Abstract
Inorganic nanoparticles provide multipurpose platforms for a broad range of delivery applications. Intrinsic nanoscopic properties provide access to unique magnetic and optical properties. Equally importantly, the structural and functional diversity of gold, silica, iron oxide, and lanthanide-based nanocarriers provide unrivalled control of nanostructural properties for effective transport of therapeutic cargos, overcoming biobarriers on the cellular and organismal level. Taken together, inorganic nanoparticles provide a key addition to the arsenal of delivery vectors for fighting disease and improving human health.
Keywords: drug delivery, inorganic nanoparticles, gold, silica, iron oxide, lanthanide upconversion particles
1. Introduction
Nanotechnology provides a potent tool for delivery and programmed release of therapeutics. Many therapeutics suffer from poor stability, inability to cross the cell membrane, and rapid clearance in vivo [1,2]. The biomimetic size and tunable properties of nanomaterials provide unique advantages for therapeutic delivery agents. [3,4,5]. A broad range of inorganic nanoparticles have been explored as carriers to deliver therapeutic cargos into living systems for the controlled, targeted management of diseases like cancer [6,7].
Today, three main classes of therapeutic are of central interest for the treatment of disease: small molecule drugs, nucleic acids, and recombinant proteins [8,9,10]. Small molecule drug delivery is a broad field that has been explored extensively for many applications, including inhibition of cellular processes [11,12], enhanced cell signaling [13,14], and targeted cytotoxicity [15]. Delivery and controlled release of these materials provides a strong value-added proposition, with the potential to maximize efficacy and minimize off-target effects [16].
Many diseases are considered ‘undruggable’ using standard small molecule therapeutics [17]. Many of these challenges, however, can be addressed using biomacromolecular therapeutics [18]. Nucleic acid therapeutics are one broad class, and include plasmid DNA (pDNA) and messenger RNA (mRNA), which induce expression of proteins in the cell [19], as well as RNA interference (RNAi) techniques such as small interfering RNA (siRNA) [20] and micro RNA (miRNA) [21], which ‘knock down’ protein expression. Nucleic acids are highly charged, making them essentially impermeable to the cell membrane [22]. They are also relatively fragile, and benefit from nanocarrier-mediated protection against endogenous nucleases [23].
Proteins present a second family of biomacromolecular therapeutics [24]. Both native and engineered proteins can function directly in the cell and can induce or suppress specific biological processes [25]. Proteins are generally large and do not generally penetrate the cell membrane on their own. [26,27,28]. While peptide (e.g. cell-penetrating peptides) [29] and other bioconjugation strategies can enable delivery into cells, these strategies often result in endosmal entrapment and degradation [30]. Nanocarriers provide access to critically important tools for effecting delivery of large and highly charged nucleic acids and protein species across the otherwise impermeable cell membrane, as well as the benefits of targeting and controlled release.
The different delivery challenges presented by small molecules, nucleic acids and proteins necessitate distinct strategies for complexation/conjugation and delivery [27]. Nanomaterials derived from gold, iron, silica, and lanthanides feature a range of unique physio-chemical characteristics and structural capabilities distinct from the properties of their bulk materials that have made them promising carrier platforms for therapeutics (Fig. 1) [31,32,44]. Many of these attributes, such as high surface-volume ratio [33], long-term stability [34], and optical responsiveness [35], make these materials promising candidates for drug loading and targeted release [36,37]. Certain types of nanocarrier technology have also significantly enhanced delivery efficiency for all three classes of cargo through triggered or localized release [38].
Fig. 1.
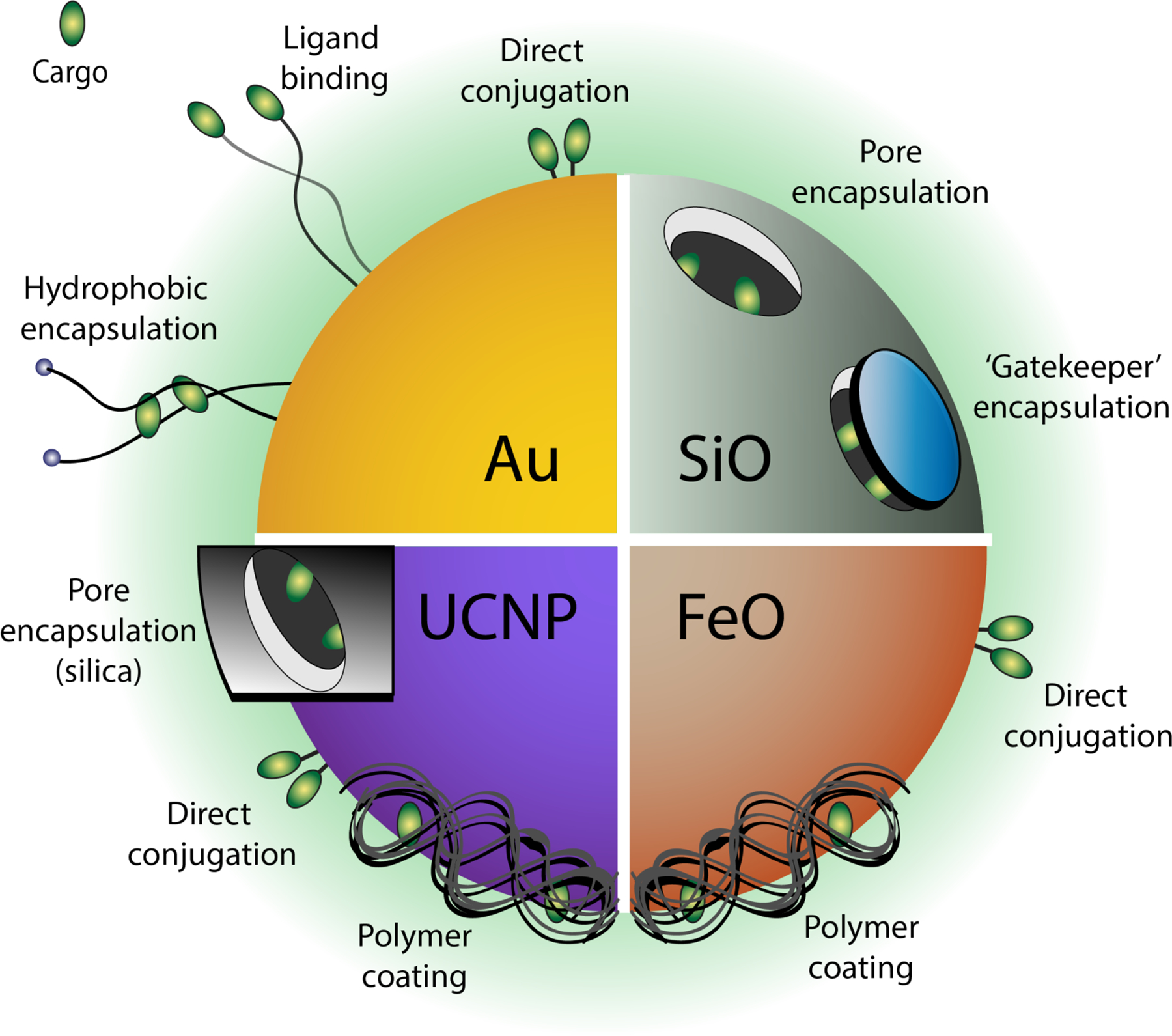
Gold, silica, iron oxide, and upconversion nanoparticle (UCNP)-based platforms provide diverse structural features for covalent or non-covalent conjugation with therapeutic cargo, as shown through representative modalities.
In this review, we highlight the evolution of inorganic nanoparticles for delivery applications, from earlier landmarks to more recent clinical translation. We will also discuss challenges still faced with inorganic nanoparticles, and finally provide our perspective on the opportunities presented by this expansive and fertile field of research. The range of nanomaterials employed for delivery is vast. For this review we have focused on four major classes of materials: gold [39], silica [40], iron oxides [41], and lanthanides [42,43].
2. Gold Particles
AuNPs emerged in the late 1990s as attractive candidates for delivery of diverse payloads [38]. Gold has since proven a versatile and useful core material for delivery applications; it can be formed into monodisperse nanostructures [44] with a high degree of specificity, is essentially chemically inert and nontoxic [45], and can be functionalized with a wide variety of ligand species and chemical moieties [46,47].
Gold can be directly conjugated using thiolated (-SH) molecules to form stable monolayer-protected particles (Fig. 2) in an interaction that is partially covalent (~35%) and mostly electrostatic (~65%) [48,49]. The monolayer stabilizes the core and can be tailored with a range of functionalities to provide effective cellular uptake, controlled payload release, and cell-specific targeting [50]. Direct covalent attachment to the gold core can be used to deliver thiolated fluorophores [51] or small molecule drugs into the cell and into tumor tissue [52] with improved efficacy over free drug [53]. These coverings are stable outside cells but labile intracellularly due to much higher glutathione levels inside cells than extracellularly, providing a mechanism for internal release [54]. The monolayer also allows for non-covalent loading with large amounts of pharmaceuticals, effectively rendering a ‘drug reservoir’ for controlled and sustained release [55,56].
Fig. 2.
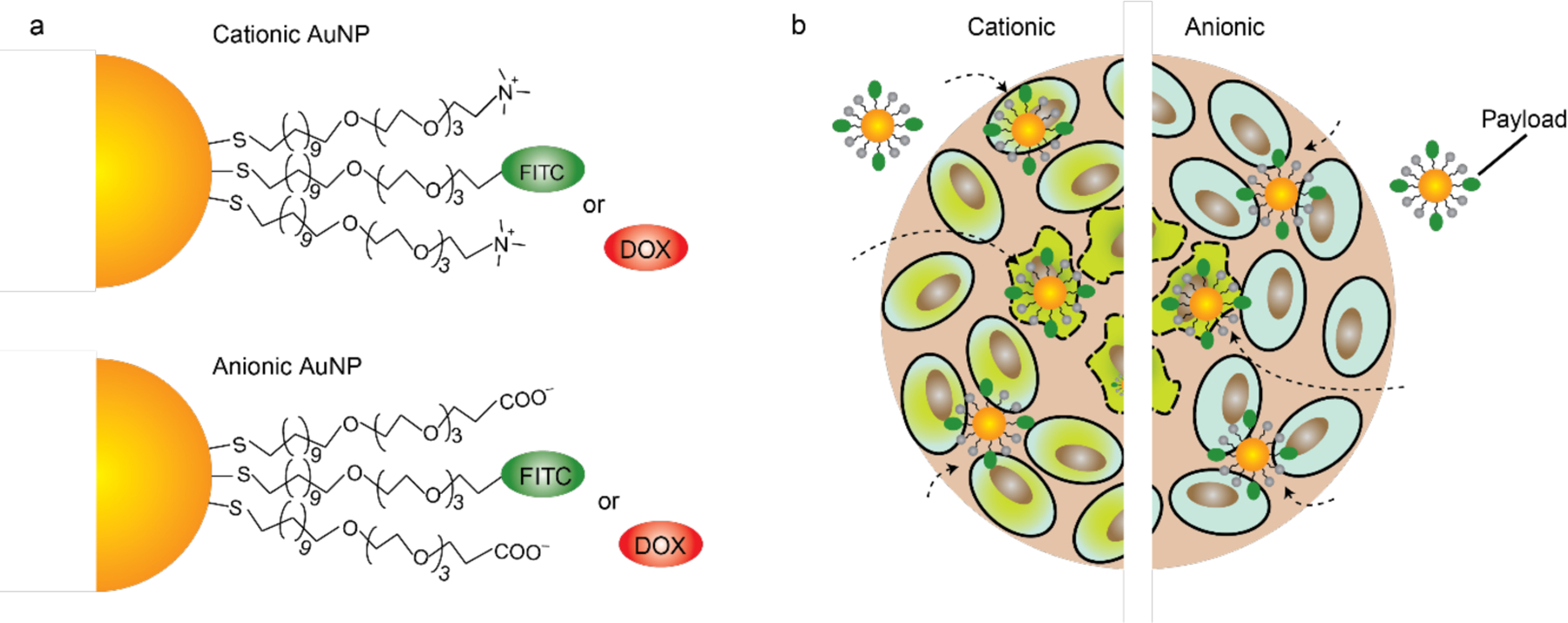
(a) Mixed monolayer-protected gold nanoparticles can be loaded with dyes or small molecule drugs, like thioalkylated FITC or Doxorubicin (Dox). (b) Cellular uptake and FITC-SH release by thiol-mediated replacement reactions within the cell. Particle uptake is dictated by surface functionality. (Adapted from ref. 52).
AuNPs are made through reduction of gold salts in the presence of an appropriate stabilizing agent, and can be synthesized with a highly controllable core size, ranging from ultrasmall (≤2 nm) to as large as 150 nm [39]. Size differences influence physio-chemical characteristics [57], pharmacokinetic behavior [58], and especially optical properties [59]; small AuNPs, including gold nanoclusters, typically have a high surface-to-volume ratio, and can exhibit photoemission (gold nanoclusters (AuNCs, typically < 2 nm), while larger particles show a characteristic surface plasmon resonance [60, 61, 62]. Numerous strategies utilize the advantageous physio-chemical properties of gold nanomaterials to provide robust platforms for intracellular delivery of everything from small molecule drugs [39] to large biomolecules like proteins [27] and pDNA [50].
2.1. Covalent AuNP cargo attachment
Small AuNCs and AuNPs can penetrate cells without disrupting the cell membrane structure [63,64]. Ultrasmall particles also benefit from a tunable biodistribution [65,66], as well as enhanced uptake into the nucleus [67,68] and into tumors [69,70], making them excellent candidates for drug delivery vehicles. Early work demonstrated intracellular delivery of a thiolated small molecule dye covalently bound to a 2 nm AuNP gold core [71]. AuNPs are efficient quenchers of fluorescence, providing the AuNP platform with a robust ‘turn-on’ fluorescence signal to monitor delivery (Fig. 3). Similar approaches using ultrasmall AuNPs enhance small molecule drug delivery for photodynamic therapy [72,73] as well as chemotherapy [74] and at the time of this writing are undergoing clinical trials for treatment of childhood brain cancer [75]. Covalent drug conjugation to a photocleavable head group is a versatile alternative approach to photo-regulated drug delivery [76,77]. ‘Caging’ the anticancer drug 5-fluorouracil to the ligand through a photo-responsive o-nitrobenzyl linkage followed by UV-A radiation triggered photolytic cleavage and drug release, providing effective in vitro anticancer activity [78].
Fig. 3.
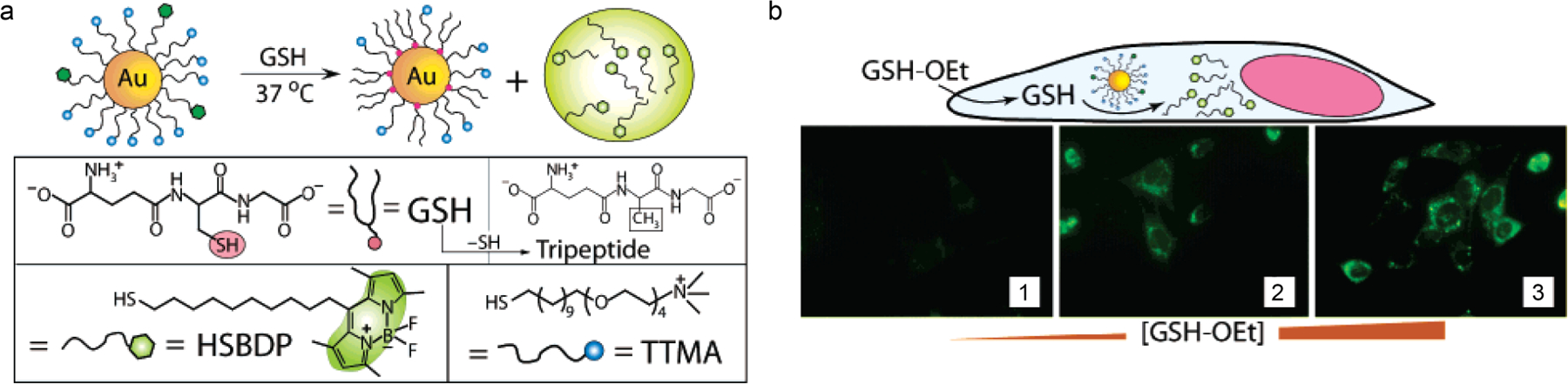
(a) Schematic depiction of AuNP carrier and glutathione-mediated surface monolayer exchange reaction to release the dye. (b) Schematic representation and fluorescence images using glutathione (GSH-OEt) as an external stimulus to trigger release of HSBDP from AuNPs. GSH-OEt concentrations were varied from 0, 5, and 20 mM in panels 1, 2, and 3, respectively (Adapted from ref. 71).
The optical behavior of gold nanoparticles ≥ 2 nm is based on plasmonic properties and gives AuNPs the ability to absorb and scatter light with extraordinary efficiency [79]. Plasmonic properties can be tuned to absorb specific wavelengths based on shape and aspect ratio [80]. AuNPs of mid-to-large core size differ from their ultrasmall counterparts in several ways, one of the most notable being efficient light-to-heat conversion. This property of AuNPs was originally used to destroy cancerous tissue through conversion of NIR irradiation into heat for photothermal therapy [81,82]. AuNPs for photothermal therapy can be combined with other treatment strategies, providing opportunities for multimodal cancer treatment [83,84,85,86]. However, the high-power density of irradiation and poor selectivity remain challenges for this approach [87]. Today, the photothermal properties of AuNPs (in particular hollow nanoshells) are being investigated in the clinic as a treatment for prostate cancer [88].
Peptide conjugation to particle surfaces can serve multiple purposes. First, peptide presentation on the particle surface can provide targeting elements for a specific cell type [89, 90, 91, 92]. Work by Russell utilized AuNPs conjugated with a phthalocyanine dye, polyethylene glycol (PEG) for stability, and HER-2 antibody to provide breast cancer cell targeting [93]. PEG conjugation is a common approach to increasing ‘stealth’ character of nanomaterials otherwise identified as foreign by the body [94,95,96,97]. These studies resulted in selective cancer cell death in cultured cells. Peptides with therapeutic activity can also be conjugated to the particle surface for intracellular delivery [98]. Recently, 2 nm AuNPs bound to small antigenic peptides have been used in clinical trials as an immunotherapeutic treatment for type I diabetes, a promising step forward in medical nanotechnology [99,100].
Nucleic acid strands can be readily modified and bound to gold nanoparticle cores [101] in a selective and cooperative manner, most commonly through thiol moieties [102,103,104]. Mirkin synthesized a class of polyvalent nucleic acid AuNPs (13–15 nm core) with thiolated oligonucleotides. These particles exhibited optical properties governed by aggregate size and were initially used as a diagnostic method to detect DNA [105,106]. They also covalently conjugated 13 nm gold core AuNPs with thiolated antisense oligonucleotides as a gene interference strategy, first using the oligonucleotides themselves [107], and then using complementarily bound siRNA strands (Fig. 4) [108], demonstrating tunable gene knockdown using both approaches [109]. This conjugation strategy is not limited to DNA; thiolated siRNA for example, can also be stably conjugated directly to the gold core [110,111] or attached to polymer-modified gold cores [112,113]. This bioconjugation has notably been shown to be dependent on AuNP shape [114]. Mirkin and Paller demonstrated the capability of spherical nucleic acid-13 nm core AuNP conjugates dispersed in moisturizing ointment to penetrate the skin and down-regulate gene targets responsible for insulin resistance in diabetic mice [115,116]. Conboy and Murthy utilized covalent conjugation to 15 nm gold core for delivery of the CRISPR/Cas9 gene repair machinery with their ‘CRISPR-Gold’ platform [117]. Thiolated complementary olignonucleotides bound to the core held the ‘donor’ DNA needed for gene repair, while the Cas9/RNA complex was encapsulated by a cationic polymer. These studies demonstrated efficient genetic repair, with follow-up studies resulting in therapeutic gene correction of a murine muscular dystrophy model [118].
Fig. 4.
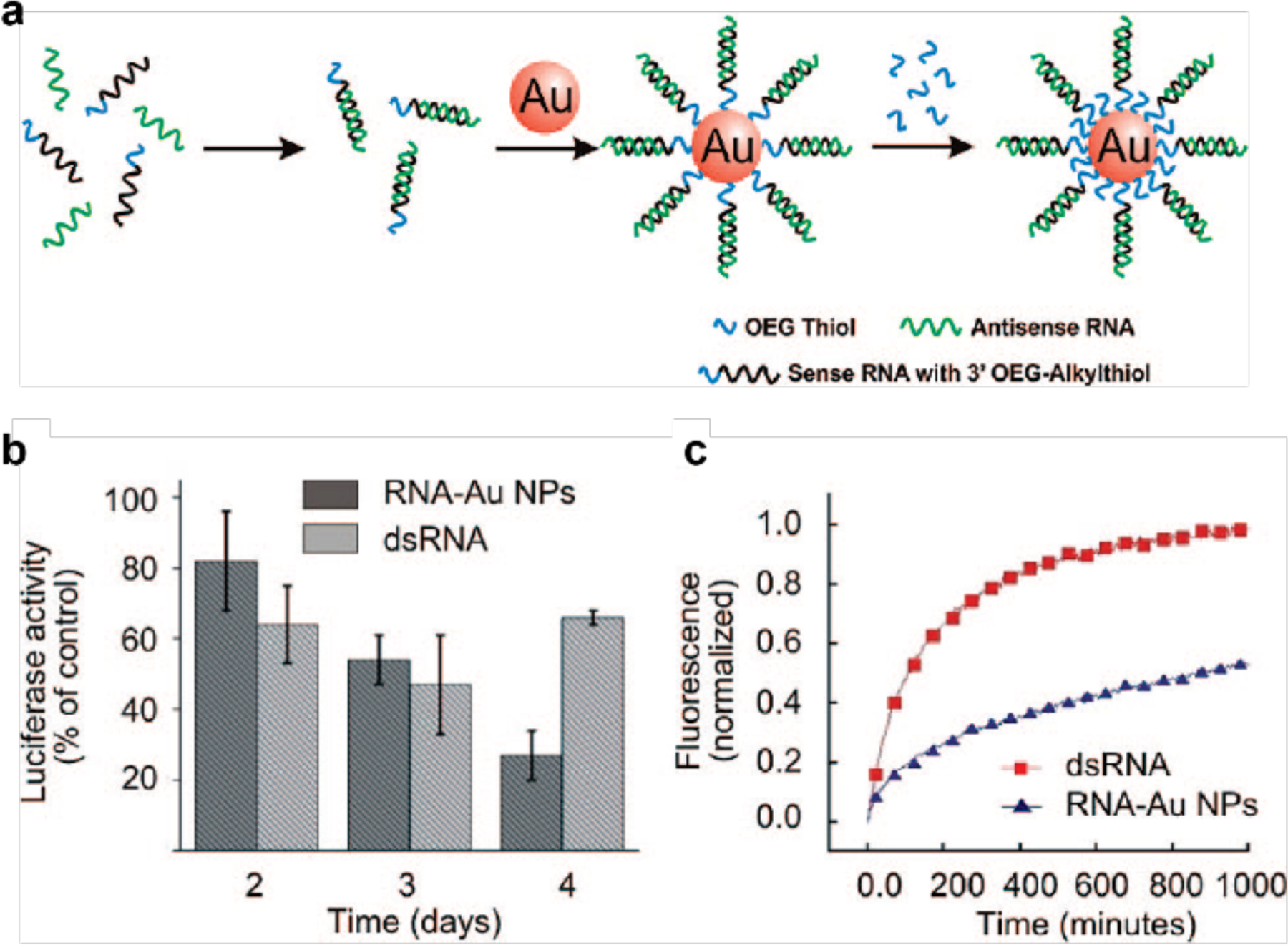
(a) Schematic depiction of the synthesis of polyvalent RNA-AuNP conjugates. (b) Knockdown of luciferase expression in HeLa cells over 4 days using polyvalent RNA–AuNP conjugates (3 nmol/L nanoparticle (NP) concentration, ~100 nmol/l RNA duplex concentration) or double-stranded (ds) RNA (100 nmol/l). (c) Stability of RNA–AuNPs. Comparison of the stability of cyanine 5-labeled double-stranded (ds)RNA (red) and RNA–AuNPs (blue) in 10% serum. The increase in fluorescence intensity demonstrates the distance-dependent release of the fluorophore from the gold core, which is an efficient quencher of the fluorescence. (Adapted from ref. 107).
2.2. Non-covalent AuNP cargo complexation
Non-covalent cargo encapsulation provides an alternative to covalent delivery strategies that avoids the challenges associated with covalent conjugation arising from modification of the cargo, and issues related to detachment [55,119,120]. Drug encapsulation within a hydrophobic pocket such as that present in PEG-conjugated (PEGylated) AuNPs can provide significant loading capacity. Burda reported 5 nm core PEGylated AuNPs with a phthalocyanine photosensitizer small molecule encapsulated within the PEG layer. Intravenous injection provided tumor localization to a much greater extent than free drug [121].
The AuNP ligand monolayer also provides opportunity to encapsulate and deliver drugs. Hydrophobic small molecule anticancer drugs can be loaded into the monolayer of 2 nm core AuNPs for delivery to cancer cells [122]. In this work, AuNP ligands were comprised of hydrophobic alkanethiol chains with zwitterionic head groups, to prevent non-specific adhesion. Several hydrophobic anticancer drugs, including bodipy, tamoxifen, and β-lapachone were encapsulated and demonstrated in vitro anticancer activity significantly greater than free drug alone [123]. Monolayer encapsulation is a versatile strategy; biorthogonal catalysts, such as transition metal catalysts, have also been delivered. These cargos provide an alternative approach to delivery, through localized ‘prodrug’ activation, with the potential for selective intra/extracellular drug activation, based on ligand functionality [124,125,126,127].
Mixed-monolayer AuNPs have the ability to stabilize the interface between immiscible fluids, and form Pickering emulsions [128]. Self-assembled capsules have been demonstrated extensively for encapsulation and delivery, and benefit from the advantages of incorporated AuNPs [129]. Rotello reported 2 nm AuNP-stabilized capsules (NPSCs) with highly controllable physical properties that could encapsulate and deliver small molecule drugs [130]. Follow-up studies demonstrated these systems for delivery of siRNA [131,132,133] through a combination of encapsulation and lateral interaction at the NPSC surface. In this work, siRNA that silenced TNF-α expression was delivered to macrophages LPS-challenged mice. In vivo studies showed directed delivery of NPSCs to the spleen after intravenous administration, with 70% gene silencing, demonstrating NPSC platforms as efficient vectors for immunomodulation in treatment of inflammation (Fig. 5). Macrophages are the first line phagocytes of the innate immune system and are being investigated as therapeutic targets using AuNPs for treatment of several diseases, including inflammation and cancer [134,135,136].
Figure 5.
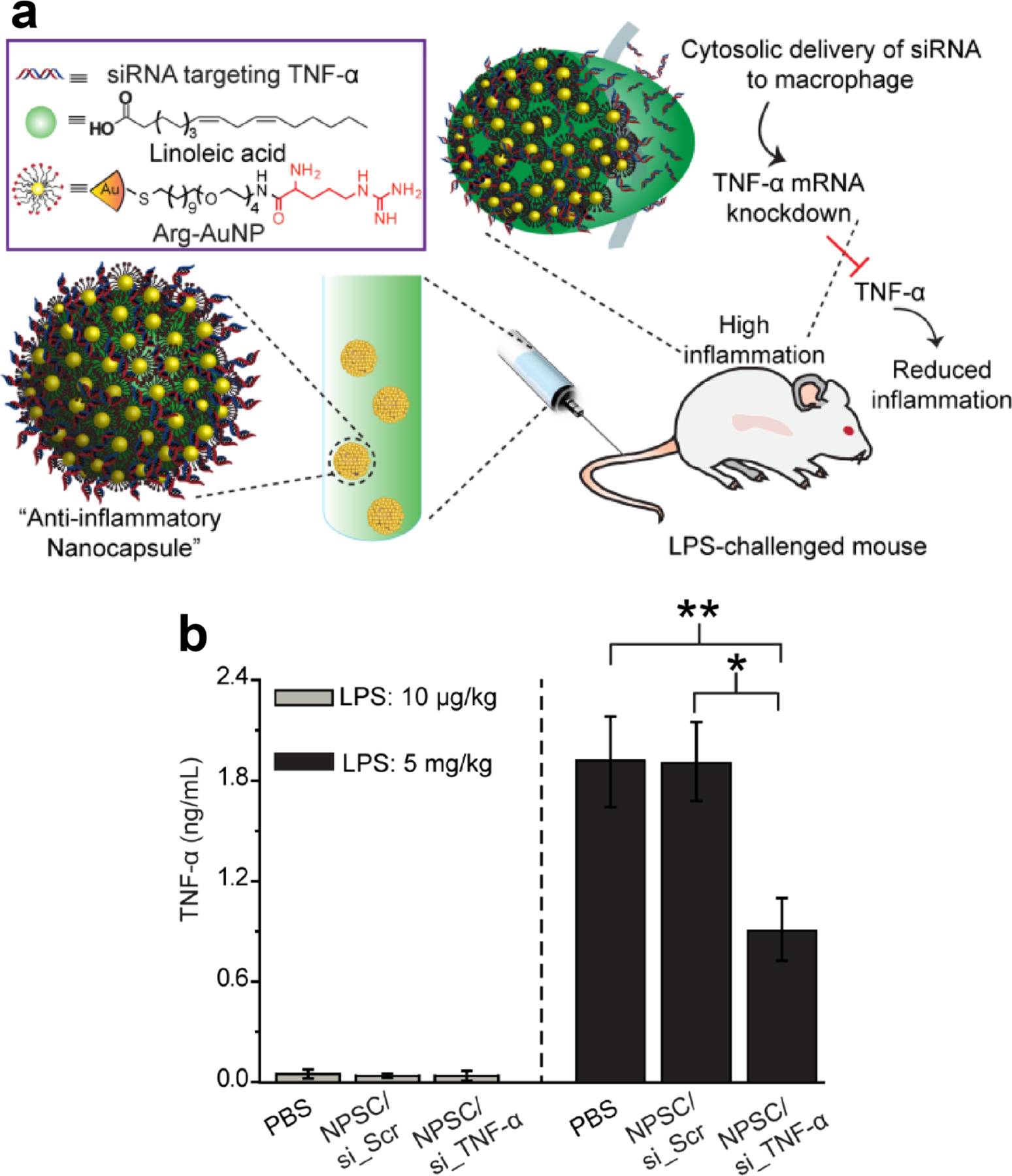
(a) Schematic of NPSC/siRNA-mediated in vivo TNF-α silencing in LPS-induced inflammation. The anti-inflammatory NPSC was prepared by assembling TNF-α targeted siRNA with arginine functionalized AuNPs, with the ensemble self -assembled onto the surface of fatty acid nanodroplets to form a NPSC/siRNA nanocomplex. (b) in vivo delivery of NPSC/si_TNF-α decreased serum TNF-α production from LPS-induced inflammation. (Adapted from ref. 132)
The AuNP monolayer can be tailored to interact with a diverse range of targets, including large biomolecules through electrostatic interaction. One study prepared cationic 2 nm core AuNPs capable of electrostatic surface recognition of a large anionic protein, β-galactosidase. This recognition was used first for intracellular binding and inactivation of the protein [137], and later for intracellular delivery of the same protein [138]. However, this approach relied on inefficient endosomal uptake and escape pathways. In later work, Rotello reported direct cytosolic protein delivery using supramolecular protein-AuNP complexes. Electrostatic interaction between cationic AuNPs and poly(glutamic acid)-tagged proteins generated hierarchically-structured complexes that delivered protein cargo of various sizes and isoelectric points (pI) into the cytosol of mammalian cells [139,140]. This highly effective cytosolic delivery system avoids the endosomal entrapment challenges faced by other strategies [141] and demonstrate the unique structural and dynamic properties that can be obtained using AuNPs [10,39]. This AuNP platform was notably used for intracellular delivery of the CRISPR/Cas9 gene editing machinery, providing efficient (30%) knockout of the PTEN gene in HeLa cells [142]. This approach was effective in vivo as well, as demonstrated by efficient (>8%) knockout of the PTEN gene in splenic macrophages, following systemic administration of the CRISPR/Cas9-AuNP assemblies in BALB/c mice (Fig. 6) [143].
