Summary
To survive, mammalian cells must adapt to environmental challenges. While the cellular response to mild stress has been widely studied, how cells respond to severe stress remains unclear. We show here that under severe hyperosmotic stress, cells enter a transient hibernation-like state in anticipation of recovery. We demonstrate this Adaptive Pausing Response (APR) is a coordinated cellular response that limits ATP supply and consumption through mitochondrial fragmentation and widespread pausing of mRNA translation. This pausing is accomplished by ribosome stalling at translation initiation codons, which keeps mRNAs poised to resume translation upon recovery. We further show that recovery from severe stress involves ISR (Integrated Stress Response) signaling that permits cell cycle progression, resumption of growth, and reversal of mitochondria fragmentation. Our findings indicate cells can respond to severe stress via a hibernation-like mechanism that preserves vital elements of cellular function under harsh environmental conditions.
Keywords: ribosome stalling, stress, mTOR, ISR, mitochondria, hypertonic, translation, ATF4, neMito mRNAs
Graphical Abstract

eTOC:
Jobava et al. show that near-lethal environmental stress induces a hibernation-like state of severe mitochondrial fragmentation with 80S ribosomes stalled at the start codons of a select group of mRNAs. Induction of ISR during removal of stress reverses the hibernation-like state and promotes a return to homeostasis.
Introduction
Cells adapt to different stressors by disrupting the translational apparatus and altering the translatome to selectively express a subset of factors governing stress responses (Roux and Topisirovic, 2018). This is accompanied by downregulation of total protein synthesis, which helps maintain the cellular energy balance. Two major pathways that lead to translational reprogramming in response to stress are the integrated stress response (ISR) and mechanistic/mammalian target of rapamycin (mTOR) pathway (Costa-Mattioli and Walter, 2020; Roux and Topisirovic, 2018). The ISR involves signaling to eukaryotic translation initiation factor 2 (eIF2) culminating in phosphorylation of its α subunit (Costa-Mattioli and Walter, 2020). This impairs recycling of eIF2:GDP to eIF2:GTP by the guanine nucleotide exchange factor eIF2B, thus limiting Met-tRNAi delivery to the ternary complex. mTOR, a serine/threonine kinase component of distinct complexes mTORC1 and mTORC2, integrates extracellular stimuli and intracellular cues to promote cellular growth and proliferation (Liu and Sabatini, 2020). In response to stress, mTOR signaling is downregulated, altering levels and/or functions of translational components and associated factors (Roux and Topisirovic, 2018). Stressors, therefore, can induce the ISR and reduce mTORC1 activity to establish adaptive translational programs wherein selective translation of target mRNAs can be conferred by specific 5’-UTR features (Hinnebusch et al., 2016). Reports have indicated multiple mechanisms account for stress-induced perturbations in translation, including phase separation (Franzmann et al., 2018; Iserman et al., 2020) and alternate initiation factors (Sendoel et al., 2017; Starck et al., 2012) (Jeong et al., 2019; Meyer et al., 2015). These have been studied in mild and moderate stress conditions, but strategies used to survive extreme stress are unknown.
In response to increased extracellular osmolarity (hyperosmotic stress), cells initially shrink, disrupting mitochondria structure (Copp et al., 2005) and decreasing protein synthesis (Saikia et al., 2012). The osmoadaptive response induces expression of chaperones and proteins that promote cellular uptake of compatible osmolytes to restore cell volume and function (Burg et al., 2007; Grady et al., 2014; Izumi et al., 2015). We have shown that increased stress intensity inhibits osmoadaptation and activates proinflammatory gene expression (Farabaugh et al., 2020). Previous studies suggested cells can survive extreme hyperosmotic conditions (Saikia et al., 2014) in neurodegenerative diseases (Motori et al., 2020) and cancer (Hangauer et al., 2017; Picco et al., 2017).
Here we investigated the cellular response to severe increase of extracellular osmolarity. We made the striking observation that severe stress induces a hibernation-like cellular state in anticipation of recovery, which we named the Adaptive Pausing Response (APR). The hallmark of the APR is widespread inhibition of mRNA translation initiation accompanied by widespread ribosome pausing at initiation codons. We further showed this pausing keeps cells poised to resume activity upon recovery from stress, characterized by ISR signaling, ultimate progression of the cell cycle, resumption of growth, and reversal of stress-induced mitochondria fragmentation. Our findings show mammalian cells preserve vital functions in response to severe environmental stress, and reveal the necessity of cellular plasticity to survive harsh environments.
Results
Increasing intensity of hyperosmotic stress prevents adaptive recovery of protein synthesis
We examined the proliferation of mouse embryonic fibroblasts (MEFs) in response to increasing hyperosmotic stress (500, 600, and 700 mOsm). In response to 500 mOsm stress, cells osmoadapted (Burg et al., 2007) and resumed proliferation. In contrast, cell proliferation was halted at 600 mOsm, and survival gradually decreased in 700 mOsm (Fig. 1A), indicating cells can survive severe hyperosmotic stress for a limited time. We next determined the rates of protein synthesis in MEFs exposed to increasing extracellular osmolarity (Fig. 1B and S1A). Exposure to mild (500 mOsm) stress for 30 min decreased protein synthesis by 72%, which returned to normal levels with establishment of osmoadaptation (Fig. S1A and 1B). Moderate (600 mOsm) and severe (700 mOsm) stress intensities decreased protein synthesis by more than 80% after 2 h with no recovery (Figs. 1B and S1A). Inhibition of protein synthesis correlated with increased eIF2α phosphorylation and mTORC1 substrate p70 (S6K) dephosphorylation (Fig. 1C), suggesting both eIF2α phosphorylation-mediated decrease in ternary complex availability and mTORC1 inhibition contribute to decreased protein synthesis in response to stress. However, the difference in survival between 500 mOsm and 600 or 700 mOsm suggests higher stress intensities trigger mechanisms to delay cell death.
Figure 1. Inhibition of mRNA translation with increasing hyperosmotic stress intensity.
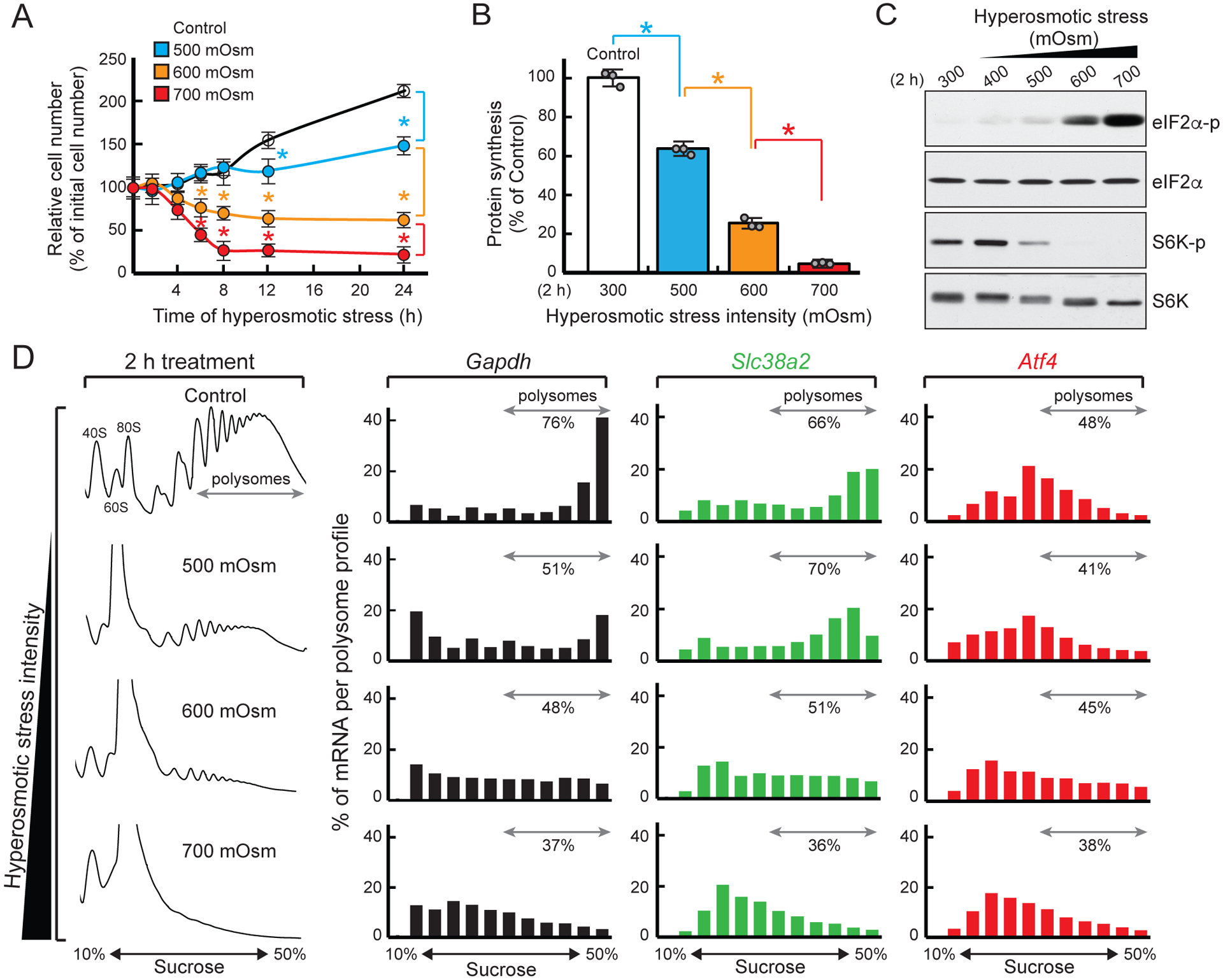
MEFs treated with the indicated hyperosmotic stress conditions were analyzed by (A) cell counting, (B) protein synthesis assays, (C) Western blotting, and (D) polysome profiling. Means ± SEM of triplicate determinations are shown. *p < 0.01
To compare adaptation mechanisms, we analyzed translation of osmoadaptive and stress-related mRNAs. We monitored polysomal distribution of mRNAs encoding master regulators of the cellular stress response: the osmoadaptive amino acid transporter SNAT2/SLC38A2 (Burg et al., 2007) and the ISR transcription factor ATF4 (Andreev et al., 2015). Polysome profile analysis revealed a loss of polysomes as stress intensity increased, with concomitant accumulation of monosomes (Fig. 1D). While some polysomes were preserved in 600 mOsm, 700 mOsm stress caused almost complete dissociation of ribosomes from mRNAs. The housekeeping mRNA Gapdh shifted to smaller polysomes, 80S, and ribosome-free fractions with increasing intensity. In contrast, Slc38a2 mRNA was translated efficiently in cells exposed to mild stress, but its association with ribosomes declined at severe stress intensities (Fig 1D). Despite high levels of eIF2α phosphorylation, known to increase Atf4 mRNA translation under other stress conditions (Andreev et al., 2015), translation of Atf4 was attenuated at high intensity hyperosmotic stress (Fig. 1D).
Hyperosmotic stress induces a DNA damage response culminating in cell cycle arrest (Dmitrieva and Burg, 2008). We observed a replicative stress response (Fig. S1C) evidenced by elevated phosphorylation of serine/threonine protein kinase checkpoint kinase 1 (CHK1) (Bartek and Lukas, 2003) during recovery from 700 mOsm stress, but not during stress treatment itself. This signal was only activated transiently and rapidly decreased after 3 h recovery (Fig. S1C). Hence, recovery from hyperosmotic stress immediately activates a DNA replication checkpoint, possibly to allow time for repair of damaged DNA, while also facilitating synthesis of p21 and cyclin D1 (Fig. S1D). Increased p21 levels induce a G1 and G2/M phase block, whereas the later increase in cyclin D1 promotes progression to S phase (Abbas and Dutta, 2009). Notably, during recovery from severe stress, mTORC1 was reactivated (Fig. S1D), accompanied by a decline in eIF2α phosphorylation (Fig. S1C). These findings suggest cells halt protein synthesis and growth in response to severe hyperosmotic stress. Similar results were obtained when the media osmolarity was raised by addition of NaCl (Fig. S1E–G).
Increasing stress intensity involves reshaping of the translatome
We next performed ribosome profiling to analyze the distribution of ribosome footprints on mRNAs to understand whether translation regulation is involved in restricting cellular function under severe stress (Ingolia et al., 2009; Steitz, 1969). Ribosome-bound and cytoplasmic RNAs were isolated from cells treated with various stress intensities, and libraries were prepared for next-generation sequencing. We evaluated fold change of RNAs and ribosome footprints compared to untreated controls, and classified mRNAs into seven groups (Fig. 2A and Supp. File 1). This analysis indicated relative differences in mRNA translation within each condition while controlling for inhibition of protein synthesis. We observed a loss of ribosome-associated mRNAs (increase in riboocp-down group) in non-adaptive stress conditions (Fig. 2A), suggesting increased stress intensity leads to decreased association of mRNAs with ribosomes via inhibition of translation of select mRNAs. Gapdh and Atf4 mRNAs shifted from the no-change (NC) group in 500 and 600 mOsm stress conditions to the riboocp-down group in 700 mOsm stress (Fig. 2A). In contrast, Slc38a2 (Fig. 1D) shifted from the congruent up group in 500 mOsm to the NC group in 600 and 700 mOsm stress. These examples demonstrate a hierarchy in selective mRNA translation during stress.
Figure 2. Adaptation to increasing stress intensity involves differential enrichment of ribosome-associated mRNAs.
