Abstract
Three recent renal denervation studies in both drug-naïve and drug-treated hypertensive patients demonstrated a significant reduction of ambulatory blood pressure compared with respective sham control groups. Improved trial design, selection of relevant patient cohorts, and optimized interventional procedures have likely contributed to these positive findings. However, substantial variability in the blood pressure response to renal denervation can still be observed and remains a challenging and important problem. The International Sympathetic Nervous System Summit was convened to bring together experts in both experimental and clinical medicine to discuss the current evidence base, novel developments in our understanding of neural interplay, procedural aspects, monitoring of technical success, and others. Identification of relevant trends in the field and initiation of tailored and combined experimental and clinical research efforts will help to address remaining questions and provide much-needed evidence to guide clinical use of renal denervation for hypertension treatment and other potential indications.
Keywords: blood pressure, cardiovascular disease, hypertension, renal denervation, sympathetic nervous system
The initial results from the Symplicity HTN-1 (1) and HTN-2 (2) trials moved catheter-based renal denervation (RDN) on to center stage in the cardiovascular field. In these initial trials, RDN was demonstrated to be safe and effective in lowering blood pressure (BP) in subjects with resistant hypertension via a reduction in renal and central neural sympathetic activity (3,4). However, the sham-controlled Symplicity HTN-3 trial (5) failed to demonstrate superiority of RDN in reducing BP compared with a sham group at 6 months post-procedure. These unexpected findings have been discussed extensively and controversially in the published literature. The failure of the trial was attributed to several possible confounding factors, including issues related to patient selection and medication adherence, suboptimal procedural performance, and operator experience (6,7). The Symplicity HTN-3 results significantly affected other research studies and clinical trials in the field. It was not rectified until the multicenter, randomized-controlled DENERHTN (Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension) study showed a clear signal for supremacy of RDN and a standardized stepped-care antihypertensive treatment approach over the same standardized stepped-care antihypertensive treatment alone (8). Importantly, the prevalence of nonadherence to antihypertensive drugs at 6 months was high (w50%), but was not different in the renal denervation and control groups (9) in this study.
Nonadherence to antihypertensive medications is highly variable over time in clinical trials (10,11). Tomaszewski et al. (12) demonstrated that a significant proportion of patients in a specialist center show at least some degree of nonadherence that correlates with their BP. Furthermore, patients with undeclared/unrecognized nonadherence frequently undergo numerous additional diagnostic tests in specialist centers to identify the causes of their apparent poor response to antihypertensive medications (12). The SPYRAL HTN-ON MED (Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomized trial) proof-of-concept randomized trial (13) showed adherence rates of ~60% and variation for individual patients throughout the study.
A recent study suggested that patients with apparent treatment-resistant hypertension should be formally evaluated for nonadherence prior to any further changes in medication. Indeed, the investigators observed a mean drop in 24-h ambulatory BP monitoring of 19/9 mm Hg after supervised administration of antihypertensive medications, highlighting the potential utility of “directly observed therapy” clinics (14). Poor adherence with prescribed medication in general may be considered an expression of patient’s preference, which could potentially be overcome by alternative one-off interventions such as RDN (15,16).
More than a decade after the publication of the original proof-of-concept study, 4 important recent trials have sparked renewed interest in RDN: the DENERHTN trial (8), the SPYRAL HTN-OFF MED (Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications [SPYRAL HTN-OFF MED]: a randomized, sham-controlled, proof-of-concept trial) (15), and RADIANCE-HTN SOLO (Endovascular ultrasound renal denervation to treat hypertension [RADIANCE-HTN SOLO]: a multicenter, international, single-blind, randomized, sham-controlled trial) (16) trials in drug-naïve hypertensive patients, as well as the SPYRAL HTN-ON MED trial (13) in hypertensive patients on concurrent antihypertensive therapy. All demonstrated a convincing and clinically significant reduction of ambulatory BP in comparison with respective sham control groups (Figure 1). Evidence is therefore now available from 3 consecutive and adequately designed, randomized, sham-controlled trials confirming the BP-lowering efficacy of catheter-based RDN approaches (17).
FIGURE 1. Results From Recent Renal Denervation Randomized, Sham-Controlled Clinical Trials.
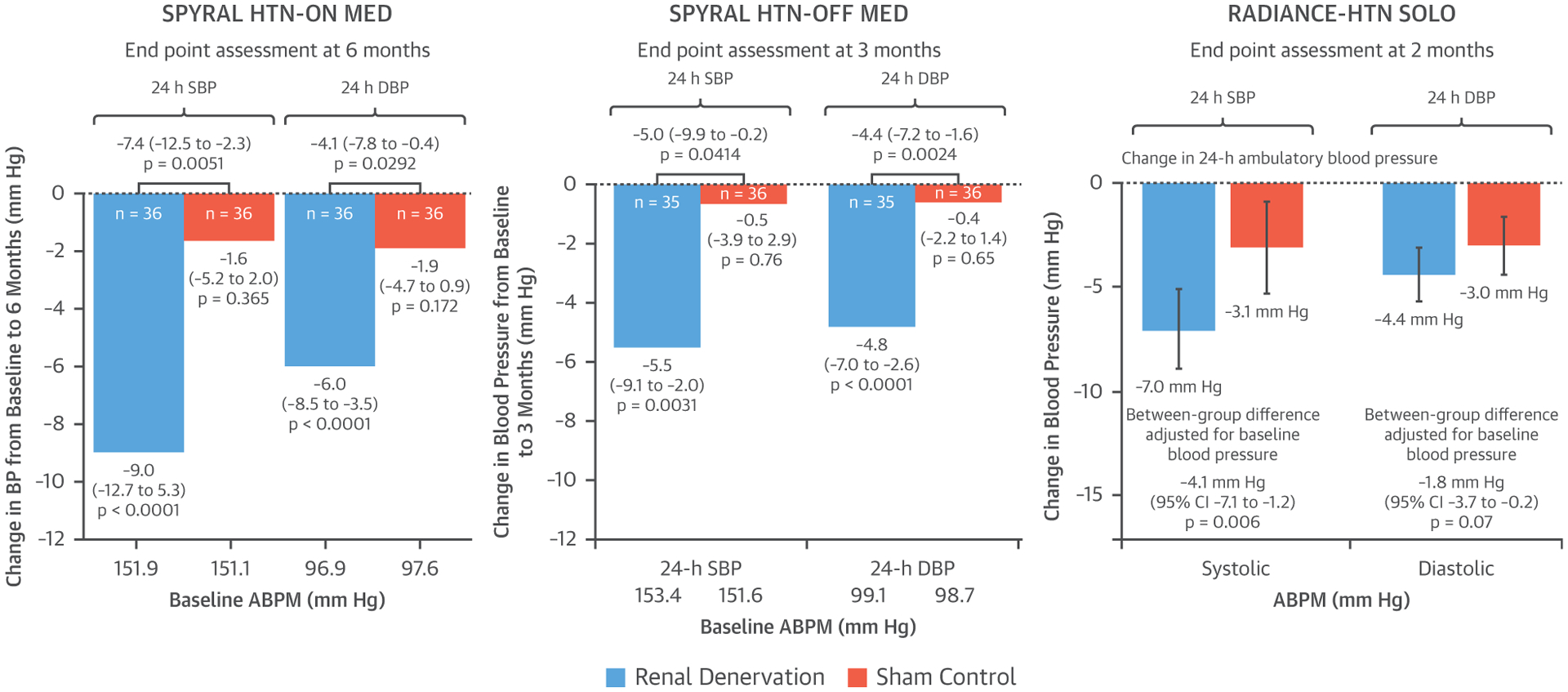
Comparison of changes in 24-h systolic blood pressure (SBP) and diastolic blood pressure (DBP) in renal denervation versus sham-control groups in 3 recent randomized, sham-controlled clinical trials. Reprinted with permission from Kandzari et al. (13), Townsend et al. (15), and Azizi et al (16). ABPM = ambulatory blood pressure monitoring; BP = blood pressure; CI = confidence interval; SPYRAL HTN-ON MED = Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomized trial; SPYRAL HTN-OFF MED = Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomized, sham-controlled, proof-of-concept trial; RADIANCE-HTN SOLO = Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicenter, international, single-blind, randomized, sham-controlled trial.