Fig. 6.
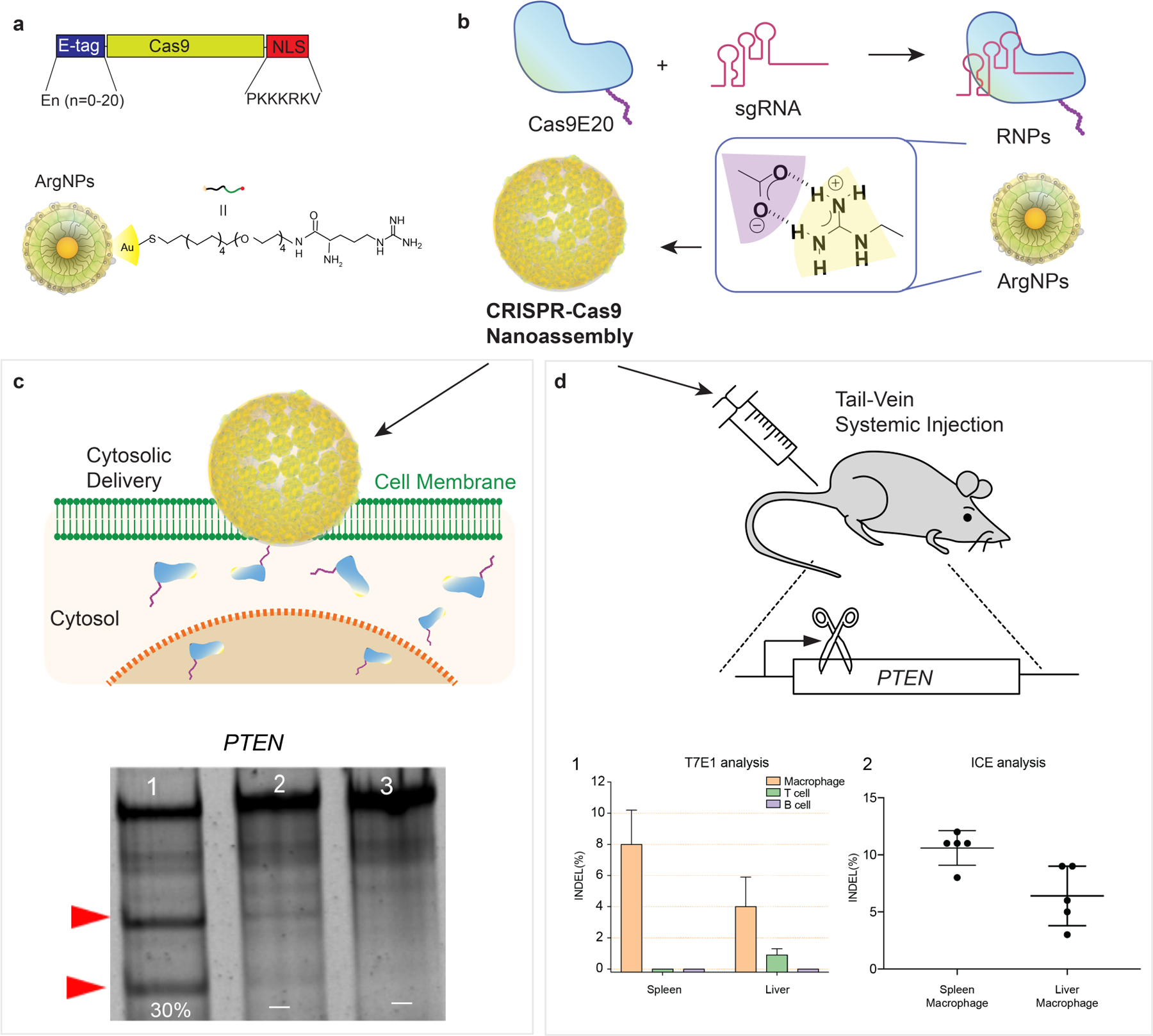
(a) Schematic depiction of E-tagged Cas9 (Cas9E20) protein construct and arginine-functionalized AuNPs (ArgNPs). (b) Cas9E20 was pre-assembled with single guide RNA (sgRNA) to form ribonucleoprotein (RNP) complexes. Cas9E20 RNPs self-assembled with ArgNPs to escalate particle protein nanoassemblies. (c) AuNP-protein nanoassemblies associated with the cell membrane and delivered protein through a membrane fusion-type mechanism in cultured HeLa cells. Gene edited was evaluated by T7 endonuclease I (T7E1) assay in experimental samples (1), with no editing observed in controls with no ArgNPs (2) and cell only (3). (d) Nanoassemblies administered to the tail vein of BALB/c mice exhibited gene editing in the spleen and liver, as determined by T7E1 assay and Interference of CRISPR Edits (ICE) sequencing analysis. (Adapted from refs. 142 and 143).
Cationic small AuNPs can non-covalently bind nucleic acids through electrostatic interactions with the highly anionic phosphate backbone [144,145]. Early work demonstrated the ability of mixed monolayer AuNPs to complex [146] with pDNA and transfect mammalian cells with low toxicity [147]. Translation of these platforms to in vivo applications [148,149,150] has shown promising results, with potential advantages over viral carriers in terms of safety and efficacy [151,152,153].
Multilayer assembly is an encapsulation approach that electrostatically complexes cargo within complementarily charged coatings [154,155]. Tung employed multilayered 40 nm core AuNPs fabricated with poly-L-lysine and siRNA (up to 4 layers) in alternating coatings to provide a protease-degradable siRNA carrier [156]. The authors observed a gene knock-down effect correlated with the number of siRNA layers. Co-assembly with the organic transfection reagent polyethylene imine (PEI) is a commonly used approach to promote uptake of AuNPs for delivery of siRNA or miRNA [157]. Incorporation is often done through multilayering of PEI and RNA [158], but supramolecular complexation and delivery has also been demonstrated using AuNPs with dendritic ligands [145]. In recent work, Liang et al. developed self-assembled and crosslinked clusters based on 2 nm AuNPs, containing an anticancer oligonucleotide, into a sunflower-like superstructure that dissociated and enhanced cell uptake upon irradiation with near-IR (NIR) light [159]. This strategy notably utilized the uptake capabilities of small AuNPs as well as the photo-responsive properties of larger gold clusters to achieve targeted delivery in vivo [160].
The optical responsiveness of gold nanomaterials has garnered especial interest in gold nanorods (AuNRs), elongated Au-based nanoscale materials with optical properties that can be finely tuned through the aspect ratio of the rods [161,162,163]. Upon wavelength-specific irradiation, AuNRs generate heat, potentially providing for localized payload release [164,165,166]. AuNRs have been extensively explored for delivery applications [167], perhaps most notably of nucleic acids including DNA [168,169,170,171] and siRNA [172]. In 2008, Murphy encapsulated a model hydrophobic small molecule in the surfactant bilayer bound to the nanorods, demonstrating their high drug loading capacity [166]. More recently they have been explored as delivery vehicles for nucleic acids. Wei demonstrated the electrostatic adsorption of dithiocarbamate-modified siRNA duplexes to a AuNR surface, minimizing premature siRNA desorption and release [173]. These carriers released their cargo upon NIR irradiation and demonstrated significant knockdown of a target gene related to metastatic ovarian cancer. AuNRs provide for efficient non-covalent cargo loading and benefit from their photo-responsiveness, however they also tend to suffer from aggregation, and sufficient purification from surfactants and other chemicals used in their fabrication can be a challenge [174].
2.3. Gold nanoclusters
Gold nanoclusters (AuNCs) are smaller gold-based nanomaterials consisting of tens to hundreds of gold atoms. The properties of AuNCs bridge the gap between nanoparticles and atoms, as they possess unique properties distinct from their bulk counterparts [175]. Unlike AuNPs, AuNCs are mixed-valence species that have discrete energy levels and show multiple absorption bands. These photophysical properties can allow for fluorescence in the NIR region [176] and for other unique photodynamic properties [177] that position AuNCs as potential vehicles for delivery.
Cui et al reported a facile method of assembling monodisperse and stable self-assembled nanoparticles (NPs) in water using chlorin e6 (Ce6) molecules to cross-link AuNCs. These GNCs-Ce6 NPs were conjugated with CD3 antibody to create a cell-specific drug delivery system for cytokine-induced killer cells (CIK). This system exhibited high tumor-targeting efficiency and excellent therapeutic efficacy toward MGC-803 tumor-bearing mice [178]. Antibody-based tumor targeting can be extended to include multiple targeting elements [179,180,181]. Chen developed a AuNC platform for doxorubicin (dox) delivery to tumors, using a dual-targeting strategy. AuNCs were covalently conjugated with both a peptide specific for integrins on the surface of tumor tissues (cRGD) and an aptamer with high affinity to nucleolin overexpressed in the cytoplasm of tumor cells (Apt), for extra- and intracellular tumor targeting, respectively (Fig. 7) [182]. dox was immobilized onto the dual targeting platform and was shown to release and trigger tumor cell death in both cultured cell models and in vivo, with enhanced accumulation in the tumor.
Fig. 7.
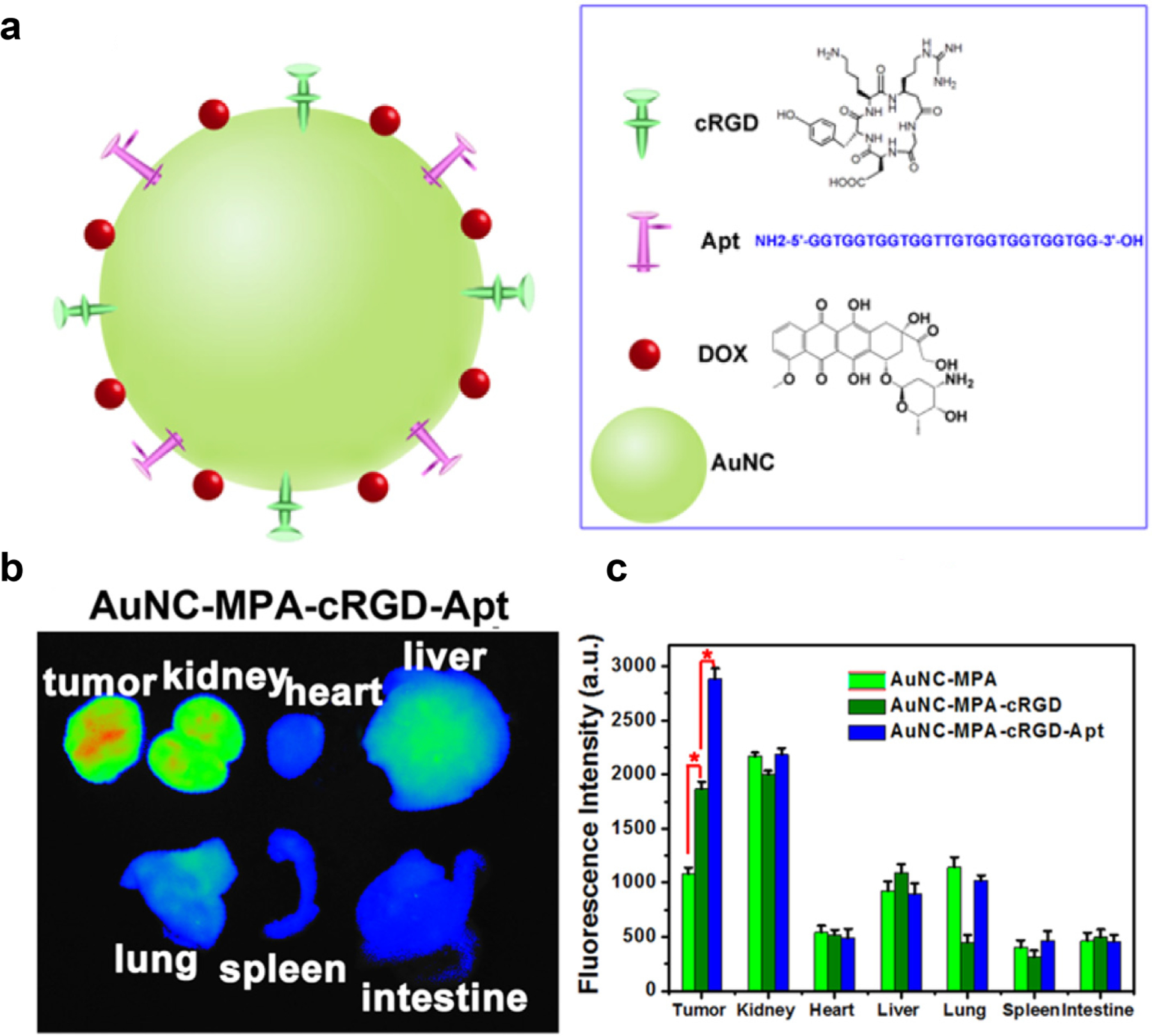
(a) Schematic structure of dox-loaded antibody/aptamer (cRGD/Apt)-conjugated AuNC. (b) ex vivo fluorescence images of isolated organs from tumor-bearing mice at 8 h post-injection. (c) Fluorescence intensity of isolated organs at 8 h post-injection with different sample formulations (Adapted from ref. 182).
Like AuNPs, cationic AuNCs can electrostatically complex nucleic acids to enhance cellular delivery. In a recent study, Jiang [183] complexed siRNA targeted at NGF (a tumor-associated gene) with < 3 nm cationic AuNCs. The authors demonstrated enhanced siRNA stability in serum, enhanced cellular uptake and gene silencing in cells, and tumor accumulation in vivo. Notably, AuNCs with cores < 3 nm often exhibit robust fluorescence, enabling imaging applications. Wang [184] demonstrated carborane NCs that provide accurate tumor imaging and long-term accumulation in tumor sites by the EPR effect.
AuNPs and AuNCs are both versatile platforms that benefit from their stability and tunable surface functionalization. AuNP-based delivery platforms are also among the few that have reported for direct cytosolic delivery, critical for future development of efficient therapeutic delivery systems for biologics [139,142]. Gold is inherently inert and has even been called the ‘noblest’ of metals [185]. Work by Xu [186] demonstrated that even direct incubation of gold nanoparticles with zebrafish embryos had minimal effect on development. Despite being mostly accumulated in the liver and spleen of animal models, gold nanomaterials did not induce any hepatic or renal toxicity [187]. Although some uncertainty in regards to the biological fate of the gold nanomaterials is to be considered, there are ways to overcome this limitation by controlling the surface properties such as surface charge [66] which will be critical to the future advancement of gold-based delivery platforms. [143,188].
3. Silica Nanoparticles
Silica-based nanocarriers offer many advantages in terms of loading capacity and can be easily functionalized for a variety of applications [189,190,191]. Silica nanomaterials and especially porous nanoparticles have gained interest as delivery platforms for their potential to address key therapeutic issues including drug solubility and extended drug release rate [192,193]. Mesoporous silica nanoparticles (MSNPs) are nanoscale silica particles with a honeycomb-like structure featuring hollow channels and are the most commonly used silica-based delivery vehicles [31,194]. The generation of MSNP structures was advanced considerably in the early 1990s by with scientists at Mobil Oil working to develop a new family of molecular sieves [195]. MSNPs have since become attractive delivery vehicles due to their chemical inertness, thermal stability, extended cargo loading, and tunable structure [196,197,198]. The synthesis of MSNPs generally relies on a cationic surfactant to provide a template for the base-catalyzed sol-gel process (Fig. 8) [199]. The process itself and choice of template are highly controllable, so resulting MSNPs can be finely tuned for particle size, pore quantity, and pore radius [200,201]. MSNPs generally encapsulate cargo within their pores through non-covalent binding, but delivery strategies can also benefit from rationally designed covalent surface modifications to provide targeted or controlled cargo release [197].
Fig. 8.
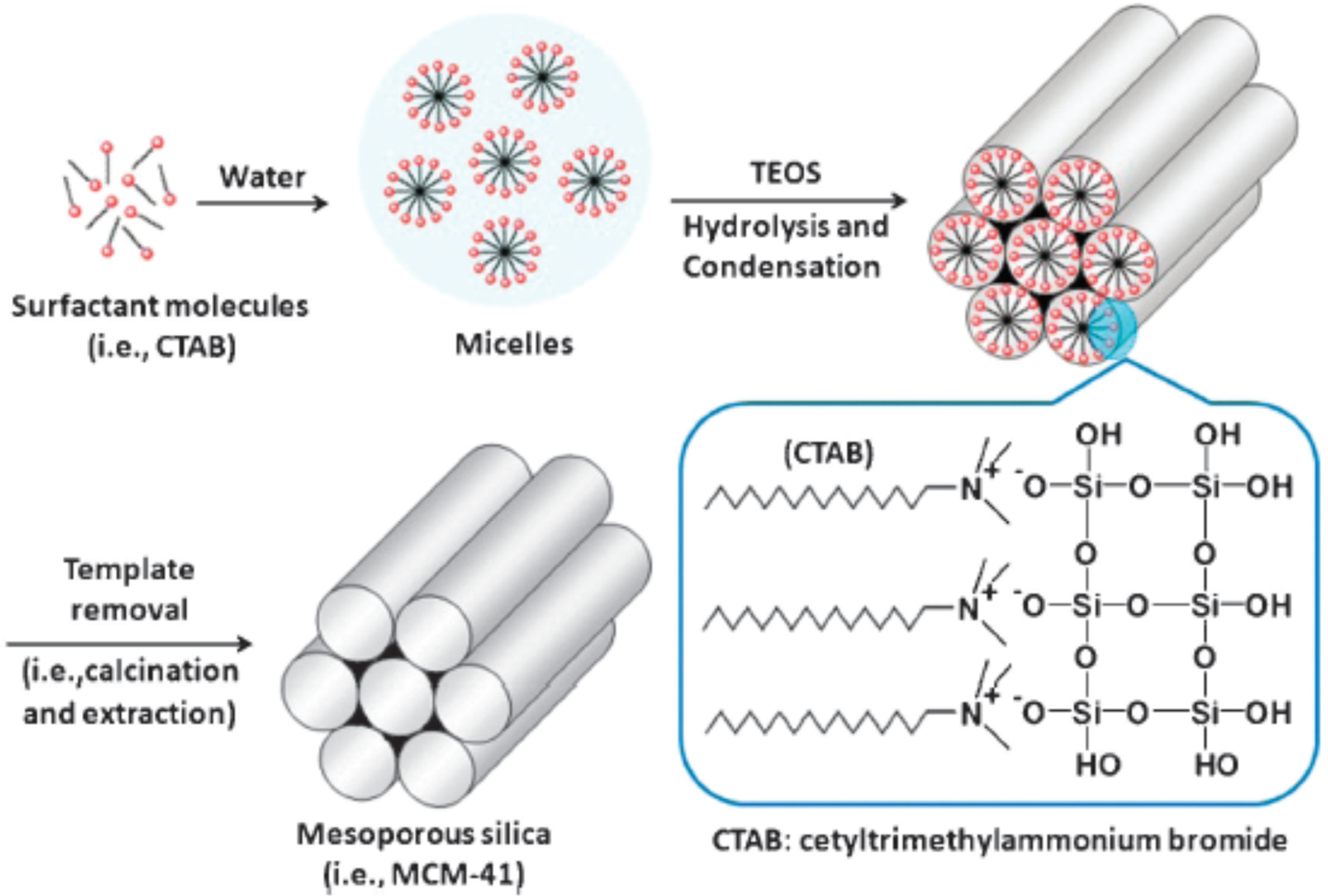
General synthetic scheme for preparation of MSNPs. (Adapted from ref 202).
3.1. Non-covalent cargo encapsulation by MSNPs
Pore size and surface functionalization play crucial roles in the drug loading capacity and tunable release rate of MSNPs [202]. Typically, cargo molecules are loaded in MSNPs through weak non-covalent interactions, such as hydrogen bonding, physical adsorption, electrostatic interaction, or aromatic stacking [203]. Altering pore characteristics can significantly influence charge density and steric effects within the pore, modulating weak electrostatic interactions, and impacting cargo release [204,205]. MSNPs with small pores can provide tunable release rate for small molecule drugs: in early studies, Pérez-Pariente reported that by employing different pore sizes, release of pore-loaded ibuprofen could be tuned from burst release to long-term sustained release [206]. In later work by Tian, MSNPs with pore sizes ranging from 3 nm to 10 nm were loaded with the chemotherapeutic drug paclitaxel [207]. The release rate of paclitaxel loaded MSNPs was measured in solution and in cultured cells, and it was found that larger pores provided higher loading capacity, faster release rate, and greater in vitro anticancer activity. Later however, Yin’s in vivo antitumor experiments added complexity to these conclusions by demonstrating that MSNs with a pore size of ~5 nm induced the most apoptosis and showed best tumor reduction in H22 tumor bearing mice [208].
Recently the ability of MSNPs with small pores to isolate encapsulated materials from the environment has been used for biorthogonal applications [209,210,211]. Mascareñas and coworkers reported a hollow ‘nanoreactor’ consisting of 3 nm pore MSNPs as a nano-shell, doped with an inner layer of palladium nanoparticles [212]. These nanoreactors facilitated palladium-catalyzed in situ de-caging reactions and Suzuki-Miyaura intermolecular cross-couplings in living systems, providing a promising platform for biorthogonal prodrug activation [213,214].
Biomolecules such as proteins and nucleic acids are multi-nanometer scale, and thus require larger pore sizes. Lin used MSNPs with a large average pore diameter (5.4 nm) to deliver cell membrane-impermeable protein cytochrome c into mammalian cells through endocytosis [215]. The authors observed that enzymes adsorbed and released from MSNPs retained their catalytic activity, suggesting that loading did not impact protein structure. As mentioned above, pore size can be used to regulate release of proteins. Shi prepared a series of MSNPs with varying average pore size (ranging from 2.7 to 4.6 nm) to evaluate encapsulation of cytochrome c [216]. The authors observed increased loading capacity up to 4.6 nm pore size, indicating the importance of pore size in efficient loading of protein cargo.
Small nucleic acids can be loaded into MSNP pores using hydrogen bonding, but only if the repulsive negative charges of the nucleic acids and the MSNP surface are shielded from each other [217]. In early studies, Zink and Tamanoi [218] developed PEI-coated 100 nm MSNPs with ~2.5 nm pores to encapsulate and deliver functional siRNA to knock-down enhanced green fluorescent protein (eGFP) and functional genes related to signaling in cultured PANC-1 human cancer cells. Gu and Xia similarly packaged functional siRNA into MSNPs featuring ~3.7 nm pores and then mixed them with PEI to form a polymer layer on the external surface [219]. Delivery to reporter cell lines demonstrated efficient knockdown of target genes eGFP and Bcl-2. MSNP pore sizes can also be expanded to tens of nanometers to accommodate larger nucleic acids. Min reported that compared to smaller pore sizes, ‘ultralarge’ pore size (> 15 nm) provided higher pDNA loading capacity and was able to protect luciferase pDNA (4.8 kbp) from degradation (Fig. 9) [220].
Fig. 9.
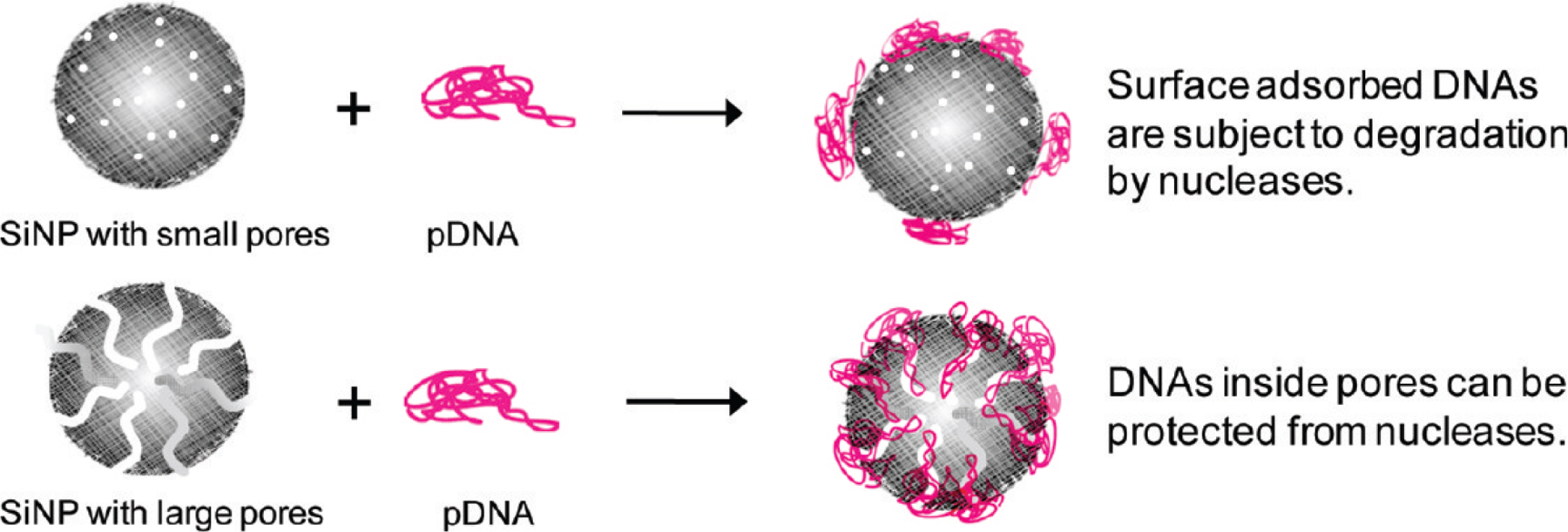
Monodisperse MSNPs with large pores provide higher loading capacity for pDNA and better protection from nucleases compared to those with small pores. (Adapted from ref. 220).
Surface modification provides an avenue for stimuli-responsive delivery, using ‘gatekeeper’ moieties. Gatekeeper molecules are generally bound to the outside of the pore, and sterically block cargo release until they are detached. Joo and Ryu [221] developed a gatekeeping strategy by capping drug-loaded MSNP pores with disulfide cross-linkable polymers that would degrade in the cytosol, triggering drug release. The authors reported high loading capacity of dox and cisplatin, with a varied cytotoxic response upon intracellular release. Tan and Zhao [222] developed similar MSNP platforms for targeted delivery of dox to MDA-MB-231 xenograft model breast cancer in vivo models. MSNPs loaded with dox were gated with amino-β-cyclodextrin bridged by cleavable disulfide bonds and grafted with folate tumor targeting moieties. The drug cargo was released after delivery by the decrease in intracellular pH in endosomes and increased intracellular glutathione levels. After intravenous injection, significant decrease in tumor growth was seen in treated mice over 30 days without decrease in body weight (Fig. 10).
Figure 10.