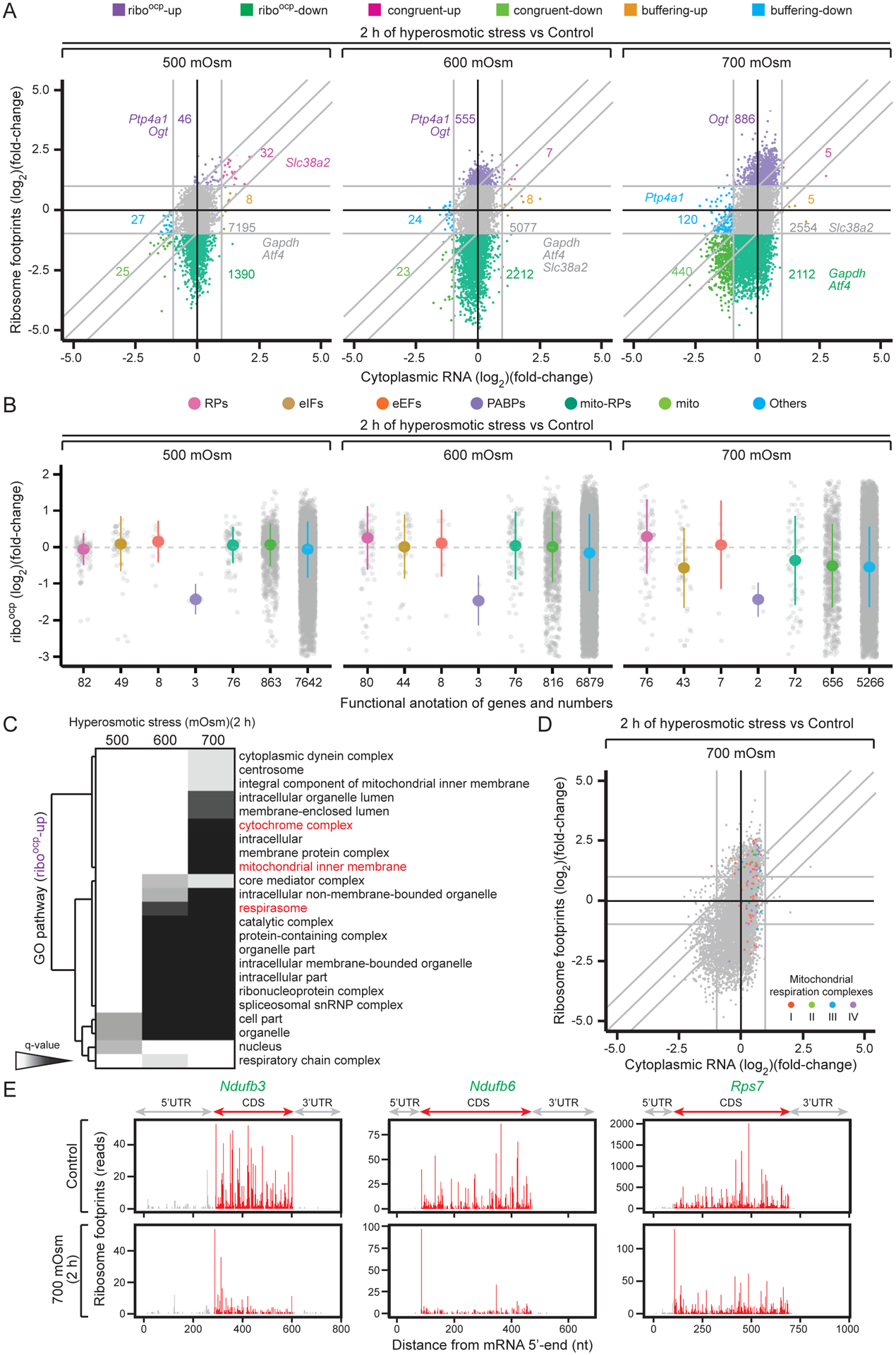
(A) Scatter plot comparing fold changes of ribosomal footprints (y-axis) and cytoplasmic RNA levels (x-axis) in MEFs exposed to the indicated hyperosmotic stress intensities. (B) Distribution of select mRNAs according to changes in their riboocp (ribosome footprints normalized to corresponding mRNA levels). The number of mRNAs in each group is indicated on the x-axis. (C) Hierarchical clustering of q-values associated with enriched GO terms of the riboocp-up groups. (D) Scatterplot comparing changes in ribosome footprints and cytoplasmic RNA levels of mRNAs encoding mitochondrial respiration complexes. (E) Ribosome footprints across select mRNA sequences. Footprints in the coding region (CDS) are highlighted in red, and in the untranslated regions (UTRs) in gray.
To better understand selective translation during adaptive pausing, we examined translation of mRNAs regulated by mTOR activity, such as 5’-terminal pyrimidine tract (TOP) mRNAs, mRNAs translationally inhibited upon treatment with the mTOR inhibitor Torin-1 (Thoreen et al., 2012), and nuclear mRNAs encoding mitochondrial proteins (neMito mRNAs) (Calvo et al., 2016; Morita et al., 2013). These mRNAs were largely in the NC group in 500 and 600 mOsm stress (Fig. S2A and Supp. File 2). In 700 mOsm, we observed a shift of some mTOR-related and neMito mRNAs to the riboocp-up group, suggesting selection for translation initiation under severe stress (Fig. S2A and Supp. File 2). We further broke down mTOR-related mRNAs by function. Except for three PABPs, mTOR-related mRNAs were not present in low-ribosome occupancy groups in 500 and 600 mOsm stress (Fig. 2B and Supp. File 2), suggesting these mRNAs are translated during severe stress, while other mTOR-related and neMito mRNAs are less inhibited (riboocp-up), likely via alternative mechanisms (Roux et al., 2007).
Pathway analysis of the riboocp-up groups across these stress intensities (Supp. File 1) identified mRNAs in the GO category “respirasome” as enriched in 600 and 700 mOsm stress (Fig. 2C). Enrichment of “cytochrome complex” and “mitochondria inner membrane”-encoding mRNAs was observed at 700 mOsm, suggesting continuous reprogramming of mRNA translation with increasing stress intensity to meet shifting needs. This was further supported by KEGG pathway analysis of the riboocp-up group, revealing differential enrichment in mild and severe stress conditions (Fig. S2B).
We next examined the distribution of mRNAs encoding mitochondrial respiration complexes (I-IV) and related factors in severe stress. We identified 85 mRNAs (almost all of those in complexes I-III, and half of those in complex IV) distributed in the NC and riboocp-up groups in the stress translatome (Fig. 2D). However, this distribution only compares relative translation within a translatome, not to unstressed cells. We therefore examined the distribution of footprints within individual mRNAs encoding mitochondrial respiration complexes in the riboocp-up group. In two examples (Ndufb3 and Ndufb6), ribosome footprints were distributed in the open reading frames (ORFs) in control cells, indicating efficient translation. In response to severe stress, we observed a sharp increase in ribosome pausing at the translation initiation codon and fewer reads in ORFs (Fig. 2E). mTOR-related mRNAs from the NC group such as rpS7 also showed a dramatic decrease in footprints in the ORF (Fig. 2E). This suggests adaptive pausing during severe hyperosmotic stress may halt ribosomes at the translation initiation codons of select mRNAs.
Adaptive pausing is marked by translatome and mRNA abundance changes of select mRNAs
Pathway analysis of the riboocp-up mRNAs in different stress intensities suggested remodeling of translation selectivity. Of the mRNAs in the riboocp-up group at 500 mOsm, 57% remained in the same category at 600 mOsm, and of those in this group at 600 mOsm, 74% remained at 700 mOsm. In cells exposed to mild stress, the relative number of ribosome footprints increased in 46 mRNAs without concomitant upregulation of their mRNA levels (riboocp-up, Fig. 2A). Among the mRNAs with the highest change in ribosome occupancy were Ptp4a1 (encoding a protein tyrosine phosphatase that regulates TGF-β signaling (Sacchetti et al., 2017), and important for hyperosmotic stress signaling (Tew et al., 2011)), and Ogt (encoding O-Linked N-Acetylglucosamine (GlcNAc) Transferase, which post-translationally modifies proteins involved in the stress response (Groves et al., 2013)) (Figs. 2A and S2C). Both mRNAs remained associated with ribosomes in high stress intensities, in support of their functions in the stress response (Groves et al., 2013; Hardy et al., 2019).
In mild stress conditions, 32 mRNAs were in the congruent up group, which had both significantly increased mRNA levels and ribosome footprints (Fig. 2A). KEGG pathway analysis revealed enrichment in terms such as ‘TNF signaling’, ‘MAPK signaling’, and ‘IL-17 signaling’ (Fig. S2B). Negative feedback regulators of p38 kinase and NF-κB signaling (Dusp1, Nfkb1a, Tnfaip3, and Ier3) were among the top mRNAs in these pathways, consistent with termination of inflammation as the cells progress to an osmoadaptive state (Farabaugh et al., 2020). These data suggest progression to osmoadaptation involves changes in mRNA abundance and translational control to promote osmoadaptive gene expression and inhibit that of inflammatory genes. In contrast, stress-induced adaptive pausing revealed “ribosome” and “oxidative phosphorylation” as predominant KEGG pathways (Fig. S2B). Finally, APR at 700 mOsm was marked by 440 mRNAs in the congruent down group (Fig. 2A), which were primarily in the NC and riboocp-down groups in 500 and 600 mOsm stress conditions. This suggests increasing stress intensity applies additional layers of translational and mRNA abundance controls, engaging limited cellular resources for translation of the most needed mRNAs in anticipation of recovery.
Ribosomes stall at translation initiation codons of specific mRNAs during severe hyperosmotic stress
To obtain a genome-wide view of ribosome pausing at translation initiation codons, we evaluated average ribosome occupancy around authentic translation initiation AUG and translation termination codons (Fig. 3A). We found elevated ribosome occupancy at initiation codons, but not at termination codons, during severe stress. Ribosome occupancy was changed at the P-site but not at the A-site, suggesting ribosome movement halted when the AUG codon is in the P-site. Analysis showed that, on average, ribosomes spend more time at the initiation codon in isoosmolar conditions, consistent with this being a slow step in the transition from initiation to elongation (Wang et al., 2019). Pausing at initiation codons increased with stress intensity (Fig. 3B), while pausing at other codons was similar between isoosmolar and stress conditions. Interestingly, 60% of mRNAs with paused ribosomes contained footprints at AUG (and non-cognate initiation) codons downstream of the authentic translation initiation codon (Fig. 3C). As authentic initiation codons can be skipped due to leaky scanning of the 40S subunit in stress conditions characterized by decreased ternary complex availability (Andreev et al., 2015), the observed pausing events within the main ORFs may be the result of failed translation initiation events.
Figure 3. Sustained inhibition of protein synthesis during severe stress is accompanied by ribosome pausing at translation initiation codons of select mRNAs.

MEFs exposed to the indicated stress conditions were analyzed for (A) mean density of ribosomal footprints relative to the translation initiation codon (top row) and translation termination codon (bottom row). (B) Heat map depicting ribosome occupancies on the different codons (left) based on their relative location on the mRNAs (x-axis). (C) Scatter plots comparing ribosome occupancies of different codons (codon occupancy) in ORFs, excluding authentic translation initiation codons. (D) Percentage of paused and non-paused mRNAs in each group after 700 mOsm stress for 2 h. (E) Distribution of footprints on Sat1 mRNA. (F) Distribution of individual mRNAs on polysome profiles. Data are representative of three biological replicates. (G) Western blot analysis and quantification for indicated proteins in sucrose gradient fractions. Data are representative and quantification is inclusive of three independent experiments. *p < 0.01
To compare relative pausing between different mRNA groups engaged with ribosomes, we calculated a pausing index for individual mRNAs by dividing the number of ribosome reads at translation initiation codons by reads in the remainder of the ORF (Supp. File 3). Paused mRNAs (pausing index ≥ 2) were identified among each mRNA group (Fig. 3D), suggesting ribosome pausing at translation initiation codons marks suspension of translation initiation. Lower enrichment in paused mRNAs in the riboocp-up group (Fig. 3D) is consistent with better translation of select mRNAs during severe stress. A striking example of a non-paused mRNA in the riboocp-up group at all stress intensities is Sat1, which encodes polyamine metabolism spermidine/spermine N1-acetyl transferase 1 (Brett-Morris et al., 2014) (Fig. 3E). Translation of Sat1 mRNA increased in mild stress, and some translating ribosomes were maintained in the ORF even in severe stress (Figs. 3E and 2E). We further found enrichment of genes of the “ribosome” and “oxidative phosphorylation” pathways in the 700 mOsm riboocp-up group in both paused and non-paused mRNAs (Fig. S3A), suggesting the degree of pausing differs in those mRNAs translated during severe stress.
We hypothesized that paused mRNAs with very low translation (riboocp-down group) would be enriched on 80S ribosomes in severe stress and this distribution would be similar to the effect of the translation initiation inhibitor harringtonine, which allows runoff of polyribosomes and freezes initiation at monosomes (Ingolia et al., 2011). We focused on the mRNA Rad50 encoding a DNA repair protein (Dmitrieva and Burg, 2008) in the NC group in 500 and 600 mOsm stress conditions. During severe stress, Rad50 mRNA was enriched in fractions corresponding to 80S ribosomes, indistinguishable from harringtonine-treated samples (Fig. 3F). In contrast, the paused mRNA Hnrnpa1 in the NC group at 700 mOsm was associated with ribosomes heavier than 80S, suggesting some translation of Hnrnpa1 was maintained (Fig. 3F). These data suggest translation initiation of select mRNAs is halted during severe stress, perhaps due to the slowed first step in translation elongation.
To identify the cause of ribosome pausing, we investigated aminoacylation of initiator Met-tRNA (Met-tRNAi) during severe hyperosmotic stress. No change in aminoacylation was observed in any stress intensity (Fig. S3B), suggesting there is no limitation of Met-tRNAi for translation initiation. We next investigated a recently reported rate-limiting step in translation initiation, release of the translation initiation factor eIF5B (Wang et al., 2019) following 80S assembly on the initiation codon at the P site and before the first elongator tRNA is delivered to the A site (Lee et al., 2002). In isoosmolar conditions, eIF5B migrated in polysome profiles with 40S ribosomes, while at 700 mOsm, eIF5B shifted to fractions corresponding with 80S ribosomes. These data suggest ribosome stalling at translation initiation codons during severe hyperosmotic stress may involve slowed release of eIF5B from the initiating ribosome (Fig. 3G).
Stress duration-dependent checkpoints dictate recovery kinetics
To understand the significance of ribosome pausing after cells exit the APR, we evaluated whether return of cells to isoosmolar conditions coincided with reversal of pausing at translation initiation sites. Indeed, paused mRNAs associated with more ribosomes early during recovery (Fig. 4A). To confirm that the degree of pausing on mRNAs during stress reflects efficiency in initiating translation during recovery, we compared the top 20% (high pausing index) and bottom 20% (low pausing index) of paused mRNAs (Supp. File 4). We found mRNAs with a higher degree of pausing were preferentially translated upon recovery (Fig. S4A), exemplified by the distribution of ribosome footprints in mitochondrial respiration complex mRNAs Uqcrsf1 (top 20%) and Ndufv1 (bottom 20%). Ribosome pausing at the translation initiation codon recovered after 2 h, with ribosome footprints in these mRNAs returning to control levels (Fig. S4B). This paralleled the return of the riboocp values of respirasome-associated mRNAs to the levels of untreated cells (Fig. 4B). These data indicate that ribosome pausing occurs on mRNAs initiating translation during severe stress, and paused mRNAs are translated ahead of non-paused mRNAs during recovery. Therefore, APR may prioritize some mRNAs important for survival to quickly resume their translation during recovery from stress. As stress also caused significant leaky scanning followed by translation initiation pausing (Fig. 3C), recovery may yield N-terminally truncated polypeptides with potentially toxic properties. The presence and function of such products during recovery will require further investigation.
Figure 4. Pausing at translation initiation codon is stress duration-dependent and reversed by the return to isoosmolar media.

MEFs treated with hyperosmolar media for the indicated times were analyzed for (A) cumulative distribution (y-axis) as a function of the fold change of riboocp values (x-axis) for mRNAs that were either paused (red) or non-paused (cyan) after 2 h of 700 mOsm stress. Riboocp values were calculated as ribosome footprints normalized to corresponding mRNA levels. (B) Relative changes of the riboocp values of neMito mRNAs. (C) Scatter plot comparing fold changes of ribosomal footprints (y-axis) and cytoplasmic RNA levels (x-axis). Comparisons were made against values from 700 mOsm stress (2 h). (D) Protein synthesis levels. Means ± SEM for triplicate determinations are shown. *p < 0.05. (E) Heat map depicting ribosome occupancies on different codons (y-axis) based on their relative location on mRNAs (x-axis).