With pivotal trials now underway for several RDN devices, it is perhaps pertinent for the hypertension community to develop strategies on exactly how to best allocate the limited resources to ensure the availability of RDN for those individuals who are likely to benefit most. To achieve this goal, intensive research efforts are required to address the most critical unresolved issues (Central Illustration). These issues and related recent research findings are discussed in the following text.
CENTRAL ILLUSTRATION. Areas of Ongoing Research in the Field of Renal Denervation.
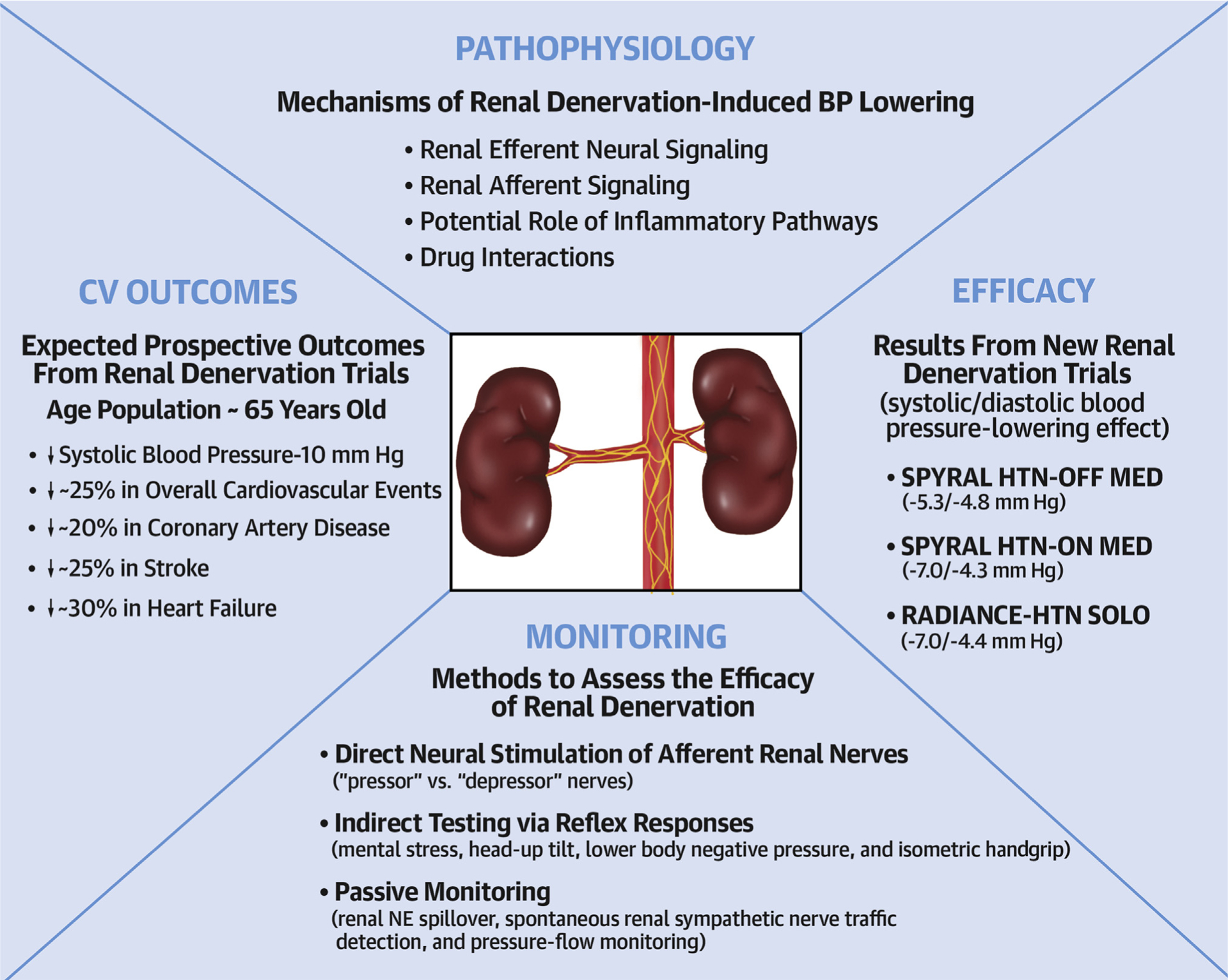
BP = blood pressure; NE = norepinephrine; RADIANCE-HTN SOLO = Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicenter, international, single-blind, randomized, sham-controlled trial; SPYRAL HTN-ON MED = Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomized trial; SPYRAL HTN-OFF MED = Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomized, sham-controlled, proof-of-concept trial.
MECHANISMS OF RDN-INDUCED BP LOWERING
RENAL EFFERENT NEURAL SIGNALING.
The renal efferent sympathetic nerves are mostly adrenergic (18). Norepinephrine release mediates vasoconstriction of the renal vessels, as well as sodium and water reabsorption at renal tubular epithelial cells, and renin release from juxtaglomerular cells (19).
Interference with the neural drive to the kidneys by global suppression of central sympathetic outflow through chronic electrical activation of the carotid baroreflex (20) or by renal-specific sympatho-inhibition through RDN has been shown to abolish hypertension (21). RDN increases renal sodium and water excretion by suppressing sympathetically mediated renin secretion and/or renal tubular sodium reabsorption (22). However, Foss et al. (23) found no differences in daily or cumulative sodium and water balance between sham and RDN rats. This is consistent with previous reports that RDN decreases BP in normotensive Sprague Dawley rats independent of sodium balance or renin release (24,25). It is important to note that RDN did not affect the salt sensitivity of arterial pressure in Sprague Dawley rats (22) or Dahl salt-sensitive (DS) rats. Combined with their findings that afferent renal nerves do not play a role in the DS rat model, these data point toward reduced activity of the renin-angiotensin system and possibly reduced renal vascular resistance being involved in the antihypertensive effect of RDN in the DS rat (26). In a canine model of obesity hypertension, the abolition of hypertension by RDN emphasized the importance of increased renal sympathetic nerve activity in the pathogenesis of obesity-induced hypertension, and highlighted the role of renal-specific suppression of sympathetic activity in mediating the antihypertensive effects of baroreflex activation. The chronic neurohormonal responses to prolonged baroreflex activation and bilateral RDN in this experimental model suggested that the inhibition of the renin-angiotensin system contributes to the sustained BP-lowering during both global and renal-specific sympathoinhibition (21).
The rostral ventrolateral medulla (RVLM) is an important regulator of efferent renal sympathetic nerve activity (RSNA). The level of RSNA is reliant on the neuronal activity in sympathetic premotor nuclei in the brainstem and hypothalamus, including the RVLM and rostral ventromedial medulla as well as the paraventricular nucleus. The notable reduction in BP after the destruction of premotor neurons in the RVLM highlights its importance (27). Inhibition of RSN signaling after RDN with downstream effects on the renin-angiotensin system cascade are likely contributors to the BP-lowering efficacy.
RENAL AFFERENT SIGNALING.
