Abstract
Purpose:
Long-term outcomes after external beam radiation therapy (EBRT) and radiofrequency ablation (RFA) for medically inoperable early stage non-small cell lung cancer (NSCLC) are not well-known.
Materials and Methods:
Patients with medically inoperable early stage NSCLC were enrolled in a prospective single-arm, phase 2 study between June 2007 and October 2008 and were treated with RFA followed by EBRT. Radiation was delivered using hypofractionated RT (HFRT) (70.2 Gy in 26 fractions) or stereotactic body RT (SBRT) (54 Gy in 3 fractions).
Results:
Twelve patients were evaluable; ten patients were treated with HFRT. The cumulative incidence of local progression at 5 years was 16.7% (95% CI: 0–37.8). Median progression-free survival was 37.8 months (95% CI: 11.1-not reached [NR]) and median overall survival was 53.6 months (95% CI: 21.0-NR). There were no mortalities within 30-days following RFA and no grade ≥4 toxicity.
Conclusion:
Combination RFA+EBRT appears feasible with favorable long-term local control. However, since SBRT alone has similar or better rates of control, we do not recommend routine combined RFA and EBRT.
Introduction:
At the time this study was conceived in 2007, modern prospective study results were lacking on the best utilization of RT for inoperable early stage NSCLC. External beam radiation therapy (EBRT) had been employed using different techniques including conventionally fractionated RT (CFRT) (1.8–2.0 Gy delivered daily), hypofractionated RT (HFRT) (>2.0 Gy/fx), and stereotactic body radiotherapy (SBRT) (ablative dose of radiation in a highly conformal approach). CFRT, even with escalated total doses of 70–80 Gy, led to high rates of local progression (LP).1 The CALGB 39904 study examined escalating doses of HFRT and found LP occurring in 3 of 39 (8%) patients after a median follow-up (MFU) of 53 months.2 Early results with SBRT were promising with 2-year local control (LC) rates of 95%.3
While RT techniques were evolving, other techniques such as radiofrequency ablation (RFA) were increasingly utilized. Information regarding the use of RFA in early stage NSCLC was limited but LC and safety profile were encouraging.4 It was hypothesized that combination RFA and EBRT may lead to improved LC. Dupuy et al. used CT-guided RFA followed by conventional RT (66 Gy) and reported a median overall survival (OS) of 24 months with only 2 of 24 patients having primary tumor progression after a MFU of 27 months.5 Based on this experience, Wake Forest Baptist Medical University initiated a prospective phase 2 study in 2007 to examine the combination of RFA and EBRT for patients with early stage NSCLC. Limited data regarding the combination of these modalities exist and we present the long-term outcomes form this study.
Materials and Methods:
Patients with biopsy-proven NSCLC, T1N0 or T2N0 (by AJCC 6th Edition) with a maximum tumor size of 3.5 cm were enrolled. Negative nodal status was determined via [18F] fluorodeoxyglucose positron emission tomography (PET) and/or negative mediastinal assessment using endobronchial ultrasound (EBUS). Patients were evaluated by a thoracic surgeon and either declined surgery or were inoperable due to comorbidities. Patients with prior chest irradiation were excluded. This study was approved by the IRB and registered on ClinicalTrials.gov as XXXX. Prior to enrollment patients underwent mandatory work up including a biopsy of the primary tumor, pulmonary function tests (PFTs), coagulation studies, and staging imaging including contrasted computed tomography (CT) chest and PET/CT. The target accrual was 35 patients; 13 patients were enrolled and the study was closed early due to slow accrual.
Ablation occurred within 21 days of study registration. A radiofrequency generator and expandable probes (Boston Scientific, Natick MA) were used with a co-axial guide when possible. The RF electrode was advanced through the chest wall and lung to the target lesion under CT guidance. Treatment was delivered as per standard institutional protocol until a major increase in impedance occurred. The location of the ablation probe and impact of the procedure on the surrounding parenchyma was monitored with serial CT imaging.