To further understand gene regulation during recovery, we analyzed the trancriptome and translatome upon recovery from severe hyperosmotic stress (Fig. 4C). During recovery, the distribution of the riboocp values suggested massive reversal of protein synthesis inhibition (Figs. 2A and 4C). However, of the 550 mRNAs with decreased riboocp during recovery (Fig. 4C), 85% were in the riboocp-up group after 2 h of stress. Reprogramming of mRNA abundance was evident in the congruent up group (103 mRNAs), which was enriched in genes of the ‘transcription’ and ‘cellular signaling’ pathways (Fig. S4C). Interestingly, 93% of these were in the congruent down group in severe stress (Figs. 2A and 4C). A similar observation was made for the buffering up group (72 mRNAs, Fig. 4C), mRNAs of which were primarily in the congruent and buffering down groups in severe stress. These data suggest on exiting the APR cells reverse translational reprogramming induced on severe stress, while a transcription and signaling program promotes a return to homeostasis. This is further supported by the increase of mRNAs in the congruent up and buffering up groups after 2 h of recovery (Fig. 4C). These data suggest the early response in recovery from the APR is focused on regulation of translation, while the late response involves congruent changes in the early response translatome.
Programs that function upon exit from the APR have been previously described in adaptation to chronic ER stress (Guan et al., 2017). In contrast to this adaptation, recovery from the APR is a transient state preceding the return to homeostasis. We put forth two hypotheses: (i) adaptive pausing develops in a stress duration-dependent manner, and (ii) exiting the adaptive pausing state likely shares elements with the cellular response of adaptation to chronic ER stress. To test the first, cells were exposed to 700 mOsm stress for 15 min, which inhibited protein synthesis by 95% (Fig. 4D). The subcodon resolution of ribosome distribution showed clearance of ribosomes at the 5’-end of ORFs and accumulation near stop codons (Fig. 4E). Since the average elongation rate in mammalian cells is 5–6 codons/s (Ingolia et al., 2011; Wu et al., 2016), even ORFs as long as 6 kb should be devoid of ribosomes by 6–7 min, suggesting severe hyperosmotic stress also slows translation elongation (Burg et al., 2007, Teige et al., 2001). The most prominent group of mRNAs with ribosomes in the 3’-ends of ORFs were those with ORFs longer than 1 kb (Supp. File 1) such as Rad50 and Fn1 (encoding fibronectin) (Fig. S4D). Global pausing at translation initiation codons after 2 h of stress was not evident after 15 min of stress (Fig. 4E), suggesting ribosome pausing on translation initiation codons in response to severe hyperosmotic stress is duration-dependent and that there is a temporal component to selective mRNA translation initiation during severe stress.
We next compared gene regulation in response to short stress and recovery. Hyperosmotic stress for 15 min induced dramatic translational reprogramming (Fig. S4E and Supp. File 1). The overall patterns observed in the transcriptome/translatome of cells recovering from short durations of severe stress were similar to those in cells recovering from longer durations (Fig. S4E). Translational recovery of 2,047 mRNAs during early recovery was followed by increased mRNA abundance (69 mRNAs congruent up and 69 buffering up). However, both the number of mRNAs and the magnitude of increase were smaller in cells recovering from 15 min stress compared to cells recovering from 2 h stress (Figs. 4C and S4E), suggesting the duration of severe stress dictates the threshold of gene regulation during recovery. This converges on our second hypothesis: recovery from both severe hyperosmotic stress and adaptation to chronic ER stress have duration-dependent adaptation programs. The early response involves translational control, while the late response involves a new transcriptome in part derived from the early response-regulated translatome.
Hyperosmotic stress duration dictates adaptation programs promoting a return to homeostasis
We hypothesized recovery following adaptive pausing would involve induction of stress adaptation prior to resumption of growth, and thus be slower than recovery from short stress. To test this, we compared changes in the transcriptomes and translatomes between control and recovering cells in 700 mOsm stress for 15 min (short) or 2 h (long), followed by recovery for 2 h (Fig. 5A). Upon recovery from short stress, cells showed nearly complete return to homeostasis, with 36 mRNAs in the riboocp down group and 50 mRNAs in the combined congruent up and riboocp-up groups (Fig. 5A). In contrast, after recovery from long stress, cells maintained riboocp-down (927 mRNAs), riboocp-up (185 mRNAs), and congruent up (186 mRNAs) stress response patterns. The 50 mRNAs that had higher ribosome association during recovery from short stress showed enrichment of genes in “Cytokine-cytokine receptor interactions”, “IL-17 signaling pathway”, “TNF signaling pathway”, and “MAPK signaling pathway” (Fig 5B), consistent with proinflammatory signaling promoting growth as previously described in other cell injury models (Werner and Grose, 2003). Furthermore, the pro-growth signaling was evident from the association of proliferation mRNA Ereg (Singh et al., 2016), mitochondria oxidative capacity mRNA Angptl4 (Guo et al., 2014), and growth factor mRNA Vegf (Apte et al., 2019) having the highest fold changes in ribosome occupancy (Fig. 5A). The 371 mRNAs that displayed higher association with ribosomes after recovery from 2 h stress showed enrichment in “Transcriptional misregulation in cancer”, “MAPK signaling pathway”, and “TNF and FoxO signaling pathways” (Fig. 5B). Interestingly, these mRNAs encoded many transcription factors (Fig. 5A), suggesting during recovery from prolonged stress, the synthesis of transcription factors facilitates gene expression reprogramming. A prominent ISR program was evident, as Atf4, Atf5, Atf3, and Chop were among these 371 mRNAs, in addition to downstream targets such as the cystine transporter Slc7a11 (Koppula et al., 2018) and the E3 ligase mRNA Herpud1 (Li et al., 2018). Consistent with a regulated transition from the APR to homeostasis is the presence of mRNAs associated with termination of inflammatory signaling (Nfkbia and Tnfaip3) and dual-specificity phosphatases (Dusps 1, 2, 4, 6, 8, 10, 14, and 16) which function in termination of ERK/MAPK signaling and apoptosis inhibition (Lang and Raffi, 2019). Therefore, cells exiting the APR enter a prosurvival stress-adaptation program that combines transcriptional and translational controls to regulate gene expression.
Figure 5. Re-shaping of the translatome/transcriptome in recovery from severe stress is determined by stress duration.
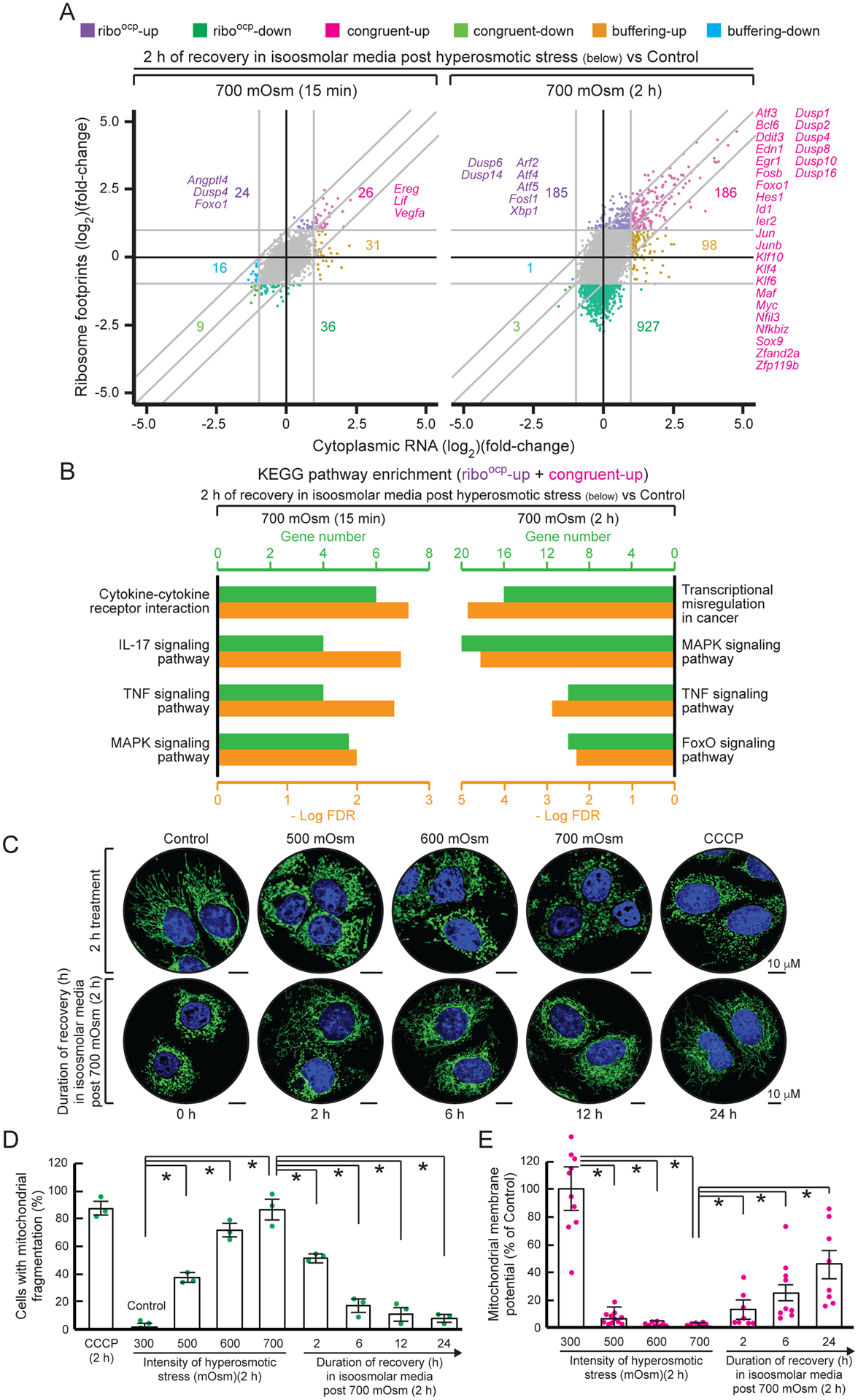
MEFs treated for the indicated times were analyzed by (A) scatter plot comparing fold changes of ribosomal footprints (y-axis) and cytoplasmic RNA levels (x-axis) compared to control (isoosmotic) conditions. (B) KEGG enrichment pathway analysis. (C) Representative confocal microscopy images of mitochondria (TOM20, green) and nuclei (Hoechst, blue). Treatment for 2 h with CCCP served as a positive control for mitochondrial fragmentation. Scale bar: 10 μm. (D) Quantification of cells with fragmented mitochondria. (E) Mitochondrial membrane potential. Means ± SEM of triplicate determinations are shown. *p < 0.01
We also examined mitochondrial morphology during recovery from the APR. Exposure of MEFs to increasing stress intensity caused mitochondrial fragmentation, with 40% of cells having fragmented mitochondria in mild stress and almost 90% at severe stress (Fig. 5C–D), comparable to treatment with the positive control mitochondrial uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP) (Lou et al., 2007). When cells were reverted to isoosmolar media after 2 h of severe stress, mitochondrial fragmentation decreased to 50% after 2 h and almost completely recovered by 24 h (Figs. 5C–D). We also observed a stress intensity-dependent decrease in mitochondrial membrane potential (MMP) in MEFs followed by a gradual increase during recovery (Fig. 5E). The decrease in MMP further supports that negative feedback-mediated protein synthesis inhibition halts the effort of cells in severe stress to maintain functionality by initiating translation of a select group of mRNAs. These data show translational pausing during severe hyperosmotic stress is paralleled by changes in mitochondrial morphology and function.
Slow kinetics of recovery from severe stress reveal stress-adaptation mechanisms
We next investigated protein synthesis rates during recovery from severe stress (Fig. 6A). Incorporation of [35S]-Met/Cys into proteins was gradually restored, reaching approximately 40% by 6 h and almost 100% by 12 h (Fig. S5A). The slow kinetics of protein synthesis recovery were inconsistent with reactivation of mTOR, as shown by p70 (S6K) phosphorylation (Fig. 6B). In contrast, gradual recovery of global protein synthesis correlated precisely with recovery of eIF2B activity (Fig. 6C), which, severely inhibited upon stress, remained inhibited for the first 2 h of recovery and only returned to normal levels thereafter (Fig. 6C). Although the ISR was not active during hyperosmotic stress, it was activated during recovery from severe stress (Fig. 6B). Induction of both ATF4 and GADD34 increased significantly upon recovery. GADD34 is a subunit of the PP1 phosphatase, which dephosphorylates eIF2α (Novoa et al., 2001). In agreement with previous studies (Guan et al., 2017), low levels of eIF2α phosphorylation were sufficient to limit eIF2B activity and induce the ISR during recovery (Fig. 6B). Polysome profile analysis confirmed the increased association of Atf4 mRNA with polysomes after 2 h of recovery (Fig. 6D). Therefore, recovery from severe hyperosmotic stress has similarities to adaptation to chronic ER stress, i.e. the ISR (Costa-Mattioli and Walter, 2020). These data suggest the pausing state induced by severe hyperosmotic stress suspends stress-induced adaptive mechanisms despite decreased eIF2B activity. In contrast, adaptive mechanisms are transiently activated during recovery from pausing, supporting the importance of the ISR.
Figure 6. ISR is a hallmark of the recovery from adaptive pausing in response to severe stress.

MEFs treated with the indicated stress conditions were analyzed by (A) protein synthesis assays, (B) Western blotting, (C) eIF2B activity assays, (D) polysome profiles, (E) cell survival assays, (F) mitochondria fragmentation assays, and (G) flow cytometry of propidium iodide-stained cells in the presence or absence of GADD34/PP1 inhibitor sephin1. Percentage of cells in each cell cycle phase are indicated. (H) Temporal cellular responses to severe hyperosmotic stress. The diagonal arrow indicates the development of APR (graded red color) as cells transition through different states (represented by quadrants). The processes that associate with APR development are listed at the right. Means ± SEM for triplicate determinations are shown. *p < 0.01
Induction of GADD34/PP1 during recovery from severe stress promotes cell survival
We next investigated whether the ISR is absent during recovery from short stress. We observed recovery of protein synthesis was swift after 15 min (Fig. 4D), in contrast to slow recovery after 2 h stress. Similarly, we did not observe expression of ISR genes (Fig. S5B), but did observe accumulation of CCAAT Enhancer Binding Protein β (CEBPβ) LIP (Li et al., 2008) (Fig. S5B), suggesting entry and exit from the APR uniquely prepare cells to recover once stress abates.
As GADD34 has been shown to have functions independent of its eIF2α dephosphorylation activity, we investigated the role of GADD34 in recovery of protein synthesis following severe hyperosmotic stress (Harding et al., 2009). Gadd34−/− cells had a similar response as WT cells in regulation of protein synthesis (Figs. S5C and 1B)and of mTOR substrate phosphorylation in increasing stress intensity and recovery (Figs. 6B and S5D). During recovery, GADD34 deficiency inhibited restoration of protein synthesis, consistent with sustained phosphorylated eIF2α (Figs. S5D and S5E). These data suggest the induction of GADD34 during recovery from severe stress promotes recovery of protein synthesis via dephosphorylation of eIF2α, supporting that the ISR promotes gradual recovery of protein synthesis upon exit from the APR.