Recently, Tsai et al. (28) demonstrated in ambulatory canines that bilateral RDN, possibly via interrupting afferent renal innervation, led to substantial brain stem and bilateral stellate ganglion remodeling at 8 weeks post-procedure. These changes were associated with reduced 18FDG-uptake in the brainstem, left stellate ganglion nerve activity, and atrial tachyarrhythmia events. The authors concluded that neural remodeling in the brain stem and stellate ganglion may partially explain the described anti-arrhythmic effects of RDN (28).
Trans-synaptic degeneration is a phenomenon in the central and peripheral nervous system that may remain active both at the level of the insult and in remote brain structures for as long as 1 year after a trauma (29). These progressive alterations may underlie some of the long-term functional consequences after initial injury (i.e., RDN) as shown in Figure 2, which summarizes the various direct and indirect connections between renal sympathetic nerves and the stellate ganglion. Meckler et al. (30) showed that approximately 10% of renal sympathetic neurons in cats originated from the thoracic chain ganglia. In view of the connections between these 2 structures, RDN may directly result in retrograde cell death of the stellate ganglion. Furthermore, the application of fluorescent dyes in the renal nerves results in fluorescent labeling of the sympathetic cell bodies in paravertebral and prevertebral ganglia (31–33).
FIGURE 2. Schematic of Possible Connections Among Different Neural Structures.
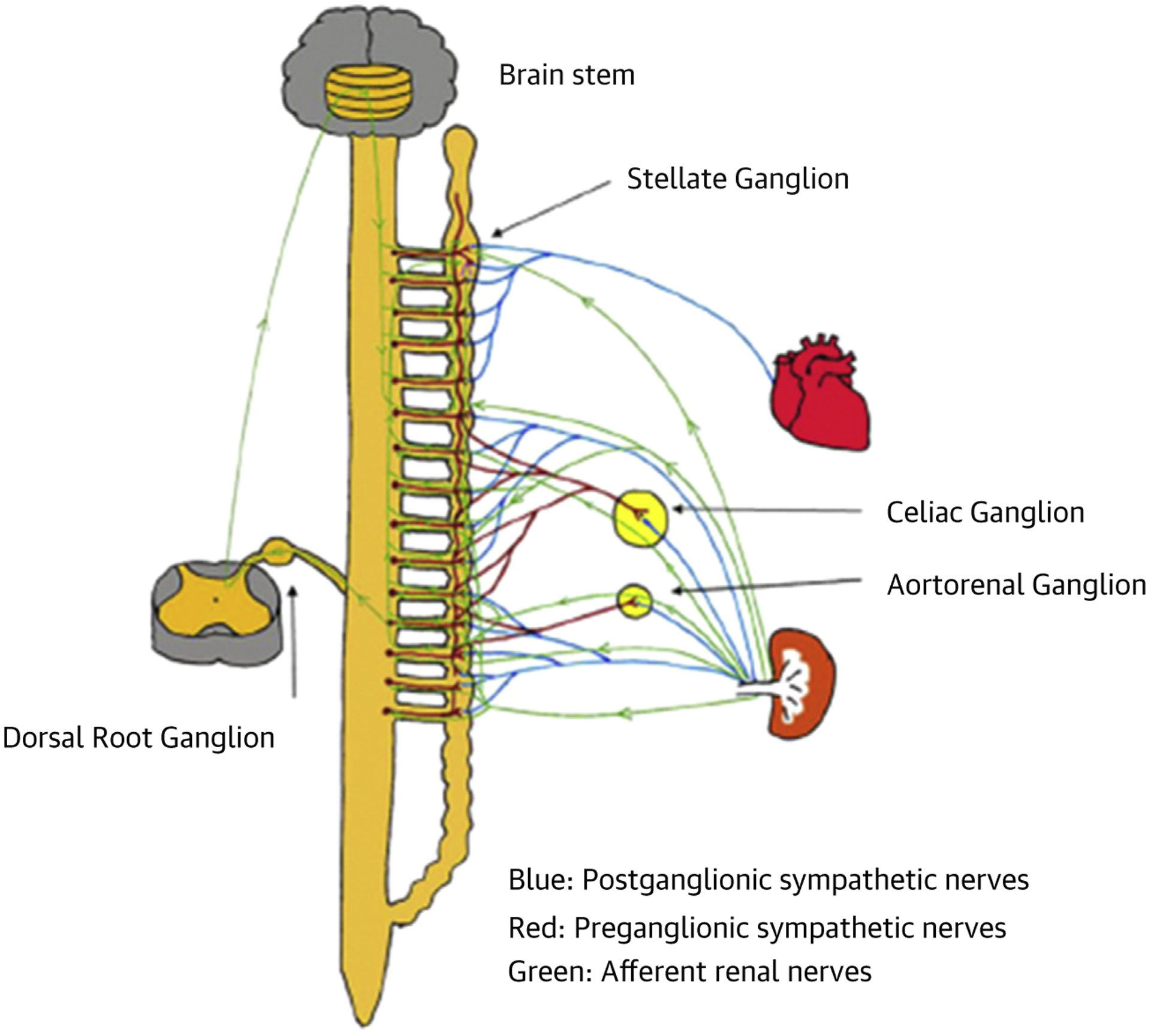
There are multiple pathways through which renal denervation can directly and indirectly result in brain stem and stellate ganglion remodeling and reduced nerve activity. These include inhibition of afferent nerve signaling (green lines) and retrograde trans-synaptic degeneration (blue lines) (28).
Because the sympathetic preganglionic neurons that project to the stellate ganglion are dispersed in spinal cord segments T1 to T10 (34), there is ample opportunity to inter-relate with the preganglionic cells that link indirectly with sympathetic nerve fibers surrounding the renal arteries. However, it is likely that some other pathways contribute to the trans-synaptic degeneration (28), because the ganglion cells of renal afferent nerves situated in thoracic and lumbar spine dorsal root ganglia also link to the posterior and lateral hypothalamic nuclei and the locus coeruleus (35,36). Overall, these findings indicate that persistent effects of RDN may well be mediated by remodeling of critical brain stem areas and the stellate ganglia.
METHODS TO ASSESS THE EFFICACY OF RDN
Unlike coronary interventions, current RDN devices provide no feedback to the interventionalist regarding technical success of the procedure. As a consequence, the degree of denervation varies widely (37), and inadequate renal denervation was likely implicated in the failure of Symplicity HTN-3 to show a BP benefit beyond that of a sham control procedure (5,6). Despite more recent positive clinical trials (13,15,16) intraprocedural validation of adequate renal sympathetic nerve ablation remains a fundamental challenge in the field.
Validation of successful RDN in preclinical studies has been achieved in a number of ways, including assessment of histopathology, direct neural stimulation, reflex elicitation, and passive monitoring (Figure 3). Although histopathology is considered the gold standard for validation of RDN in preclinical studies, this approach is unsuitable in patients. Three other modalities are the subject of exciting new developments that may translate to the clinic to provide intraprocedural validation of RDN.
FIGURE 3. Modalities of Procedural Monitoring.
