Abstract
Objective:
We developed a decision analytic model to evaluate the impact of a preoperative Staphylococcus aureus decolonization bundle on surgical site infections (SSIs), health-care–associated costs (HCACs), and deaths due to SSI.
Methods:
Our model population comprised US adults undergoing elective surgery. We evaluated 3 self-administered preoperative strategies: (1) the standard of care (SOC) consisting of 2 disinfectant soap showers; (2) the “test-and-treat” strategy consisting of the decolonization bundle including chlorhexidine gluconate (CHG) soap, CHG mouth rinse, and mupirocin nasal ointment for 5 days) if S. aureus was found at any of 4 screened sites (nasal, throat, axillary, perianal area), otherwise the SOC; and (3) the “treat-all” strategy consisting of the decolonization bundle for all patients, without S. aureus screening. Model parameters were derived primarily from a randomized controlled trial that measured the efficacy of the decolonization bundle for eradicating S. aureus.
Results:
Under base-case assumptions, the treat-all strategy yielded the fewest SSIs and the lowest HCACs, followed by the test-and-treat strategy. In contrast, the SOC yielded the most SSIs and the highest HCACs. Consequently, relative to the SOC, the average savings per operation was $217 for the treat-all strategy and $123 for the test-and-treat strategy, and the average savings per per SSI prevented was $21,929 for the treat-all strategy and $15,166 for the test-and-treat strategy. All strategies were sensitive to the probability of acquiring an SSI and the increased risk if SSI if the patient was colonized with SA.
Conclusion:
We predict that the treat-all strategy would be the most effective and cost-saving strategy for preventing SSIs. However, because this strategy might select more extensively for mupirocin-resistant S. aureus and cause more medication adverse effects than the test-and-treat approach or the SOC, additional studies are needed to define its comparative benefits and harms.
Staphylococcus aureus is the most common cause of surgical site infection (SSI).1 A large percentage (25%–33%) of adults in the US population are colonized with S. aureus.2,3 Such S. aureus carriers are at 2–10 times greater risk of SSI than noncarriers.2,3 Consequently, screening for and decolonization of S. aureus carriers is a recommended strategy for SSI prevention in selected surgical patients.4–7 However, optimal S. aureus screening and decolonization approaches remain undefined, and practices are highly variable. This is due in part to the scarcity of randomized clinical trial data in outpatients and to doubts that patients will apply decolonization medications as reliably or effectively at home as would nurses in the hospital, as well as concerns about side effects, costs, and possible selection for antimicrobial-resistant bacteria.6,8,9
Previous cost-effectiveness analyses concluded that pre-operative S. aureus screening and decolonization of S. aureus carriers, or administration of an S. aureus decolonization regimen to all preoperative patients (without assessing S. aureus carrier status), are potentially cost-effective strategies for SSI prevention.10–13 However, these studies used decolonization efficacy estimates based on earlier trials in which hospital inpatients underwent supervised S. aureus decolonization, which is inapplicable to current surgical practice in the United States, where most surgical patients are admitted from home on the day of their scheduled surgery.
To overcome these limitations of the available evidence, we undertook a novel cost-effectiveness analysis based on the results of our recent randomized controlled clinical trial of a self-applied 3-component preoperative S. aureus decolonization bundle.14 In that trial, preoperative 427 outpatients were screened for S. aureus carriage at 4 body sites (nares, throat, axillae, and perianal area), and 121 S. aureus carriers were randomized to either the standard of care (SOC) consisting of 2 preoperative antiseptic soap showers (n=53 participants) or the decolonization bundle consisting of 5 days of self-administered nasal mupirocin, chlorhexidine gluconate (CHG) bathing, and CHG mouthwash (n=57 participants). The trial demonstrated a 47% difference (95% confidence interval [CI], 29.1%–65.7%) in the eradication of S. aureus at all screened sites between the decolonization bundle (eradication in 41 of 57 patients, 71.9%) and the SOC (eradication in 13 of 52 patients, 24.5%).14 The types of surgical patients in this trial were general surgery (17.3%), neurologic surgery (20%), orthopedic surgery (56.4%), and urologic surgery (6.4%).14 However, the trial was powered to evaluate the decolonization bundle only for S. aureus eradication, not SSI prevention. Given the low incidence of SSI (~2%–3%), evaluation for SSI prevention would have required a much larger sample size.15
Because the “test-and-treat” strategy used in our decolonization trial would miss some S. aureus carriers (ie, due to the imperfect sensitivity of screening cultures and to newly acquired colonization between screening and surgery) and would impose screening costs and complexity, we envisioned a third approach in which all preoperative patients would receive the decolonization bundle without screening (ie, regardless of S. aureus carrier status).14,16,17 Thus, we conducted a formal analysis of the costs and benefits of 3 different SSI prevention strategies: the SOC, “test and treat,” and “treat all.”