To assess the function of ISR during recovery, we determined the impact of GADD34 on cell survival. Consistent with inhibited recovery of protein synthesis, Gadd34−/− cells had decreased survival (Fig. 6E) and high mitochondria fragmentation (Fig. 6F) during recovery. These data suggest GADD34/PP1 is required for cell survival and cell cycle re-entry during recovery from severe stress. We next determined the progression of the cell cycle in the presence of SEPHIN1 (Das et al., 2015), an inhibitor of PP1/GADD34-phosphatase activity (Fig. 6G), which can decrease osmoadaptation (Krokowski et al., 2017). We found hyperosmotic stress induced a shift of cells from the G1/S phases to the G2/M phase of the cell cycle for the first 2 h of recovery; this was followed by resumption of the G1 phase, but the reduction of cells in the S phase persisted until 6 h. The number of cells in G2/M phase remained high during this period. After 6 h of recovery the cells returned to normal cell cycle progression as the number of cells in S phase increased. The presence of SEPHIN1 did not prevent the reduction in the number of cells in S phase from 2–6 h of recovery, but cells were unable to return to normal cell cycle progression, and instead maintained inhibition of S phase entry. We conclude inhibition of GADD34/PP1 causes G1 phase cell cycle arrest (Fig. 6G). It is unknown how GADD34 promotes S phase entry, but it is likely via its function in recovery of protein synthesis inhibition and amino acid transporter trafficking (Cano-Crespo et al., 2019; Krokowski et al., 2017). We propose the induction of ISR during recovery from severe hyperosmotic stress promotes a return to normal growth and survival via GADD34/PP1. The similar states of the cell cycle at 2 h of severe stress and after 2 h recovery further support that induction of the ISR and other gene expression programs is due to recovery from the APR, not cell cycle-mediated gene expression.
Finally, we found recovery of protein synthesis via eIF2α dephosphorylation independent of mTORC1 activity was important for reversal of mitochondria fragmentation (Fig. 7). Severe stress impacted mitochondria fragmentation in a time-dependent manner, with 15 min of stress showing half the mitochondria fragmentation of 2 h of stress (Fig. S6A). This suggests the APR may be a prosurvival mechanism to inhibit mitochondria fragmentation and cell death. Consistent with this, we identified time- and stress intensity-dependent cleavage of the mitochondrial protein OPA1 (Del Dotto et al., 2017) (Fig. S6B–D). Opa1 mRNA was translationally repressed and paused at 2 h of stress and quickly associated with polyribosomes during recovery (Fig. S6E). OPA1 protein synthesis during recovery was dependent on eIF2α dephosphorylation by GADD34/PP1 (Fig. S6F) and important for recovery from severe stress (Fig. S6G). Therefore ISR-mediated dephosphorylation of eIF2α restores mitochondrial morphology (Fig. 7). We conclude that the APR delays cell death and preserves the ability of cells to selectively restart mRNA translation during recovery from severe stress (Fig. 6H).
Figure 7. Restored mitochondria morphology during recovery from severe stress requires reversal of mRNA translation inhibition caused by the signaling of eIF2α phosphorylation.

(A) Representative confocal microscopy images of mitochondria (TOM20, green) and nuclei (Hoechst, blue) in MEFs treated as indicated. Scale bar: 10 μm. (B) Quantification of cells with fragmented mitochondria after indicated treatments. Means ± SEM of triplicate determinations are shown. *p < 0.01. Protein synthesis was inhibited during recovery from 700 mOsm stress via the use of chemicals inhibiting translation initiation (Hippuristanol and Harringtonine) or eIF2B activity (Salubrinal). (C) Western blot analysis of cells treated with 700 mOsm for 2 h and allowed to recover in isoosmolar media with or without Torin 1.
Discussion
In response to hyperosmotic stress, cells undergo cell cycle arrest followed by either adaptation (Arsenijevic et al., 2013) or apoptosis (Farabaugh et al., 2020). We show here cells exposed to severe hyperosmotic stress induce a duration-dependent hibernation-like inhibition of cellular processes, which delays cell death in anticipation of stress recovery. This APR is characterized by inhibition of mRNA translation initiation and mitochondria fragmentation. Selective translation initiation of a subset of mRNAs enriched in protein synthesis and mitochondria functions support cytosol-mitochondria communication. Ribosome pausing at initiation codons was identified as a unique feature of APR. Cells initiated the ISR response during recovery from stress, which allowed cell cycle progression and resumption of growth. We propose that severe environmental stress induces translational silencing, yet preserves vital elements of cellular function via layered regulation of mRNA translation.
The discovery of adaptive pausing shows that cells use different mechanisms to adapt to stress based on duration and intensity. Although it is remarkable that in mild hyperosmotic stress cells reprogram the transcriptome and translatome to maintain homeostasis, it is even more astonishing that cells have the plasticity to continue reshaping their translatome as stress intensity increases. We showed that translational control is a major contributor to this plasticity. With varying stress intensities, we observed differential enrichment of cellular pathways in mRNAs that remained associated with ribosomes, as well as a hierarchy in the efficiency of translation initiation and ribosome pausing during severe stress. The enrichment and persistent association of mRNAs involved in oxidative phosphorylation and protein synthesis with ribosomes during severe stress is consistent with a low-energy metabolic state in preparation for swift recovery. It was interesting that of the nuclear-encoded mRNAs for oxidative phosphorylation complex subunits, only the complex I subunit mRNA Ndufv2 had low ribosome occupancy in stress conditions, indicating lower efficiency translation compared to other subunits. Complex I is a major source of ROS production, which can be harmful to cells, especially in conditions of weakened anti-oxidant defense mechanisms (Snezhkina et al., 2019). This low translation efficiency of Ndufv2 mRNA may indicate a defense mechanism against superoxide radical production, supported by the finding that NDUFV2 is at lower levels in longer-lived animals, correlating with lower mitochondrial ROS and reduced symptoms of aging (Mota-Martorell et al., 2020).
The characterization of APR during severe hyperosmotic stress revealed elements unique from heat shock (Richter et al., 2010). In contrast to translation initiation pausing observed in APR, in response to severe heat shock (44°C for 2 h), a translation elongation block is observed within the first 60 nucleotides of most mRNAs (Shalgi et al., 2013). This translational pausing is caused by decreased activity and levels of chaperone proteins, in agreement with increased nascent protein ubiquitination (Aprile-Garcia et al., 2019). In the heat shock response, translation of mRNAs encoding ISR factors ATF4 and ATF5 escape translation elongation pausing, and were translated along with other factors important for the heat shock response (Shalgi et al., 2013). In contrast, in the APR, Atf4 and Atf5 are translationally repressed, and the reprogramming of transcription is evident during recovery. Other examples of adaptive ribosome stalling were recently described for nutrient deprivation and UV radiation. It was shown that the pro-survival ISR is induced via activated protein kinases SAPK and GCN2 via mechanisms that involve colliding ribosomes (Wu et al., 2020). These differences suggest the APR is a unique adaptive cellular state of severe environmental stress, reminiscent more of hibernation than an active adaptive stress response to maintain cellular function. Similar to hibernation, the APR reversibly suppresses mitochondrial metabolism to survive extreme conditions (Mathers et al., 2017; Staples, 2014).
A notable feature of the APR is the hierarchy of translational efficiency and initiation codon pausing among mRNAs. The latter associated with decreased mTOR activity and increased eIF2α phosphorylation, both inhibitory for mRNA translation. Surprisingly, among mRNAs with higher ribosome occupancy were mRNAs known to be severely inhibited by mTOR inhibition (Thoreen et al., 2012). Downregulated mRNAs had longer 5’-UTRs, higher GC content, and more stable secondary structures (Fig. S7). Analysis of initiation codons within uORFs indicated that high osmolarity tended to increase initiation at sites with weak Kozak context, and to inhibit initiation at AUG codons or those with strong Kozak context by stabilizing the 80S ribosome (Figs. S8). These data suggest that the hierarchy in translation initiation may reflect a hierarchy in eIF4F-mediated mRNA translation, supported by recent literature indicating that there are at least eight different eIF4F complexes involved in translation initiation (Robert et al., 2020). These findings are also supported by an elegant report in yeast, in which the authors showed that acute oxidative stress or nutrient limitation revealed subpopulations of mRNAs with a hierarchy of translation initiation and differential eIF4F depletion (Costello et al., 2017). These reports are consistent with model systems demonstrating mRNA competition for translation initiation (Godefroy-Colburn and Thach, 1981), and support a model of differential stability of eIF4F complexes with mRNAs in response to acute inhibition of protein synthesis (Costello et al., 2017). It remains for future studies to determine the signaling pathways that lead to differential ribosome recruitment by APR-translated mRNAs.
We have shown that a potential mechanism of APR is the slow release of eIF5B from the assembled ribosome. The cause of such a slow release is not known, however, it is logical to expect that the decreased function of eIF2 beyond a certain threshold (due to eIF2α phosphorylation) in delivering the met-tRNAi at ATG initiation codons may play a role. Both eIF5B (Shin et al., 2002; Terenin et al., 2008) and eIF2A have been shown to bind met-tRNAi and promote binding to the 40S ribosome under inhibition of eIF2 activity (Komar and Merrick, 2020). In yeast, eIF2A is genetically linked with eIF5B (Komar et al., 2005; Zoll et al., 2002) and accumulates on 80S ribosomes due to the slow transition of the 80S ribosome to the elongation cycle. Therefore, eIF5B and eIF2A appear as possible candidates for ATG translation initiation pausing under severe eIF2α phosphorylation. In agreement with this, eIF2A (but not eIF2α or eIF4A), shifted to 80S ribosomes during severe stress (Fig. 3G). We therefore propose that under severe inhibition of eIF2 activity, alternative ATG initiation mechanisms may lead to the APR or dictate the selectivity of APR. For example, mRNAs which use the little remaining active eIF2 may not pause in favor of those which use the eIF2A, eIF5B, or other mechanisms of ATG initiation.
The initiation of the ISR during recovery from the APR was distinct from cellular adaptation to chronic ER stress (Guan et al., 2017). Similar to adaptation to chronic ER stress, Atf4 was translationally upregulated when eIF2B activity was low, and in turn induced expression of Chop and Gadd34. In contrast to chronic ER stress, the recovery of protein synthesis was dependent on recovery of the eIF2B activity via dephosphorylation of eIF2α. In this manner, the recovery from the APR is similar to the recovery from transient ER stress, in which the eIF2α kinase transiently causes a decrease of eIF2B activity. The requirement of GADD34 may also involve mechanisms shown previously in progression to osmoadaptation, such as GADD34/PP1-mediated plasma membrane protein trafficking and osmoprotective protein localization (Krokowski et al., 2017).
The translational control mechanism described here may be applicable in other extreme environmental stress conditions, such as hibernation in mammals. Remarkably, in mammalian hibernation, neurons lose their ability to generate long term potentiation (LTP), but they maintain an arousal state (Horowitz and Horwitz, 2019). Similar observations were recently made for mouse neurons that regulate the response to hypothermia and nutrient restriction during torpor (Hrvatin et al., 2020). The latter represents an adaptive energy-conserving prosurvival response in which a subpopulation of neurons remain responsive to permit termination of torpor. These observations suggest that neuroplasticity is essential for surviving extreme stress conditions. Interestingly, both hibernation and torpor associate with increased phosphorylation of eIF2α (Frerichs et al., 1998) and inactivation of eEF2 (Yamada et al., 2019).
The findings in this study provide insights into the cellular response to extreme environments, and reveal an unexpected response preserving the ability to recover once that environment changes. In addition to the resemblance of the APR to physiological hibernation and torpor, these findings are also significant to diseases such as cancer. Therapeutic strategies to kill cancer cells aim to induce cell death in dividing cells with high nutrient demands and deregulated ROS levels (Truitt et al., 2015). However, in response to chemotherapy, cancer cells can become dormant and develop resistance (Hangauer et al., 2017). Molecular signatures of dormant cells include inactive mTOR signaling (Kim et al., 2013), activation of the p38 kinase pathway, eIF2α phosphorylation (Ranganathan et al., 2006), and hypoxia-induced signaling (Butturini et al., 2019). Interestingly, the cellular response to hypoxia mimics the hibernation response (Stone et al., 2019). Although the APR is evident in the response to increased extracellular osmolarity, it likely plays a role in other physiological and pathological cellular responses.
Limitations of the Study
A limitation of the study is that the mechanism(s) of ribosome pausing on translation initiation codons during severe hyperosmotic stress remain unclear. This study focused on the significance of ribosome pausing in recovery from severe stress. Potential mechanisms may be revealed by purification of the paused ribosomes and characterization of the protein and RNA components by cryo-EM. Another limitation is that we used thresholds to assign mRNA groups. Other differential analysis tools (such as DEseq2) gave similar results, and biochemical data and validation analysis confirmed the regulation of individual mRNAs. However, we cannot exclude the possibility that much larger numbers of genes can be assigned to the defined mRNA groups.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Maria Hatzoglou (maria.hatzoglou@case.edu).
Materials availability
All unique/stable reagents generated in this study are available from the Lead Contact without restriction.
Data and code availability
Unprocessed western blot and microscopy images have been deposited at Mendeley Data (http://dx.doi.org/10.17632/yfgndyv8bh.1) and are publicly available as of the date of publication. Sequencing data has been deposited at GEO (GSE157519).
No original code has been generated for this publication.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell Lines, Cell Culture Treatments and Chemicals
The MEFs used were described previously (Scheuner et al., 2001). The cells were grown in high glucose Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific, 11960044) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, 26140079), 1X Penicillin-Streptomycin-Glutamine (Thermo Fisher Scientific, 10378016) at 37°C and 5% CO2. For all experiments, cells were subcultured 24 hours prior to the experiment so that cells were ~80% confluent at the time of the experiment. GADD34−/− MEFs were a gift from D. Ron (Novoa et al., 2003). Hyperosmotic stress was induced with Sorbitol (Sigma-Aldrich, S1876). Other chemicals used in this study include Cycloheximide (CHX) (100 μg/mL) (Sigma-Aldrich C7698), Harringtonine (2 μg/mL) (LKT Laboratories, H0169), Sephin1 (Apexbio, A8708), Sal 003 (1 μM) (Tocris Bioscience, 3657), Torin 1 (250 nM) (Tocris Bioscience, 4247), Hippuristanol (1 μM) (a generous gift from Dr. Junichi Tanaka, University of the Ryukyus, Japan) (Bordeleau et al., 2006) and CCCP (10 μM) (Sigma-Aldrich, C2759).