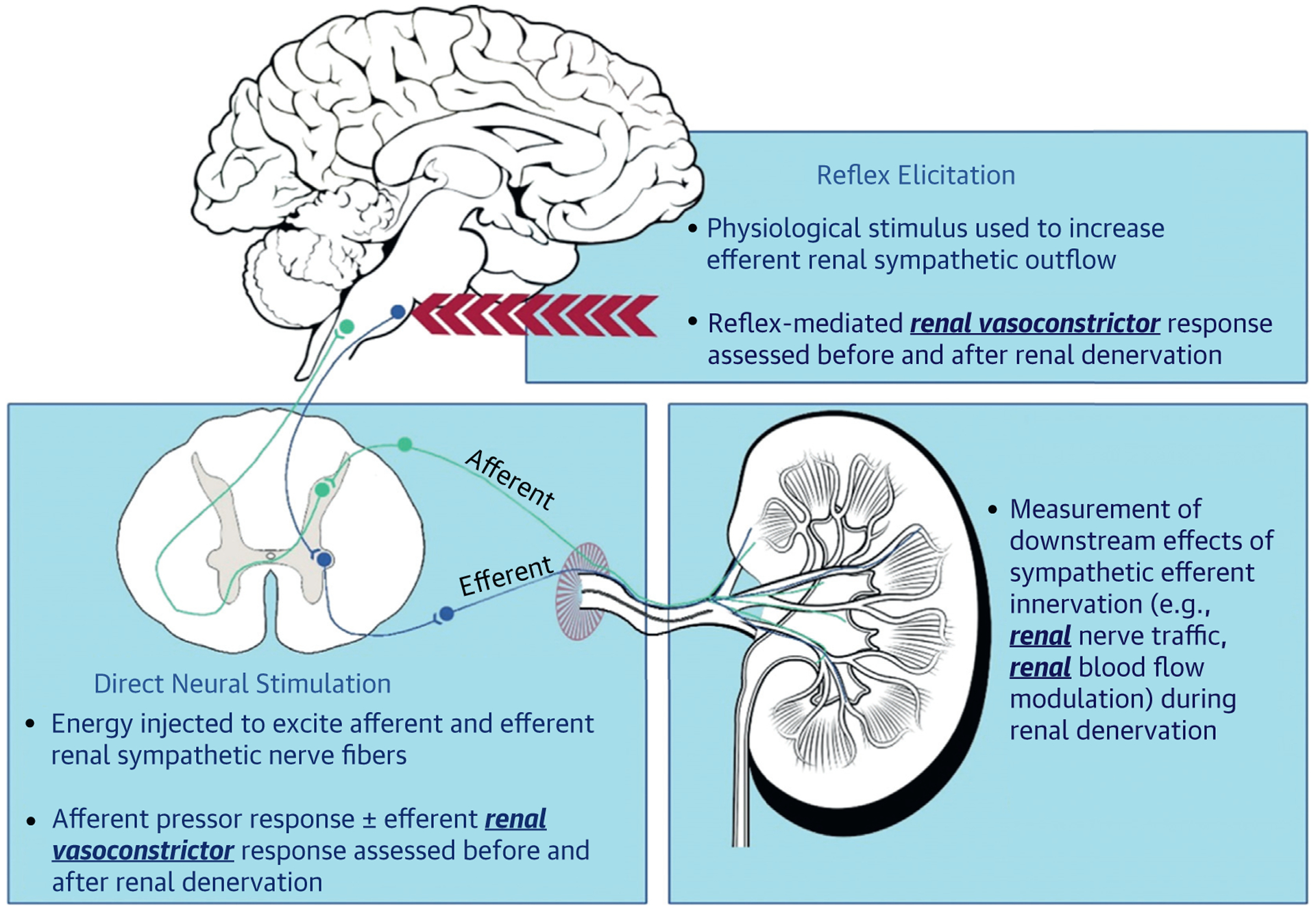
The promising 3 modalities of procedural monitoring (direct neural stimulation, reflex elicitation, and passive monitoring) evaluated by new technologies, providing intraprocedural validation of renal denervation.
DIRECT NEURAL STIMULATION OF AFFERENT RENAL NERVES.
Although the BP variability could merely be due to differences in the level of RDN that has been achieved in individual patients (i.e., “completeness” of nerve damage), another possibility is that some of these renal nerve fibers may act as “pressor nerves,” that is, promoting increases in BP when activated, whereas activation of sympatho-inhibitory afferent “depressor nerves” may lead to a fall in BP. Predominant ablation of pressor nerves would be expected to produce the largest fall in BP, whereas ablation of depressor nerve fibers might result in no effect or even a rise in BP after RDN. The net effect on BP may therefore vary substantially. Although currently mainly a theoretical working concept without exact knowledge about spatial arrangement of these fibers and other anatomic and physiological features, identification of potential “ideal” ablation sites appears highly attractive.
It is possible to activate renal nerves using a catheter placed in the renal artery (38). Sites in the renal artery that upon stimulation do not exert an acute BP rise (or even a BP fall) may reflect areas of convergence of sympatho inhibitory (depressor) nerves, the ablation of which should ideally be avoided. In contrast, those sites at which stimulation results in a clear BP rise (pressor nerves) would be considered preferential ablation sites. Clearly, with unselected RDN, the possibility of ablating sympatho-inhibitory depressor (cold spot) or neutral (neutral spot) nerve fibers seems likely and may counteract BP-lowering effects achieved by ablating pressor nerves (hot spots) (39) (Figure 4).
FIGURE 4. Theoretical Framework for Selective Versus Nonselective Renal Denervation.
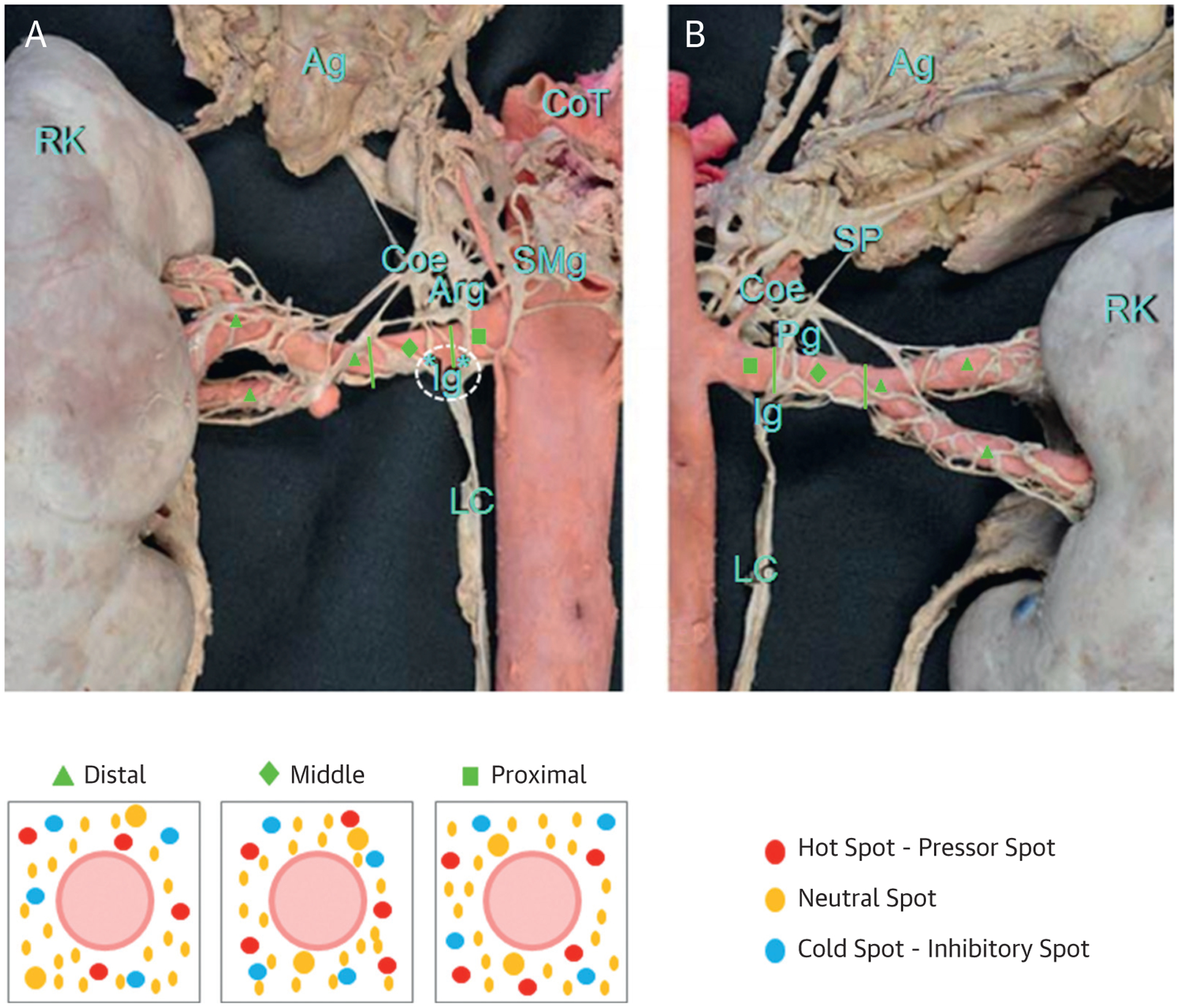
(Top) The right renal artery sympathetic innervation in (A) anterior and (B) posterior views divided in 3 segments: proximal (green squares), middle (green diamonds), and distal (green triangles). (Bottom) A schematic concept for selective versus global renal denervation: red dots represent “hot spots”—pressor spots. These are nerves that when stimulated increase BP. They are the ideal target for renal denervation. Blue dots represent “cold spots”—inhibitory spots, which lower BP when stimulated. The yellow dots represent the majority of nerve fibers, which are neutral in their contribution to BP control and do not show hemodynamic effects when stimulated. *Connection between ganglia. Adapted with permission from Fudim et al. (39) Mompeo et al. (68). Ag = adrenal gland; Arg = aorticorenal ganglion; Coe = coeliac ganglion; CoT = coeliac trunk; Ig = renal inferior ganglion; LC = contribution of the lumbar chain to the renal plexus; Pg = renal posterior ganglion; RK = right kidney; SMg = superior mesenteric ganglion; SP = thoracic splanchnic nerves.
