Abstract
Background:
Previous research has suggested that vigorous physical activity (VPA) during adolescence and early adulthood is associated with ALS. The National ALS Registry (Registry) collects physical activity data from persons with ALS.
Objective:
To examine the association between vigorous VPA and early onset ALS, defined as a diagnosis before age 60, among patients enrolled in the Registry. VPA was defined as engaging in dynamic exercise for at least 10 minutes in a session that caused heavy sweating or large increases in breathing or heart rate.
Methods:
A cross-sectional study was conducted of 5,463 ALS patients with VPA history and 956 ALS patients who never engaged in VPA. Patient characteristics were collected via online surveys in the following areas: demographic, lifetime VPA history, and initial onset of symptoms. General linear modeling was used to estimate mean age of diagnosis and to compute 95% confidence intervals.
Results:
Patients who reported engaging in VPA at least moderately (three times a week) during early adulthood were more likely to have an ALS diagnosis earlier compared to patients who did not (p<0.0001). After controlling for year of birth, statistically significant associations between those reporting VPA at age 15–24 and 25–34 and diagnosis of ALS earlier (p=0.0009, p=0.0144 respectively).
Conclusion:
Patients with ALS who had a history of VPA before age 35, were significantly more likely to be diagnosed with ALS before age 60 compared to patients with ALS who never engaged vigorously. More research is needed in the relationship between VPA and early onset ALS.
Keywords: Amyotrophic lateral sclerosis, motor neuron disease, physical activity, exercise
Introduction
Amyotrophic lateral sclerosis (ALS) is a complex multifactorial neurodegenerative disease defined by the loss of upper and lower motor neurons, typically resulting in death within 2–5 years from diagnosis [1, 2]. Despite ALS being defined in 1869 [3], the actual risk factors and causes for the disease remain largely unknown. Conservative estimates suggest that in the United States (US) over 16,000, or 5.2/100,000 people, lived with ALS in 2016 [2] and approximately 5,000, or 1.5/100,000, are diagnosed annually [4].
Over the years, investigations have examined numerous risk factors for ALS, including vigorous physical activity (VPA). VPA can induce oxidative stress [5], which has been proposed as a possible mechanism to explain associations between physical activity and ALS [5–7]. Studies of physical activity in varsity and professional athletes, as well as military personnel, have also shown increased physical activity as a one of the possible risk factors for ALS[8–15]. In addition, participation in sports that require strenuous physical activity, such as professional soccer and American football, is thought to be a risk factor for ALS [16]. Other studies have shown no association or inconclusive results regarding leisure time physical activity [17, 18].
Here, we evaluate associations between VPA and the age at ALS onset in a large group of US patients enrolled in the National ALS Registry. The National ALS Registry is the largest population-based registry for ALS in the US. [4]. Advantages of using participants from this registry include the wide phenotypic differences in a national population [19, 20]. Having a better understanding of ALS risk factors pertaining to history of VPA and age of diagnosis may assist clinicians in making more rapid diagnoses, which could lead to earlier therapeutic interventions.
Methods
The National ALS Registry
In October 2010, the US federal Agency for Toxic Substances and Disease Registry (ATSDR), part of the Centers for Disease Control and Prevention (CDC), launched the congressionally-mandated, population-based National ALS Registry (Registry) to help clarify the epidemiology of ALS in the US [21]. While details about the Registry’s objectives are presented elsewhere [2], briefly, the Registry’s purpose is to quantify the incidence and prevalence of ALS in the US, describe the patient demographics, and examine potential risk factors [22]. Similarly, the Registry’s methods also have been previously described [23]. Briefly, cases from both the national administrative databases and the web portal are merged and de-duplicated to ensure that individuals are not counted twice. To verify ALS status within the web portal, ATSDR adopted the six validation questions from the US Department of Veterans Affairs ALS registry that have been proven to be reliable indicators for accurate ALS diagnoses [24]. Informed consent was obtained under a protocol approved by the Institutional Review Board of the Centers for Disease Control and Prevention.
The Registry’s web portal also allows participants to complete brief online surveys about ALS risk factors and experience various including physical activity. Currently, there are 17 survey modules available, shown in Supplementary Table 1 [25]. These surveys were designed and validated by the ALS Consortium of Epidemiologic Studies (ACES) at Stanford University [26] [27] and are structured such that participants can answer the questions without having to involve a healthcare provider. The Registry’s web portal is voluntary with no recruitment or requirement to participate. Patients may register in the web portal but never complete any surveys while others may complete all 17 surveys. These data are likely slanted towards a younger and better educated patient sample group [28]. To-date, over 90,000 surveys have been completed representing the largest, most geographically diverse collection of ALS risk factor data available.
A directed acyclic graph (DAG) has been constructed to identify covariates for the association of VPA status and age at the ALS diagnosis [29]. The proposed DAG for VPA (at any age) included race, sex, physically active jobs (defined as a job where little to no sedentary activity is involved), body mass index (BMI) at age 40, military status, family history of ALS, and site of disease onset (Figure). Military service is a collider that we attempt to control with use of inverse probability weighting and the rest of the variables are confounders (race, occupation, BMI, family history of ALS and site of onset) that we attempted to control for via multivariate modeling. Military service, while a potential collider, did not change the outcome of the modeling, therefore was not included in the analyses.
Figure.