RT was initiated within a median of 36 days (range 27–60). Out of a great deal of caution for the safety of combining the two treatments, a planned delay between RFA and SBRT of approximately 5 weeks was recommended in the protocol. CT simulation was performed using a wingboard or SBRT immobilization device for planned HFRT or SBRT, respectively. Treatment planning included 4-dimensional respiratory timed imaging in which an internal target volume (ITV) was created to encompass the maximum intensity projection of the tumor during all phases of respiration (abdominal compression was utilized for SBRT when tolerated). The planning target volume (PTV) was defined using a uniform expansion from the ITV of 0.5 cm. Treatment was delivered using beam energies of 6 or 10 MV and pre-treatment portal images or cone-beam CT imaging were obtained. For HFRT, 7020 cGy was delivered over 26 daily fractions of 270 cGy. For lesions felt amenable to SBRT, 5400 cGy in 3 fractions was delivered using SBRT techniques with heterogeneity corrections.
Response determination was made with use of a modified RECIST criteria.6 Time-to-event outcomes were calculated from the date of study registration. Cumulative incidence (CI) of LP (CILP) was estimated using competing risk methodology (death without LP as the competing risk). LP was defined as progression within the PTV, OS as time to death or last follow-up (right censor), and progression-free survival (PFS) as the time to any disease progression (local, regional and/or distant), death, or last follow-up. OS and PFS were estimated using the Kaplan-Meier method. Safety and toxicity were evaluated using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. Statistical analyses were performed using R version 3.6 (R Foundation for Statistical Computing, Vienna, Austria).
Results:
Thirteen patients were enrolled between June 2007 and October 2008; one patient was not treated per protocol and was excluded from analysis. MFU was 51.7 months (95% CI: 12.3–130.9). Table 1 lists the clinical and treatment characteristics of the analyzed patients. Baseline FDG-PET mean SUV for the cohort was 6.0. Following RFA treatment mean SUV was 2.1 and following irradiation it was 2.5. Response based on modified RECIST found a complete or partial response in 67% of patients and stable disease in 33%. Five-year CILP at 5 years was 16.7% (95% CI: 10.8–37.8) (Figure 1). The competing risk of death without LP at 5 years was 41.7% (95% CI: 13.8–69.6). Three