Methods
Model overview
We developed a decision analytic model to evaluate the impact of a novel preoperative decolonization bundle on reducing SSIs, healthcare-associated costs (HCACs), and death due to SSI. The decision tree was built in OpenTree (Fig. 1) and was converted to R code.18 Analyses were performed using R Studio version 3.1.3 software (R Core Development Team, Vienna, Austria).
Fig. 1.
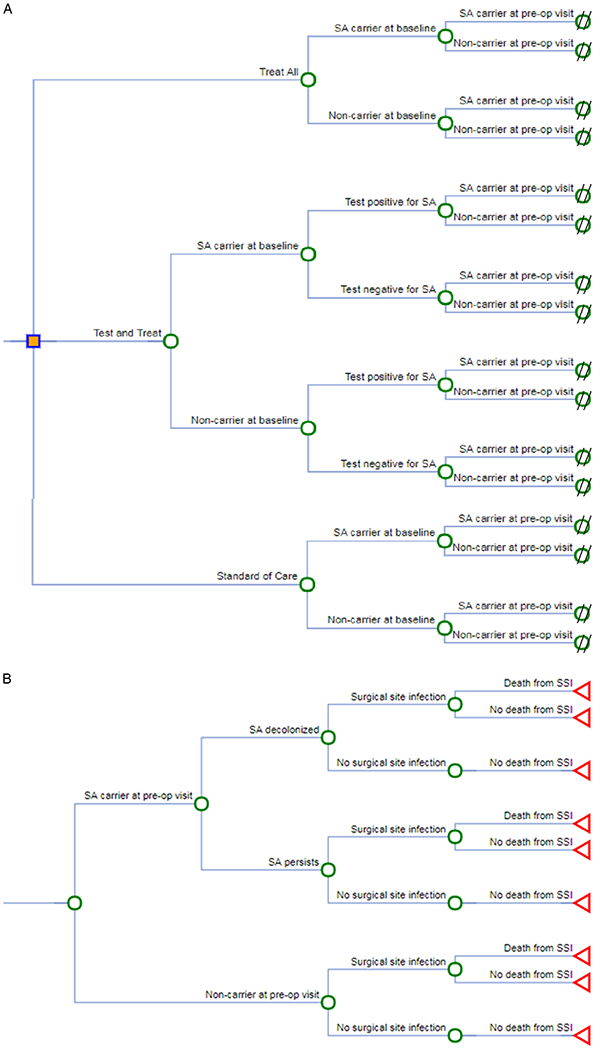
Decision analytic trees. The trees were designed to evaluate the impact of a preoperative (pre-op) decolonization bundle on reducing surgical site infections (SSIs), healthcare-associated costs, and death due to SSI. Carrier and noncarrier refer to carriage of Staphylococcus aureus (SA). (A) 3 strategies. (B) Subtree of strategy efficacy.
Population modeled
Our model population included men and women aged 18 years or older in the United States who were to undergo elective surgery, including orthopedic, urologic, neurologic, colorectal, cardiovascular, and general surgery.
Strategies modeled
We evaluated 3 preoperative treatment strategies: (1) the SOC; (2) the test-and-treat strategy consisting of administration of the decolonization bundle to surgical patients who screen positive for S. aureus at any of 4 swabbed body sites (ie, nasal, throat, axillary, and perianal area); and (3) a treat-all strategy consisting of administration of the decolonization bundle to all surgical patients. The self-administered decolonization bundle consisted of daily showering with CHG soap, twice daily gargling with CHG mouth rinse, and twice daily application of mupirocin nasal ointment, for 5 days prior to surgery. The SOC consisted of 2 self-administered disinfectant soap showers prior to surgery: 1 the night before and 1 the morning of surgery.