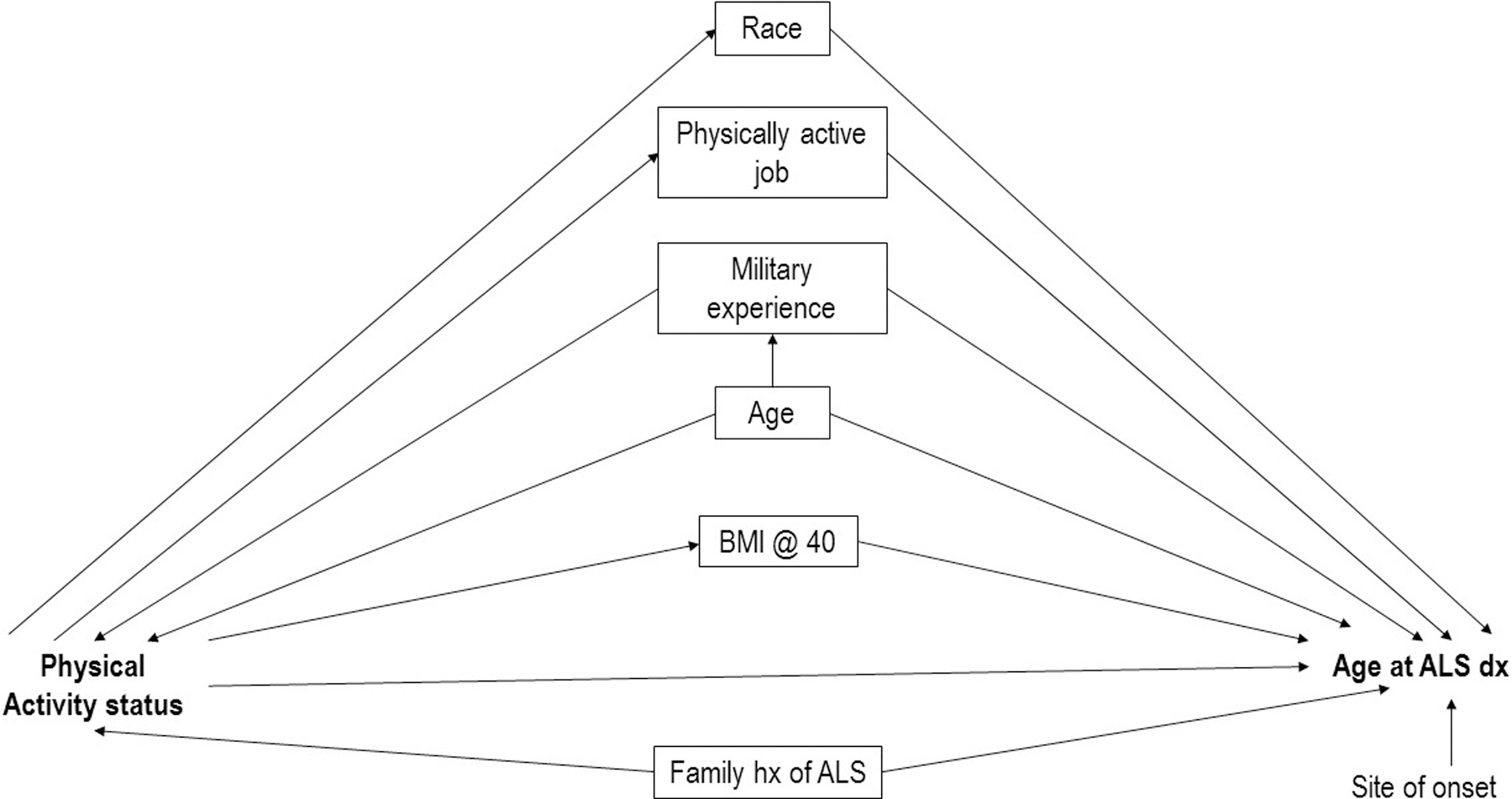
Directed acyclic graph (DAG) showing the associations between physical activity status and age at ALS diagnosis in the Na˙ ALS Registry, 2010 – 2018.
Physical Activity Survey Module
The Registry physical activity survey was pulled from the Global Physical Activity Questionnaire (GPAQ) by the World Health Organization released in 2002 [30]. Lifetime physical activity questionnaires including the GPAQ have been evaluated and validated [31, 32]. The Registry’s physical activity survey module launched on October 19, 2010. The purpose of the module is to examine the patients’ vigorous physical activity throughout their lifetime up to and after diagnosis. The survey contains 7 questions and covers time periods of physical activity and duration of physical activity as shown in Supplementary Table 2 [33]. VPA was defined to the patients as vigorous leisure-time exercise for at least 10 minutes per session evidenced by heavy sweating, and the number of times engaged in vigorous exercise during different ages by week, month, or year. For each age category (15–24 years, 25–34 years, 35–44 years, 45–54 years, 55–64 years, and 65+ years) VPA was reported, the number of sessions were categorized: sedentary (<1 session/week), minimal (1–2 sessions/week), moderate (3–4 sessions/week), and heavy (5+ sessions/week). As the module was initiated from the start of the Registry, all enrollees were able to voluntary participate. Therefore, this analysis covers from October 19, 2010 to December 31, 2018, the most recent year data was available.