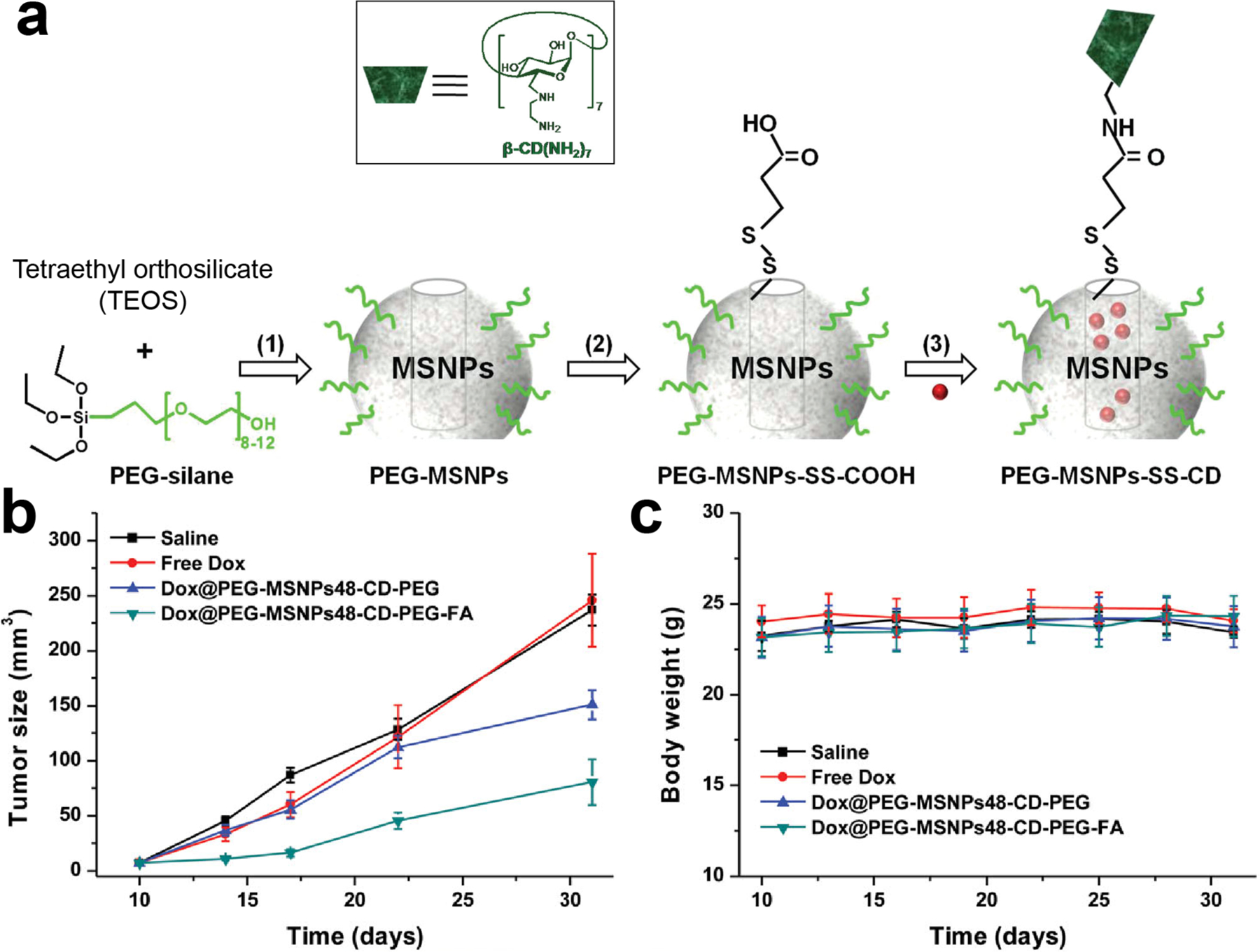
(a) Schematic representation of the cargo-loaded PEG-MSNPs-SS-CD prior to folate conjugation. (b) Tumor volume changes from different treatment groups. (c) Body weight changes of MDA-MB-231 tumor-bearing mice.
A variety of non-covalent and covalent gatekeeping strategies have been developed for triggered cargo release, more of which will be highlighted in the following section [223].
3.2. Covalent surface modification of MSNPs
Covalent modification of the MSNP surface can greatly influence cargo loading, protein adsorption, and rate of release [224,225]. MSNP surfaces are typically negatively charged but can be covalently modified to have cationic surfaces. These modified cationic MSNPs can bind with highly negatively charged cargos, generating complexes with potential for delivery. Shi used MSNPs covalently grafted with cationic rhodamine B to enhance encapsulation of the negatively charged cardiovascular drug salvianolic acid B [226]. The authors reported protection of encapsulated drug from degradation, a sustained rate of release, and increased uptake over free drug in LX-2 hepatic cells. Similar approaches to modify MSNP surfaces for adsorption and delivery of nucleic acids have included a variety of other cationic macromolecules as modifiers, including PEI [227,228], dendrimers [229], and lipids [230]. Work by Cristini and Brinker [231] examined the biological fate of MSNPs with varied sizes, surface chemistries and routes of administration through single photon emission computed tomography integrated with computed tomography (SPECT/CT) imaging, with tandem mathematical modeling used to interpret the results. These studies revealed cationic MSNPs with surface exposed amines (PEI) are rapidly sequestered into the liver and spleen but show less total excretion than MSNPs with surface-shielded amines.
Covalently bound gatekeeper moieties can provide carrier stability, and potentially stimuli-responsive release. Stimuli-responsive gatekeepers provide a method for targeted delivery at a desired locale through endogenous or exogenous stimuli [232,233]. Several families of covalent gatekeepers have been used for controlled release, including cyclodextrins [234], azobenzenes [235,236], and complementary DNA [237]. In a notable study, Zhu developed dox-loaded MSNPs gated with a DNA hybrid complementary to miR-21 miRNA [238]. Upon binding miR-21, conformational changes in the DNA hybrid released dox and effectively killed HeLa cells. Interestingly the DNA hybrid also provided miR-21 silencing, inducing apoptosis and providing complementary chemotherapeutic mechanism.
Stimuli-responsive polymers provide versatile gatekeepers for release of payloads under disease-relevant environmental conditions like the low pH found in tumors [239] and bacterial biofilms [240]. Chu and coworkers [241] developed a pH-responsive release system for delivery of the anticancer cytokine protein TNF-α. Hollow MSNPs loaded with TNF-α were conjugated with pH-sensitive chitosan for controlled release in the acidic tumor microenvironment (Fig. 11). Chitosan was further conjugated with ErbB 2 antibody to provide enhanced breast cancer cell targeting. Under acidic conditions, the chitosan amino groups were protonated, stretching the polymers and triggering TNF-α release. These platforms eliminated 70% of MCF-7 cells in vitro, and upon intraperitoneal administration to mice with implanted MCF-7 tumors, reduced tumor weight by 50% in vivo.
Fig. 11.
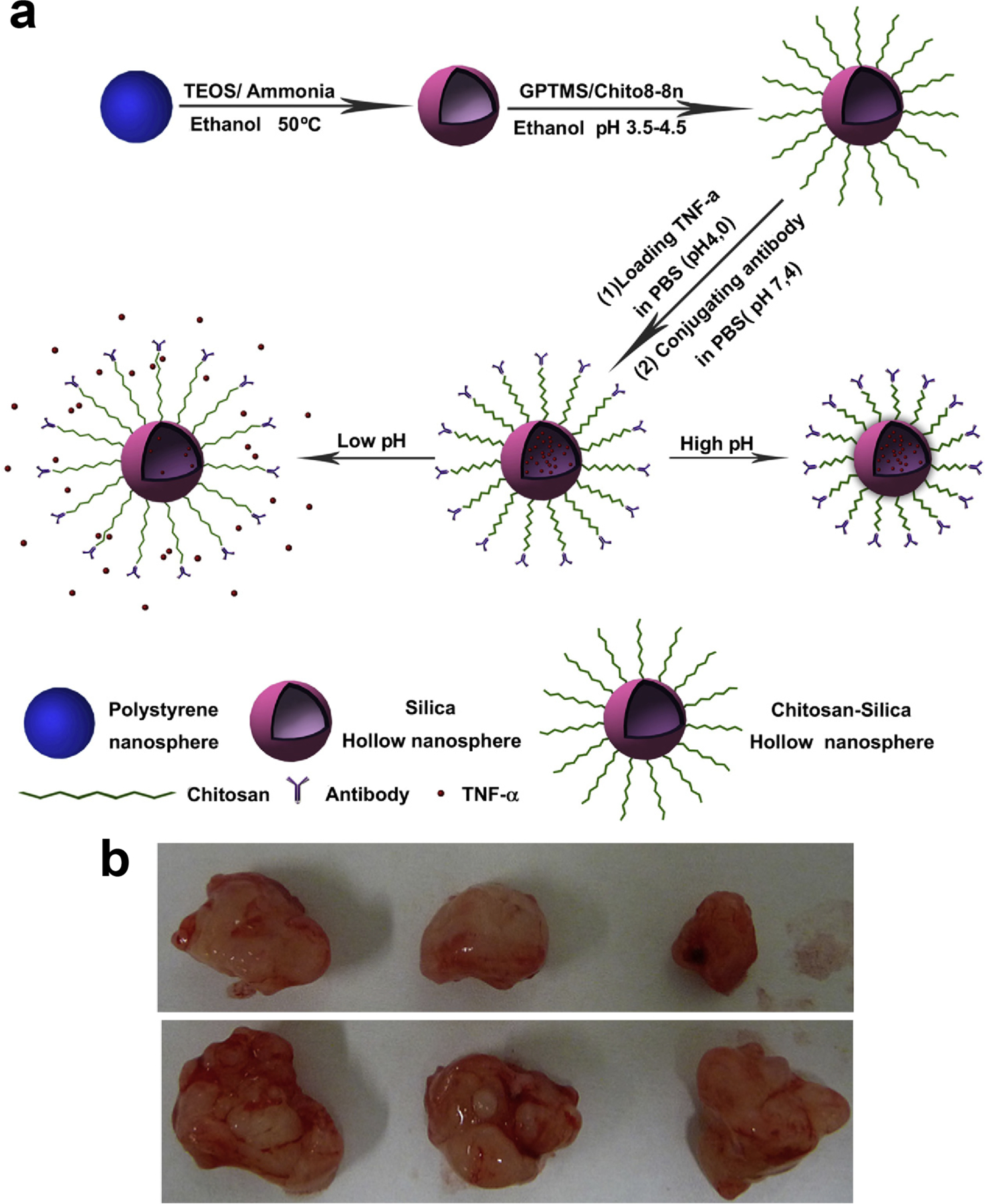
(a) Schematic diagram illustrating the formation of nanocarriers (MSNP-chitosan-TNF-a conjugated with antibody) and the drug release behavior at different pH values. This structure blocks and restricts drugs release from the hollow interior. (b) Photos of tumors collected from mice in Nano group; nanocarriers composed of CS-SiO2,HNPs, TNF-a and antibody were injected into the mice (top); PBS (pH 7.4) only was injected into the mice (bottom). (Adapted from ref.241).
Conformational changes in engineered gatekeeping polymers can be triggered by exogenous stimuli including ultrasound waves and light [242]. Vallet-Regi [243] designed thermally responsive co-polymer-grafted MSNPs for ultrasound-triggered small molecule delivery. Thermally triggered conformational changes in the polymer allowed the MSNP pores to be loaded with small molecule therapeutic cargo at 4°C and to encapsulate their cargo at 37 °C. Triggered by ultrasound waves, conformational changes in polymer tetrahydropyranyl moieties on the surface of the MSNP increased hydrophilicity and concomitantly the exposure of carboxylates, resulting in payload release (Fig. 12). dox-loaded MSNP hybrids showed significant efficacy relative to free drug in prostate adenocarcinoma (LNCaP) cells. Recent work by Zink [244] has applied ultrasound-stimulation for MRI-guided MSNP platforms capable of triggered spatiotemporal small molecule release. These vectors were demonstrated for delivery of an MRI contrast agent in a proof-of-concept study but provide opportunity for future image-guided theragnostic applications. Related work by Jokerst [245] examined MRI-guided MSNPs for delivery of the pro-survival protein insulin-like growth factor (IGF) in cultured cells.
Fig. 12.
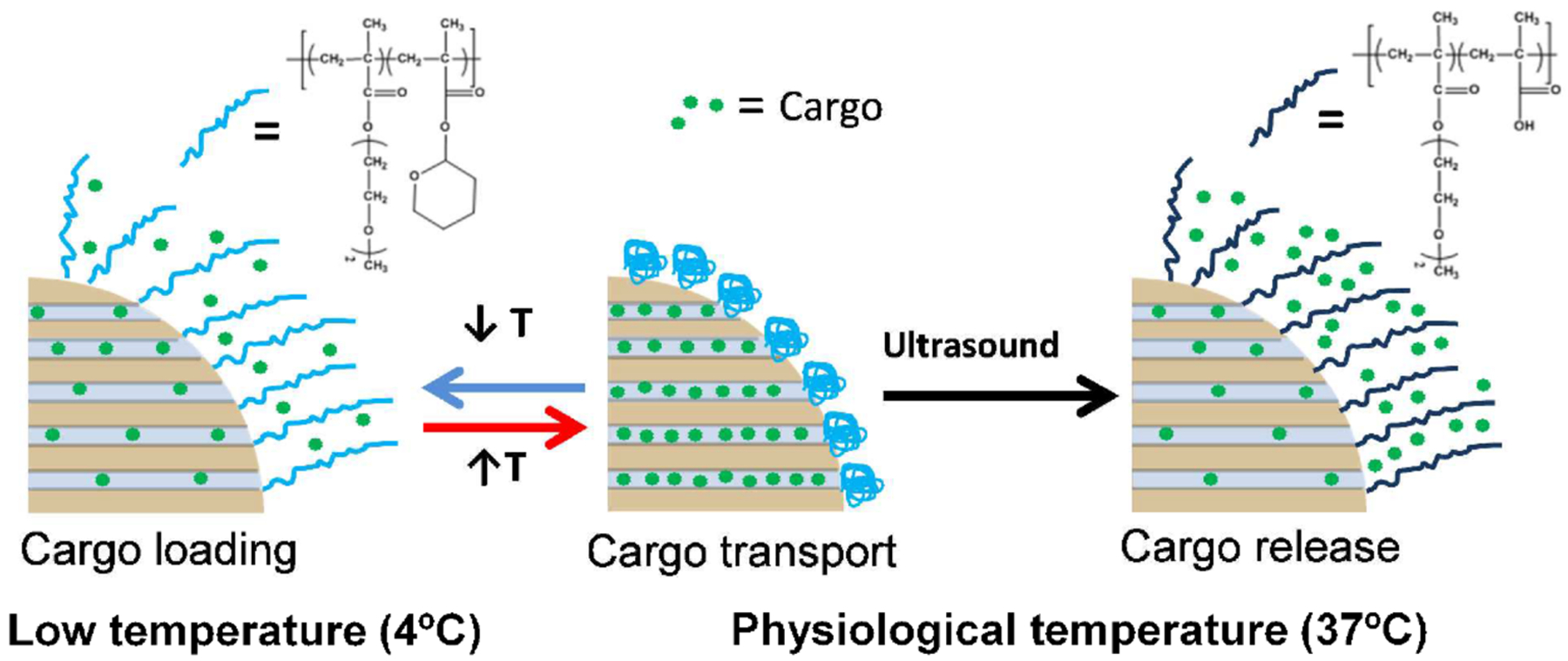
Scheme of polymeric ultrasound sensitive “nano-gate” on MSNPs. Loading was achieved at lower temperature. Ultrasound waves triggered cargo release at physiological temperature. (Adapted from ref 243).
Ultrasound has been used for targeted disruption of microbubbles for delivery. Zhang and coworkers [246] adopted this strategy for delivery of MSNP-loaded cargo. A small molecule chemotherapeutic (Tanshinone IIA) was encapsulated in folate-conjugated MSNPs that were then incorporated within microbubble surfaces. These microbubbles protected the MSNPs from non-specific release of drug and enhanced circulation time. Ultrasound disrupted the microbubble structure and provided drug release from the MSNP at the desired locale, providing ~30% tumor growth suppression in vivo after intravenous administration to H22-tumor bearing mice.
Photoisomeric moieties such as azobenzene are useful gatekeeper moieties that undergo isomerization upon exposure to specific wavelengths of light [247,248]. In recent work, Fu [249] developed dox-loaded MSNPs capable of triggered release by both acidic pH and exposure to UV light. dox-loaded MSNPs were covalently conjugated with supramolecular switches consisting of hydrazone-based bonds, azobenzene moieties, and α-cyclodextrins to sterically block drug release from the pores. Trans-azobenzene has a strong host-guest interaction with cyclodextrin, while the cis-state inhibits interaction due to changes in polarity and steric hindrance. Acidic pH led to rapid hydrolysis of the hydrazone-based bonds, while exposure to UV light (365 nm) triggered cis-trans isomerization of the azobenzene moiety. The authors demonstrated that either stimulus alone was sufficient to trigger cargo release, and demonstrated delivery through phagocytosis, and efficient killing of MCF-7 human breast cancer cells.
UV-triggered delivery strategies are of course limited by the poor tissue penetration of UV light compared with longer wavelength radiation (e.g. NIR) [250]. Several NIR light-responsive platforms have been developed including MSNPs embedded with upconversion nanoparticles (such as lanthanide nanoparticles) [251] or iron oxide nanoparticles [252,253,254,255]. These platforms will be further discussed in later sections.
A major challenge facing clinical translation of MSNPs is the surface-exposed silanol groups on the particle surface. Silanol moieties interact with surface phospholipids on red blood cells, potentially causing hemolysis [256]. MSNPs have also been implicated in promoting the growth of malignant melanoma through altered ROS production in vivo [257]. Coupled with this is the concern of accumulation in the body; much recent research has focused on understanding the biodegradability of silica-based nanocarriers [258,259], even as a tumor-specific delivery strategy [260]. Notable work by Maggini and De Cola [261] explored ‘breakable’ MSNPs held together by disulfide linkages that degraded in the cytosol, providing a biodegradable carrier vehicle. This vehicle was also shown to be capable of a functional protein, cytochrome c to cultured cells [262]. Despite these challenges the advantages of porous silica nanocarriers have positioned them as competitive platforms for delivery of both small molecules and biologics.
4. Iron Oxide Nanoparticles
Iron oxide nanoparticles feature inherent magnetic properties coupled with tunable size and functionality that provide excellent potential for therapeutic applications [263]. The two predominant forms of iron oxide nanoparticles used for biomedical applications are maghemite (γ-Fe2O3) and magnetite (Fe3O4), due to their relative colloidal stability [264–268]. Magnetite has a ferromagnetic structure arising from alternating lattices of Fe(II) and Fe(III) [269]. Nanoparticles composed only of magnetite with a mesoporous structure have been demonstrated for encapsulation and delivery of small molecule drugs [270,271]. Maghemite has the same lattice structure as magnetite and exhibits similar ferromagnetic properties, however all iron atoms are in Fe(III) oxidation state. Fe(III) ions are commonly found in the human body, and are less toxic than Fe(II) that can effect Fenton chemistry, making maghemite a favorable core material [272].
Iron oxide nanoparticle delivery vectors typically consist of a magnetic core, an outer coating (often a polymer or metal) to provide a surface for grafting, and finally surface functionality (Fig. 13). Cargo may be bound to surface moieties, or alternatively can be encapsulated either inside the particle shell or within co-embedded mesoporous particles. Stimuli-responsive elements for encapsulation can provide triggered cargo release, for synergistic combination with the hyperthermic properties of the iron oxide [273,274].
Fig. 13.
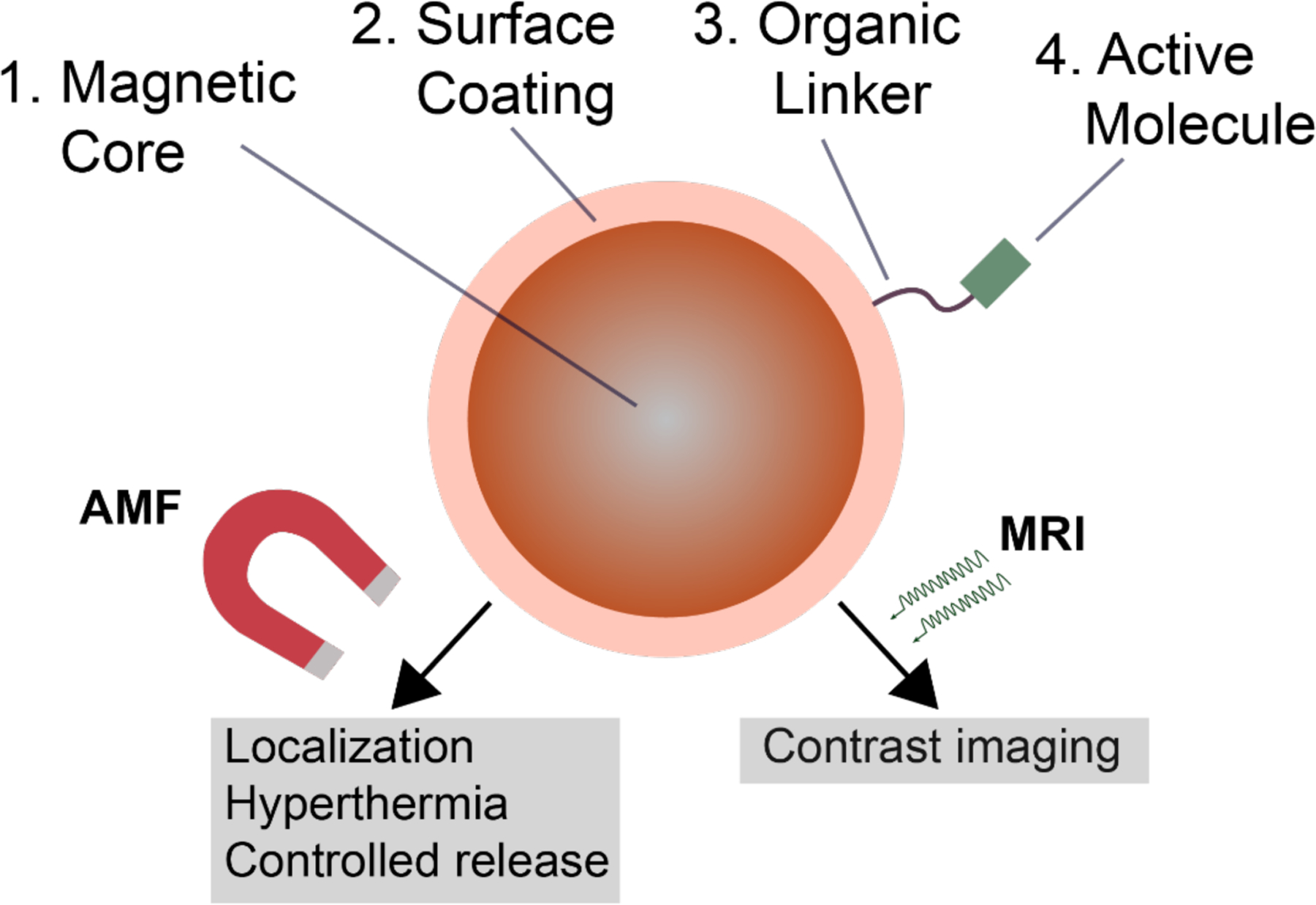
Schematic representation of typical magnetic iron oxide nanoparticle structure, with potential applications for delivery, theragnostics, and imaging.
Superparamagnetic iron oxide nanoparticles (SPIONs) are a special class of iron oxide particles that display superparamagnetic properties, i.e. the ability to strongly magnetize when the material is exposed to an alternating magnetic field (AMF) no residual magnetization when the magnetic field is removed. Notably, this is an effect of size; superparamagnetism usually arises only in small (< 30 nm) ferromagnetic particles or larger particles with nanodomains [275,276]. SPIONs have been explored extensively in the past decade for their ability to penetrate and kill biofilms, as well as their utility in magnetic resonance imaging (MRI) [277]. Biological fate, labelling efficiency, and magnetic characteristics heavily affect the efficiency of SPION-based MRI contrast agents, as notably reported by Lévy [278].
One of the main applications of iron oxide nanoparticles historically has been in magnetic hyperthermia. Upon excitation with an alternating current magnetic field, iron oxide nanoparticles generate localized heat due to Néel [279] and Brown [280] relaxation pathways. First proposed by therapeutically by Gilchrist [281] in the 1950s, iron oxide nanoparticles provide localized heat sources for destroying diseased tissue [282] or dispersal of biofilms [283,284]. SPIONs in particular generate a significant amount of heat under an applied magnetic field (42–45 °C) [285] and have been used for cancer treatment through magnetic hyperthermia [286–290]. Taratula [291] recently developed magnetic nanoclusters composed of cobalt- and manganese-doped hexagonal iron oxide nanoparticles encapsulated in PEG-based polymer nanocarriers for hyperthermia treatment of ovarian cancer (Fig. 14). The authors reported accumulation at the tumor site after intravenous injection and increased local temperature at the tumor up to 44 °C, resulting in inhibited tumor growth. Magnetic nanoparticles have also been utilized by several groups for cancer detection and ablation through tandem MRI and hyperthermic treatment after localization at the tumor site [292–298]. Today these properties are used synergistically with therapeutics, as discussed here.
Fig. 14.
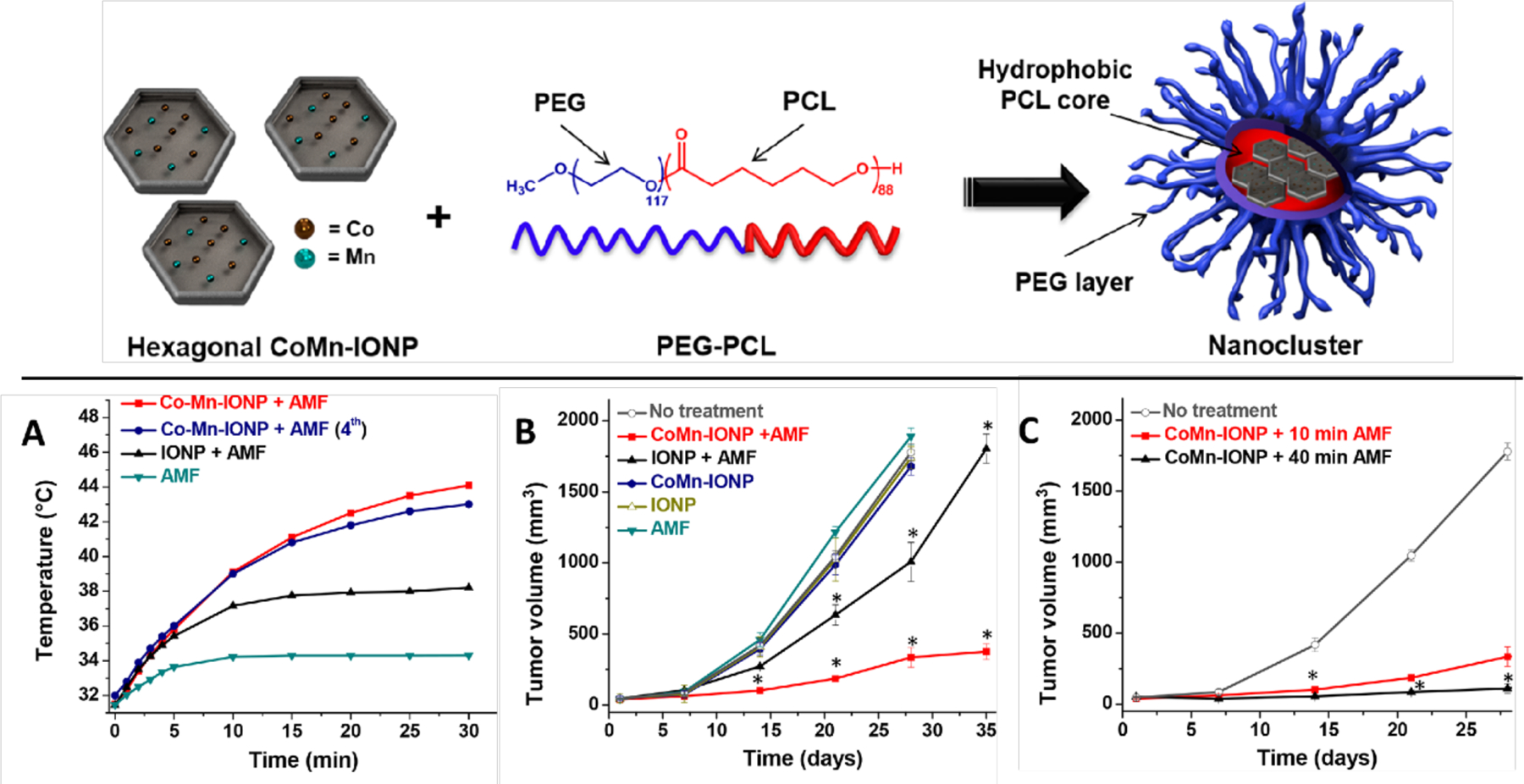
(top) Schematic depiction of hexagonal cobalt- and manganese-doped iron oxide nanoparticles encapsulated within polymeric nanocarriers. (bottom) (a) Representative intratumoral temperature profiles during AMF (420 kHz, 26.9 kA/m) exposure of mice injected with a single dose of 5% dextrose (AMF), cobalt and manganese-doped iron oxide nanoclusters (Co-Mn-IONP), and IONP nanoclusters. The navy curve shows the intratumoral temperature during the fourth cycle in a mouse that was treated with CoMn-IONP nanoclusters (6 mg Fe/kg) and AMF once a week for 4 weeks. (b) Tumor growth profiles of mice with ES-2 xenografts after four cycles of the following treatments; *p < 0.05 when compared with nontreated animals. (c) Tumor growth profiles of mice with ES-2 xenografts after treatment with AMF for 10 min. or 40 min. (Adapted from ref. 291).
Widder and Senyi first demonstrated the use of magnetic fields to manipulate nanoparticles for drug delivery in the late 1970s [299,300]. Magnetic targeting involves the attachment of therapeutic cargo to magnetic nanomaterials, followed by injection and guidance of these particles to the site of interest, providing localized drug delivery at a controllable rate [301,302,303]. This directed motion can provide targeting to regions that are traditionally difficult to deliver to, such as the brain [304]. Work by Polyak [305] demonstrated that small magnetic nanoparticles could be localized to brain tumor tissue in murine glioma models, while larger particles (1 μm) could not. Iron oxide nanoparticles can be magnetically directed to a disease site, tracked via contrast imaging, heated at the effected sites, and provide triggered drug release [306,307,308]. Several strategies have been taken for attachment of therapeutic cargo to these nanoparticles, to provide enhanced stability, targeting, and in some cases, controlled release.
