Abstract
Objective
The coronavirus disease 2019 (COVID-19) pandemic has had a serious impact on health all over the world. Cancer patient, whose immunity is often compromised, faces a huge challenge. Currently, some COVID-19 vaccines are being developed and applied on general population; however, whether cancer patients should take COVID-19 vaccine remains unknown. Our study aimed to explore the knowledge, attitude, acceptance, and predictors of intention to receive the COVID-19 vaccine among cancer patients in Eastern China.
Methods
A cross-sectional study was conducted in Eastern China from June 17th to September 3rd, 2021. Patients were selected using a convenience sampling method. A self-report questionnaire was developed to assess knowledge about the COVID-19 vaccine, attitude towards the vaccine and acceptance of the vaccine; following a review of similar studies previously published in the scientific literature, multivariate logistic regression analysis was used to determine the predictors associated with COVID-19 vaccine acceptance.
Results
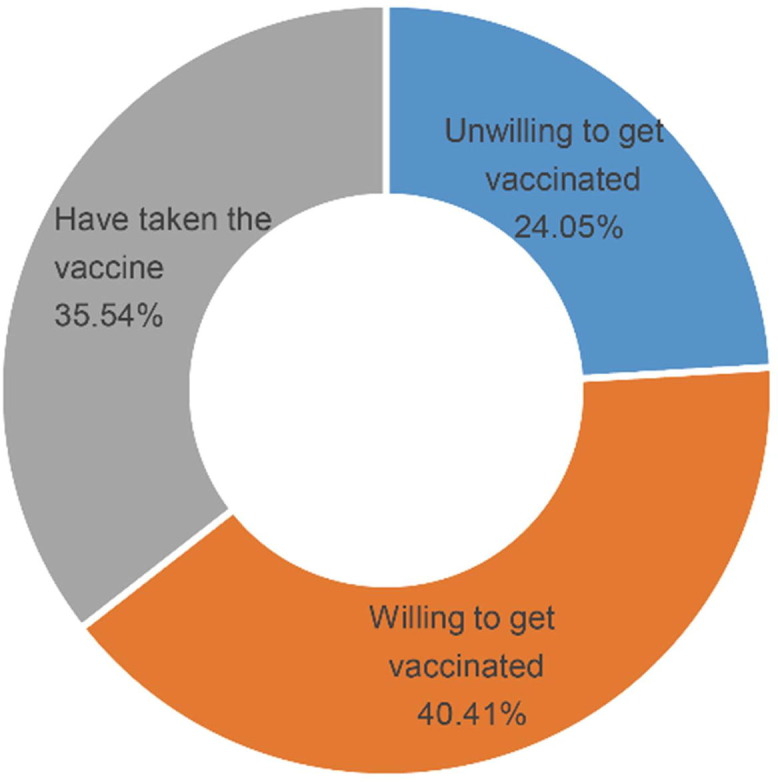
A total of 2158 cancer patients were enrolled in this study. The rate of vaccine hesitancy was 24.05% (519/2158); further, among the participants of vaccine acceptance, 767 had taken COVID-19 vaccine (35.54%), and 872 were willing to get vaccinated (40.01%). A total of 24 variables including demographic characteristics, clinical status of cancer, impact of COVID-19 pandemic on study participants, patients’ knowledge about the COVID-19 vaccine, and attitude towards the vaccine, had significant differences between the “vaccine hesitancy” population and “vaccine acceptance” population. Multivariate logistic regression analysis indicated that parameters including alcohol consumption (odds ratio [OR] = 1.849; 95% confidence interval [CI]: 1.375–2.488; P-reference [P-Ref] < 0.001 vs non-drinkers), income impacted by COVID-19 pandemic (OR = 1.930, 2.037 and 2.688 for mild, moderate, and severe impact, respectively; all P-Ref < 0.01 vs no impact), knowledge of how the vaccine was developed (OR = 1.616; 95% CI: 1.126–2.318; P-Ref = 0.009 vs unknown), believing in the safety of the vaccine (OR = 1.502; 95% CI: 1.024–2.203; P-Ref = 0.038 vs denying the safety of vaccine), willingness to pay for the vaccine (OR = 3.042; 95% CI: 2.376–3.894; P-Ref < 0.001 vs unwilling), and willingness to recommend families and friends to get vaccinated (OR = 2.744; 95% CI: 1.759–4.280; P-Ref < 0.001 vs do not recommend) were contributors to vaccine acceptance. While such as being retired (OR = 0.586; 95% CI: 0.438–0.784; P-Ref < 0.001 vs unemployed), undergoing multiple therapies of cancer (OR = 0.408; 95% CI: 0.221–0.753; P-Ref = 0.004 vs no ongoing treatment), and worrying that the vaccine might deteriorate the prognosis of cancer (OR = 0.393; 95% CI: 0.307–0.504; P-Ref < 0.001 vs might not) were contributors to vaccine hesitancy.
Conclusion
This study provided preliminary estimates of the rates of vaccine acceptance and vaccine hesitancy among cancer patients in Eastern China. The intention to receive the COVID-19 vaccine was impacted by factors such as patient occupation, alcohol consumption, and some parts of knowledge about and attitude towards COVID-19 vaccine. It is recommended to develop individualized vaccination plans that meet the healthcare needs of cancer patients.
Keywords: COVID-19, Cancer, Vaccine, Knowledge, Attitude, Predictors, Intention, Vaccine hesitancy
1. Introduction
Currently, the prevalence of coronavirus disease-19 (COVID-19) is a serious threat to socio-economic development and public health worldwide [1]. As of September 19th, 2021, globally, the reported cumulative number of COVID-19 cases exceeded 228 million, and the number of deaths was almost 4.6 million [2]. The World Health Organization (WHO) declared the COVID-19 outbreak to be a pandemic on 11 March 2020 [3], [4].
When the COVID-19 outbroke in China at the beginning of 2020, the Chinese government took urgent action to control its prevalence. During this battle, traditional Chinese medicine (TCM), as an important part of the complementary and alternative medicine, played a vital role in China: it was adopted as one of the main therapies in the treatment plan announced by the National Health Commission of the People’s Republic of China [5], and applied on 90% of Chinese COVID-19 patients [6]. Practice has proved that the combination of TCM and Western medicine is the most powerful weapon for the prevention and treatment of COVID-19, providing a new idea and new method for the global fight against this epidemic [7], [8], [9]. A meta-analysis showed that TCM not only can promote the recovery of patients in mild stage, but also is an important auxiliary treatment method for those in severe and extremely severe stage [10]. A retrospective study also proved that TCM could help Western medicine treatments to reduce the negative conversion time of fecal nucleic acid and the duration of negative conversion of pharyngeal-fecal nucleic acid [11].
Although TCM has been proved to be a good way to cure COVID-19 patients, it is believed that vaccination is still the only way to ultimately control the prevalence of COVID-19 pandemic [12]. Since the genetic sequence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which caused COVID-19, was published, many vaccines have been developed [13], [14], [15], [16], and, as of the writing of this manuscript, the WHO has approved two Chinese vaccines for emergency use against COVID-19 [17].
The cancer patient population is a special group of people who are at high risk of serious and lethal complications from COVID-19 [18], [19]. Due to a lack of data on the safety and efficacy of vaccines in cancer patients [20], [21], [22], there is still no clinical guideline on whether cancer patient should take COVID-19 vaccine. On April 27, 2021, the European Society for Medical Oncology launched an initiative to prioritize the vaccination of patients with cancer, based on the extrapolation of safety and effectiveness data for other vaccines among cancer patients and COVID-19 vaccination in non-cancer patients; however, they also affirmed the necessity to monitor side effects [23]. The United States (US) National Comprehensive Cancer Network recommended that patients with active cancer and those under treatment should be prioritized for vaccination and should be immunized when any vaccine had been authorized for use by the US Food and Drug Administration [24]. Further, a prospective observational study suggested that cancer patients should receive the second dose of COVID-19 vaccine earlier than the general population [25]. Some researchers believed that, among cancer patients, the benefits of vaccination would outweigh the risks [26].
Vaccination is an important tool for reducing the spread of an infectious disease through a population, but hesitancy in vaccination has been one of the biggest obstacles to controlling the COVID-19 pandemic. “Vaccine hesitancy” refers to the reluctance or refusal to receive a vaccination despite the availability of a vaccine, and the WHO identified it as one of the top ten health threats in the world [27].
Recently, several studies have explored the attitudes of cancer patients towards receiving the COVID-19 vaccine. A French cross-sectional study showed that about 16.6% of cancer patients were unwilling to get vaccinated at the beginning of the vaccination campaign [28]. In Italy, it was reported that 11.2% of cancer patients refused vaccination [29]. In Poland, up to 23.46% of cancer patients refused to get vaccinated, while 16.22% were undecided about whether they should receive the vaccine [30]. The rate of vaccination hesitancy is higher among cancer patients than among the general population. For example, a Chinese cross-sectional survey reported that only 8.7% of healthy adults were unwilling to receive the vaccine [31]; in Australia, 6% of the population refused the vaccine [32], and in Japan, 11.0% of respondents refused to take the COVID-19 vaccine [33]. Lack of faith in the efficacy and safety of vaccines is often cited as a main factor in vaccine hesitancy in various countries [34], [35], [36], [37], [38]. Some researchers pointed out that gender, age, race, level of education, marital status, and knowledge about COVID-19 may also be associated with vaccine acceptance [39], [40], [41].