Data Analysis
Selected demographic characteristics including sex, race, age at diagnosis, and military history were abstracted for those who completed the physical activity survey module. All surveys are completed by participants after their ALS diagnosis. Race was defined by standard US definitions: a primary race was noted and if more than one race was chosen, participants were categorized as non-white. BMI was calculated using standard formula: BMI = weight (lb) / [height (in)]2 × 703 [34]. The main predictor variable for this analysis was age(s) at which VPA was reported and the outcome was age of ALS diagnosis. Age at diagnosis is treated as a continuous variable as well as dichotomous using the break point at age 60; while ALS can affect people at any age, sporadic cases typically start around 60 years [25]. Participants are removed from the analysis once they are diagnosed with ALS. Student’s t-Test was used to examine differences in report of VPA by age category and mean age of ALS diagnosis. Generalized linear models were used to compare how the covariates affect age at diagnosis as a continuous variable. The models included sex, race, BMI at the time the patient entered the registry and at age 40, family history of ALS, physically active job(s), (i.e. a job that is not sedentary or requires sitting for a long period of time), military history and initial site of onset. Due to the fact that leisure physical activity became popular in the early 1970’s [35], year of birth (born before 1950 vs after 1950) was added as a covariate to control for the exposure some of the younger participants may have received. While VPA can accompany military history, which can lead to a potential selection bias for military history in the VPA group, inverse probability weighting was used initially. Inverse probability weighting is a statistical technique for calculating statistics standardized to a different population. After weights were applied to the analyses for military history, no significant difference was found, therefore the weighting was removed. All data analysis was performed using SAS 9.4 [36].
Results
Participants were 18 years or older, had been diagnosed with ALS by a neurologist and registered via the online portal at the National ALS Registry. Some 8,739 participants registered between October 19, 2010 and December 31, 2018 and completed at least one of the 17 surveys. Of these, 6,419 (73.5%) completed the physical activity survey module as well as basic demographic information. Demographic characteristics of these 6,419 participants are displayed in Table 1. Approximately 85% of the participants answered that they had engaged in VPA, at some point in their lifetime. Among those reporting ever participating in VPA, 60% were diagnosed between 50 and 69 years and male, and over 95% were white. The ever-VPA group was more likely to have a higher BMI at age 40, be born at or after 1950, familial ALS, and a military history when compared to the never VPA group. (Table 1). Of the registrants who completed the demographic survey but did not complete the physical activity survey (n=1,356), were slightly more likely to be overweight/have obesity at age 40 as well as at registration (data not shown).
Table 1.
Demographic characteristics among US adults with ALS who responded to the National ALS Registry’s Physical Activity Survey (October 19, 2010–December 31, 2018)
| Characteristic | Vigorous Physical Activity | Never Vigorous Physical Activity | p-value | ||
|---|---|---|---|---|---|
| N = 5,463 | % | N = 956 | % | ||
| Year Registered | 0.2347 | ||||
| 2010 | 637 | 11.7 | 102 | 10.7 | |
| 2011 | 543 | 9.9 | 101 | 10.6 | |
| 2012 | 535 | 9.8 | 87 | 9.1 | |
| 2013 | 747 | 13.7 | 130 | 13.6 | |
| 2014 | 736 | 13.5 | 159 | 16.6 | |
| 2015 | 671 | 12.3 | 119 | 12.5 | |
| 2016 | 630 | 11.5 | 96 | 10 | |
| 2017 | 471 | 8.6 | 71 | 7.4 | |
| 2018 | 493 | 9 | 91 | 9.5 | |
| Age at Diagnosis | <0.0001 | ||||
| 18–39 | 366 | 6.7 | 42 | 4.4 | |
| 40–49 | 888 | 16.3 | 108 | 11.3 | |
| 50–59 | 1,678 | 30.7 | 242 | 25.3 | |
| 60–69 | 1,780 | 32.6 | 345 | 36.1 | |
| 70–79 | 662 | 12.1 | 186 | 19.5 | |
| 80+ | 86 | 1.6 | 33 | 3.5 | |
| Missing | 3 | 0.1 | 0 | - | |
| Year of Birth | <0.0001 | ||||
| Before 1950 | 1,854 | 34 | 451 | 47.3 | |
| 1950 or later | 3,597 | 66 | 503 | 52.7 | |
| Gender | <0.0001 | ||||
| Male | 3,443 | 63.1 | 408 | 42.8 | |
| Female | 2,017 | 36.9 | 546 | 57.2 | |
| Race | 0.8214 | ||||
| Non-White | 249 | 4.6 | 42 | 4.4 | |
| White | 5,214 | 95.4 | 914 | 95.6 | |
| BMI at Registration | 0.0188 | ||||
| Below/Ideal Weight | 2,281 | 42.1 | 437 | 46.2 | |
| Overweight/Obese | 3,137 | 57.9 | 509 | 53.8 | |
| BMI at Age 40 | 0.0044 | ||||
| Below/Ideal Weight | 2,189 | 42.1 | 435 | 47.2 | |
| Overweight/Obese | 3,005 | 57.9 | 487 | 52.8 | |
| Change in weight from age 40 to registration | 0.0101 | ||||
| < 25 lbs. | 2,205 | 42.4 | 433 | 47 | |
| ≥ 25 lbs. | 2,994 | 57.6 | 489 | 53 | |
| Family History of ALS | 0.0216 | ||||
| Sporadic | 5,196 | 94.9 | 926 | 96.7 | |
| Familal | 277 | 5.1 | 32 | 3.3 | |
| Physically active job | 0.2164 | ||||
| No | 4,256 | 80 | 755 | 81.7 | |
| Yes | 1,067 | 20 | 169 | 18.3 | |
| Military History | <0.0001 | ||||
| No | 4,246 | 77.8 | 814 | 85.1 | |
| Yes | 1,214 | 22.2 | 142 | 14.8 | |
The mean number of VPA sessions per week among the 5,463 participants who recorded having ever engaged in VPA stratified by gender are presented in Table 2. All participants reported a decline in the number of VPA sessions over time regardless of gender (5.2 to 3.5 VPA sessions/week). Male participants who reported VPA at age 15 – 24 years averaged 5.6 VPA sessions/week compared to same-aged females who averaged 4.3 VPA sessions/week (p<0.0001). Males and females who reported VPA by age 65 both averaged 3.5 VPA sessions/week and was not statistically significant (p=0.6016).