METHOD DETAILS
Protein Extraction and Western Blot Analysis
Cells were washed twice with ice-cold phosphate-buffer saline (PBS) and scraped into lysis buffer (50 mM Tris-HCl at pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% IGEPAL, 0.1% SDS, and 0.5% sodium deoxycholate) supplemented with EDTA-free protease inhibitor (Roche, 04693159001) and PhosSTOP phosphatase inhibitor (Roche, 04906837001) (1 tablet of each per 10 mL), and sonicated briefly 10 times (2 RMS). After centrifugation (12000 × g, 10 min, 4°C), proteins were quantified using the DC Protein Assay Kit (Bio-Rad, 500–0113 and 500–0114). Equal amounts of protein (10–20 μg) were analyzed by Western blotting. Primary antibodies used in this study are listed in Key Resources Table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-ATF4 | Santa Cruz Biotechnology | Cat# sc-200; RRID: AB_2058752 |
| Mouse monoclonal anti-eIF2α | Santa Cruz Biotechnology | Cat# sc-133227; RRID: AB_2096505 |
| Rabbit monoclonal anti-eIF2α-phospho (Ser51) | Abcam | Cat# ab32157; RRID: AB_732117 |
| Rabbit polyclonal anti-GADD34 | Santa Cruz Biotechnology | Cat# sc-825; RRID: AB_2168847 |
| Mouse monoclonal anti-GAPDH | Santa Cruz Biotechnology | Cat# sc-32233; RRID: AB_627679 |
| Rabbit polyclonal anti-RPS5 | This study | Custom-made, N/A |
| Rabbit polyclonal anti-S6K | Cell Signaling Technology | Cat# 9202; RRID: AB_331676 |
| Rabbit polyclonal anti-S6K-phospho (Thr389) | Cell Signaling Technology | Cat# 9205; RRID: AB_330944 |
| Mouse monoclonal anti-CHOP | Cell Signaling Technology | Cat# 2895; RRID: AB_2089254 |
| Rabbit monoclonal anti-4E-BP1 | Cell Signaling Technology | Cat# 9644; RRID:AB_2097841 |
| Rabbit monoclonal anti-p21 | Abcam | Cat# ab188224; RRID:AB_2734729 |
| Rabbit monoclonal anti-CYCLIN D1 | Cell Signaling Technology | Cat# 55506; RRID:AB_2827374 |
| Rabbit monoclonal anti-CEBP Beta | Abcam | Cat# ab32358, RRID:AB_726796 |
| Mouse monoclonal anti-CHK1 | Cell Signaling Technology | Cat# 2360; RRID: AB_2080320 |
| Rabbit monoclonal anti-CHK1-phospho (Ser345) | Cell Signaling Technology | Cat# 2348; RRID: AB_331212 |
| Mouse monoclonal anti-eIF5B | Santa Cruz Biotechnology | Cat# Sc-393564 |
| Rabbit polyclonal anti-TOM20 | Proteintech | Cat# 11802–1-AP RRID: B_2207530 |
| Mouse monoclonal anti-OMA1 | Santa Cruz Biotechnology | Cat# Sc-515788 |
| Mouse monoclonal anti-OPA1 | BD Transduction Laboratories | Cat# 612606 RRID:AB_399888 |
| Rabbit polyclonal anti-YME1L1 | Proteintech | Cat# 11510–1-AP RRID:AB_2217459 |
| Mouse monoclonal anti-MFN1 | Abcam | Cat# ab126575 RRID:AB_11141234 |
| Mouse monoclonal anti-MFN2 | Santa Cruz | Cat# sc-515647 RRID:AB_2811176 |
| Mouse monoclonal anti-FLAG M2 | Sigma-Aldrich | Cat# F1804 RRID:AB_262044 |
| Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-11034 RRID:AB_2576217 |
| Bacterial and Virus Strains | ||
| N/A | ||
| Chemicals,peptides,recombinant proteins | ||
| Penicillin-Streptomycin-Glutamine (100X) | Thermo Fisher Scientific | Cat# 10378016 |
| high glucose Dulbecco’s modified Eagle’s medium (DMEM) | Thermo Fisher Scientific | Cat# 11960044 |
| Gibco™ Fetal Bovine Serum, qualified | Thermo Fisher Scientific | Cat# 26140079 |
| cOmplete™, Mini, EDTA-free Protease Inhibitor Cocktail | Roche | Cat# 04693159001 |
| PhosSTOP™ | Roche | Cat# 04906837001 |
| Harringtonine | LKT Laboratories | Cat# H0169 |
| Hippuristanol | (Bordeleau et al., 2006) | N/A |
| Sal 003 | Tocris Bioscience | Cat# 3657 |
| Sorbitol | Sigma-Aldrich | Cat# S1876 |
| Sephin1 | Apexbio | Cat# A8708 |
| EXPRE35S35S Protein Labeling Mix | PerkinElmer | Cat# NEG072007MC |
| TRIzol Reagent | Thermo Fisher Scientific | Cat# 15596018 |
| TRIzol LS Reagent | Thermo Fisher Scientific | Cat# 10296028 |
| Cycloheximide | Sigma-Aldrich | Cat# C7698 |
| Trichloroacetic acid (TCA) | Sigma-Aldrich | Cat# T0699 |
| IGEPAL | Sigma-Aldrich | Cat# I8896 |
| Torin 1 | Tocris Bioscience | Cat# 4247 |
| RNase Inhibitor, Murine | NEB | Cat# M0314L |
| Ambion™ RNase I, cloned, 100 U/μL | Thermo Fisher Scientific | Cat# AM2295 |
| SYBR™ Gold Nucleic Acid Gel Stain | Thermo Fisher Scientific | Cat# S11494 |
| Novex™ TBE-Urea Gels, 15%, 10 well | Thermo Fisher Scientific | Cat# EC6885BOX |
| Novex™ TBE-Urea Gels, 10%, 10 well | Thermo Fisher Scientific | Cat# EC6875BOX |
| Novex™ TBE Gels, 8%, 10 well | Thermo Fisher Scientific | Cat# EC6215BOX |
| Corning® Costar® Spin-X® centrifuge tube filters | Sigma | Cat# CLS8160 |
| GlycoBlue™ Coprecipitant | Thermo Fisher Scientific | Cat# AM9515 |
| T4 Polynucleotide Kinase | NEB | Cat# M0201S |
| T4 RNA ligase truncated KQ | NEB | Cat# M0373 |
| Carbonyl Cyanide Chlorophenylhydrazone (CCCP) | Sigma-Aldrich | Cat# C2759 |
| 5´ DNA Adenylation Kit | NEB | Cat# E2610S |
| SuperScript™ III Reverse Transcriptase | Thermo Fisher Scientific | Cat# 18080044 |
| CircLigase™ ssDNA Ligase | Lucigen | Cat# CL4111K |
| MyOne streptavidin C1 DynaBeads | Thermo Fisher Scientific | Cat# 65001 |
| NEBNext® Multiplex Oligos for Illumina® | NEB | Cat# E7335S |
| NEBNext® Ultra™ II Q5® Master Mix | NEB | Cat# M0544S |
| puromycin | Thermo Fisher Scientific | Cat#A1113803 |
| Guanosine 5′-Diphosphate, Trisodium Salt, [8,5′−3H] | PerkinElmer | Cat# NET96600 |
| Glass microfiber filters (Whatman) | GE Healthcare Life Sciences | Cat# 1822–025 |
| Cellulose Nitrate Membrane Filters (Whatman) | GE Healthcare Life Sciences | Cat# 7184–002 |
| Purified eIF2 from rabbit reticulocytes | This study | N/A |
| RNase A, DNase and protease-free | Thermo Fisher Scientific | Cat# EN0531 |
| Propidium iodide | Sigma-Aldrich | Cat# P4170 |
| GeneScreen Plus Hybridization Transfer Membrane | PerkinElmer | Cat# NEF986001PK |
| Spectroline™ XL-1500 | Thermo Fisher Scientific | Cat# 11-992-90 |
| ATP, [γ−32P]- 6000Ci/mmol 10mCi/ml | PerkinElmer | Cat # NEG002Z250UC |
| ULTRAhyb™-Oligo | Thermo Fisher Scientific | Cat# AM8663 |
| Normal Goat Serum | Thermo Fisher Scientific | Cat# 10000C |
| Hoechst 33342 Solution | Thermo Fisher Scientific | Cat# 62249 |
| Faramount, Aqueous Mounting Medium | DAKO | Cat# S3025 |
| Tetramethylrhodamine, Methyl Ester, Perchlorate (TMRM) | Thermo Fisher Scientific | Cat# T668 |
| X-tremeGENE™ 9 DNA Transfection Reagent | Roche | Cat# 6365779001 |
| Hexadimethrine bromide | Sigma-Aldrich | Cat# H9268 |
| puromycin | Thermo Fisher Scientific | Cat# A1113803 |
| Critical Commercial Assays | ||
| DC Protein Assay Kit | Bio-Rad | Cat# 500–0113 and 500–0114 |
| SuperScript III First-Strand Synthesis SuperMix | Thermo Fisher Scientific | Cat# 11752250 |
| VeriQuest SYBR Green qPCR Master Mix | Thermo Fisher Scientific | Cat# 756001000RXN |
| StepOnePlus Real-Time PCR System with Tower | Thermo Fisher Scientific | Cat# 4376599 |
| CellTiter-Glo Luminescent Cell Viability Assay | Promega | Cat# G7570 |
| TruSeq Stranded Total RNA Library Prep Gold | Illumina | Cat# 20020598 |
| Deposited Data | ||
| NGS data | This study | GSE157519 |
| Raw imaging and gel data are deposited at Mendeley Data | This study | http://dx.doi.org/10.17632/yfgndyv8bh.1 |
| Experimental Models: Cell Lines | ||
| Wild type MEFs | (Scheuner et al., 2001) | N/A |
| GADD34 KO MEFs | (Novoa et al., 2003) | N/A |
| HEK293T cells | ATCC | Cat# CRL-3216, RRID: CVCL_0063 |
| Experimental Models: Organisms/Strains | ||
| N/A | ||
| Oligonucleotides | ||
| oligos for Ribosome footprinting | Integrated DNA Technologies | See Table S1 |
| probe for Northern blot | Integrated DNA Technologies | See Table S1 |
| qPCR primers | Integrated DNA Technologies | See Table S1 |
| Recombinant DNA | ||
| Plasmid expressing shRNA against Opa1 | Sigma-Aldrich | Cat# TRCN0000091111 |
| MISSION® pLKO.1-puro Non-Target shRNA Control Plasmid | Sigma-Aldrich | Cat# SHC016 |
| psPAX2 | Addgene | Cat# 12260 |
| pMD2.G | Addgene | Cat# 12259 |
| OPA1 plasmids | Trifunovic lab | N/A |
| Software and Algorithms | ||
| String database | https://string-db.org/ | |
| Bowtie | Langmead et al., 2009 | http://bowtie-bio.sourceforge.net/index.shtml |
| R | The R Project for Statistical Computing | https://www.r-project.org/ |
| Cutadapt | Martin, 2011 | http://cutadapt.readthedocs.io/en/stable/index.html |
| Other | ||
Gene Knockdown Experiments
Plasmid expressing shRNA against OPA1 (Sigma-Aldrich, TRCN0000091111) and MISSION® pLKO.1-puro Non-Target shRNA Control Plasmid DNA (Sigma-Aldrich, SHC016)) were purchased from Sigma-Aldrich. HEK 293T cells (150 mm dishes, 50% confluency) (ATCC, CRL-3216) were transfected with 12.5 μg shRNA expressing vector: 8 μg viral packaging plasmid (psPAX2 (Addgene,12260)): 4.5 μg viral envelope plasmid (pMD2.G (Addgene,12259)) ratio with X-tremeGENE™ 9 DNA Transfection Reagent (Roche, 6365779001) according to the manufacturer’s instructions. Media was collected after 24, 48 and 72 hours and filtered through 0.45 μM filter. The filtrate was diluted with the full media (1:5 ratio) supplemented with Hexadimethrine bromide (Sigma-Aldrich, H9268) at 10μg/ml and added to MEFs for 12 h followed by 12 h in the growth media without viral particles. The procedure was repeated once more and cells were allowed to recover one day in the growth media. After this, cells were subcultured and grown in media containing 30 μg/ml puromycin (Thermo Fisher Scientific, A1113803). After 3–4 days of the selection with puromycin cells were used for experiments. Knockdown efficiency was verified using Western blot analysis.
Plasmids and transient transfection experiment
Plasmids expressing human OPA1 were generous gifts of Dr. Aleksandra Trifunovic. Both wild-type and mutant plasmids have 3XFLAG tag at the C-terminus. Mutant plasmid has OMA1 clevage site removed. Transient transfection experiments were done using X-tremeGENE™ 9 DNA Transfection Reagent according to the manufacturer’s instructions.
Measurement of protein synthesis rates via metabolic labeling with 35S-Met/Cys
Cells on 24-well plates were treated with appropriate stress conditions, followed by incubation with 35S-Met/Cys (30 μCi/mL EXPRE35S35S Protein Labeling Mix, PerkinElmer, NEG072007MC) for an additional 30 min. After quickly washing with ice-cold PBS twice, cells were incubated with 5% trichloroacetic acid (TCA)/1 mM cold methionine for 10 min on ice (repeated three times) and lysed in 200 μL of 1 N NaOH/0.5% sodium deoxycholate for 1 h. Incorporation of radioactive amino acids was determined by liquid scintillation counting and normalized to the cellular protein content (quantified with DC Protein Assay (Bio-Rad)).
Flow cytometric analysis of cell cycle via propidium iodide staining of DNA
After treatment, cells were trypsinized, pelleted at 850 × g, and washed with PBS. Cells were fixed by incubation with 70% ethanol overnight at 4°C. The cells were treated with 100 μg/ml RNase A (Thermo Fisher Scientific, EN0531). Cells were stained with 50 μg/ml propidium iodide (Sigma, P4170). Staining was analyzed using a FACSAria™ II instrument. ModFit LT was used to fit a model to the data.
CellTiter-Glo and proliferation assays
Cell viability was measured with CellTiter-Glo Luminescent Cell Viability Assay (Promega, G7570) following the manufacturer’s instructions. For the cell viability assay performed in Figure1A, 5 × 105 cells were seeded on 60 mm dishes. 24 h post-seeding, cells were treated with 500, 600, or 700 mOsm hyperosmotic media and imaged at 2, 4, 6, 8, 12, and 24 h after treatment. The number of cells still attached to the plate in each field of view at 20X magnification were determined manually from at least 3 images with over 250 cells total. This number of cells was expressed as the percentage of cells counted at the same specifications immediately prior to treatment.