To promote the global COVID-19 vaccination programs, it is critical to understand the concerns and hesitancy about the COVID-19 vaccine among cancer patients. However, until now, no data addressing this question for cancer patients in China had been published.
Eastern China, covering provinces of Shanghai, Jang Su, Zhejiang, Anhui, Jiangxi, Shangdong, and Fujian, is one of the regions with the high incidence and mortality of cancer in China [42]; it is also among the areas that have high incidence of COVID-19 in China [43]. Therefore, we conducted an online survey among cancer patients in Eastern China, to document this population’s knowledge about and attitude towards the COVID-19 vaccine; we also evaluated the rate of vaccine acceptance, and analyzed parameters that were predictive of a cancer patient’s willingness to receive the COVID-19 vaccine.
2. Methods and materials
2.1. Study design and participants
A cross-sectional, web-based, anonymous survey was conducted among cancer patients in Eastern China, from June 17th to September 3rd, 2021. The study was conducted using the “Questionnaire Star,” a paid website that helps generate, distribute, and retrieve electronic questionnaires for mobile platforms [44]. Cancer patients over the age of 18 years were invited to participate in the study, and those with cognitive disabilities were excluded.
Ethical approval
This study was approved by the Institutional Review Board of Changhai Hospital, Naval Medical University, China (CCHEC2013-119) and Chaohu Hospital, Anhui Medical University, China (KYXM-20210501). All participants were informed about the purpose of the study, the study procedures, and their rights. Furthermore, all participants were informed that only anonymized data would be used in this study. The written informed consent of all participants was obtained.
2.2. Questionnaire
The self-report questionnaire was developed to assess knowledge about the COVID-19 vaccine, attitude towards the vaccine and acceptance of the vaccine, following a review of similar studies previously published in the scientific literature [30], [44], [45], [46], [47], [48], [49]. The questionnaire was developed in English and then translated into Chinese. Subsequently, it was translated back into English to check for compatibility.
The questionnaire included four parts. (1) Basic information: the demographic characteristics (gender, age, body mass index, education, average monthly income, marital status, residence, occupation, current smoking status, and current drinking status), clinical status of cancer (cancer type, time since cancer diagnosis, presence of metastasis, family history of cancer, ongoing treatment, and complications), and the impact of COVID-19 pandemic on participants (whether the daily life and income are influenced by COVID-19, whether the regular medical treatment of cancer is hampered by COVID-19, and the risk of COVID-19 infection). (2) The intention to receive vaccination: participants were first asked to report whether they had taken COVID-19 vaccines; then, if the answer was no, they were asked whether they were willing to receive a COVID-19 vaccination; participants who had received the COVID-19 vaccine or were willing to be vaccinated were considered to belong to the “vaccine acceptance” group, and those who had not received the vaccine or were unwilling to be vaccinated were considered to be part of the “vaccine hesitancy” group. (3) Knowledge about COVID-19 vaccines: participants were asked to report their trusted information sources about COVID-19 vaccines, how long it takes for the COVID-19 vaccine to start working after vaccination, how the COVID-19 vaccines were developed, how safe the COVID-19 vaccines are, and whether they thought that COVID-19 vaccines would trigger allergies. (4) Attitude towards COVID-19 vaccine: participants were asked to respond to the following prompts: “is the COVID-19 vaccine effective,” “will you encourage your parents and friends to get vaccinated,” “are you willing to get the COVID-19 vaccine, even if you have to pay for it,” “will the COVID vaccine worsen the prognosis of cancer,” and “is it necessary to wear a mask after getting COVID-19 vaccine.”
2.3. Data analyses and sample size estimation
Data analyses were performed using SPSS version 22.0 (SPSS Inc, Chicago, Illinois, USA). Socio-demographic characteristics and responses to the questionnaire were treated as categorical data and presented as numbers and percentages. A chi-squared test was used to evaluate the relationships between the independent variables (basic information, knowledge about the COVID-19 vaccine, and attitude towards the COVID-19 vaccine) and the outcome variable (the intention to receive vaccination). Variables with a P value < 0.1 in the univariate analysis (chi-squared test) were included in the multivariate logistic regression analyses to identify variables that were correlated with a participant’s intention to receive the COVID-19 vaccine. For these variables, the odds ratio (OR) and 95% confidence interval (CI) were reported, and a P value < 0.05 was considered to be statistically significant.
According to the Sample Size Guidelines for Logistic Regression from Observational Studies with Large Population [50], a minimum sample size of 500 is necessary to properly conduct a logistic regression; further, when considering the number of independent variables in the final model of logistic regression (i), the minimum sample size (n) can be estimated as: n = 100 + 50i. In this study, as indicated in section 2.3, the maximum number of potential independent variables in the final logistic regression model is 30, including 20 basic information variables and 10 attitude and knowledge variables; the question about trusted sources of COVID-19 vaccine information was intended to understand the participants’ information sources and was not included in the analysis of predictors. Therefore, in this survey, the minimum necessary sample size was estimated to be 1600, and a greater sample size would add to the robustness of the results.
To balance the time limitation and the accuracy of statistics, the study was implemented as follows: during a two-week period, the study populations were initially recruited using a convenience sampling method in 28 hospitals located in Eastern China (each province of Eastern China had at least one hospital); next, the study population was broadened via patient-patient spreading. If, at this point, we received more than 1600 valid questionnaire responses, data collection for the study would be considered complete. Otherwise, another two-week period would have been allocated to recruit more participants.
3. Results
3.1. The characteristic of participants and COVID-19 vaccination status
A total of 2170 cancer patients were invited to participate in this online survey; of these, 12 participants returned incomplete questionnaires. Ultimately, 2158 cancer patients completed the study, and the overall effective response rate of this survey was 99.4%.
As shown in Fig. 1 , among the 2158 participants, 519 were unwilling to receive the COVID-19 vaccine. Therefore, the rate of vaccine hesitancy was 24.05% (519/2158). Among the participants willing to be vaccinated, 767 had already received the COVID-19 vaccine (35.54%), and 872 were willing to be vaccinated (40.41%).
Fig. 1.
The acceptance status of coronavirus disease 2019 vaccines among cancer patients in Eastern China (N = 2158).
The demographic characteristics of participants showed that there were significant between-group differences (“vaccine hesitancy” group vs “vaccine acceptance” group) in the variables of age, marital status, education level, occupation, and current drinking status (all P ≤ 0.001, Table 1 ). Among cancer patients over 40 years of age, married, with less than a high-school education, retired, and non-drinking, the proportion of vaccine hesitancy was higher than that of vaccine acceptance).
Table 1.
Demographic characteristics of participants.