Table 2.
Mean Vigorous Physical Activity sessions per week among US adults with ALS who responded to the National ALS Registry’s Physical Activity Survey (October 19, 2010–December 31, 2018)
| Ages | Total VPA sessions /week | Male VPA sessions /week | Female VPA sessions /week | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (95% CI) | ||||||||
| n | n | n | ||||||
| 15–24 years | 5,463 | 4.3 (4.1, 4.3) | 3,441 | 5.0 (4.9, 5.2) | 2,022 | 3.2 (3.1, 3.4) | <0.0001 | |
| 25–34 years | 5,298 | 3.8 (3.7, 3.9) | 3,329 | 4.2 (4.0, 4.5) | 1,969 | 3.0 (2.9, 3.2) | <0.0001 | |
| 35–44 years | 5,168 | 3.5 (3.4, 3.6)) | 3,138 | 3.9 (3.8, 4.1) | 2,030 | 2.9 (2.7, 3.0) | <0.0001 | |
| 45–54 years | 4,560 | 3.2 (3.1, 3.3) | 2,755 | 3.6 (3.5, 3.8) | 1,805 | 2.7 (2.6, 2.9) | <0.0001 | |
| 55–64 years | 3,092 | 2.8 (2.7, 2.9) | 1,782 | 3.1 (2.9, 3.2) | 1,310 | 2.4 (2.3, 2.6) | <0.0001 | |
| 65+ years | 673 | 2.3 (2.2, 2.4) | 388 | 2.5 (2.3, 2.6) | 285 | 2.0 (1.8, 2.2) | 0.008 | |
A description of VPA through early/mid-adulthood (15–24 years and 25–34 years) among participants is displayed in Table 3. The participants were measured on the amount of moderate or heavy VPA they engaged in during at age 15–24 years and 25–34 years. The categories are 1) VPA less than 3 times per session before age 35, for each age group 2) VPA at least 3 sessions per week between the ages 15–24 years but not 25–34 years, 3) VPA at least 3 sessions per week between the ages 25–34 years but not 15–24 years, and 4) VPA at least 3 sessions per week both between the ages 15–24 and 25–34 years. Males were more likely to engage in VPA moderately or heavy than females (p<0.0001). Over 55% of ALS participants with military history had engaged in moderate or heavy VPA at age 15–24 years and 25–34 years compared to those without military history (p<0.0001). Over 56% of ALS participants that were diagnosed before age 60 engaged in VPA moderately or heavy in both adolescence as well as early/mid adulthood (p<0.0001). Also, ALS participants born in 1950 or later were more likely to have engaged in VPA moderately or heavy compared to participants born before 1950 (p<0.0001).
Table 3.
Frequency of cumulative VPA through early adulthood among US adults with ALS who responded to the National ALS Registry’s Physical Activity Survey (October 19, 2010–December 31, 2018)
| Characteristic | VPA less than 3 sessions per week for both age groups 15–24 years and 25–34 years | VPA at least 3 sessions per week ages 15–24 but not 25–34 years | VPA at least 3 sessions per week ages 25–34 but not 15–24 years | VPA at least 3 sessions per week during both age groups 15–24 years and 25–34 years | p-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N = 2,109 | % | N = 1,138 | % | N = 148 | % | N = 3,227 | % | ||
| Gender | <0.0001 | ||||||||
| Male | 946 | 24 | 680 | 17.2 | 58 | 1.5 | 2,260 | 70.0 | |
| Female | 1,163 | 43.4 | 458 | 17.1 | 90 | 3.4 | 967 | 36.1 | |
| Race | 0.1994 | ||||||||
| White | 2,008 | 95.0 | 1,085 | 95.3 | 146 | 98.7 | 3,085 | 95.6 | |
| Non-White | 106 | 5.0 | 54 | 4.7 | n | n | 143 | 4.4 | |
| Physically active job | <0.0001 | ||||||||
| No | 1,670 | 32.3 | 936 | 2.3 | 120 | 2.3 | 2,438 | 47.2 | |
| Yes | 374 | 29.2 | 184 | 14.4 | 26 | 2 | 698 | 54.5 | |
| BMI at Age 40 | <0.0001 | ||||||||
| Below/Ideal Weight | 988 | 36.4 | 461 | 17 | 84 | 3.1 | 1,180 | 43.5 | |
| Overweight/Obesity | 1,038 | 28.9 | 599 | 16.7 | 58 | 1.6 | 1,901 | 52.86 | |
| Military | <0.0001 | ||||||||
| No | 1,778 | 34 | 884 | 16.9 | 125 | 2.4 | 2,429 | 46.7 | |
| Yes | 338 | 24.2 | 259 | 18.5 | 23 | 1.7 | 777 | 55.6 | |
| Age at ALS Diagnosis | <0.0001 | ||||||||
| ≥ 60 years old | 1,247 | 38.8 | 586 | 18.2 | 80 | 2.5 | 1,304 | 40.5 | |
| < 60 years old | 862 | 25.3 | 552 | 16.2 | 68 | 2 | 1,924 | 56.5 | |
| Year of Birth | <0.0001 | ||||||||
| Pre-1950 | 1,002 | 41.3 | 442 | 18.2 | 53 | 2.2 | 927 | 38.2 | |
| 1950 or later | 1,104 | 26.4 | 695 | 16.6 | 95 | 2.3 | 2,294 | 54.8 | |
The mean age at diagnosis for ALS within the study by amount of VPA by age group is displayed in Table 4. Participants who engaged in VPA at least 3 sessions per week, from ages 15 to 24 years, had a mean diagnosis age of 56.8 years while those with fewer than 3 VPA sessions per week had a mean diagnosis age of 60.5 years (p<0.0001). This trend continues and is especially notable for those less than 35 years and until the age group is 55–64 years. Among those who report engaging in VPA at 55–64 years compared to those who did not report VPA at the same age, there was no statistically significant difference in age of ALS diagnosis(p=0.7257).