4.1. Functionalization of Magnetic Nanomaterials by Covalent Conjugation
Covalent linkages can be used for conjugation of small molecules and biologics to iron oxide particles. Alexiou [309] used a covalent strategy for delivery of cisplatin, developing 4.5 nm magnetite SPIONs coated with cisplatin-bound hyaluronic acid. The authors observed two-stage drug release in vitro, with burst release in the first 30 minutes, followed by slow release for a 48-hour time span. The Kim group [310,311] utilized thermally crosslinked magnetite SPIONs featuring PEG and carboxylic acid moieties to engineer a co-delivery system for a protein and the small molecule chemotherapeutic paclitaxel. Magnetically responsive host-guest complexes were generated between a polymerized β-cyclodextrin conjugate (pCD) and paclitaxel, with enhanced anticancer activity in CT26 tumor-bearing mice relative to the free drug (Fig. 15).
Fig. 15.
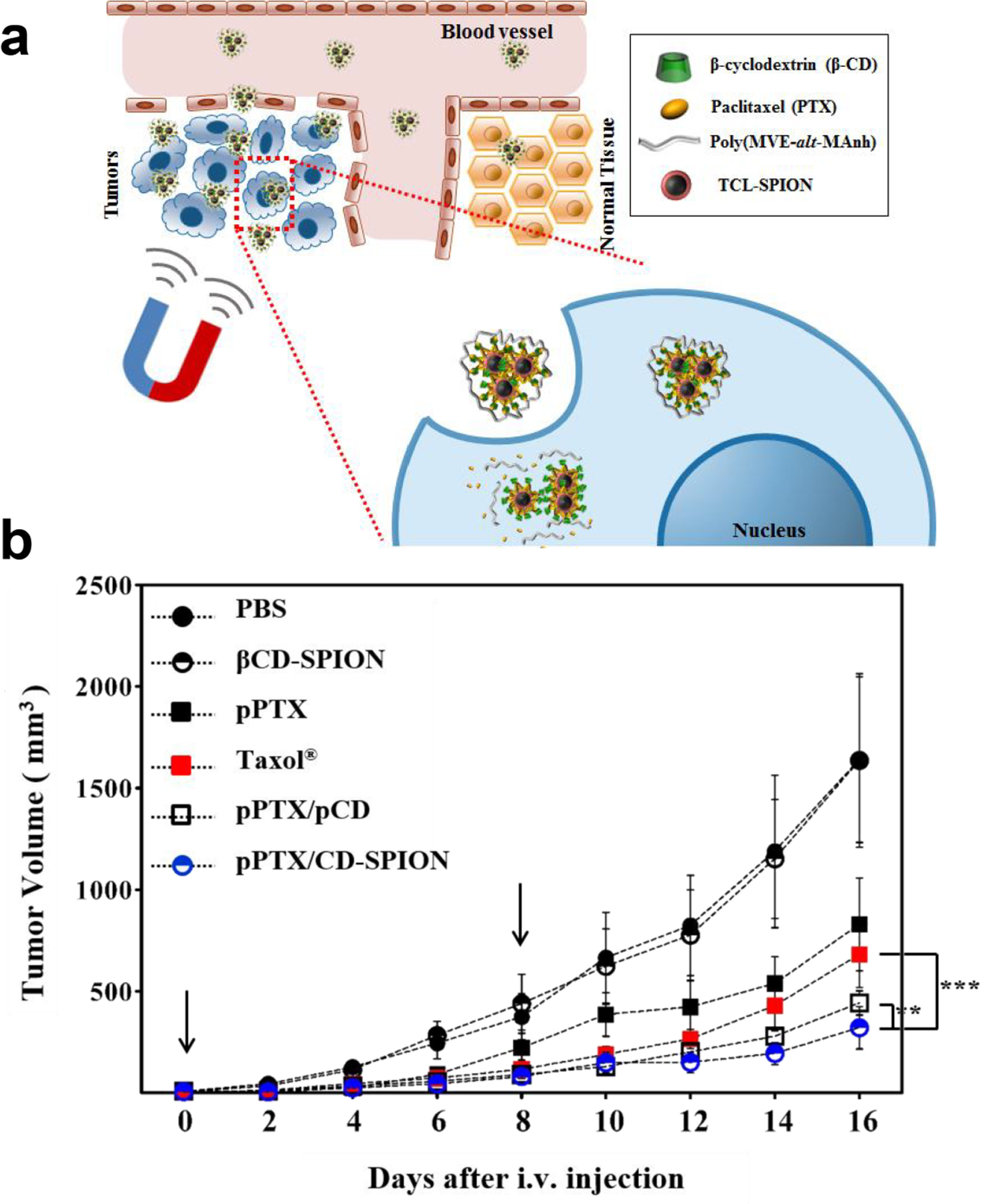
(a) Schematic illustration of polymerized β-cyclodextrin and paclitaxel conjugated to SPIONs. Delivery complexes were magnetically guided to the tumor site. (b) CT26 tumor growth profile for different treatment groups. Data represent mean ± S.E.M. from n=4 (*P<0.05; **P<0.01; ***P<0.001). Each arrow represents the time of intravenous injection of samples. (Adapted from ref. 311).
Covalent modification has also been utilized for delivery of biologics including DNA [312]. Licandro recently reported the covalent conjugation of SPIONs with peptide nucleic acids (PNAs), synthetic polyamide mimics of natural DNA and RNA [313]. PNAs were functionalized with terminal maleimide moieties, allowing conjugation with SPION surface-exposed thiol groups through Michael addition. These particles exhibited hyperthermic and superparamagnetic properties, making them promising carriers for delivery.
In recent years, iron oxide technologies have been commercialized for tumor-targeted delivery of chemotherapeutic delivery with hyperthermic tumor ablation. Chemicell GmbH developed TargetMAG nanoparticles that feature a multidomain magnetite core and a crosslinked starch matrix with terminal cations, that can be loaded with dox [314] or cisplatin. Hilger [315] demonstrated that bound cisplatin would desorb in PBS with hyperthermal (42 °C) or thermal ablative (60 °C) temperatures used for therapeutic approaches. A 20–40 nm iron oxide nanoparticle (MagNaGel) hydrogel featuring different targeting ligands is also produced by Alnis Biosciences for loading and delivery of chemotherapeutic agents [316]. In conjunction with MRI and inductive heating, these platforms provide multimodal treatment for cancer.
4.2. Non-Covalent Conjugation
Electrostatic attachment is useful for iron oxide nanoparticle-mediated delivery of biologics, particularly nucleic acids. Many of these approaches rely on surface-engineered cationic iron oxide nanoparticles that can electrostatically interact with anionic nucleic acid cargos. Scherer [317] demonstrated non-viral gene delivery to cultured cells using PEI-coated SPIONs with sizes ranging from 400–1000 nm. Application of a magnetic field in vitro provided an increase in efficiency and speed of transfection over several commercial reagents. This method, known as magnetofection, was adapted by several groups following Scherer’s initial report as a simple method to deliver DNA [318], antisense oligonucleotides [319] and siRNA to cultured cells, with applicability for ex vivo applications.
PEI-modified iron oxide particles have been used for delivery in vivo. Dou [320] recently utilized PEI-coated SPIONs modified with galactose for the siRNA-mediated treatment of hepatocellular carcinoma. Intravenous tail vein injection to C57BL/6 murine luciferin-expressing hepatic tumor models demonstrated efficient hepatic tumor localization, with fluorescence knockdown using luciferin-silencing siRNA, and inhibiting tumor growth through silencing of the c-Met gene. Addition of a permanent magnet placed on the skin above the tumor for 2 hours post-injection did not significantly affect efficiency. Delivery of pDNA in vivo has also been approached using similar methods. Zhang [321] reported high gene expression levels in a C6 xenograft mouse model following intravenous injection of pDNA-conjugated SPIONs. These SPION particles were surface-grafted with PEG-PEI for biocompatibility and bound with chitosan to allow for pDNA condensation. Transfection of eGFP-encoded pDNA in vivo revealed high expression levels in tumor sites after 48 hours, as confirmed by MRI imaging. Later work by Sersa [322] aimed at adenocarcinoma treatment in BALB/c (TS/A tumors) and C57Bl/6 (B16F1 tumors) murine models through cancer immuno-gene therapy and SPION-mediated gene delivery. SPIONs modified with PEI and endosomolytic polyacrylic acid (PAA) were injected intratumorally, and magnetically stimulated to deliver to cells in a process considered in vivo magnetofection. These studies revealed high expression levels first of GFP in a model study, and then immunostimulatory cytokine interleukin 12 (IL-12) in experimental work. Tumor IL-12 expression resulted in a significant decrease in tumor growth over a course of 20 days (Fig. 16).
Fig. 16.
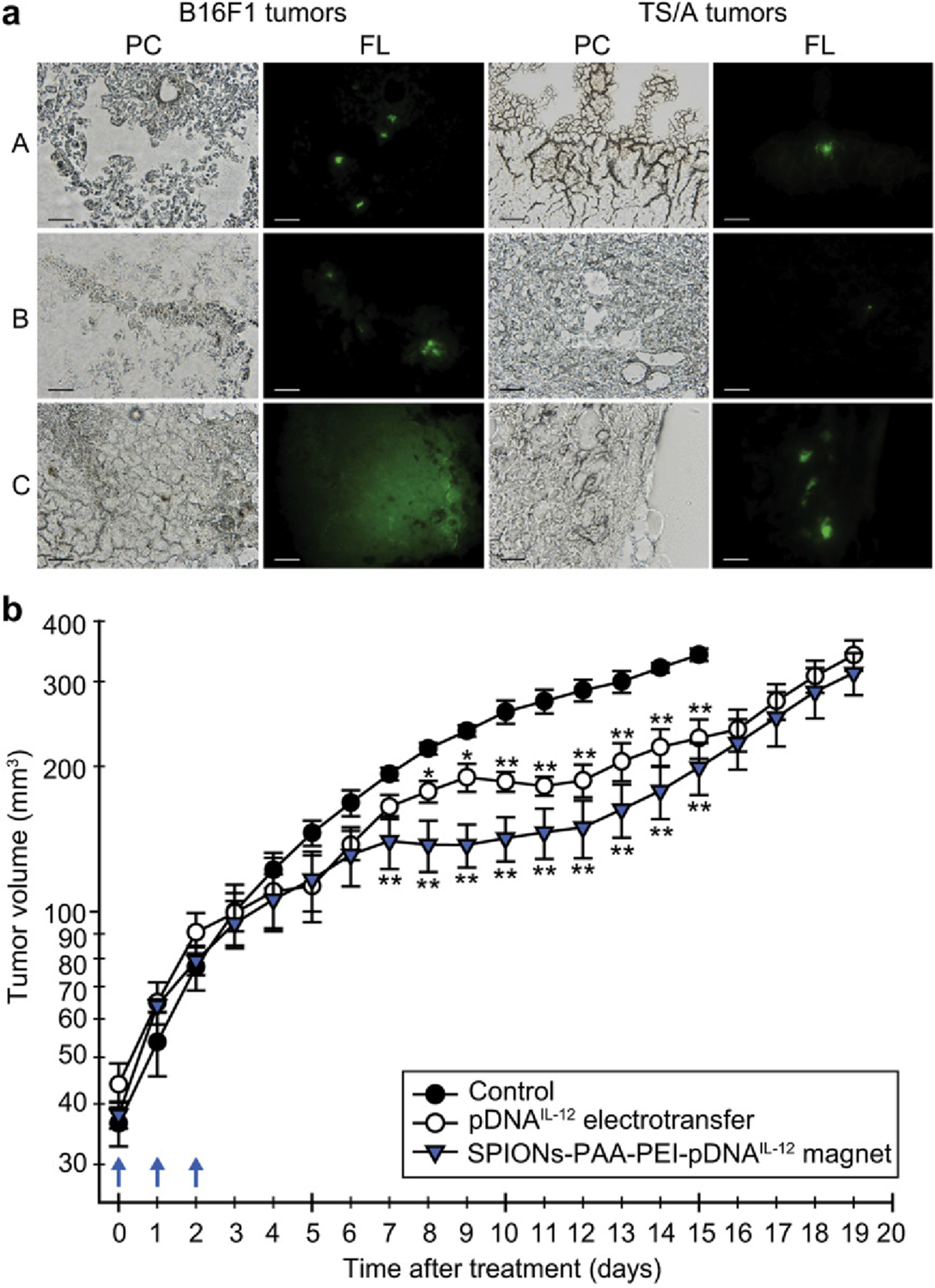
Magnetofection of tumors with pDNAGFP or pDNAIL-12 using SPIONs-PAA-PEI: (a) Micrographs of frozen murine melanoma B16F1 and mammary adenocarcinoma TS/A tumor sections under phase contrast (PC) and epi-fluorescence illumination (FL) after transfection with pDNAGFP using (A) magnetofection, (B) SPIONs-PAA-PEI in the absence of magnetic field, (C) electrotransfer. Scale bar, 50 mm. (b) Antitumor effect of intratumoral administration of pDNAIL-12 on TS/A mammary adenocarcinoma tumors after magnetofection with SPIONs-PAA-PEI and electrotransfer. Blue arrows represent three consecutive treatments with pDNAIL-12. Tumor growth delay was calculated on the 10th day. (Adapted from ref. 304).
Micellar lipid coatings have been used to adsorb and layer nucleic acids. Anderson reported a technique for iron oxide nanoparticle coating using nucleic acid-loaded lipidoids [323]. These 50–100 nm particles encapsulated siRNA and plasmid DNA with high efficiency, and demonstrated efficient in vitro transfection, with future potential for magnetically guided treatment and magnetic hyperthermia in vivo. Later work by Chertok [324] demonstrated lymph node targeting using size-controlled lipidoid-iron oxide nanoparticles, suggesting a promising route for design of theranostic platforms.
Iron oxide nanoparticles have been utilized to some extent for recombinant protein delivery as well, however the relatively large size and complex surfaces of proteins makes interfacial recognition with iron oxide particles challenging. Silica-embedded iron oxide particles have been demonstrated in the past as enhanced MRI contrast agents [325]. Khashab [326] utilized mesoporous silica nanoparticles to overcome this issue, using biodegradable silica-iron oxide propylamine nanohybrids featuring ultra large pores capable of protecting and delivering a large protein (mTFP-Ferritin, ~530 kDa) to cancer cells (Fig. 17). These large proteins were electrostatically immobilized into mesoporous cavities of the particles and were released under acidic pH or magnetic stimuli in HeLa cells.
Fig. 17.
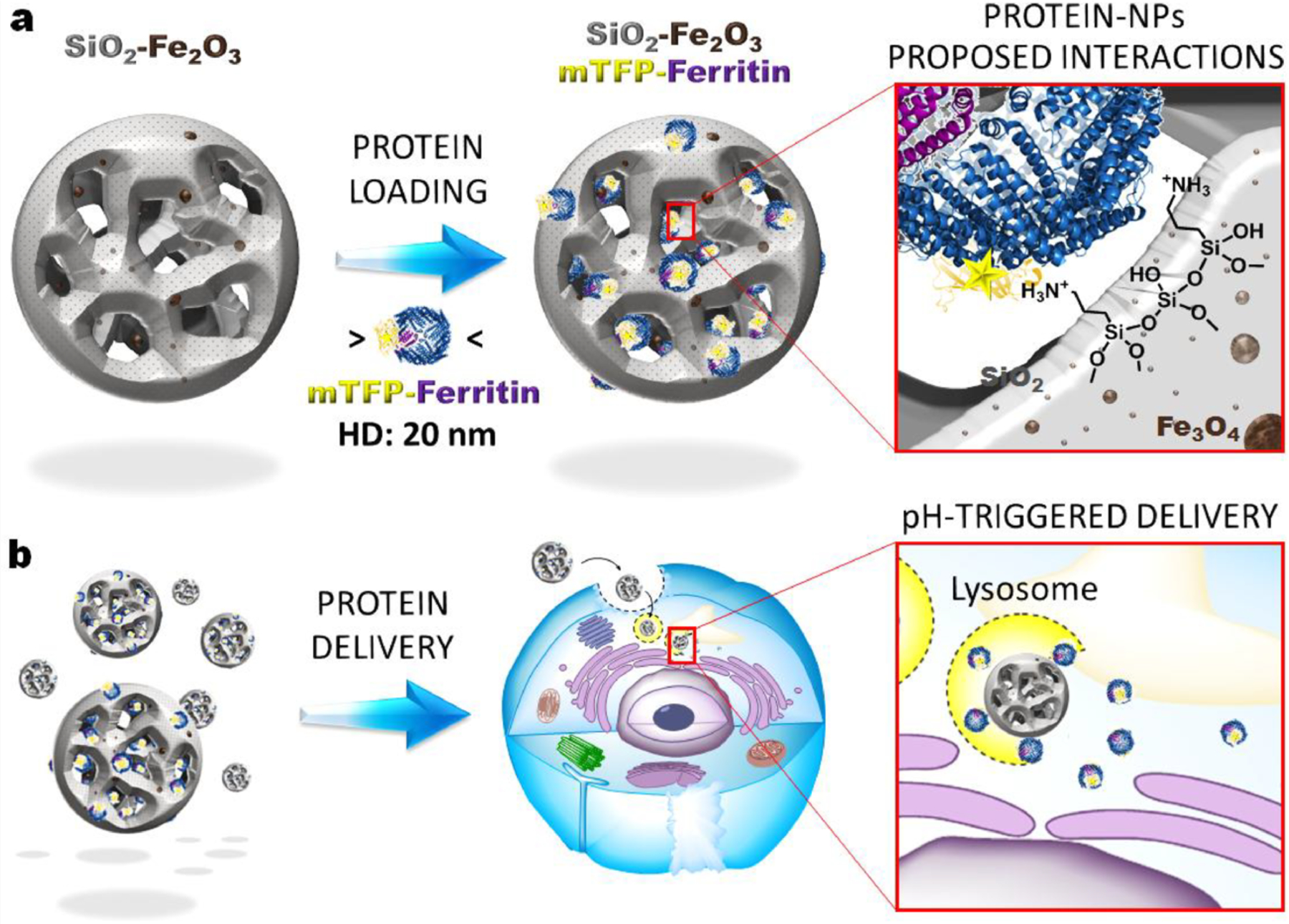
Schematic illustration of silica-iron oxide propylamine nanocomposites, with (a) proposed loading interactions for large proteins with the particles and (b) representation of pH-driven intracellular delivery. (Adapted from ref. 326).
Hyperthermic applications are challenging due to natural thermoregulatory processes make it difficult to locally increase temperature in the body, and hyperthermic heating is dependent on particle concentration at the target site [327]. At the time of writing, the only clinically available AMF system, NanoActivator® (MagForce AG, Germany) is capable of operating frequency up to 100 kHz [328]. Limited data is available regarding clinically tolerable AMF dosage [329,330], and future studies must critically evaluate tumor temperature. Magnetic field depth is another important consideration, and several studies have implanted internal magnets using minimally invasive surgery [331,332]. Finally, the degradation of iron oxide particles in the body, and the potential accumulation of magnetic nanoparticles and their by-products in tissues and organs [333] has elicited concerns over toxicity [334]. Dissociated iron oxide particles promote the formation of reactive oxygen species and hydroxyl radicals, and as a result may lead to cytotoxicity as well as impaired cell metabolism and an increase in apoptosis [335]. Despite the several advantages that iron oxide nanoparticles offer, it is important to consider ways to overcome iron ion-induced toxicity. Toxicity of these nanomaterials to a large extent is concentration as well as exposure time dependent [336]. in mouse models that at lower concentrations these particles can be cleared from the body without significant toxicity [337]. Therefore, iron oxide nanoparticles have a potential for nanomedical applications if we understand and mitigate potential risks.
5. Lanthanide Upconversion Particles
As discussed above, light-triggered drug delivery is a promising strategy to spatiotemporally control the drug release in vivo, boosting local effective drug accumulation while minimizing side effects [32]. However, most light-triggered drug carriers require short wavelength UV/visible light, resulting in high phototoxicity with limited penetration depth in vivo [338,339]. Lanthanide upconversion nanoparticles (UCNPs) are a unique class of optical nanomaterials that absorb and convert low-energy near-infrared (NIR) photons into high-energy UV/visible light through a nonlinear anti-Stokes process [340,341] Multiple lanthanide-doped UCNPs have been studied, and today ytterbium (Yb3+), erbium (Er3+), thulium (Tm3+), and holmium (Ho3+) are the most widely used lanthanide dopants [341]. UNCPs offer several advantages such as low toxicity and deep light penetration in tissues, making them excellent candidates for controlling in situ drug release via photochemical processes [342,343].
Three common approaches have been developed to load photosensitive compounds onto UCNPs for drug delivery, with perhaps the most common being encapsulation within a mesoporous silica shell [344]. Compound loading has also been accomplished using covalent conjugation [345,346,347] and non-covalent adsorption [348,349,350] to the particle. Drug release can then be controlled by three mechanisms, namely i) bond cleavage between the molecule and the carrier; ii) destruction of the carrier, or iii) photoisomerization.
5.1. Covalent UCNP cargo attachment
UCNPs have been employed as a NIR-triggered delivery vehicle by covalently binding drug molecules onto the nanoparticle surface to control the drug release [351–353]. O-nitrobenzyl (ONB) esters are the most commonly used photosensitive compounds for modification as they will undergo irreversible deformation when triggered by UV/visible light [354]. Krull [355] conjugated a chemotherapeutic agent 5-fluorouracil (5-FU) to core-shell UCNPs (~20 nm) via an ONB photolabile linker. Upconverted UV and blue light from the UCNPs matches the absorption profile of caged 5-FU and cleaves the drug-UCNPs linker using a NIR laser (Fig. 18). In a similar manner, the Hartman group [356] developed a photo-responsive drug delivery system by attaching dox directly onto the surface exposed UCNPs (LiYF4:Tm3+/Yb3+) through a nitrobenzyl derivative. The authors observed ~40% drug release after exposure to NIR irradiation.
Fig. 18.
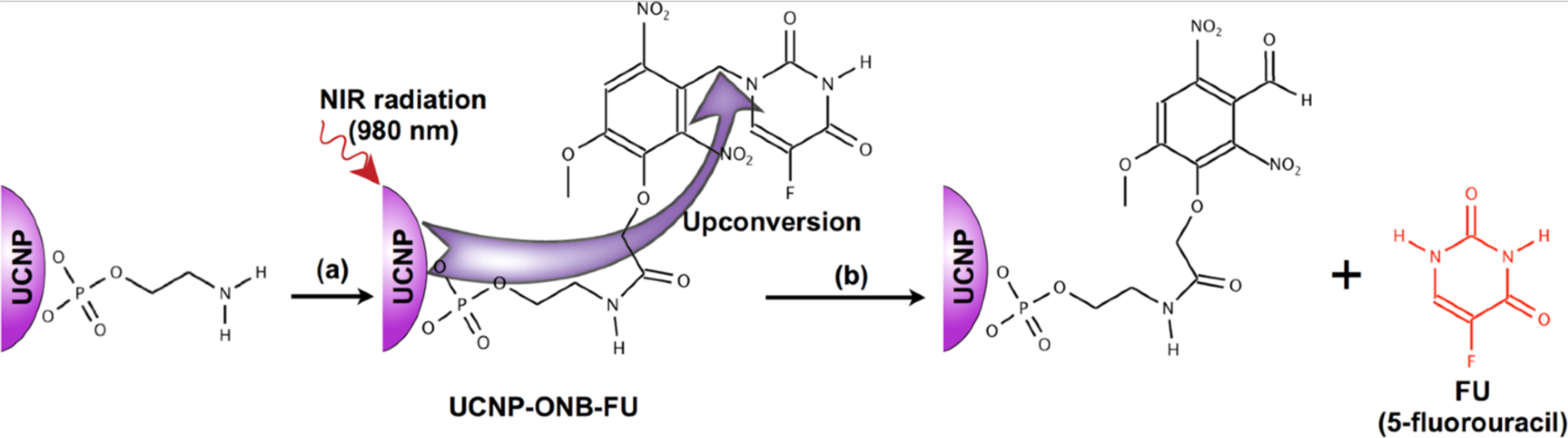
NIR excitation (980 nm) of the UCNPs resulted in upconverted UV emission at 364 nm used for photocleavage subsequent release of 5-fluorouracil from the UCNP surface. (Adapted from ref. 355).
In a theranostic approach, the Lin group [357] conjugated dox to the surface of UCNPs (NaYF4: Yb/Tm) by acid-labile hydrazone-based bonds (~25 nm) to generate a luminescence-monitored drug delivery system. In the design, the spectral overlap between emission of donor UCNPs (452 nm and 477 nm) and the broad absorbance of acceptor dox (480 nm) enabled luminescence resonance energy transfer (LRET) to quench the upconversion luminescence of UCNPs under NIR irradiation. This quenching effect was used as a probe to confirm the dox conjugation and monitor drug release in vitro. More recently, this group attached dox onto BaGdF5: Yb/Tm-UCNPs using the same hydrazone linker to develop a multifunctional nanoplatform (sub-10nm) for simultaneous diagnosis and therapy [358]. This UCNP exhibited strong upconversion fluorescence after delivery to cultured cells under NIR irradiation and can be used as well for T1-weighted magnetic resonance and X-ray computed tomography imaging. Peptide conjugation to the UCNP surface was used in the targeted delivery of siRNA to cancer cells. Ye [359] functionalized the silica coated UCNPs (NaYF4: Yb/Tm) with an Anti-Her2 antibody by reaction of its terminal amino group via carbodiimide chemistry (~30 nm). Under NIR irradiation, UCNPs enabled the real-time tracking of siRNA due to their superior optical properties. The silencing effect of siRNA on luciferase gene was studied in vitro and ~50% down-regulation was observed. Recently, covalent conjugation strategies for UCNPs have included quantum dots [360], fluorescent proteins [361], and fluorophores [362] and focusing largely on photodynamic therapy, theragnostics [363] and imaging [364].
5.2. Non-covalent cargo attachment to UCNPs
By incorporating appropriate photosensitive moieties into polymer structure, photochemical reactions such bond cleavage can result in disassembly of the carrier due to photoinduced structural and/or property changes [365]. UCNPs can be encapsulated into polymer micelles to act as internal UV/visible light sources upon NIR irradiation, with the upconverted light activating photoreactions to trigger micelle disruption and release co-loaded hydrophobic drugs [366]. Almutairi [367] utilized cresol monomers functionalized with ONB groups to fabricate micelles (ranging in size from 0.3 μm to 1 μm) that can degrade through a cascade of cyclization and rearrangement reactions in response to upconverted UV light by UCNPs (Fig. 19). Cleavage of ONB moieties triggered by upconverted UV light rapidly increased water permeability and facilitated cargo release.
Fig. 19.
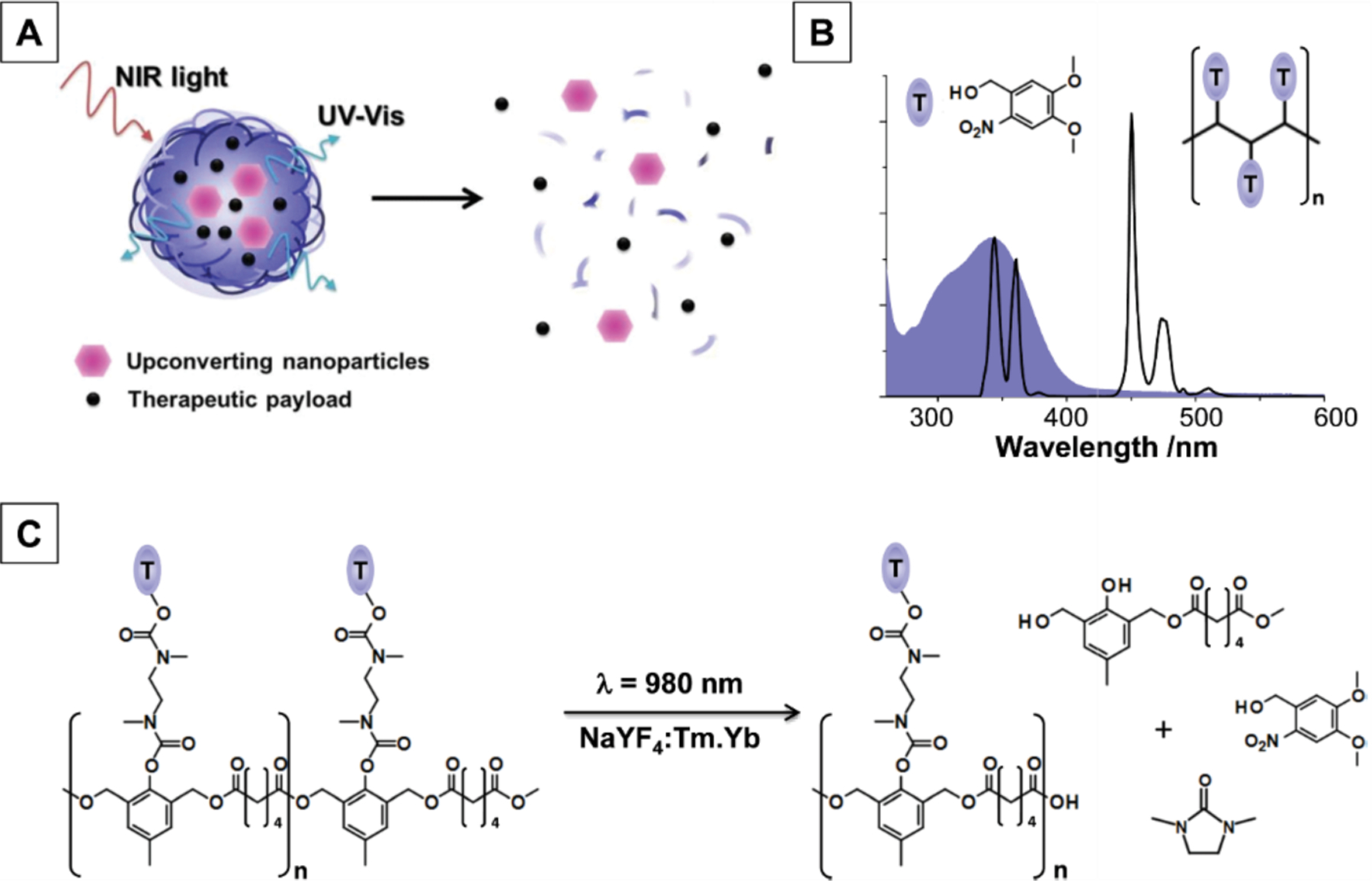
Schematic representation: (a) upconverted luminescence triggers degradation and release from light-sensitive nanoparticles; (b) spectral overlap between the UV emission profile of NaYF 4 :Yb.Tm core-shell UCNPs (black trace) and the absorption spectrum (shaded blue) of ONB triggering groups; (c) photochemical mechanism of light-triggered degradation. (Adapted from ref. 367).