patients, all treated with RFA+HFRT, experienced LP at 6.8, 49.7, and 135.4 months. Two of these patients also had developed distant metastases at the time of their LP. The patient with LP alone at 135.4 months was successfully treated with a second course of HFRT but a year later did develop further LP as well as distant metastases and is still alive on systemic therapy. Among patients treated with RFA+HFRT (n=10), 5-year CILP was 20.0% (95% CI: 12.6–44.8). Overall median PFS was 37.8 months (95% CI: 11.1-not reached [NR]) and median OS was 53.6 months (95% CI: 21.0-NR).
Table 1:
Patient and Treatment Characteristics
| Patient Characteristics | n (%) | |
|---|---|---|
| Age at diagnosis (median [IQR]) | 71 [60–93] | |
| Gender | Male | 8 (67) |
| Female | 4 (33) | |
| Race | Caucasian | 11 (92) |
| African American | 1 (8.3) | |
| Ethnicity | Non-Hispanic | 12 (100) |
| ECOG | 0 | 7 (59) |
| 1 | 4 (33) | |
| 2 | 1 (8) | |
| Smoking History | Current | 8 (67) |
| Former | 4 (33) | |
| Pack Years (mean [SD]) | 67.4 [30.5] | |
| Histology | Squamous Cell Carcinoma | 2 (17) |
| Adenocarcinoma | 9 (75) | |
| NSCLC, NOS | 1 (8) | |
| Lesion size (mean [SD]) | 2.0 [0.6] | |
| Location | RUL | 3 (25) |
| RML | 2 (17) | |
| LUL | 7 (58) | |
| Stage (AJCC 6th edition) | IA | 11 (92) |
| IB | 1 (8) | |
| Dose-fractionation RT used | 70.2 Gy/26 fx | 10 (83) |
| 54 Gy/3 fx | 2 (17) | |
| Pre-Tx PET SUV (mean [SD]) | 6.0 [2.5] | |
| Post-RFA PET SUV (mean [SD]) | 2.1 [2.0] | |
| Post-RT PET SUV (mean [SD]) | 2.5 [2.6] | |
| Response (RECIST) | CR | 2 (17) |
| PR | 6 (50) | |
| SD | 4 (33) | |
| Local Progression | No | 9 (75) |
| Yes | 3 (25) | |
| Regional Progression | No | 7 (58) |
| Yes | 5 (42) | |
| Distant Progression | No | 4 (33) |
| Yes | 8 (67) |
ECOG = Eastern Cooperative Oncology Group; RT = Radiotherapy; PET = Positron Emission Tomography
Figure 1:
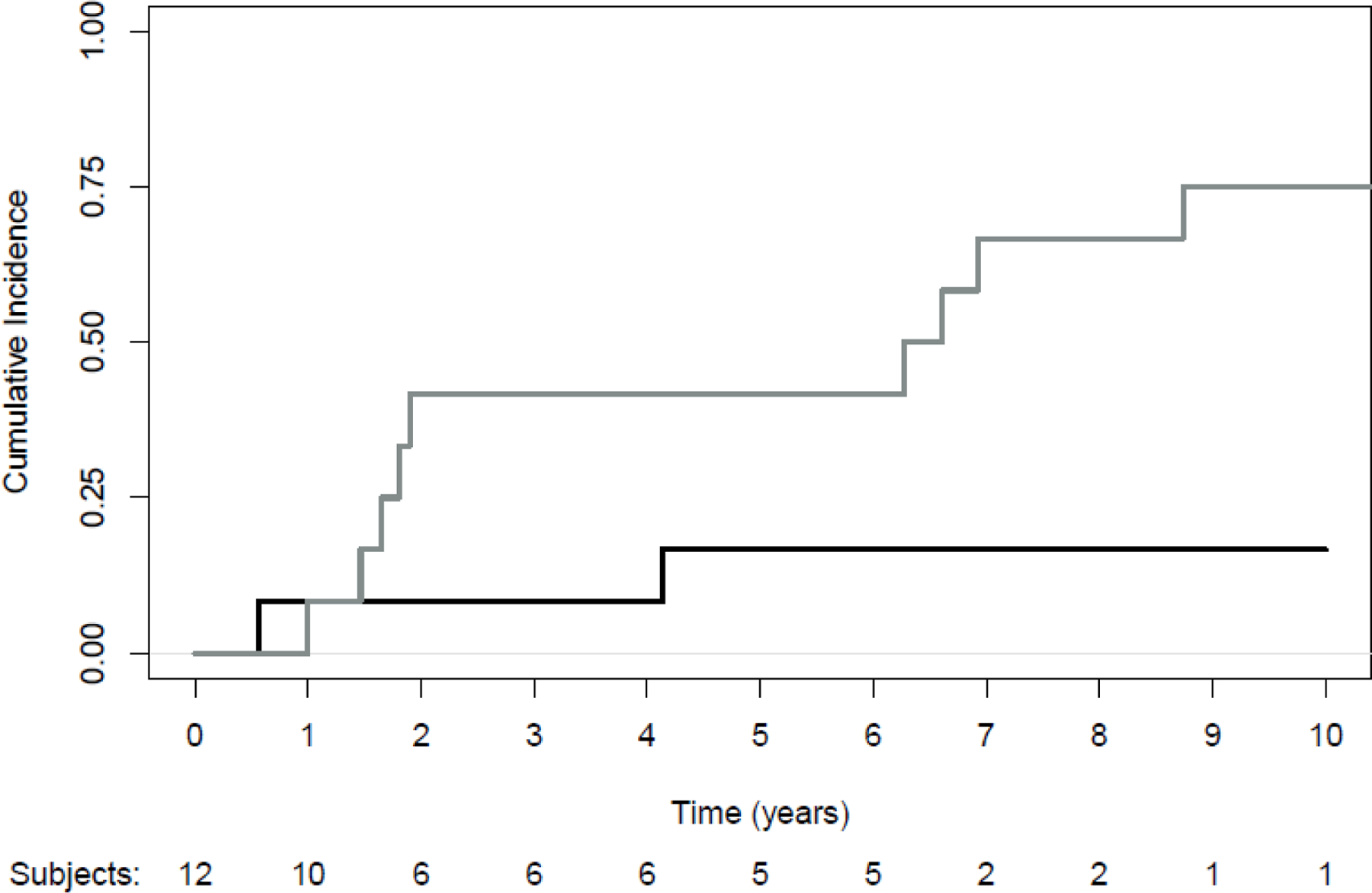
Cumulative Incidence of Local Progression with Death as a Competing Risk. Black line: progression; Grey line: death without progression
The adverse events and the associated grade of toxicity using CTCAEv3.0 are summarized in Table 2. There were no mortalities within 30-days following RFA. One patient required repeat RFA after the initial procedure related hemorrhage. One patient developed a grade 3 full thickness thermal burn due to a complication during the RFA procedure. There were no grade ≥2 acute pneumonitis and overall no grade ≥4 toxicity in the study.