Model parameters
Model parameters were derived primarily from the Kline et al14 randomized controlled trial of the decolonization bundle for eradicating S. aureus from the 4 screened body sites (Table 1). Parameters not available from the trial were obtained from the literature and expert opinion. The prevalence of S. aureus at any of the 4 screened body sites was estimated from the trial, and plausible were ranges obtained from the literature to account for lower prevalence estimates from studies in which fewer body sites were swabbed.19 We assumed that all patients attended a baseline appointment at which 1 of the 3 treatment strategies was assigned and screening was performed (if applicable). The average number of days between the baseline appointment and surgery was estimated to be ~ 60 days.14 We assumed that patients had a low but nonzero probability of spontaneously acquiring or clearing S. aureus between treatment assignment and preoperative treatment administration.14 Spontaneous acquisition and clearance rates were derived from a longitudinal study of community-dwelling adult outpatients who underwent nasal swab culturing at 1 and 2 months post baseline and subsequently every 2 months for a median of 2 years.17
Table 1.
Parameter Estimates for Decision Analytic Model
| Parameter | Base-Case Value, % (Range) | Reference(s) |
|---|---|---|
| Prevalence of Staphylococcus aureus colonizationa | 30.0 (15.0–36.0) | Kline et al,14 Wertheim et al,19 Tong et al,28 Botelho-Nevers et al9 |
| Monthly probability of natural S. aureus clearance | 3.6 (2.5–6.7) | Miller et al17 |
| Monthly probability of S. aureus acquisition | 1.5 (1.3–1.8) | Miller et al17 |
| S. aureus culture sensitivity | 88.0 (82.0–92.0) | Luteijn et al16 |
| S. aureus culture specificity | 95.0 (91.0–97.0) | Luteijn et al16 |
| Probability of SSI given no colonization with S. aureus | 2.0 (1.0–5.0) | Magill et al,15 Urban et al.29 |
| RR of SSI given colonization with S. aureus | 4.5 (2.0–10.0)b | Simor20 Bode et al21 |
| Bundle efficacy | 71.9 (58.5–83.0) | Kline et al14 |
| Standard of care efficacy | 24.5 (13.8–38.3) | Kline et al14 |
| Probability of death from SSI | 1.0 (0.3–5.0) | Berger et al,23 Kirkland et al30 |
Note. SSI, surgical site infection; RR, relative risk.
Nasal, throat, axillary, and/or perianal area.
This is a multiplier of probability of SSI given no colonization with S. aureus (relative risk).
The efficacies of the decolonization bundle and the SOC in eliminating S. aureus from all 4 sites were obtained from the recent trial.14 Adherence to treatment assignment was not modeled explicitly, given that treatment efficacy estimates were based on the results of the trial, in which 80%–90% of the decolonization-bundle group participants reported applying >80% of prescribed medication doses and 100% of the SOC group participants reported taking both preoperative showers.14 Patients with S. aureus colonization were assumed to be at 4.5-fold greater risk of acquiring an SSI relative to patients without S. aureus colonization.20,21 The baseline risk of SSI among patients without S. aureus colonization was estimated from a multistate prevalence survey in which the medical records of hospitalized patients were reviewed for documentation of SSI.15 Estimates for sensitivity and specificity of culture screening were derived from a systematic review and meta-analysis of methicillin-resistant S. aureus detection assays.16
Costs
The analytic perspective of this study was the medical sector. As such, only direct costs pertaining to healthcare were included in the model (Table 2). Costs associated with the treatment strategies (ie, decolonization bundle, SOC, and screening cultures) were obtained from the trial.14 We assumed that, on average, a nurse needed 30 minutes per patient to collect swabs for screening and to report patient results. The median wage for a registered nurse was obtained from the US Bureau of Labor Statistics.22 The plausible range for the attributable cost of an SSI was obtained from the Centers for Disease Control (CDC) evidence-based guidelines for the prevention of SSI and a study of healthcare claims following major elective surgery.7,23 All costs were adjusted to 2016 US dollars using the Consumer Price Index.24 Costs were not discounted, due to the limited model time horizon (see the following section). Patients under the test-and-treat strategy who lacked S. aureus carriage but falsely screened positive (estimated as 3%) accrued the same decolonization-bundle treatment costs as did actual S. aureus carriers.14 Patients with S. aureus colonization who falsely screened negative (estimated as 12%) accrued the same SOC treatment costs as non–S. aureus carriers.16
Table 2.
