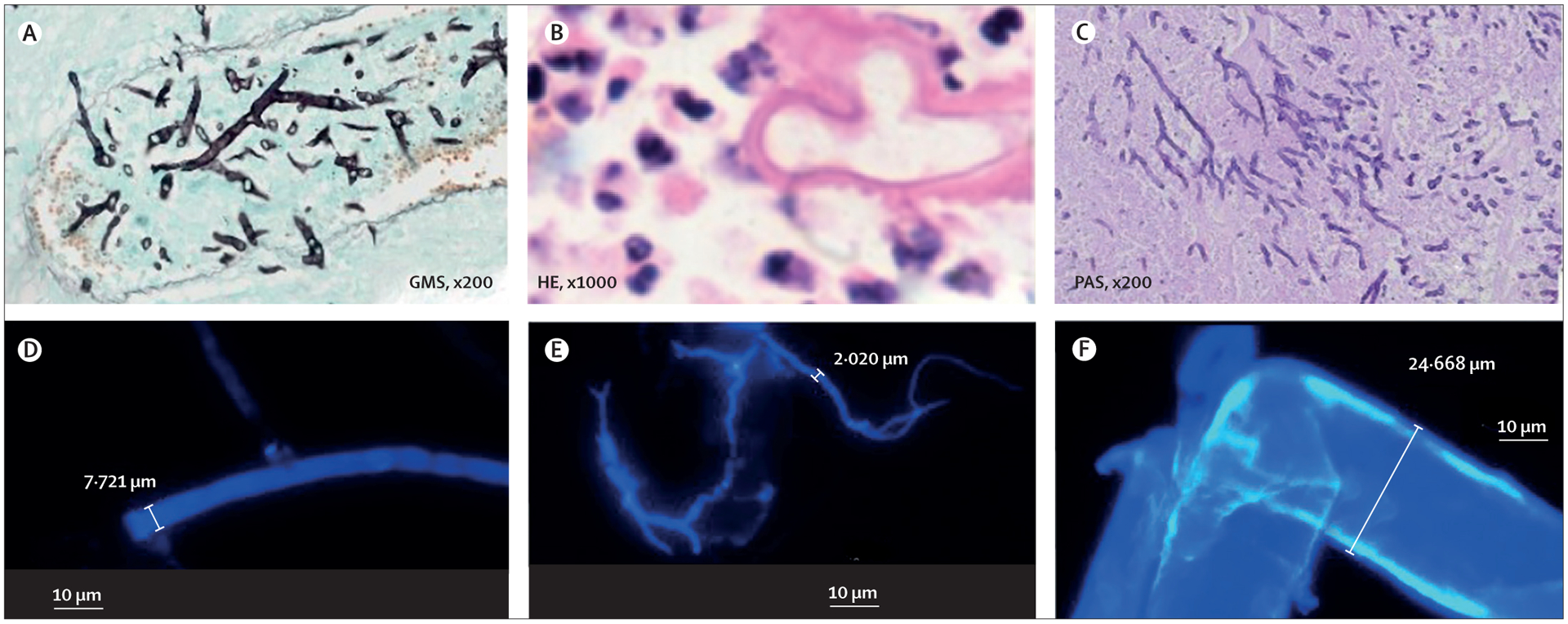
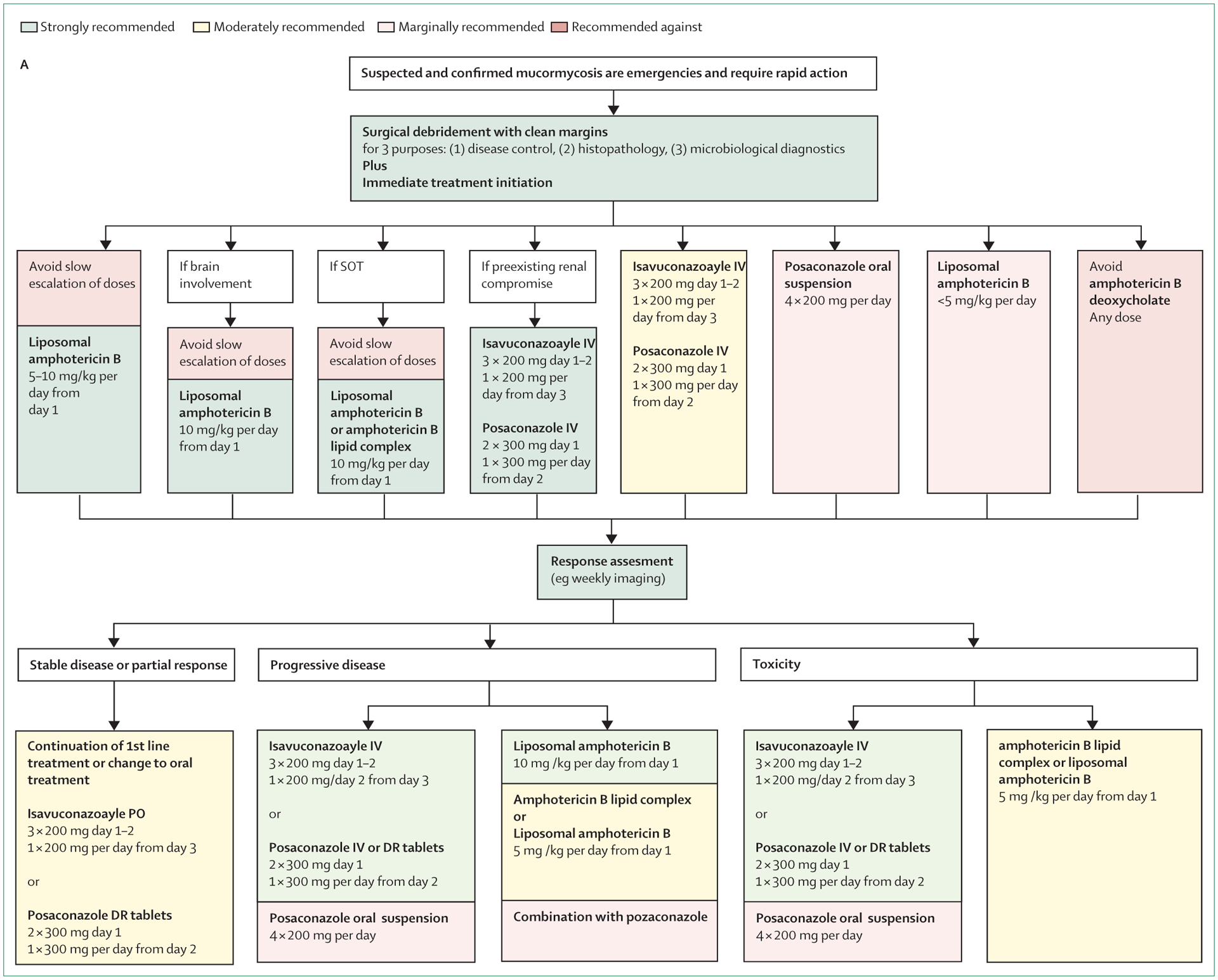
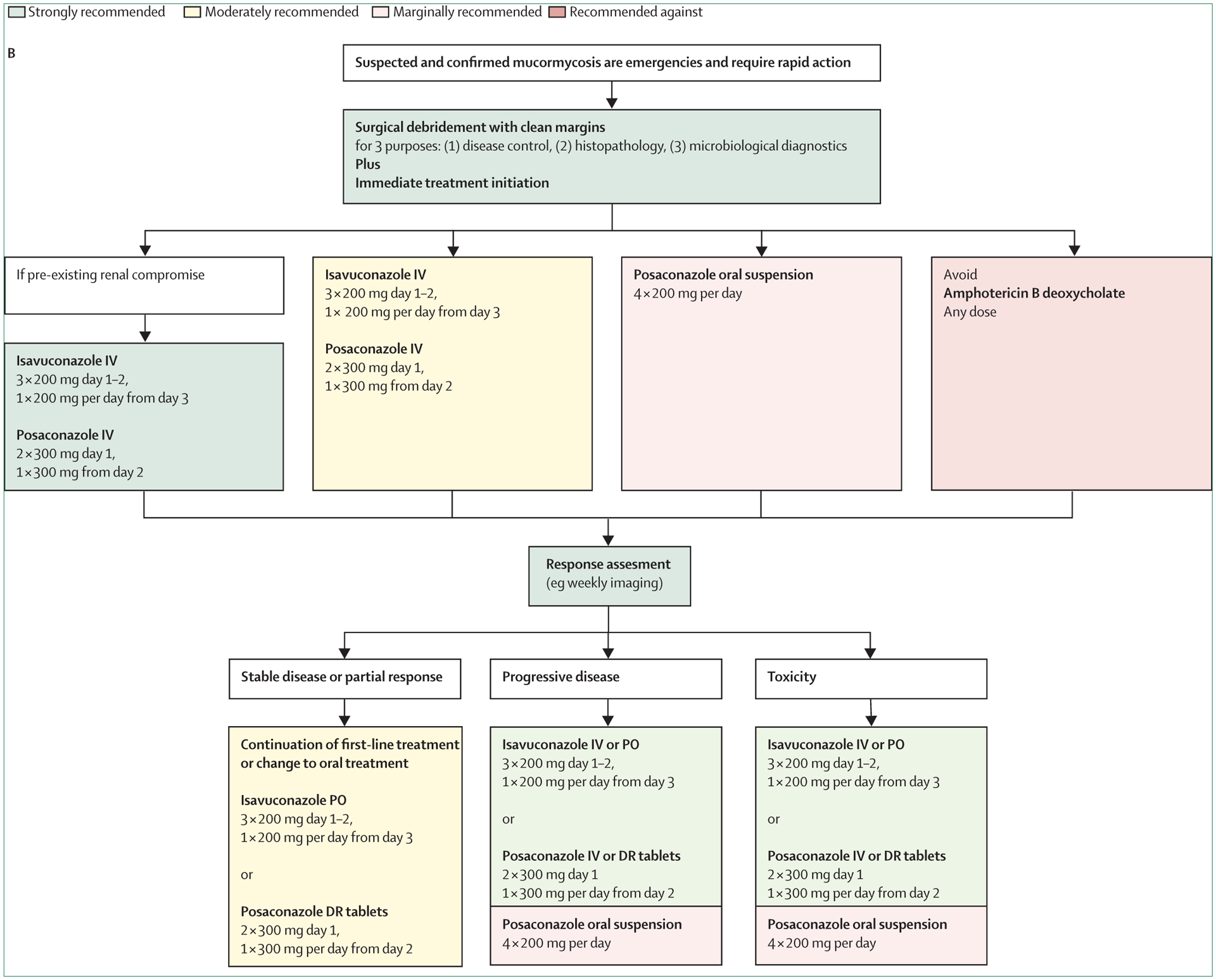
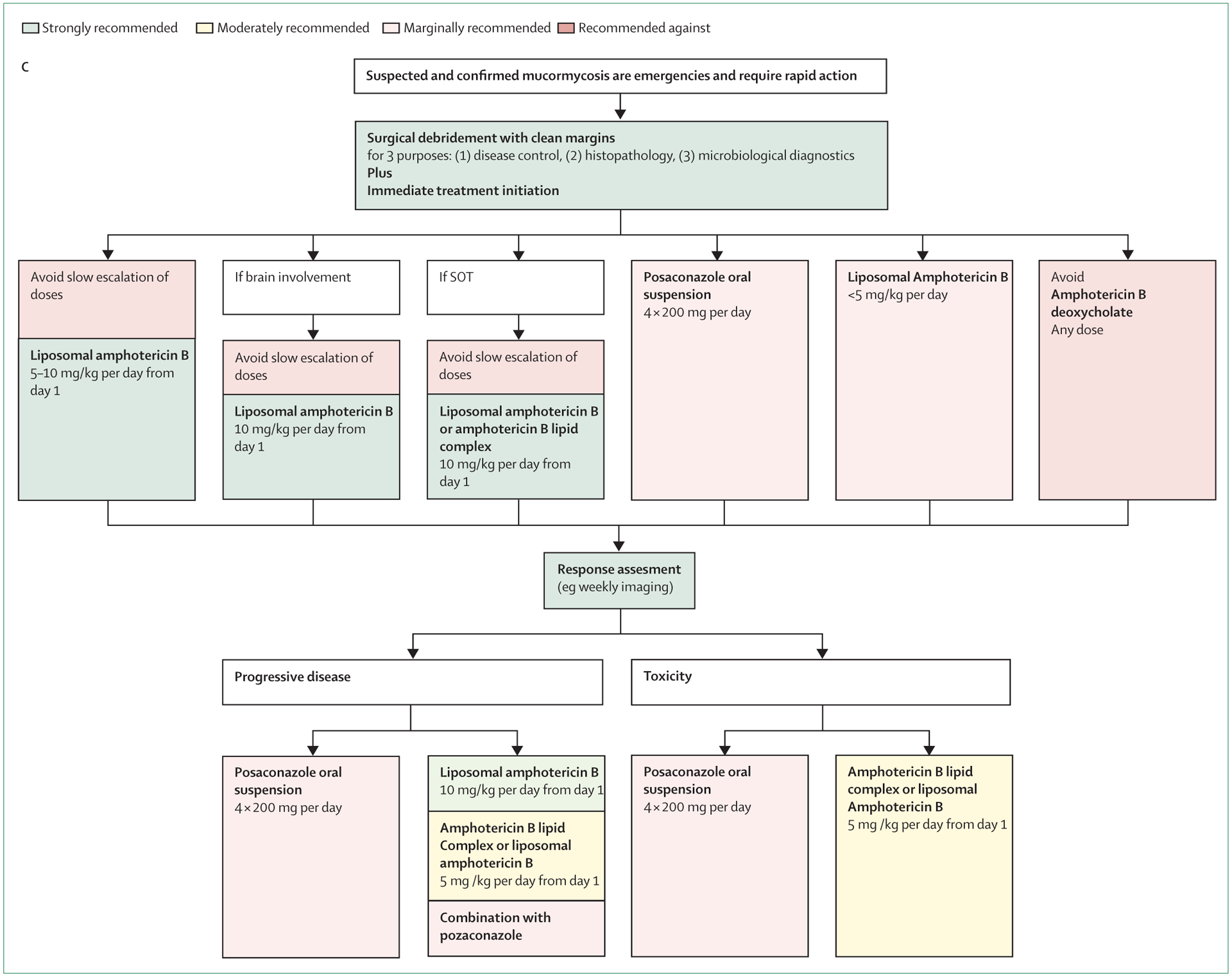
Oliver A Cornely
Oliver A Cornely
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Ana Alastruey-Izquierdo
Ana Alastruey-Izquierdo
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Dorothee Arenz
Dorothee Arenz
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Sharon C A Chen
Sharon C A Chen
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Eric Dannaoui
Eric Dannaoui
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Bruno Hochhegger
Bruno Hochhegger
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Martin Hoenigl
Martin Hoenigl
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Henrik E Jensen
Henrik E Jensen
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Katrien Lagrou
Katrien Lagrou
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Russell E Lewis
Russell E Lewis
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Sibylle C Mellinghoff
Sibylle C Mellinghoff
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Mervyn Mer
Mervyn Mer
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Zoi D Pana
Zoi D Pana
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Danila Seidel
Danila Seidel
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Donald C Sheppard
Donald C Sheppard
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Roger Wahba
Roger Wahba
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Murat Akova
Murat Akova
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Alexandre Alanio
Alexandre Alanio
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Abdullah M S Al-Hatmi
Abdullah M S Al-Hatmi
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Sevtap Arikan-Akdagli
Sevtap Arikan-Akdagli
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Hamid Badali
Hamid Badali
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Ronen Ben-Ami
Ronen Ben-Ami
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Alexandro Bonifaz
Alexandro Bonifaz
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Stéphane Bretagne
Stéphane Bretagne
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Elio Castagnola