Lu et al. (40) reported that renal nerve stimulation in both proximal and middle regions of the renal artery in dogs promptly increased systolic BP >10 mm Hg. However, during stimulation of the distal portions of the renal artery, BP did not increase. Only the proximal “responsive” sites were ablated and resulted in a systolic BP reduction of 24 mm Hg at 3 months of follow-up (p = 0.001 vs. baseline). As a result, targeted selective ablation not only prevented similar BP response with stimulation at the previously responsive sites, but also attenuated the response to stimulus at the ipsilateral midrenal arterial sites, suggesting that afferent renal nerves are likely to play an important role in BP reduction.
Recently, Tsioufis et al. (41) reported a first-in-man study, in which 20 hypertensive patients underwent renal nerve stimulation using the ConfidenHT system. Bilateral renal nerve stimulations were performed at 3 to 4 sites/artery at 2 and 4 mA, respectively. Stimulation with 2 mA resulted in a maximum increase of 8 ± 6 mm Hg in systolic BP (based on 119 stimulations; p < 0.001) while stimulating with 4 mA resulted in a maximum increase in stimulus-evoked BP of 10 ± 8 mm Hg (based on 61 stimulations; p < 0.001). The mean increase in systolic BP did not vary between mid, distal, or branch sites when stimulating at 2 mA but was significantly higher at ostial (23 ± 14 mm Hg) than in nonostial locations (9 ± 7 mm Hg) when stimulating at 4 mA (p = 0.003). This suggests that renal nerve stimulation might help in optimizing treatment effects (41).
In the SPYRAL HTN-OFF and HTN-ON MED trials, a large number of ablations were performed safely (>40 ablations) in the main renal artery and its branches, and this approach appeared to be effective and consistent in lowering BP. However, these findings do not oppose the concept of “hot spots,” as the higher number of ablated sites may improve the chance of hitting pressor nerves. Moreover, the successful RDN achieved through a circular lesion in the main renal artery by the endovascular ultrasound system used in RADIANCE-HTN SOLO suggests that interruption of the renal nerves in any segment of the renal artery may be sufficient to achieve RDN or it has deeper penetration beyond the artery.
INDIRECT TESTING VIA REFLEX RESPONSES.
Whereas direct renal nerve stimulation uses energy, reflex elicitation uses a physiological stimulus to excite the efferent renal sympathetic nerves and thus, reflex-mediated renal vasoconstriction before and after RDN is used to assess intervention success. In patients, renal sympathetic neural vasoconstriction has been shown in response to mental stress (42), head-up tilt (43), and lower body negative pressure (44), and recent reports of isometric handgrip (45) exercise-driven renal vasoconstriction in patients undergoing RDN are promising.
Contrasting from direct neural stimulation, reflex elicitation (42–45) is not painful, and thus, can be performed in conscious patients. It also assesses the integrity of the entire efferent limb of the renal sympathetic innervation, avoiding complexities arising from relationships between stimulation and denervation loci. However, this modality also has limitations, as repeatability of reflexes may be impacted by habituation, sensitization, and intraprocedural sedative and analgesic medications.
PASSIVE MONITORING.
Passive monitoring techniques detect spontaneous efferent renal sympathetic nerve activity or its downstream effects, which are attenuated after successful RDN. The classic example is renal norepinephrine spillover, which was used to demonstrate renal sympathoexcitation in hypertensive patients (46) and to validate achieved RDN in a small group of patients (37). Although renal norepinephrine spillover is unsuitable to be used broadly for intraprocedural monitoring, new technologies based on passive monitoring approaches are being developed. One early-stage device detects spontaneous renal sympathetic nerve traffic from within the renal artery lumen (Autonomix Medical, Doylestown, Pennsylvania) (47). Additionally, to overcome the strong and confounding influence of autoregulatory mechanisms on traditional measures of renal vascular tone (48,49), novel means of renal sympathetic vascular control are under investigation for intraprocedural feedback (48,50,51).
Unlike other modalities, passive monitoring does not require a stimulus-response profile, which is inherently time-consuming and challenging to reproduce. However, passive monitoring techniques can be influenced by any factor that changes renal sympathetic outflow, including patient anxiety, autonomic reflexes, and pharmacological agents used for analgesia and sedation.
DO RENAL NERVES REGROW FOLLOWING RDN?
In 2013, Mulder et al. (52) reported that substance P and calcitonin gene-related peptide content of sensory nerves in the denervated kidney gradually returned toward that of the innervated kidney over a period of 12 weeks after RDN. This followed a similar time course as the return of neuropeptide Y and tyrosine hydroxylase content in sympathetic nerves. Whether restoration of neurotransmitter content demonstrated herein is associated with restoration of function of both efferent sympathetic and afferent sensory nerves remains to be demonstrated (52).
Booth et al. (53) reported that in sheep, functional reinnervation was demonstrated by the finding that electrical stimulation of the whole renal nerve resulted in normal afferent and efferent renal nerve responses, in contrast to the lack of responses acutely post-RDN.
Anatomical and biochemical studies indicated that 1 week after catheter-based RDN, the renal levels of markers for sympathetic efferent and afferent sensory nerves were significantly reduced, but by 11 months post-RDN, the levels had returned to pre-RDN levels, suggestive of reinnervation of both the sensory renal afferent nerves and the sympathetic efferent nerves (53). It is currently unknown whether the control of the renal vasculature, renin release, and sodium excretion in response to changes in RSNA is normal in the reinnervated kidney and whether there are changes in the central neural pathways controlling RSNA following RDN. However, Singh et al. (54) demonstrated in a hypertensive chronic kidney disease sheep model that BP, renin, and sodium excretion responses to blood loss were markedly blunted 2 and 5 months post-RDN.