Costs for Decision Analytic Model
| Parameter | Base-Case Value, $ (Range) | Reference(s) |
|---|---|---|
| Cost of SOC treatment | 9.00 (6.75–11.25) | Kline et al14 |
| Cost of bundle treatment | 25.33 (19.00–31.66) | Kline et al14 |
| Cost of Staphylococcus aureus screening culturea | 63.58 (54.63.26–81.46) | Kline et al14 |
| Cost of surgical site infection | 23,573 (10,209–34,174) | Berger et al,23 Berrios-Torres et al7 |
| Spine surgery | (42,845–91,999)b | Emohare et al26 |
| Total knee arthroplasty | (27,154–84,043)b | Kurtz et al27 |
| Total hip arthroplasty | (33,999–105,025)b | Kurtz et al27 |
Note. SOC, standard of care.
Includes labor.
Used for secondary base-case analysis only.
Model outcomes and analysis
We assessed the model outcomes of SSIs, deaths due to SSI, and direct costs for each treatment strategy using the base-case values of model parameters. A time horizon of 150 days was modeled, based on the assumed time between baseline visit and surgery, plus 90 days post surgery to capture outcomes directly related to surgery.1 Using the SOC as the reference, we calculated the number of SSIs prevented, the number of deaths from SSI prevented, and the total savings under the treat-all and the test-and-treat strategies.
We conducted a cost-effectiveness analysis in which the SOC was the reference strategy. If a strategy was more costly and less effective than an adjacent strategy, it was considered a less favorable approach.25 We conducted 1-way sensitivity analyses of model parameters to examine the impact of extreme values on predicted SSIs per 10,000 population. We also conducted a probabilistic sensitivity analysis in which we sampled values randomly from parameter distributions to account for the uncertainty in the parameter estimates used in the model. In total, 10,000 sets of parameter values were sampled for the probabilistic sensitivity analysis.
In addition to conducting a base-case analysis with an average SSI cost for general surgery, we conducted a sensitivity analysis using surgery-specific SSI costs. This analysis accounted for the higher costs of SSIs associated with certain procedures, including spinal surgery, total knee arthroplasty (TKA), and total hip arthroplasty (THA).26,27 We used the patient distribution from Kline et al14 to determine the percentage of all surgical patients undergoing these procedures. We assessed both extremes of the ranges of these surgery-specific SSI costs. The general surgery SSI cost was applied to patients undergoing a procedure of any type other than those listed above.
Mupirocin resistance and medication side effects modeled
We used estimates from the recent Kline et al14 trial to estimate for each strategy the number of patients who would experience an adverse drug event or develop mupirocin resistance.
Results
Base case
Under base-case assumptions, the treat-all strategy prevented the most SSIs and resulted in the lowest healthcare-associated costs, followed by the test-and-treat strategy. Compared to the treat-all and test-and-treat strategies, the SOC was the least favorable because it resulted in both the most SSIs and the highest healthcare-associated costs. The base-case results from the decision analytic model are shown in Table 3.
Table 3.
Base-Case Results per 10,000 Population Undergoing Surgery
| Variable | Decolonization Strategy |
||
|---|---|---|---|
| SOC | Test and Treat | Treat All | |
| Surgical site infections, no. | 358 | 277 | 259 |
|
| |||
| Prevented SSIs, no. | N/A | 81 | 99 |
|
| |||
| Deaths due to SSI, no. | 4 | 3 | 3 |
|
| |||
| Prevented deaths due to SSI, no. | N/A | 1 | 1 |
|
| |||
| Total cost savings, $ | N/A | 1,235,114 | 2,178,307 |
Note. SOC, standard of care; N/A, not applicable; SSI, surgical site infection.
For the 2 more favorable strategies, the average savings per patient were $217 for the treat-all strategy and $123 for the test-and-treat strategy, a difference of $94 per patient, whereas the average savings per SSI prevented were $21,929 for the treat-all strategy and $15,166 for the test-and-treat strategy. Compared to the test-and-treat strategy, the treat-all strategy prevented 18 more SSIs per 10,000 patients undergoing surgery. In a threshold analysis based on the decolonization bundle, the efficacy thresholds below which the particular strategy would no longer be cost saving were 42% for the test-and-treat strategy and 28% for the treat-all strategy. For comparison purposes, the efficacies for S. aureus eradication in the Kline et al14 study were 71.9% for the decolonization bundle and 24.5% for the SOC.
Inclusion of surgery-specific costs for spinal surgery, TKA, and THA increased the total savings for the treat-all and the test-and-treat strategies. Specifically, the ranges of savings per SSI prevented were $30,694–$58,626 for the treat-all strategy and $23,931–$51,863 for the test-and-treat strategy. The ranges of savings per patient undergoing surgery were $305–$582 for the treat-all strategy and $195–$422 for the test-and-treat strategy.