| Item | All participants(N = 2158) | Intention to receive COVID-19 vaccine | ||
|---|---|---|---|---|
| Vaccine hesitancy(n = 519) | Vaccine acceptance(n = 1639) | P value | ||
| Age (year) | < 0.001 | |||
| < 40 | 357 (16.54%) | 46 (8.86%) | 311 (18.97%) | |
| 40–70 | 1443 (66.87%) | 365 (70.33%) | 1078 (65.77%) | |
| > 70 | 358 (16.59%) | 108 (20.81%) | 250 (15.25%) | |
| Gender | 0.546 | |||
| Female | 1103 (51.11%) | 259 (49.90%) | 844 (51.49%) | |
| Male | 1055 (48.89%) | 260 (50.10%) | 795 (48.51%) | |
| BMI (kg/m2) | 0.729 | |||
| < 18.5 | 233 (10.80%) | 59 (11.37%) | 174 (10.62%) | |
| ≥ 18.5, < 24 | 1257 (58.25%) | 303 (58.38%) | 954 (58.21%) | |
| ≥ 24, < 28 | 530 (24.56%) | 129 (24.86%) | 401 (24.47%) | |
| ≥ 28 | 138 (6.39%) | 28 (5.39%) | 110 (6.71%) | |
| Marital status | 0.001 | |||
| Unmarried | 160 (7.41%) | 18 (3.47%) | 142 (8.66%) | |
| Married | 1889 (87.53%) | 469 (90.37%) | 1420 (86.64%) | |
| Divorced | 47 (2.18%) | 15 (2.89%) | 32 (1.95%) | |
| Widowed | 62 (2.87%) | 17 (3.28%) | 45 (2.75%) | |
| Residence | 0.202 | |||
| Rural | 910 (42.17%) | 206 (39.69%) | 704 (42.95%) | |
| Urban | 1248 (57.83%) | 313 (60.31%) | 935 (57.05%) | |
| Education level | 0.001 | |||
| ≤ Senior high school | 1507 (69.83%) | 393 (75.72%) | 1114 (67.97%) | |
| College degree | 319 (14.78%) | 69 (13.29%) | 250 (15.25%) | |
| ≥ Bachelor’s degree | 332 (15.38%) | 57 (10.98%) | 275 (16.78%) | |
| Occupation | < 0.001 | |||
| Unemployed | 819 (37.95%) | 176 (33.91%) | 643 (39.23%) | |
| Employed | 623 (28.87%) | 84 (16.18%) | 539 (32.89%) | |
| Retired | 716 (33.18%) | 259 (49.90%) | 457 (27.88%) | |
| Average monthly income (CNY) | 0.652 | |||
| < 3000 | 1204 (55.79%) | 293 (56.45%) | 911 (55.58%) | |
| 3000–8000 | 753 (34.89%) | 183 (35.26%) | 570 (34.78%) | |
| > 8000 | 201 (9.31%) | 43 (8.29%) | 158 (9.64%) | |
| Current smoking status | 0.571 | |||
| No | 1726 (79.98%) | 420 (80.92%) | 1306 (79.68%) | |
| Yes | 432 (20.02%) | 99 (19.08%) | 333 (20.32%) | |
| Current drinking status | < 0.001 | |||
| No | 1609 (74.56%) | 420 (80.92%) | 1189 (72.54%) | |
| Yes | 549 (25.44%) | 99 (19.08%) | 450 (27.46%) | |
Data are presented as number (percentage). P values were calculated via chi-squared tests between the “vaccine hesitancy” and “vaccine acceptance” groups. Participants who had already received the COVID-19 vaccine or were willing to be vaccinated were included in the “vaccine acceptance” group, and those who had not received or were unwilling to receive the COVID-19 vaccine were included in the “vaccine hesitancy” group. BMI: body mass index; CNY: China Yuan; COVID-19: coronavirus disease 2019.
Among the six variables of clinical cancer status, the majority of both the “vaccine hesitancy” (435/519; 83.82%) and “vaccine acceptance” (1362/1639; 83.10%) participants were free of cancer-related complications (P = 0.736, Table 2 ), while significant between-group differences were observed in the proportions of cancer type, time since cancer diagnosis, ongoing treatment, family history of cancer, and metastasis (all P < 0.01, Table 2). Percentage of vaccine hesitancy was greater than that of vaccine acceptance among patients who reported suffering from digestive tract cancer, gynecologic cancer, and multiple types of cancer. When referred to ongoing treatment, participants undergoing TCM or multiple therapies held a higher percentage of vaccine hesitancy than vaccine acceptance, while those undergoing other therapies or without any treatment were more likely to accept vaccine. Besides, participants having been diagnosed with cancer more than one year prior to the questionnaire, with a family history of cancer or metastasis also tended to be vaccine hesitancy.
Table 2.
Clinical status of participants.
| Item | All participants(N = 2158) | Intention to receive COVID-19 vaccine | ||
|---|---|---|---|---|
| Vaccine hesitancy(n = 519) | Vaccine acceptance(n = 1639) | P value | ||
| Type of cancer | < 0.001 | |||
| Head and neck cancer | 203 (9.41%) | 44 (8.48%) | 159 (9.70%) | |
| Respiratory and thoracic cancer | 579 (26.83%) | 136 (26.20%) | 443 (27.03%) | |
| Digestive tract cancer | 703 (32.58%) | 194 (37.38%) | 509 (31.06%) | |
| Urogenital caner | 136 (6.30%) | 25 (4.82%) | 111 (6.77%) | |
| Gynecologic cancer | 325 (15.06%) | 84 (16.18%) | 241 (14.70%) | |
| Other type of cancer | 152 (7.04%) | 15 (2.89%) | 137 (8.36%) | |
| Multiple types of cancer | 60 (2.78%) | 21 (4.05%) | 39 (2.38%) | |
| Time since cancer diagnosis (year) | 0.002 | |||
| < 1 | 1085 (50.28%) | 223 (42.97%) | 862 (52.59%) | |
| ≥ 1, < 3 | 692 (32.07%) | 192 (36.99%) | 500 (30.51%) | |
| ≥ 3, < 5 | 186 (8.62%) | 52 (10.02%) | 134 (8.18%) | |
| ≥ 5 | 195 (9.04%) | 52 (10.02%) | 143 (8.72%) | |
| Ongoing treatment | < 0.001 | |||
| None | 176 (8.16%) | 17 (3.28%) | 159 (9.70%) | |
| Surgery* | 420 (19.46%) | 63 (12.14%) | 357 (21.78%) | |
| Radiotherapy | 82 (3.80%) | 17 (3.28%) | 65 (3.97%) | |
| Chemotherapy | 330 (15.29%) | 77 (14.84%) | 253 (15.44%) | |
| Immunological and molecular-targeted therapy | 67 (3.10%) | 15 (2.89%) | 52 (3.17%) | |
| Traditional Chinese medicine | 144 (6.67%) | 43 (8.29%) | 101 (6.16%) | |
| Other therapy | 20 (0.93%) | 3 (0.58%) | 17 (1.04%) | |
| Multiple therapies | 919 (42.59%) | 284 (54.72%) | 635 (38.74%) | |
| Family history of cancer | 0.005 | |||
| No | 1895 (87.81%) | 437 (84.20%) | 1458 (88.96%) | |
| Yes | 263 (12.19%) | 82 (15.80%) | 181 (11.04%) | |
| Complication | 0.736 | |||
| No | 1797 (83.27%) | 435 (83.82%) | 1362 (83.10%) | |
| Yes | 361 (16.73%) | 84 (16.18%) | 277 (16.90%) | |
| Metastasis | 0.001 | |||
| No | 1609 (74.56%) | 358 (68.98%) | 1251 (76.33%) | |
| Yes | 549 (25.44%) | 161 (31.02%) | 388 (23.67%) | |
Data are presented as number (percentage). P values were calculated via chi-squared tests between the “vaccine hesitancy” and “vaccine acceptance” groups. Participants who had received or were willing to receive the COVID-19 vaccine were included in the “vaccine acceptance” group, and those who had not received or were unwilling to receive the COVID-19 vaccine were included in the “vaccine hesitancy” group. * Surgery including procedures such as excision, transarterial chemoembolization, and microwave ablation. COVID-19: coronavirus disease 2019.
As shown in Table 3 , the impact of the COVID-19 pandemic had effects on participants: the majority participants reported that they were at low to high risk of infection (1747/2158; 80.95%), and that the COVID-19 pandemic had a mild to severe impact on their regular medical treatment for cancer (1877/2158; 86.98%), their daily life (1918/2158; 88.88%), and their income (1626/2158; 75.35%). The degree of risk and impact that participants reported experiencing were significantly different between “vaccine hesitancy” and “vaccine acceptance” groups (all P ≤ 0.001, Table 3). Among participants who believed they were at risk of COVID-19 infection and who reported pandemic-related impacts on their lives, a greater proportion belonged to the “vaccine acceptance” group than the “vaccine hesitancy” group. Among participants who believed that they had not been impacted by the COVID-19 pandemic or that their regular medical treatment had not been interrupted, there were lower proportion of vaccine acceptance and greater proportion of vaccine hesitancy; when the degree of impact was mild, the proportion of vaccine acceptance overwhelmed vaccine hesitancy; however, when the degree was moderate or severe, the proportion was similar among the two intentions.
Table 3.
Impact of COVID-19 pandemic on study participants.
| Item | All participants(N = 2158) | Intention to receive COVID-19 vaccine | ||
|---|---|---|---|---|
| Vaccine hesitancy(n = 519) | Vaccine acceptance(n = 1639) | P value | ||
| Risk of COVID-19 infection | 0.001 | |||
| Unknown | 411 (19.05%) | 130 (25.05%) | 281 (17.14%) | |
| Low | 893 (41.38%) | 194 (37.38%) | 699 (42.65%) | |
| Medium | 440 (20.39%) | 105 (20.23%) | 335 (20.44%) | |
| High | 414 (19.18%) | 90 (17.34%) | 324 (19.77%) | |
| Impact of COVID-19 pandemic on regular medical treatment of cancer | < 0.001 | |||
| None | 281 (13.02%) | 97 (18.69%) | 184 (11.23%) | |
| Mild | 517 (23.96%) | 96 (18.50%) | 421 (25.69%) | |
| Moderate | 627 (29.05%) | 153 (29.48%) | 474 (28.92%) | |
| Severe | 733 (33.97%) | 173 (33.33%) | 560 (34.17%) | |
| Impact of COVID-19 pandemic on daily life | < 0.001 | |||
| None | 240 (11.12%) | 92 (17.73%) | 148 (9.03%) | |
| Mild | 526 (24.37%) | 99 (19.08%) | 427 (26.05%) | |
| Moderate | 693 (32.11%) | 169 (32.56%) | 524 (31.97%) | |
| Severe | 699 (32.39%) | 159 (30.64%) | 540 (32.95%) | |
| Impact of COVID-19 pandemic on income | < 0.001 | |||
| None | 532 (24.65%) | 208 (40.08%) | 324 (19.77%) | |
| Mild | 539 (24.98%) | 103 (19.85%) | 436 (26.60%) | |
| Moderate | 542 (25.12%) | 113 (21.77%) | 429 (26.17%) | |
| Severe | 545 (25.25%) | 95 (18.30%) | 450 (27.46%) | |
Data are presented as number (percentage). P values were calculated via chi-squared tests between the “vaccine hesitancy” and “vaccine acceptance” groups. Participants who had received or were willing to receive the COVID-19 vaccine were included in the “vaccine acceptance” group, and those who had not received or were unwilling to receive the COVID-19 vaccine were included in the “vaccine hesitancy” group. COVID-19: coronavirus disease 2019.
