Abstract
Antibiotic resistance has become a major global issue. Understanding the molecular mechanisms underlying microbial adaptation to antibiotics is of keen importance to fight Antimicrobial Resistance (AMR). Aminoglycosides are a class of antibiotics that target the small subunit of the bacterial ribosome, disrupting translational fidelity and increasing the levels of misfolded proteins in the cell. In this work, we investigated the role of VchM, a DNA methyltransferase, in the response of the human pathogen Vibrio cholerae to aminoglycosides. VchM is a V. cholerae specific orphan m5C DNA methyltransferase that generates cytosine methylation at 5’-RCCGGY-3’ motifs. We show that deletion of vchM, although causing a growth defect in absence of stress, allows V. cholerae cells to cope with aminoglycoside stress at both sub-lethal and lethal concentrations of these antibiotics. Through transcriptomic and genetic approaches, we show that groESL-2 (a specific set of chaperonin-encoding genes located on the second chromosome of V. cholerae), are upregulated in cells lacking vchM and are needed for the tolerance of vchM mutant to lethal aminoglycoside treatment, likely by fighting aminoglycoside-induced misfolded proteins. Interestingly, preventing VchM methylation of the four RCCGGY sites located in groESL-2 region, leads to a higher expression of these genes in WT cells, showing that the expression of these chaperonins is modulated in V. cholerae by DNA methylation.
Author summary
Bacteria are organisms with a remarkable ability to adapt to several stress conditions, including to the presence of antibiotics. The molecular mechanisms underlying such adaptation lead, very often, to phenomena like antimicrobial tolerance and resistance, responsible for the frequent failure of antibiotic treatment. The study of these molecular mechanisms is thus an important tool to understand development of antimicrobial resistance in bacteria. In this work, we show that abrogating cytosine DNA methylation in Vibrio cholerae increases its tolerance to aminoglycosides, a class of antibiotics that cause protein misfolding. DNA methylation is known to affect gene expression and regulate several cellular processes in bacteria. Here we provide evidence that DNA methylation also has a more direct role in controlling antibiotic susceptibility in bacteria. Consequently, the study of bacterial DNA methyltransferases and DNA methylation should not be overlooked when addressing the problem of antimicrobial tolerance/resistance.
Introduction
In the past decades, the over/misuse and large-scale production of antibiotics has created a serious ecological problem with important consequences for the emergence of antimicrobial resistance (AMR). In fact, a large proportion of the antibiotics ingested are released intact in the environment [1,2] and found at trace levels or as gradients in various environments [3,4]. Hence, in these environments, one can find the presence of very low doses of drugs commonly referred as subMIC, i.e. under the MIC (Minimal Inhibitory Concentration). Although not enough to kill or prevent the growth of bacterial populations, subMIC doses of antibiotics are proposed to work as signaling molecules [5] and trigger important stress mechanisms that often result in development of antibiotic resistance [4,6–8]. We have previously shown that subMIC of antibiotics, such as aminoglycosides, trigger common and specific stress responses in Gram-negative bacteria [9,10].
Aminoglycosides (AGs) are positively charged molecules that bind 16S rRNA at the 30S ribosomal subunit and negatively affect translation. Specifically, AGs (e.g. tobramycin, streptomycin, kanamycin, gentamicin and neomycin) are known to disrupt translational fidelity and increase the levels of mistranslation, i.e. the misincorporation of certain amino acids in proteins [11,12]. In turn, high levels of mistranslation result in the production and accumulation of aberrant proteins in the cell, which contribute to the collapse of important cell processes and ultimately lead to cell death [13,14].
V. cholerae is a water-borne gram-negative bacterium, human pathogen and the causative agent of cholera disease. As part of its life cycle, V. cholerae often transits between the human gut and the external environment where it can find low doses of antibiotics. During our studies to better understand adaption of V. cholerae to aminoglycosides [15], we observed that a mutant of a V. cholerae’s specific DNA methyltransferase (vca0198—VchM) was less susceptible to aminoglycosides than its isogenic WT strain, suggesting that DNA methylation could play a role in V. cholerae adaptation to AGs. vchM codes for an Orphan m5C DNA methyltransferase that causes DNA methylation at 5’-RCCGGY-3’ motifs [16]. DNA methylation is catalyzed by enzymes called DNA methyltransferases (DNA MTases) that transfer a methyl group from S-adenosyl-L methionine (SAM) to adenine and cytosine in specific DNA motifs [17,18]. As a result, one can find the existence of small amounts of N6-methyl-adenine (6mA), C5-methyl-cytosine (5mC) and N4-methyl-cytosine (4mC) in the DNA of both eukaryotes and prokaryotes. In bacteria, the existence of such modified DNA bases have been shown to play a critical role in processes such as protection against invasive DNA, DNA replication and repair, cell cycle regulation and control of gene expression [19–23].
While it was previously proposed that VchM plays a role in the cell envelope stress response of V. cholerae [23], no link between this DNA MTase and antibiotic stress has yet been established. Here, we show that deletion of vchM (although causing a growth defect in absence of stress) allows V. cholerae cells to better deal with the effect of aminoglycosides. In fact, not only the vchM mutant is a better competitor during growth in presence of subMIC doses of aminoglycosides, it is also more tolerant to killing by lethal doses of these antibiotics. Transcriptome analysis of a ΔvchM strain revealed the upregulation of groESL-2 genes, a specific set of chaperonin-encoding genes located on the second chromosome of V. cholerae. High expression of groESL-2 genes (but not of chromosome one groESL-1 homologues) determines the higher tolerance of ΔvchM to lethal AG treatment, suggesting a new and specific role of groESL-2 in managing AG-mediated proteotoxic stress. Interestingly, we observed the presence of four VchM motifs in groESL-2 region. Preventing methylation of all these sites in the WT strain by disrupting such motifs results in increased expression of these genes. Intriguingly, the high expression of groESL-2 does not seem to contribute to the competitive advantage of the ΔvchM strain grown under subMIC AG which suggests the involvement of additional players in the global response of ΔvchM to aminoglycosides.
Results
V. cholerae cells lacking vchM cope better with subMIC doses of AGs
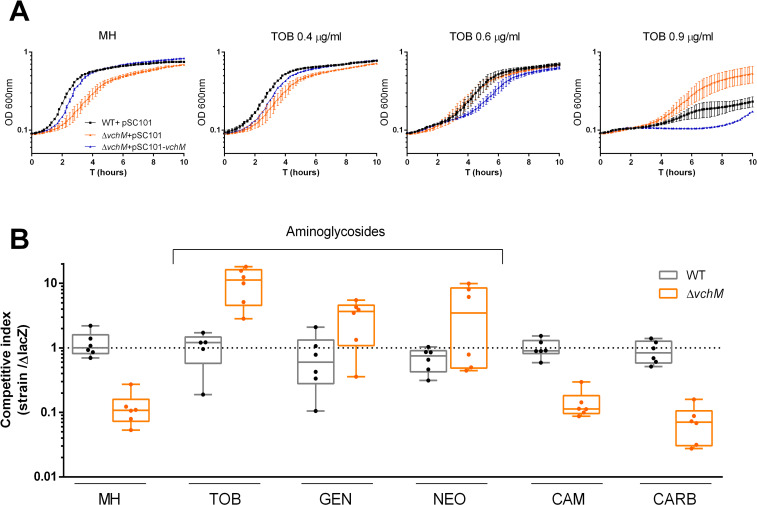
In order to explore a possible role of vchM in the response of V. cholerae O1 El Tor N16961 to aminoglycosides, we constructed an in-frame deletion mutant of vchM by allelic replacement with an antibiotic resistance cassette, and compared its growth to the isogenic wild-type (WT) strain, in rich media, with or without increasing concentrations of subMIC tobramycin (Fig 1). As previously described [23], this mutant exhibits a reduced doubling rate when grown in monoculture in antibiotic free rich media. However, the difference in growth between WT and ΔvchM strains observed in absence of antibiotics becomes gradually more negligible with increasing concentrations of subMIC TOB. At higher concentrations (90% of the MIC), ΔvchM even displays a clear advantage over the WT (Fig 1A). Importantly, a ΔvchM strain harboring a low-copy number plasmid with vchM gene under the control of its own promoter behaves as the WT strain in absence of tobramycin and even slightly worse in presence of higher doses of this drug (Fig 1A), showing that the observed growth phenotypes are due to the absence of vchM.