One-way sensitivity analyses
In a 1-way sensitivity analyses for the outcome of SSIs, all 3 strategies were highly sensitive to both the probability of acquiring an SSI and the risk of SSI given colonization with S. aureus, but they were relatively less sensitive to treatment efficacy. Parameters pertaining to SSI risk similarly had the greatest impact on costs. When the prevalence of S. aureus carriage was set at the lowest plausible value (15%), the SOC remained less favorable than the treat-all and the test-and-treat strategies. However, when the relative risk of SSI among S. aureus-colonized patients was <3.5 and the prevalence of S. aureus was ≤15% or the risk of SSI was ≤1%, the test-and-treat strategy became more costly than the SOC while remaining more effective in preventing SSIs. With test-and-treat strategy, the use of the highest plausible values for culture sensitivity and specificity increased the savings per infection prevented by $363 and the number of infections prevented per 10,000 population undergoing surgery by 4. Under no combination of extreme screening or treatment costs was the test-and-treat strategy less expensive than the treat-all strategy.
Probabilistic sensitivity analysis
In 10,000 simulations of the decision analytic model, based on the different parameter sets that were generated for probabilistic sensitivity analysis, the median savings per infection prevented were $20,355 (IQR, $14,488–$26,615) for the treat-all strategy and $14,480 (IQR, $8,103–$21,407) for the test-and-treat strategy. The respective median numbers of infections prevented per 10,000 population undergoing surgery were 164 (IQR, 81–281) for the treat-all strategy and 120 (IQR, 70–223) for the test-and-treat strategy. The respective median numbers of deaths prevented per 10,000 population undergoing surgery were 4 (IQR, 2–8) for the treat-all strategy and 3 (IQR, 2–6) for the test-and-treat strategy. The most costly strategy was the SOC in 91% of simulations and the test-and-treat strategy in the remaining 9% of simulations. In 100% of simulations, the treat-all strategy both prevented the most SSIs and was least costly.
Mupirocin resistance and adverse drug events
Using estimates from the recent trial, the number of instances of mupirocin-resistant S. aureus per 10,000 patients treated would be 52 for the treat-all strategy and 46 for the test-and-treat strategy, whereas the SOC would yield no mupirocin resistance (Fig. 2).14 Adverse drug events from applied medications would be most numerous in the treat-all strategy, with 3,460 patients per 10,000 treated experiencing at least 1 adverse drug event, compared to 3,039 for the test-and-treat strategy and 2,860 for the SOC (Fig. 2). These include all participant-reported adverse drug events from the recent trial (as recorded by patients in a drug diary), including minor complaints such as dry skin and unpleasant taste.14 Because no serious adverse drug events occurred in the recent trial,14 none were projected here.
Fig. 2.
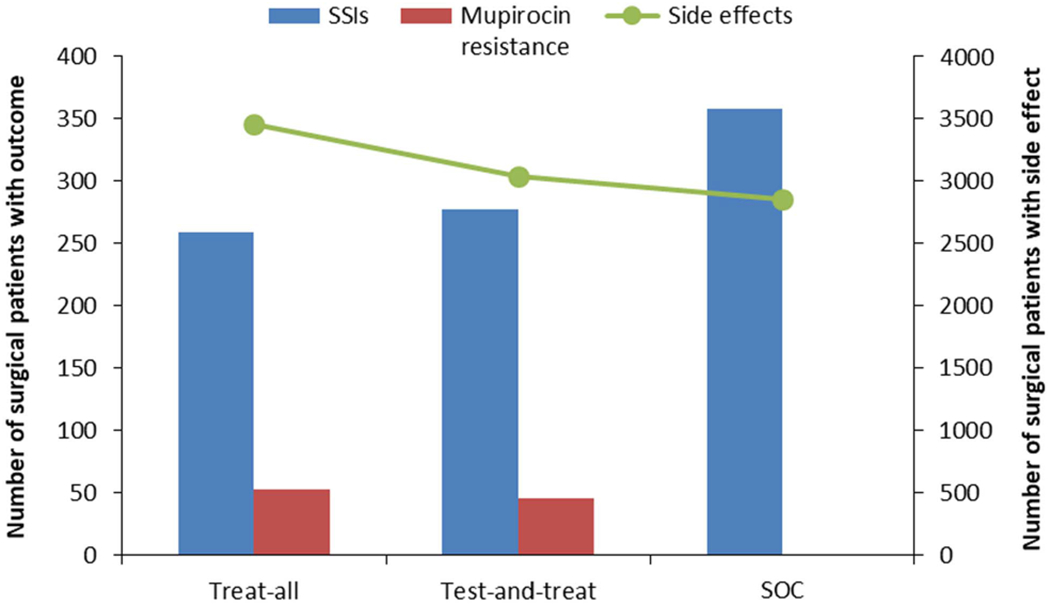
Surgical site infections (SSIs), muprocin resistance, and side effects from medications modeled for a population of 10,000 surgical patients. Modeled patients assigned to 1 of 3 preoperative treatment strategies: “treat all,” “test and treat,” and the standard of care (SOC).