3.2. Knowledge about and attitude towards the COVID-19 vaccine
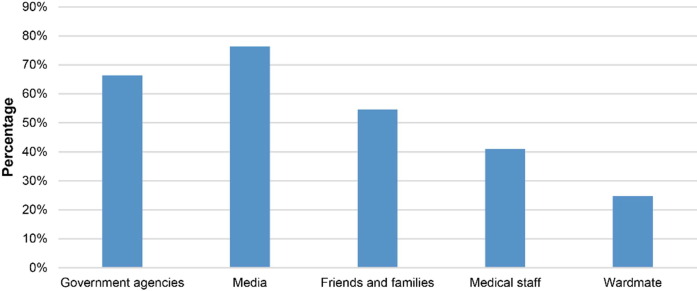
For the participants in this study, the most-trusted sources of information about the COVID-19 vaccine were media (1649/2158; 76.41%), followed by government agencies (1433/2158; 66.40%), and friends and families (1178/2158; 54.59%), while only a small proportion of participants had learned about the COVID-19 vaccine from medical staff (885/2158, 41.01%) or ward mates (533/2158; 24.70%, Fig. 2 ).
Fig. 2.
The sources of trusted information about coronavirus disease 2019 vaccines (N = 2158).
As shown in Table 4 , participants’ knowledge about and attitude towards the COVID-19 vaccine had significant differences between the “vaccine hesitancy” and “vaccine acceptance” groups (P ≤ 0.001 for each of the ten relevant questions). In terms of knowledge, only 39.30% of participants clearly knew the onset time of COVID-19 vaccine after vaccination (848/2158), and the majority did not know how the COVID-19 vaccine was developed (1767/2158; 81.88%). Comfortingly, more than half participants thought the COVID-19 vaccine was safe (1833/2158; 84.94%) and could be useful in controlling this pandemic (1497/2158; 69.37%). The proportions of positive and passive answers to the question “will the COVID-19 vaccine trigger allergy?” was close among all participants (47.82% vs 52.18%). In terms of attitude, the majority of participants believed that the COVID-19 vaccine was effective (882/2158, 87.21%), thought that the vaccine would not worsen prognosis of cancer (1475/2158, 68.35%), and believed that a mask was still needed after getting the COVID-19 vaccine (1600/2158, 74.14%). Approximately 69.09% of participants were willing to pay for the vaccine (1491/2158), and 92.35% of participants were willing to recommend their families and friends to get vaccinated (1993/2158).
Table 4.
Participants’ knowledge about and attitude towards the COVID-19 vaccine.
| Item | All participants(N = 2158) | Intention to receive COVID-19 vaccine | ||
|---|---|---|---|---|
| Vaccine hesitancy(n = 519) | Vaccine acceptance(n = 1639) | P value | ||
| When does the COVID-19 vaccine start working after vaccination? | < 0.001 | |||
| Unknown | 696 (32.25%) | 233 (44.89%) | 463 (28.25%) | |
| Immediately after the first dose | 269 (12.47%) | 43 (8.29%) | 226 (13.79%) | |
| Immediately after the second dose | 345 (15.99%) | 65 (12.52%) | 280 (17.08%) | |
| Fourteen days after the second dose | 848 (39.30%) | 178 (34.30%) | 670 (40.88%) | |
| Do you know about how the COVID-19 vaccine was developed? | < 0.001 | |||
| No | 1767 (81.88%) | 466 (89.79%) | 1301 (79.38%) | |
| Yes | 391 (18.12%) | 53 (10.21%) | 338 (20.62%) | |
| Will the COVID-19 vaccine be useful in controlling the COVID-19 pandemic? | < 0.001 | |||
| No | 661 (30.63%) | 216 (41.62%) | 445 (27.15%) | |
| Yes | 1497 (69.37%) | 303 (58.38%) | 1194 (72.85%) | |
| Is the COVID-19 vaccine safe? | < 0.001 | |||
| No | 325 (15.06%) | 127 (24.47%) | 198 (12.08%) | |
| Yes | 1833 (84.94%) | 392 (75.53%) | 1441 (87.92%) | |
| Will the COVID-19 vaccine trigger allergy? | 0.001 | |||
| No | 1126 (52.18%) | 239 (46.05%) | 887 (54.12%) | |
| Yes | 1032 (47.82%) | 280 (53.95%) | 752 (45.88%) | |
| Is the COVID-19 vaccine effective? | < 0.001 | |||
| No | 276 (12.79%) | 95 (18.3%) | 181 (11.04%) | |
| Yes | 1882 (87.21%) | 424 (81.7%) | 1458 (88.96%) | |
| Will you encourage your parents and friends to get vaccinated? | < 0.001 | |||
| No | 165 (7.65%) | 86 (16.57%) | 79 (4.82%) | |
| Yes | 1993 (92.35%) | 433 (83.43%) | 1560 (95.18%) | |
| Will the COVID vaccine worsen prognosis of cancer? | < 0.001 | |||
| No | 1475 (68.35%) | 266 (51.25%) | 1209 (73.76%) | |
| Yes | 683 (31.65%) | 253 (48.75%) | 430 (26.24%) | |
| Is it necessary to wear a mask after getting the COVID-19 vaccine? | < 0.001 | |||
| No | 558 (25.86%) | 107 (20.62%) | 451 (27.52%) | |
| Yes | 1600 (74.14%) | 412 (79.38%) | 1188 (72.48%) | |
| Are you willing to get the COVID-19 vaccine, even if you must pay for it? | < 0.001 | |||
| No | 667 (30.91%) | 279 (53.76%) | 388 (23.67%) | |
| Yes | 1491 (69.09%) | 240 (46.24%) | 1251 (76.33%) | |
Data are presented as number (percentage). P values were calculated via chi-squared tests between the “vaccine hesitancy” and “vaccine acceptance” groups. Participants who had received or were willing to receive the COVID-19 vaccine were included in the “vaccine acceptance” group, and those who had not received or were unwilling to receive the COVID-19 vaccine were included in the “vaccine hesitancy” group. COVID-19: coronavirus disease 2019.
The proportion of vaccine hesitancy was higher than that of vaccine acceptance among the participants who did not know the onset time of enhanced immunity following the COVID-19 vaccine, did not know the process by which the vaccine had been developed, regarded the vaccine as unsafe, ineffective, or useless in controlling the panic, thought that the vaccine could trigger allergy or worsen the prognosis of cancer, thought the vaccination could not release them from mask use, were reluctant to pay for vaccination, or were not willing to recommend vaccination to their friends and families.
3.3. Predictors of COVID-19 vaccine attitude
A total of 24 variables with P values < 0.1 were present in the univariate analysis (chi-squared test, Table 1, Table 2, Table 3, Table 4). The multivariate logistic regression analyses of these 24 variables (Table 5 ) showed that, after adjusting for the other variables, nine variables contributed significantly to the participants’ intention towards receiving the COVID-19 vaccine (all P-Log < 0.05), including “occupation,” “current drinking status,” “ongoing treatment of cancer,” “impact of COVID-19 pandemic on income,” “do you know about how the COVID-19 vaccine was developed,” “is the COVID-19 vaccine safe,” “will you encourage your friends and family to get vaccinated,” “will the COVID vaccine worsen the prognosis of cancer,” and “are you willing to get the COVID-19 vaccine, even if you have to pay for it.”
Table 5.