Zhao’s group [368] developed a photosensitive micelle composed of hydrophilic polyethylene oxide and a hydrophobic polymethacrylate modified with ONB groups. The upconverted UV light photocleaved the ONB groups and thus released the drug by destabilization of micelles. In follow-up work, this group developed a hybrid UCNP-hydrogel system which allows the use of NIR light to induce gel-sol transition and release large, inactive biomacromolecules on demand, after which bioactivity could be recovered [368]. In a proof-of-concept study, they entrapped the enzyme trypsin and UCNPs into the hydrogel which has a crosslinked hybrid polyacrylamide-poly(ethylene-glycol) structure held together by photosensitive ONB groups and observed an immediate release of protein upon exposure to NIR light.
Incorporating UCNPs into mesoporous silica nanoparticles (MSNPs) is a common approach to non-covalent drug loading. Wu [369] employed the previously mentioned MSNP gatekeeping strategy using blue-light-cleavable ruthenium (Ru) complex to control drug release. UCNPs were first coated with porous silica and then loaded with dox. A Ru-based complex was covalently grafted onto UCNPs, encapsulating dox within the pores. that was released after 5h exposure to NIR irradiation. Cell culture studies showed a significant inhibition to the growth of HeLa cells after incubation for 3–6 h followed by NIR irradiation. Lanthanide-doped UCNPs coated with MSNPs were also used to deliver siRNA. Ju [370] loaded siRNA and photosensitizer hypocrellin A (HA) into MSNP pores and then wrapped the obtained complexes with PEG polymer ‘tape’ using an ONB-based photocleavable linker (Fig. 17c). Under NIR irradiation, the UV light emitted by UCNPs broke photocleavable linkers to release siRNA and activate HA to generate reactive oxygen species (ROS), disrupting the endosomal membrane. This combination of two photosensitive moieties led to a significant enhancement in gene silencing.
As discussed previously [235,236], photoisomerizable molecules such as azobenzene undergo spatial conformation changes reversibly under UV/visible illumination [371]. Changing between the two isomers can be used as a switch to control drug release [372]. In 2013, Shi [373] reported NIR-induced drug delivery using UCNPs coated with an azobenzene-modified mesoporous silica shell (Fig. 20) (~54 nm). Since the azobenzene molecular impeller is only activated under upconverted UV light, the amount of the released drug could be controlled through tuning the intensity and/or time duration of NIR light. Studies in vitro confirmed that the dox released in cytoplasm could migrate into nucleoplasm to kill the cancer cells.
Fig. 20.
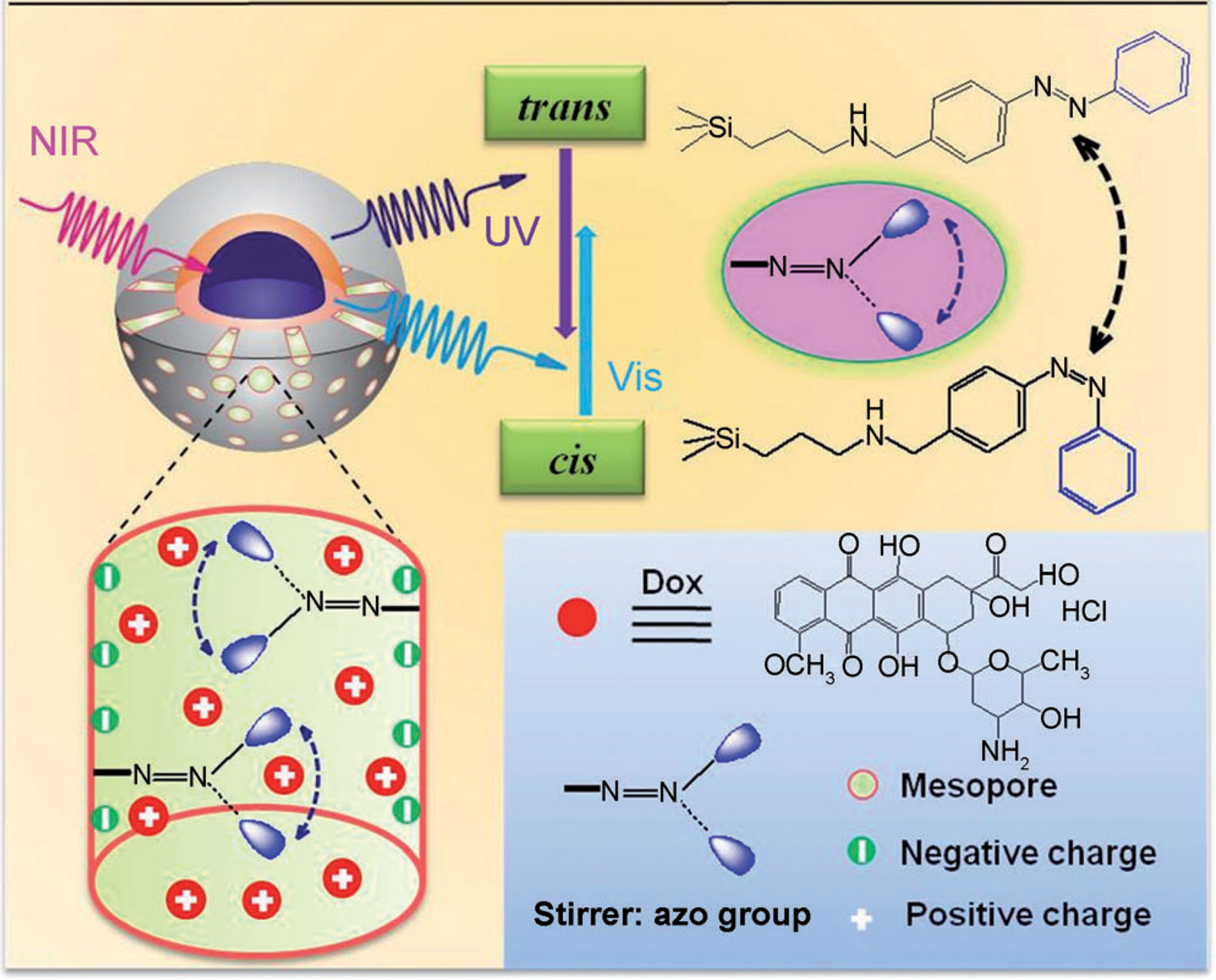
Schematic illustration of NIR light-triggered dox release by making use of the upconversion properties of UPNCs and trans–cis photoisomerization of azobenzene molecules grafted on a mesoporous silica layer. (Adapted from ref. 373).
The trans-cis transformation of azobenzene was later adapted for UCNP-mediated siRNA delivery by Gong [374]. In this study, siRNA strands tagged with azobenzene were complexed onto UCNPs modified with cyclodextrin via a host-guest interaction. This NIR-activated UCNPs core emitted UV light which efficiently isomerized azobenzene to the cis state, thus releasing siRNA as a result of unmatched host-guest pairs. This group further conjugated GE11 and TH peptides onto the carrier surface to facilitate the cellular uptake and endosomal/lysosomal escape of the nanoparticles. The amount of released siRNA was controlled by adjusting NIR irradiation time, demonstrating 85% siRNA release within 20min.
A major challenge of delivery with NIR-absorbing UCNPs is the strong absorption of water molecules at 980 nm. UCNPs have a limited number of available excitation wavelengths, with 980 nm being one of the most common. Irradiation at this wavelength will cause the surrounding solution to heat, potentially causing a complication for in vivo applications [375]. However, studies have shown an appreciable lack of toxicity from in vivo administration of UCNPs, encouraging their biomedical use [376]. The versatile chemical nature and low toxicity of UCNPs lends them great promise for future delivery applications.
6. Conclusions and perspective
Inorganic nanoparticles offer a versatile, multimodal approach to the intracellular delivery of therapeutic molecules. With a vast diversity of therapeutic targets comes a similar diversity of therapeutic cargo- from small molecule drugs to large multimeric proteins, effective treatment of disease requires a wide range of delivery vehicles featuring a broad spectrum of structure and activity properties. Inorganic nanoparticles in their many forms offer structural versatility that can accommodate these different cargos. Each class of inorganic nanoparticle offers unique structural properties, each with their own potential advantages such as stimuli-responsive release, favorable biodistribution, and photothermal reactivity [377]. Table 1 summarizes the notable advantages and challenges of each inorganic material in the context of therapeutics delivery, with focus on clinical translatability.
Table 1. Comparison of inorganic materials for delivery purposes.
Summarized advantages and potential issues of gold, silica, iron oxide, and UCNP platforms for in vivo delivery of therapeutics, with key references for delivery of each form of therapeutic cargo.
| Material | Advantages | Challenges | References |
|---|---|---|---|
| Gold | tunable size and shape, optical reactivity, flexible monolayer design, inherently non-toxic | uncertain biological fate, nuanced synthetic process |
Small molecule 71–78, 81–86, 88, 122–127, 130–136, 165 Protein 93, 99, 100, 138–140, 142, 143 Nucleic acid 105–118, 146–153, 156, 159, 160, 173 |
| Silica | high loading capacity, controllable release rate, flexible platforms for triggered release | toxicity from surface-exposed silanol groups, increased ROS production |
Small molecule 225, 242, 243, 245, 248, 205–213, 220, 221 Protein 230, 240, 244, 214, 215 Nucleic acid 237, 217–219 |
| Iron Oxide | hyperthermic properties, potential for contrast imaging or magnetic localization | particle accumulation/degradation in vivo produces toxic byproducts |
Small molecule 308, 309, 313–315 Protein 310, 325 Nucleic acid 311, 312, 316–323 |
| Lanthanide Upconversion Particles | NIR-triggered release for localized drug release, low toxicity | limited available excitation wavelengths, need for silica components |
Small molecule 348–355, 363, 364, 365, 367, 371 Protein 366 Nucleic acid 356, 368, 372 |
NIR: Near-infrared radiation
ROS: Reactive oxygen species
Challenges remain to the development of clinically translatable delivery vehicles, partly because hurdles exist in clinical applications that are overlooked in fundamental research [378]. One of these is entry into the cytosol [379,380]. Many delivery platforms enter the cell through endosomal uptake. Recent studies have shown that <10% of carriers escape from degradative endo/lysosomal pathways, making endosomal delivery and escape an extremely inefficient process [381,382]. Another challenge remaining in delivery is localization at the disease site in vivo. Nanocarriers in general exhibit a degree of colloidal stability in circulation [383] due to their small size. Specific delivery to a cell type or organ remains challenging, however. Particles above a certain size will invariably accumulate in the major clearance organs of the mononuclear phagocyte system, such as liver and spleen [384,385,386]. Moreover cellular uptake has been shown to decrease with increasing particle size, but increase significantly with increased cationic surface charge [387]. Surface functionalization with specific chemical moieties or recognition elements can promote cell type-specific delivery, but proteins in the blood bind nanoparticle surfaces electrostatically, potentially skewing biodistribution and masking surface-exposed recognition elements [388,389].
Nanomaterial shape also dictates organ-level biodistribution, tissue penetration, and cellular uptake. The shape and aspect ratio of MSNPs have been shown to significantly effect tissue residence time and cell internalization rate [390,391] with small, spherical particles exhibiting the most favorable trends of circulation and tissue residence. Similar results have been demonstrated with PEGylated polystyrene-core nanoparticles [392], and gold nanoparticles of various sizes and shapes [393,394]. Importantly, particle shape has been shown to directly relate to uptake in macrophage cells, suggesting critical implications in terms of clearance from the body [393]. While small, spherical particles generally exhibit enhanced uptake and greater circulation time, this can prove a challenging target for nanocarrier development. Furthermore, rationally designed surface functionality may provide benefits to localized delivery beyond what can be achieved by relying on size alone.
Stimuli-responsive materials provide a promising avenue for localized delivery and have demonstrated promising results in numerous inorganic nanoparticle-based platforms, especially using NIR light-triggered release or assisted accumulation through magnetism. Notably however several reported systems took advantage of the enhanced permeability and retention, or EPR effect for increased particle accumulation in tumor tissue in vivo. This effect has been observed in murine models and may not directly apply to humans [395]. Finally, although many inorganic nanoparticle platforms exhibit low toxicity on a cellular level, accumulation in vivo can exacerbate toxic effects, making degradability and clearance critical safety measures in carrier design [396].
The approaches highlighted herein demonstrate the immense progress made toward development of efficacious delivery platforms in the past decade alone. Inorganic nanomaterials provide versatile scaffolds with high surface-to-volume ratio for cargo loading, generally low toxicity, and highly tunable structural properties. From proof-of-concept to systems with real clinical relevance, gold, silica, iron oxide, and UCNP delivery platforms have been advanced significantly to overcome the challenges faced in therapeutics delivery. Inorganic nanoparticle-based platforms remain a front-line approach for delivery and with continued advancement will doubtlessly see extensive clinical application in the future.
Acknowledgement and Funding Sources
This research was supported by the NIH (EB022641 and AI134770 to V.M.R), and the NSF (CHE-1808199).
References
- [1].Ojea-Jimenez I, Comenge J, Garcia-Fernandez L, Megson Z, Casals E, Puntes V, Engineered Inorganic Nanoparticles for Drug Delivery Applications, Curr. Drug Metab 14 (2013) 518–530. [DOI] [PubMed] [Google Scholar]
- [2].Fadeel B, Garcia-Bennett AE, Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications, Adv. Drug Deliv. Rev 62 (2010) 362–374. [DOI] [PubMed] [Google Scholar]
- [3].Zhao Y, Bai C, Brinker CJ, Chi L, Dawson KA, Gogotsi Y, Halas NJ, Lee ST, Lee T, Liz-Marzán L, Miller JF, Mitra S, Nel AE, Nordlander P, Parak WJ, Rowan A, Rogach AL, Rotello VM, Tang BZ, Wee ATS, Weiss PS, Nano as a rosetta stone: The global roles and opportunities for nanoscience and nanotechnology, ACS Nano 13 (2019) 10853–10855. [DOI] [PubMed] [Google Scholar]
- [4].Rotello VM, Advanced drug delivery reviews theme issue, Adv. Drug Deliv. Rev 60 (2008) 1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Montenegro JM, Grazu V, Sukhanova A, Agarwal S, de la Fuente JM, Nabiev I, Greiner A, Parak WJ, Controlled antibody/(bio-) conjugation of inorganic nanoparticles for targeted delivery, Adv. Drug Deliv. Rev 65 (2013) 677–688. [DOI] [PubMed] [Google Scholar]
- [6].Navya PN, Kaphle A, Srinivas SP, Bhargava SK, Rotello VM, Daima HK, Current trends and challenges in cancer management and therapy using designer nanomaterials, Nano Converge 6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pearce AK, O’Reilly RK, Insights into Active Targeting of Nanoparticles in Drug Delivery: Advances in Clinical Studies and Design Considerations for Cancer Nanomedicine, Bioconjug. Chem 30 (2019) 2300–2311. [DOI] [PubMed] [Google Scholar]
- [8].Giri K, Shameer K, Zimmermann MT, Saha S, Chakraborty PK, Sharma A, Arvizo RR, Madden BJ, McCormick DJ, Kocher JPA, Bhattacharya R, Mukherjee P, Understanding protein-nanoparticle interaction: A new gateway to disease therapeutics, Bioconjug. Chem 25 (2014) 1078–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Panyam J, Labhasetwar V, Biodegradable nanoparticles for drug and gene delivery to cells and tissue, Adv. Drug Deliv. Rev 55 (2003) 329–347. [DOI] [PubMed] [Google Scholar]
- [10].Ray M, Lee Y-W, Scaletti F, Yu R, Rotello VM, Intracellular delivery of proteins by nanocarriers, Nanomed 12 (2017) 941–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Warner KD, Hajdin CE, Weeks KM, Principles for targeting RNA with drug-like small molecules, Nat. Rev. Drug Discov 17 (2018) 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang J, Yang PL, Gray NS, Targeting cancer with small molecule kinase inhibitors, Nat. Rev. Cancer 9, (2009) 28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Deng J, Grande F, Neamati N, Small Molecule Inhibitors of Stat3 Signaling Pathway, Curr. Cancer Drug Targets, 7 (2007) 91–107. [DOI] [PubMed] [Google Scholar]
- [14].McCormick F, Small-molecule inhibitors of cell signaling, Curr. Op. in Biotechnol 11 (2000) 593–597. [DOI] [PubMed] [Google Scholar]
- [15].Singh AP, Biswas A, Shukla A, Maiti P, Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles, Signal Transduct. Target. Ther 4 (2019) 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lombardo D, Kiselev MA, Caccamo MT, Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine, J. Nanomater 2019 (2019) 1–26. [Google Scholar]
- [17].Dandapani S, Marcaurelle LA, Grand Challenge Commentary: Accessing new chemical space for 'undruggable' targets, Nat. Chem. Biol 6 (2010) 861–863. [DOI] [PubMed] [Google Scholar]
- [18].Dang CV, Reddy EP, Shokat KM, Soucek L, Drugging the “undruggable” cancer targets, Nat. Rev. Cancer 17 (2017) 502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lou B, De Koker S, Lau CYJ, Hennink WE, Mastrobattista E, MRNA Polyplexes with Post-Conjugated GALA Peptides Efficiently Target, Transfect, and Activate Antigen Presenting Cells, Bioconjug. Chem 30 (2018) 461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vaidya AM, Sun Z, Ayat N, Schilb A, Liu X, Jiang H, Sun D, Scheidt J, Qian V, He S, Gilmore H, Schiemann WP, Lu ZR, Systemic Delivery of Tumor-Targeting siRNA Nanoparticles against an Oncogenic LncRNA Facilitates Effective Triple-Negative Breast Cancer Therapy, Bioconjug. Chem 30 (2019) 907–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Catalanotto C, Cogoni C, Zardo G, MicroRNA in control of gene expression: An overview of nuclear functions, Int. J. Mol. Sci 17 (2016) 1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yang NJ, Hinner MJ, Getting across the cell membrane: an overview for small molecules, peptides, and proteins, Methods Mol. Biol 1266 (2015) 29–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Sokolova V, Epple M, Inorganic Nanoparticles as Carriers of Nucleic Acids into Cells, Angew. Chemie Int. Ed 47 (2008) 1382–1395. [DOI] [PubMed] [Google Scholar]
- [24].Liu X, Wu F, Ji Y, Yin L, Recent Advances in Anti-cancer Protein/Peptide Delivery, Bioconjug. Chem 30 (2019) 305–324. [DOI] [PubMed] [Google Scholar]
- [25].Leader B, Baca QJ, Golan DE, Protein therapeutics: a summary and pharmacological classification, Nat. Rev. Drug Discov 7 (2008) 21–39. [DOI] [PubMed] [Google Scholar]
- [26].Reineke TM, Raines RT, Rotello VM, Delivery of Proteins and Nucleic Acids: Achievements and Challenges, Bioconjug. Chem 30 (2019) 261–262. [DOI] [PubMed] [Google Scholar]
- [27].Scaletti F, Hardie J, Lee YW, Luther DC, Ray M, Rotello VM, Protein delivery into cells using inorganic nanoparticle-protein supramolecular assemblies, Chem. Soc. Rev 47 (2018) 3421–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee Y-W, Luther DC, Kretzmann JA, Burden A, Jeon T, Zhai S, Rotello VM, Protein Delivery into the Cell Cytosol using Non-Viral Nanocarriers, Theranostics 9 (2019) 3280–3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kristensen M, Nielsen LH, Zor K, Boisen A, Christensen MV, Berthelsen J, Mørck Nielsen H, Cellular Effects and Delivery Propensity of Penetratin Is Influenced by Conjugation to Parathyroid Hormone Fragment 1–34 in Synergy with pH, Bioconjug. Chem 29 (2018) 371–381. [DOI] [PubMed] [Google Scholar]
- [30].Brock R, The uptake of arginine-rich cell-penetrating peptides: Putting the puzzle together, Bioconjug. Chem 25 (2014) 863–868. [DOI] [PubMed] [Google Scholar]
- [31].Grumezescu AM, Nanobiomaterials in Galenic Formulations and Cosmetics: Applications of Nanobiomaterials, Elsevier Inc., (2016). [Google Scholar]
- [32].Li H, Wang X, Huang D, Chen G, Recent advances of lanthanide-doped upconversion nanoparticles for biological applications, Nanotechnology 31 (2020) 072001. [DOI] [PubMed] [Google Scholar]
- [33].Tang F, Li L, Chen D, Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery, Adv. Mater 24 (2012) 1504–1534. [DOI] [PubMed] [Google Scholar]
- [34].Requejo KI, Liopo AV, Derry PJ, Zubarev ER, Improving the Shape Yield and Long-Term Stability of Gold Nanoprisms with Poly(vinylpyrrolidone), Langmuir 35 (2019) 9777–9784. [DOI] [PubMed] [Google Scholar]
- [35].Austin LA, MacKey MA, Dreaden EC, El-Sayed MA, The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery, Arch. Toxicol 88 (2014) 1391–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ehlerding EB, Chen F, Cai W, Biodegradable and Renal Clearable Inorganic Nanoparticles, Adv. Sci 3 (2016) 1500223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rosenblum D, Joshi N, Tao W, Karp JM, Peer D, Progress and challenges towards targeted delivery of cancer therapeutics, Nat. Commun 9 (2018) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rana S, Bajaj A, Mout R, Rotello VM, Monolayer coated gold nanoparticles for delivery applications, Adv. Drug Deliv. Rev 64 (2012) 200–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ghosh P, Han G, De M, Kim CK, Rotello VM, Gold nanoparticles in delivery applications, Adv. Drug Deliv. Rev 60 (2008) 1307–1315. [DOI] [PubMed] [Google Scholar]
- [40].Trewyn BG, Giri S, Slowing II, Lin VS-Y, Mesoporous silicananoparticle based controlled release, drug delivery, and biosensor systems, Chem. Comm 31 (2007) 3236–3245. [DOI] [PubMed] [Google Scholar]
- [41].Mahmoudi M, Sant S, Wang B, Laurent S, Sen T, Superparamagnetic iron oxide nanoparticles (SPIONs): Development, surface modification and applications in chemotherapy, Adv. Drug Deliv. Rev 63 (2011) 24–46. [DOI] [PubMed] [Google Scholar]
- [42].Shen J, Zhao L, Han G, Lanthanide-doped upconverting luminescent nanoparticle platforms for optical imaging-guided drug delivery and therapy, Adv. Drug Deliv. Rev 65 (2013) 744–755. [DOI] [PubMed] [Google Scholar]
- [43].Bünzli JCG, Lanthanide Photonics: Shaping the Nanoworld, Trends Chem 1 (2019) 751–762. [Google Scholar]
- [44].Luo Z, Hou J, Menin L, Ong QK, Stellacci F, Evolution of the Ligand Shell Morphology during Ligand Exchange Reactions on Gold Nanoparticles, Angew. Chemie - Int. Ed 56 (2017) 13521–13525. [DOI] [PubMed] [Google Scholar]
- [45].Umair M, Javed I, Rehman M, Madni A, Javeed A, Ghafoor A, Ashraf M, Nanotoxicity of Inert Materials: The Case of Gold, Silver and Iron 19 (2016) 161–180. [DOI] [PubMed] [Google Scholar]
- [46].Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD, Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity, Small 1 (2005) 325–327. [DOI] [PubMed] [Google Scholar]
- [47].Colangelo E, Comenge J, Paramelle D, Volk M, Chen Q, Lévy R, Characterizing self-assembled monolayers on gold nanoparticles, Bioconjug. Chem 28 (2017) 11–22. [DOI] [PubMed] [Google Scholar]
- [48].Pakiari AH, Jamshidi Z, Nature and strength of MS bonds (M = Au, Ag, and Cu) in binary alloy gold clusters, J. Phys. Chem. A 114 (2010) 9212–9221. [DOI] [PubMed] [Google Scholar]
- [49].Gupta A, Moyano DF, Parnsubsakul A, Papadopoulos A, Wang L-S, Landis RF, Das R, Rotello VM, Ultrastable and Biofunctionalizable Gold Nanoparticles, ACS Appl. Mater. Interfaces 8 (2016) 14096–14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Han G, Ghosh P, De M, Rotello VM, Drug and gene delivery using gold nanoparticles, Nanobiotechnology 3 (2007) 40–45. [Google Scholar]
- [51].Bidoggia S, Milocco F, Polizzi S, Canton P, Saccani A, Sanavio B, Krol S, Stellacci F, Pengo P, Pasquato L, Fluorinated and charged hydrogenated alkanethiolates grafted on gold: Expanding the diversity of mixed-monolayer nanoparticles for biological applications, Bioconjug. Chem 28 (2017) 43–52. [DOI] [PubMed] [Google Scholar]
- [52].Kim B, Han G, Toley BJ, Kim CK, Rotello VM, Forbes NS, Tuning payload delivery in tumour cylindroids using gold nanoparticles, Nat. Nanotechnol 5 (2010) 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Dreaden EC, Mwakwari SC, Sodji QH, Oyelere AK, El-Sayed MA, Tamoxifen-poly(ethylene glycol)-thiol gold nanoparticle conjugates: Enhanced potency and selective delivery for breast cancer treatment, Bioconjug. Chem 20 (2009) 2247–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hassan SSM, Rechnitz GA, Determination of Glutathione and Glutathione Reductase with a Silver Sulfide Membrane Electrode, Anal. Chem 54 (1982) 1972–1976. [Google Scholar]
- [55].Duncan B, Kim C, Rotello VM, Gold nanoparticle platforms as drug and biomacromolecule delivery systems, J. Control. Release 148 (2010) 122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Elci G, Yesilbag Tonga G, Yan B, Kim ST, Kim CS, Jiang Y, Saha K, Moyano DF, Marsico ALM, Rotello VM, Vachet RW, Dual-Mode Mass Spectrometric Imaging for Determination of in Vivo Stability of Nanoparticle Monolayers, ACS Nano 11 (2017) 7424–7430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Bergen JM, von Recum HA, Goodman TT, Massey AP, Pun SH, Gold Nanoparticles as a Versatile Platform for Optimizing Physicochemical Parameters for Targeted Drug Delivery, Macromol. Biosci 6 (2006) 506–516. [DOI] [PubMed] [Google Scholar]
- [58].Sonavane G, Tomoda K, Makino K, Biodistribution of colloidal gold nanoparticles after intravenous administration: Effect of particle size, Colloid Surface B 66 (2008) 274–280. [DOI] [PubMed] [Google Scholar]
- [59].Huang X, El-Sayed MA, Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy, J. Adv. Res 1 (2010) 13–28. [Google Scholar]
- [60].Donati G, Plasmonic photoemission, Nat. Photonics 11 (2017) 72–72. [Google Scholar]
- [61].Goswami N, Yao Q, Luo Z, Li J, Chen T, Xie J, Luminescent Metal Nanoclusters with Aggregation-Induced Emission, J. Phys. Chem. Lett 7 (2016) 962–975. [DOI] [PubMed] [Google Scholar]
- [62].Ringe E, Langille MR, Sohn K, Zhang J, Huang J, Mirkin CA, Van Duyne RP, Marks LD, Plasmon Length: A Universal Parameter to Describe Size Effects in Gold Nanoparticles, J. Phys. Chem. Lett 3 (2012) 1479–1483. [DOI] [PubMed] [Google Scholar]
- [63].Leifert A, Pan-Bartnek Y, Simon U, Jahnen-Dechent W, Molecularly stabilised ultrasmall gold nanoparticles: Synthesis, characterization and bioactivity, Nanoscale 5 (2013) 6224–6242. [DOI] [PubMed] [Google Scholar]
- [64].Kumar A, Ma H, Zhang X, Huang K, Jin S, Liu J, Wei T, Cao W, Zou G, Liang XJ, Gold nanoparticles functionalized with therapeutic and targeted peptides for cancer treatment, Biomaterials 33 (2012) 1180–1189. [DOI] [PubMed] [Google Scholar]
- [65].Schmid G, Kreyling WG, Simon U, Toxic effects and biodistribution of ultrasmall gold nanoparticles, Arch. Toxicol 91 (2017) 3011–3037. [DOI] [PubMed] [Google Scholar]
- [66].Elci G, Jiang Y, Yan B, Kim ST, Saha K, Moyano DF, Tonga GY, Jackson LC, Rotello VM, Vachet RW, Surface Charge Controls the Suborgan Biodistributions of Gold Nanoparticles, ACS Nano 10 (2016) 5536–5542. [DOI] [PubMed] [Google Scholar]
- [67].Leifert A, Pan-Bartnek Y, Simon U, Jahnen-Dechent W, Molecularly stabilised ultrasmall gold nanoparticles: Synthesis, characterization and bioactivity, Nanoscale 5 (2013) 6224–6242. [DOI] [PubMed] [Google Scholar]
- [68].Dykman LA, Khlebtsov NG, Uptake of Engineered Gold Nanoparticles into Mammalian Cells, Chem. Rev 114 (2014) 1258–1288. [DOI] [PubMed] [Google Scholar]
- [69].Huang K, Ma H, Liu J, Huo S, Kumar A, Wei T, Zhang X, Jin S, Gan Y, Wang PC, He S, Zhang X, Liang XJ, Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo, ACS Nano 6 (2012) 4483–4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lee KYJ, Lee GY, Lane LA, Li B, Wang J, Lu Q, Wang Y, Nie S, Functionalized, Long-Circulating, and Ultrasmall Gold Nanocarriers for Overcoming the Barriers of Low Nanoparticle Delivery Efficiency and Poor Tumor Penetration, Bioconjug. Chem 28 (2017) 244–252. [DOI] [PubMed] [Google Scholar]