Discussion
In this study, we developed a decision analytic model to evaluate the impact on healthcare-associated costs, SSIs, and deaths due to SSI of a preoperative decolonization bundle directed toward S. aureus colonization in outpatient surgery clinic patients. To our knowledge, this study is the first to use clinical trial data to inform a cost-effectiveness analysis of this 3-component, self-applied, outpatient decolonization bundle in SSI prevention. Based on the decolonization efficacy estimates from the recent trial, and other parameters taken from the literature, our decision analytic model showed that screening and decolonization with the decolonization bundle (ie, the test-and-treat strategy) is cost-saving. Moreover, a treat-all strategy in which all preoperative patients receive the decolonization bundle (regardless of S. aureus status) was cost-saving compared to both the SOC and the test-and-treat strategy.14
Despite the limited evidence from randomized clinical trials involving outpatients that preoperative S. aureus screening and decolonization prevents SSIs, several studies involving hospitalized patients have demonstrated potential benefits with this approach.21,31–36 The recent Kline et al14 trial involving outpatients that served as the basis for the present cost-effectiveness analysis demonstrated that the 3-drug decolonization bundle was significantly more effective than 2 showers in eradicating S. aureus carriage from 4 body sites prior to surgery. Although the trial demonstrated that patients were able to apply the multidrug, multidose regimen reliably and effectively at home, it did not assess the clinical outcome of SSIs. The results of our decision analytic model provide evidence that supports conducting a larger clinical trial to assess experimentally the impact of this decolonization bundle on SSIs.
From a medical cost perspective, our findings support offering the decolonization bundle to all preoperative elective surgery patients for self-administration at home for 5 days prior to surgery. However, this approach would expose non–S. aureus carriers to medication unnecessarily, with attendant risks of side effects (eg, allergic reactions and irritated or dry skin) and possible selection for resistance to CHG and/or mupirocin, as can occur in S. aureus with prolonged administration of these drugs.37,38 In addition, only S. aureus carriers would be at risk for developing these drug-resistant S. aureus strains.
An additional consideration for the treat-all strategy is that patients would not know their S. aureus carrier status and consequently might be less motivated to apply the decolonization-bundle medications compared to patients who test positive for S. aureus in the test-and-treat strategy. Decreased adherence to the decolonization-bundle medications would be expected to lower the efficacy of the decolonization bundle. Our threshold analysis, however, suggested that resistance (or other factors, including nonadherence to the decolonization bundle) would need to reduce the efficacy of the decolonization bundle to <28% (from the 71.9% observed in the recent trial) before the treat-all strategy would no longer be cost-saving in comparison to the SOC.14
The potential long-term adverse health impacts resulting from the unintended consequence of widespread S. aureus decolonization efforts need to be balanced against the costs and deaths caused by S. aureus SSIs.23,26,27,39 Our analysis suggests that this balance may differ by surgery type. Total joint replacement and spine surgeries can result in deep SSIs that lead to prolonged hospitalization and disability. Our analysis showed that the savings per SSI prevented by the treat-all strategy increased by 40%–167% for THA and TKA procedures compared to general surgery. We acknowledge that the mix of surgical patients across institutions varies widely. Each institution would need to consider case mix when deciding what strategy to use.
From a societal perspective, the human and heath economic burden of SSIs should be balanced against the costs, potential side effects, and selection for S. aureus resistant to mupirocin or CHG that could result from the decolonization bundle. A clinical trial to study these outcomes in the real-world setting of busy surgical clinics, surgery centers, and acute-care hospitals is warranted.
Financial support.