Fig 1. V. cholerae N16961 ΔvchM is less susceptible to subMIC aminoglycosides A.
Growth curves in absence (MH) or presence of subMIC doses of tobramycin. Bars represent SD (n = 3) B. In vitro competitions of WT and mutant strains against isogenic ΔlacZ reference strain in absence or presence of different antibiotics at subMIC concentrations (TOB, 0.6 μg/ml; GEN, 0.5 μg/ml; NEO, 2.0 μg/ml; CAM, 0.4 μg/ml; CARB, 2.5 μg/ml). Box plots indicate the median and the 25th and 75th percentiles; whiskers indicate the min and max values (n = 6).
Next, we asked whether the growth phenotype observed in monocultures was translatable to a higher relative fitness in co-cultures in the presence of subMIC doses of tobramycin and other AGs. For that, we competed both WT and ΔvchM strains (both lacZ+) with an isogenic ΔlacZ mutant (initial ratio of 1:1), in MH or MH supplemented with subMIC concentrations (50% MIC) of the aminoglycosides tobramycin (TOB), gentamicin (GEN) and neomycin (NEO). We assessed relative fitness by plating cultures after 20 hours of growth and counting the final proportion of lacZ+/lacZ- colonies. Competition of WT against the lacZ- mutant served as a control to account for any effect of lacZ deletion on growth. Supporting the previous results in monocultures, ΔvchM is outcompeted by the lacZ mutant in MH (≈10-fold difference) (Fig 1B). More importantly, in presence of low concentrations of aminoglycosides, ΔvchM is either equally competitive or even displays a clear growth advantage over the reference strain (Fig 1B). Additionally, in order to test whether these results hold for drugs other than aminoglycosides we performed competitions in the presence of chloramphenicol (CAM) and the beta-lactam carbenicillin (CARB). Unlike AGs, the presence of low concentrations of these drugs did not increased the relative fitness of the ΔvchM mutant.
Altogether, these results confirm that lack of vchM in V. cholerae negatively impacts growth in antibiotic-free media [23] but confers a selective advantage to V. cholerae in presence of subMIC doses of AGs (Fig 1). In order to test if deleting vchM affects the MIC of these drugs, we measured the MIC of both WT and ΔvchM mutant and found no difference (Table 1).
Table 1. MICs (μg/ml) of the different antibiotics tested for V. cholerae N16961 WT and ΔvchM strains.
| Strain | TOB | GEN | NEO | CAM | CARB |
|---|---|---|---|---|---|
| WT | 1–2 | 1 | 4 | 1 | 15 |
| ΔvchM | 1–2 | 1 | 4 | 1 | 7.5 |
TOB, tobramycin; GEN, gentamicin; NEO, neomycin; CAM, chloramphenicol; CARB, carbenicillin
VchM deficiency promotes higher tolerance to lethal doses of aminoglycosides
It has been previously shown that certain mutations affect functions conferring bacterial populations a tolerant phenotype towards a specific drug [24]. Such bacterial populations can transiently withstand lethal doses of that drug without necessarily any impact on the MIC of the population [24].
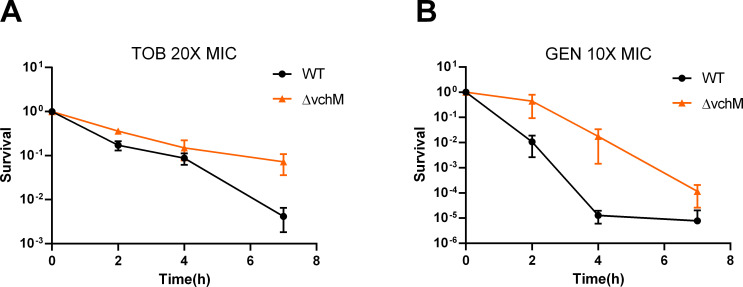
To continue exploring the different susceptibility of the ΔvchM strain to aminoglycosides we assessed the survival rate of V. cholerae WT and ΔvchM strains during treatment with lethal doses of tobramycin and gentamicin at 20x and 10x the MIC, respectively (Fig 2). Given the inherent growth defect of VchM deficiency, we performed time-dependent killing curves on stationary phase cells, where both WT and ΔvchM strains are no longer actively growing, excluding a possible link between growth rate and aminoglycoside lethality as previously shown [25]. Strikingly, survival to both antibiotics was increased 10–1000 fold in the ΔvchM mutant, suggesting that the absence of VchM allows V. cholerae to transiently withstand lethal doses of these aminoglycosides (Fig 2). We additionally tested the tolerance of exponential phase cells of WT and ΔvchM mutant strains to lethal concentrations of tobramycin and observed similar results (S1 Fig).
Fig 2. ΔvchM strain is more tolerant to lethal aminoglycoside treatment.
Survival of stationary-phase WT and ΔvchM cells exposed to lethal doses of tobramycin (TOB) (A), and gentamicin (GEN) (B). Survival represents the number of bacteria (CFU/mL) after treatment divided by the initial number of bacteria prior treatment. Time in the X-axis represents the duration of the antibiotic treatment in hours. Means and SD are represented, n = 3.
One crucial aspect that determines the efficacy of aminoglycoside treatment is the uptake of these drugs by the bacterial cell. This process is energy dependent and requires a threshold membrane potential [26]. We used a previously reported assay that measures cell fluorescence after incubation with fluorescent-marked neomycin (neo-cy5) [27] as a proxy for aminoglycoside uptake. We did not observe any difference in fluorescence between WT and ΔvchM mutant strains (S2 Fig). Thus, differential uptake of aminoglycosides is unlikely the reason for the increased tolerance to these drugs in ΔvchM.
A specific set of chaperonins is upregulated in ΔvchM cells
To understand the high tolerance to aminoglycosides observed in ΔvchM, we performed RNA-seq on stationary phase cells of WT and ΔvchM strains grown in rich, stress-free media. The analysis of the transcriptome of ΔvchM V. cholerae O1 El Tor N16961 strain reveals the significant upregulation (fold change ≥ 2, p<0.01) and downregulation (fold change ≤ -2, p<0.01) of 68 and 53 genes, respectively (S1 Table). Among the differentially expressed, we found four genes directly involved in protein folding to be upregulated in ΔvchM strain. Those are the molecular chaperones GroEL and co-chaperonins GroES (Table 2). In many bacterial species, GroEL and its co-chaperonin GroES form a molecular machine essential for folding of large newly synthesized proteins also helping re-folding of proteins damaged by proteotoxic stress [28]. Interestingly, overexpression of GroES and GroEL proteins was found to promote short-term tolerance to aminoglycoside-induced protein misfolding in E. coli [29].