Conversely, Mauriello et al. (55) reported evidence of neural sprouting as early as 5 months after human kidney transplantation. In all likelihood, nerve sprouting stems from sympathetic ganglia, because neural regeneration appears to be limited to sympathetic efferent nerve fibers. Complete periadventitial renal nerve regeneration in hypertensive subjects to reach levels seen in native arteries, being paralleled by worsening of hypertension-related lesions in distal arterioles, seems to be achieved within 24 months following renal transplantation. Indeed, nerve density in kidney transplant arteries tends to reach levels similar to those in native renal arteries (55).
Reinnervation in humans following catheter-based RDN has not been demonstrated, which raises the question of underlying mechanisms capable of chronically maintaining a sustained decrease in BP despite potential reinnervation. Reduced renal vascular resistance, decreased intrarenal levels of renin, reduced RSNA burst size and burst incidence, changes in central autonomic nuclei, and others are possible explanations that require further exploration.
THE POTENTIAL ROLE OF INFLAMMATORY PATHWAYS.
Some studies propose that renal inflammation may be directly triggered by amplified RSNA, as there is much evidence that inflammation of the vasculature, brain, and kidneys contributes to chronic increases in BP (56–58). Recently, a study reported that angiotensin II (AngII)–induced hypertension in mice is decreased by RDN and that this is paralleled by reduced renal inflammation independently of the BP (59). Because specific ablation of renal afferent nerves had no effect on the pathogenesis of hypertension in this study, it was suggested that the antihypertensive effect of RDN was due to ablation of efferent renal sympathetic nerve-mediated renal inflammation (59). Similarly, Banek et al. (60) also reported that RDN attenuates hypertension and renal inflammation in the rat deoxycorticosterone acetate (DOCA)-salt model of hypertension. Importantly, they also stated that resting afferent renal nerve discharge is elevated in DOCA-salt rats, and they hypothesized that this is caused by an increase in certain inflammatory cytokines in the kidney (60). Another finding was that afferent-specific renal nerve ablation attenuated the development of hypertension in this model to the same degree as total RDN (60). The authors concluded that although renal inflammation may have its onset in efferent renal sympathetic nerves, hypertension was driven by augmented afferent renal nerve traffic, probably secondary to renal inflammation (60). A subsequent study from the same group demonstrated that afferent-specific renal nerve ablation also decreased arterial pressure in the established phase of DOCA-salt hypertension to the same degree as total RDN (61). However, neither method of ablation reversed renal inflammation in the established phase of this model, suggesting the presence of other mechanisms responsible for the inflammation.
Although there is a shortage of clinical studies directly measuring renal inflammation after RDN, there are several studies in which markers of peripheral inflammation were evaluated. Kampmann et al. (62) reported similar cardiovascular and inflammatory responses in hypertensive humans who underwent catheter-based RDN, where all measured circulating inflammatory cytokines (tumor necrosis factor-α, interleukin [IL]-6, and IL-1β) remained un-affected 6 months post-treatment, despite a significant decrease in arterial pressure. In contrast to these results, a clinical report from Zaldivia et al. (63) described a reduction in circulating inflammatory cytokines (MCP-1, IL-1β, tumor necrosis factor-α, and IL-12) several months after catheter-based RDN in hypertensive subjects. Further clinical and preclinical investigations are necessary to elucidate the anti-inflammatory effect of RDN.
DRUG INTERACTIONS
The recent 3 sham-controlled trials, SPYRAL HTN-OFF MED and RADIANCE SOLO (in which patients were not on concurrent medication), and SPYRAL HTN-ON MED (in which 29% of individuals were using 1 drug, 18% were taking 2 pills, and 53% were using 3 types of antihypertensive medications), demonstrated a significant baseline-adjusted fall in ambulatory BP, although background treatment and baseline BP levels differed between them (Table 1). Similar BP responses in SPYRAL HTN-OFF and RADIANCE-HTN SOLO suggest that multiple lesions in the main renal artery and its branches is equivalent to circumferential lesion to main renal artery only, albeit the depth of lesions may be relevant in this context. Further studies are needed to elucidate if spironolactone and/or clonidine cause any drug interaction with RDN or if their response can predict responders to RDN, as clonidine reduced BP (p < 0.001) and plasma noradrenaline concentrations (p < 0.05) significantly 7 and 30 days after therapy onset. The BP-lowering effects of clonidine are related to the centrally mediated and/or direct suppression of peripheral noradrenergic activity, indicating the utility of clonidine in cases of hypertension where the sympathetic nervous system is hyperactive (64). Thus, the premise that patients with greater sympathetic dependence (clonidine-sensitive) may represent a more susceptible population for RDN.
TABLE 1.
Baseline-Adjusted Fall in ABPM
| 24-h ABPM | ||||
|---|---|---|---|---|
| Trial | Systolic | p Value | Diastolic | p Value |
| SPYRAL HTN-ON MED | −7.0 mm Hg (−12.0 to −2.1) | 0.0059 | −4.3 mm Hg (−7.8 to −0.8) | 0.0174 |
| RADIANCE-HTN SOLO | −7.0 ± 8.6 mm Hg (−7.1 to −1.2) | 0.006 | − 4.4 ± 5.8 mm Hg (−3.7 to −0.2) | 0.07 |
| SPYRAL HTN-OFF MED | −5.3 mm Hg (−8.6 to −2.0) | 0.0020 | −4.8 mm Hg (−6.8 to −2.8) | 0.001 |
Values are mean (95% confidence interval) or mean ± SD (95% confidence interval).
ABPM = ambulatory blood pressure measurements; CI = confidence interval; RADIANCE-HTN SOLO = Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicenter, international, single-blind, randomized, sham-controlled trial; SPYRAL HTN-ON MED = Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomized trial; SPYRAL HTN-OFF MED = Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomized, sham-controlled, proof-of-concept trial.
POTENTIAL EFFECT ON CARDIOVASCULAR OUTCOMES
Reduction in target organ damage by pharmacological treatment is closely linked to a decrease in the time-averaged BP it causes. In a meta-analysis including 613,815 patients from 122 studies, reduction of office systolic BP by 10 mm Hg was related to a reduction in cardiovascular events by 20%, overall mortality by 13%, coronary artery disease by 17%, stroke by 27%, and heart failure by 28%, respectively (65). In the HOPE-3 study, patients with baseline office systolic BP of >143.5 mm Hg had a reduction of systolic BP by −5.8/−3.0 mm Hg (due to pharmacological therapy) associated with a 28% lower incidence of cardiovascular events compared with the placebo group (66). In another meta-analysis of 147 randomized trials comprising 464,000 patients, a reduction in 10 mm Hg systolic and 5 mm Hg diastolic office BP was related to a decrease of coronary heart disease and stroke events by ~22 and 41%, respectively, depending on the age of the patient (67).
Although not proven by prospective outcome trials in the context of RDN, a ~10 mm Hg decrease in office systolic BP achieved in RDN trials in which the average age of the population was ~65 years, if maintained long-term, would be associated with a reduction in cardiovascular events by ~25%, especially heart failure and stroke.
SUMMARY
The positive results from SPYRAL HTN-OFF MED, RADIANCE SOLO, and SPYRAL HTN-ON MED reignited the RDN field. However, several unsolved issues remain to be addressed appropriately, including identifying those patients who may benefit most, defining the durability of effects on BP and safety in the long-term, determining the mechanisms of the BP reduction to optimize response, and developing tests and technologies to establish the extent of renal artery denervation, ideally at the time of intervention.
Filling in these blanks will help to advance the field further and ultimately determine the clinical utility of RDN. Here, we have summarized some of the most relevant issues identified by a group of clinical and experimental scientists to facilitate this critical task.
HIGHLIGHTS.
Sympathetic nervous system activation is a key contributor to elevated BP.
Catheter-based RDN reduces BP in both treated and drug naïve patients.