This work was supported by the Agency for Healthcare Research and Quality (AHRQ grant no. 1R03HS022912-01), the National Institutes of Health (NIH grant no. UL1TR000114); by matching funds and pilot study funds from the University of Minnesota and the University of Minnesota Medical Center; and by the Office of Research and Development, US Department of Veterans Affairs.
Footnotes
Conflicts of interest. All authors report no conflict of interest relevant to this article.
PREVIOUS PRESENTATION: This work was reported in preliminary form as a poster at the SHEA 2018 Spring Meeting in Portland, Oregon, on April 18, 2018, Abstract 10081, Poster 209.
References
- 1.Hidron A, Edwards J, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011. [DOI] [PubMed] [Google Scholar]
- 2.Perl TM, Golub JE. New approaches to reduce Staphylococcus aureus nosocomial infection rates: treating S. aureus nasal carriage. Ann Pharmacother 1998;32:S7–S16. [DOI] [PubMed] [Google Scholar]
- 3.Wenzel RP, Perl TM. The significance of nasal carriage of Staphylococcus aureus and the incidence of postoperative wound infections. J Hosp Infect 1995;31:13–24. [DOI] [PubMed] [Google Scholar]
- 4.Ban KA, Minei JP, Laronga C, et al. American college of surgeons and surgical infection society: surgical site infection guidelines, 2016 update. Am Coll Surg 2017;224:59–74. [DOI] [PubMed] [Google Scholar]
- 5.Global guidelines for the prevention of surgical site infection. World Health Organization; website. http://www.who.int/gpsc/ssi-guidelines/en/. Published 2016. Accessed July 21, 2017. [PubMed] [Google Scholar]
- 6.Parvizi J, Shohat N, Gehrke T. Prevention of periprosthetic joint infection: new guidelines. Bone Joint J 2017;99-B 4 Supple B:3–10. [DOI] [PubMed] [Google Scholar]
- 7.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention guideline for the prevention of surgical site infection, 2017. JAMA Surg 2017;152:784–791. [DOI] [PubMed] [Google Scholar]
- 8.Chlebicki M, Safdar N, O’Horo JC. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis. Am J Infect Control 2013;41:167–173. [DOI] [PubMed] [Google Scholar]
- 9.Botelho-Nevers E, Gagnaire J, Verhoeven PO, et al. Decolonization of Staphylococcus aureus carriage in 2016. Med Mai Infect 2016:1–6. [DOI] [PubMed] [Google Scholar]
- 10.Courville XF, Tomek IM, Kirkland KB, et al. Cost-effectiveness of preoperative nasal mupirocin treatment in preventing surgical site infection in patients undergoing total hip and knee arthroplasty: a cost-effectiveness analysis. Infect Control Hosp Epidemiol 2012:33:152–159. [DOI] [PubMed] [Google Scholar]
- 11.Wassenberg MWM, De Wit GA, Bonten MJM. Cost-effectiveness of preoperative screening and eradication of Staphylococcus aureus carriage. PLoS One 2011;6:e14815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young LS, Winston LG. Preoperative use of mupirocin for the prevention of healthcare-associated Staphylococcus aureus infections: a cost-effectiveness analysis. Infect Control Hosp Epidemiol 2006;27:1304–1312. [DOI] [PubMed] [Google Scholar]
- 13.Noskin GA, Rubin RJ, Schentag JJ, et al. Budget impact analysis of rapid screening for Staphylococcus aureus colonization among patients under-going elective surgery in US hospitals. Infect Control Hosp Epidemiol 2008;29:16–24. [DOI] [PubMed] [Google Scholar]
- 14.Kline S, Neaton J, Lynfield R, et al. Effectiveness of screening and decolonization of S. aureus in surgery outpatients. Infect Control Hosp Epidemiol 2018. July 24:1–9. doi: 10.1017/ice.2018.151. [DOI] [Google Scholar]
- 15.Magill S, Edwards J, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. Nat Rev Microbiol 2014;370:1198–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luteijn JM, Hubben GAA, Pechlivanoglou P, et al. Diagnostic accuracy of culture-based and PCR-based detection tests for methicillin-resistant Staphylococcus aureus: a meta-analysis. Clin Microbiol Infect 2010;17:146–154. [DOI] [PubMed] [Google Scholar]