Predictors of intention to receive the COVID-19 vaccine among cancer patients.
| Variable | Intention to receive COVID-19 vaccine | OR (95% CI) | P-Ref | P-Log | |
|---|---|---|---|---|---|
| Vaccine hesitancy(n = 519) | Vaccine acceptance(n = 1639) | ||||
| Age (year) | 0.329 | ||||
| <40 | 46 | 311 | Ref | / | |
| 40–70 | 365 | 1078 | 0.715 (0.456–1.122) | 0.144 | |
| >70 | 108 | 250 | 0.761 (0.449–1.292) | 0.313 | |
| Marital status | 0.239 | ||||
| Unmarried | 18 | 142 | Ref | / | |
| Married | 469 | 1420 | 0.634 (0.333–1.207) | 0.165 | |
| Divorced | 15 | 32 | 0.383 (0.150–0.980) | 0.045 | |
| Widowed | 17 | 45 | 0.549 (0.223–1.353) | 0.193 | |
| Education level | 0.783 | ||||
| ≤ Senior high school | 393 | 1114 | Ref | / | |
| College degree | 69 | 250 | 0.965 (0.671–1.387) | 0.847 | |
| ≥ Bachelor’s degree | 57 | 275 | 0.861 (0.566–1.310) | 0.485 | |
| Occupation | < 0.001 | ||||
| Unemployed | 176 | 643 | Ref | / | |
| Employed | 84 | 539 | 1.446 (0.995–2.102) | 0.053 | |
| Retired | 259 | 457 | 0.586 (0.438–0.784) | < 0.001 | |
| Current drinking status | < 0.001 | ||||
| No | 420 | 1189 | Ref | / | |
| Yes | 99 | 450 | 1.849 (1.375–2.488) | < 0.001 | |
| Type of cancer | 0.401 | ||||
| Other type of cancer | 15 | 137 | Ref | / | |
| Head and neck cancer | 44 | 159 | 0.712 (0.346–1.467) | 0.357 | |
| Respiratory and thoracic cancer | 136 | 443 | 0.631 (0.323–1.232) | 0.177 | |
| Digestive tract cancer | 194 | 509 | 0.592 (0.307–1.140) | 0.117 | |
| Urogenital caner | 25 | 111 | 1.048 (0.472–2.329) | 0.908 | |
| Gynecologic cancer | 84 | 241 | 0.641 (0.321–1.282) | 0.209 | |
| Multiple types of cancer | 21 | 39 | 0.682 (0.275–1.692) | 0.410 | |
| Time since cancer diagnosis (year) | 0.577 | ||||
| < 1 | 223 | 862 | Ref | / | |
| ≥ 1, < 3 | 192 | 500 | 0.962 (0.736–1.258) | 0.777 | |
| ≥ 3, < 5 | 52 | 134 | 1.136 (0.742–1.741) | 0.557 | |
| ≥ 5 | 52 | 143 | 1.267 (0.836–1.920) | 0.265 | |
| Ongoing treatment for cancer | 0.001 | ||||
| None | 17 | 159 | Ref | / | |
| Surgery* | 63 | 357 | 0.863 (0.449–1.658) | 0.658 | |
| Radiotherapy | 17 | 65 | 0.544 (0.234–1.266) | 0.158 | |
| Chemotherapy | 77 | 253 | 0.567 (0.294–1.096) | 0.091 | |
| Immunological and molecular-targeted therapy | 15 | 52 | 0.585 (0.243–1.410) | 0.232 | |
| Traditional Chinese medicine | 43 | 101 | 0.583 (0.282–1.207) | 0.146 | |
| Other therapy | 3 | 17 | 0.883 (0.203–3.843) | 0.868 | |
| Multiple therapies | 284 | 635 | 0.408 (0.221–0.753) | 0.004 | |
| Family history of cancer | 0.719 | ||||
| No | 437 | 1458 | Ref | / | |
| Yes | 82 | 181 | 0.939 (0.668–1.321) | 0.719 | |
| Metastasis of cancer | 0.160 | ||||
| No | 358 | 1251 | Ref | / | |
| Yes | 161 | 388 | 0.828 (0.636–1.077) | 0.160 | |
| Risk of COVID-19 infection | 0.716 | ||||
| Unknown | 130 | 281 | Ref | / | |
| Low | 194 | 699 | 1.047 (0.750–1.463) | 0.786 | |
| Medium | 105 | 335 | 1.219 (0.826–1.799) | 0.319 | |
| High | 90 | 324 | 1.172 (0.784–1.752) | 0.438 | |
| Impact of COVID-19 pandemic on regular medical treatment of cancer | 0.320 | ||||
| None | 97 | 184 | Ref | / | |
| Mild | 96 | 421 | 1.306 (0.751–2.271) | 0.345 | |
| Moderate | 153 | 474 | 0.875 (0.490–1.563) | 0.651 | |
| Severe | 173 | 560 | 1.002 (0.559–1.796) | 0.994 | |
| Impact of COVID-19 pandemic on daily life | 0.485 | ||||
| None | 92 | 148 | Ref | / | |
| Mild | 99 | 427 | 1.116 (0.635–1.963) | 0.702 | |
| Moderate | 169 | 524 | 0.802 (0.436–1.475) | 0.478 | |
| Severe | 159 | 540 | 0.945 (0.503–1.777) | 0.861 | |
| Impact of COVID-19 pandemic on income | < 0.001 | ||||
| None | 208 | 324 | Ref | / | |
| Mild | 103 | 436 | 1.930 (1.325–2.81) | 0.001 | |
| Moderate | 113 | 429 | 2.037 (1.382–3.002) | < 0.001 | |
| Severe | 95 | 450 | 2.688 (1.791–4.035) | < 0.001 | |
| When does the COVID-19 vaccine start working after vaccination? | 0.080 | ||||
| Unknown | 233 | 463 | Ref | / | |
| Immediately after the first dose | 43 | 226 | 1.524 (1.002–2.317) | 0.049 | |
| Immediately after the second dose | 65 | 280 | 1.468 (1.016–2.120) | 0.041 | |
| Fourteen days after the second dose | 178 | 670 | 1.277 (0.966–1.689) | 0.086 | |
| Do you know about how the COVID-19 vaccine was developed? | 0.009 | ||||
| No | 466 | 1301 | Ref | / | |
| Yes | 53 | 338 | 1.616 (1.126–2.318) | 0.009 | |
| Will the COVID-19 vaccine be useful in controlling the COVID-19 pandemic? | 0.439 | ||||
| No | 216 | 445 | Ref | / | |
| Yes | 303 | 1194 | 1.116 (0.845–1.474) | 0.439 | |
| Is the COVID-19 vaccine safe? | 0.038 | ||||
| No | 127 | 198 | Ref | / | |
| Yes | 392 | 1441 | 1.502 (1.024–2.203) | 0.038 | |
| Will the COVID-19 vaccine trigger allergy? | 0.062 | ||||
| No | 239 | 887 | Ref | / | |
| Yes | 280 | 752 | 0.791 (0.617–1.012) | 0.062 | |
| Is the COVID-19 vaccine effective? | 0.422 | ||||
| No | 95 | 181 | Ref | / | |
| Yes | 424 | 1458 | 0.847 (0.564–1.271) | 0.422 | |
| Will you encourage your parents and friends to get vaccinated? | < 0.001 | ||||
| No | 86 | 79 | Ref | / | |
| Yes | 433 | 1560 | 2.744 (1.759–4.280) | < 0.001 | |
| Will the COVID vaccine worsen the prognosis of cancer? | < 0.001 | ||||
| No | 266 | 1209 | Ref | / | |
| Yes | 253 | 430 | 0.393 (0.307–0.504) | < 0.001 | |
| Is it necessary to wear a mask after getting the COVID-19 vaccine? | 0.053 | ||||
| No | 107 | 451 | Ref | / | |
| Yes | 412 | 1188 | 0.731 (0.532–1.003) | 0.053 | |
| Are you willing to get the COVID-19 vaccine, even if you must pay for it? | < 0.001 | ||||
| No | 279 | 388 | Ref | / | |
| Yes | 240 | 1251 | 3.042 (2.376–3.894) | < 0.001 | |
P-Log: the P value indicates whether the variable contributes significantly to the occurrence of “vaccine acceptance”; P-Ref: the P value indicates whether the adjusted OR of particular sub-category is significant when compared with the reference category. * Surgery including procedures such as excision, transarterial chemoembolization, and microwave ablation. COVID-19: coronavirus disease 2019; OR: odds ratio; CI: confidence interval; Ref: reference category.
As these nine variables suggested (Table 5), cancer patients who consumed alcohol (OR = 1.849; 95% CI: 1.375–2.488, P-reference [P-Ref] < 0.001 vs non-drinkers), whose incomes were impacted by the COVID-19 pandemic (OR = 1.930, 2.037 and 2.688 for mild, moderate and severe impact; all P-Ref < 0.01 vs no impact), knew how the vaccine was developed (OR = 1.616; 95% CI: 1.126–2.318; P-Ref = 0.009 vs unknown), believed in the safety of the vaccine (OR = 1.502; 95% CI: 1.024–2.203; P-Ref = 0.038 vs denying the safety of vaccine), were willing to pay for the vaccine (OR = 3.042; 95% CI: 2.376–3.894; P-Ref < 0.001 vs unwilling), and would recommend families and friends to get vaccinated (OR = 2.744; 95% CI: 1.759–4.28; P-Ref < 0.001 vs do not recommend) were more likely to accept the COVID-19 vaccine.