Improved trial design, patient selection, and optimized procedural approaches contributed to these positive findings.
Future research will focus on reducing the variability of the BP response and indications beyond hypertension.
Acknowledgments
All authors received honoraria and travel support from Medtronic to present at “The SNS Summit” held in Barcelona in June 2018. Dr. Esler has been supported in part by the Victorian Government’s Operational Infrastructure Support Program; has received salary support from an Australian Government NHMRC Senior Principal Research Fellowship; and has been a member of the Advisory Boards of SyMap and Medtronic. Dr. Banek is supported by National Institutes of Health grant K99 HL141650. Drs. Böhm and Mahfoud are supported by Deutsche Gesellschaft für Kardiologie, Deutsche Hochdruckliga, and Deutsche Forschungsgemeinschaft (SFB TRR 219); and have received grant support and personal fees from Medtronic and Recor Medical. Dr. Böhm has received research support and speaker fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Servier, Medtronic, Vifor, and Novartis. Dr. Denton is supported by an NHMRC research fellowship; and has received travel support from Medtronic. Dr. Everett is supported in part by National Institutes of Health grant R42DA043391 and a Charles Fisch Cardiovascular Research Award endowed by Dr. Suzanne B. Knoebel of the Krannert Institute of Cardiology. Dr. Mahfoud has received research grants from and served on the Speakers Bureau of Medtronic and Recor. Dr. Paton was supported by the British Heart Foundation and the Health Research Council of New Zealand. Dr. Schmieder has received speaker fees, consultancy, and advisory board fees from Ablative Solutions, Medtronic, Recor, and ROX Medical. Dr. Pellegrino has received honoraria and travel support from Medtronic. Dr. Schlaich is supported by an NHMRC Research Fellowship; and has received consulting fees and/or travel and research support from Medtronic, Abbott, Novartis, Servier, Pfizer, and Boehringer Ingelheim.
ABBREVIATIONS AND ACRONYMS
- DOCA
deoxycorticosterone acetate
- DS
Dahl salt-sensitive
- RDN
renal denervation
- RSNA
renal sympathetic nerve activity
- RVLM
rostral ventrolateral medulla
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
REFERENCES
- 1.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 2009;373:1275–81. [DOI] [PubMed] [Google Scholar]
- 2.Esler MD, Krum H, Sobotka PA, et al. , for the Symplicity HTN-2 Investigators. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet 2010;376:1903–9. [DOI] [PubMed] [Google Scholar]
- 3.Donazzan L, Mahfoud F, Ewen S, et al. Effects of catheter-based renal denervation on cardiac sympathetic activity and innervation in patients with resistant hypertension. Clin Res Cardiol 2016; 105:364–71. [DOI] [PubMed] [Google Scholar]
- 4.Hering D, Marusic P, Walton AS, et al. Sustained sympathetic and blood pressure reduction 1 year after renal denervation in patients with resistant hypertension. Hypertension 2014;64:118–24. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt DL, Kandzari DE, O’Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014;370:1393–401. [DOI] [PubMed] [Google Scholar]
- 6.Kandzari DE, Bhatt DL, Brar S, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J 2015;36:219–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahfoud F, Schmieder RE, Azizi M, et al. Proceedings from the 2nd European Clinical Consensus Conference for device-based therapies for hypertension: state of the art and considerations for the future. Eur Heart J 2017;38:3272–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet 2015;385:1957–65. [DOI] [PubMed] [Google Scholar]
- 9.Azizi M, Pereira H, Hamdidouche I, et al. Adherence to antihypertensive treatment and the blood pressure-lowering effects of renal denervation in the renal denervation for hypertension (DENERHTN) Trial. Circulation 2016;134:847–57. [DOI] [PubMed] [Google Scholar]
- 10.Brinker S, Pandey A, Ayers C, et al. Therapeutic drug monitoring facilitates blood pressure control in resistant hypertension. J Am Coll Cardiol 2014; 63:834–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jung O, Gechter JL, Wunder C, et al. Resistant hypertension? Assessment of adherence by toxicological urine analysis. J Hypertens 2013;31: 766–74. [DOI] [PubMed] [Google Scholar]
- 12.Tomaszewski M, White C, Patel P, et al. High rates of non-adherence to antihypertensive treatment revealed by high-performance liquid chromatography-tandem mass spectrometry (HP LC-MS/MS) urine analysis. Heart 2014;100: 855–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kandzari DE, Bohm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018;391:2346–55. [DOI] [PubMed] [Google Scholar]
- 14.Hameed MA, Tebbit L, Jacques N, Thomas M, Dasgupta I. Non-adherence to antihypertensive medication is very common among resistant hypertensives: results of a directly observed therapy clinic. J Hum Hypertens 2016;30:83–9. [DOI] [PubMed] [Google Scholar]
- 15.Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 2017;390:2160–70. [DOI] [PubMed] [Google Scholar]
- 16.Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018;391: 2335–45. [DOI] [PubMed] [Google Scholar]
- 17.Schlaich MP, Kiuchi MG, Esler MD. Renal denervation-ready for prime time!? The steep SPYRAL stairs to RADIANCE in hypertension treatment. Hypertension 2018;72:287–90. [DOI] [PubMed] [Google Scholar]
- 18.Morrow AL, Creese I. Characterization of alpha-1-adrenergic receptor subtypes in rat-brain—a reevaluation of [H-3] Wb4104 and [H-3] Prazosin Binding. Mol Pharmacol 1986;29:321–30. [PubMed] [Google Scholar]
- 19.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Compr Physiol 2011;1:731–67. [DOI] [PubMed] [Google Scholar]
- 20.Lohmeier TE, Iliescu R, Dwyer TM, Irwin ED, Cates AW, Rossing MA. Sustained suppression of sympathetic activity and arterial pressure during chronic activation of the carotid baroreflex. Am J Physiol Heart Circ Physiol 2010;299:H402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohmeier TE, Iliescu R, Liu B, Henegar JR, Maric-Bilkan C, Irwin ED. Systemic and renal-specific sympathoinhibition in obesity hypertension. Hypertension 2012;59:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiBona GF, Esler M. Translational medicine: the antihypertensive effect of renal denervation. Am J Physiol-Regul Integr Comp Physiol 2010; 298:R245–53. [DOI] [PubMed] [Google Scholar]
- 23.Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 2013;61:806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacob F, Ariza P, Osborn JW. Renal denervation chronically lowers arterial pressure independent of dietary sodium intake in normal rats. Am J Physiol-Heart Circ Physiol 2003;284:H2302–10. [DOI] [PubMed] [Google Scholar]
- 25.Jacob F, LaBine BG, Ariza P, Katz SA, Osborn JW. Renal denervation causes chronic hypotension in rats: Role of beta(1)-adrenoceptor activity. Clin Exp Pharmacol P 2005;32:255–62. [DOI] [PubMed] [Google Scholar]
- 26.Foss JD, Fink GD, Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol-Regul Integr Comp Physiol 2016;310:R262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guertzenstein PG, Silver A. Fall in blood pressure produced from discrete regions of the ventral surface of the medulla by glycine and lesions. J Physiol 1974;242:489–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai WC, Chan YH, Chinda K, et al. Effects of renal sympathetic denervation on the stellate ganglion and brain stem in dogs. Heart Rhythm 2017;14:255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bramlett HM, Dietrich WD. Progressive damage after brain and spinal cord injury: patho-mechanisms and treatment strategies. Prog Brain Res 2007;161:125–41. [DOI] [PubMed] [Google Scholar]