- [71].Hong R, Han G, Fernández JM, Kim BJ, Forbes NS, Rotello VM, Glutathione-mediated delivery and release using monolayer protected nanoparticle carriers, J. Am. Chem. Soc 128 (2006) 1078–1079. [DOI] [PubMed] [Google Scholar]
- [72].Vines JB, Yoon JH, Ryu NE, Lim DJ, Park H, Gold nanoparticles for photothermal cancer therapy, Front. Chem 7 (2019) 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Hone DC, Walker PI, Evans-Gowing R, FitzGerald S, Beeby A, Chambrier I, Cook MJ, Russell DA, Generation of cytotoxic singlet oxygen via phthalocyaninel-stabilized gold nanoparticles: A potential delivery vehicle for photodynamic therapy, Langmuir 18 (2002) 2985–2987. [Google Scholar]
- [74].Zhang X, Chibli H, Mielke R, Nadeau J, Ultrasmall gold-Doxorubicin conjugates rapidly kill apoptosis-resistant cancer cells, Bioconjug. Chem 22 (2011) 235–243. [DOI] [PubMed] [Google Scholar]
- [75].Midatech Pharma US Inc. (2018) MTX110 by Convection-Enhanced Delivery in Treating Participants with Newly-Diagnosed Diffuse Intrinsic Pontine Glioma (PNOC015). (Clinicaltrials.gov Identifier NCT03566199). [Google Scholar]
- [76].Jiménez-Balsa A, Pinto S, Quartin E, Lino MM, Francisco V, Ferreira L, Nanoparticles Conjugated with Photocleavable Linkers for the Intracellular Delivery of Biomolecules, Bioconjug. Chem 29 (2018) 1485–1489. [DOI] [PubMed] [Google Scholar]
- [77].Liu Q, Zhan C, Kohane DS, Phototriggered Drug Delivery Using Inorganic Nanomaterials, Bioconjug. Chem 28 (2017) 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Agasti SS, Chompoosor A, You CC, Ghosh P, Kim CK, Rotello VM, Photoregulated release of caged anticancer drugs from gold nanoparticles, J. Am. Chem. Soc 131 (2009) 5728–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Jain PK, Lee KS, El-Sayed IH, El-Sayed MA, Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine, J. Phys. Chem. B 110 (2006) 7238–7248. [DOI] [PubMed] [Google Scholar]
- [80].Wang L, Zhang J, Wang X, Huang Q, Pan D, Song S, Fan C, Gold nanoparticle-based optical probes at target-responsive DNA structures, Gold Bull 41 (2008) 37–41. [Google Scholar]
- [81].Abadeer NS, Murphy CJ, Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles, J. Phys. Chem. C 120 (2016) 9, 4691–4716. [Google Scholar]
- [82].Chadwick SJ, Salah D, Livesey PM, Brust M, Volk M, Singlet oxygen generation by laser irradiation of gold nanoparticles, J. Phys. Chem. C 120 (2016) 10647–10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Cheng X, Sun R, Yin L, Chai Z, Shi H, Gao M, Light-Triggered Assembly of Gold Nanoparticles for Photothermal Therapy and Photoacoustic Imaging of Tumors In Vivo, Adv. Mater 29 (2017) 1604894. [DOI] [PubMed] [Google Scholar]
- [84].Hameed S, Mo S, Mustafa G, Bajwa SZ, Khan WS, Dai Z, Immunological Consequences of Nanoparticle-Mediated Antitumor Photoimmunotherapy, Adv. Ther (2019) 1900101. [Google Scholar]
- [85].Liu Y, Bhattarai P, Dai Z, Chen X, Photothermal therapy and photoacoustic imaging: Via nanotheranostics in fighting cancer, Chem. Soc. Rev 48 (2019) 2053–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ye W, Li H, Li X, Fan X, Jin Q, Ji J, mRNA Guided Intracellular Self-Assembly of DNA–Gold Nanoparticle Conjugates as a Precise Trigger to Up-Regulate Cell Apoptosis and Activate Photothermal Therapy, Bioconjug. Chem 30 (2019) 1763–1772. [DOI] [PubMed] [Google Scholar]
- [87].Nieves DJ, Li Y, Fernig DG, Lévy R, Photothermal raster image correlation spectroscopy of gold nanoparticles in solution and on live cells, R. Soc. Open Sci 2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Nanospectra Biosciences, Inc. (2019) MRI/US Fusion Imaging and Biopsy in Combination with Nanoparticle Directed Focal Therapy for Ablation of Prostate Tissue. (Clinicaltrails.gov Identifier NCT02680535). [Google Scholar]
- [89].Kim J, Cao L, Shvartsman D, Silva EA, Mooney DJ, Targeted delivery of nanoparticles to ischemic muscle for imaging and therapeutic angiogenesis, Nano Lett 11 (2011) 694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Kodiha M, Wang YM, Hutter E, Maysinger D, Stochaj U, Off to the organelles - killing cancer cells with targeted gold nanoparticles, Theranostics 5 (2015) 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Chen F, Ma K, Madajewski B, Zhuang L, Zhang L, Rickert K, Marelli M, Yoo B, Turker MZ, Overholtzer M, Quinn TP, Gonen M, Zanzonico P, Tuesca A, Bowen MA, Norton L, Subramony JA, Wiesner U, Bradbury MS, Ultrasmall targeted nanoparticles with engineered antibody fragments for imaging detection of HER2-overexpressing breast cancer, Nat. Commun 9 (2018) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Jha S, Ramadori F, Quarta S, Biasiolo A, Fabris E, Baldan P, Guarino G, Ruvoletto M, Villano G, Turato C, Gatta A, Mancin F, Pontisso P, Scrimin P, Binding and uptake into human hepatocellular carcinoma cells of peptide-functionalized gold nanoparticles, Bioconjug. Chem 28 (2017) 222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Stuchinskaya T, Moreno M, Cook MJ, Edwards DR, Russell DA, Targeted photodynamic therapy of breast cancer cells using antibody-phthalocyanine-gold nanoparticle conjugates, Photochem. Photobiol. Sci 10 (2011) 822–831. [DOI] [PubMed] [Google Scholar]
- [94].Paciotti GF, Zhao J, Cao S, Brodie PJ, Tamarkin L, Huhta M, Myer LD, Friedman J, Kingston DGI, Synthesis and Evaluation of Paclitaxel-Loaded Gold Nanoparticles for Tumor-Targeted Drug Delivery, Bioconjug. Chem 27 (2016) 2646–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Du Y, Xia L, Jo A, Davis RM, Bissel P, Ehrich MF, Kingston DGI, Synthesis and Evaluation of Doxorubicin-Loaded Gold Nanoparticles for Tumor-Targeted Drug Delivery, Bioconjug. Chem 29 (2018) 420–430. [DOI] [PubMed] [Google Scholar]
- [96].Perrett AJ, Dickinson RL, Krpetić Ž, Brust M, Lewis H, Eperon IC, Burley GA, Conjugation of PEG and gold nanoparticles to increase the accessibility and valency of tethered RNA splicing enhancers, Chem. Sci 4 (2013) 257–265. [Google Scholar]
- [97].Karakoti AS, Das S, Thevuthasan S, Seal S, PEGylated inorganic nanoparticles, Angew. Chemie - Int. Ed 50 (2011) 1980–1994. [DOI] [PubMed] [Google Scholar]
- [98].Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine, Angew. Chem- Int. Ed 49 (2010) 3280–3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Dul M, Nikolic T, Stefanidou M, McAteer MA, Williams P, Mous J, Roep BO, Kochba E, Levin Y, Peakman M, Wong FS, Dayan CM, Tatovic D, Coulman SA, Birchall JC, Conjugation of a peptide autoantigen to gold nanoparticles for intradermally administered antigen specific immunotherapy, Int. J. Pharm 562 (2019) 303–312. [DOI] [PubMed] [Google Scholar]
- [100].Cardiff University (2019) Enhanced Epidermal Antigen Specific Immunotherapy Trial -1 (EE-ASI-1) (ClinicalTrials.gov Identifier: NCT02837094). [Google Scholar]
- [101].Letsinger RL, Elghanian R, Viswanadham G, Mirkin CA, Use of Steroid Cyclic Disulfide Anchor in Constructing Gold Nanoparticle-Oligonucleotide Conjugates, Bioconjug. Chem 11 (2000) 289–291. [DOI] [PubMed] [Google Scholar]
- [102].Ding Y, Jiang Z, Saha K, Kim CS, Kim ST, Landis RF, Rotello VM, Gold Nanoparticles for Nucleic Acid Delivery, Molec. Ther 22 (2014), 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Niemeyer CM, Ceyhan B, Hazarika P, Oligofunctional DNA–Gold Nanoparticle Conjugates, Angew. Chemie Int. Ed 42 (2003) 5766–5770. [DOI] [PubMed] [Google Scholar]
- [104].Mirkin CA, Letsinger RL, Micic RC, Storhoff JJ, A DNA-based method for rationally assembling nanoparticles into macroscopic materials, Nature 382 (1996) 607–609. [DOI] [PubMed] [Google Scholar]
- [105].Storhoff JJ, Lazarides AA, Mucic RC, Mirkin CA, Letsinger RL, Schatz GC, What controls the optical properties of DNA-linked gold nanoparticle assemblies?, J. Am. Chem. Soc 122 (2000) 4640–4650. [Google Scholar]
- [106].Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA, Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles, Science (80-.) 277 (1997) 1078–1081. [DOI] [PubMed] [Google Scholar]
- [107].Rosi NL, Giljohann DA, Thaxton CS, Lytton-Jean AKR, Han MS, Mirkin CA, Oligonucleotide-modified gold nanoparticles for infracellular gene regulation, Science (80-.) 312 (2006) 1027–1030. [DOI] [PubMed] [Google Scholar]
- [108].Giljohann DA, Seferos DS, Prigodich AE, Patel PC, Mirkin CA, Gene regulation with polyvalent siRNA-nanoparticle conjugates, J. Am. Chem. Soc 131 (2009) 2072–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Giljohann DA, Seferos DS, Patel PC, Millstone JE, Rosi NL, Mirkin CA, Oligonucleotide loading determines cellular uptake of DNA-modified gold nanoparticles, Nano Lett 7 (2007) 3818–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Barnaby SN, Lee A, Mirkin CA, Probing the Inherent Stability of siRNA Immobilized on Nanoparticle Constructs, Proc. Natl. Aca. Sci 111 (2014) 9739–9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Oishi M, Nakaogami J, Ishii T, Nagasaki Y, Smart PEGylated gold nanoparticles for the cytoplasmic delivery of siRNA to induce enhanced gene silencing, Chem. Lett 35 (2006) 1046–1047. [Google Scholar]
- [112].Lee JS, Green JJ, Love KT, Sunshine J, Langer R, Anderson DG, Gold, poly(β-amino ester) nanoparticles for small interfering RNA delivery, Nano Lett 9 (2009) 2402–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kim HJ, Takemoto H, Yi Y, Zheng M, Maeda Y, Chaya H, Hayashi K, Mi P, Pittella F, Christie RJ, Toh K, Matsumoto Y, Nishiyama N, Miyata K, Kataoka K, Precise engineering of siRNA delivery vehicles to tumors using polyion complexes and gold nanoparticles, ACS Nano 8 (2014) 8979–8991. [DOI] [PubMed] [Google Scholar]
- [114].Morgan E, Wupperfeld D, Morales D, Reich N, Shape Matters: Gold Nanoparticle Shape Impacts the Biological Activity of siRNA Delivery, Bioconjug. Chem 30 (2019) 853–860. [DOI] [PubMed] [Google Scholar]
- [115].Zheng D, Giljohann DA, Chen DL, Massich MD, Wang X-Q, Iordanov H, Mirkin CA, Paller AS, Topical Delivery of siRNA-based Spherical Nucleic Acid Nanoparticle Conjugates for Gene Regulation, Proc. Natl. Aca. Sci. U. S. A 109 (2012) 11975–11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Randeria PS, Seeger MA, Wang XQ, Wilson H, Shipp D, Mirkin CA, Paller AS, siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown, Proc. Natl. Acad. Sci. U. S. A 112 (2015) 5573–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Lee K, Conboy M, Park HM, Jiang F, Kim HJ, Dewitt MA, Mackley VA, Chang K, Rao A, Skinner C, Shobha T, Mehdipour M, Liu H, Huang WC, Lan F, Bray NL, Li S, Corn JE, Kataoka K, Doudna JA, Conboy I, Murthy N, Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair, Nat. Biomed. Eng 1 (2017) 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Lee B, Lee K, Panda S, Gonzales-Rojas R, Chong A, Bugay V, Park HM, Brenner R, Murthy N, Lee HY, Nanoparticle delivery of CRISPR into the brain rescues a mouse model of fragile X syndrome from exaggerated repetitive behaviours, Nat. Biomed. Eng 2 (2018) 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Lucarini M, Franchi P, Pedulli GF, Gentilini C, Polizzi S, Pengo P, Scrimin P, Pasquato L, Effect of core size on the partition of organic solutes in the monolayer of water-soluble nanoparticles: An ESR investigation, J. Am. Chem. Soc 127 (2005) 16384–16385. [DOI] [PubMed] [Google Scholar]
- [120].Love JC, Estroff LA, Kriebel JK, Nuzzo RG, Whitesides GM, Self-assembled monolayers of thiolates on metals as a form of nanotechnology, Chem. Rev 105 (2005) 1103–1169. [DOI] [PubMed] [Google Scholar]
- [121].Cheng Y, Samia AC, Meyers JD, Panagopoulos I, Fei B, Burda C, Highly efficient drug delivery with gold nanoparticle vectors for in vivo photodynamic therapy of cancer, J. Am. Chem. Soc 130 (2008) 10643–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Kim CK, Ghosh P, Pagliuca C, Zhu ZJ, Menichetti S, Rotello VM, Entrapment of hydrophobic drugs in nanoparticle monolayers with efficient release into cancer cells, J. Am. Chem. Soc 131 (2009) 1360–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kapur A, Medina SH, Wang W, Palui G, Schneider JP, Mattoussi H, Intracellular Delivery of Gold Nanocolloids Promoted by a Chemically Conjugated Anticancer Peptide, ACS Omega 3 (2018) 12754–12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Tonga GY, Jeong Y, Duncan B, Mizuhara T, Mout R, Das R, Kim ST, Yeh YC, Yan B, Hou S, Rotello VM, Supramolecular regulation of bioorthogonal catalysis in cells using nanoparticle-embedded transition metal catalysts, Nat. Chem 7 (2015) 597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Das R, Landis RF, Tonga GY, Cao-Milán R, Luther DC, Rotello VM, Control of Intra- versus Extracellular Bioorthogonal Catalysis Using Surface-Engineered Nanozymes, ACS Nano 13 (2019) 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Fuhrmann G, Chandrawati R, Parmar PA, Keane TJ, Maynard SA, Bertazzo S, Stevens MM, Engineering Extracellular Vesicles with the Tools of Enzyme Prodrug Therapy, Adv. Mater 30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Zhang X, Liu Y, Gopalakrishnan S, Castellanos-Garcia L, Li G, Malassine M, Uddin I, Huang R, Luther DC, Vachet RW, Rotello VM, Intracellular Activation of Bioorthogonal Nanozymes through Endosomal Proteolysis of the Protein Corona, ACS Nano, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Glogowski E, He J, Russell TP, Emrick T, Mixed monolayer coverage on gold nanoparticles for interfacial stabilization of immiscible fluids, Chem. Commun (2005) 4050–4052. [DOI] [PubMed] [Google Scholar]
- [129].Torchilin VP, Recent advances with liposomes as pharmaceutical carriers, Nat. Rev. Drug Discov 4 (2005) 145–160. [DOI] [PubMed] [Google Scholar]
- [130].Yang XC, Samanta B, Agasti SS, Jeong Y, Zhu ZJ, Rana S, Miranda OR, Rotello VM, Drug delivery using nanoparticle-stabilized nanocapsules, Angew. Chemie - Int. Ed 50 (2011) 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Jiang Y, Tang R, Duncan B, Jiang Z, Yan B, Mout R, Rotello VM, Direct cytosolic delivery of siRNA using nanoparticle-stabilized nanocapsules, Angew. Chemie - Int. Ed 54 (2015) 506–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Jiang Y, Hardie J, Liu Y, Ray M, Luo X, Das R, Landis RF, Farkas ME, Rotello VM, Nanocapsule-mediated cytosolic siRNA delivery for anti-inflammatory treatment, J. Control. Release 283 (2018) 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Hardie J, Jiang Y, Tetrault ER, Ghazi PC, Yesilbag Tonga G, Farkas ME, Rotello VM, Simultaneous Cytosolic Delivery of a Chemotherapeutic and siRNA using Nanoparticle-Stabilized Nanocapsules, Nanotechnol 27 (2016) 374001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].M Pallares R, Choo P, Cole LE, Mirkin CA, Lee A, Odom TW, Manipulating Immune Activation of Macrophages by Tuning the Oligonucleotide Composition of Gold Nanoparticles, Bioconjug. Chem 30 (2019) 2032–2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Ray M, Lee Y-W, Hardie J, Yesilbag Tonga G, Farkas ME, Rotello VM, CRISPRed Macrophages for Cell-Based Cancer Immunotherapy, Bioconjug. Chem 29 (2018) 445–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Moyano DF, Liu Y, Ayaz F, Hou S, Puangploy P, Duncan B, Osborne BA, Rotello VM, Immunomodulatory Effects of Coated Gold Nanoparticles in LPS-Stimulated In Vitro and In Vivo Murine Model Systems, Chem 1 (2016) 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Verma A, Simard JM, Worrall JWE, Rotello VM, Tunable reactivation of nanoparticle-inhibited beta-galactosidase by glutathione at intracellular concentrations, J. Am. Chem. Soc 126 (2004) 13987–13991. [DOI] [PubMed] [Google Scholar]
- [138].Ghosh P, Yang X, Arvizo R, Zhu ZJ, Agasti SS, Mo Z, Rotello VM, Intracellular delivery of a membrane-impermeable enzyme in active form using functionalized gold nanoparticles, J. Am. Chem. Soc 132 (2010) 2642–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Mout R, Ray M, Tay T, Sasaki K, Yesilbag Tonga G, Rotello VM, General Strategy for Direct Cytosolic Protein Delivery via Protein-Nanoparticle Co-engineering, ACS Nano 11 (2017) 6416–6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Mout R, Yesilbag Tonga G, Wang LS, Ray M, Roy T, Rotello VM, Programmed Self-Assembly of Hierarchical Nanostructures through Protein-Nanoparticle Coengineering, ACS Nano 11 (2017) 3456–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Stewart MP, Lorenz A, Dahlman J, Sahay G, Challenges in carrier-mediated intracellular delivery: moving beyond endosomal barriers, 8 (2016) 465–478. [DOI] [PubMed] [Google Scholar]
- [142].Mout R, Ray M, Yesilbag Tonga G, Lee YW, Tay T, Sasaki K, Rotello VM, Direct Cytosolic Delivery of CRISPR/Cas9-Ribonucleoprotein for Efficient Gene Editing, ACS Nano 11 (2017) 2452–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Lee Y, Mout R, Luther DC, Liu Y, Castellanos-García L, Burnside AS, Ray M, Tonga GY, Hardie J, Nagaraj H, Das R, Phillips EL, Tay T, Vachet RW, Rotello VM, In Vivo Editing of Macrophages through Systemic Delivery of CRISPR-Cas9-Ribonucleoprotein-Nanoparticle Nanoassemblies, Adv. Ther 2 (2019) 1900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Sandhu KK, McIntosh CM, Simard JM, Smith SW, Rotello VM, Gold nanoparticle-mediated transfection of mammalian cells, Bioconjug. Chem 13 (2002) 3–6. [DOI] [PubMed] [Google Scholar]
- [145].Kim ST, Chompoosor A, Yeh YC, Agasti SS, Solfiell DJ, Rotello VM, Dendronized gold nanoparticles for siRNA delivery, Small 8 (2012) 3253–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Boal AK, Rotello VM, Fabrication and self-optimization of multivalent receptors on nanoparticle scaffolds, J. Am. Chem. Soc 122 (2000) 734–735. [Google Scholar]
- [147].Goodman CM, McCusker CD, Yilmaz T, Rotello VM, Toxicity of gold nanoparticles functionalized with cationic and anionic side chains, Bioconjug. Chem 15 (2004) 897–900. [DOI] [PubMed] [Google Scholar]
- [148].Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG, Non-viral vectors for gene-based therapy, Nat. Rev. Genet 15 (2014) 541–555. [DOI] [PubMed] [Google Scholar]
- [149].Wang W, Li W, Ma N, Steinhoff G, Non-Viral Gene Delivery Methods | BenthamScience, Curr. Pharm. Biotechnol 14 (2013) 46–60. [PubMed] [Google Scholar]
- [150].Yi Y, Kim HJ, Zheng M, Mi P, Naito M, Kim BS, Min HS, Hayashi K, Perche F, Toh K, Liu X, Mochida Y, Kinoh H, Cabral H, Miyata K, Kataoka K, Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells, J. Control. Release 295 (2019) 268–277. [DOI] [PubMed] [Google Scholar]
- [151].Shan Y, Luo T, Peng C, Sheng R, Cao A, Cao X, Shen M, Guo R, Tomás H, Shi X, Gene delivery using dendrimer-entrapped gold nanoparticles as nonviral vectors, Biomaterials 33 (2012) 3025–3035. [DOI] [PubMed] [Google Scholar]
- [152].Kudgus RA, Szabolcs A, Khan JA, Walden CA, Reid JM, Robertson JD, Bhattacharya R, Mukherjee P, Inhibiting the Growth of Pancreatic Adenocarcinoma In Vitro and In Vivo through Targeted Treatment with Designer Gold Nanotherapeutics, PLoS One 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Arvizo RR, Saha S, Wang E, Robertson JD, Bhattacharya R, Mukherjee P, Inhibition of tumor growth and metastasis by a self-therapeutic nanoparticle, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 6700–6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Guo S, Huang Y, Jiang Q, Sun Y, Deng L, Liang Z, Du Q, Xing J, Zhao Y, Wang PC, Dong A, Liang XJ, Enhanced gene delivery and siRNA silencing by gold nanoparticles coated with charge-reversal polyelectrolyte, ACS Nano 4 (2010) 5505–5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Gittins DI, Caruso F, Multilayered Polymer Nanocapsules Derived from Gold Nanoparticle Templates, Adv. Mater 12 (2000) 1947–1949. [Google Scholar]
- [156].Lee SK, Han MS, Asokan S, Tung CH, Effective gene silencing by multilayered siRNA-coated gold nanoparticles, Small 7 (2011) 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Yu M, Lei B, Gao C, Yan J, Ma PX, Optimizing surface-engineered ultra-small gold nanoparticles for highly efficient miRNA delivery to enhance osteogenic differentiation of bone mesenchymal stromal cells, Nano Res 10 (2017) 49–63. [Google Scholar]
- [158].Lytton-Jean AKR, Langer R, Anderson DG, Five Years of siRNA Delivery: Spotlight on Gold Nanoparticles, Small 7 (2011) 1932–1937. [DOI] [PubMed] [Google Scholar]
- [159].Huo S, Gong N, Jiang Y, Chen F, Guo H, Gan Y, Wang Z, Herrmann A, Liang XJ, Gold-DNA nanosunflowers for efficient gene silencing with controllable transformation, Sci. Adv 5 (2019) eaaw6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Browning LM, Huang T, Xu XHN, Real-time in vivo imaging of sizedependent transport and toxicity of gold nanoparticles in zebrafish embryos using single nanoparticle plasmonic spectroscopy, Interface Focus 3 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Khanal BP, Zubarev ER, Gram-Scale Synthesis of Isolated Monodisperse Gold Nanorods, Chem. - A Eur. J 25 (2019) 1595–1600. [DOI] [PubMed] [Google Scholar]
- [162].Schmucker AL, Harris N, Banholzer MJ, Blaber MG, Osberg KD, Schatz GC, Mirkin CA, Correlating Nanorod Structure with Experimentally Measured and Theoretically Predicted Surface Plasmon Resonance, ACS Nano 9 (2010) 5453–5463. [DOI] [PubMed] [Google Scholar]
- [163].Jones MR, Osberg KD, Macfarlane RJ, Langille MR, Mirkin CA, Templated Techniques for the Synthesis and Assembly of Plasmonic Nanostructures, Chem. Rev 111 (2011) 3736–3827. [DOI] [PubMed] [Google Scholar]
- [164].Kim K, Jo MC, Jeong S, Palanikumar L, Rotello VM, Ryu JH, Park MH, Externally controlled drug release using a gold nanorod contained composite membrane, Nanoscale 8 (2016) 11949–11955. [DOI] [PubMed] [Google Scholar]
- [165].Zhang Z, Wang J, Nie X, Wen T, Ji Y, Wu X, Zhao Y, Chen C, Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods, J. Am. Chem. Soc 136 (2014) 7317–7326. [DOI] [PubMed] [Google Scholar]
- [166].Alkilany AM, Frey RL, Ferry JL, Murphy CJ. Gold nanorods as nanoadmicelles: 1-naphthol partitioning into a nanorod-bound surfactant bilayer, Langmuir 24 (2008) 10235–10239. [DOI] [PubMed] [Google Scholar]
- [167].Vigderman L, Khanal BP, Zubarev ER, Functional Gold Nanorods: Synthesis, Self-Assembly, and Sensing Applications, Adv. Mater 24 (2012) 4811–4841. [DOI] [PubMed] [Google Scholar]
- [168].Chen CC, Lin YP, Wang CW, Tzeng HC, Wu CH, Chen YC, Chen CP, Chen LC, Wu YC. DNA-gold nanorod conjugates for remote control of localized gene expression by near infrared irradiation, J. Am. Chem. Soc 128 (2006) 3709–3715. [DOI] [PubMed] [Google Scholar]
- [169].Wijaya A, Schaffer SB, Pallares IG, Hamad-Schifferli K, Selective release of multiple DNA oligonucleotides from gold nanorods, ACS Nano 3 (2009) 80–86. [DOI] [PubMed] [Google Scholar]
- [170].Xiao Z, Ji C, Shi J, Pridgen EM, Frieder J, Wu J, Farokhzad OC, DNA Self-Assembly of Targeted Near-Infrared-Responsive Gold Nanoparticles for Cancer Thermo-Chemotherapy, Angew. Chemie Int. Ed 51 (2012) 11853–11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Takahashi H, Niidome Y, Yamada S. Controlled release of plasmid DNA from gold nanorods induced by pulsed near-infrared light, Chem. Commun (2005) 2247–2249. [DOI] [PubMed] [Google Scholar]
- [172].Somin EL, Sasaki DY, Perroud TD, Yoo D, Patel KD, Lee LP, Biologically functional cationic phospholipid-gold nanoplasmonic carriers of RNA, J. Am. Chem. Soc 131 (2009) 14066–14074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Wang J, Thomas M, Lin P, Cheng JX, Matei DE, Wei A, SiRNA Delivery Using Dithiocarbamate-Anchored Oligonucleotides on Gold Nanorods, Bioconjug. Chem 30 (2019) 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Alkilany AM, Thompson LB, Boulos SP, Sisco PN, Murphy CJ, Gold nanorods: Their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions, Adv. Drug Deliv. Rev 64 (2012) 190–199. [DOI] [PubMed] [Google Scholar]
- [175].Chakraborty I, Pradeep T, Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles, Chem. Rev 117 (2017) 8208–8271. [DOI] [PubMed] [Google Scholar]
- [176].Mishra D, Lobodin V, Zhang C, Aldeek F, Lochner E, Mattoussi H, Gold-doped silver nanoclusters with enhanced photophysical properties, Phys. Chem. Chem. Phys 20 (2018) 12992–13007. [DOI] [PubMed] [Google Scholar]
- [177].Zhang C, Li C, Liu Y, Zhang J, Bao C, Liang S, Wang Q, Yang Y, Fu H, Wang K, Cui D, Gold Nanoclusters-Based Nanoprobes for Simultaneous Fluorescence Imaging and Targeted Photodynamic Therapy with Superior Penetration and Retention Behavior in Tumors, Adv. Funct. Mater (2015) 1314–1325. [Google Scholar]
- [178].Xia F, Hou W, Liu Y, Wang W, Han Y, Yang M, Zhi X, Li C, Qi D, Li T, Martinez J Fuente de la, Zhang C, Song J, Cui D, Cytokine induced killer cells-assisted delivery of chlorin e6 mediated self-assembled gold nanoclusters to tumors for imaging and immuno-photodynamic therapy, Biomaterials 170 (2018) 1–11. [DOI] [PubMed] [Google Scholar]
- [179].Kedmi R, Veiga N, Ramishetti S, Goldsmith M, Rosenblum D, Dammes N, Hazan-Halevy I, Nahary L, Leviatan-Ben-Arye S, Harlev M, Behlke M, Benhar I, Lieberman J, Peer D, A modular platform for targeted RNAi therapeutics, Nat. Nanotechnol 13 (2018) 214–219. [DOI] [PubMed] [Google Scholar]