Elio Castagnola
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Methee Chayakulkeeree
Methee Chayakulkeeree
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Arnaldo L Colombo
Arnaldo L Colombo
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Dora E Corzo-León
Dora E Corzo-León
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Lubos Drgona
Lubos Drgona
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Andreas H Groll
Andreas H Groll
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Jesus Guinea
Jesus Guinea
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Claus-Peter Heussel
Claus-Peter Heussel
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Ashraf S Ibrahim
Ashraf S Ibrahim
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Souha S Kanj
Souha S Kanj
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Nikolay Klimko
Nikolay Klimko
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Michaela Lackner
Michaela Lackner
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Frederic Lamoth
Frederic Lamoth
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Fanny Lanternier
Fanny Lanternier
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Cornelia Lass-Floerl
Cornelia Lass-Floerl
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Dong-Gun Lee
Dong-Gun Lee
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Thomas Lehrnbecher
Thomas Lehrnbecher
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Badre E Lmimouni
Badre E Lmimouni
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Mihai Mares
Mihai Mares
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Georg Maschmeyer
Georg Maschmeyer
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Jacques F Meis
Jacques F Meis
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Joseph Meletiadis
Joseph Meletiadis
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
C Orla Morrissey
C Orla Morrissey
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Marcio Nucci
Marcio Nucci
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Rita Oladele
Rita Oladele
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Livio Pagano
Livio Pagano
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Alessandro Pasqualotto