However, being retired (OR = 0.586; 95% CI: 0.438–0.784; P-Ref < 0.001 vs unemployed), undergoing multiple treatments for cancer (OR = 0.408; 95% CI: 0.221–0.753; P-Ref = 0.004 vs no ongoing treatment), and worrying that the vaccine might worsen the prognosis of cancer (OR = 0.393; 95% CI: 0.307–0.504; P-Ref < 0.001 vs might not) are the catalyst for vaccine hesitancy (Table 5).
4. Discussion
With all the difficulty in managing COVID-19 pandemic worldwide, it is undeniable that TCM as a unique therapy contributed a lot during the quick and effective control of COVID-19 prevalence in China—during the past two years, TCM was deeply involved in the whole process of diagnosis and treatment of patients with COVID-19, and exerted superior advantage in promoting the treating effects when combined with Western medicine, which was regarded as a perfect anti-COVID-19 “combined punch” in China [51], [52], [53].
Despite the success of TCM application in the pandemic control in China, vaccination is still an inevitable way to wind up the COVID-19 pandemic. The National Health Commission of the Peoples’ Republic of China published a technical guideline for the inoculation with COVID-19 vaccines on March 29, 2021 [54]. Since then, the Chinese government has actively promoted vaccination campaigns, encouraging people to get the COVID-19 vaccine as soon as possible.
This study preliminarily revealed the predictors of intention to receive COVID-19 vaccine among cancer patients in Eastern China. Based on the results of this study, the following suggestions are recommended to be considered before making vaccination plans for cancer patients.
4.1. Clarifying the impact of COVID-19 vaccine on cancer progression is the premise of advocating vaccination for cancer patients
Eastern China is an area with a high cancer incidence as well as being strongly affected by the COVID-19 pandemic. In this study, among the 2158 cancer patients from Eastern China, 24.05% were “vaccine hesitancy” towards the COVID-19 vaccine (Fig. 1). The multivariate logistic regression analyses indicated that the concern that the COVID-19 vaccine could worsen the prognosis of their cancer treatment was one of the main predictors for vaccine hesitancy (Table 5). These results are similar to data from other countries: in Tunisia, 15.5% cancer patients thought the COVID-19 vaccine could impact cancer treatment outcomes or treatment efficacy [55]; in the US, among cancer patients who refused to take the COVID-19 vaccine, 56.3% reported being concerned about the compatibility of the COVID-19 vaccine and their cancer treatment [56].
Further, this study found that 69.37% of cancer patients believed that the COVID-19 vaccine would effectively control the COVID-19 epidemic. However, the acceptance rate was much lower than a previous anonymous cross-sectional survey among adults conducted in China (89.5%) [31]. Actually, the distrust towards the COVID-19 vaccine seems to be a worldwide phenomenon: in Korea, among cancer patients who were willing to take the COVID-19 vaccine, only 46.7% trusted its effectiveness [57]; in Poland, 44.4% of cancer patients questioned the usefulness of COVID-19 vaccines [58].
The efficacy of the vaccine is critical to controlling the epidemic and has caused widespread concern. A previous cross-sectional study including the general population, medical students, and healthcare workers showed that approximately 41.2% of the study population was willing to receive a COVID-19 vaccine with an efficacy of 50% or more, 60.6% with an efficacy of 70% or more, and 79.6% with an efficacy of 90% or more [59]. Similar results were found in low- and middle-income countries [60].
Therefore, although the safety and efficacy of COVID-19 vaccines seem to be acknowledged by the general population, among cancer patients the support is less enthusiastic. In fact, the risk of vaccine about COVID-19 infection has been partially focused: a study of the mass vaccination in Israel found that the BNT162b2 vaccine was not associated with an elevated risk of most of the adverse events considered, but the risk of potentially serious adverse events was significantly increased after SARS-COV-2 infection [61].
Cancer patients comprise a special group, whose immune function and health status are different from those of the general public. Since most cancer patients are middle-aged or elderly, their reactivity to the vaccine may also be different. A retrospective study of 326 cancer patients found that although the BNT162b2 vaccine was safe and effective in cancer patients undergoing regular treatment, the antibody titers of these patients were significantly lower than in the general population, especially among those undergoing chemotherapy [62].
Therefore, considering the intention of cancer patients to receive the COVID-19 vaccine and the effectiveness and safety of the vaccine itself, clarifying the impact of COVID-19 vaccine on the progression of cancer is the premise of advocating vaccination for this special population.
4.2. Individualized vaccination plans/recommendations should be drawing up for cancer patients with different demographic and health characteristics
According to the results from this study, cancer patients’ occupation, the nature of their ongoing treatment, the impact of the COVID-19 pandemic on their income, and their individual knowledge about and attitude towards the COVID-19 vaccine are all factors that impact their intention to receive the vaccine (Table 5). The vaccination intention of cancer patients is the real reflection of their vaccine demand. Therefore, individualized recommendations are more suitable for convincing cancer patients to get vaccinated.
Firstly, for retired cancer patients living in areas with low risk of COVID-19 infection, vaccination does not need to be recommended with urgency, since their risk of infection is low while the adverse impact of COVID-19 vaccine may overwhelm its benefits in this population.
Secondly, for patients in the stable phase of cancer management, who are still working, and live in an area with a high risk of COVID-19 exposure, and meanwhile have no allergic diseases, vaccination should be prioritized. For these individuals, vaccination could provide protection from COVID-19 infection, and there are few chances of vaccination negatively impacting their cancer treatment.
In addition, for cancer patients who want to be vaccinated due to the impact of the COVID-19 pandemic on their life and income, a comprehensive evaluation of the cancer progression and the risk of COVID-19 infection is necessary before receiving the vaccination. When the benefit of COVID-19 vaccination overwhelms its potential risk to cancer progression, vaccination is recommended, otherwise vaccination should be delayed.
4.3. Public information and guidance are important ways to attract eligible patients to complete vaccination
At present, the Chinese government regularly releases information about COVID-19 infections and vaccination rates, promotes vaccination decisions, and provides free vaccines to Chinese citizens. Numerous media outlets also actively publicize the benefits of vaccination. The COVID-19 epidemic has been brought under control in China, and the number of daily confirmed cases is very small.
This shows that government guidance plays an important role in elevating the vaccination willingness of the general public, improving the vaccination rate and controlling the spread of the COVID-19 epidemic.
In this study, we found that for cancer patients the most reliable sources of information about the COVID-19 vaccine were the media (76.41%), government agencies (66.40%), and discussions with friends and family (54.59%). These findings suggest that appropriate publicity and guidance from the government and media help cancer patients to accept the COVID-19 vaccine. In fact, cancer patients are a special group. Whether individual patients should be vaccinated needs to be comprehensively evaluated by the physicians and clinicians who manage cancer progression, rather than relying on the decisions of the patients themselves. In this regard, developing a system for caregivers to determine the suitability of COVID-19 vaccination for individual cancer patients will be of more use to this population than the general public health guidelines supplied by the government and echoed by the media.
Globally, the knowledge about the COVID-19 vaccine itself, especially its formulation and manufacture, is an important element that may affect the acceptance of the vaccine. With the development of the global pandemic, the COVID-19 vaccine has a huge market demand. Many new manufacturers have entered the market, and the development of vaccines has become the focus of global attention. In our study, only 18.12% of the respondents said they were aware of the process behind the development of the COVID-19 vaccines (Table 4), which was much lower than in Ethiopia (73.6%) [47]. Further logistic regression analysis shows that whether an individual understands the vaccine development process and whether an individual believes the vaccine is safe are two of the main factors that affect the vaccination intention of a cancer patient (Table 5). This shows that although the Chinese government and media are actively publicizing the advantages of the COVID-19 vaccine and advocating the general public to get vaccinated as soon as possible, the content and depth of the publicity need to be strengthened. In future publicity and guidance, providing comprehensive and systematic information about the source of the vaccine and its production process should be emphasized, to improve vaccination acceptance.
4.4. Multi-population, multi-region surveys with large sample size will help to better plan vaccination
Although this is the first survey in China to investigate the knowledge, attitude, and intention to receive the vaccination among cancer patients, due to the limitations of time, manpower and material resources, this study still has some deficiencies.
First, due to the short time-window for questionnaire development, the questionnaire used in our study was only partially validated through a small-scale pilot study. Second, this survey was only conducted among cancer patients in Eastern China, and therefore may not represent the status of cancer patients all over China. Third, the research was a cross-sectional study which could not infer the causality. Further longitudinal studies are needed to verify any causalities. Fourth, the online survey we used did not include individuals who could not access to the internet, like the old and the poor. Fifth, our research was conducted only in China, which limits the ability to extrapolate our findings to the global population. Fortunately, our findings were similar to those in other recent studies conducted in other countries [30]. Sixth, given the rapid and unpredictable progression of the COVID-19 pandemic, the willingness of cancer patients to accept the COVID-19 vaccine may change over time. Seventh, due to the time limitation, our study was based on convenience sampling method, and therefore, the sample size may not uniformly distributed among all the seven provinces of Eastern China. Finally, the data collected from this self-reported questionnaire may not fully reflect the attitudes of this population towards the COVID-19 vaccines.