Table 4.
Mean Age at Diagnosis by frequency of vigorous physical activity, among US adults with ALS who responded to the National ALS Registry’s Physical Activity Survey (October 19, 2010–December 31, 2018)
| VPA Age group | N | Mean Age of Diagnosis | 95% CI | Pooled Pr > |t| |
|---|---|---|---|---|
| 15–24 years old | ||||
| < 3 times/week | 1,573 | 60.5 | (59.9, 61.0) | |
| ≥ 3 times/week | 4,162 | 56.8 | (56.4, 57.1) | <0.0001 |
| 25–34 years old | ||||
| < 3 times/week | 1,992 | 60.0 | (59.5, 60.5) | |
| ≥ 3 times/week | 3,580 | 56.8 | (56.4, 57.1) | <0.0001 |
| 35–44 years old | ||||
| < 3 times/week | 2,111 | 59.7 | (59.2, 60.2) | |
| ≥ 3 times/week | 3,260 | 58.4 | (58.1, 58.8) | <0.0001 |
| 45–54 years old | ||||
| < 3 times/week | 1,958 | 61.7 | (61.3, 62.1) | |
| ≥ 3 times/week | 2,605 | 60.9 | (60.6, 61.2) | 0.001 |
| 55–64 years old | ||||
| < 3 times/week | 1,453 | 65.4 | (65.1, 65.8) | |
| ≥ 3 times/week | 1,639 | 65.5 | (65.2, 65.8) | 0.7257 |
The crude and adjusted mean age of diagnosis by ages of physical activity are shown in Table 5. In the unadjusted model, participants who engaged in VPA at least 3 sessions per week from 15 to 54 years were statistically different from those who did in VPA less than 3 sessions per week. After adjusting for year of birth, gender, weight change, BMI, race, military status, physically active job, and site of onset, the only physical activity groups that showed a significant association between age at ALS diagnosis were those who reported VPA at age 15–24 years and 25–34 years (p=0.0009, p=0.0144). There was no statistically significant association between age at ALS diagnosis and report of engaging in VPA after age 34.
Table 5.
Generalized Linear Modeling Analyses for the Mean age of ALS Diagnosis by frequency of Vigorous Physical Activity and age, among US adults with ALS who responded to the National ALS Registry’s Physical Activity Survey (October 19, 2010–December 31, 2018)
| Unadjusted Model | Adjusted Model * | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Variable | Parameter Estimate | Mean Age of ALS Diagnosis | t Value | Pr > |t| | Parameter Estimate | Mean Age of Diagnosis | t Value | Pr > |t| |
| Reported Age of VPA ^ | ||||||||
| Ages 15 – 24 years | −3.7231 | 57.8 | −11.12 | <0.0001 | −1.2133 | 58.5 | −3.31 | 0.0009 |
| Ages 25 – 34 years | −3.2222 | 57.9 | −10.5 | <0.0001 | −0.0722 | 59.6 | −2.45 | 0.0144 |
| Ages 35 – 44 years | −1.2556 | 58.9 | −4.43 | <0.0001 | −0.0123 | 59.9 | −0.04 | 0.965 |
| Ages 45 – 54 years | −0.8486 | 61.2 | −3.3 | 0.001 | 0.1742 | 61.5 | 0.69 | 0.4902 |
| Ages 55 – 64 years | 0.0842 | 65.5 | 0.35 | 0.7257 | 0.2453 | 65.5 | 1.03 | 0.3053 |
- Adjusted model, controlling for year of birth, gender, weight change, BMI, race, military status, physically active job, and site of onset
VPA is moderate or heavy - at least 3 sessions per week, compared to less than 3 sessions per week
Discussion
ALS is generally considered a complex multifactorial disease with unknown etiologies which likely incorporate both genetic and environmental factors [37] (Figure). The role of physical activity in the etiology of ALS is likely complex, with it being one of many patient factors that may play a causative role. Physical activity has demonstrated health benefits for the general population such as lower blood pressure, reduced risk for heart disease and strokes, and reduced obesity [38–40]. The authors of this study are not suggesting that physical activity should be avoided. The benefits of regular physical activity outweigh the lack of PA over the course of a lifetime. Rather, the role of VPA as a risk factor for ALS is still inconclusive and needs to be explored further [16]. Some previously published studies have shown no link or an inverse association between ALS and routine physical activity [41, 42]. In contrast, these data from the National ALS Registry show patients who had VPA more than 3 times per week when they were young were significantly more likely to be diagnosed before age 60 compared to ALS patients who never engaged in physical activity vigorously. One reason this may have occurred is due to the age breakdowns we used throughout the analysis. We did not consider only varsity athletes or combine exercise throughout the patients’ lifetime to calculate VPA. In its place, we broke down the ages throughout the patients’ lifetime to enhance when VPA or oxidative stress may play a role in ALS diagnosis.