Alessandro Pasqualotto
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Atul Patel
Atul Patel
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Zdenek Racil
Zdenek Racil
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Malcolm Richardson
Malcolm Richardson
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Emmanuel Roilides
Emmanuel Roilides
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Markus Ruhnke
Markus Ruhnke
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Seyedmojtaba Seyedmousavi
Seyedmojtaba Seyedmousavi
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Neeraj Sidharthan
Neeraj Sidharthan
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Nina Singh
Nina Singh
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
János Sinko
János Sinko
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Anna Skiada
Anna Skiada
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Monica Slavin
Monica Slavin
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Rajeev Soman
Rajeev Soman
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Brad Spellberg
Brad Spellberg
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
William Steinbach
William Steinbach
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Ban Hock Tan
Ban Hock Tan
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Andrew J Ullmann
Andrew J Ullmann
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Jörg J Vehreschild
Jörg J Vehreschild
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Maria J G T Vehreschild
Maria J G T Vehreschild
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Thomas J Walsh
Thomas J Walsh
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
P Lewis White
P Lewis White
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Nathan P Wiederhold
Nathan P Wiederhold
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Theoklis Zaoutis
Theoklis Zaoutis
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1,
Arunaloke Chakrabarti
Arunaloke Chakrabarti
1Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany (O A Cornely MD, D Arenz PhD, J J Vehreschild MD, M J G T Vehreschild MD, S C Mellinghoff MD, D Seidel PhD); German Centre for Infection Research (DZIF) partner site Bonn-Cologne, Cologne, Germany (O A Cornely, J J Vehreschild, M J G T Vehreschild); CECAD Cluster of Excellence, University of Cologne, Cologne, Germany (O A Cornely, D Arenz, S C Mellinghoff, D Seidel); Clinical Trials Center Cologne, University Hospital of Cologne, Cologne, Germany (O A Cornely); Mycology Reference Laboratory, National Centre for Microbiology, Instituto de Salud Carlos III, Madrid, Spain (A Alastruey-Izquierdo PhD); Centre for Infectious Diseases and Microbiology Laboratory Services, New South Wales Health Pathology, and the Department of Infectious Diseases, Westmead Hospital, School of Medicine, University of Sydney, Sydney, NSW, Australia (S C-A Chen PhD); Université Paris-Descartes, Faculté de Médecine, APHP, Hôpital Européen Georges Pompidou, Unité de Parasitologie-Mycologie, Service de Microbiologie, Paris, France (E Dannaoui MD); Radiology, Hospital São Lucas da Pontificia Universidade Catolica do Rio Grande do Sul (PUCRS), Escola de Medicina, Porto Alegre, Brazil (B Hochhegger MD); Radiology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre, Brazil (B Hochhegger); Section of Infectious Diseases and Tropical Medicine and Division of Pulmonology, Medical University of Graz, Graz, Austria (M Hoenigl MD); Division of Infectious Diseases and Global Public Health, Department of Medicine, University of California San Diego, San Diego, USA (M Hoenigl); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (H E Jensen PhD); Department of Microbiology, Immunology and Transplantation, KU Leuven and Clinical Department of Laboratory Medicine and National Reference Center for Mycosis, University Hospitals Leuven, Leuven, Belgium (K Lagrou PharmD); Infectious Diseases Clinic, Sant’Orsola-Malpighi Hospital, Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy (R E Lewis PharmD); Divisions of Critical Care and Pulmonology, Department of Medicine, Charlotte Maxeke Johannesburg Academic Hospital and Faculty of Health Sciences University of the Witwatersrand, Johannesburg, South Africa (M Mer MD); Infectious Diseases Unit, 3rd Department of Paediatrics, Faculty of Medicine, Aristotle University School of Health Sciences, Thessaloniki, Greece; Hippokration General Hospital, Thessaloniki, Greece (Z D Pana MD; E Roilides MD); Division of Infectious Diseases, Department of Medicine, Microbiology and Immunology, McGill University, Montreal, Quebec, Canada (D C Sheppard MD); Department of General, Visceral and Cancer Surgery, University Hospital of Cologne, Cologne, Germany (R Wahba MD); Department of Medical Microbiology, Postgraduate Institute of Medical Education & Research, Chandigarh, India (A Chakrabarti MD); Department of Infectious Diseases, Hacettepe University School of Medicine, Ankara, Turkey (M Akova MD); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, CNRS UMR2000, Parasitology-Mycology Laboratory, Lariboisière, Saint-Louis, Fernand Widal Hospitals, Assistance Publique-Hôpitaux de Paris (AP-HP), Université de Paris, Paris, France (A Alanio MD, S Bretagne MD); Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands (A M S Al-Hatmi PhD); Centre of Expertise in Mycology RadboudUMC/Canisius Wilhelmina Hospital, Nijmegen, The Netherlands (A M S Al-Hatmi); Ministry of Health, Directorate General of Health Services, Ibri, Oman (A M S Al-Hatmi PhD); Department of Medical Microbiology, Hacettepe University School of Medicine, Sıhhiye Ankara, Turkey (S Arikan-Akdagli MD); Department of Medical Mycology/Invasive Fungi Research Center (IFRC), School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran (H Badali PhD, S Seyedmousavi PhD); Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel (R Ben-Ami MD); Infectious Diseases Unit, Tel Aviv Medical Center, Tel- Aviv, Israel (R Ben-Ami); Dermatology Service & Mycology Department, Hospital General de México “Dr. Eduardo Liceaga”, Mexico City, Mexico (A Bonifaz MD); Infectious Diseases Unit, Istituto Giannina Gaslini Children’s Hospital, Genoa, Italy (E Castagnola MD); Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (M Chayakulkeeree MD); Special Mycology Laboratory, Division of Infectious Diseases, Department of Medicine, Universidade Federal de São Paulo (UNIFESP), São Paulo, Brazil (A L Colombo MD); Department of Epidemiology and Infectious Diseases, Hospital General Dr Manuel Gea González, Mexico City, Mexico (D E Corzo-Leon MD); Medical Mycology and Fungal Immunology/Wellcome Trust Strategic Award