Table 2. Protein folding and stabilization genes upregulated in ΔvchM (fold change > 2, p-value < 0.01).
| Locus | Name | Fold change (ΔvchM/WT) | Annotation |
|---|---|---|---|
| vc2665 | groEL-1 | 2.24 | Chaperonin, 60-kDa subunit |
| vca0820 | groEL-2 | 3.54 | Chaperonin, 60-kDa subunit |
| vc2664 | groES -1 | 2.60 | Chaperonin, 10-kDa subunit |
| vca0819 | groES-2 | 6.78 | Chaperonin, 10-kDa subunit |
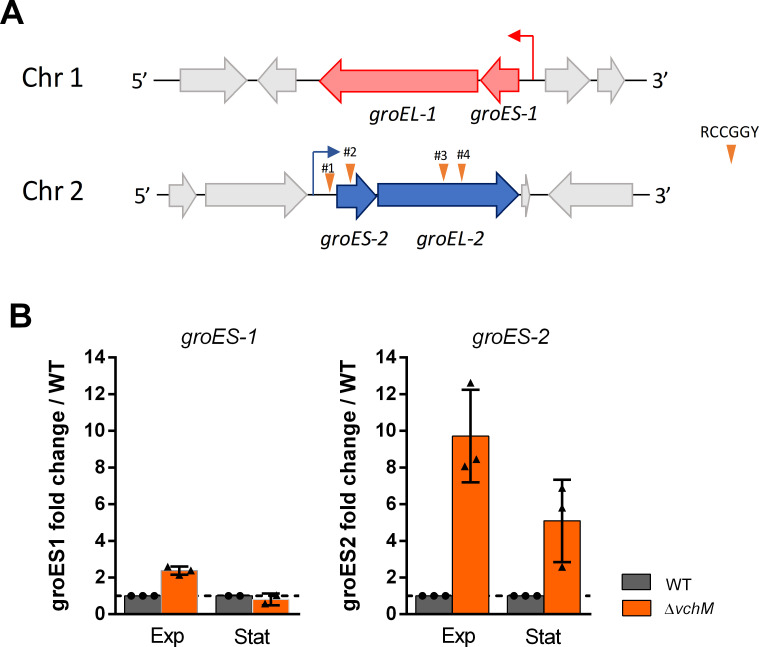
V. cholerae is one of, at least, seven Vibrio species harboring two copies of groES–groEL (groESL) bicistronic operons [30]. Whereas groESL-1 is encoded in chromosome 1 (vc2664-vc2665), groESL-2 is located in chromosome 2 (vca0819-0820) (Fig 3A) [30]. Based on our RNA-seq data, the latter manifested a larger fold change (Table 2). In order to confirm differential expression of these genes in ΔvchM, we measured groES-1 and groES-2 relative gene expression in exponential and stationary phase cells of WT and mutant strains, using digital qRT-PCR with the housekeeping gyrA gene as reference [31]. The results confirm a higher induction of groES-2 genes in both exponential and stationary phase ΔvchM cells with a fold change (over the WT) of ca. 10X and 5X, respectively (Fig 3B). However, groES-1 fold change was only slightly increased in exponential and unnoticeable in stationary phase.
Fig 3. groESL-2 operon is upregulated in ΔvchM strain.
A. Schematic representation of both groESL operons in V. cholerae. The four RCCGGY sites present along the groESL-2 region are represented by the inverted orange triangles. B. Fold change (ΔvchM/WT) of the relative expression levels of groES-1 and groES-2 in cultures at exponential phase (Exp, OD600 ≈ 0.3) or stationary phase (Stat, OD600 ≈ 1.8–2.0). Means and SD are represented, n = 3.
Induction of groESL genes is usually associated to perturbations in proteostasis which leads to activation of the heat-shock response [32]. Indeed, expression of both groESL-1 and groESL-2 is controlled by the heat-shock alternative sigma factor RpoH in V. cholerae [33]. However, the upregulation of groESL-2 genes in ΔvchM cells is likely independent of heat-shock activation as i) we do not observe any other genes of the heat-shock regulon being upregulated in the mutant (for example, dnaKJ/grpE, clpB, ibpAB) and ii) expression of groESL-1 is not as increased as expression of groESL-2 (Fig 3B).
Altogether, these results confirm that in absence of VchM, expression of groESL-2 genes is markedly increased in V. cholerae and suggest that regulation of groESL-2 operon in the ΔvchM mutant can be independent of heat-shock response.
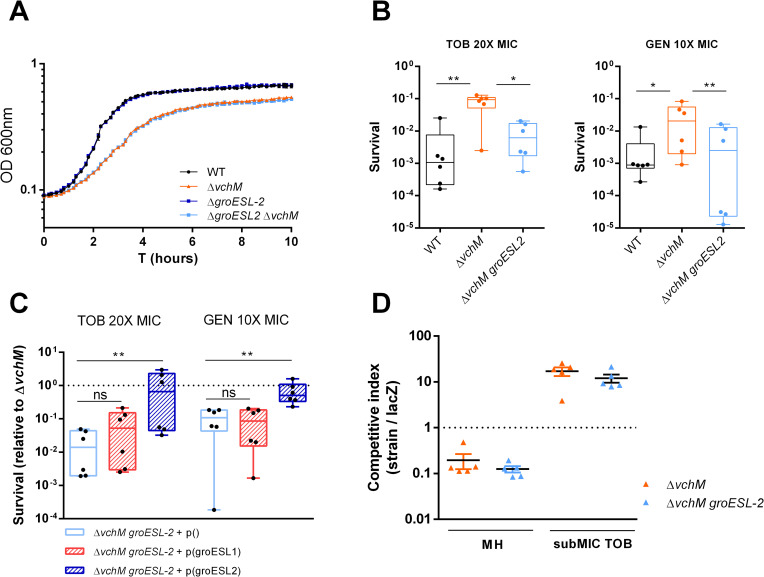
Deletion of groESL-2 operon abolishes ΔvchM high tolerance to lethal doses of tobramycin
In bacteria that harbor a single copy of this operon, GroESL are essential proteins for cell viability [34]. However, possible redundancy between groESL-1 and groESL-2 could allow for the deletion of one or the other operon in V. cholerae. Thus, we attempted to delete groESL-1 and groESL-2 from V. cholerae WT and ΔvchM strains. While ΔgroESL-2 and ΔvchM groESL-2 strains were easily obtained, we could not manage to delete groESL-1 in any background despite several attempts. Moreover, deletion of groESL-2 did not affect the growth of V. cholerae in rich medium (Fig 4A). Respectively, GroES-1 and GroEL-1 share 80% and 87% amino acid identity with the only and essential GroES and GroEL proteins of E. coli, but lower (66% and 76%) amino acid identity with GroES-2 and GroEL-2 (S3 Fig). These observations suggest that i) GroESL-1 (but not GroESL-2) is essential for V. cholerae viability and ii) GroESL-1 is probably the main housekeeping chaperonin system while the divergent GroESL-2 could act synergistically in response to high levels of misfolding or having specific substrates upon protein damage caused by specific stresses. Surprisingly, competition of ΔgroESL-2 with a lacZ- strain shows that loss of these proteins is not detrimental for growth of V. cholerae in presence of subMIC TOB (S4A Fig). Similarly, survival of the ΔgroESL-2 strain to lethal doses of TOB does not differ from that of the WT (S4B Fig). However, these genes are intrinsically highly expressed in ΔvchM strain, where they may confer a selective advantage in presence of AG stress. In this case, deletion of groESL-2 in ΔvchM background would affect the mutant’s tolerance. Indeed, when we compared the survival to lethal AG treatment of ΔvchM to that of a ΔvchM groESL-2 double mutant, we found that the absence of groESL-2 abolishes high tolerance to tobramycin and gentamicin in ΔvchM (Fig 4B), without affecting growth in absence of stress (Fig 4A). These results show that the higher expression of groESL-2 is required for the high tolerance of the ΔvchM mutant to lethal AG treatment. Importantly, we assessed whether groESL-2 locus is part of V. cholerae’s intrisinc stress response to AGs, and we observed induction of this locus by both subMIC and lethal concentrations of tobramycin (S5 Fig)”
Fig 4. groESL-2 is needed for the increased tolerance of ΔvchM to lethal AG treatment.