Analysis of tRNA charging by gel electrophoresis
After respective treatments, RNA was isolated with Trizol according to the manufacturer’s instructions, except that RNA was dissolved in 10 mM sodium acetate (pH 5)/1 mM EDTA. To prepare deacylated tRNAs, RNA was incubated in 0.2 M Tris-HCl (pH 9.5) for 1 h at 37°C, followed by precipitation with ethanol and resuspension in 10 mM sodium acetate (pH 5)/1 mM EDTA. 10 μg RNA was mixed with equal volume acid urea sample buffer (0.1 M sodium acetate (pH 5), 8 M urea, 0.05% bromophenol blue, 0.05% xylene cyanol FF). RNA was resolved by an acid urea polyacrylamide gel (12% polyacrylamide (19:1 acrylamide/bisacrylamide), 0.1 M sodium acetate (pH 5), 8 M urea) at 500 V over 24 h at 4°C in 0.1 M sodium acetate (pH 5) buffer. GENIE® blotter (Idea Scientific, 4003) was used to transfer to GeneScreen Plus Hybridization Transfer Membrane (PerkinElmer, NEF986001PK) for 30 min in 1X TBE buffer (0.089 M Tris Base, 0.089 M Boric Acid, 0.002 M EDTA). The membrane was UV crosslinked (120 mjoules/cm2) using a Stratalinker Spectroline™ XL-1500 (Thermo Fisher Scientific, 11-992-90). Probe was labeled with T4 PNK (NEB M0201S) at 37°C for 1 h in a reaction containing 20 pmol probe, 1X T4 PNK Reaction Buffer, 50 pmol [γ−32P]-ATP (7000 Ci/mmol, 150 mCi/ml) (PerkinElmer, NEG002Z250UC). For hybridization, ULTRAhyb™-Oligo (Thermo Fisher Scientific, AM8663) was used according to the manufacturer’s instructions.
Measuring in vitro guanine nucleotide exchange factor (GEF) activity of eIF2B
GEF activity of the cellular extracts was assayed as previously described (Kimball et al., 1989; Guan et al., 2017). Briefly, after washing twice with ice-cold PBS, cells were lysed in the homogenization buffer (45 mM HEPES-KOH at pH 7.4, 0.375 mM MgOAc, 75 μM EDTA, 95 mM KOAc, 10% glycerol, 1 mM DTT, 2.5 mg/mL digitonin, protease and phosphatase inhibitors (Roche Applied Science; 1 tablet per 10 mL)). Cell lysates were incubated on ice for 15 min, vortexed occasionally, passed six times through a 26-gauge needle, and centrifuged at 13,000 rpm for 15 min at 4°C. After determining protein concentration using the DC Protein Assay (Bio-Rad), 100 μg was used for the assay. eIF2 (purified from rabbit reticulocyte lysate) was incubated with [3H]-GDP for 10 min at 30°C in buffer (62.5 mM MOPS at pH 7.4, 125 mM KCl, 1.25 mM DTT, 0.25 mg/mL bovine serum albumin (BSA)). The complex was stabilized by the addition of 2.5 mM MgOAc and incubated with nonradioactive GDP (0.1 mg/mL)-supplemented lysate at 30°C. At 0, 2, 4, and 6 min, aliquots of the mixture were removed and filtered through nitrocellulose filters. The amount of eIF2α-[3H]-GDP complex bound to the nitrocellulose filters was assessed by liquid scintillation counting. The eIF2B activity was calculated as the rate of exchange of [3H]-GDP for nonradioactive GDP.
Ribosome footprinting analysis
Three 150 mm plates of MEFs (80% confluency) were treated with the indicated intensity of hyperosmotic stress. To avoid potential artifacts of pre-treating live cells with cycloheximide (Gerashchenko and Gladyshev, 2014), cycloheximide was added to the lysis buffer only after cells were harvested as previously described (Calviello et al., 2016; Darnell et al., 2018; Gerashchenko and Gladyshev, 2014; Mao et al., 2019). Cells were washed three times with ice-cold PBS and scraped in 1 mL lysis buffer (10 mM HEPES pH 7.4, 5 mM MgCl2,100 mM KCl,1% Triton-X,100 μg/mL cycloheximide, 2 mM DTT, RNase inhibitor (1:100 dilution, NEB, M0314L), protease inhibitor (1 tablet per 10 mL, Roche, 04693159001). The lysate was passed four times through a 26 gauge needle and centrifuged at 1300 × g for 10 min. Lysate (OD A260 = 10) was loaded on a linear 10%–50% sucrose gradient (gradient buffer was the same as the lysis buffer, lacking only Triton-X and Protease and RNase inhibitors) and centrifuged at 4°C at 31,000 rpm for 2.5 h using an SW 32 Ti rotor (Beckman Coulter, 369694). The gradients were fractionated using a Teledyne ISCO Density Gradient Fractionation System and 12 fractions were collected. The fractions from (including) the 80S peak to the bottom of the gradient were combined. 300 μL of the combined fractions was digested with 5 μL RNase I (Thermo Fisher Scientific , AM2295) for 1 h at 4°C (with slow rotation on a nutator). Immediately, Trizol LS (Thermo Fisher Scientific, 10296028) was added, and RNA isolated according to the manufacturer’s instructions.
10 μg of total RNA isolated from the combined fractions was resolved by 15% TBE-Urea gel (Thermo Fisher Scientific, EC6885BOX) and visualized with SYBR™ Gold Nucleic Acid Gel Stain (Thermo Fisher Scientific, S11494); the 28–30 nt region was excised from the gel. The RNA was eluted in 400 μL RNA elution buffer (EB) at 4°C overnight (RNA EB: 0.3M sodium acetate (NaOAc, pH 5.5), 1 mM EDTA, 0.4 U/μL RNase inhibitor). Gel debris was removed using Corning® Costar® Spin-X® centrifuge tube filters (Sigma-Aldrich, CLS8160). RNA was precipitated with 4 μL GlycoBlue™ Coprecipitant (Thermo Fisher Scientific, AM9515), 40 μL sodium acetate, and 1 mL ethanol overnight at −80°C. Recovered footprints were dephosphorylated in a 15 μL reaction mixture (1 μL T4 PNK (NEB, M0201S), 10X T4 PNK buffer, 0.4 U/μL RNase inhibitor) at 37°C for 1 h followed by heat inactivation at 65°C for 10 min. Dephosphorylated RNA was precipitated as described above and recovered in 4 μL of water. Pre-adenylated DNA linker (DNA oligo purchase from IDT was adenylated using 5´ DNA Adenylation Kit (NEB, E2610S)) was ligated to the 3’-end of RNA in a 20 μL reaction (25% PEG8000, 1X T4 RNA ligase buffer, 1 mM DTT, 0.4 U/μL murine RNase inhibitor, 300 U T4 RNA ligase truncated KQ (NEB, M0373S), 1 μM pre-adenylated DNA linker) for 6 h at 25°C followed by overnight ethanol precipitation as described above. Ligated footprints were resolved by 10% TBE-Urea Gel (Thermo Fisher Scientific, EC6875BOX), purified, eluted in the RNA EB at 4°C overnight, precipitated at −80°C as described above and resuspended in 10.5 μL water. 1 μL of 2.5 μM RT primer was added and heated at 65°C for 5 min, cooled on ice and incubated in 20 μL RT mix (300 U Superscript III (Thermo Fisher Scientific, 18080044), 1X RT buffer, 500 μM dNTPs, 5 mM DTT) for 30 min at 55°C, then heat inactivated at 70°C for 15 min. RT product was precipitated with ethanol as described above, resuspended in 10 μL water and resolved by 10% TBE-Urea gel. cDNA was isolated and eluted overnight in DNA elution buffer (DNA EB: 0.3 M NaCl, 1 mM EDTA), precipitated overnight, recovered and used for the 20 μL circularization reaction (100 U CircLigase™ ssDNA Ligase (Lucigen, CL4111K), 50 mM ATP, 2.5 mM MnCl2, 1X Circligase buffer) at 60°C for 2 h. rRNA depletion was performed according to Ingolia et al. (Ingolia et al., 2012). Circularized, rRNA-depleted cDNA was used in the PCR using NEBNext® Ultra™ II Q5® Master Mix (NEB, M0544S) and NEBNext® Multiplex Oligos for Illumina® (NEB, E7335S). Half of the cDNA was used to determine the optimal PCR cycle number. The PCR products were resolved by 8% TBE gel (Thermo Fisher Scientific, EC6215BOX). The other half of the cDNA was amplified by the determined PCR cycle, resolved by 8% TBE gel, and PCR products were cut, eluted in DNA EB, precipitated as described above, and recovered in 15 μL water. The quality and quantity of libraries were confirmed using DNA High Sensitivity Bioanalyzer (Agilent). Equal amounts of libraries were mixed and sequenced using NextSeq 550 (Illumina). The quality control of the ribosome footprinting libraries is provided in the Figure S8.
To determine cytoplasmic RNA levels, total RNA was isolated from the cytoplasmic lysate (input that was loaded on the sucrose gradients for the abovementioned ribosome footprinting analysis) using Trizol LS according to the manufacturer’s instructions, except that precipitation with isopropanol was conducted overnight at −80°C. 1 μg RNA was used to make libraries with TruSeq Stranded Total RNA Library Prep Gold (Illumina, 20020598) according to the manufacturer’s instructions. The quality and quantity of libraries were confirmed using DNA High Sensitivity Bioanalyzer (Agilent) and sequenced using PE150, HiSeq 4000 sequencing platform at the Novogene Corporation Inc.
Alignment of sequencing reads.
The 3′ adapters and low quality bases were trimmed using Cutadapt (https://doi.org/10.14806/ej.17.1.200). Trimmed reads with length <15 nucleotides were excluded. The remaining reads were mapped to the mouse transcriptome using Bowtie (Langmead et al., 2009) with parameters: -a --best -m1 --strata. To construct the transcriptome, the annotation file from the ENSEMBL database (GRCm38) was used. For each gene, the mRNA with the longest CDS was selected. In the case of equal CDS length, the longest transcript was used. For read alignment, a maximum of two mismatches were permitted. To avoid ambiguity, reads that were mapped to multiple positions were excluded.
Aggregation plot of footprint reads near the start and stop codons.
The ribosome P-site was defined as positons +12, +13, and +14 from the 5’-end of the reads (the first positon of the reads is recorded as 0). Footprint reads in the CDS were counted for each mRNA. mRNAs with total reads in the CDS <32 or having empty codons (no observed reads) >90% were excluded. Ribosome footprint counts at individual mRNA sites were normalized by the average count of the CDS. The normalized footprint counts with the same distance from the start or stop codon were then averaged over the whole transcriptome. We applied a threshold, i.e. total read count >32 in this study, to filter out the mRNAs that are inactive in translation. In addition, we repeated our analysis by using different thresholds (>16, >32, >64 or >128), and confirmed that the initiation pausing revealed by aggregation plot remains unchanged.
Estimation of ribosome occupancy on mRNAs.
For each mRNA, a RPKM (Reads Per Kilobase of transcript, per Million mapped reads) value was calculated, and used to measure the relative ribosome occupancy on individual mRNAs. mRNAs with RPKM values < 1 were excluded. For parallel paired-end RNA-seq, a FPKM (Fragments Per Kilobase of transcript per Million mapped reads) value was calculated. Riboocp was calculated by dividing ribosome occupancy by the corresponding mRNA level. mRNAs with fold change of ribosome occupancy or mRNA level between samples higher than 2 (up-regulated) or lower than 0.5 (downregulated) were defined as the groups of mRNAs with differences in ribosome occupancy or mRNA level. Of note, ribosome occupancy was evaluated with the inclusion of the initiation codon of mRNAs. We observed negligible effects on the evaluation of the changes in ribosome occupancy between the classified mRNAs in groups when the initiation codon was excluded. We classified mRNAs into seven groups in each stress condition: (i) ribosome occupancy (riboocp) up or (ii) ribosome occupancy down, wherein the ribosome footprint changes exceeded the changes in mRNA levels; (iii) congruent up and (iv) congruent down, exhibiting parallel changes up or down, respectively, in both mRNA levels and ribosome footprints; (v) buffering up or (vi) buffering down, if the changes in mRNA levels exceeded changes in ribosome footprints; and (vii) no change (NC), including the remaining mRNAs which did not exhibit enough change to reach the threshold for the six regulated groups.
We also used (Love et al., 2014) DESeq2 (Fig. S12) to identify mRNAs with significant changes (FDR < 0.05 and the estimated fold change is higher than 2-fold). As a result, when cells were treated with 500 or 600 mOsm, mRNAs with up- or down- regulated translation defined by DESeq2 are similar to mRNA groups defined by a threshold-only method (>2 or <1/2). When cells were treated with 700 mOsm, DESeq2 tends to find more up-regulated mRNAs, and less down-regulated (2417 up- and 2080 down- regulated). With the fact that DESeq2 or other differential analysis tools do not work well when there is global change (transcriptome) of expression levels, and that our biochemistry data demonstrate a global reduction of protein synthesis, it is likely that DESeq2 overestimates translation levels of most mRNAs when cells were treated with 700 mOSM, leading to a higher number of up-regulated mRNAs. Although the threshold-only method has a similar problem, that is, RPKM values and the fold change represent a relative rather than an absolute translation level which may be skewed when translation is globally down, the results based on the threshold-only method can be explained well by our biochemistry data. We therefore defined mRNA groups only based on a threshold in the main manuscript.
Calculation of codon occupancy.
For each mRNA, ribosome footprint counts at individual codons of the CDS were normalized to the average count of the CDS. Similar to the aggregation plot, mRNAs with read count<32 were not included. The normalized ribosome occupancies at the same codons were then averaged over the whole transcriptome. The average ribosome occupancy at codons was defined as codon occupancy. To estimate ribosome occupancy in different CDS regions, the CDS was divided into 100 bins, and ribosome occupancies at 61 codons (the three stop codons were not included) within the same bin were averaged over the whole transcriptome.
Classification of RNAs into groups.
We evaluated the fold change of RNA levels and ribosome footprints as compared to untreated controls, and classified mRNAs into seven groups in each stress condition (Supplemental File 1): (i) ribosome occupancy (riboocp) up or (ii) ribosome occupancy down, wherein the ribosome footprint changes exceeded the changes in mRNA levels; (iii) congruent up and (iv) congruent down, exhibiting parallel changes up or down, respectively, in both mRNA levels and ribosome footprints; (v) buffering up or (vi) buffering down, if the changes in mRNA levels exceeded changes in ribosome footprints; and (vii) no change (NC), including the remaining mRNAs which did not exhibit enough change to reach the threshold for the six regulated groups (Figure 2A and Supplemental File 1).
Calculation of pausing index.
For each mRNA, a pausing index at the AUG codon was calculated. We defined the pausing index as the ratio of ribosome footprint reads at AUG codons over the other 60 codons. Upon stress, mRNAs with pausing index increases up to 2-fold or higher were defined as paused mRNAs, and the remaining mRNAs were defined as the non-paused mRNAs. We defined the initiation pausing index as the ratio of ribosome footprint reads at the authentic initiation sites over the mean reads of the CDS. Because the majority of mRNAs showed dramatically increased initiation pausing (fold change of initiation pausing index > 2) upon stress treatment, the mRNAs with initiation pausing were defined as those with fold changes of initiation pausing index at the top 20%. As a control, the bottom 20% mRNAs were defined as the non-paused mRNAs.
RNA secondary structure analysis.
We used a sliding window of 30 nucleotides with a step of 3 nucleotides to calculate RNA minimum fold free energy (MFE) along the 5’ UTR of mRNA. For each sliding window, MFE was calculated using the default parameters of ViennaRNA (Lorenz et al., 2011). For each mRNA, an average value of MFE was calculated by averaging MFE values in all sliding windows within 5’ UTR of the mRNA.
uORF prediction.