Program, Aberdeen Fungal Group, University of Aberdeen, King’s College, Aberdeen, UK (D E Corzo-Leon MD); Oncohematology Clinic, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia (L Drgona MD); InfectiousDisease Research Program, Department of Paediatric Hematology/Oncology and Center for Bone Marrow Transplantation, University Children’s Hospital Münster, Münster, Germany (A H Groll MD); Clinical Microbiology and Infectious Diseases, Hospital General Universitario Gregorio Marañón, Madrid, Spain (J Guinea PharmD); Instituto de Investigación v Sanitaria Gregorio Marañón, Madrid, Spain (J Guinea); Medicine Department, School of Medicine, Universidad Complutense de Madrid, Madrid, Spain (J Guinea PharmD); Diagnostic and Interventional Radiology, Thoracic Clinic, University Hospital Heidelberg, Heidelberg, Germany (C-P Heussel MD); Division of Infectious Diseases, Los Angeles Biomedical Research Institute at Harbor-University of California at Los Angeles (UCLA) Medical Center, Torrance, CA, USA (A S Ibrahim PhD); Department of Internal Medicine, Division of Infectious Diseases, American University of Beirut Medical Center, Beirut, Lebanon (S S Kanj MD); Department of Clinical Mycology, Allergology and Immunology, North Western State Medical University, St Petersburg, Russia (N Klimko MD); Division of Hygiene and Medical Microbiology, Department of Hygiene, Microbiology and Public Health, Medical University Innsbruck, Innsbruck, Austria (M Lackner MD, C Lass-Floerl MD); Infectious Diseases Service, Department of Medicine and Institute of Microbiology, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth MD); Institute of Microbiology, Department of Laboratories, Lausanne University Hospital, Lausanne, Switzerland (F Lamoth); Institut Pasteur, National Reference Center for Invasive Mycoses and Antifungals, Department of Mycology, Paris Descartes University, Necker-Enfants Malades University Hospital, Department of Infectious Diseases and Tropical Medicine, Centre d’Infectiologie Necker-Pasteur, Institut Imagine, AP-HP, Paris, France (Lanternier MD); Division of Infectious Diseases, Department of Internal Medicine, Catholic Hematology Hospital, College of Medicine, The Catholic University of