A. Growth curves in MH medium. Means and SD are represented, n = 3. B. Survival of stationary-phase WT, ΔvchM and ΔvchM ΔgroESL-2 cells exposed to lethal aminoglycoside treatment for 7 hours. Box plots indicate the median and the 25th and 75th percentiles; whiskers indicate the min and max values (n = 6 from two independent experiments). C. Survival (after 7 hours AG treatment) of ΔvchM groESL-2 double mutant harboring an empty plasmid or a plasmid expressing either groESL-1 or groESL-2, relative to survival of the ΔvchM with the control plasmid. Box plots indicate the median and the 25th and 75th percentiles; whiskers indicate the min and max values (n = 6 from two independent experiments). In B and C statistically significant differences were determined using Friedman’s test with Dunn’s post-hoc test for multiple comparisons. * P<0.05, ** P<0.01, ns = not significant. D. In vitro competitions of ΔvchM and ΔvchM groESL-2 double mutant strains against isogenic ΔlacZ reference strain in absence or presence of subMIC TOB, 0.6 μg/ml; Error bars indicate SD (n = 6).
We then tested whether the high tolerance of this mutant relies on general higher levels of chaperonins or if it specifically linked to GroESL-2 chaperonins. We thus tried to complement the ΔvchM groESL-2 mutant by ectopically expressing groESL-1 or groESL-2 and assessed survival to lethal doses of AGs. Strikingly, only overexpression of groESL-2 is able to promote survival levels similar to those observed in ΔvchM (Fig 4C), suggesting a specific role for GroESL-2 in managing AG-mediated proteotoxic stress in V. cholerae cells lacking VchM. Interestingly, we observed no difference in the relative fitness of ΔvchM and ΔvchM groESL-2 mutants in competitions in presence of subMIC doses of tobramycin, which shows that groESL-2 it is not implicated in ΔvchM higher relative fitness during growth in subMIC AGs (Fig 4D).
VchM controls groESL-2 expression in part through direct DNA methylation
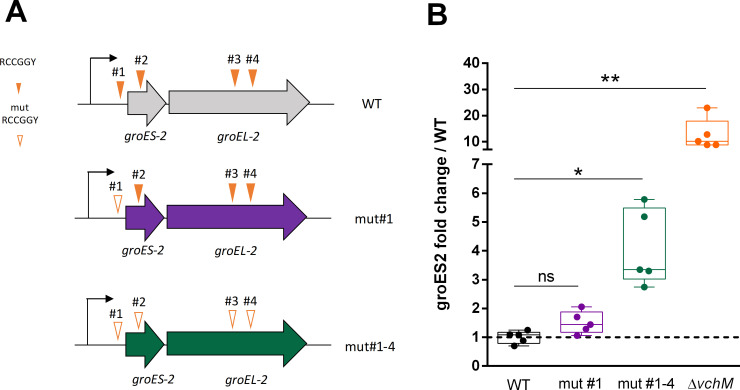
Knowing the role of VchM in regulating gene expression in V. cholerae [23], we asked whether VchM controls groESL expression directly through DNA methylation. VchM methylates the first cytosine in 5’-RCCGGY-3’ motifs [16]. This prompted us to search for such motifs in both groESL operons. While we couldn’t detect any of these sites along the groESL-1 locus, we found a total of four VchM motifs in groESL-2 region: motif #1 within the 5’ UTR of the operon, 47 bp away from the initiation codon; motif #2 is within the coding region of groES-2 while motifs #3 and #4 are located within the coding region of groEL-2 (Fig 5A). We hypothesized that the methylation state of these motifs could modulate the transcription of groESL-2 genes. To test this, we generated a mutant by replacing all RCCGGY motifs in groESL-2 region by non-consensus motifs but maintaining the amino acid sequence of GroESL-2 proteins intact (Fig 5A, mut#1–4). Additionally, we created a mutant where only the RCCGGY #1 was altered in order to investigate if this site, for being in the regulatory region of this operon, had a stronger contribution in modulating gene expression (Fig 5A, mut#1). We then measured groES-2 expression in both mutants and observed that disruption of RCCGGY #1 lead to a very weak increase in groES-2 expression relative to the WT, while disruption of all four sites led to a significantly higher expression of this gene (Fig 5B), although not as high as in the ΔvchM mutant. We additionally tested groEL-2 expression and observed similar results (S6 Fig). Supporting our hypothesis that this regulation is methylation-dependent, we did not observe any difference in groES-2 or groEL-2 expression when we mutated sites #1–4 in the ΔvchM background (S6 Fig). It is worth mentioning that, in these experiments, the expression of groES-2 in the ΔvchM strain was consistently higher than in the WT mut#1–4 (Fig 5B) suggesting that an additional factor (e.g. an unknown DNA methylation-sensitive transcription factor), in synergy with the methylation of RCCGGY sites, controls expression of groES-2. Nonetheless, overall these results show that a specific set of chaperonin encoding genes is under the control of DNA cytosine methylation in V. cholerae, linking DNA methylation to modulation of chaperonin expression and tolerance to antibiotics.
Fig 5. Disrupted VchM sites in groESL-2 region leads to increased gene expression in the WT.
A. Schematic representation of mutants with abrogated VchM sites. B. Relative expression of groES-2 in the different strains at OD600 of 1.0. Box plots indicate the median and the 25th and 75th percentiles; whiskers indicate the min and max values (n = 5). Statistical significance was determined by Kruskal-Wallis test with Dunn’s post-hoc test for multiple comparisons. * P<0.05, ** P<0.01, ns = not significant.
Discussion
Antimicrobial resistance (AMR) is currently one of the biggest threats to global health [35]. It is thus urgent to not only find new and alternative ways to fight bacterial infections but also to understand how bacteria adapt to the presence of antibiotics and study the molecular mechanisms they use to circumvent antibiotic action.
In this study, we establish a previously unknown link between VchM-mediated DNA methylation and aminoglycoside susceptibility in the human pathogen V. cholerae. VchM is a relatively understudied orphan DNA methyltransferase only found in V. cholerae species, known to methylate the first cytosine at 5’-RCCGGY-3’ DNA motifs [16,23]. VchM is necessary for the optimal growth of V. cholerae, both in vitro and in vivo, and it was shown to repress the expression of a gene important for cell envelope stability through direct DNA methylation [23].
Here we show that despite the growth defect in stress-free medium, cells lacking VchM are also less susceptible to aminoglycoside toxicity. Specifically, we show that these cells have a higher relative fitness in presence of low AG concentrations. The reason for this can be inferred from the growth curves in presence of subMIC TOB (Fig 1A) where it is clear that small increments in TOB concentration lead to a higher toxicity in the WT strain when compared to the ΔvchM. Moreover, even though the MIC values for the tested AGs are the same in both strains, ΔvchM displays a higher tolerance to lethal aminoglycoside treatment (Fig 2).
Aminoglycosides are a well-known class of antimicrobial drugs that cause disruption of the translation process and consequently protein misfolding [14,36]. The exact mechanism underlying the bactericidal activity of aminoglycosides has been subject of debate in the literature [37] but it is generally accepted that killing by AGs involves i) the uptake of the AG into the cytoplasm [11,38,39] and ii) membrane disruption mediated by insertion of misfolded proteins in the membrane as consequence of AG binding to the ribosomes and disruption of translational fidelity [11–13,40]. Indeed, mechanisms modulating aminoglycoside tolerance/resistance in different bacterial species (in exponential or stationary phase) have been shown to be associated either to AG uptake [41–44] or to translational fidelity and proteostasis [14,29,40,45]. Our results revealed a higher relative abundance of groESL-2 transcripts in bacterial cells lacking VchM, which led us to hypothesize that such increased expression of these chaperonins could underlie the high tolerance to AGs observed in this mutant, as it had been previously observed in E. coli [29]. In fact, we show that stationary phase cells lacking both vchM and groESL-2 genes have similar or even lower tolerance to lethal AG treatment compared to the WT strain. However, we could not observe a significant increase in tolerance upon overexpression of groESL-2 in the WT strain (S7 Fig), suggesting that high groESL-2 levels alone do not explain the high tolerance to lethal AG treatment. Instead, it is possible that high levels of GroESL-2 chaperone system counteract AG-mediated misfolding of specific substrates present only in cells devoid of VchM.