Patients in this National ALS Registry study that engaged in VPA were more likely to be overweight (57%) at registration. This may be explained by the initial site of onset (limb onset is the most common) which may reduce physical activity if patients have lower limb weakness [43] as well as the implementation of a caloric/protein rich diet after diagnosis (as decreased survival correlates with a reduced body mass) [44].
Demographically, the Registry’s data are skewed towards a higher socio-economic status (SES) when compared to the general population. This difference is evident in a higher proportion of whites (males and females) enrolling in the Registry as well as those with an occupation that are more likely to require use of computers and internet access. In addition, physical activity and dietary behaviors are linked to racial and ethnic disparities with minorities having less vigorous physical activity and poorer dietary behaviors than whites [45] which may also skew the results of the analyses.
Enrollees in the Registry were more likely to engage in vigorous leisure-time sessions between the ages 15–44 (males and females) when compared to adults in the U.S. in the same age group (3 sessions per week) [46]. Regular physical activity helps to offset the generation of free radicals in the body; however, VPA where the body is taxed can also induce oxidative stress and increased inflammation [47] [48]. Since increased inflammation has been associated with an increased risk for ALS [49] [50], the role of VPA at young ages and ALS needs to be further explored. However, the patients who engaged in physical activity later in life, were less likely to be diagnosed with ALS before age 60. From our data, the percentage of this ALS population who engaged in physical activity when they were 45–54 years was consistent to a similar population [51]. This elevation of VPA in ALS patients could be explained by a higher SES patient cohort found in the Registry and further analysis is needed. A higher SES in general is associated with increased physical activity [52].
The benefits of physical activity outweigh the risks of little or no physical activity. One question that needs to be analyzed further is whether too much VPA or the frequency of these vigorous sessions at a younger age is a risk factor for ALS, especially males as they age. It is more likely that physical activity is one potential risk factor, is one component of many unknown variables such as environmental exposures in a multifactorial disease etiology for ALS, but more research is necessary.
A major study limitation is that the physical activity survey was not answered by a random sample from the database, and it is likely that some biases are introduced by the self-identification process. We attempted to address this using inverse probability weights, but that may not account for all the unknown variables related to participation. Participants with internet access are presumably more likely to participate; this may skew the population towards a younger, demographically white, and better educated patient sample. The participants in this study were mostly (63%) between the ages of 50 and 69, which is a somewhat larger proportion than what is seen in the National ALS Registry as a whole (48.2%) [2], and clearly contrasting with the overall U.S. population (24.4%) [53]. The portion of younger participants is overrepresented in this sample and the oldest age group is underrepresented compared to the data in the National ALS Registry (Table 1). Racial diversity appears to be underrepresented in the sample with only 4.5% being nonwhite as compared to 12.1% in the Registry as a whole [2, 54]. Potential reasons for these discrepancies include lower access to computers that are required for self-registration; reduced awareness of the Registry perhaps due to lower use of ALS specialty clinics; and reduced participation by Western residents, a region comprising a substantial non-white population [55]. Another possible study limitation is recall bias. Participants were asked to enter dates and ages from childhood through their ALS diagnosis as well as ALS symptoms before diagnosis. It is possible participants incorrectly estimated the date, ages, and symptoms resulting in possibly driving the odds towards the null if the errors were random. Further, answering surveys is voluntary and not everyone who registered took this survey. A final limitation is duplication of participants. While every attempt is taken to include only unique registrants in our analyses, there remains the possibility that a few duplicates would be included.
Conclusion
ALS has a multifactorial disease etiology; therefore, it is not surprising that many unknowns still exist about this rare condition. The National ALS Registry is a multi-faceted platform that advances research by evaluating potential risk factors, recruiting for national clinical trials and studies, funding research, and collecting as well as disseminating biospecimens and data nationally and internationally.
The Registry data presented here show VPA over one’s lifetime may play a role in the development of ALS; however, further investigation is warranted. This study is consistent with results of other reported research on smaller, less geographically diverse populations [16, 47, 48]. Better characterization of risk factors for ALS can assist clinicians in making referrals to an ALS specialist resulting in earlier diagnosis, which could lead to earlier therapeutic interventions.
Supplementary Material
Acknowledgements
The authors are grateful to those living with ALS who give their valuable time to contribute important health data to researchers. Without their help, these findings, and countless others, would not be possible.
Funding information
The funding for this paper comes from CDC/National ALS Registry.
Footnotes
Publisher's Disclaimer: Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the Agency for Toxic Substances and Disease Registry, the Centers for Disease Control and Prevention, and/or the US Department of Health and Human Services.
Declaration of interest
The CDC/ATSDR authors have no declarations of interest. Dr. Factor-Litvak has no declarations of interest linked to the study.