Korea, Seocho-gu, Seoul, Korea (D-G Lee MD); Division of Paediatric Haematology and Oncology, Hospital for Children and Adolescents, Johann Wolfgang Goethe-University, Frankfurt, Germany (T Lehrnbecher MD); School of Medicine and Pharmacy, University Mohammed the fifth, Hay Riad, Rabat, Morocco (B E Lmimouni MD); Laboratory of Antimicrobial Chemotherapy, Ion Ionescu de la Brad University, Iași, Romania (M Mares PhD); Department of Hematology, Oncology and Palliative Care, Klinikum Ernst von Bergmann, Potsdam, Germany (G Maschmeyer MD); Department of Medical Microbiology and Infectious Diseases, Centre of Expertise in Mycology Radboudumc/Canisius Wilhelmina Hospital, Nijmegen, Netherlands (J F Meis MD); Clinical Microbiology Laboratory, Attikon University Hospital, National and Kapodistrian University of Athens, Athens, Greece (J Meletiadis PhD); Department of Medical Microbiology and Infectious Diseases, Erasmus Medical Center, Rotterdam, The Netherlands (J Meletiadis PhD); Department of Infectious Diseases, Alfred Health & Monash University, Melbourne, Australia (C O Morrissey PhD); Department of Internal Medicine, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil (M Nucci MD); Department of Medical Microbiology & Parasitology, College of Medicine, University of Lagos, Yaba, Lagos, Nigeria (R Oladele MD); Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, UK (R Oladele MD); Department of Hematology, Fondazione Policlinico Universitario A. Gemelli –IRCCS– Universita Cattolica del Sacro Cuore, Roma, Italy (L Pagano MD); Federal University of Health Sciences of Porto Alegre, Hospital Dom Vicente Scherer, Porto Alegre, Brazil (A Pasqualotto MD); Infectious Diseases Clinic, Vedanta Institute of Medical Sciences, Navarangpura, Ahmeddabad, India (A Patel MD); Institute of Hematology and Blood Transfusion, Prague, Czech Republic (Z Racil MD); UK NHS Mycology Reference Centre, Manchester University NHS Foundation Trust, Manchester, UK (M Richardson PhD); Hämatologie & Internistische Onkologie, Lukas-Krankenhaus Bünde, Onkologische Ambulanz, Bünde, Germany (M Ruhnke MD); Center of Expertise in Microbiology, Infection Biology and Antimicrobial Pharmacology, Tehran, Iran (S Seyedmousavi PhD); Molecular