- [180].Dammes N, Peer D, Monoclonal antibody-based molecular imaging strategies and theranostic opportunities, Theranostics 10 (2020) 938–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].García-Fernández L, Garcia-Pardo J, Tort O, Prior I, Brust M, Casals E, Lorenzo J, Puntes VF, Conserved effects and altered trafficking of Cetuximab antibodies conjugated to gold nanoparticles with precise control of their number and orientation, Nanoscale 9 (2017) 6111–6121. [DOI] [PubMed] [Google Scholar]
- [182].Chen D, Li B, Cai S, Wang P, Peng S, Sheng Y, He Y, Gu Y, Chen H, Dual targeting luminescent gold nanoclusters for tumor imaging and deep tissue therapy, Biomaterials 100 (2016) 1–16. [DOI] [PubMed] [Google Scholar]
- [183].Lei Y, Tang L, Xie Y, Xianyu Y, Zhang L, Wang P, Hamada Y, Jiang K, Zheng W, Jiang X, Gold nanoclusters-assisted delivery of NGF siRNA for effective treatment of pancreatic cancer, Nat. Commun 8 (2017) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Wang J, Chen L, Ye J, Li Z, Jiang H, Yan H, Stogniy MY, Sivaev IB, Bregadze VI, Wang X, Carborane Derivative Conjugated with Gold Nanoclusters for Targeted Cancer Cell Imaging, Biomacromolecules 18 (2017) 1466–1472. [DOI] [PubMed] [Google Scholar]
- [185].Hammer B, Norskov JK, Why gold is the noblest of all the metals, Nature 376 (1995) 238–240. [Google Scholar]
- [186].Browning LM, Lee KJ, Huang T, Nallathamby PD, Lowman JE, Xu XN, Random walk of single gold nanoparticles in zebrafish embryos leading to stochastic toxic effects on embryonic developments, Nanoscale 1 (2009) 138–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Bailly AL, Correard F, Popov A, Tselikov G, Chaspoul F, Appay R, Al-Kattan A, Kabashin AV, Braguer D, Esteve MA, In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles, Sci. Rep 9 (2019) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Sikora KN, Hardie JM, Castellanos-Garcia LJ, Liu Y, Reinhardt BM, Farkas ME, Rotello VM, Vachet RW, Dual Mass Spectrometric Tissue Imaging of Nanocarrier Distributions and their Biochemical Effects, Anal. Chem 92 (2020) 2011–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Castillo RR, Lozano D, Vallet-Regí M, Building Block Based Construction of Membrane-Organelle Double Targeted Nanosystem for Two-Drug Delivery, Bioconjug. Chem 29 (2018) 3677–3685. [DOI] [PubMed] [Google Scholar]
- [190].Daglioglu C, Okutucu B, Synthesis and Characterization of AICAR and DOX Conjugated Multifunctional Nanoparticles as a Platform for Synergistic Inhibition of Cancer Cell Growth, Bioconjug. Chem 27 (2016) 1098–1111. [DOI] [PubMed] [Google Scholar]
- [191].Yang D, Yao X, Dong J, Wang N, Du Y, Sun S, Gao L, Zhong Y, Qian C, Hong H, Design and Investigation of Core/Shell GQDs/hMSN Nanoparticles as an Enhanced Drug Delivery Platform in Triple-Negative Breast Cancer, Bioconjug. Chem 29 (2018) 2776–2785. [DOI] [PubMed] [Google Scholar]
- [192].Jeelani PG, Mulay P, Venkat R, Ramalingam C, Multifaceted Application of Silica Nanoparticles. A Review, Silicon 1 (2019). [Google Scholar]
- [193].Barbé C, Bartlett J, Kong L, Finnie K, Lin HQ, Larkin M, Calleja S, Bush A, Calleja G, Silica Particles: A Novel Drug-Delivery System 16 (2004) 1959–1966. [Google Scholar]
- [194].Slowing II, LO J. Vivero-Escoto, C.-W. Wu, V.S.-Y. Lin, Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers, Adv. Drug Deliv. Rev 60 (2008) 1278–288. [DOI] [PubMed] [Google Scholar]
- [195].Slowing II, Vivero-Escoto JL, Trewyn BG, Lin VSY, Mesoporous silica nanoparticles: Structural design and applications, J. Mater. Chem 20 (2010) 7924–7937. [Google Scholar]
- [196].Tarn D, Ashley CE, Xue M, Carnes EC, Zink JI, Brinker CJ, Mesoporous Silica Nanoparticle Nanocarriers: Biofunctionality and Biocompatibility, Acc. Chem. Res 46 (2013) 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Chen W, Glackin CA, Horwitz MA, Zink JI, Nanomachines and Other Caps on Mesoporous Silica Nanoparticles for Drug Delivery, Acc. Chem. Res 52 (2019) 1531–1542. [DOI] [PubMed] [Google Scholar]
- [198].Hai L, Jia X, He D, Zhang A, Wang T, Cheng H, He X, Wang K, Dna-functionalized hollow mesoporous silica nanoparticles with dual cargo loading for near-infrared-responsive synergistic chemo-photothermal treatment of cancer cells, ACS Appl. Nano Mater 1 (2018) 3486–3497. [Google Scholar]
- [199].Valtchev V, Tosheva L, Porous Nanosized Particles: Preparation, Properties, and Applications, Chem. Rev 113 (2013) 6734–6760. [DOI] [PubMed] [Google Scholar]
- [200].Li Z, Barnes JC, Bosoy A, Stoddart JF, Zink JI, Mesoporous silica nanoparticles in biomedical applications, Chem. Soc. Rev 41 (2012) 2590–2605. [DOI] [PubMed] [Google Scholar]
- [201].Yang K-N, Zhang C-Q, Wang W, Wang PC, Zhou J-P, Liang X-J, pH-responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment, Cancer Biol. Med 11 (2014) 34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Yang P, Gai S, Lin J, Functionalized mesoporous silica materials for controlled drug delivery, Chem Soc. Rev 41 (2012) 3679–98. [DOI] [PubMed] [Google Scholar]
- [203].Tang S, Huang X, Chen X, Zheng N, Hollow Mesoporous Zirconia Nanocapsules for Drug Delivery, Advanced Functional Materials 20 (2010) 2442–2447. [Google Scholar]
- [204].Niu Y, Yu M, Meka A, Liu Y, Zhang J, Yang Y, Yu C, Understanding the contribution of surface roughness and hydrophobic modification of silica nanoparticles to enhanced therapeutic protein delivery, J. Mater. Chem. B 4 (2016) 212. [DOI] [PubMed] [Google Scholar]
- [205].Niu Y, Yu M, Zhang J, Yang Y, Xu C, Yeh M, Taran E, Hou JJC, Gray PP, Yu C, Synthesis of silica nanoparticles with controllable surface roughness for therapeutic protein delivery, J. Mater. Chem. B 3 (2015) 8477. [DOI] [PubMed] [Google Scholar]
- [206].Vallet-Regi M, Ramila A, del Real RP, Perez-Pariente J, A New Property of MCM-41: Drug Delivery System, Chem. Mater 13 (2001) 308–311. [Google Scholar]
- [207].Jia L, Shen J, Li Z, Zhang D, Zhang Q, Duan C, Liu G, Zheng D, Tian X, Successfully tailoring the pore size of mesoporous silica nanoparticles: Exploitation of delivery systems for poorly water-soluble drugs, International Journal of Pharmaceutics 439 (2012) 81–91. [DOI] [PubMed] [Google Scholar]
- [208].Li J, Shen S, Kong F, Jiang T, Tang C, Yin C, Effects of pore size on in vitro and in vivo anticancer efficacies of mesoporous silica nanoparticles, RSC Adv 8 (2018) 24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Ambrogio MW, Thomas CR, Zhao Y-L, Zink JI, Stoddart JF, Mechanized Silica Nanoparticles: A New Frontier in Theranostic Nanomedicine Acc. Chem. Res 44 (2011) 903–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Tonga GY, Saha K, Rotello VM, 25th Anniversary Article: Interfacing Nanoparticles and Biology: New Strategies for Biomedicine, Adv. Mater 26 (2014) 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Zhang X, Huang R, Gopalakrishnan S, Cao-Milán R, Rotello VM, Bioorthogonal Nanozymes: Progress towards Therapeutic Applications, Trends Chem 1 (2019) 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Destito P, Castillo AS, Couceiro JR, Lopez F, Duarte MAC, Mascarenas JL, Hollow nanoreactors for Pd-catalyzed Suzuki-Miyaura coupling and O-propargyl cleavage reactions in bio-relevant aqueous media, Chem. Sci 10 (2019) 2598–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Rubio-Ruiz B, Weiss JT, Unciti-Broceta A, Efficient Palladium-Triggered Release of Vorinostat from a Bioorthogonal Precursor, J. Med. Chem 59 (2016) 9974–9980. [DOI] [PubMed] [Google Scholar]
- [214].Adam C, Pérez-López AM, Hamilton L, Rubio-Ruiz B, Bray TL, Sieger D, Brennan PM, Unciti-Broceta A, Bioorthogonal Uncaging of the Active Metabolite of Irinotecan by Palladium-Functionalized Microdevices, Chem. - A Eur. J 24 (2018) 16783–16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Slowing II, Trewyn BG, Lin VSY, Mesoporous silica nanoparticles for intracellular delivery of membrane-impermeable proteins, J. Am. Chem. Soc 129 (2007) 8845–8849. [DOI] [PubMed] [Google Scholar]
- [216].Gu J, Huang K, Zhu X, Li Y, Wei J, Zhao W, Liu C, Shi J, Sub-150nm mesoporous silica nanoparticles with tunable pore sizes and well-ordered mesostructure for protein encapsulation, J. Colloid Interface Sci 407 (2013) 236–242. [DOI] [PubMed] [Google Scholar]
- [217].Kamegawa R, Naito M, Miyata K, Functionalization of silica nanoparticles for nucleic acid delivery, Nano Research 11 (2018) 5219–5239. [Google Scholar]
- [218].Hom C, Lu J, Liong M, Luo H, Li Z, Zink JI, Tamanoi F, Mesoporous silica nanoparticles facilitate delivery of siRNA to shutdown signaling pathways in mammalian cells, Small 6 (2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Li X, Xie QR, Zhang JW Xia H. Gu, The packaging of siRNA within the mesoporous structure of silica nanoparticles, Biomater 32 (2011) 9546–9556. [DOI] [PubMed] [Google Scholar]
- [220].Kim MH, Na HK, Kim YK, Ryoo SR, Cho HS, Lee KE, Jeon H, Ryoo R, Min DH, Facile synthesis of monodispersed mesoporous silica nanoparticles with ultralarge pores and their application in gene delivery, ACS Nano 5 (2011) 3568–3576. [DOI] [PubMed] [Google Scholar]
- [221].Palanikumar L, Choi ES, Cheon JY, Joo SH, Ryu JH, Noncovalent Polymer-Gatekeeper in Mesoporous Silica Nanoparticles as a Targeted Drug Delivery Platform, Adv. Funct. Mater 25 (2015) 957–965. [Google Scholar]
- [222].Zhang Q, Wang X, Li PZ, Nguyen KT, Wang XJ, Luo Z, Zhang H, Tan NS, Zhao Y, Biocompatible, Uniform, and Redispersible Mesoporous Silica Nanoparticles for Cancer-Targeted Drug Delivery In Vivo, Adv. Funct. Mater 24 (2014) 2450–2461. [Google Scholar]
- [223].Wen J, Yang K, Liu F, Li H, Xu Y, Sun S, Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems, Chem. Soc. Rev 46 (2017) 6024–6045. [DOI] [PubMed] [Google Scholar]
- [224].Ahmadi E, Dehghannejad N, Hashemikia S, Ghasemneja M, Tabedordbar H, Synthesis and surface modification of mesoporous silica nanoparticles and its application as carriers for sustained drug delivery, Drug Deliv 21 (2014) 3. [DOI] [PubMed] [Google Scholar]
- [225].de Oliveira JFA, Scheffer F, Landis RF, Teixeira-Neto E, Rotello VM, Cardoso MB, Dual Functionalization of Nanoparticles for Generating Corona-Free and Non-Cytotoxic Silica Nanoparticles, ACS Appl. Mater. Interfaces 10 (2018) 41917–41923. [DOI] [PubMed] [Google Scholar]
- [226].He Q, Zhang J, Chen F, Guo L, Zhu Z, Shi J, An anti-ROS/hepatic fibrosis drug delivery system based on savianolic acid B loaded mesoporous silica nanoparticles, Biomaterials 31 (2010) 7785– 7796. [DOI] [PubMed] [Google Scholar]
- [227].Xia T, Kovochich M, Liong M, Meng H, Kabehie S, George S, Zink JI, Nel AE, Polyethyleneimine Coating Enhances the Cellular Uptake of Mesoporous Silica Nanoparticles and Allows Safe Delivery of siRNA and DNA Constructs, ACS Nano 3 (2019) 3273–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Kapilov-Buchman Y, Lellouche E, Michaeli S, Lellouche JP, Unique surface modification of silica nanoparticles with polyethylenimine (PEI) for siRNA delivery using cerium cation coordination chemistry, Bioconjug. Chem 26 (2015) 880–889. [DOI] [PubMed] [Google Scholar]
- [229].Radu DR, Lai CY, Jeftinjia K, Rowe EW, Jeftinjia S, Lin VSY, A Polyamidoamine Dendrimer-Capped Mesoporous Silica Nanosphere-Based Gene Transfection Reagent, J. Am. Chem. Soc 126 (2004) 13216–13217. [DOI] [PubMed] [Google Scholar]
- [230].Liu J, Naughton AS, Brinker CJ, Silica nanoparticle supported lipid bilayers for gene delivery, Chem. Commun 34 (2009) 5100–5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Dogra P, Adolphi NL, Wang Z, Lin Y-S, Butler KS, Durfee PN, Croissant JG, Noureddine A, Coker EN, Bearer EL, Cristini V, Brinker CJ. Establishing the effects of mesoporous silica nanoparticle properties on in vivo disposition using imaging-based pharmacokinetics, Nat. Commun 9 (2018) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Cheng CA, Deng T, Lin FC, Cai Y, Zink JI, Supramolecular Nanomachines as Stimuli-Responsive Gatekeepers on Mesoporous Silica Nanoparticles for Antibiotic and Cancer Drug Delivery, Theranostics 9 (2019), 3341–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Mura S, Nicolas J, Couvreur P, Stimuli-responsive nanocarriers for drug delivery, Nature Materials. 12 (2013) 991–1003. [DOI] [PubMed] [Google Scholar]
- [234].Park C, Lee K, Kim C, Photoresponsive Cyclodextrin-Covered Nanocontainers and Their Sol-Gel Transition Induced by Molecular Recognition, Angew. Chem 48 (2009) 1275–1278. [DOI] [PubMed] [Google Scholar]
- [235].Yuan Q, Zhang Y, Chen T, Zhao Z, Zhang X, Li Z, Yan C-H, Tan W, Photon-Manipulated Drug Release from a Mesoporous Nanocontainer Controlled by Azobenzene-Modified Nucleic Acid, ACS Nano 6 (2012) 6337–6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Tarn D, Ferris DP, Barnes JC, Ambrogio MW, Stoddart JF, Zink JI, A reversible light-operated nanovalve on mesoporous silica nanoparticles, Nanoscale 6 (2014) 3335–3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Zhang P, He Z, Wang C, Chen J, Zhao J, Zhu X, Li C-Z, Min Q, Zhu J-J, In Situ Amplification of Intracellular MicroRNa with MNAzyme Nanodevices for Multiplexed Imaging, Logic Operation, and Controlled Drug Release, ACS Nano 9 (2015) 789–798. [DOI] [PubMed] [Google Scholar]
- [238].Zhang P, Cheng F, Zhou R, Cao J, Li J, Burda C, Min Q, Zhu J-J, DNA-Hybrid-Gated Multifuctional Mesoporous Silica Nanocarriers for Dual-Targeted and MicroRNA-Responsive Controlled Drug Delivery, Angew. Chem 53 (2014) 2371–2375. [DOI] [PubMed] [Google Scholar]
- [239].Tang H, Zhao W, Yu J, Li Y, Zhao C, Recent development of pH-responsive polymers for cancer nanomedicine, Molecules 24 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Gupta A, Das R, Yesilbag Tonga G, Mizuhara T, Rotello VM, Charge-Switchable Nanozymes for Bioorthogonal Imaging of Biofilm-Associated Infections, ACS Nano 12 (2018) 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Deng Z, Zhen Z, Hu X, Wu S, Xu Z, Chu PK, Hollow chitosan-silica nanospheres as pH-sensitive targeted delivery carriers in breast cancer therapy, Biomaterials 32 (2011) 4976–4986. [DOI] [PubMed] [Google Scholar]
- [242].Kauscher U, Holme MN, Björnmalm M, Stevens MM, Physical stimuli-responsive vesicles in drug delivery: Beyond liposomes and polymersomes, Adv. Drug Deliv. Rev 138 (2019) 259–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Paris JL, Cabanas MV, Manzano M, Vallet-Regi M, Polymer-Grafted Mesoporous Silica Nanoparticles as Ultrasound-Responsive Drug Carriers, ACS Nano 9 (2015) 11023–11033. [DOI] [PubMed] [Google Scholar]
- [244].Cheng CA, Chen W, Zhang L, Wu HH, Zink JI, A Responsive Mesoporous Silica Nanoparticle Platform for Magnetic Resonance Imaging-Guided High-Intensity Focused Ultrasound-Stimulated Cargo Delivery with Controllable Location, Time, and Dose, J. Am. Chem. Soc 141 (2019) 17670–17684. [DOI] [PubMed] [Google Scholar]
- [245].Kempen PJ, Greasley S, Parker KA, Campbell JL, Chang HY, Jones JR, Sinclair R, Gambhir SS, Jokerst JV, Theranostic Mesoporous Silica Nanoparticles Biodegrade after Pro-Survival Drug Delivery and Ultrasound/Magnetic Resonance Imaging of Stem Cells, Theranostics 5 (2015) 631–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Lv Y, Cao Y, Li P, Liu J, Chen H, Hu W, Zhang L, Ultrasound-Triggered Destruction of Folate-Functionalized Mesoporous Silica Nanoparticle-Loaded Microbubble for Targeted Tumor Therapy, Adv. Healthc. Mater 6 (2017). [DOI] [PubMed] [Google Scholar]
- [247].Wen J, Yang K, Liu F, Li H, Xu Y, Sun S, Diverse gatekeepers for mesoporous silica nanoparticle based drug delivery systems, Chem. Soc. Rev 46 (2017) 6024–6045. [DOI] [PubMed] [Google Scholar]
- [248].Linsley CS, Wu BM, Recent advances in light-responsive on-demand drug-delivery systems, Ther. Deliv 8 (2017) 89–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Wang M, Wang T, Wang D, Jiang W, Fu J, Acid and light stimuli-responsive mesoporous silica nanoparticles for controlled release, J. Mater. Sci 54 (2019) 6199–6211. [Google Scholar]
- [250].Ash C, Dubec M, Donne K, Bashford T, Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods, Lasers Med. Sci 32 (2017) 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Liu J, Bu W, Pan L, Shi J, NIR-Triggered Anticancer Drug Delivery by Upconverting Nanoparticles with Integrated Azobenzene-Modified Mesoporous Silica, Angew. Chem 52 (2013) 4375–4379. [DOI] [PubMed] [Google Scholar]
- [252].Thomas CR, Ferries DP, Lee J-H, Choi E, Cho MH, Kim ES, Stoddart JF, Shin J-S, Cheon J, Zink JI, Noninvasive Remote-Controlled Release of Drug Molecules in Vitro Using Magnetic Actuation of Mechanized Nanoparticles, J. Am. Chem. Soc 132 (2010) 10623–10625. [DOI] [PubMed] [Google Scholar]
- [253].Omar H, Croissant JG, Alamoudi K, Alsaiari S, Alradwan I, Majrashi MA, Anjum DH, Martins P, Laamarti R, Eppinger J, Moosa B, Almalik A, Kashab NM, Biodegradable Magnetic Silica@Iron Oxide Nanovectors with Ultra-Large Mesopores for High Protein Loading, Magnetothermal Release, and Delivery, Journal of Controlled Release 259 (2017) 187–194. [DOI] [PubMed] [Google Scholar]
- [254].Xiong L, Bi J, Tang Y, Qiao S-Z, Magnetic Core-Shell Silica Nanoparticles with Large Radial Mesopores for siRNA Delivery, Small 12 (2016) 4735–4742. [DOI] [PubMed] [Google Scholar]
- [255].Chen Y, Wang X, Liu T, Zhang DS, Wang Y, Gu H, Di W, Highly effective antiangiogenesis via magnetic mesoporous silica-based siRNA vehicle targeting the VEGF gene for orthotopic ovarian cancer therapy, Int. J. Nanomedicine 10 (2015) 2579–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Bharti C, Gulati N, Nagaich U, Pal A, Mesoporous silica nanoparticles in target drug delivery system: A review, Int. J. Pharm. Investig 5 (2015) 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Huang X, Zhuang J, Teng X, Li L, Chen D, Yan X, Tang F, The promotion of human malignant melanoma growth by mesoporous silica nanoparticles through decreased reactive oxygen species, Biomaterials 31 (2010) 6142–6153. [DOI] [PubMed] [Google Scholar]
- [258].Paris JL, Baeza A, Vallet-Regí M, Overcoming the stability, toxicity, and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics, Expert Opin. Drug Deliv 16 (2019) 1095–1112. [DOI] [PubMed] [Google Scholar]
- [259].Croissant JG, Fatieiev Y, Khashab NM, Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles, Adv. Mater 29 (2017) 1604634. [DOI] [PubMed] [Google Scholar]
- [260].Yu L, Chen Y, Lin H, Du W, Chen H, Shi J, Ultrasmall mesoporous organosilica nanoparticles: Morphology modulations and reDox-responsive biodegradability for tumor-specific drug delivery, Biomater 161 (2018) 292–305. [DOI] [PubMed] [Google Scholar]
- [261].Maggini L, Cabrera I, Ruiz-Carretero A, Prasetyanto EA, Robinet E, De Cola L, Breakable mesoporous silica nanoparticles for targeted drug delivery, Nanoscale 13 (2016) 7240–7247. [DOI] [PubMed] [Google Scholar]
- [262].Prasetyanto EA, Bertucci A, Septiadi D, Corradini R, Castro-Hartmann P, De Cola L, Breakable Hybrid Organosilica Nanocapsules for Protein Delivery, Angew. Chem 55 (2016) 3323–3327. [DOI] [PubMed] [Google Scholar]
- [263].Partain BD, Unni M, Rinaldi C, Allen KD, The clearance and biodistribution of magnetic composite nanoparticles in healthy and osteoarthritic rat knees, J. Control. Release 321 (2020) 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Hufschmid R, Teeman E, Mehdi BL, Krishnan KM, Browning ND, Observing the colloidal stability of iron oxide nanoparticles in situ, Nanoscale 11 (2019) 13098–13107. [DOI] [PubMed] [Google Scholar]
- [265].Azcona P, Zysler R, Lassalle V, Simple and novel strategies to achieve shape and size control of magnetite nanoparticles intended for biomedical applications, Colloids Surfaces A Physicochem. Eng. Asp 504 (2016) 320–330. [Google Scholar]
- [266].Illés E, Szekeres M, Kupcsik E, Tóth IY, Farkas K, Jedlovszky-Hajdú A, Tombácz E, PEGylation of surfacted magnetite core-shell nanoparticles for biomedical application, Colloids Surfaces A Physicochem. Eng. Asp 460 (2014) 429–440. [Google Scholar]
- [267].Múzquiz-Ramos EM, Guerrero-Chávez V, Macías-Martínez BI, López- Badillo CM, García-Cerda LA, Synthesis and characterization of maghemite nano- particles for hyperthermia applications, Ceram. Int 41 (2015) 397–402. [Google Scholar]
- [268].Silva MF, Winkler Hechenleitner AA, de Oliveira DMF, Agüeros M, Peñalva R, Irache JM, et al. , Optimization of maghemite-loaded PLGA nanospheres for biomedical applications, Eur. J. Pharm. Sci 49 (2013) 343–351. [DOI] [PubMed] [Google Scholar]
- [269].McBain SC, Yiu HHP, Dobson J, Magnetic nanoparticles for gene and drug delivery, Int. J. Nanomedicine, 3 (2008) 169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Guo S, Li D, Zhang L, Li J, Wang E, Monodisperse mesoporous superparamagnetic single-crystal magnetite nanoparticles for drug delivery, Biomaterials 30 (2009) 1881–1889. [DOI] [PubMed] [Google Scholar]
- [271].Huang Z, Tang F, Preparation, structure, and magnetic properties of mesoporous magnetite hollow spheres, J. Colloid Interface Sci 281 (2005) 432–436. [DOI] [PubMed] [Google Scholar]
- [272].He WL, Feng Y, Li XL, Wei YY, Yang XE, Availability and toxicity of Fe(II) and Fe(III) in Caco-2 cells, J. Zhejiang Univ. Sci. B 9 (2008) 707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Glover AL, Bennett JB, Pritchett JS, Nikles SM, Nikles DE, Nikles JA, Brazel CS, Magnetic Heating of Iron Oxide Nanoparticles and Magnetic Micelles for Cancer Therapy, IEEE Trans. Magn 49 (2013) 231–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Hu Y, Hu H, Yan J, Zhang C, Li Y, Wang M, Tan W, Liu J, Pan Y, Multifunctional Porous Iron Oxide Nanoagents for MRI and Photothermal/Chemo Synergistic Therapy, Bioconjug. Chem 29 (2018) 1283–1290. [DOI] [PubMed] [Google Scholar]
- [275].El-Sherbiny IM, El-Sayed M, Reda A, Superparamagnetic Iron Oxide Nanoparticles (SPIONs) as Multifunctional Cancer Theranostics, Magnet. Nanoheterostruct (2020) 223–241 [Google Scholar]
- [276].Orel VE, Tselepi M, Mitrelias T, Zabolotny M, Shevchenko A, Rykhalskyi A, Orel VB, Burlaka A, Lukin S, Kyiashko V, Barnes CHW, The comparison between superparamagnetic and ferromagnetic iron oxide nanoparticles for cancer nanotherapy in the magnetic resonance system, Nanotechnology 30 (2019) 415701. [DOI] [PubMed] [Google Scholar]
- [277].Billotey C, Wilhelm C, Devaud M, Bacri JC, Bittoun J, Gazeau F, Cell internalization of anionic maghemite nanoparticles: Quantitative effect on magnetic resonance imaging, Magn. Reson. Med 49 (2003) 646–654. [DOI] [PubMed] [Google Scholar]
- [278].Ashraf S, Taylor A, Sharkey J, Barrow M, Murray P, Wilm B, Poptani H, Rosseinsky MJ, Adams DJ, Lévy R, in vivo fate of free and encapsulated iron oxide nanoparticles after injection of labelled stem cells, Nanoscale Adv 1 (2019) 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Fortin JP, Gazeau F, Wilhelm C, Intracellular heating of living cells through Néel relaxation of magnetic nanoparticles, Eur. Biophys. J 37 (2008) 223–228. [DOI] [PubMed] [Google Scholar]
- [280].Abenojar EC, Wickramasinghe S, Bas-Concepcion J, Samia ACS, Structural effects on the magnetic hyperthermia properties of iron oxide nanoparticles, Prog. Nat. Sci. Mater. Int 26 (2016) 440–448. [Google Scholar]
- [281].Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB, Selective inductive heating of lymph nodes, Ann. Surg 146 (1957) 596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].GIUSTINI AJ, PETRYK AA, CASSIM SM, TATE JA, BAKER I, HOOPES PJ, MAGNETIC NANOPARTICLE HYPERTHERMIA IN CANCER TREATMENT, Nano Life 01 (2010) 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Nguyen TK, Duong HTT, Selvanayagam R, Boyer C, Barraud N, Iron oxide nanoparticle-mediated hyperthermia stimulates dispersal in bacterial biofilms and enhances antibiotic efficacy, Sci. Rep 5 (2015) 18385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Gul S, Khan SB, Rehman IU, Khan MA, Khan MI, A Comprehensive Review of Magnetic Nanomaterials Modern Day Theranostics, Front. Mater 6 (2019) 179. [Google Scholar]
- [285].Mamiya H, Jeyadevan B, Hyperthermic effects of dissipative structures of magnetic nanoparticles in large alternating magnetic fields, Sci. Rep 1 (2011) 157–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Chatterjee DK, Diagaradjane P, Krishnan S, Nanoparticle-mediated hyperthermia in cancer therapy, Ther. Deliv 2 (2011) 1001–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Soleymani M, Khalighfard S, Khodayari S, Khodayari H, Kalhori MR, Hadjighassem MR, Shaterabadi Z, Alizadeh AM, Effects of multiple injections on the efficacy and cytotoxicity of folate-targeted magnetite nanoparticles as theranostic agents for MRI detection and magnetic hyperthermia therapy of tumor cells, Sci. Rep 10 (2020) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Mérida F, Rinaldi C, Juan EJ, Torres-Lugo M, In vitro ultrasonic potentiation of 2-phenylethynesulfonamide/magnetic fluid hyperthermia combination treatments for ovarian cancer, Int. J. Nanomedicine 15 (2020) 419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Rivera-Rodriguez A, Chiu-Lam A, Morozov VM, Ishov AM, Rinaldi C, Magnetic nanoparticle hyperthermia potentiates paclitaxel activity in sensitive and resistant breast cancer cells, Int. J. Nanomedicine 13 (2018) 4771–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Court KA, Hatakeyama H, Wu SY, Lingegowda MS, Rodríguez-Aguayo C, López-Berestein G, Ju-Seog L, Rinaldi C, Juan EJ, Sood AK, Torres-Lugo M, HSP70 inhibition synergistically enhances the effects of magnetic fluid hyperthermia in ovarian cancer, Mol. Cancer Ther 16 (2017) 966–976. [DOI] [PubMed] [Google Scholar]