Despite the above shortcomings, this study reveals willingness of cancer patients to receive the COVID-19 vaccine and exposes some of the factors that contribute to their attitudes. Thus, it has value for developing future vaccination initiatives for cancer patients in China. As a research precedent, this study proposes that a multi-population, multi-region survey with large sample size will be helpful to the correct planning and implementation of vaccination programs and should be undertaken.
5. Conclusion
Our study revealed the knowledge about, attitude towards, and intention to receive the COVID-19 vaccine among cancer patients in Eastern China: the rate of vaccine hesitancy was 24%; the main contributors to vaccine hesitancy were being retired, undergoing multiple treatments for cancer, and worrying that the vaccine might worsen the prognosis of one’s cancer treatment; however, alcohol consumption, having one’s income impacted by the COVID-19 pandemic, knowing how the vaccine was developed, believing in the safety of vaccine, being willing to pay for the vaccine, and being willing to recommend that friends and family receive the vaccine were contributors to vaccine acceptance.
When advocating for vaccination among cancer patients, we recommend incorporating the specific details of each patient and their personal needs into the development of an individualized vaccination plan, balancing the public health needs of controlling the COVID-19 pandemic against the best clinical practices for managing the progression of disease in cancer patients.
Funding
This study was supported by National Administration of Traditional Chinese Medicine’s Traditional Chinese Medicine Inheritance and Innovation “Hundreds and Thousands” Talent Project (Qihuang Project) Qihuang Scholars Support Fund, and National Natural Science Foundation of China (No. 82030117).
Please cite this article as: Hong J, Xu XW, Yang J, Zheng J, Dai SM, Zhou J, Zhang QM, Ruan Y, Ling CQ. Knowledge about, attitude and acceptance towards, and predictors of intention to receive the COVID-19 vaccine among cancer patients in Eastern China: A cross-sectional survey. J Integr Med. 2021; Epub ahead of print.
Author contributions
CQL, JH and YR designed the study. YR and JH performed the study and their analyses. XWX, JY, JZ, SMD, JZ and QMZ were responsible for data collection. YR performed data analyses. All authors participated in data interpretation, manuscript review and writing. YR and JH were responsible for preparation of the Tables and Figures. All authors contributed to the scientific discussion of the data and of the manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgement
The authors would like to thank all the participants in this work.
References
- 1.Chakraborty I., Maity P. COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci Total Environ. 2020;728:138882. doi: 10.1016/j.scitotenv.2020.138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. COVID-19 weekly epidemiological update. (2021-09-21) [2021-09-27]. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---21-september-2021.
- 3.Yan E., Lai D.W.L., Lee V.W.P. Predictors of intention to vaccinate against COVID-19 in the general public in Hong Kong: findings from a population-based, cross-sectional survey. Vaccines (Basel) 2021;9(7):696. doi: 10.3390/vaccines9070696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin Y., Yang H., Ji W., Wu W., Chen S., Zhang W., et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4):372. doi: 10.3390/v12040372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health Commission of the People’s Republic of China. Notice on issuing the diagnosis and treatment plan for pneumonia infected by novel coronavirus (trial eighth edition). (2021-04-14) [2021-09-12]. http://www.nhc.gov.cn/xcs/zhengcwj/202104/7de0b3837c8b4606a0594aeb0105232b.shtml.
- 6.National Health Commission of the People’s Republic of China. Document of the press conference of State Council Information Office on March 23, 2020. (2020-03-23) [2021-09-12]. http://www.nhc.gov.cn/xcs/yqfkdt/202003/3ae4901e428b46ea90065f29e56cdbd1.shtml.
- 7.Ling C.-Q. Traditional Chinese medicine is a resource for drug discovery against 2019 novel coronavirus (SARS-CoV-2) J Integr Med. 2020;18(2):87–88. doi: 10.1016/j.joim.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J., Yang W., Liu Y., Lu C., Ruan L., Zhao C., et al. Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): a single-center, open-label, randomized controlled trial. Phytomedicine. 2021;91:153671. doi: 10.1016/j.phymed.2021.153671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z.-J., Wu W.-Y., Hou J.-J., Zhang L.-L., Li F.-F., Gao L., et al. Active constituents and mechanisms of Respiratory Detox Shot, a traditional Chinese medicine prescription, for COVID-19 control and prevention: network-molecular docking-LC-MSE analysis. J Integr Med. 2020;18(3):229–241. doi: 10.1016/j.joim.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan A.Y., Gu S., Alemi S.F. Research Group for Evidence-based Chinese Medicine. Chinese herbal medicine for COVID-19: current evidence with systematic review and meta-analysis. J Integr Med. 2020;18(5):385–394. doi: 10.1016/j.joim.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Xue Y., Chen X., Wu J.-M., Su Z.-J., Sun M., et al. Effects of Tanreqing Capsule on the negative conversion time of nucleic acid in patients with COVID-19: a retrospective cohort study. J Integr Med. 2021;19(1):36–41. doi: 10.1016/j.joim.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai L., Gao G.F. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21(2):73–82. doi: 10.1038/s41577-020-00480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H., Zhang Y., Huang B., Deng W., Quan Y., Wang W., et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell. 2020;182(3):713–721.e9. doi: 10.1016/j.cell.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang S., Li Y., Dai L., Wang J., He P., Li C., et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021;21(8):1107–1119. doi: 10.1016/S1473-3099(21)00127-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee N., McGeer A. The starting line for COVID-19 vaccine development. Lancet. 2020;395(10240):1815–1816. doi: 10.1016/S0140-6736(20)31239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallapaty S. WHO approval of Chinese CoronaVac COVID vaccine will be crucial to curbing pandemic. Nature. 2021;594(7862):161–162. doi: 10.1038/d41586-021-01497-8. [DOI] [PubMed] [Google Scholar]
- 18.Liang W., Guan W., Chen R., Wang W., Li J., Xu K.e., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu C., Zhao Y., Okwan-Duodu D., Basho R., Cui X. COVID-19 in cancer patients: risk, clinical features, and management. Cancer Biol Med. 2020;17(3):519–527. doi: 10.20892/j.issn.2095-3941.2020.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corti C., Curigliano G. Commentary: SARS-CoV-2 vaccines and cancer patients. Ann Oncol. 2021;32(4):569–571. doi: 10.1016/j.annonc.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapani D., Curigliano G. COVID-19 vaccines in patients with cancer. Lancet Oncol. 2021;22(6):738–739. doi: 10.1016/S1470-2045(21)00250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai A., Gainor J.F., Hegde A., Schram A.M., Curigliano G., Pal S., et al. COVID-19 vaccine guidance for patients with cancer participating in oncology clinical trials. Nat Rev Clin Oncol. 2021;18(5):313–319. doi: 10.1038/s41571-021-00487-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garassino M.C., Vyas M., de Vries E.G.E., Kanesvaran R., Giuliani R., Peters S., et al. The ESMO Call to Action on COVID-19 vaccinations and patients with cancer: Vaccinate. Monitor. Educate. Ann Oncol. 2021;32(5):579–581. doi: 10.1016/j.annonc.2021.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Comprehensive Cancer Network. Cancer and COVID-19 vaccination (version 4.0). (2021-08-30) [2021-09-12]. https://www.nccn.org/docs/default-source/covid-19/2021_covid-19_vaccination_guidance_v4-0.pdf?sfvrsn=b483da2b_70.
- 25.Monin L., Laing A.G., Muñoz-Ruiz M., McKenzie D.R., del Molino del Barrio I., Alaguthurai T., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;22(6):765–778. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang J.K., Zhang T., Wang A.Z., Li Z. COVID-19 vaccines for patients with cancer: benefits likely outweigh risks. J Hematol Oncol. 2021;14(1):38. doi: 10.1186/s13045-021-01046-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Ten threats to global health in 2019. (2021) [2021-09-27]. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019.