V. cholerae harbors two copies of groESL operon in its genome, thus belonging to the group of 30% of bacterial species that contains multiple copies of these chaperonins [46,47]. An interesting question to ask is whether these extra copies of chaperonins are functionally redundant or have a more specialized role in the cell, as it had been observed for Myxococcus xanthus [46–48]. Supporting the latter hypothesis, we show here that the high tolerance observed in ΔvchM is dependent on the high expression of groESL-2 but not on the high expression of groESL-1 (Fig 4B). Amino acid identity comparison between these proteins suggests that Vc GroESL-1 is likely the orthologue of the housekeeping GroESL of E. coli whereas Vc GroESL-2, thought to have appeared by duplication in V. cholerae [30], differ equally from both (S3 Fig). Thus, we speculate that V. cholerae GroESL-2 constitutes an alternative chaperone system capable of helping the folding of specific substrates important for survival to specific stresses. The existence of an alternative groESL-2 locus in V. cholerae that is needed for aminoglycoside tolerance only in certain contexts is a new finding and it paves the way for the study of potential alternative roles of otherwise thought redundant chaperonin systems in bacteria. It would be interesting, in future studies, to assess in depth how GroESL-2 interacts with specific substrates and which stress conditions demand one of the chaperonins in preference to the other.
Interestingly, even though essential for the higher tolerance to lethal aminoglycoside treatment, groESL-2 is not involved in the increased relative fitness of the ΔvchM mutant in presence of subMIC doses of aminoglycosides (Fig 4C). This suggests that the mechanisms operating in ΔvchM cells that increase their relative fitness during growth in subMIC AGs are not the same that increase their tolerance to lethal doses of these drugs. In fact, it has been recently shown that the type of translation errors occurring at lower streptomycin (another AG) concentrations differ from those found in high concentrations of this aminoglycoside, with the latter being associated to a higher misfolding propensity [12]. Thus, it seems plausible that, in ΔvchM cells, the higher expression of groESL-2 is likely to be more important at high concentrations of AGs, when the abundance of misfolded proteins tend to increase. The mechanisms driving ΔvchM higher relative fitness at lower doses of aminoglycosides remain to be elucidated in future work.
DNA methylation controls gene expression through modulation of protein-DNA interactions [49]. In most of the cases, the methylated base interferes with the binding of transcription factors and/or the RNA polymerase at the regulatory region of a gene, affecting transcription [50–52]. However, there is also evidence that the presence of methylated DNA bases that occur along the coding region of genes could also directly affect their expression in bacteria, even though the precise mechanism is still unknown [20,22,23]. In eukaryotes, cytosine methylation tends to repress gene expression. A recent study shedding light on how cytosine methylation affect DNA mechanical properties shows that cytosine methylation stabilizes the DNA helix and slows transcription in eukaryotic cells [53]. Thus, a similar m5C-mediated transcriptional hindrance is likely to happen also in prokaryotes. Here we support this view by showing that abrogation of VchM-dependent methylation of cytosines at the four RCCGGY motifs in groESL-2 region increased its expression in WT cells (Fig 5B). However, this in unlikely the sole mechanism responsible for the high expression of groESL-2 genes in ΔvchM cells, as this mutant has even higher expression levels of groESL-2. This suggests that the pleiotropic effects resulting from VchM deficiency also affect, indirectly, the expression of these genes maybe through regulation of a methylation sensitive transcription factor. Thus, VchM controls groESL-2 expression in part in a direct and in an indirect way.
Our work shows that a V. cholerae deletion mutant of the orphan DNA methyltransferase VchM have a general higher tolerance towards aminoglycosides. It remains to be explored whether V. cholerae WT cells can modulate VchM expression and, consequently, alter the levels of cytosine methylation. Bisulfite sequencing analysis of V. cholerae genome shows that all cytosines within RCCGGY motifs were methylated in V. cholerae, during exponential and stationary phases, with the exception of three of these sites which had been previously shown to be constantly undermethylated in this species [54]. However, these studies were conducted in cells cultured in LB stress-free media or collected from frozen rabbit cecal fluid, and thus may not reflect the m5C profile of V. cholerae during other stress conditions. Moreover, bisulfite sequencing allows for cytosine methylation analysis of the total population at a specific time and thus it is not suitable to detect potential transient changes in small subpopulations of cells. Such changes could be mediated, for example, by altering the levels of VchM through gene expression. Little is known about vchM regulation but it was recently shown that the V. cholerae quorum sensing low density transcriptional regulator AphA is able to bind the vchM region [55] leaving the possibility that vchM may be regulated by quorum sensing. Moreover, vchM was previously found to be differentially expressed between different stages of human infection [56], suggesting the possibility that modulation of cytosine methylation levels can be adaptative during V. cholerae’s life cycle. In line with our work, lowering VchM levels could lead to a trade-off, where low m5C levels would be detrimental for fitness in stress-free contexts, but highly advantageous in presence of specific stress conditions, such as antibiotic exposure.
With this study, we show for the first time that in V. cholerae (and potentially other clinically relevant species) the high expression of specialized chaperonins are crucial in certain conditions where specific proteins may be affected by AGs. Moreover, we show here that there is a link between chaperonin expression and DNA cytosine methylation, which has not previously been observed, thus linking DNA methylation levels with response to proteotoxic stress.
Materials and methods
Strains, media and culture conditions
V. cholerae was routinely cultured at 37°C in Mueller-Hinton (MH) medium. Plasmids were introduced in V. cholerae by electrotransformation. Strains containing the pSC101 plasmid were grown in presence of 100 μg/mL carbenicilin for plasmid maintenance. All V. cholerae mutant strains are derived from Vibrio cholerae serotype O1 biotype El Tor strain N16961 hapR+. Mutants were constructed by homologous recombination after natural transformation or with a conjugative suicide plasmid as previously described [15,57–59]. Primers, strains and plasmids used in this study, and their constructions, are listed in S2 Table. For routine cloning we used chemically competent E. coli One Shot TOP10 (Invitrogen). All strains and plasmids were confirmed by sanger sequencing.
Mutation of RCCGGY sites #1–4 in groESL-2 region
In order to mutate all four RCCGGY sites present in groESL-2 (vca0819-0820) region we generated a DNA fragment (S2 Table) with these sites containing the following nucleotide changes: #1- ACCGGC changed to ATCGGC; #2- ACCGGC changed to ACGGGC; #3- GCCGGC changed to GCGGGC and #4- ACCGGC changed to ACGGGC. This fragment was then introduced in V. cholerae at the endogenous locus by allelic replacement as described in S2 Table.
Growth curves
Overnight cultures from single colonies were diluted 1:100 in Mueller-Hinton (MH) rich media or MH + subMIC antibiotics at different concentrations, in 96-well microplates. OD600 was measured in a Tecan Infinite plate reader at 37°C, with shaking for 20 hours. Measurements were taken every 10 minutes.
MIC determination
MICs were determined by microtiter broth dilution method [60] with an initial inoculum size of 105 CFUs/mL. The MIC was interpreted as the lowest antibiotic concentration preventing visible growth.
Neo-Cy5 uptake
Quantification of fluorescent neomycin (Neo-cy5) uptake was performed as described [61]. Neo-cy5 is an aminoglycoside coupled to the fluorophore Cy5, and has been shown to be active against Gram- bacteria [27]. Briefly, overnight cultures were diluted 100-fold in rich MOPS (Teknova EZ rich defined medium). When the bacterial cultures reached an OD600 of 0.25, they were incubated with 0.4 μM of Cy5 labeled Neomycin for 15 minutes at 37°C. 10 μL of the incubated culture were then used for flow cytometry, diluting them in 250 μL of PBS before reading fluorescence. Flow cytometry experiments were performed as described [62]. For each experiment, 100000 events were counted on the Miltenyi MACSquant device.