- [291].Albarqi HA, Wong LH, Schumann C, Sabei FY, Korzun T, Li X, Hansen MN, Dhagat P, Moses AS, Taratula O, Taratula O, Biocompatible Nanoclusters with High Heating Efficiency for Systemically Delivered Magnetic Hyperthermia, ACS Nano 13 (2019) 6383–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Tay ZW, Chandrasekharan P, Chiu-Lam A, Hensley DW, Dhavalikar R, Zhou XY, Yu EY, Goodwill PW, Zheng B, Rinaldi C, Conolly SM, Magnetic particle imaging-guided heating in vivo using gradient fields for rbitary localization of magnetic hyperthermia therapy, ACS Nano 12 (2018) 3699–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Sivakumar B, Aswathy RG, Romero-Aburto R, Mitcham T, Mitchel KA, Nagaoka Y, Bouchard RR, Ajayan PM, Maekawa T, Sakthikumar DN, Highly versatile SPION encapsulated PLGA nanoparticles as photothermal ablators of cancer cells and as multimodal imaging agents, Biomater. Sci 5 (2015) 432–443. [DOI] [PubMed] [Google Scholar]
- [294].Alkilany AM, Abulateefeh SR, Murphy CJ, Facile Functionalization of Gold Nanoparticles with PLGA Polymer Brushes and Efficient Encapsulation into PLGA Nanoparticles: Toward Spatially Precise Bioimaging of Polymeric Nanoparticles, Part. Part. Syst. Charact 36 (2018) 1800414. [Google Scholar]
- [295].Bogart LK, Pourroy G, Murphy CJ, Puntes V, Pellegrino T, Rosenblum D, Peer D, Lévy R, Nanoparticles for imaging, sensing, and therapeutic intervention, ACS Nano 8 (2014) 3107–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Liu G, Deng J, Liu F, Wang Z, Peer D, Zhao Y, Hierarchical theranostic nanomedicine: MRI contrast agents as a physical vehicle anchor for high drug loading and triggered on-demand delivery, J. Mater. Chem. B 6 (2018) 1995–2003. [DOI] [PubMed] [Google Scholar]
- [297].El-Boubbou K, Ali R, Bahhari HM, Alsaad KO, Nehdi A, Boudjelal M, Alkushi A, Magnetic Fluorescent Nanoformulation for Intracellular Drug Delivery to Human Breast Cancer, Primary Tumors, and Tumor Biopsies: Beyond Targeting Expectations, Bioconjug. Chem 27 (2016) 1471–1483. [DOI] [PubMed] [Google Scholar]
- [298].Manigandan A, Handi V, Sundaramoorthy NS, Dhandapani R, Radhakrishnan J, Sethuraman S, Subramanian A, Responsive Nanomicellar Theranostic Cages for Metastatic Breast Cancer, Bioconjug. Chem 29 (2018) 275–286. [DOI] [PubMed] [Google Scholar]
- [299].Widder KJ, Senyei AE, Scarpelli GD, Magnetic microspheres: a model system of site specific drug delivery in vivo, Proc. Soc. Exp. Biol. Med 158 (1978) 141–6. [DOI] [PubMed] [Google Scholar]
- [300].Senyei A, Widder K, Czerlinski G, Magnetic guidance of drug-carrying microspheres, J. Appl. Phys 49 (1978) 3578–3583. [Google Scholar]
- [301].Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergermann C, Erhardt W, Wagenpfeil S, Lübbe AS, Locoregional cancer treatment with magnetic drug targeting, Cancer Res 60 (2000) 6641–8. [PubMed] [Google Scholar]
- [302].Lübbe AS, Alexiou C, Bergermann C, Clinical applications of magnetic drug targeting, J. Surg Res 95 (2001) 200–6. [DOI] [PubMed] [Google Scholar]
- [303].Fuller EG, Scheutz GM, Jimenez A, Lewis P, Savliwala S, Liu S, Sumerlin BS, Rinaldi C, Theranostic nanocarriers combining high drug loading and magnetic particle imaging, Int. J. Pharm 572 (2019) 118796. [DOI] [PubMed] [Google Scholar]
- [304].Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L, Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases, J. Control. Release 235 (2016) 34–47. [DOI] [PubMed] [Google Scholar]
- [305].Pulfer SK, Ciccotto SL, Gallo JM, Distribution of small magnetic particles in brain tumor-bearing rats, J. Neurooncol 41(1999), 99–105. [DOI] [PubMed] [Google Scholar]
- [306].Vangijzegem T, Stanicki D, Laurent S, Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics, Expert Opin. Drug Deliv 16 (2019) 69–78. [DOI] [PubMed] [Google Scholar]
- [307].Alexiou C, Arnold W, Klein RJ, Parak FG, Hulin P, Bergermann C, Erhardt W, Wagenpfeil S, Lübbe AS, Locoregional cancer treatment with magnetic drug targeting, Cancer. Res 60 (2000) 6641–8. [PubMed] [Google Scholar]
- [308].Lübbe AS, Alexiou C, Bergermann C, Clinical applications of magnetic drug targeting, J. Surg Res 95 (2001) 200–6. [DOI] [PubMed] [Google Scholar]
- [309].Unterweger H, Tietze R, Janko C, Zaloga J, Lyer S, Dürr S, Taccardi N, Goudouri O-M, Hoppe A, Eberbeck D, Schubert DW, Boccaccini AR, Alexiou C, Development and characterization of magnetic iron oxide nanoparticles with a cisplatin-bearing polymer coating for targeted drug delivery, Int. J. Nanomedicine 9 (2014) 3659–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Yu MK, Jeong YY, Park J, Park S, Kim JW, Min JJ, Kim K, Jon S, Drug-loaded superparamagnetic iron oxide nanoparticles for combined cancer imaging and therapy in vivo, Angew. Chem. Int. Ed 47 (2008) 5362–5365. [DOI] [PubMed] [Google Scholar]
- [311].Jeon H, Kim J, Lee YM, Kim J, Choi HW, Lee J, Park H, Kang Y, Kim IS, Lee BH, Hoffman AS, Kim WJ, Poly-paclitaxel/cyclodextrin-SPION nano-assembly for magnetically guided drug delivery system, J. Control Release 231 (2016) 68–76 [DOI] [PubMed] [Google Scholar]
- [312].Magro M, Martinello T, Bonaiuto E, Gomiero C, Baratella D, Zoppellaro G, Cozza G, Patruno M, Zboril R, Vianello F, Covalently bound DNA on naked iron oxide nanoparticles: Intelligent colloidal nano-vector for cell transfection, Biochim. Biophys. Acta - Gen. Subj 1861 (2017) 2802–2810. [DOI] [PubMed] [Google Scholar]
- [313].Galli M, Guerrini A, Cauteruccio S, Thakare P, Dova D, Orsini F, Arosio P, Carrara C, Sangregorio C, Lascialfari A, Maggioni D, Licandro E, Superparamagnetic iron oxide nanoparticles functionalized by peptide nucleic acids, RSC Adv 7 (2017) 15500–15512. [Google Scholar]
- [314].Steinfeld U, Pauli C, Kaltz N, Bergemann C, Lee HH, T lymphocytes as potential therapeutic drug carrier for cancer treatment, Int. J. Pharm 311 (2006) 229–236. [DOI] [PubMed] [Google Scholar]
- [315].Kettering M, Zorn H, Bremer-Streck S, Oehring H, Zeisberger M, Bergemann C, Hergt R, Halbhuber K-J, Kaiser WA, Hilger I, Characterization of iron oxide nanoparticles adsorbed with cisplatin for biomedical applications, Phys. Med. Biol 54 (2009) 5109–5121. [DOI] [PubMed] [Google Scholar]
- [316].Sunderland CJ, Steiert M, Talmadge JE, Derfus AM, Barry SE, Targeted nanoparticles for detecting and treating cancer, Drug Dev. Res 67 (2006) 70–93. [Google Scholar]
- [317].Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, Gänsbacher B, Plank C, Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo, Gene Ther 9 (2002) 102–9. [DOI] [PubMed] [Google Scholar]
- [318].Gersting SW, Schillinger U, Lausier J, Nicklaus P, Rudolph C, Plank C, Reinhardt D, Rosenecker J, Gene delivery to respiratory epithelial cells by magnetofection, J. Gene. Med 6 (2004) 913–22. [DOI] [PubMed] [Google Scholar]
- [319].Krötz F, de Wit C, Söhn HY, Zahler S, Gloe T, Pohl U, Plank C, Magnetofection-a highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo, Mol. Ther 7 (2003) 700–10. [DOI] [PubMed] [Google Scholar]
- [320].Yang Z, Duan J, Wang J, Liu Q, Shang R, Yang X, Lu P, Xia C, Wang L, Dou K, Superparamagnetic iron oxide nanoparticles modified with polyethylenimine and galactose for siRNA targeted delivery in hepatocellular carcinoma therapy, Int. J. Nanomedicine 13 (2018) 1851–1865. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [321].Kievit FM, Veiseh O, Bhattarai N, Fang C, Gunn JW, Lee D, Ellenbogen RG, Olson JM, Zhang M, PEI–PEG–Chitosan-Copolymer-Coated Iron Oxide Nanoparticles for Safe Gene Delivery: Synthesis, Complexation, and Transfection, Adv. Funct. Mater 24 (2009) 2244–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Prijic S, Prosen L, Cemazar M, Scancar J, Romih R, Lavrencak J, Bregar VB, Coer A, Krzan M, Znidarsic A, Sersa G, Surface modified magnetic nanoparticles for immuno-gene therapy of murine mammary adenocarcinoma, Biomater 33 (2012) 4379–4391. [DOI] [PubMed] [Google Scholar]
- [323].Jiang S, Eltoukhy AA, Love KT, Langer R, Anderson DG, Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery, Nano Lett 13 (2013) 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [324].Clauson RM, Chen M, Scheetz LM, Berg B, Chertok B, Size-Controlled Iron Oxide Nanoplatforms with Lipidoid-Stabilized Shells for Efficient Magnetic Resonance Imaging-Trackable Lymph Node Targeting and High-Capacity Biomolecule Display, ACS Appl. Mater. Interfaces 10 (2018) 20281–20295. [DOI] [PubMed] [Google Scholar]
- [325].Yan F, Xu H, Anker J, Kopelman R, Ross B, Rehemtulla A, Reddy R, Synthesis and characterization of silica-embedded iron oxide nanoparticles for magnetic resonance imaging, J. Nanosci. Nanotechnol 4 (2004) 72–76. [DOI] [PubMed] [Google Scholar]
- [326].Omar H, Croissant JG, Alamoudi K, Alsaiari S, Alradwan I, Majrashi MA, Anjum DH, Martins P, Laamarti R, Eppinger J, Moosa B, Almalik A, Khashab NM, Biodegradable magnetic silica@iron oxide nanovectors with ultra-large mesopores for high protein loading, magnetothermal release, and delivery, J. Control Release 259 (2017) 187–194. [DOI] [PubMed] [Google Scholar]
- [327].Chang D, Lim M, Goos JACM, Qiao R, Ng YY, Mansfeld FM, Jackson M, Davis TP, Kavallaris M, Biologically targeted magnetic hyperthermia: Potential and limitations, Front. Pharmacol 9 (2018) 831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Jordan A, Scholz R, Wust P, Fahling H, Felix R, Magnetic fluid hyperthermia (MFH): cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles, J. Magn. Magn. Mater 201 (1999) 413–419. [Google Scholar]
- [329].Dutz S, Hergt R, Magnetic nanoparticle heating and heat transfer on a microscale: basic principles, realities and physical limitations of hyperthermia for tumour therapy, Int. J. Hyperther 29 (2013) 790–800. [DOI] [PubMed] [Google Scholar]
- [330].Obaidat IM, Issa B, Haik Y, Magnetic properties of magnetic nanoparticles for efficient hyperthermia, Nanomater 5 (2015) 63–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Cass ND, Honce JM, O’Dell AL, Gubbels SP, First MRI with new cochlear implant with rotatable internal magnet system and proposal for standardization of reporting magnet-related artifact size, Otol. Neurotol 40 (2019) 883–891. [DOI] [PubMed] [Google Scholar]
- [332].Fernández-Pacheco R, Valdivia JG, Ibarra MR, Magnetic Nanoparticles for Local Drug Delivery Using Magnetic Implants, Micro and Nano Technol. In Bioanal 544 (2009) 559–569. [DOI] [PubMed] [Google Scholar]
- [333].Mahmoudi M, Hofmann H, Rothern-Rutishauser B, Petri-Fink A, Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles, Chem. Rev 112 (2012) 2323–2338. [DOI] [PubMed] [Google Scholar]
- [334].Pisanic II TR, Blackwell JD, Shubayev VI, Finones RR, Jin S, Nanotoxicity of iron oxide nanoparticle internalization in growing neurons, Biomater 28 (2007) 2572–2581. [DOI] [PubMed] [Google Scholar]
- [335].Solanki A, Kim JD, Lee KB, Nanotechnology for regenerative medicine: nanomaterials for stem cell imaging, Nanomedicine 3 (2008) 567–78. [DOI] [PubMed] [Google Scholar]
- [336].Patil RM, Thorat ND, Shete PB, Bedge PA, Gavde S, Joshi MG, Tofail SAM, Bohara RA, Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles, Biochem. Biophys. Reports 13 (2018) 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Arami H, Khandhar A, Liggitt D, Krishnan KM, In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles, Chem. Soc. Rev 44 (2015) 8576–8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Weissleder R A clearer vision for in vivo imaging, Nat. Biotechnol 19 (2001) 316–317. [DOI] [PubMed] [Google Scholar]
- [339].Li C, Liu J, Alonso S, Li F, Zhang Y, Upconversion nanoparticles for sensitive and in-depth detection of Cu 2+ ions, Nanoscale 4 (2012) 6065–6071. [DOI] [PubMed] [Google Scholar]
- [340].Auzel F, Upconversion and Anti-Stokes Processes with f and d Ions in Solids, Chem. Rev 104 (2004) 139–173. [DOI] [PubMed] [Google Scholar]
- [341].Li H, Hao S, Yan C, Chen G, Synthesis of multicolor core/shell NaLuF4:Yb3+/Ln3+ @CaF2 upconversion nanocrystals, Nanomaterials 7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [342].Wang C, Cheng L, Liu Z, Upconversion Nanoparticles for Photodynamic Therapy and Other Cancer Therapeutics, Theranostics 3 (2013) 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [343].Wen S, Zhou J, Zheng K, Bednarkiewicz A, Liu X, Jin D, Advances in highly doped upconversion nanoparticles, Nat. Commun 9 (2018) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [344].Yang Y, Velmurugan B, Liu X, Xing B, NIR photoresponsive crosslinked upconverting nanocarriers toward selective intracellular drug release, Small 9 (2013) 2937–2944. [DOI] [PubMed] [Google Scholar]
- [345].Dai Y, Xiao H, Liu J, Yuan Q, Ma P, Yang D, Li C, Cheng Z, Hou Z, Yang P, Lin J, In vivo multimodality imaging and cancer therapy by near-infrared light-triggered trans -platinum pro-drug-conjugated upconverison nanoparticles, J. Am. Chem. Soc 135 (2013) 18920–18929. [DOI] [PubMed] [Google Scholar]
- [346].Carling CJ, Nourmohammadian F, Boyer JC, Branda NR, Remote-control photorelease of caged compounds using near-infrared light and upconverting nanoparticles, Angew. Chemie - Int. Ed 49 (2010) 3782–3785. [DOI] [PubMed] [Google Scholar]
- [347].Yang Y, Liu F, Liu X, Xing B, NIR light controlled photorelease of siRNA and its targeted intracellular delivery based on upconversion nanoparticles, Nanoscale 5 (2013) 231–238. [DOI] [PubMed] [Google Scholar]
- [348].Zhang L, Lu Z, Bai Y, Wang T, Wang Z, Chen J, Ding Y, Yang F, Xiao Z, Ju S, Zhu J, He N, PEGylated denatured bovine serum albumin modified water-soluble inorganic nanocrystals as multifunctional drug delivery platforms, J. Mater. Chem. B 1 (2013) 1289–1295. [DOI] [PubMed] [Google Scholar]
- [349].Zhao L, Peng J, Huang Q, Li C, Chen M, Sun Y, Lin Q, Zhu L, Li F, Near-Infrared Photoregulated Drug Release in Living Tumor Tissue via Yolk-Shell Upconversion Nanocages, Adv. Funct. Mater 24 (2014) 363–371. [Google Scholar]
- [350].Dai Y, Ma P, Cheng Z, Kang X, Zhang X, Hou Z, Li C, Yang D, Zhai X, Lin J, Up-Conversion Cell Imaging and pH-Induced Thermally Controlled Drug Release from NaYF4:Yb3+/Er3+@ Hydrogel Core−Shell Hybrid Microspheres, ACS Nano 6 (2012) 3327–3338. [DOI] [PubMed] [Google Scholar]
- [351].Wong PT, Tang S, Cannon J, Chen D, Sun R, Lee J, Phan J, Tao K, Sun K, Chen B, Photocontrolled Release of DOX Conjugated through a Thioacetal Photocage in Folate-Targeted Nanodelivery Systems, Bioconjug. Chem 28 (2017) 3016–3028. [DOI] [PubMed] [Google Scholar]
- [352].Michael Dcona M, Yu Q, Capobianco JA, Hartman MCT, Near infrared light mediated release of Doxorubicin using upconversion nanoparticles, Chem. Commun 51 (2015) 8477–8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Ruggiero E, Garino C, Mareque-Rivas JC, Habtemariam A, Salassa L, Upconverting Nanoparticles Prompt Remote Near-Infrared Photoactivation of Ru(II)-Arene Complexes, Chem. - A Eur. J 22 (2016) 2801–2811. [DOI] [PubMed] [Google Scholar]
- [354].Aujard I, Benbrahim C, Gouget M, Ruel O, Baudin JB, Neveu P, Jullien L, O-nitrobenzyl photolabile protecting groups with red-shifted absorption: Syntheses and uncaging cross-sections for one- And two-photon excitation, Chem. - A Eur. J 12 (2006) 6865–6879. [DOI] [PubMed] [Google Scholar]
- [355].Fedoryshin LL, Tavares AJ, Petryayeva E, Doughan S, Krull UJ, Near-infrared-triggered anticancer drug release from upconverting nanoparticles, ACS Appl. Mater. Interfaces 6 (2014) 13600–13606. [DOI] [PubMed] [Google Scholar]
- [356].Michael Dcona M, Yu Q, Capobianco JA, Hartman MCT, Near infrared light mediated release of Doxorubicin using upconversion nanoparticles, Chem. Commun 51 (2015) 8477–8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [357].Dai Y, Yang D, Ma P, Kang X, Zhang X, Li C, Hou Z, Cheng Z, Lin J, Doxorubicin conjugated NaYF4: Yb3+/Tm3+ Nanoparticles for therapy and sensing of drug delivery by luminescence resonance energy transfer, Biomaterials 33 (2012) 8704–8713. [DOI] [PubMed] [Google Scholar]
- [358].Yang D, Dai Y, Liu J, Zhou Y, Chen Y, Li C, Ma P, Lin J, Ultra-small BaGdF5-based upconversion nanoparticles as drug carriers and multimodal imaging probes, Biomaterials 35 (2014) 2011–2023. [DOI] [PubMed] [Google Scholar]
- [359].Jiang S, Zhang Y, Lim KM, Sim EKW, Ye L, NIR-to-visible upconversion nanoparticles for fluorescent labeling and targeted delivery of siRNA., Nanotechnology 20 (2009) 155101. [DOI] [PubMed] [Google Scholar]
- [360].Zhang D, Wen L, Huang R, Wang H, Hu X, Xing D, Mitochondrial Specific Photodynamic Therapy by Rare-Earth Nanoparticles Mediated Near-Infrared Graphene Quantum Dots. Biomaterials 153 (2018) 14–26. [DOI] [PubMed] [Google Scholar]
- [361].Liang L, Lu Y, Zhang R, Care A, Ortega TA, Deyev SM, Qian Y, Zvyagin AV, Deep-Penetrating Photodynamic Therapy with KillerRed Mediated by Upconversion Nanoparticles. Acta Biomater 51 (2017) 461–470. [DOI] [PubMed] [Google Scholar]
- [362].Tajon CA, Yang H, Tian B, Tian Y, Ercius P, Schuck PJ, Chan M, Cohen BE, Photostable and efficient upconverting nanocrystal-based chemical sensors. Optical Mater 84 (2019) 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [363].Gulzar A, Xu J, Yang D, Xu L, He F, Gai S, Yang P, Nano-Graphene Oxide-UCNP-Ce6 Covalently Constructed Nanocomposites for NIR-Mediated Bioimaging and PTT/PDT Combinatorial Therapy, Dalt. Trans 47 (2018) 3931–3939. [DOI] [PubMed] [Google Scholar]
- [364].Dhal S, Pal K, Banerjee I, Giri S, Upconversion Nanoparticle Incorporated Oleogel as Probable Skin Tissue Imaging Agent. Chem. Eng. J 379 (2020) 122272. [Google Scholar]
- [365].Karimi M, Sahandi Zangabad P, Baghaee-Ravari S, Ghazadeh M, Mirshekari H, Hamblin MR, Smart Nanostructures for Cargo Delivery: Uncaging and Activating by Light, J. Am. Chem. Soc 139 (2017) 4584–4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Yang Y, Velmurugan B, Liu X, Xing B, NIR photoresponsive crosslinked upconverting nanocarriers toward selective intracellular drug release, Small 9 (2013) 2937–2944. [DOI] [PubMed] [Google Scholar]
- [367].Viger ML, Grossman M, Fomina N, Almutairi A, Low power upconverted near-IR light for efficient polymeric nanoparticle degradation and cargo release, Adv. Mater 25 (2013) 3733–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [368].Yan B, Boyer JC, Branda NR, Zhao Y, Near-infrared light-triggered dissociation of block copolymer micelles using upconverting nanoparticles, J. Am. Chem. Soc 133 (2011) 19714–19717. [DOI] [PubMed] [Google Scholar]
- [369].He S, Krippes K, Ritz S, Chen Z, Best A, Butt HJ, Mailänder V, Wu S, Ultralow-intensity near-infrared light induces drug delivery by upconverting nanoparticles, Chem. Commun 51 (2015) 431–434. [DOI] [PubMed] [Google Scholar]
- [370].Zhang Y, Ren K, Zhang X, Chao Z, Yang Y, Ye D, Dai Z, Liu Y, Ju H, Photo-tearable tape close-wrapped upconversion nanocapsules for near-infrared modulated efficient siRNA delivery and therapy, Biomaterials 163 (2018) 55–66. [DOI] [PubMed] [Google Scholar]
- [371].Yao C, Wang P, Li X, Hu X, Hou J, Wang L, Zhang F, Near-Infrared-Triggered Azobenzene-Liposome/Upconversion Nanoparticle Hybrid Vesicles for Remotely Controlled Drug Delivery to Overcome Cancer Multidrug Resistance, Adv. Mater 28 (2016) 9341–9348. [DOI] [PubMed] [Google Scholar]
- [372].Barhoumi A, Liu Q, Kohane DS, Ultraviolet light-mediated drug delivery: Principles, applications, and challenges, J. Control. Release 219 (2015) 31–42. [DOI] [PubMed] [Google Scholar]
- [373].Liu J, Bu W, Pan L, Shi J, NIR-triggered anticancer drug delivery by upconverting nanoparticles with integrated azobenzene-modified mesoporous silica, Angew. Chemie - Int. Ed 52 (2013) 4375–4379. [DOI] [PubMed] [Google Scholar]
- [374].Chen G, Ma B, Xie R, Wang Y, Dou K, Gong S, NIR-induced spatiotemporally controlled gene silencing by upconversion nanoparticle-based siRNA nanocarrier, J. Control. Release 282 (2018) 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [375].Bettinelli M, Carlos L, Liu X, Lanthanide-doped upconversion nanoparticles, Phys. Today 68 (2015) 38–44. [Google Scholar]
- [376].Cheng L, Yang K, Shao M, Lu X, Liu Z, In vivo pharmacokinetics, long-term biodistribution and toxicology study of functionalized upconversion nanoparticles in mice, Nanomed 6 (2011) 1327–1340. [DOI] [PubMed] [Google Scholar]
- [377].Mura S, Couvreur P, Nanotheranostics for personalized medicine, Adv. Drug Deliv. Rev 64 (2012) 1394–1416. [DOI] [PubMed] [Google Scholar]
- [378].Dai Z, Man NK, Parak WJ, Smith B, Sumer BD, Tang BZ, Van Hest J, Rotello VM, Translational Research: Bridging the Gap between Fundamental Research and the Clinic, Bioconjug. Chem 30 (2019) 2989–2990. [DOI] [PubMed] [Google Scholar]
- [379].Stewart MP, Sharei A, Ding X, Sahay G, Langer R, Jensen KF, In vitro and ex vivo strategies for intracellular delivery, Nature 538 (2016) 183–192. [DOI] [PubMed] [Google Scholar]
- [380].Smith SA, Selby LI, Johnston APR, Such GK, The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery, 30 (2019) 263–272. [DOI] [PubMed] [Google Scholar]
- [381].Rehman ZU, Hoekstra D, Zuhorn IS, Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: Real-time visualization of transient membrane destabilization without endosomal lysis, ACS Nano 7 (2013) 3767–3777. [DOI] [PubMed] [Google Scholar]
- [382].Dinh AT, Pangarkar C, Theofanous T, Mitragotri S, Understanding intracellular transport processes pertinent to synthetic gene delivery via stochastic simulations and sensitivity analyses, Biophys. J 92 (2007) 831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [383].Wang W, Ji X, Du L, Mattoussi H, Enhanced Colloidal Stability of Various Gold Nanostructures Using a Multicoordinating Polymer Coating, J. Phys. Chem. C 121 (2017) 22901–22913. [Google Scholar]
- [384].Tsoi KM, MacParland SA, Ma X-Z, Spetzler VN, Echeverri J, Ouyang B, Fadel SM, Sykes EA, Goldaracena N, Kaths JM, Conneely JB, Alman BA, Selzner M, Ostrowski MA, Adeyi OA, Zilman A, McGilvray ID, Chan WCW, Mechanism of hard-nanomaterial clearance by the liver, Nat. Mater 15 (2016) 1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [385].Zhang Y-N, Poon W, Tavares AJ, McGilvray ID, Chan WCW, Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination, J. Contr. Release, 240 (2016) 332–348. [DOI] [PubMed] [Google Scholar]
- [386].Alric C, Miladi I, Kryza D, Taleb J, Lux F, Bazzi R, Billotey C, Janier M, Perriat P, Roux S, Tillement O, The biodistribution of gold nanoparticles designed for renal clearance, Nanoscale 5 (2013) 5930–5939. [DOI] [PubMed] [Google Scholar]
- [387].Jiang Y, Huo S, Mizuhara T, Das R, Lee Y-W, Hou S, Moyano DF, Duncan B, Liang X-J, Rotello VM, The interplay of size and surface functionality on the cellular uptake of sub-10 nm gold nanoparticles, ACS Nano 9 (2015) 9986–9993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [388].Nam J, Won N, Bang J, Jin H, Park J, Jung S, Jung S, Park Y, Kim S, Surface engineering of inorganic nanoparticles for imaging and therapy, Adv. Drug Deliv. Rev 65 (2013) 622–648. [DOI] [PubMed] [Google Scholar]
- [389].Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE, Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy, Adv. Drug Deliv. Rev 61 (2009) 428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [390].Zhao Y, Wang Y, Ran F, Cui Y, Liu C, Zhao Q, Gao Y, Wang D, Wang S, A comparison between sphere and rod nanoparticles regarding their in vivo biological behavior and pharmacokinetics, Sci. Rep 7 (2017) 4131–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [391].Huang X, Li L, Liu T, Hao N, Liu H, Chen D, Tang F, The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo, ACS Nano 5 (2011) 5390–5399. [DOI] [PubMed] [Google Scholar]
- [392].Kaga S, Truong NP, Esser L, Senyschyn D, Sanyal A, Sanyal R, Quinn JF, Davis TP, Kaminskas LM, Whittaker MR, Influence of size and shape on the biodistribution of nanoparticles prepared by polymerization-induced self-assembly, Biomacromolecules 18 (2017) 3963–3970. [DOI] [PubMed] [Google Scholar]
- [393].Xie X, Liao J, Shao X, Li Q, Lin Y, The effect of shape on cellular uptake of gold nanoparticles in the forms of stars, rods, and triangles, Sci. Rep 7 (2017) 5356–5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [394].Carnovale C, Bryant G, Shukla R, Bansal V, Identifying trends in gold nanoparticle toxicity and uptake: size, shape, capping ligand, and biological corona, ACS Omega 4 (2019) 242–256. [Google Scholar]
- [395].Danhier F, To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine?, J. Control. Release 244 (2016) 108–121. [DOI] [PubMed] [Google Scholar]
- [396].Zhou H, Ge J, Miao Q, Zhu R, Wen L, Zeng J, Gao M, Biodegradable Inorganic Nanoparticles for Cancer Theranostics: Insights into the Degradation Behavior, Bioconjug. Chem 31 (2020) 315–331. [DOI] [PubMed] [Google Scholar]