- 30.Meckler RL, Weaver LC. Comparison of the distributions of renal and splenic neurons in sympathetic ganglia. J Auton Nerv Syst 1984;11:189–200. [DOI] [PubMed] [Google Scholar]
- 31.Sripairojthikoon W, Wyss JM. Cells of origin of the sympathetic renal innervation in rat. Am J Physiol 1987;252:F957–63. [DOI] [PubMed] [Google Scholar]
- 32.Gattone VH 2nd., Marfurt CF, Dallie S. Extrinsic innervation of the rat kidney: a retrograde tracing study. Am J Physiol 1986;250:F189–96. [DOI] [PubMed] [Google Scholar]
- 33.Ferguson M, Ryan GB, Bell C. Localization of sympathetic and sensory neurons innervating the rat kidney. J Auton Nerv Syst 1986;16:279–88. [DOI] [PubMed] [Google Scholar]
- 34.Pilowsky P, Llewellyn-Smith IJ, Minson J, Chalmers J. Sympathetic preganglionic neurons in rabbit spinal cord that project to the stellate or the superior cervical ganglion. Brain Res 1992;577:181–8. [DOI] [PubMed] [Google Scholar]
- 35.Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension 1995;25: 878–82. [DOI] [PubMed] [Google Scholar]
- 36.Jansen AS, Wessendorf MW, Loewy AD. Transneuronal labeling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res 1995; 683:1–24. [DOI] [PubMed] [Google Scholar]
- 37.Esler M Illusions of truths in the Symplicity HTN-3 trial: generic design strengths but neuroscience failings. J Am Soc Hypertens 2014;8:593–8. [DOI] [PubMed] [Google Scholar]
- 38.de Jong MR, Adiyaman A, Gal P, et al. Renal nerve stimulation-induced blood pressure changes predict ambulatory blood pressure response after renal denervation. Hypertension 2016;68:707–14. [DOI] [PubMed] [Google Scholar]
- 39.Fudim M, Sobotka AA, Yin YH, et al. Selective vs. global renal denervation: a case for less is more. Curr Hypertens Rep 2018;20:37. [DOI] [PubMed] [Google Scholar]
- 40.Lu J, Wang Z, Zhou T, et al. Selective proximal renal denervation guided by autonomic responses evoked via high-frequency stimulation in a preclinical canine model. Circ Cardiovasc Interv 2015; 8:e001847. [DOI] [PubMed] [Google Scholar]
- 41.Tsioufis KP, Feyz L, Dimitriadis K, et al. Safety and performance of diagnostic electrical mapping of renal nerves in hypertensive patients. Euro-Intervention 2018;14:e1334–42. [DOI] [PubMed] [Google Scholar]
- 42.Ray CA, Carter JR. Effects of aerobic exercise training on sympathetic and renal responses to mental stress in humans. Am J Physiol Heart Circ Physiol 2010;298:H229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conboy EE, Fogelman AE, Sauder CL, Ray CA. Endurance training reduces renal vasoconstriction to orthostatic stress. Am J Physiol Renal Physiol 2010;298:F279–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hansen JM, Abildgaard U, Fogh-Andersen N, et al. The transplanted human kidney does not achieve functional reinnervation. Clin Sci (Lond) 1994;87:13–20. [DOI] [PubMed] [Google Scholar]
- 45.Burchell A, Hinton T, Hart E, Nightingale A, Paton J, Baumbach A. Assessment of the procedural efficacy of renal denervation by measurement of efferent renal nerve function. J Hum Hypertens 2017;31:677–8. [Google Scholar]
- 46.Esler M, Jennings G, Lambert G. Noradrenaline release and the pathophysiology of primary human hypertension. Am J Hypertens 1989;2:140S–6S. [DOI] [PubMed] [Google Scholar]
- 47.Autonomix. Available at: https://www.autonomixmed.com/. Accessed April 26, 2019.
- 48.Schiller AM, Pellegrino PR, Zucker IH. Eppur si muove: the dynamic nature of physiological control of renal blood flow by the renal sympathetic nerves. Auton Neurosci 2017;204:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev 2015;95:405–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pellegrino P, Schiller AM, Wang H, Chatzizisis Y, Zucker I. Functional renal denervation decreases renal vascular control quantified by pressure-flow monitoring in swine (abstr). ACC18. J Am Coll Cardiol 2018;71:A1179. [Google Scholar]
- 51.Finegold J, Broyd C, Shun-shin M, et al. Systematic evaluation of haemodynamic parameters to predict haemodynamic responders to renal artery denervation. Paper presented at: Euro PCR; Paris, France; 2016. [Google Scholar]
- 52.Mulder J, Hokfelt T, Knuepfer MM, Kopp UC. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am J Physiol Regul Integr Comp Physiol 2013;304:R675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Booth LC, Nishi EE, Yao ST, et al. Reinnervation of renal afferent and efferent nerves at 5.5 and 11 months after catheter-based radio-frequency renal denervation in sheep. Hypertension 2015;65:393–400. [DOI] [PubMed] [Google Scholar]
- 54.Singh RR, Sajeesh V, Booth LC, et al. Catheter-based renal denervation exacerbates blood pressure fall during hemorrhage. J Am Coll Cardiol 2017;69:951–64. [DOI] [PubMed] [Google Scholar]
- 55.Mauriello A, Rovella V, Borri F, et al. Hypertension in kidney transplantation is associated with an early renal nerve sprouting. Nephrol Dial Transplant 2017;32:1053–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Miguel C, Rudemiller NP, Abais JM, Mattson DL. Inflammation and hypertension: new understandings and potential therapeutic targets. Curr Hypertens Rep 2015;17:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ Res 2015;116: 1022–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zubcevic J, Santisteban MM, Pitts T, et al. Functional neural-bone marrow pathways: implications in hypertension and cardiovascular disease. Hypertension 2014;63:e129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xiao L, Kirabo A, Wu J, et al. Renal denervation prevents immune cell activation and renal inflammation in angiotensin II-induced hypertension. Circ Res 2015;117:547–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banek CT, Knuepfer MM, Foss JD, et al. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 2016;68:1415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banek CT, Gauthier MM, Baumann DC, et al. Targeted afferent renal denervation reduces arterial pressure but not renal inflammation in established DOCA-salt hypertension in the rat. Am J Physiol-Regul Integr Comp Physiol 2018;314:R883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kampmann U, Mathiassen ON, Christensen KL, et al. Effects of renal denervation on insulin sensitivity and inflammatory markers in nondiabetic patients with treatment-resistant hypertension. J Diabetes Res 2017;2017:6915310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaldivia MT, Rivera J, Hering D, et al. Renal denervation reduces monocyte activation and monocyte-platelet aggregate formation: an anti-inflammatory effect relevant for cardiovascular risk. Hypertension 2017;69:323–31. [DOI] [PubMed] [Google Scholar]
- 64.Chernow B, Lake CR, Zaloga GP, Coleman MD, Ziegler MG. Effect of clonidine on sympathetic nervous-system activity in patients with essential-hypertension. Int J Clin Pharm Res 1983;3:9–15. [PubMed] [Google Scholar]
- 65.Ettehad D, Emdin CA, Kiran A, et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–67. [DOI] [PubMed] [Google Scholar]
- 66.Yusuf S, Lonn E, Pais P, et al. Blood-pressure and cholesterol lowering in persons without cardiovascular disease. N Engl J Med 2016;374: 2032–43. [DOI] [PubMed] [Google Scholar]
- 67.Law MR, Morris JK, Wald NJ. Use of blood pressure lowering drugs in the prevention of cardiovascular disease: meta-analysis of 147 randomised trials in the context of expectations from prospective epidemiological studies. BMJ 2009; 338:b1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mompeo B, Maranillo E, Garcia-Touchard A, Larkin T, Sanudo J. The gross anatomy of the renal sympathetic nerves revisited. Clin Anat 2016;29:660–4. [DOI] [PubMed] [Google Scholar]