Competitions experiments
Overnight cultures from single colonies of lacZ- and lacZ+ strains were washed in PBS (Phosphate Buffer Saline) and mixed 1:1 (500μl + 500μl). At this point 100μl of the mix were serial diluted and plated in MH agar supplemented with X-gal (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) at 40 μg/mL to assess T0 initial 1:1 ratio. At the same time, 10 μl from the mix were added to 2 mL of MH or MH supplemented with subMIC tobramycin at 0.6μg/mL and incubated with agitation at 37°C for 20 hours. Cultures were then diluted and plated in MH agar plates supplemented with X-gal. Plates were incubated overnight at 37°C and the number of blue and white CFUs was assessed. Competitive index was calculated by dividing the number of blue CFUs (lacZ+ strain) by the number of white CFUs (lacZ- strain) and normalizing this ratio to the T0 initial ratio.
Survival assays
Bacterial cultures from single colonies were cultured at 37°C for 16 h with agitation in 10 mL of MH medium. Aliquots from these cultures were removed, serial diluted and plated in MH agar plates to assess CFUs formation prior antibiotic treatment (T0). In addition, 5 mL of these aliquots were subjected to antibiotic treatment and incubated with agitation at 37°C. At the indicated time points, 500uL of these cultures were collected, washed in PBS, serial diluted and plated in MH agar plates. The plates were then incubated overnight at 37°C. Survival at each time point was determined by dividing the number of CFUs/mL at that time point by the number of CFUs/mL prior treatment. Antibiotics were used at the following final concentrations: 20 μg/mL Tobramycin (TOB) and 10 μg/mL Gentamicin (GEN). Experiments were repeated at least two to three times.
Digital qRT-PCR
For RNA extraction, overnight cultures of three biological replicates of strains of interest were diluted 1:1000 in MH media and grown with agitation at 37°C until an OD600 of 0.3 (exponential phase) or an OD600 of 1.0 or 2.0 (stationary phase). 0.5 mL of these cultures were centrifuged and supernatant removed. Pellets were homogenized by resuspension with 1.5 mL of cold TRIzol Reagent. Next, 300 μL chloroform were added to the samples following mix by vortexing. Samples were then centrifuged at 4°C for 10 minutes. Upper (aqueous) phase was transferred to a new 2mL tube and mixed with 1 volume of 70% ethanol. From this point, the homogenate was loaded into a RNeasy Mini kit (Quiagen) column and RNA purification proceeded according to the manufacturer’s instructions. Samples were then subjected to DNase treatment using TURBO DNA-free Kit (Ambion) according to the manufacturer’s instructions. RNA concentration of the samples was measured with NanoDrop spectrophotometer and diluted to a final concentration of 1–10 ng/μL.
qRT-PCR reactions were prepared with 1 μL of diluted RNA samples using the qScript XLT 1-Step RT-qPCR ToughMix (Quanta Biosciences, Gaithersburg, MD, USA) within Sapphire chips. Digital PCR was conducted on a Naica Geode (programmed to perform the sample partitioning step into droplets, followed by the thermal cycling program suggested in the user’s manual. Primer and probe sequences used in digital qRT-PCR reaction are listed in S3 Table. Image acquisition was performed using the Naica Prism3 reader. Images were then analyzed using Crystal Reader software (total droplet enumeration and droplet quality control) and the Crystal Miner software (extracted fluorescence values for each droplet). Values were normalized against expression of the housekeeping gene gyrA as previously described [31].
RNA-seq
For RNA extraction, overnight cultures of three biological replicates of WT and ΔvchM strains were diluted 1:100 in MH medium and grown with agitation at 37°C until cultures reach an OD600 of 2.0. Total RNA extraction, library preparation, sequencing and analysis were performed as previously described [63]. The data for this RNA-seq study has been submitted in the GenBank Sequence Read Archive (SRA) under project number PRJNA509113.
Supporting information
(PDF)
(PDF)
(PDF)
Survival of exponential phase cells (OD600 0.3–0.4) of WT and ΔvchM strains exposed to lethal doses of tobramycin (TOB) for a period of 3.5 hours. The Y-axis represents the log10 CFUs/mL after 3.5 hours growth with 0, 3, 5 or 10 μg/mL of Tobramycin (3x, 5x or 10x higher than the MIC, respectively). Means and SD are represented, n = 3.
(TIF)
Percentage of neo-cy5 positive cells analyzed by flow cytometry after incubation with fluorescent marked neomycin. Means and SD are represented, n = 3.
(TIF)
Amino acid identity between GroES and GroEL proteins of E. coli MG1655 (Eco) and V. cholerae O1 El Tor N16961 (Vch) computed by BLASTP. Values represent percentage identity between proteins.
(TIF)
A. In vitro competitions of WT and ΔgroESL-2 strains against isogenic ΔlacZ reference strain in absence or presence of tobramycin (TOB) at 0.6 μg/mL; n = 3, error bars indicate SD. B. Survival of stationary-phase WT and ΔgroESL-2 cells exposed to 20X MIC of tobramycin. n = 3, error bars indicate SD.
(TIF)
Relative expression of groES-2 in WT cells growing in MH, MH with subMIC tobramycin (≈ 20% MIC) or exponential phase cells following lethal TOB treatment (5X MIC) for a period of 30 minutes. n = 3, error bars indicate SD.
(TIF)
Relative expression of groES-2 and groEL-2 genes in the indicated strains grown at OD600 1.0. n = 3, error bars indicate SD.
(TIF)
Survival (after 7 hours TOB treatment) of WT strain carrying a control plasmid or a plasmid overexpressing groESL-2 genes. n = 6, error bars indicate SD.
(TIF)
Acknowledgments
We are thankful to Manon Lang for her valuable help with the neo-cy5 uptake experiments, Dominique Fourmy for the gift of neo-Cy5, and Sebastian Aguilar Pierlé for help with RNAseq analysis. We thank Claudia Chica and Christophe Becavin (Bioinformatics and Biostatistics Hub, Department of computational biology, USR 3756 CNRS, Institut Pasteur) for DNA bisulfite sequencing analysis on stationary phase cultures. We also thank Evelyne Krin for help with molecular cloning procedures and João Gama for helpful comments on the manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by the Institut Pasteur (D. M., Z. B.), the Centre National de la Recherche Scientifique (D.M) (CNRS-UMR 3525), the Fondation pour la Recherche Médicale (D.M, FRM Grant No. DBF20160635736), ANR Unibac (D. M., ANR-17-CE13-0010-01) and Institut Pasteur grant PTR 245-19 (Z. B.). A. C. is part of the Pasteur - Paris University (PPU) International PhD Program, which has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement No 665807. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liu YC, Huang WK, Huang TS, Kunin CM. Detection of antimicrobial activity in urine for epidemiologic studies of antibiotic use. J Clin Epidemiol. 1999. doi: 10.1016/s0895-4356(99)00027-x [DOI] [PubMed] [Google Scholar]
- 2.Haggard BE, Bartsch LD. Net Changes in Antibiotic Concentrations Downstream from an Effluent Discharge. J Environ Qual. 2009. doi: 10.2134/jeq2007.0540 [DOI] [PubMed] [Google Scholar]
- 3.Fick J, Söderström H, Lindberg RH, Phan C, Tysklind M, Larsson DGJ. Contamination of surface, ground, and drinking water from pharmaceutical production. Environ Toxicol Chem. 2009. doi: 10.1897/09-073.1 [DOI] [PubMed] [Google Scholar]
- 4.Andersson DI, Hughes D. Microbiological effects of sublethal levels of antibiotics. Nature Reviews Microbiology. 2014. doi: 10.1038/nrmicro3270 [DOI] [PubMed] [Google Scholar]
- 5.Davies J, Spiegelman GB, Yim G. The world of subinhibitory antibiotic concentrations. Curr Opin Microbiol. 2006;9: 445–453. doi: 10.1016/j.mib.2006.08.006 [DOI] [PubMed] [Google Scholar]