Table 2:
Adverse Events
| Toxicity | Grade (CTCAEv3.0) | n (%) |
|---|---|---|
| Procedure Related Pneumothorax | 0 | 4 (33) |
| 1 | 4 (33) | |
| 2 | 4 (33) | |
| Procedure Related Infection | No | 12 (100) |
| Hemorrhage | 0 | 8 (67) |
| 1 | 3 (25) | |
| 2 | 1 (8) | |
| Skin | 0 | 5 (42) |
| 1 | 6 (50) | |
| 2 | 0 (0) | |
| 3 | 1 (8) | |
| Gastrointestinal | 0 | 9 (75) |
| 1 | 2 (17) | |
| 2 | 1 (8) | |
| Pneumonitis | 0 | 2 (17) |
| 1 | 10 (83) | |
| Post-Tx ECOG | 0 | 5 (42) |
| 1 | 5 (42) | |
| 2 | 1 (8) | |
| 3 | 1 (8) |
ECOG = Eastern Cooperative Oncology Group
Discussion:
The results of this trial are consistent with other studies that have investigated a combination of RFA and EBRT.5,7 These studies utilized RFA+CFRT while our study treated patients in a unique manner with RFA+HFRT (70.2 Gy in 26 fractions) or RFA+SBRT (54 Gy in 3 fractions), with a BED (α/β = 10) of 89.2 and 151.2, respectively. In addition to the CALGB 39904 study mentioned previously, a retrospective study reporting on stage I NSCLC patients treated with the same hypofractionated course of RT that was used in our study found a LP of 25% after a MFU of 43 months.2,8 Two clinical trials (TROG 09.02/CHISEL and SPACE) that enrolled patients with early-stage medically inoperable NSCLC have demonstrated improvements in disease control, reduction of local failure, and improved toxicity with SBRT compared to CFRT.9,10 Both of these trials began enrolling within 1–2 years of the current study era but were more recently reported with 2 to 3 year disease control outcomes. The 2-year local control with SBRT was 89% on the CHISEL trial and 3-year local control with SBRT was 86.4% on the SPACE trial. In addition, a phase 2 study, NCIC CTG BR.25, treated patients with hypofractionated radiation with 60 Gy in 15 fractions and found a 2-year primary tumor control of 87.4%.11 Longer term control data has not been published to the best of our knowledge. However, when comparing the results of our study against these others which have demonstrated high local control with HFRT alone, it calls into the question whether combination with RFA provides additional benefits to a patient treated with HFRT alone.
At the time this study was conceived and initiated, the large prospective SBRT trials of patients with inoperable early stage lung cancer had not yet been reported. Since that time, SBRT has been shown in a variety of dose-fractionation schemes to be the standard of care for this patient population with high rates of LC (>85–90%) and minimal grade ≥3 toxicity.12–14 A systematic review and pooled analysis compared SBRT and RFA in patients with inoperable stage I NSCLC demonstrated a 5-year LC rate of RFA to be 42% which was significantly lower than that for SBRT (88%) (p<0.001).15 Though the CILP found in our study of 16.7% is good, it does not appear to be an improvement to the rates of control seen in patients treated with SBRT alone. While most prospective SBRT series report 1–3 year outcomes, long term outcomes from RTOG 0236 showed 5-year primary and lobar progression of 20%, but primary of only 7.3%.16 Another phase 2 study, RTOG 0618, examined the use of SBRT in operable patients found a 4-year primary and lobar progression of only 4%.17
Conclusion:
Combination RFA+EBRT appears feasible with 5-year CILP of 16.7%. However, SBRT alone has become the standard treatment for inoperable early stage NSCLC. We would not recommend routine use of combined modalities when SBRT alone has similar or better control at 5 years and with decreased toxicity such as pneumothorax. Regardless, long term outcomes from this study may guide clinical practice in rare scenarios that may necessitate salvage RT after RFA or in re-irradiation cases requiring de-escalated RT dose.