References
- 1.Kiernan MC, et al. , Amyotrophic lateral sclerosis. Lancet, 2011. 377(9769): p. 942–55. [DOI] [PubMed] [Google Scholar]
- 2.Mehta P., et al. , Prevalence of Amyotrophic Lateral Sclerosis - United States, 2015. MMWR Morb Mortal Wkly Rep, 2018. 67(46): p. 1285–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deux CJA, Deux cas d’atrophie musculaire progressive avec lesions de la susstance grise et des faisceaux antero-lateraux de la moelle epiniere. Arch Physiol neurol Pathol, 1869. 2. [Google Scholar]
- 4.Wagner L., et al. , State and metropolitan area-based amyotrophic lateral sclerosis (ALS) surveillance. Amyotroph Lateral Scler Frontotemporal Degener, 2015. 17(1–2): p. 128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harwood CA, McDermott CJ, and Shaw PJ, Physical activity as an exogenous risk factor in motor neuron disease (MND): a review of the evidence. Amyotroph Lateral Scler, 2009. 10(4): p. 191–204. [DOI] [PubMed] [Google Scholar]
- 6.Longstreth WT, et al. , Hypotheses to explain the association between vigorous physical activity and amyotrophic lateral sclerosis. Med Hypotheses, 1991. 34(2): p. 144–8. [DOI] [PubMed] [Google Scholar]
- 7.Vanacore N., et al. , Job strain, hypoxia and risk of amyotrophic lateral sclerosis: Results from a death certificate study. Amyotroph Lateral Scler, 2010. 11(5): p. 430–4. [DOI] [PubMed] [Google Scholar]
- 8.Strickland D., et al. , Physical activity, trauma, and ALS: a case-control study. Acta Neurol Scand, 1996. 94(1): p. 45–50. [DOI] [PubMed] [Google Scholar]
- 9.Scarmeas N., et al. , Premorbid weight, body mass, and varsity athletics in ALS. Neurology, 2002. 59(5): p. 773–5. [DOI] [PubMed] [Google Scholar]
- 10.Beghi E., et al. , Amyotrophic lateral sclerosis, physical exercise, trauma and sports: results of a population-based pilot case-control study. Amyotroph Lateral Scler, 2010. 11(3): p. 289–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehman EJ, et al. , Neurodegenerative causes of death among retired National Football League players. Neurology, 2012. 79(19): p. 1970–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chio A., et al. , Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain, 2005. 128(Pt 3): p. 472–6. [DOI] [PubMed] [Google Scholar]
- 13.Weisskopf MG, et al. , Prospective study of military service and mortality from ALS. Neurology, 2005. 64(1): p. 32–7. [DOI] [PubMed] [Google Scholar]
- 14.Chio A., et al. , ALS in Italian professional soccer players: the risk is still present and could be soccer-specific. Amyotroph Lateral Scler, 2009. 10(4): p. 205–9. [DOI] [PubMed] [Google Scholar]
- 15.Huisman MH, et al. , Family history of neurodegenerative and vascular diseases in ALS: a population-based study. Neurology, 2011. 77(14): p. 1363–9. [DOI] [PubMed] [Google Scholar]
- 16.Lacorte E., et al. , Physical activity, and physical activity related to sports, leisure and occupational activity as risk factors for ALS: A systematic review. Neurosci Biobehav Rev, 2016. 66: p. 61–79. [DOI] [PubMed] [Google Scholar]
- 17.Veldink JH, et al. , Physical activity and the association with sporadic ALS. Neurology, 2005. 64(2): p. 241–5. [DOI] [PubMed] [Google Scholar]
- 18.Huisman MH, et al. , Lifetime physical activity and the risk of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry, 2013. 84(9): p. 976–81. [DOI] [PubMed] [Google Scholar]
- 19.Chio A., et al. , Extensive genetics of ALS: a population-based study in Italy. Neurology, 2012. 79(19): p. 1983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Chalabi A., et al. , Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol, 2016. 15(11): p. 1182–94. [DOI] [PubMed] [Google Scholar]
- 21.Service UPH, ALS registry act. 110th congress. Public Law., U.P.H. Service, Editor. 2008, US Public Health Service: Washington DC. p. 110–373. [Google Scholar]
- 22.Brooks, B.R., El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and the El Escorial “Clinical limits of amyotrophic lateral sclerosis” workshop contributors. J Neurol Sci, 1994. 124 Suppl: p. 96–107. [DOI] [PubMed] [Google Scholar]
- 23.Antao VC and Horton DK, The National Amyotrophic Lateral Sclerosis (ALS) Registry. J Environ Health, 2012. 75(1): p. 28–30. [PMC free article] [PubMed] [Google Scholar]
- 24.Allen KD, et al. , The National Registry of Veterans with amyotrophic lateral sclerosis. Neuroepidemiology, 2008. 30(3): p. 180–90. [DOI] [PubMed] [Google Scholar]
- 25.Cedarbaum JM, et al. , The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci, 1999. 169(1–2): p. 13–21. [DOI] [PubMed] [Google Scholar]
- 26.Horton DK, Mehta P, and Antao VC, Quantifying a nonnotifiable disease in the United States: the National Amyotrophic Lateral Sclerosis Registry model. JAMA, 2014. 312(11): p. 1097–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Services, U.D.o.H.a.H., US department of health and human services regions., U.D.o.H.a.H. Services, Editor. 2017: Washington DC. [Google Scholar]
- 28.Bryan L., et al. , Preliminary Results of National Amyotrophic Lateral Sclerosis (ALS) Registry Risk Factor Survey Data. PLoS One, 2016. 11(4): p. e0153683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenland S, Pearl J, and Robins JM, Causal diagrams for epidemiologic research. Epidemiology, 1999. 10(1): p. 37–48. [PubMed] [Google Scholar]
- 30.Armstrong T, Bull F., Development of the World Health Organization Global Physical Activity Questionnaire (GPAQ). J Public Health 2006(14): p. 5.