- 6.Wistrand-Yuen E, Knopp M, Hjort K, Koskiniemi S, Berg OG, Andersson DI. Evolution of high-level resistance during low-level antibiotic exposure. Nat Commun. 2018. doi: 10.1038/s41467-018-04059-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jørgensen KM, Wassermann T, Jensen PØ, Hengzuang W, Molin S, Høiby N, et al. Sublethal ciprofloxacin treatment leads to rapid development of high-level ciprofloxacin resistance during long-term experimental evolution of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013. doi: 10.1128/AAC.00493-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gullberg E, Cao S, Berg OG, Ilbäck C, Sandegren L, Hughes D, et al. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011. doi: 10.1371/journal.ppat.1002158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baharoglu Z, Mazel D. Vibrio cholerae triggers SOS and mutagenesis in response to a wide range of antibiotics: A route towards multiresistance. Antimicrob Agents Chemother. 2011. doi: 10.1128/AAC.01549-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baharoglu Z, Babosan A, Mazel D. Identification of genes involved in low aminoglycoside-induced SOS response in Vibrio cholerae: A role for transcription stalling and Mfd helicase. Nucleic Acids Res. 2014. doi: 10.1093/nar/gkt1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis BD. Mechanism of bactericidal action of aminoglycosides. Microbiological Reviews. 1987. doi: 10.1128/mr.51.3.341-350.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wohlgemuth I, Garofalo R, Samatova E, Günenç AN, Lenz C, Urlaub H, et al. Translation error clusters induced by aminoglycoside antibiotics. Nat Commun. 2021. doi: 10.1038/s41467-021-21942-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohanski MA, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. Mistranslation of Membrane Proteins and Two-Component System Activation Trigger Antibiotic-Mediated Cell Death. Cell. 2008;135: 679–690. doi: 10.1016/j.cell.2008.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling J, Cho C, Guo LT, Aerni HR, Rinehart J, Söll D. Protein Aggregation Caused by Aminoglycoside Action Is Prevented by a Hydrogen Peroxide Scavenger. Mol Cell. 2012;48: 713–722. doi: 10.1016/j.molcel.2012.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Negro V, Krin E, Pierlé SA, Chaze T, Gianetto QG, Kennedy SP, et al. RadD contributes to R-Loop avoidance in Sub-MIC tobramycin. MBio. 2019. doi: 10.1128/mBio.01173-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Banerjee S, Chowdhury R. An orphan {DNA} (cytosine-5-)-methyltransferase in Vibrio cholerae. Microbiology. 2006;152: 1055–1062. doi: 10.1099/mic.0.28624-0 [DOI] [PubMed] [Google Scholar]
- 17.Fujimoto D, Srinivasan PR, Borek E. On the Nature of the Deoxyribonucleic Acid Methylases. Biological Evidence for the Multiple Nature of the Enzymes. Biochemistry. 1965. doi: 10.1021/bi00888a041 [DOI] [PubMed] [Google Scholar]
- 18.Casadesús J, Low D. Epigenetic Gene Regulation in the Bacterial World. Microbiol Mol Biol Rev. 2006. doi: 10.1128/MMBR.00016-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sánchez-Romero MA, Casadesús J. The bacterial epigenome. Nature Reviews Microbiology. 2020. doi: 10.1038/s41579-019-0286-2 [DOI] [PubMed] [Google Scholar]
- 20.Kumar S, Karmakar BC, Nagarajan D, Mukhopadhyay AK, Morgan RD, Rao DN. N4-cytosine DNA methylation regulates transcription and pathogenesis in Helicobacter pylori. Nucleic Acids Res. 2018. doi: 10.1093/nar/gky126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estibariz I, Overmann A, Ailloud F, Krebes J, Josenhans C, Suerbaum S. The core genome m5C methyltransferase JHP1050 (M.Hpy99III) plays an important role in orchestrating gene expression in Helicobacter pylori. Nucleic Acids Res. 2019;47: 2336–2348. doi: 10.1093/nar/gky1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Militello KT, Simon RD, Qureshi M, Maines R, van Horne ML, Hennick SM, et al. Conservation of Dcm-mediated cytosine DNA methylation in Escherichia coli. FEMS Microbiol Lett. 2012. doi: 10.1111/j.1574-6968.2011.02482.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao MC, Zhu S, Kimura S, Davis BM, Schadt EE, Fang G, et al. A Cytosine Methytransferase Modulates the Cell Envelope Stress Response in the Cholera Pathogen. PLoS Genet. 2015;11: e1005666. doi: 10.1371/journal.pgen.1005666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brauner A, Fridman O, Gefen O, Balaban NQ. Distinguishing between resistance, tolerance and persistence to antibiotic treatment. Nature Reviews Microbiology. 2016. doi: 10.1038/nrmicro.2016.34 [DOI] [PubMed] [Google Scholar]
- 25.Haugan MS, Løbner-Olesen A, Frimodt-Møller N. Comparative activity of ceftriaxone, ciprofloxacin, and gentamicin as a function of bacterial growth rate probed by Escherichia coli chromosome replication in the mouse peritonitis model. Antimicrob Agents Chemother. 2019. doi: 10.1128/AAC.02133-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakae R, Nakae T. Diffusion of aminoglycoside antibiotics across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 1982. doi: 10.1128/AAC.22.4.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabeti Azad M, Okuda M, Cyrenne M, Bourge M, Heck MP, Yoshizawa S, et al. Fluorescent Aminoglycoside Antibiotics and Methods for Accurately Monitoring Uptake by Bacteria. ACS Infect Dis. 2020. doi: 10.1021/acsinfecdis.9b00421 [DOI] [PubMed] [Google Scholar]
- 28.Hartl FU, Hayer-Hartl M. Protein folding. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science. 2002;295: 1852–1858. doi: 10.1126/science.1068408 [DOI] [PubMed] [Google Scholar]
- 29.Goltermann L, Good L, Bentin T. Chaperonins fight aminoglycoside-induced protein misfolding and promote short-term tolerance in Escherichia coli. J Biol Chem. 2013. doi: 10.1074/jbc.M112.420380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chowdhury N, Kingston JJ, Brian Whitaker W, Carpenter MR, Cohen A, Fidelma Boyd E. Sequence and expression divergence of an ancient duplication of the chaperonin groESEL operon in Vibrio species. Microbiol (United Kingdom). 2014;160: 1953–1963. doi: 10.1099/mic.0.079194-0 [DOI] [PubMed] [Google Scholar]
- 31.Lo Scrudato M, Blokesch M. The regulatory network of natural competence and transformation of Vibrio cholerae. PLoS Genet. 2012. doi: 10.1371/journal.pgen.1002778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richter K, Haslbeck M, Buchner J. The Heat Shock Response: Life on the Verge of Death. Molecular Cell. 2010. doi: 10.1016/j.molcel.2010.10.006 [DOI] [PubMed] [Google Scholar]
- 33.Slamti L, Livny J, Waldor MK. Global gene expression and phenotypic analysis of a Vibrio cholerae rpoH deletion mutant. J Bacteriol. 2007. doi: 10.1128/JB.01297-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fayet O, Ziegelhoffer T, Georgopoulos C. The groES and groEL heat shock gene products of Escherichia coli are essential for bacterial growth at all temperatures. J Bacteriol. 1989;171: 1379–1385. doi: 10.1128/jb.171.3.1379-1385.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Neill J. Tackling a Crisis for the Health and Wealth of Nations. In: The Review on Antimicrobial Resistance. 2015. doi: 10.1038/510015a [DOI] [PubMed] [Google Scholar]
- 36.Magnet S, Blanchard JS. Molecular insights into aminoglycoside action and resistance. Chem Rev. 2005;105: 477–497. doi: 10.1021/cr0301088 [DOI] [PubMed] [Google Scholar]
- 37.Baquero F, Levin BR. Proximate and ultimate causes of the bactericidal action of antibiotics. Nature Reviews Microbiology. 2021. doi: 10.1038/s41579-020-00443-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ezraty B, Vergnes A, Banzhaf M, Duverger Y, Huguenot A, Brochado AR, et al. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science. 2013. doi: 10.1126/science.1238328 [DOI] [PubMed] [Google Scholar]