Funding:
The authors wish to acknowledge the support of the Wake Forest Baptist Comprehensive Cancer Center Biostatistics Shared Resource and Clinical Protocol and Data Management Shared Resource, supported by the National Cancer Institute’s Cancer Center Support Grant award number P30CA012197. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Cole R. Steber: none
Ryan T. Hughes: none
James Urbanic: none
Hollins Clark: none
Jeffrey Petty: none
A William Blackstock: none
Michael K. Farris: none
Clinical Trial Information: ClinicalTrials.gov Identifier: NCT00499447
Data Availability:
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References:
- 1.Bradley JD, Wahab S, Lockett MA, Perez CA, Purdy JA. Elective nodal failures are uncommon in medically inoperable patients with Stage I non-small-cell lung carcinoma treated with limited radiotherapy fields. Int J Radiat Oncol Biol Phys. 2003;56(2):342–347. [DOI] [PubMed] [Google Scholar]
- 2.Bogart JA, Hodgson L, Seagren SL, et al. Phase I study of accelerated conformal radiotherapy for stage I non-small-cell lung cancer in patients with pulmonary dysfunction: CALGB 39904. J Clin Oncol. 2010;28(2):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833–4839. [DOI] [PubMed] [Google Scholar]
- 4.Pennathur A, Luketich JD, Abbas G, et al. Radiofrequency ablation for the treatment of stage I non-small cell lung cancer in high-risk patients. J Thorac Cardiovasc Surg. 2007;134(4):857–864. [DOI] [PubMed] [Google Scholar]
- 5.Dupuy DE, DiPetrillo T, Gandhi S, et al. Radiofrequency ablation followed by conventional radiotherapy for medically inoperable stage I non-small cell lung cancer. Chest. 2006;129(3):738–745. [DOI] [PubMed] [Google Scholar]
- 6.Herrera LJ, Fernando HC, Perry Y, et al. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg. 2003;125(4):929–937. [DOI] [PubMed] [Google Scholar]
- 7.Grieco CA, Simon CJ, Mayo-Smith WW, DiPetrillo TA, Ready NE, Dupuy DE. Percutaneous image-guided thermal ablation and radiation therapy: outcomes of combined treatment for 41 patients with inoperable stage I/II non-small-cell lung cancer. J Vasc Interv Radiol. 2006;17(7):1117–1124. [DOI] [PubMed] [Google Scholar]
- 8.Hughes RT, Helis CA, Soike MH, Levine BJ, Farris M, Blackstock AW. Moderately Hypofractionated Radiotherapy Alone for Stage I-IIB Non-small Cell Lung Cancer. Cureus. 2019;11(6):e4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20(4):494–503. [DOI] [PubMed] [Google Scholar]
- 10.Nyman J, Hallqvist A, Lund J, et al. SPACE - A randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121(1):1–8. [DOI] [PubMed] [Google Scholar]
- 11.Cheung P, Faria S, Ahmed S, et al. Phase II study of accelerated hypofractionated three-dimensional conformal radiotherapy for stage T1–3 N0 M0 non-small cell lung cancer: NCIC CTG BR.25. J Natl Cancer Inst. 2014;106(8). [DOI] [PubMed] [Google Scholar]
- 12.Timmerman R, Paulus R, Galvin J, et al. Stereotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA. 2010;303(11):1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Videtic GMM, Hu C, Singh AK, et al. A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927). International Journal of Radiation Oncology*Biology*Physics. 2015;93(4):757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bezjak A, Paulus R, Gaspar LE, et al. Safety and Efficacy of a Five-Fraction Stereotactic Body Radiotherapy Schedule for Centrally Located Non-Small-Cell Lung Cancer: NRG Oncology/RTOG 0813 Trial. J Clin Oncol. 2019;37(15):1316–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bi N, Shedden K, Zheng X, Kong FS. Comparison of the Effectiveness of Radiofrequency Ablation With Stereotactic Body Radiation Therapy in Inoperable Stage I Non-Small Cell Lung Cancer: A Systemic Review and Pooled Analysis. Int J Radiat Oncol Biol Phys. 2016;95(5):1378–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Timmerman RD, Hu C, Michalski JM, et al. Long-term Results of Stereotactic Body Radiation Therapy in Medically Inoperable Stage I Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4(9):1287–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Timmerman RD, Paulus R, Pass HI, et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol. 2018;4(9):1263–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.