We used Ribo-seq data to predict uORFs with robust translation. For each mRNA, we first extracted all possible uORFs, i.e., mRNA regions starting with ATG, GTG, CTG or TTG, and ending with TAG, TGA or TAA. The potential uORFs with total ribo-seq reads higher than 15 and the fraction of in-frame reads higher than 60% were defined as uORFs with robust translation. The overlapping uORFs located to the same coding frame were merged into a single one, by selecting the stop codon located at the most 5’ end of transcript, and the start codon which maximizes the fraction of in-frame reads.
Code availability.
All statistical analyses were performed using R. The heatmap plots were made by R package heatmap.2. All procedures but sequencing mapping were completed using custom Perl scripts, which are available upon request.
Pathway analysis.
For KEGG pathway analysis, the corresponding list of genes were fed into the STRING database (https://string-db.org/), and the pathways with the lowest FDR values were plotted. GO term analyses were performed with the GOEnrichment tool from Galaxy (Afgan et al., 2018) using a Benjamimi-Hochberg multiple test correction with a P-value cut off of 0.01 to generate a summarized list of enriched terms. PANTHER GO-Slim (Mi et al., 2019) was used as the gene ontology file along with the Mouse Genome Informatics GO annotation file, format 2.1, GO version 2020-03-23 (Ashburner et al., 2000; The Gene Ontology, 2019). Hierarchical clustering of q-values associated with each enriched GO term was performed using the NG-CMH Builder (Ward agglomeration, Euclidean distance) (Broom et al., 2017).
Polysome profile analysis
The lysate preparation and centrifugation conditions were identical to those described in the ribosome footprinting analysis. 12 fractions (1.2 mL) were collected, and RNA from each fraction was isolated using TRIzol LS reagent. An equal volume of RNA from each fraction was used for cDNA synthesis using the SuperScript™ III First-Strand Synthesis SuperMix for qRT-PCR (Thermo Fisher Scientific, 11752250). The relative quantity of specific mRNAs was measured by reverse transcriptase (RT)-quantitative polymerase chain reaction (qPCR) using VeriQuest SYBR Green qPCR Master Mix (Thermo Fisher Scientific, 756001000RXN) with the StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, 4376599). For the list of primers see Table S1. For the studies of the migration of translation initiation factors, 1% formaldehyde was added to the lysis buffer and quenched with glycine (0.25 M) after 20 min on ice. Lysates were layered on 5%–20% sucrose gradients and centrifuged at 20,000 rpm for 20 h using an SW 32 Ti rotor. Twelve equal-size fractions were collected and precipitated with TCA (10%) at 4°C overnight. Precipitated proteins were washed twice with the wash buffer (volume 5:1 of acetone:1 M Tris-HCl at pH 7.4), dried, and dissolved in 1X SDS-PAGE sample buffer (50 mM Tris-HCl at pH 6.8, 5% β-mercaptoethanol, 2% SDS, 10% glycerol, 0.05% bromophenol blue). An equal volume of the dissolved proteins from each fraction was run on SDS-PAGE, transferred to membranes, and immunoblotted with specific antibodies. Polysome profile tracers were compared by the evaluation of the area under the curves (AUC, www.originlab.com). For example, in 500 mOsm we observed 33% decrease of AUC as compared to control when the AUC of fractions 5–12(corresponding to polysomes) were compared. This method of analysis confirms the decrease of protein synthesis observed with increased extracellular osmolarity.
Mitochondrial membrane potential measurement
MEF cells were cultured on coverslips overnight, followed by treatment with hyperosmotic stress and refreshed in isoosmolar media as indicated. After treatment, cells were incubated with 0.5 μM tetramethylrhodamine (TMRM; a cell permeant fluorescent red-orange dye as mitochondrial membrane potential indicator) (Thermo Fisher Scientific,T668) and Hoechst dye (Thermo Fisher Scientific, 62249) for 30 min at 37°C. After staining, cells were washed 3 times with warm PBS and fixed on coverslips with mounting reagent (DAKO, S3025). Cell images were taken by confocal microscopy (Fluoview FV100, Olympus) followed with ImageJ quantification of total TMRM signal and cell numbers from each image. Mitochondrial membrane potential from each image was calculated as the total TMRM signal normalized to the total cell number. At least 100 cells per image were counted in at least 4 fields per coverslip. Three replicates were analyzed for each indicated treatment.
Mitochondrial morphology and fragmentation measurement
MEFs were cultured on coverslips overnight followed by treatment with hyperosmotic stress and recovery in isoosmolar media as indicated. After treatment, cells were fixed in 4% paraformaldehyde for 30 min and permeabilized with 0.1% Triton X-100. After blocking with 2% normal goat serum (Thermo Fisher Scientific, 10000C), fixed cells were incubated overnight at 4°C with anti-TOM20 primary antibodies (Proteintech,11802–1-AP) followed with 3 PBS washes. Secondary antibody with Alexa Fluor 488 (Thermo Fisher Scientific, A −11034) was applied to the coverslips followed by nuclei staining with Hoechst dye. After staining, cells were washed 3 times with warm PBS and mounted with mounting reagent. Cell images were taken by confocal microscopy (Olympus, Fluoview FV100) followed by ImageJ quantification of total cell numbers and cells with fragmented mitochondria from each image. At least 10 images were taken from each coverslip, and at least 100 cells per treatment were counted in the analysis. In this set of experiments, treatment of cells for 2 h with 10 μM of Carbonyl Cyanide Chlorophenylhydrazone (CCCP; Sigma-Aldrich, C2759) served as a positive control due to its known ability to severely induce mitochondrial fragmentation.
QUANTIFICATION AND STATISTICAL ANALYSIS
At least three independent experiments or biological replicates were used to generate all quantitative data and presented as mean ± SEM. All statistics is included in the Figure Legends. For all Ribo-seq datasets, translation levels are estimated based on footprint reads in the CDS region. For all RNA-seq datasets, mRNA expression levels are estimated based on sequencing reads on mRNAs, including 5’ UTR and 3’ UTR. All Ribo-seq and RNA-seq data analyses are performed using house-hold Perl scripts, unless otherwise stated. For all Ribo-seq and RNA-seq, Wilcoxon rank test was performed, using the software R, to estimate statistical significance of the changes in translation or mRNA expression levels. Two biological replicates of all Ribo-seq and RNA-seq samples are used.
Supplementary Material
Supplemental File 1: Quantitative analysis of changes in ribosome occupancies in response to increasing stress intensity. Related to Figs. 2A, 2C, 4C, and 5A.
The following sheets are included in this order:
(1) 500 mOsm 2 h vs Control (Fig. 2A)
(2) 600 mOsm 2 h vs Control (Fig. 2A)
(3) 700 mOsm 2 h vs Control (Fig. 2A)
(4) Enriched GO Terms (Fig. 2C)
(5) 700 mOsm 2 h recovery 15 min vs 700 mOsm 2 h (Fig. 4C)
(6) 700 mOsm 2 h recovery 2 h vs 700 mOsm 2 h (Fig. 4C)
(7) 700 mOsm 15 min recovery 2 h vs Control (Fig. 5A)
(8) 700 mOsm 2 h recovery 2 h vs Control (Fig. 5A)
Supplemental File 2: Quantitative analysis of changes in ribosome occupancies in response to increasing stress intensity. Related to Figs. 2B and S2A.
The following sheets are included in this order:
(1) 500 mOsm 2 h vs Control (Fig. 2B)
(2) 600 mOsm 2 h vs Control (Fig. 2B)
(3) 700 mOsm 2 h vs Control (Fig. 2B)
(4) 500 mOsm 2 h vs Control (Fig. S2A)
(5) 600 mOsm 2 h vs Control (Fig. S2A)
(6) 700 mOsm 2 h vs Control (Fig. S2A)
Supplemental File 3: Quantitative analysis of changes of ribosome pausing at initiation codons across different stress intensities. Related to Figs. 3D and 4A.
Supplemental File 4: Evaluation of pausing events at initiation codons and their relevance to translation. Related to STAR Methods and Fig. S4A.
Highlights:
Cells exposed to extremely harsh environments preserve the ability to recover
Severe stress induces dramatic mitochondrial fragmentation
Severe stress induces a hibernation-like state entailing ribosome pausing
Exit from the hibernation-like state requires induction of ISR
Acknowledgements
This work was supported by NIH R01DK053307, R01DK060596, and R01DK113196 to MH; CDDRCC pilot grant DK097948 to MH; NIGMS R01GM107331 to DDL; National science center (Poland) grant 2018/30/E/NZ1/00605 to DK; R21CA227917, R01GM1222814, and HHMI Faculty Scholar (55108556) to S.-B.Q; Canadian Institutes of health research (CIHR; MOP-363027) and Joint Canada-Israel Health Research Program (JCIHRP) [108589-001] to IT who is a senior research scholar of FRQ-S; YZ supported in part by R01CA230453; D.H. and X.Q. supported by R01AG065240 and R01NS115903. WCM. is supported by GM128981-0A1. AT is supported by DFG-SFB 1218-269925409 and TR1018/8-1. R01GM135362 TO HAC. The manuscript was edited by Kenneth Farabaugh.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests
The authors declare no competing interests.
References:
- Abbas T, and Dutta A (2009). p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 9, 400–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afgan E, Baker D, Batut B, van den Beek M, Bouvier D, Cech M, Chilton J, Clements D, Coraor N, Gruning BA, et al. (2018). The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res 46, W537–W544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev DE, O’Connor PB, Fahey C, Kenny EM, Terenin IM, Dmitriev SE, Cormican P, Morris DW, Shatsky IN, and Baranov PV (2015). Translation of 5’ leaders is pervasive in genes resistant to eIF2 repression. Elife 4, e03971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprile-Garcia F, Tomar P, Hummel B, Khavaran A, and Sawarkar R (2019). Nascent-protein ubiquitination is required for heat shock-induced gene downregulation in human cells. Nat Struct Mol Biol 26, 137–146. [DOI] [PubMed] [Google Scholar]
- Apte RS, Chen DS, and Ferrara N (2019). VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 176, 1248–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsenijevic T, Vujovic A, Libert F, Op de Beeck A, Hebrant A, Janssens S, Gregoire F, Lefort A, Bolaky N, Perret J, et al. (2013). Hyperosmotic stress induces cell cycle arrest in retinal pigmented epithelial cells. Cell Death Dis 4, e662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. (2000). Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25, 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartek J, and Lukas J (2003). Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3, 421–429. [DOI] [PubMed] [Google Scholar]
- Bordeleau ME, Mori A, Oberer M, Lindqvist L, Chard LS, Higa T, Belsham GJ, Wagner G, Tanaka J, and Pelletier J (2006). Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat Chem Biol 2, 213–220. [DOI] [PubMed] [Google Scholar]
- Brett-Morris A, Wright BM, Seo Y, Pasupuleti V, Zhang J, Lu J, Spina R, Bar EE, Gujrati M, Schur R, et al. (2014). The polyamine catabolic enzyme SAT1 modulates tumorigenesis and radiation response in GBM. Cancer Res 74, 6925–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom BM, Ryan MC, Brown RE, Ikeda F, Stucky M, Kane DW, Melott J, Wakefield C, Casasent TD, Akbani R, et al. (2017). A Galaxy Implementation of Next-Generation Clustered Heatmaps for Interactive Exploration of Molecular Profiling Data. Cancer Res 77, e23–e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MB, Ferraris JD, and Dmitrieva NI (2007). Cellular response to hyperosmotic stresses. Physiol Rev 87, 1441–1474. [DOI] [PubMed] [Google Scholar]
- Butturini E, Carcereri de Prati A, Boriero D, and Mariotto S (2019). Tumor Dormancy and Interplay with Hypoxic Tumor Microenvironment. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calviello L, Mukherjee N, Wyler E, Zauber H, Hirsekorn A, Selbach M, Landthaler M, Obermayer B, and Ohler U (2016). Detecting actively translated open reading frames in ribosome profiling data. Nat Methods 13, 165–170. [DOI] [PubMed] [Google Scholar]
- Calvo SE, Clauser KR, and Mootha VK (2016). MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res 44, D1251–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano-Crespo S, Chillaron J, Junza A, Fernandez-Miranda G, Garcia J, Polte C, L R.d.l.B., Ignatova Z, Yanes O, Zorzano A, et al. (2019). CD98hc (SLC3A2) sustains amino acid and nucleotide availability for cell cycle progression. Sci Rep 9, 14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copp J, Wiley S, Ward MW, and van der Geer P (2005). Hypertonic shock inhibits growth factor receptor signaling, induces caspase-3 activation, and causes reversible fragmentation of the mitochondrial network. Am J Physiol Cell Physiol 288, C403–415. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, and Walter P (2020). The integrated stress response: From mechanism to disease. Science 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello JL, Kershaw CJ, Castelli LM, Talavera D, Rowe W, Sims PFG, Ashe MP, Grant CM, Hubbard SJ, and Pavitt GD (2017). Dynamic changes in eIF4F-mRNA interactions revealed by global analyses of environmental stress responses. Genome Biol 18, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell AM, Subramaniam AR, and O’Shea EK (2018). Translational Control through Differential Ribosome Pausing during Amino Acid Limitation in Mammalian Cells. Mol Cell 71, 229–243 e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das I, Krzyzosiak A, Schneider K, Wrabetz L, D’Antonio M, Barry N, Sigurdardottir A, and Bertolotti A (2015). Preventing proteostasis diseases by selective inhibition of a phosphatase regulatory subunit. Science 348, 239–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva NI, and Burg MB (2008). Analysis of DNA breaks, DNA damage response, and apoptosis produced by high NaCl. Am J Physiol Renal Physiol 295, F1678–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh KT, Krokowski D, Guan BJ, Gao Z, Gao XH, Wu J, Jobava R, Ray G, de Jesus TJ, Bianchi MG, et al. (2020). PACT-mediated PKR activation acts as a hyperosmotic stress intensity sensor weakening osmoadaptation and enhancing inflammation. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmann TM, Jahnel M, Pozniakovsky A, Mahamid J, Holehouse AS, Nuske E, Richter D, Baumeister W, Grill SW, Pappu RV, et al. (2018). Phase separation of a yeast prion protein promotes cellular fitness. Science 359. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Smith CB, Brenner M, DeGracia DJ, Krause GS, Marrone L, Dever TE, and Hallenbeck JM (1998). Suppression of protein synthesis in brain during hibernation involves inhibition of protein initiation and elongation. Proc Natl Acad Sci U S A 95, 14511–14516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerashchenko MV, and Gladyshev VN (2014). Translation inhibitors cause abnormalities in ribosome profiling experiments. Nucleic Acids Res 42, e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godefroy-Colburn T, and Thach RE (1981). The role of mRNA competition in regulating translation. IV. Kinetic model. J Biol Chem 256, 11762–11773. [PubMed] [Google Scholar]
- Grady CR, Knepper MA, Burg MB, and Ferraris JD (2014). Database of osmoregulated proteins in mammalian cells. Physiol Rep 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves JA, Lee A, Yildirir G, and Zachara NE (2013). Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis. Cell Stress Chaperones 18, 535–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan BJ, van Hoef V, Jobava R, Elroy-Stein O, Valasek LS, Cargnello M, Gao XH, Krokowski D, Merrick WC, Kimball SR, et al. (2017). A Unique ISR Program Determines Cellular Responses to Chronic Stress. Mol Cell 68, 885–900 e886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Li SY, Ji FY, Zhao YF, Zhong Y, Lv XJ, Wu XL, and Qian GS (2014). Role of Angptl4 in vascular permeability and inflammation. Inflamm Res 63, 13–22. [DOI] [PubMed] [Google Scholar]
- Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, et al. (2017). Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature 551, 247–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Scheuner D, Chen JJ, Kaufman RJ, and Ron D (2009). Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 alpha (eIF2alpha) dephosphorylation in mammalian development. Proc Natl Acad Sci U S A 106, 1832–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S, Kostantin E, Wang SJ, Hristova T, Galicia-Vazquez G, Baranov PV, Pelletier J, and Tremblay ML (2019). Magnesium-sensitive upstream ORF controls PRL phosphatase expression to mediate energy metabolism. Proc Natl Acad Sci U S A 116, 2925–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG, Ivanov IP, and Sonenberg N (2016). Translational control by 5’-untranslated regions of eukaryotic mRNAs. Science 352, 1413–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz JM, and Horwitz BA (2019). Extreme Neuroplasticity of Hippocampal CA1 Pyramidal Neurons in Hibernating Mammalian Species. Front Neuroanat 13, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrvatin S, Sun S, Wilcox OF, Yao H, Lavin-Peter AJ, Cicconet M, Assad EG, Palmer ME, Aronson S, Banks AS, et al. (2020). Neurons that regulate mouse torpor. Nature 583, 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Brar GA, Rouskin S, McGeachy AM, and Weissman JS (2012). The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat Protoc 7, 1534–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Ghaemmaghami S, Newman JR, and Weissman JS (2009). Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324, 218–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia NT, Lareau LF, and Weissman JS (2011). Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell 147, 789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iserman C, Desroches Altamirano C, Jegers C, Friedrich U, Zarin T, Fritsch AW, Mittasch M, Domingues A, Hersemann L, Jahnel M, et al. (2020). Condensation of Ded1p Promotes a Translational Switch from Housekeeping to Stress Protein Production. Cell 181, 818–831 e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi Y, Yang W, Zhu J, Burg MB, and Ferraris JD (2015). RNA-Seq analysis of high NaCl-induced gene expression. Physiol Genomics 47, 500–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SJ, Park S, Nguyen LT, Hwang J, Lee EY, Giong HK, Lee JS, Yoon I, Lee JH, Kim JH, et al. (2019). A threonyl-tRNA synthetase-mediated translation initiation machinery. Nat Commun 10, 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Jung Y, Wang J, Joseph J, Mishra A, Hill EE, Krebsbach PH, Pienta KJ, Shiozawa Y, and Taichman RS (2013). TBK1 regulates prostate cancer dormancy through mTOR inhibition. Neoplasia 15, 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar AA, Gross SR, Barth-Baus D, Strachan R, Hensold JO, Goss Kinzy T, and Merrick WC (2005). Novel characteristics of the biological properties of the yeast Saccharomyces cerevisiae eukaryotic initiation factor 2A. J Biol Chem 280, 15601–15611. [DOI] [PubMed] [Google Scholar]
- Komar AA, and Merrick WC (2020). A Retrospective on eIF2A-and Not the Alpha Subunit of eIF2. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppula P, Zhang Y, Zhuang L, and Gan B (2018). Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun (Lond) 38, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokowski D, Guan BJ, Wu J, Zheng Y, Pattabiraman PP, Jobava R, Gao XH, Di XJ, Snider MD, Mu TW, et al. (2017). GADD34 Function in Protein Trafficking Promotes Adaptation to Hyperosmotic Stress in Human Corneal Cells. Cell Rep 21, 2895–2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R, and Raffi FAM (2019). Dual-Specificity Phosphatases in Immunity and Infection: An Update. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M, and Salzberg SL (2009). Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Pestova TV, Shin BS, Cao C, Choi SK, and Dever TE (2002). Initiation factor eIF5B catalyzes second GTP-dependent step in eukaryotic translation initiation. Proc Natl Acad Sci U S A 99, 16689–16694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bevilacqua E, Chiribau CB, Majumder M, Wang C, Croniger CM, Snider MD, Johnson PF, and Hatzoglou M (2008). Differential control of the CCAAT/enhancer-binding protein beta (C/EBPbeta) products liver-enriched transcriptional activating protein (LAP) and liver-enriched transcriptional inhibitory protein (LIP) and the regulation of gene expression during the response to endoplasmic reticulum stress. J Biol Chem 283, 22443–22456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xie Y, Hao J, Liu J, Ning Y, Tang Q, Ma M, Zhou H, Guan S, Zhou Q, et al. (2018). ER-localized protein-Herpud1 is a new mediator of IL-4-induced macrophage polarization and migration. Exp Cell Res 368, 167–173. [DOI] [PubMed] [Google Scholar]
- Liu GY, and Sabatini DM (2020). mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol 21, 183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz R, Bernhart SH, Zu Siederdissen CH, Tafer H, Flamm C, Stadler PF, and Hofacker IL (2011). ViennaRNA Package 2.0. Algorithms for molecular biology 6, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou PH, Hansen BS, Olsen PH, Tullin S, Murphy MP, and Brand MD (2007). Mitochondrial uncouplers with an extraordinary dynamic range. Biochem J 407, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love MI, Huber W, and Anders S (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Dong L, Liu XM, Guo J, Ma H, Shen B, and Qian SB (2019). m(6)A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat Commun 10, 5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers KE, McFarlane SV, Zhao L, and Staples JF (2017). Regulation of mitochondrial metabolism during hibernation by reversible suppression of electron transport system enzymes. J Comp Physiol B 187, 227–234. [DOI] [PubMed] [Google Scholar]
- Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB, and Jaffrey SR (2015). 5’ UTR m(6)A Promotes Cap-Independent Translation. Cell 163, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Ebert D, Huang X, and Thomas PD (2019). PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res 47, D419–D426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Gravel SP, Chenard V, Sikstrom K, Zheng L, Alain T, Gandin V, Avizonis D, Arguello M, Zakaria C, et al. (2013). mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab 18, 698–711. [DOI] [PubMed] [Google Scholar]
- Mota-Martorell N, Jove M, Pradas I, Sanchez I, Gomez J, Naudi A, Barja G, and Pamplona R (2020). Low abundance of NDUFV2 and NDUFS4 subunits of the hydrophilic complex I domain and VDAC1 predicts mammalian longevity. Redox Biol 34, 101539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motori E, Atanassov I, Kochan SMV, Folz-Donahue K, Sakthivelu V, Giavalisco P, Toni N, Puyal J, and Larsson N-G (2020). Neuronal metabolic rewiring promotes resilience to neurodegeneration caused by mitochondrial dysfunction. Science Advances 6, eaba8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, and Ron D (2001). Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 153, 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, and Ron D (2003). Stress-induced gene expression requires programmed recovery from translational repression. EMBO J 22, 1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picco N, Gatenby RA, and Anderson ARA (2017). Stem Cell Plasticity and Niche Dynamics in Cancer Progression. IEEE Trans Biomed Eng 64, 528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganathan AC, Adam AP, Zhang L, and Aguirre-Ghiso JA (2006). Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol Ther 5, 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DW, Tay AS, Sundaram JR, Lee IC, Chen Q, George SE, Nicchitta CV, and Shenolikar S (2016). Complementary Roles of GADD34- and CReP-Containing Eukaryotic Initiation Factor 2alpha Phosphatases during the Unfolded Protein Response. Mol Cell Biol 36, 1868–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, and Buchner J (2010). The heat shock response: life on the verge of death. Mol Cell 40, 253–266. [DOI] [PubMed] [Google Scholar]
- Robert F, Cencic R, Cai R, Schmeing TM, and Pelletier J (2020). RNA-tethering assay and eIF4G:eIF4A obligate dimer design uncovers multiple eIF4F functional complexes. Nucleic Acids Res 48, 8562–8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, and Blenis J (2007). RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem 282, 14056–14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, and Topisirovic I (2018). Signaling Pathways Involved in the Regulation of mRNA Translation. Mol Cell Biol 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti C, Bai Y, Stanford SM, Di Benedetto P, Cipriani P, Santelli E, Piera-Velazquez S, Chernitskiy V, Kiosses WB, Ceponis A, et al. (2017). PTP4A1 promotes TGFbeta signaling and fibrosis in systemic sclerosis. Nat Commun 8, 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia M, Jobava R, Parisien M, Putnam A, Krokowski D, Gao XH, Guan BJ, Yuan Y, Jankowsky E, Feng Z, et al. (2014). Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells from apoptosis during osmotic stress. Mol Cell Biol 34, 2450–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia M, Krokowski D, Guan BJ, Ivanov P, Parisien M, Hu GF, Anderson P, Pan T, and Hatzoglou M (2012). Genome-wide identification and quantitative analysis of cleaved tRNA fragments induced by cellular stress. J Biol Chem 287, 42708–42725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, and Kaufman RJ (2001). Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7, 1165–1176. [DOI] [PubMed] [Google Scholar]
- Sendoel A, Dunn JG, Rodriguez EH, Naik S, Gomez NC, Hurwitz B, Levorse J, Dill BD, Schramek D, Molina H, et al. (2017). Translation from unconventional 5’ start sites drives tumour initiation. Nature 541, 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalgi R, Hurt JA, Krykbaeva I, Taipale M, Lindquist S, and Burge CB (2013). Widespread regulation of translation by elongation pausing in heat shock. Mol Cell 49, 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin BS, Maag D, Roll-Mecak A, Arefin MS, Burley SK, Lorsch JR, and Dever TE (2002). Uncoupling of initiation factor eIF5B/IF2 GTPase and translational activities by mutations that lower ribosome affinity. Cell 111, 1015–1025. [DOI] [PubMed] [Google Scholar]
- Singh B, Carpenter G, and Coffey RJ (2016). EGF receptor ligands: recent advances. F1000Res 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snezhkina AV, Kudryavtseva AV, Kardymon OL, Savvateeva MV, Melnikova NV, Krasnov GS, and Dmitriev AA (2019). ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid Med Cell Longev 2019, 6175804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples JF (2014). Metabolic suppression in mammalian hibernation: the role of mitochondria. J Exp Biol 217, 2032–2036. [DOI] [PubMed] [Google Scholar]
- Starck SR, Jiang V, Pavon-Eternod M, Prasad S, McCarthy B, Pan T, and Shastri N (2012). Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I. Science 336, 1719–1723. [DOI] [PubMed] [Google Scholar]
- Steitz JA (1969). Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature 224, 957–964. [DOI] [PubMed] [Google Scholar]
- Stone LLH, Chappuis E, Marquez M, McFalls EO, Kelly RF, and Butterick T (2019). Mitochondrial Respiratory Capacity is Restored in Hibernating Cardiomyocytes Following Co-Culture with Mesenchymal Stem Cells. Cell Med 11, 2155179019834938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenin IM, Dmitriev SE, Andreev DE, and Shatsky IN (2008). Eukaryotic translation initiation machinery can operate in a bacterial-like mode without eIF2. Nat Struct Mol Biol 15, 836–841. [DOI] [PubMed] [Google Scholar]
- Tew SR, Vasieva O, Peffers MJ, and Clegg PD (2011). Post-transcriptional gene regulation following exposure of osteoarthritic human articular chondrocytes to hyperosmotic conditions. Osteoarthritis Cartilage 19, 1036–1046. [DOI] [PubMed] [Google Scholar]
- The Gene Ontology C (2019). The Gene Ontology Resource: 20 years and still GOing strong. Nucleic Acids Res 47, D330–D338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, and Sabatini DM (2012). A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 485, 109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt ML, Conn CS, Shi Z, Pang X, Tokuyasu T, Coady AM, Seo Y, Barna M, and Ruggero D (2015). Differential Requirements for eIF4E Dose in Normal Development and Cancer. Cell 162, 59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Johnson AG, Lapointe CP, Choi J, Prabhakar A, Chen DH, Petrov AN, and Puglisi JD (2019). eIF5B gates the transition from translation initiation to elongation. Nature 573, 605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S, and Grose R (2003). Regulation of wound healing by growth factors and cytokines. Physiol Rev 83, 835–870. [DOI] [PubMed] [Google Scholar]
- Wu B, Eliscovich C, Yoon YJ, and Singer RH (2016). Translation dynamics of single mRNAs in live cells and neurons. Science 352, 1430–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Peterson A, Zinshteyn B, Regot S, and Green R (2020). Ribosome Collisions Trigger General Stress Responses to Regulate Cell Fate. Cell 182, 404–416 e414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Kamata T, Nawa H, Sekijima T, and Takei N (2019). AMPK activation, eEF2 inactivation, and reduced protein synthesis in the cerebral cortex of hibernating chipmunks. Sci Rep 9, 11904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoll WL, Horton LE, Komar AA, Hensold JO, and Merrick WC (2002). Characterization of mammalian eIF2A and identification of the yeast homolog. J Biol Chem 277, 37079–37087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental File 1: Quantitative analysis of changes in ribosome occupancies in response to increasing stress intensity. Related to Figs. 2A, 2C, 4C, and 5A.
The following sheets are included in this order:
(1) 500 mOsm 2 h vs Control (Fig. 2A)
(2) 600 mOsm 2 h vs Control (Fig. 2A)
(3) 700 mOsm 2 h vs Control (Fig. 2A)
(4) Enriched GO Terms (Fig. 2C)
(5) 700 mOsm 2 h recovery 15 min vs 700 mOsm 2 h (Fig. 4C)
(6) 700 mOsm 2 h recovery 2 h vs 700 mOsm 2 h (Fig. 4C)
(7) 700 mOsm 15 min recovery 2 h vs Control (Fig. 5A)
(8) 700 mOsm 2 h recovery 2 h vs Control (Fig. 5A)
Supplemental File 2: Quantitative analysis of changes in ribosome occupancies in response to increasing stress intensity. Related to Figs. 2B and S2A.
The following sheets are included in this order:
(1) 500 mOsm 2 h vs Control (Fig. 2B)
(2) 600 mOsm 2 h vs Control (Fig. 2B)
(3) 700 mOsm 2 h vs Control (Fig. 2B)
(4) 500 mOsm 2 h vs Control (Fig. S2A)
(5) 600 mOsm 2 h vs Control (Fig. S2A)
(6) 700 mOsm 2 h vs Control (Fig. S2A)
Supplemental File 3: Quantitative analysis of changes of ribosome pausing at initiation codons across different stress intensities. Related to Figs. 3D and 4A.
Supplemental File 4: Evaluation of pausing events at initiation codons and their relevance to translation. Related to STAR Methods and Fig. S4A.
Data Availability Statement
Unprocessed western blot and microscopy images have been deposited at Mendeley Data (http://dx.doi.org/10.17632/yfgndyv8bh.1) and are publicly available as of the date of publication. Sequencing data has been deposited at GEO (GSE157519).
No original code has been generated for this publication.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