- 28.Barrière J., Gal J., Hoch B., Cassuto O., Leysalle A., Chamorey E., et al. Acceptance of SARS-CoV-2 vaccination among French patients with cancer: a cross-sectional survey. Ann Oncol. 2021;32(5):673–674. doi: 10.1016/j.annonc.2021.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Noia V., Renna D., Barberi V., Di Civita M., Riva F., Costantini G., et al. The first report on coronavirus disease 2019 (COVID-19) vaccine refusal by patients with solid cancer in Italy: early data from a single-institute survey. Eur J Cancer. 2021;153:260–264. doi: 10.1016/j.ejca.2021.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brodziak A., Sigorski D., Osmola M., Wilk M., Gawlik-Urban A., Kiszka J., et al. Attitude of patients with cancer towards vaccinations-results of online survey with special focus on the vaccination against COVID-19. Vaccines (Basel) 2021;9(5):411. doi: 10.3390/vaccines9050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J., Jing R., Lai X., Zhang H., Lyu Y., Knoll M.D., et al. Acceptance of COVID-19 vaccination during the COVID-19 pandemic in China. Vaccines (Basel) 2020;8(3):482. doi: 10.3390/vaccines8030482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards B., Biddle N., Gray M., Sollis K., Di Gennaro F. COVID-19 vaccine hesitancy and resistance: correlates in a nationally representative longitudinal survey of the Australian population. PLoS One. 2021;16(3):e0248892. doi: 10.1371/journal.pone.0248892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nomura S., Eguchi A., Yoneoka D., Kawashima T., Tanoue Y., Murakami M., et al. Reasons for being unsure or unwilling regarding intention to take COVID-19 vaccine among Japanese people: a large cross-sectional national survey. Lancet Reg Health West Pac. 2021;14:100223. doi: 10.1016/j.lanwpc.2021.100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Del Riccio M., Boccalini S., Rigon L., Biamonte M.A., Albora G., Giorgetti D., et al. Factors influencing SARS-CoV-2 vaccine acceptance and hesitancy in a population-based sample in Italy. Vaccines (Basel) 2021;9(6):633. doi: 10.3390/vaccines9060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu B., Gao X., Zhang X., Hu Y., Yang H., Zhou Y.H. Real-world acceptance of COVID-19 vaccines among healthcare workers in perinatal medicine in China. Vaccines (Basel) 2021;9(7):704. doi: 10.3390/vaccines9070704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J., Wen X., Guo Q.i., Ji M., Zhang F., Wagner A.L., et al. Sensitivity to COVID-19 vaccine effectiveness and safety in Shanghai, China. Vaccines (Basel) 2021;9(5):472. doi: 10.3390/vaccines9050472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Syed Alwi S.A.R., Rafidah E., Zurraini A., Juslina O., Brohi I.B., Lukas S. A survey on COVID-19 vaccine acceptance and concern among Malaysians. BMC Public Health. 2021;21(1):1129. doi: 10.1186/s12889-021-11071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wirawan GBS, Mahardani PNTY, Cahyani MRK, Laksmi NLPSP, Januraga PP. Conspiracy beliefs and trust as determinants of COVID-19 vaccine acceptance in Bali, Indonesia: cross-sectional study. Pers Individ Dif 2021;180:110995. [DOI] [PMC free article] [PubMed]
- 39.Urrunaga-Pastor D., Bendezu-Quispe G., Herrera-Añazco P., Uyen-Cateriano A., Toro-Huamanchumo C.J., Rodriguez-Morales A.J., et al. Cross-sectional analysis of COVID-19 vaccine intention, perceptions and hesitancy across Latin America and the Caribbean. Travel Med Infect Dis. 2021;41:102059. doi: 10.1016/j.tmaid.2021.102059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ciardi F., Menon V., Jensen J.L., Shariff M.A., Pillai A., Venugopal U., et al. Knowledge, attitude and perceptions of COVID-19 vaccination among healthcare workers of an inner-city hospital in New York. Vaccines (Basel) 2021;9(5):516. doi: 10.3390/vaccines9050516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Babicki M., Mastalerz-Migas A. Attitude toward vaccination against COVID-19 in Poland. A longitudinal study performed before and two months after the commencement of the population vaccination programme in Poland. Vaccines (Basel) 2021;9(5):503. doi: 10.3390/vaccines9050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen W., Zheng R., Baade P.D., Zhang S., Zeng H., Bray F., et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 43.Kang D., Choi H., Kim J.-H., Choi J. Spatial epidemic dynamics of the COVID-19 outbreak in China. Int J Infect Dis. 2020;94:96–102. doi: 10.1016/j.ijid.2020.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han K., Francis M.R., Zhang R., Wang Q., Xia A., Lu L., et al. Confidence, acceptance and willingness to pay for the COVID-19 vaccine among migrants in Shanghai, China: a cross-sectional study. Vaccines (Basel) 2021;9(5):443. doi: 10.3390/vaccines9050443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumari A., Ranjan P., Chopra S., Kaur D., Upadhyay A.D., Kaur T., et al. Development and validation of a questionnaire to assess knowledge, attitude, practices, and concerns regarding COVID-19 vaccination among the general population. Diabetes Metab Syndr. 2021;15(3):919–925. doi: 10.1016/j.dsx.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher K.A., Bloomstone S.J., Walder J., Crawford S., Fouayzi H., Mazor K.M. Attitudes toward a potential SARS-CoV-2 vaccine: a survey of U.S. adults. Ann Intern Med. 2020;173(12):964–973. doi: 10.7326/M20-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abebe H., Shitu S., Mose A. Understanding of COVID-19 vaccine knowledge, attitude, acceptance, and determinants of COVID-19 vaccine acceptance among adult population in Ethiopia. Infect Drug Resist. 2021;14:2015–2025. doi: 10.2147/IDR.S312116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y., Hu Z., Zhao Q., Alias H., Danaee M., Wong L.P., et al. Understanding COVID-19 vaccine demand and hesitancy: a nationwide online survey in China. PLoS Negl Trop Dis. 2020;14(12):e0008961. doi: 10.1371/journal.pntd.0008961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rzymski P., Zeyland J., Poniedziałek B., Małecka I., Wysocki J. The perception and attitude toward COVID-19 vaccines: a cross-sectional study in Poland. Vaccines (Basel) 2021;9(4):382. doi: 10.3390/vaccines9040382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bujang M.A., Sa’at N., Tg Abu Bakar Sidik T.M.I., Chien Joo L. Sample size guidelines for logistic regression from observational studies with large population: emphasis on the accuracy between statistics and parameters based on real life clinical data. Malays. J Med Sci. 2018;25(4):122–130. doi: 10.21315/mjms2018.25.4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee D.Y.W., Li Q.Y., Liu J., Efferth T. Traditional Chinese herbal medicine at the forefront battle against COVID-19: clinical experience and scientific basis. Phytomedicine. 2021;80:153337. doi: 10.1016/j.phymed.2020.153337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao M., Tian J., Zhou Y., Xu X.i., Min X., Lv Y.i., et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res. 2020;161:105126. doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiong X., Wang P., Su K., Cho W.C., Xing Y. Chinese herbal medicine for coronavirus disease 2019: a systematic review and meta-analysis. Pharmacol Res. 2020;160:105056. doi: 10.1016/j.phrs.2020.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Health Commission of People’s Republic of China. Technical guideline for the inoculation of COVID-19 vaccines. (2021-03-29) [2021-09-27]. http://www.nhc.gov.cn/jkj/s3582/202103/c2febfd04fc5498f916b1be080905771.shtml.
- 55.Mejri N., Berrazega Y., Ouertani E., Rachdi H., Bohli M., Kochbati L., et al. Understanding COVID-19 vaccine hesitancy and resistance: another challenge in cancer patients. Support Care Cancer. 2021 doi: 10.1007/s00520-021-06419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moujaess E., Zeid N.B., Samaha R., Sawan J., Kourie H., Labaki C., et al. Perceptions of the COVID-19 vaccine among patients with cancer: a single-institution survey. Future Oncol. 2021 doi: 10.2217/fon-2021-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chun J.Y., Kim S.I., Park E.Y., Park S.-Y., Koh S.-J., Cha Y., et al. Cancer patients’ willingness to take COVID-19 vaccination: a nationwide multicenter survey in Korea. Cancers (Basel) 2021;13(15):3883. doi: 10.3390/cancers13153883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brodziak A., Sigorski D., Osmola M., Wilk M., Gawlik-Urban A., Kiszka J., et al. Attitudes of patients with cancer towards vaccinations—results of online survey with special focus on the vaccination against COVID-19. Vaccines (Basel) 2021;9(5):411. doi: 10.3390/vaccines9050411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elhadi M., Alsoufi A., Alhadi A., Hmeida A., Alshareea E., Dokali M., et al. Knowledge, attitude, and acceptance of healthcare workers and the public regarding the COVID-19 vaccine: a cross-sectional study. BMC Public Health. 2021;21(1) doi: 10.1186/s12889-021-10987-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bono S.A., Faria de Moura Villela E., Siau C.S., Chen W.S., Pengpid S., Hasan M.T., et al. Factors affecting COVID-19 vaccine acceptance: an international survey among low- and middle-income countries. Vaccines (Basel) 2021;9(5):515.;9(5):515. doi: 10.3390/vaccines9050515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barda N., Dagan N., Ben-Shlomo Y., Kepten E., Waxman J., Ohana R., et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078–1090. doi: 10.1056/NEJMoa2110475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ligumsky H, Safadi E, Etan T, Vaknin N, Waller M, Croll A, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. J Natl Cancer Inst 2021; doi: 10.1093/jnci/djab174. [DOI] [PMC free article] [PubMed]