- 17.Miller RR, Walker AS, Godwin H, et al. Dynamics of acquisition and loss of carriage of Staphylococcus aureus strains in the community: the effect of clonal complex. J Infect 2014;68:426–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jalal H, Alarid-Escudero F, Hunink MGM. PS 1-58 DTree: an open source tool for building decision trees and cost-effectiveness analyses. In: 38th Annual North American Meeting of the Society for Medical Decision Making; 2016:E57. [Google Scholar]
- 19.Wertheim HFL, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis 2005;5:751–762. [DOI] [PubMed] [Google Scholar]
- 20.Simor AE. Staphylococcal decolonization: an effective strategy for prevention of infection? Lancet Infect Dis 2011;11:952–962. [DOI] [PubMed] [Google Scholar]
- 21.Bode L, Kluytmans J, W H, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med 2010;362:9–17. [DOI] [PubMed] [Google Scholar]
- 22.Occupational outlook handbook, 2016–2017. US Department of Labor Bureau of Labor Statistics; website, https://www.bls.gov/ooh/healthcare/registered-nurses.htm. Published 2017. Accessed August 1, 2017. [Google Scholar]
- 23.Berger A, Edelsberg J, Yu H, et al. Clinical and economic consequences of post-operative infections following major elective surgery in US hospitals. Surg Infect (Larchmt) 2014;15:322–327. [DOI] [PubMed] [Google Scholar]
- 24.CPI inflation calculator. US Department of Labor Bureau of Labor Statistics; website, https://data.bls.gov/cgi-bin/cpicalc.pl. Accessed August 23, 2018. [Google Scholar]
- 25.Gillian S, Neiumann P, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA 2016;316:1093–1103. [DOI] [PubMed] [Google Scholar]
- 26.Emohare O, Ledonio CG, Hill BW, et al. Cost savings analysis of intrawound vancomycin powder in posterior spinal surgery. Spine J 2014;14:2710–2715. [DOI] [PubMed] [Google Scholar]
- 27.Kurtz SM, Lau E, Watson H, et al. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty 2012;27:61–65. [DOI] [PubMed] [Google Scholar]
- 28.Tong SYC, Davis JS, Eichenberger E, et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015;28:603–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urban JA. Cost analysis of surgical site infections. Surg Infect (Larchmt) 2006;7:19–22. [DOI] [PubMed] [Google Scholar]
- 30.Kirkland KB, Briggs JP, Trivette SL, et al. The impact of surgical-site infections in the 1990’s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol 1999;20:725–730. [DOI] [PubMed] [Google Scholar]
- 31.Perl TM, Cullen JJ, Wenzel RP. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infection. New Engl J Med 2002;346:1871–1877. [DOI] [PubMed] [Google Scholar]
- 32.Segers P, Speekenbrink R, Ubbink D, et al. Prevention of nosocomial infection in cardiac surgery by decontamination of the nasopharynx and oropharynx with chlorhexidine gluconate a randomized controlled trial. JAMA 2006;296:2460–2466. [DOI] [PubMed] [Google Scholar]
- 33.Kluytmans JA, Mouton JW, Marjolein FQ. Reduction of surgical site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol 1996;17:780–784. [DOI] [PubMed] [Google Scholar]
- 34.Schweizer ML, Chiang H, Septimus E, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA 2015;313:2162–2171. [DOI] [PubMed] [Google Scholar]
- 35.Kalmeijer MD, Coertjens H, van Nieuwland-Bollen PM. Surgical site infections in orthopedic surgery: the effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis 2002;35:353–358. [DOI] [PubMed] [Google Scholar]
- 36.Konvalink A, Erret L, Fong IW. Impact of treating Staphylococcus aureus nasal carriage on wound infections in cardiac surgery. J Hosp Infect 2006;64:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irish D, Eltringham I, Teall A, et al. Control of an outbreak of an epidemic methicillin-resistant Staphylococcus aureus also resistant to mupirocin. J Hosp Infect 1998;39:19–26. [DOI] [PubMed] [Google Scholar]
- 38.Hayden MK, Lolans K, Haffenreffer K, et al. Chlorhexidine and mupirocin susceptibility of methicillin-resistant Staphylococcus aureus isolates in the REDUCE-MRSA trial. J Clin Microbiol 2016;54:2735–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noskin GA, Rubin RJ, Schentag JJ, et al. National trends in Staphylococcus aureus infection rates: impact on economic burden and mortality over a 6-year period (1998–2003). Clin Infect Dis 2007;45:1132–1140. [DOI] [PubMed] [Google Scholar]