- 31.Rudolf K., et al. , Show cards of the Global Physical Activity Questionnaire (GPAQ) - do they impact validity? A crossover study. BMC Public Health, 2020. 20(1): p. 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chasan-Taber L., et al. , Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol, 2002. 155(3): p. 282–9. [DOI] [PubMed] [Google Scholar]
- 33.Khishchenko N., et al. , Time to diagnosis in the National Registry of Veterans with Amyotrophic Lateral Sclerosis. Amyotroph Lateral Scler, 2010. 11(1–2): p. 125–32. [DOI] [PubMed] [Google Scholar]
- 34.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser, 1995. 854: p. 1–452. [PubMed] [Google Scholar]
- 35.Cooper KH, The History of Aerobics (50 Years and Still Counting). Res Q Exerc Sport, 2018. 89(2): p. 129–134. [DOI] [PubMed] [Google Scholar]
- 36.Institute, S., SAS 9.4. Cary, NC. [Google Scholar]
- 37.Eisen A., Amyotrophic lateral sclerosis is a multifactorial disease. Muscle Nerve, 1995. 18(7): p. 741–52. [DOI] [PubMed] [Google Scholar]
- 38.Warburton DER and Bredin SSD, Health benefits of physical activity: a systematic review of current systematic reviews. Curr Opin Cardiol, 2017. 32(5): p. 541–556. [DOI] [PubMed] [Google Scholar]
- 39.Amanat S., et al. , Exercise and Type 2 Diabetes. Adv Exp Med Biol, 2020. 1228: p. 91–105. [DOI] [PubMed] [Google Scholar]
- 40.Fuzeki E, Engeroff T, and Banzer W., Health Benefits of Light-Intensity Physical Activity: A Systematic Review of Accelerometer Data of the National Health and Nutrition Examination Survey (NHANES). Sports Med, 2017. 47(9): p. 1769–1793. [DOI] [PubMed] [Google Scholar]
- 41.Pupillo E., et al. , Physical activity and amyotrophic lateral sclerosis: a European population-based case-control study. Ann Neurol, 2014. 75(5): p. 708–16. [DOI] [PubMed] [Google Scholar]
- 42.Luna J., et al. , Current issues in ALS epidemiology: Variation of ALS occurrence between populations and physical activity as a risk factor. Rev Neurol (Paris), 2017. 173(5): p. 244–253. [DOI] [PubMed] [Google Scholar]
- 43.Hamidou B., et al. , Exploring the diagnosis delay and ALS functional impairment at diagnosis as relevant criteria for clinical trial enrolment. Amyotroph Lateral Scler Frontotemporal Degener, 2017. 18(7–8): p. 519–527. [DOI] [PubMed] [Google Scholar]
- 44.Kellogg J., et al. , Nutrition management methods effective in increasing weight, survival time and functional status in ALS patients: a systematic review. Amyotroph Lateral Scler Frontotemporal Degener, 2018. 19(1–2): p. 7–11. [DOI] [PubMed] [Google Scholar]
- 45.August KJ and Sorkin DH, Racial/ethnic disparities in exercise and dietary behaviors of middle-aged and older adults. J Gen Intern Med, 2011. 26(3): p. 245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhoades JA, Exercise in Adults, Age 18 and Older, in the United States, 2002: Estimates for the Noninstitutionalized Population, Quality A.f.H.R.a., Editor. 2005: Medical Expenditure Panel Survey. [Google Scholar]
- 47.Simioni C., et al. , Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget, 2018. 9(24): p. 17181–17198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawamura T and Muraoka I., Exercise-Induced Oxidative Stress and the Effects of Antioxidant Intake from a Physiological Viewpoint. Antioxidants (Basel), 2018. 7(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Y., et al. , Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: a meta-analysis study. Sci Rep, 2017. 7(1): p. 9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyon MS, et al. , Inflammation, Immunity, and amyotrophic lateral sclerosis: I. Etiology and pathology. Muscle Nerve, 2019. 59(1): p. 10–22. [DOI] [PubMed] [Google Scholar]
- 51.Kats D., et al. , Leisure-time physical activity volume, intensity, and duration from mid- to late-life in U.S. subpopulations by race and sex. The Atherosclerosis Risk In Communities (ARIC) Study. Aging (Albany NY), 2020. 12(5): p. 4592–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray TC, Rodgers WM & Fraser SN, Exploring the relationship between socioeconomic status, control beliefs and exercise behavior: a multiple mediator model. J Behav Med, 2012. 35: p. 63–73. [DOI] [PubMed] [Google Scholar]
- 53.Bureau UC, Total population 2014 American community survey 1-year, U.D.o. Commerce, Editor. 2014, US Census Bureau Washington DC. [Google Scholar]
- 54.Watanabe H., et al. , Factors affecting longitudinal functional decline and survival in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener, 2015. 16(3–4): p. 230–6. [DOI] [PubMed] [Google Scholar]
- 55.Kaye WE, et al. , Evaluating the completeness of the national ALS registry, United States. Amyotroph Lateral Scler Frontotemporal Degener, 2018. 19(1–2): p. 112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