- 39.Bruni GN, Kralj JM. Membrane voltage dysregulation driven by metabolic dysfunction underlies bactericidal activity of aminoglycosides. Elife. 2020. doi: 10.7554/eLife.58706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou J, Zhang W, Zhang H, Zhang XD, Peng B, Zheng J. Studies on aminoglycoside susceptibility identify a novel function of KsgA to secure translational fidelity during antibiotic stress. Antimicrob Agents Chemother. 2018;62: 1–13. doi: 10.1128/AAC.00853-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shan Y, Lazinski D, Rowe S, Camilli A, Lewis K. Genetic basis of persister tolerance to aminoglycosides in Escherichia coli. MBio. 2015;6: 1–10. doi: 10.1128/mBio.00078-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allison KR, Brynildsen MP, Collins JJ. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature. 2011. doi: 10.1038/nature10069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McKay SL, Portnoy DA. Ribosome hibernation facilitates tolerance of stationary-phase bacteria to aminoglycosides. Antimicrob Agents Chemother. 2015;59: 6992–6999. doi: 10.1128/AAC.01532-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall CW, Farkas E, Zhang L, Mah TF. Potentiation of Aminoglycoside Lethality by C4-Dicarboxylates Requires RpoN in Antibiotic-Tolerant Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2019. doi: 10.1128/AAC.01313-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji X, Zou J, Peng H, Stolle AS, Xie R, Zhang H, et al. Alarmone Ap4A is elevated by aminoglycoside antibiotics and enhances their bactericidal activity. Proc Natl Acad Sci U S A. 2019. doi: 10.1073/pnas.1822026116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar CMS, Mande SC, Mahajan G. Multiple chaperonins in bacteria—novel functions and non-canonical behaviors. Cell Stress Chaperones. 2015;20: 555–574. doi: 10.1007/s12192-015-0598-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goyal K, Qamra R, Mande SC. Multiple gene duplication and rapid evolution in the groEL Gene: Functional implications. J Mol Evol. 2006. doi: 10.1007/s00239-006-0037-7 [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Zhang W yan, Zhang Z, Li J, Li Z feng, gao Tan Z, et al. Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in Myxococcus xanthus DK1622. PLoS Genet. 2013. doi: 10.1371/journal.pgen.1003306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casadesus J, Low D. Epigenetic Gene Regulation in the Bacterial World. Microbiol Mol Biol Rev. 2006. doi: 10.1128/MMBR.00016-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernday AD, Braaten BA, Low DA. The mechanism by which DNA adenine methylase and PapI activate the Pap epigenetic switch. Mol Cell. 2003. doi: 10.1016/s1097-2765(03)00383-6(03)00383–6 [DOI] [PubMed] [Google Scholar]
- 51.Cota I, Sánchez-Romero MA, Hernández SB, Pucciarelli MG, García-Del Portillo F, Casadesús J. Epigenetic Control of Salmonella enterica O-Antigen Chain Length: A Tradeoff between Virulence and Bacteriophage Resistance. PLoS Genet. 2015. doi: 10.1371/journal.pgen.1005667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wion D, Casadesús J. N6-methyl-adenine: an epigenetic signal for DNA-protein interactions. Nature reviews. Microbiology. 2006. doi: 10.1038/nrmicro1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rausch C, Zhang P, Casas-Delucchi CS, Daiß JL, Engel C, Coster G, et al. Cytosine base modifications regulate DNA duplex stability and metabolism. Nucleic Acids Res. 2021. doi: 10.1093/nar/gkab509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dalia AB, Lazinski DW, Camilli A. Characterization of undermethylated sites in vibrio cholerae. J Bacteriol. 2013. doi: 10.1128/JB.02112-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haycocks JRJ, Warren GZL, Walker LM, Chlebek JL, Dalia TN, Dalia AB, et al. The quorum sensing transcription factor AphA directly regulates natural competence in Vibrio cholerae. PLoS Genet. 2019. doi: 10.1371/journal.pgen.1008362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LaRocque RC, Harris JB, Dziejman M, Li X, Khan AI, Faruque ASG, et al. Transcriptional profiling of Vibrio cholerae recovered directly from patient specimens during early and late stages of human infection. Infect Immun. 2005. doi: 10.1128/IAI.73.8.4488-4493.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baharoglu Z, Krin E, Mazel D. Connecting environment and genome plasticity in the characterization of transformation-induced {SOS} regulation and carbon catabolite control of the Vibrio cholerae integron integrase. J Bacteriol. 2012;194: 1659–1667. doi: 10.1128/JB.05982-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baharoglu Z, Krin E, Mazel D. RpoS Plays a Central Role in the SOS Induction by Sub-Lethal Aminoglycoside Concentrations in Vibrio cholerae. PLoS Genet. 2013. doi: 10.1371/journal.pgen.1003421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Val ME, Skovgaard O, Ducos-Galand M, Bland MJ, Mazel D. Genome engineering in Vibrio cholerae: A feasible approach to address biological issues. PLoS Genet. 2012. doi: 10.1371/journal.pgen.1002472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard—7th Edition. Wayne, PA:Clinical and Laboratory Standards Institute. CLSI; M7–A7. 2006. [Google Scholar]
- 61.Lang M, Krin E, Korlowski C, Sismeiro O, Varet H, Coppée J-Y, et al. Sleeping ribosomes: Bacterial signaling triggers RaiA mediated persistence to aminoglycosides. iScience. 2021;24: 103128. doi: 10.1016/j.isci.2021.103128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baharoglu Z, Bikard D, Mazel D. Conjugative {DNA} transfer induces the bacterial {SOS} response and promotes antibiotic resistance development through integron activation. {PLoS} Genet. 2010;6: e1001165. doi: 10.1371/journal.pgen.1001165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krin E, Pierlé SA, Sismeiro O, Jagla B, Dillies MA, Varet H, et al. Expansion of the SOS regulon of Vibrio cholerae through extensive transcriptome analysis and experimental validation. BMC Genomics. 2018. doi: 10.1186/s12864-018-4716-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
Survival of exponential phase cells (OD600 0.3–0.4) of WT and ΔvchM strains exposed to lethal doses of tobramycin (TOB) for a period of 3.5 hours. The Y-axis represents the log10 CFUs/mL after 3.5 hours growth with 0, 3, 5 or 10 μg/mL of Tobramycin (3x, 5x or 10x higher than the MIC, respectively). Means and SD are represented, n = 3.
(TIF)
Percentage of neo-cy5 positive cells analyzed by flow cytometry after incubation with fluorescent marked neomycin. Means and SD are represented, n = 3.
(TIF)
Amino acid identity between GroES and GroEL proteins of E. coli MG1655 (Eco) and V. cholerae O1 El Tor N16961 (Vch) computed by BLASTP. Values represent percentage identity between proteins.
(TIF)
A. In vitro competitions of WT and ΔgroESL-2 strains against isogenic ΔlacZ reference strain in absence or presence of tobramycin (TOB) at 0.6 μg/mL; n = 3, error bars indicate SD. B. Survival of stationary-phase WT and ΔgroESL-2 cells exposed to 20X MIC of tobramycin. n = 3, error bars indicate SD.
(TIF)
Relative expression of groES-2 in WT cells growing in MH, MH with subMIC tobramycin (≈ 20% MIC) or exponential phase cells following lethal TOB treatment (5X MIC) for a period of 30 minutes. n = 3, error bars indicate SD.
(TIF)
Relative expression of groES-2 and groEL-2 genes in the indicated strains grown at OD600 1.0. n = 3, error bars indicate SD.
(TIF)
Survival (after 7 hours TOB treatment) of WT strain carrying a control plasmid or a plasmid overexpressing groESL-2 genes. n = 6, error bars indicate SD.
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.