Microbiology Section, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA (S Seyedmousavi); Department of Hemato Oncology, Amrita Institute of Medical Sciences, Amrita Viswa Vidyapeetham University, Kochi, India (N Sidharthan MD); Division of Infectious Diseases, University of Pittsburgh Medical Center and VA Pittsburgh Healthcare System, Infectious Diseases Section, University of Pittsburgh, Pittsburgh, PA, USA (N Singh MD); Infectious Diseases Unit, Szent Istvan and Szent Laszlo Hospital, Budapest, Hungary (J Sinko MD); Department of Infectious Diseases, Laiko General Hospital, National and Kapodistrian University of Athens, Athens, Greece (A Skiada MD); University of Melbourne, Melbourne, VIC, Australia (M Slavin MD); The National Centre for Infections in Cancer, Peter MacCallum Cancer Centre, Parkville, Melbourne, VIC, Australia (M Slavin); P D Hinduja Hospital & Medical Research Centre, Department of Medicine, Veer Sarvarkar Marg, Mumbai, India (R Soman MD); Los Angeles County and University of Southern California (LAC+USC) Medical Center, Los Angeles, CA, USA (B Spellberg MD); Division of Pediatric Infectious Diseases, Department of Pediatrics, Duke University Medical Center, Durham, NC, USA (W Steinbach MD); Department of Infectious Diseases, Singapore General Hospital, Singapur, Singapore (B H Tan MD); Department for Internal Medicine II, University Hospital Würzburg, Würzburg, Germany (A J Ullmann MD); Department of Internal Medicine, Hematology/Oncology, Goethe University Frankfurt, Frankfurt, Germany (J J Vehreschild); Department of Internal Medicine, Infectious Diseases, Goethe University Frankfurt, Frankfurt, Germany (M J G T Vehreschild); Departments of Medicine, Pediatrics, Microbiology & Immunology, Weill Cornell Medicine, and New York Presbyterian Hospital, New York City, NY, USA (T J Walsh MD); Public Health Wales Microbiology Cardiff, UHW, Heath Park, Cardiff, UK (P L White PhD); Fungus Testing Laboratory, University of Texas Health Science Center, San Antonio, TX, USA (N P Wiederhold PharmD); and Division of Infectious Diseases, The Children’s Hospital of Philadelphia, Philadelphia, PA, USA (T Zaoutis MD)
1;
Mucormycosis ECMM MSG Global Guideline Writing Group1